Metal Chelation Therapy and Parkinson’s Disease: A Critical Review on the Thermodynamics of Complex Formation between Relevant Metal Ions and Promising or Established Drugs
Abstract
1. Introduction
2. Parkinson’s Disease and Metal Ions
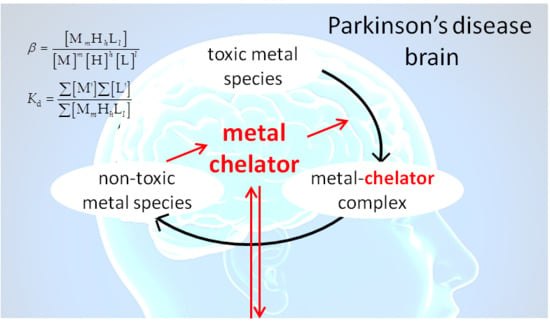
3. Metal Chelation Therapy in Parkinson’s Disease
4. The Measurement of the Stability of Metal–Ligand Complexes
5. The Metal–Ligand Speciation of Anti-Parkinson Drugs
6. Possible Usages of Speciation Data for Metal Chelation Therapy against Parkinson’s Disease
7. Concluding Remarks
Supplementary Materials
Funding
Conflicts of Interest
References
- Kalia, L.V.; Lang, A.E.; Shulman, G. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Tan, S.H.; Karri, V.; Tay, N.W.R.; Chang, K.H.; Ah, H.Y.; Ng, P.Q.; Ho, H.S.; Keh, H.W.; Candasamy, M. Emerging pathways to neurodegeneration: Dissecting the critical molecular mechanisms in Alzheimer’s disease and Parkinson’s disease. Biomed. Pharmacother. 2019, 111, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected, number of people with Parkinson disease in the most populous, nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study. Lancet Neurol. 2015, 16, 877–897. [Google Scholar]
- Dorsey, E.R.; Bloem, B. The Parkinson Pandemic-A Call to Action. JAMA Neurol. 2018, 75, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Savica, R.; Grossardt, B.R.; Bower, J.H.; Ahlskog, J.E.; Rocca, W.A. Time Trends in the Incidence of Parkinson Disease. JAMA Neurol. 2016, 73, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Scheperjans, F.; Pekkonen, E.; Kaakkola, S.; Auvinen, P. Linking smoking, coffee, urate, and Parkinson’s disease—A role for gut microbiota? J. Park. Dis. 2015, 5, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S. Environmental toxins and Parkinson’s disease. Ann. Rev. Pharmacol. Toxicol. 2014, 54, 141–164. [Google Scholar] [CrossRef]
- Van Den Eeden, S.K.; Tanner, C.M.; Bernstein, A.L.; Fross, R.D.; Leimpeter, A.; Bloch, D.A.; Nelson, L.M. Incidence of Parkinson’s disease: Variation by age, gender, and Race/Ethnicity. Am. J. Epidemiol. 2003, 157, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Stejskal, V.; Urbina, M.A.; Dadar, M.; Chirumbolo, S.; Mutter, J. Metals and Parkinson’s Disease: Mechanisms and Biochemical Processes. Curr. Med. Chem. 2018, 25, 2198–2214. [Google Scholar] [CrossRef] [PubMed]
- Savelieff, M.G.; Nam, G.; Kang, J.; Lee, H.J.; Lee, M.; Lim, M.H. Development of Multifunctional Molecules as Potential Therapeutic Candidates for Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis in the Last Decade. Chem. Rev. 2019, 119, 1221–1322. [Google Scholar] [CrossRef]
- Lhermitte, J.; Kraus, W.M.; McAlpine, D. On the occurrence of abnormal deposits of iron in the brain in parkinsonism with special reference to its localisation. J. Neurol. Psychopathol. 1924, 5, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W. Parkinson’s disease and parkinsonism: Neuropathology. Cold Spring Harb. Perspect. Med. 2012, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sofic, E.; Paulus, W.; Jellinger, K.; Riederer, P.; Youdim, M.B.H. Selective increase of iron in substantia nigra zona compacta of Parkinsonian brains. J. Neurochem. 1991, 56, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.; Paulus, W.; Grundke-Iqbal, I.; Riederer, P.; Youdim, M.B.H. Brain iron and ferritin in Parkinson’s and Alzheimer’s diseases. J. Neural Transm. Park. Dis. Dement. Sec. 1990, 2, 327–340. [Google Scholar] [CrossRef]
- Dexter, D.T.; Wells, F.R.; Lees, A.J.; Agid, F.; Agid, Y.; Jenner, P.; Marsden, C.D. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J. Neurochem. 1989, 52, 1830–1836. [Google Scholar] [CrossRef] [PubMed]
- Sofic, E.; Riederer, P.; Heinsen, H.; Beckmann, H.; Reynolds, G.P.; Hebenstreit, G.; Youdim, M.B. Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain. J. Neural Transm. 1988, 74, 199–205. [Google Scholar] [CrossRef]
- Drayer, B.P.; Burger, P.; Darwin, R.; Riederer, S.; Herfkens, R.; Johnson, G.A. Magnetic resonance imaging of brain iron. Am. J. Neuroradiol. 1986, 7, 373–380. [Google Scholar]
- Friedman, A.; Galazka-Friedman, J.; Koziorowski, D. Iron as a cause of Parkinson disease-a myth or a well established hypothesis? Park. Relat. Disord. 2009, 15, S212–S214. [Google Scholar] [CrossRef]
- Ryvlin, P.; Broussolle, E.; Piollet, H.; Viallet, F.; Khalfallah, Y.; Chazot, G. Magnetic resonance imaging evidence of decreased putamenal iron content in idiopathic Parkinson’s disease. Arch. Neurol. 1995, 52, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Q.; Chen, Y.T.; Zhang, Y.; Wang, F.R.; Yu, H.C.; Zhang, C.Y.; Jian, Z.; Luo, W.F. Iron deposition in Parkinson’s disease by quantitative susceptibility mapping. BMC Neurosci. 2019, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Dashtipour, K.; Liu, M.; Kani, C.; Dalaie, P.; Obenaus, A.; Simmons, D.; Gatto, N.M.; Zarifi, M. Iron Accumulation Is Not Homogenous among Patients with Parkinson’s Disease. Park. Dis. 2015, 2015, 324843. [Google Scholar] [CrossRef] [PubMed]
- Gumienna-Kontecka, E.; Pyrkosz-Bulska, M.; Szebesczyk, A.; Ostrowska, M. Iron Chelating Strategies in Systemic Metal Overload, Neurodegeneration and Cancer. Curr. Med. Chem. 2014, 21, 3741–3767. [Google Scholar] [CrossRef] [PubMed]
- Ayton, S.; Lei, P.; Duce, J.A.; Wong, B.X.W.; Sedjahtera, A.; Adlard, P.A.; Bush, A.I.; Finkelstein, D.I. Ceruloplasmin dysfunction and therapeutic potential for Parkinson disease. Ann. Neurol. 2013, 73, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, X.P. Does Ceruloplasmin Defend Against Neurodegenerative Diseases? Curr. Neuropharmacol. 2019, 17, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Ayton, S.; Finkelstein, D.I.; Spoerri, L.; Ciccotosto, G.D.; Wright, D.K.; Wong, B.X.W.; Adlard, P.A.; Cherny, R.A.; Lam, L.Q.; et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat. Med. 2012, 18, 291–295. [Google Scholar] [CrossRef]
- McCarthy, R.C.; Park, Y.H.; Kosman, D.J. sAPP modulates iron efflux from brain microvascular endothelial cells by stabilizing the ferrous iron exporter ferroportin. EMBO Rep. 2014, 15, 809–815. [Google Scholar] [CrossRef]
- Zecca, L.; Zucca, F.A.; Albertini, A.; Rizzio, E.; Fariello, R.G. A proposed dual role of neuromelanin in the pathogenesis of Parkinson’s disease. Neurology 2006, 67, S8–S11. [Google Scholar] [CrossRef]
- Genoud, S.; Roberts, B.R.; Gunn, A.P.; Halliday, G.M.; Lewis, S.J.G.; Ball, H.J.; Hare, J.; Double, K.L. Subcellular compartmentalisation of copper, iron, manganese, and zinc in the Parkinson’s disease brain. Metallomics 2017, 9, 1447–1455. [Google Scholar] [CrossRef]
- Davies, K.M.; Mercer, J.F.B.; Chen, N.; Double, K.L. Copper dyshomoeostasis in Parkinson’s disease: Implications for pathogenesis and indications for novel therapeutics. Clin. Sci. 2016, 130, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Mcleary, F.A.; Rcom-H’cheo, A.N.; Goulding, M.; Radford, R.A.W.; Okita, Y.; Faller, P.; Chung, R.S.; Pountney, D.L. Switching on Endogenous Metal Binding Proteins in Parkinson’s Disease. Cells 2019, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.M.; Bohic, S.; Carmona, A.; Ortega, R.; Cottam, V.; Hare, D.J.; Finberg, J.P.M.; Reyes, S.; Halliday, G.M.; Mercer, J.F.B.; et al. Copper pathology in vulnerable brain regions in Parkinson’s disease. Neurobiol. Aging 2014, 35, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Torsdottir, G.; Kristinsson, J.; Sveinbjornsdottir, S.; Snaedal, J.; Johannesson, T. Copper, ceruloplasmin, superoxide dismutase and iron parameters in Parkinson’s disease. Pharmacol. Toxicol. 1999, 85, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Dexter, D.T.; Carayon, A.; Javoy-Agid, F.; Agid, Y.; Wells, F.R.; Daniel, S.E.; Lees, A.J.; Jenner, P.; Marsden, C.D. Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain 1991, 114, 1953–1975. [Google Scholar] [CrossRef] [PubMed]
- Forsleff, L.; Schauss, A.G.; Bier, I.D.; Stuart, S. Evidence of functional zinc deficiency in Parkinson’s disease. J. Altern. Complement. Med. 1999, 5, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Falup-Pecurariu, C.; Ferreira, J.; Martinez-Martin, P.; Chaudhuri, K.R. Toxic-Induced Parkinsonism. In Movement Disorders Curricula; Springer: Vienna, Austria, 2017. [Google Scholar]
- Caudle, W.M. Occupational Metal Exposure and Parkinsonism. Adv. Neurobiol. 2017, 18, 143–158. [Google Scholar] [PubMed]
- Masaldan, S.; Bush, A.I.; Devos, D.; Rolland, A.S.; Moreau, C. Striking while the iron is hot: Iron metabolism and ferroptosis in neurodegeneration. Free Radic. Biol. Med. 2019, 133, 221–233. [Google Scholar] [CrossRef]
- Aschner, M.; Erikson, K.M.; Herrero-Hernández, E.; Tjalkens, R. Manganese and its Role in Parkinson’s Disease: From Transport to Neuropathology. Neuromol. Med. 2009, 11, 252–266. [Google Scholar] [CrossRef]
- Barnham, K.J.; Bush, A.I. Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem. Soc. Rev. 2014, 43, 6727–6749. [Google Scholar] [CrossRef]
- Chen, P.; Totten, M.; Zhang, Z.Y.; Bucinca, H.; Erikson, K.; Santamaria, A.; Bowman, A.B.; Aschner, M. Iron and manganese-related CNS toxicity: Mechanisms, diagnosis and treatment. Exp. Rev. Neurother. 2019, 19, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liang, M.C.; Soong, T.W. Nitric Oxide, Iron and Neurodegeneration. Front. Neurosci. 2019, 18, 114. [Google Scholar] [CrossRef] [PubMed]
- Dusek, P.; Litwin, T.; Czlonkowska, A. Neurologic impairment in Wilson disease. Ann. Transl. Med. 2019, 7, S64. [Google Scholar] [CrossRef] [PubMed]
- Piloni, N.E.; Perazzo, J.C.; Fernandez, V.; Videla, L.A.; Puntarulo, S. Sub-chronic iron overload triggers oxidative stress development in rat brain: Implications for cell protection. Biometals 2016, 29, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Kakhlon, O.; Cabantchik, Z.I. The labile iron pool: Characterization, measurement, and participation in cellular processes. Free Radic. Biol. Med. 2002, 33, 1037–1046. [Google Scholar] [CrossRef]
- Sian, J.; Dexter, D.T.; Lees, A.J.; Daniel, S.; Agid, Y.; JavoyAgid, F.; Jenner, P.; Marsden, C.D. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann. Neurol. 1994, 36, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W.; Koh, J.Y. Zinc and brain injury. Annu. Rev. Neurosci. 1998, 21, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Waksmundzka-hajnos, M. An attempt to elucidate the role of iron and zinc ions in development of Alzheimer’s and Parkinson’s diseases. Biomed. Pharmacother. 2019, 111, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J. Neurochem. 2007, 103, 17–37. [Google Scholar] [PubMed]
- Uversky, V.N.; Li, J.; Fink, A.L. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson’s disease and heavy metal exposure. J. Biol. Chem. 2001, 276, 44284–44296. [Google Scholar] [CrossRef] [PubMed]
- Norris, E.H.; Giasson, B.I.; Ischiropoulos, H.; Lee, V.M. Effects of oxidative and nitrative challenges on alpha-synuclein fibrillogenesis involve distinct mechanisms of protein modifications. J. Biol. Chem. 2003, 278, 27230–27240. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.M.; Giasson, B.I.; Chen, Q.; Lee, V.M.Y.; Ischiropoulos, H. Dityrosine cross-linking promotes formation of stable alpha-synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J. Biol. Chem. 2000, 275, 18344–18349. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Pountney, D.L.; Jensen, P.H.; Gai, W.P.; Voelcker, N.H. Calcium(II) selectively induces alpha-synuclein annular oligomers via interaction with the C-terminal domain. Protein Sci. 2004, 13, 3245–3252. [Google Scholar] [CrossRef] [PubMed]
- Yamin, G.; Glaser, C.B.; Uversky, V.N.; Fink, A.L. Certain metals trigger fibrillation of methionine-oxidized alpha-synuclein. J. Biol. Chem. 2003, 278, 27630–27635. [Google Scholar] [CrossRef] [PubMed]
- Friedlich, A.L.; Tanzi, R.E.; Rogers, J.T. The 5′-untranslated region of Parkinson’s disease alpha-synuclein messengerRNA contains a predicted iron responsive element. Mol. Psychiatry 2007, 12, 222–223. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.T.; Mikkilineni, S.; Cantuti-Castelvetri, I.; Smith, D.H.; Huang, X.D.; Bandyopadhyay, S.; Cahill, C.M.; Maccecchini, M.L.; Lahiri, D.K.; Greig, N. The alpha-synuclein 5′untranslated region targeted translation blockers: Anti-alpha synuclein efficacy of cardiac glycosides and Posiphen. Neural Transm. 2011, 118, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Duce, J.A.; Wong, B.X.; Durham, H.; Devedjian, J.C.; Smith, D.P.; Devos, D. Post translational changes to alpha-synuclein control iron and dopamine trafficking; a concept for neuron vulnerability in Parkinson’s disease. Mol. Neurodegener. 2017, 12, 45. [Google Scholar] [CrossRef]
- Baksi, S.; Singh, N. α-Synuclein impairs ferritinophagy in the retinal pigment epithelium: Implications for retinal iron dyshomeostasis in Parkinson’s disease. Sci. Rep. 2017, 7, 12843. [Google Scholar] [CrossRef]
- Carboni, E.; Lingor, P. Insights on the interaction of alpha-synuclein and metals in the pathophysiology of Parkinson’s disease. Metallomics 2015, 7, 395–404. [Google Scholar] [CrossRef]
- Atrián-Blasco, E.; Gonzalez, P.; Santoro, A.; Alies, B.; Faller, P.; Hureau, C. Cu and Zn coordination to amyloid peptides: From fascinating chemistry to debated pathological relevance. Coord. Chem. Rev. 2018, 371, 38–55. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Homma, T.; Fujii, J. Application of Glutathione as Anti-Oxidative and Anti-Aging Drugs. Curr. Drug Metab. 2015, 16, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Filograna, R.; Beltramini, M.; Bubacco, L.; Bisaglia, M. Anti-Oxidants in Parkinson’s Disease Therapy: A Critical Point of View. Curr. Neuropharmacol. 2016, 14, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, O.; Amit, T.; Mandel, S.; Kupershmidt, L. Neuroprotective Multifunctional Iron Chelators: From Redox-Sensitive Process to Novel Therapeutic Opportunities. Antioxid. Redox Signal. 2010, 13, 919–949. [Google Scholar] [CrossRef] [PubMed]
- Giampietro, R.; Spinelli, F.; Contino, M.; Colabufo, N.A.; Farmaco, F.; Aldo, B.; Orabona, V.; Farmaco, F.; Aldo, B.; Orabona, V. The Pivotal Role of Copper in Neurodegeneration: A New Strategy for the Therapy of Neurodegenerative Disorders. Mol. Pharm. 2018, 15, 806–820. [Google Scholar] [CrossRef]
- Bouabid, S.; Tinakoua, A.; Nouria-Ghazal, L.; Benazzouz, A. Manganese neurotoxicity: Behavioral disorders associated with dysfunctions in the basal ganglia and neurochemical transmission. J. Neurochem. 2016, 136, 677–691. [Google Scholar] [CrossRef]
- Ndayisaba, A.; Kaindlstorfer, C.; Wenning, G.K. Iron in Neurodegeneration—Cause or Consequence? Front. Neurosci. 2019, 13, 180. [Google Scholar] [CrossRef]
- Joksić, A.Š.; Katz, S.A. Chelation therapy for treatment of systemic intoxication with uranium: A review. J. Environ. Sci. Health 2015, 50, 1479–1488. [Google Scholar] [CrossRef]
- Crisponi, G.; Dean, A.; di Marco, V.; Lachowicz, J.I.; Nurchi, V.M.; Remelli, M.; Tapparo, A. Different approaches to the study of chelating agents for iron and aluminium overload pathologies. Anal. Bioanal. Chem. 2013, 405, 585–601. [Google Scholar] [CrossRef]
- Kontoghiorghe, C.N.; Kontoghiorghes, G.J. Efficacy and safety of iron-chelation therapy with deferoxamine, deferiprone, and deferasirox for the treatment of iron-loaded patients with non-transfusion-dependent thalassemia syndromes. Drug Des. Dev. Ther. 2016, 10, 465–481. [Google Scholar] [CrossRef]
- Borgna-Pignatti, C.; Cappellini, M.D.; de Stefano, P.; del Vecchio, G.C.; Forni, G.L.; Gamberini, M.R.; Ghilardi, R.; Piga, A.; Romeo, M.A.; Zhao, H.Q.; et al. Cardiac morbidity and mortality in deferoxamine- or deferiprone-treated patients with thalassemia major. Blood 2006, 107, 3733–3737. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.; Barteselli, G.; Dell’Arti, L.; Ratiglia, R.; Viola, F. Functional and Structural Abnormalities in Deferoxamine Retinopathy: A Review of the Literature. BioMed Res. Int. 2015, 2015, 249617. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.A.; Brunskill, S.J.; Doree, C.; Gooding, S.; Chowdhury, O.; Roberts, D.J. Desferrioxamine mesylate for managing transfusional iron overload in people with transfusion-dependent thalassaemia. Cochrane Database Syst. Rev. 2013, CD004450. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G. A record number of fatalities in many categories of patients treated with deferasirox: Loopholes in regulatory and marketing procedures undermine patient safety and misguide public funds? Exp. Opin. Drug Saf. 2013, 12, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Hedera, P. Clinical management of Wilson disease. Ann. Transl. Med. 2019, 7, S66. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Kim, Y.S.; Kumar, V.J. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef]
- Ward, R.J.; Dexter, D.T.; Crichton, R.R. Chelating Agents for Neurodegenerative Diseases. Curr. Med. Chem. 2012, 19, 2760–2772. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, M.T.; Chana-Cuevas, P. New Perspectives in Iron Chelation Therapy for the Treatment of Neurodegenerative Diseases. Pharmaceuticals 2018, 11, 109. [Google Scholar] [CrossRef]
- Mot, A.I.; Wedd, A.G.; Sinclair, L.; Brown, D.R.; Collins, S.J.; Brazier, M.W. Metal attenuating therapies in neurodegenerative disease. Expert Rev. Neurother. 2011, 11, 1717–1745. [Google Scholar] [CrossRef]
- Portbury, S.D.; Yévenes, L.F.; Adlard, P.A. Novel zinc-targeted therapeutic options for cognitive decline. Future Neurol. 2015, 10, 537–546. [Google Scholar] [CrossRef]
- Poujois, A.; Devedjian, J.C.; Moreau, C.; Devos, D.; Chaine, P.; Woimant, F.; Duce, J.A. Bioavailable Trace Metals in Neurological Diseases. Curr. Treat. Opt. Neurol. 2016, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Zekavat, O.R.; Bahmanjahromi, A.; Haghpanah, S.; Ebrahimi, S.; Cohan, N. The Zinc and Copper Levels in Thalassemia Major Patients, Receiving Iron Chelation Therapy. J. Pediatr. Hematol. Oncol. 2018, 40, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Kolnagou, A.; Peng, C.T.; Shah, S.V.; Aessopos, A. Safety issues of iron chelation therapy in patients with normal range iron stores including thalassaemia, neurodegenerative, renal and infectious diseases. Exp. Opin. Drug Saf. 2010, 9, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Lanza, V.; Milardi, D.; Natale, G.D.; Pappalardo, G. Repurposing of Copper(II)-chelating Drugs for the Treatment of Neurodegenerative Diseases. Curr. Med. Chem. 2018, 25, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, V.; Vecchio, G. Prochelator strategies for site-selective activation of metal chelators. J. Inorg. Biochem. 2016, 162, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Sangchot, P.; Sharma, S.; Chetsawang, B.; Porter, J.; Govitrapong, P.; Ebadi, M. Deferoxamine attenuates iron-induced oxidative stress and prevents mitochondrial aggregation and alphasynuclein translocation in SK-N-SH cells in culture. Dev. Neurosci. 2002, 24, 143–153. [Google Scholar] [CrossRef]
- Guo, C.; Hao, L.J.; Yang, Z.H.; Chai, R.; Zhang, S.; Gao, H.L.; Zhong, M.L.; Wang, T.; Li, J.Y.; Wang, Z.Y. Deferoxamine-mediated upregulation of HIF-1alpha prevents dopaminergic neuronal death via the activation of MAPK family proteins in MPTP-treated mice. Exp. Neurol. 2016, 280, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.P.; Pandey, A.; Vishwakarma, S.; Modi, G. A review on iron chelators as potential therapeutic agents for the treatment of Alzheimer’s and Parkinson’s diseases. Mol. Divers. 2018, 23, 509–526. [Google Scholar] [CrossRef]
- Devos, D.; Moreau, C.; Devedjian, J.C.; Kluza, J.; Petrault, M.; Laloux, C.; Jonneaux, A.; Ryckewaert, G.; Garcon, G.; Rouaix, N.; et al. Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid. Redox Signal. 2014, 21, 195–210. [Google Scholar] [CrossRef]
- Kaur, D.; Yantiri, F.; Rajagopalan, S.; Kumar, J.; Mo, J.Q.; Boonplueang, R.; Viswanath, V.; Jacobs, R.; Yang, L.; Beal, M.F.; et al. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: A novel therapy for Parkinson’s disease. Neuron 2003, 37, 899–909. [Google Scholar] [CrossRef]
- Tardiff, D.F.; Tucci, M.L.; Caldwell, K.A.; Caldwell, G.A.; Lindquist, S. Different 8-hydroxyquinolines protect models of tdp-43 protein, alpha-synuclein, and polyglutamine proteotoxicity through distinct mechanisms. J. Biol. Chem. 2012, 287, 4107–4120. [Google Scholar] [CrossRef] [PubMed]
- Shachar, D.B.; Kahana, N.; Kampel, V.; Warshawsky, A.; Youdim, M.B.H. Neuroprotection by a novel brain permeable iron chelator, vk-28, against 6-hydroxydopamine lession in rats. Neuropharmacology 2004, 46, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Gal, S.; Zheng, H.; Fridkin, M.; Youdim, M.B. Novel multifunctional neuroprotective iron chelator-monoamine oxidase inhibitor drugs for neurodegenerative diseases. In vivo selective brain monoamine oxidase inhibition and prevention of MPTP-induced striatal dopamine depletion. J. Neurochem. 2005, 95, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Lannfelt, L.; Blennow, K.; Zetterberg, H.; Batsman, S.; Ames, D.; Harrison, J.; Masters, C.L.; Targum, S.; Bush, A.I.; Murdoch, R.; et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease: A phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008, 7, 779–786. [Google Scholar] [CrossRef]
- Mena, N.P.; García-Beltrán, O.; Lourido, F.; Urrutia, P.J.; Mena, R.; Castro-Castillo, V.; Cassels, B.K.; Núñez, M.T. The novel mitochondrial iron chelator 5-((methylamino)methyl)-8-hydroxyquinoline protects against mitochondrial-induced oxidative damage and neuronal death. Biochem. Biophys. Res. Commun. 2015, 463, 787–792. [Google Scholar] [CrossRef]
- Cabantchik, Z.I.; Munnich, A.; Youdim, M.B.; Devos, D. Regional siderosis: A new challenge for iron chelation therapy. Front. Pharmacol. 2013, 4, 167. [Google Scholar] [CrossRef]
- Connor, J.R.; Ponnuru, P.; Wang, X.S.; Patton, S.M.; Allen, R.P.; Earley, C.J. Profile of altered brain iron acquisition in restless legs syndrome. Brain 2011, 134, 959–968. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Erxlebenb, A.; Ochockia, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. 8-Hydroxyquinolines: A review of their metal chelating properties and medicinal applications. Drug Des. Dev. Ther. 2013, 7, 1157–1178. [Google Scholar] [CrossRef]
- Jakusch, T.; Dean, A.; Oncsik, T.; Benyei, A.C.; di Marco, V.; Kiss, T. Vanadate complexes in serum: A speciation modeling study. Dalton Trans. 2010, 39, 212–220. [Google Scholar] [CrossRef]
- Kiss, T.; Jakusch, T.; Gyurcsik, B.; Lakatos, A.; Anna, É.; Sija, É. Application of modeling calculations in the description of metal ion distribution of bioactive compounds in biological systems. Coord. Chem. Rev. 2012, 256, 125–132. [Google Scholar] [CrossRef]
- Peres, T.V.; Schettinger, M.R.C.; Chen, P.; Carvalho, F.; Avila, D.S.; Bowman, A.B.; Aschner, M. Manganese-induced neurotoxicity: A review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol. Toxicol. 2016, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Pettit, L.D. Critical survey of formation constants of complexes of histidine, phenylalanine, tyrosine, L-DOPA and tryptophan. Pure Appl. Chem. 1984, 56, 247–292. [Google Scholar] [CrossRef]
- Stayte, S.; Vissel, B. Advances in non-dopaminergic treatments for Parkinson’s disease. Front. Neurosci. 2014, 8, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Charvin, D.; Medori, R.; Hauser, R.A.; Rascol, O. Therapeutic strategies for Parkinson disease: Beyond dopaminergic drugs. Nat. Rev. Drug Discov. 2018, 17, 804–822. [Google Scholar] [CrossRef]
- Reddy, D.H.; Misra, S.; Medhi, B. Advances in Drug Development for Parkinson’s Disease: Present Status. Pharmacology 2014, 93, 260–271. [Google Scholar] [CrossRef]
- McBean, G.J.; López, M.G.; Wallner, F.K. Redox-based therapeutics in neurodegenerative disease. Br. J. Pharmacol. 2017, 174, 1750–1770. [Google Scholar] [CrossRef]
- Ellis, J.M.; Fell, M.J. Current approaches to the treatment of Parkinson’s Disease. Bioorg. Med. Chem. Lett. 2017, 27, 4247–4255. [Google Scholar] [CrossRef]
- Oertel, W.; Schulz, J.B. Current and experimental treatments of Parkinson disease: A guide for neuroscientists. J. Neurochem. 2016, 139, 325–337. [Google Scholar] [CrossRef]
- Silva, A.R.; Grosso, C.; Cristina, D.; Rocha, J.M. Comprehensive review on the interaction between natural compounds and brain receptors: Benefits and toxicity. Eur. J. Med. Chem. 2019, 174, 87–115. [Google Scholar] [CrossRef]
- Aguirre, P.; Mena, N.P.; Carrasco, C.M.; Muñoz, Y.; Pérez-Henríquez, P. Iron Chelators and Antioxidants Regenerate Neuritic Tree and Nigrostriatal Fibers of MPP+/MPTP-Lesioned Dopaminergic Neurons. PLoS ONE 2015, 10, e0144848. [Google Scholar] [CrossRef] [PubMed]
- Benvenutti, R.; Marcon, M.; Reis, C.G.; Nery, L.R.; Miguel, C.; Herrmann, A.P.; Vianna, M.R.M.; Piato, A. N-acetylcysteine protects against motor, optomotor and morphological deficits induced by 6-OHDA in zebrafish larvae. PeerJ 2018, 6, e4957. [Google Scholar] [CrossRef] [PubMed]
- Botsakis, K.; Theodoritsi, S.; Grintzalis, K.; Angelatou, F.; Antonopoulos, I.; Georgiou, C.D.; Margarity, M.; Matsokis, N.A.; Panagopoulos, N.T. 17β-estradiol/N-acetylcysteine interactions enhances the neuroprotective effect on dopaminergic neurons in the weaver model of dopamine deficency. Neuroscience 2016, 320, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Jantas, D.; Greda, A.; Golda, S.; Korostynski, M.; Grygier, B.; Roman, A.; Pilc, A.; Lason, W. Neuroprotective effects of metabotropic glutamate receptor group II and III activators against MPP(+)-induced cell death in human neuroblastoma SH-SY5Y cells: The impact of cell differentiation state. Neuropharmacology 2014, 83, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Ponnazhagan, R.; Harms, A.S.; Thome, A.D.; Jurkuvenaite, A.; Gogliotti, R.; Niswenden, C.M.; Conn, P.J.; Standaert, D.G. The Metabotropic Glutamate Receptor 4 Positive Allosteric Modulator ADX88178 Inhibits Inflammatory Responses in Primary Microglia. J. Neuroimmune Pharmacol. 2016, 11, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bai, L.; He, J.; Zhong, L.; Duan, X.; Ouyang, L.; Zhu, Y.; Wang, T.; Zhang, Y.; Shi, J. Recent advances in discovery and development of natural products as source for anti-Parkinson’s disease lead compounds. Eur. J. Med. Chem. 2017, 141, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhao, G.; Fang, J.; Yuan, T.; Liu, A.; Du, G. Discovery of the neuroprotective effects of alvespimycin by computational prioritization of potential anti-parkinson agents. FEBS J. 2014, 281, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Agar, E. The role of cannabinoids and leptin in neurological diseases. Neurol. Scand. 2015, 132, 371–380. [Google Scholar] [CrossRef] [PubMed]
- More, S.V.; Choi, D. Promising cannabinoid-based therapies for Parkinson’s disease: Motor symptoms to neuroprotection. Mol. Neurodegener. 2015, 10, 1–26. [Google Scholar] [CrossRef]
- Mishra, A.; Pratap, L.; Kumar, S. Ambroxol modulates 6-Hydroxydopamine-induced temporal reduction in Glucocerebrosidase (GCase) enzymatic activity and Parkinson’s disease symptoms. Biochem. Pharmacol. 2018, 155, 479–493. [Google Scholar] [CrossRef]
- Ishay, Y.; Zimran, A.; Szer, J.; Dinur, T.; Ilan, Y.; Arkadir, D. Combined beta-glucosylceramide and ambroxol hydrochloride in patients with Gaucher related Parkinson disease: From clinical observations to drug development. Blood Cells Mol. Dis. 2018, 68, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Redenti, S.; Marcovich, I.; de Vita, T.; Zorzi, R.D.; Demitri, N.; Perez, D.I.; Bottegoni, G.; Bisignano, P.; Bissaro, M.; Moro, S.; et al. A Triazolotriazine-Based Dual GSK-3 beta/CK-1 delta Ligand as a Potential Neuroprotective Agent Presenting Two Different Mechanisms of Enzymatic Inhibition. ChemMedChem 2019, 14, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Krajnak, K.; Dahl, R. Small molecule SUMOylation activators are novel neuroprotective agents. Bioorg. Med. Chem. Lett. 2018, 28, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Hee, T.; Hyo, K.; Kim, K.; Sook, E. Novel anti-adipogenic activity of anti-malarial amodiaquine through suppression of PPAR c activity. Arch. Pharm. Res. 2017, 40, 1336–1343. [Google Scholar]
- Gay, M.; Carato, P.; Coevoet, M.; Renault, N.; Larchanché, P.; Barczyk, A.; Yous, S.; Buée, L.; Sergeant, N.; Melnyk, P. New phenylaniline derivatives as modulators of amyloid protein precursor metabolism. Bioorg. Med. Chem. 2018, 26, 2151–2164. [Google Scholar] [CrossRef]
- Tian, S.; Wang, X.; Li, L.; Zhang, X.; Li, Y.; Zhu, F.; Hou, T.; Zhen, X. Discovery of Novel and Selective Adenosine A2A Receptor Antagonists for Treating Parkinson’s Disease through Comparative Structure-Based Virtual Screening. J. Chem. Inf. Model. 2017, 57, 1474–1487. [Google Scholar] [CrossRef] [PubMed]
- Putteeraj, M.; Lim, W.L.; Teoh, S.L.; Yahaya, M.F. Flavonoids and its Neuroprotective Effects on Brain Ischemia and Neurodegenerative Diseases. Curr. Drug Targets 2018, 19, 1710–1720. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Khan, H.; D’Onofrio, G.; Šamec, D.; Shirooie, S.; Dehpour, A.R.; Argüelles, S.; Habtemariam, S.; Sobarzo-Sanchez, E. Apigenin as neuroprotective agent: Of mice and men. Pharmacol. Res. 2018, 128, 359–365. [Google Scholar] [CrossRef]
- Anusha, C.; Sumathi, T.; Leena, D.J. Protective role of apigenin on rotenone induced rat model of Parkinson’s disease: Suppression of neuroinflammation and oxidative stress mediated apoptosis. Chem. Biol. Interact. 2017, 269, 67–79. [Google Scholar] [CrossRef]
- Ali, F.; Naz, F.; Jyoti, S.; Siddique, Y.H. Health Functionality of Apigenin: A Review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- Mack, J.M.; Moura, T.M.; Lanznaster, D.; Bobinski, F.; Massari, C.M.; Sampaio, T.B.; Schmitz, A.E.; Souza, L.F.; Walz, R.; Tasca, C.I.; et al. Intranasal administration of sodium dimethyldithiocarbamate induces motor deficits and dopaminergic dysfunction in mice. Neurotoxicology 2018, 66, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Kaidoh, K.; Hiratochi, M. Duration of drug action of dopamine D2 agonists in mice with 6-hydroxydopamine-induced lesions. Neuroreport 2015, 26, 1126–1132. [Google Scholar]
- Hami, J.; Hosseini, M.; Shahi, S.; Lotfi, N.; Talebi, A.; Afshar, M. Effects of L-arginine pre-treatment in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s diseases in Balb/c mice. Iran. J. Neurol. 2015, 14, 195–203. [Google Scholar] [PubMed]
- Yang, H.; Ehm, G.; Eun, Y.; Young, J.; Lee, W.; Kim, A.; Kim, H.; Jeon, B. Liquid levodopa-carbidopa in advanced Parkinson’s disease with motor complications. J. Neurol. Sci. 2017, 377, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, I.; Lee, S.W.; Lee, H.; Lee, G.; Kim, S.; Lee, S.W.; Yoon, D.S. Quantifying L-Ascorbic Acid-Driven Inhibitory Effect on Amyloid Fibrillation. Macromol. Res. 2016, 24, 868–873. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Sun, M.F.; Jia, X.B.; Cheng, K.; Xu, Y.D.; Zhou, Z.L.; Zhang, P.H.; Qiao, C.M.; Cui, C.; Chen, X.; et al. Neuroprotective effects of Astilbin on MPTP-induced Parkinson’s disease mice: Glial reaction, α-synuclein expression and oxidative stress. Int. Immunopharmacol. 2019, 66, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Ou, Z.; Jiang, T.; Tian, Y.; Zhou, J.; Wu, L. Azilsartan ameliorates apoptosis of dopaminergic neurons and rescues characteristic parkinsonian behaviors in a rat model of Parkinson’s disease. Oncotarget 2017, 8, 24099–24109. [Google Scholar]
- Aliakbari, F.; Akbar, A.; Bardania, H.; Akbar, A.; Christiansen, G.; Otzen, D.E.; Morshedi, D. Biointerfaces Formulation and anti-neurotoxic activity of baicalein-incorporating neutral nanoliposome. Colloids Surf. B Biointerfaces 2018, 161, 578–587. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. Baicalein as a potent neuroprotective agent: A review. Biomed. Pharmacother. 2017, 95, 1021–1032. [Google Scholar] [CrossRef]
- Chen, T.K.; Li, Y.; Li, C.W.; Yi, X.; Wang, R.B.; Lee, S.M.Y.; Zheng, Y. Pluronic P85/F68 Micelles of Baicalein Could Interfere with Mithochondria to Overcome MRP2-Mediated Efflux and Offer Improved antiParkinsonian Activity. Mol. Pharm. 2014, 14, 3331–3342. [Google Scholar] [CrossRef]
- Ilm, T.; Masroor, A.; Khursheed, M.; Ahmad, I.; Jahan, I.; Ali, M.; Nayeem, S.M.; Uversky, V.N.; Hasan, R. Molecular basis of the inhibition and disaggregation of thermally-induced amyloid fibrils of human serum albumin by an anti-Parkinson’s drug, benserazide hydrochloride. J. Mol. Liq. 2019, 278, 553–567. [Google Scholar]
- Ilm, T.; Zaidi, N.; Zaman, M.; Jahan, I.; Masroor, A.; Ahmad, I.; Nayeem, S.M.; Ali, M.; Uversky, V.N. A multiparametric analysis of the synergistic impact of anti-Parkinson’s drugs on the fibrillation of human serum albumin. BBA Proteins Proteom. 2019, 1867, 275–285. [Google Scholar]
- Bacho, M.; Coelho-Cerqueira, E.; Follmer, C.; Nabavi, S.M.; Rastrelli, L.; Uriarte, E.; Sobarzo-Sanchez, E. A Medical Approach to the Monoamine Oxidase Inhibition by Using 7Hbenzo, perimidin-7-one Derivatives. Curr. Top. Med. Chem. 2017, 17, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Mathew, G.E.; Petzer, J.P.; Anel, P. Structural Exploration of Synthetic Chromones as Selective MAO-B Inhibitors: A Mini Review. J. Mater. Chem. B 2017, 20, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Brunschweiger, A.; Koch, P.; Schlenk, M.; Rafehi, M.; Radjainia, H.; Küppers, P.; Hinz, S.; Pineda, F.; Wiese, M.; Hockemeyer, J.; et al. 8-Substituted 1,3-dimethyltetrahydropyrazino[2,1-f]purinediones: Water-soluble adenosine receptor antagonists and monoamine oxidase B inhibitors. Bioorg. Med. Chem. 2016, 24, 5462–5480. [Google Scholar] [CrossRef]
- Fonseca-Fonseca, L.A.; Nuñez-Figueredo, Y.; Sánchez, J.R.; Guerra, M.W.; Ochoa-Rodríguez, E.; Verdecia-Reyes, Y.; Hernádez, R.D.; Menezes-Filho, N.J.; Costa, T.C.S.; de Santana, W.A.; et al. Neuroprotective Effects of Bikaverin on H2O2 -Induced Oxidative Stress Mediated Neuronal Damage in SH-SY5Y Cell Line. Cell. Mol. Neurobiol. 2014, 34, 973–985. [Google Scholar]
- Modi, G.; Antonio, T.; Reith, M.; Dutta, A. Structural Modifications of Neuroprotective Anti.Parkinsonian (-)-N6-(2-(4-(Biphenyl-4-yl)piperazin-1-yl)-ethyl)-N6-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine (D-264): An Effort toward the Improvement of in Vivo Efficacy of the Parent Molecule. J. Med. Chem. 2014, 57, 1557–1572. [Google Scholar] [CrossRef]
- Cao, X.; Jin, Y.; Zhang, H.; Yu, L.; Bao, X.; Li, F.; Xu, Y. The Anti-inflammatory Effects of 4-((5-Bromo-3-chloro-2-hydroxybenzyl)amino)-2-hydroxybenzoic Acid in Lipopolysaccharide-Activated Primary Microglial Cells. Inflammation 2018, 41, 530–540. [Google Scholar] [CrossRef]
- Hu, W.; Guan, L.; Dang, X.; Ren, P.; Zhang, Y. Small-molecule inhibitors at the PSD-95/nNOS interface attenuate MPP+-induced neuronal injury through Sirt3 mediated inhibition of mitochondrial dysfunction. Neurochem. Int. 2014, 79, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Dugan, L.L.; Tian, L.; Quick, K.L.; Hardt, J.I.; Karimi, M.; Brown, C.; Loftin, S.; Flores, H.; Moerlein, S.M.; Polich, J.; et al. Carboxyfullerene Neuroprotection Postinjury in Parkinsonian Nonhuman Primates. Ann. Neurol. 2014, 76, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhao, J.; Cheng, Y.; Lee, S.M.; Rong, J. N-Propargyl Caffeamide (PACA) Ameliorates Dopaminergic Neuronal Loss and Motor Dysfunctions in MPTP Mouse Model of Parkinson’s Disease and in MPP+-Induced Neurons via Promoting the Conversion of proNGF to NGF. Mol. Neurobiol. 2018, 55, 2258–2267. [Google Scholar] [CrossRef]
- Moosavi, F.; Hosseini, R.; Rajaian, H.; Silva, T.; Magalhães, D.; Saso, L.; Edraki, N.; Miri, R.; Borges, F.; Firuzi, O. Derivatives of caffeic acid, a natural antioxidant, as the basis for the discovery of novel nonpeptidic neurotrophic agents. Bioorg. Med. Chem. 2017, 25, 3235–3246. [Google Scholar] [CrossRef] [PubMed]
- Taram, F.; Winter, A.N.; Linseman, D.A. Neuroprotection Comparison of Rosmarinic Acid and Carnosic Acid in Primary Cultures of Cerebellar Granule Neurons. Molecules 2018, 23, 2956. [Google Scholar] [CrossRef] [PubMed]
- Fazili, N.A.; Naeem, A. Anti-fibrillation potency of caffeic acid against an antidepressant induced fi brillogenesis of human α-synuclein: Implications for Parkinson’ s disease. Biochimie 2015, 108, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Reith, M.E.A.; Dutta, A.K. Design, Synthesis, and Pharmacological Characterization of Carbazole Based Dopamine Agonists as Potential Symptomatic and Neuroprotective Therapeutic Agents for Parkinson’s Disease. ACS Chem. Neurosci. 2018, 10, 396–411. [Google Scholar]
- Beata, G.; Nishigaya, Y.; Hirsz-wiktorzak, K.; Rybczy, A. Interference of carbidopa and other catechols with reactions catalyzed by peroxidases. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1626–1634. [Google Scholar]
- Oliveira, M.R.; Ferreira, G.C.; Schuck, P.F. Protective effect of carnosic acid against paraquat-induced redox impairment and mitochondrial dysfunction in SH-SY5Y cells: Role for PI3K/Akt/Nrf2 pathway. Toxicol. Vitr. 2016, 32, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Yimer, E.M.; Hishe, H.Z.; Tuem, K.B. Repurposing of the β-Lactam Antibiotic, Ceftriaxone for Neurological Disorders: A Review. Front. Neurosci. 2019, 26, 236. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Renaud, J.; Tamas, A.; Tizabi, Y.; Socı, S.B.; Del-bel, E.; Raisman-vozari, R. Novel tactics for neuroprotection in Parkinson’s disease: Role of antibiotics, polyphenols and neuropeptides. Prog. Neurobiol. 2017, 155, 120–148. [Google Scholar] [CrossRef]
- Ruzza, P.; Siligardi, G.; Hussain, R.; Marchiani, A.; Islami, M.; Bubacco, L.; Delogu, G.; Fabbri, D.; Dettori, M.A.; Sechi, M.; et al. Ceftriaxone Blocks the Polymerization of α-Synuclein and Exerts Neuroprotective E ff ects in Vitro. ACS Chem. Neurosci. 2013, 5, 30–38. [Google Scholar] [CrossRef]
- Venkatesha, S.H.; Moudgil, K.D. Celastrol and Its Role in Controlling Chronic Diseases. In Anti-Inflammatory Nutraceuticals and Chronic Diseases; Springer: Cham, Switzerland, 2016; pp. 267–289. [Google Scholar]
- Choi, B.S.; Kim, H.; Lee, H.J.; Sapkota, K.; Park, S.E.; Kim, S.; Kim, S.J. Celastrol from “Thunder God Vine” Protects SH-SY5Y Cells through the preservation of mithochondrial function and inhibition of p38 MAPK in rotenone model of Parkinson’s disease. Neurochem. Res. 2014, 39, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Min, H.; Wang, D.; Gao, R.; Chang, Y.C.; Hu, F.; Meng, X. Marine-derived protein kinase inhibitors for neuroinflammatory diseases. Biomed. Eng. Online 2018, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Takeda, H.; Sakaki, H.; Kuramoto, K. Repositioning CEP-1347, a chemical agent originally developed for the treatment of Parkinson’s disease, as an anti-cancer stem cell drug. Oncotarget 2017, 8, 94872–94882. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, J.; Kang, K.S.; Lee, K.T.; Yang, H.O. Neuroprotective Effect of Chebulagic Acid via Autophagy Induction in SH-SY5Y Cells. Biomol. Ther. 2014, 22, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.M.; Li, X.Z.; Zhang, S.N.; Yang, Z.M.; Wang, K.X.; Lu, F.; Wang, C.Z.; Yuan, C.S. Acanthopanax senticosus Protects Structure and Function of Mesencephalic Mitochondria in A Mouse Model of Parkinson’s Disease. Chin. J. Integr. Med. 2018, 24, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Skandík, M.; Ra, L.; Kuniakov, M. Modulation of BV-2 microglia functions by novel quercetin pivaloyl a. Neurochem. Int. 2015, 90, 246–254. [Google Scholar]
- Wang, J.; Song, Y.; Chen, Z.; Leng, S.X. Connection between Systemic Inflammation and Neuroinflammation Underlies Neuroprotective Mechanism of Several Phytochemicals in Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2018, 2018, 1972714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, G.; Szeto, S.S.W.; Meng, C.; Quan, Q.; Huang, C.; Cui, W.; Guo, B.; Wang, Y.; Han, Y.; et al. Examining the neuroprotective effects of protocatechuic acid and chrysin on in vitro and in vivo models of Parkinson disease. Free Radic. Biol. Med. 2015, 84, 331–343. [Google Scholar] [CrossRef]
- Cheng, X.R.; Kerman, K. Electrochemical Detection of Interaction Between α-Synuclein and Clioquinol. Electroanalysis 2015, 27, 1436–1442. [Google Scholar] [CrossRef]
- Lei, P.; Ayton, S.; Appukuttan, A.T.; Volitakis, I.; Adlard, P.A.; Finkelstein, D.I.; Bush, A. Clioquinol rescues parkinsonism and dementia phenotypes of the tau knockout mouse. Neurobiol. Dis. 2015, 81, 168–175. [Google Scholar] [CrossRef]
- Feng, J.; Hu, X.; Lv, X.; Wang, B.; Lin, J.; Zhang, X. Synthesis and biological evaluation of clovamide analogues with catechol functionality as potent Parkinson’s disease agents in vitro and in vivo. Bioorg. Med. Chem. Lett. 2019, 29, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Bachhawat, P.; Chu, M.L.; Wood, M.; Ceska, T.; Sands, Z.A.; Mercier, J. Crystal structure of the adenosine A2A receptor bound to an antagonist reveals a potential allosteric pocket. Proc. Natl. Acad. Sci. USA 2017, 114, 2066–2071. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Sharma, H.; Yedlapudi, D.; Antonio, T.; Reith, M.E.A.; Dutta, A.K. Novel multifunctional dopamine D2/D3 receptors agonists with potential neuroprotection and anti-alpha synuclein protein aggregation properties. Bioorg. Med. Chem. 2016, 24, 5088–5510. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, J.; Zheng, J.; Ma, H.; Zhang, H.; Zhen, X.; Zheng, L.T.; Zhang, X. Design, synthesis and evaluation of a series of non-steroidal anti-inflammatory drug conjugates as novel neuroinflammatory inhibitors. Int. Immunopharmacol. 2015, 25, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bao, X.; Xu, S.; Yu, W.; Cao, S.; Hu, J.; Li, Y.; Wang, X.; Zhang, D.; Yu, S. A Novel Parkinson’s Disease Drug Candidate with Potent Anti-neuroinflammatory Effects through the Src Signaling Pathway. J. Med. Chem. 2016, 59, 9062–9079. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Vedachalam, S.; Luo, D.; Antonio, T.; Reith, M.E.A.; Dutta, A.K. Development of a Highly Potent D2/D3 Agonist and a Partial Agonist from Structure–Activity Relationship Study of N6-(2-(4-(1H-Indol-5yl)piperazin-1-yl)ethyl)-N6-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine Analogues: Implication in the Treatment. J. Med. Chem. 2015, 58, 9179–9195. [Google Scholar] [CrossRef]
- Attia, A.; Ahmed, H.; Gadelkarim, M.; Morsi, M.; Awad, K.; Elnenn, M.; Ghanem, E.; El-Jaafar, S.; Negida, A. Meta-Analysis of Creatine for Neuroprotection Against Parkinson’s Disease. CNS Neurol. Disord. Drug Targets 2017, 16, 169–175. [Google Scholar] [CrossRef]
- Shafaroodi, H.; Shahbek, F.; Faizi, M.; Ebrahimi, F.; Moezi, L. Creatine Revealed Anticonvulsant Properties on Chemically and Electrically Induced Seizures in Mice. Iran. J. Pharm. Res. 2016, 15, 843–850. [Google Scholar]
- Lee, D.; Ko, W.; Kim, D.; Kim, Y.; Jeong, G. Cudarflavone B Provides Neuroprotection against Glutamate-Induced Mouse Hippocampal HT22 Cell Damage through the Nrf2 and PI3K/Akt Signaling Pathways. Molecules 2014, 19, 10818–10831. [Google Scholar] [CrossRef]
- Moosavi, M.; Farrokhi, M.R.; Tafreshi, N. The effect of curcumin against 6-hydroxydopamine induced cell death and Akt/GSK disruption in human neuroblastoma cells. Physiol. Pharmacol. 2018, 22, 163–171. [Google Scholar]
- Maiti, P.; Dunbar, G.L. Use of Curcumin, a Natural Polyphenol for Targeting Molecular Pathways in Treating Age-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2018, 19, 1637. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Nehru, B. Curcumin affords neuroprotection and inhibits α-synuclein aggregation in lipopolysaccharide-induced Parkinson’ s disease model. Inflammopharmacology 2017, 26, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Ali, T.; Kim, M.W.; Jo, M.H.; Chung, J.I.; Kim, M.O. Anthocyanins Improve Hippocampus-Dependent Memory Function and Prevent Neurodegeneration via JNK/Akt/GSK3 β Signaling in LPS-Treated Adult Mice. Mol. Neurobiol. 2019, 56, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, J. Cyanidin Protects SH-SY5Y Human Neuroblastoma Cells from 1-Methyl-4-Phenylpyridinium-induced Neurotoxicity. Pharmacology 2018, 102, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Lindenbach, D.; Das, B.; Conti, M.M.; Dutta, S.M.M.A.K.; Bishop, C. D-512, a novel dopamine D2/D3 receptor agonist, demonstrates superior anti-parkinsonian efficacy over ropinirole in parkinsonian rats. Br. J. Pharmacol. 2017, 174, 3058–3071. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Rajagopalan, S.; Joshi, G.S.; Xu, L.; Luo, D.; Andersen, J.K.; Todi, S.V.; Dutta, A.K. A novel iron (II) preferring dopamine agonist chelator D-607 significantly suppresses α-syn- and MPTP-induced toxicities in vivo. Neuropharmacology 2017, 123, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Kandegedara, A.; Xu, L.; Antonio, T.; Stemmler, T.L.; Reith, M.E.A.; Dutta, A.K. A Novel Iron ( II ) Preferring Dopamine Agonist Chelator as Potential Symptomatic and Neuroprotective Therapeutic agent for Parkinson’s Disease. ACS Chem. Neurosci. 2017, 8, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Kandil, E.A.; Sayed, R.H.; Ahmed, L.A.; Abd, M.A.; Fattah, E. Modulatory Role of Nurr1 Activation and Thrombin Inhibition in the Neuroprotective Effects of Dabigatran Etexilate in Rotenone-Induced Parkinson’s Disease in Rats. Mol. Neurobiol. 2017, 55, 4078–4089. [Google Scholar] [CrossRef] [PubMed]
- Uenaka, T.; Satake, W.; Cha, P.; Hayakawa, H.; Baba, K.; Jiang, S.; Kobayashi, K.; Kanagawa, M.; Okada, Y.; Mochizuki, H.; et al. In silico drug screening by using genome-wide association study data repurposed dabrafenib, an anti-melanoma drug, for Parkinson’s disease. Hum. Mol. 2018, 27, 3974–3985. [Google Scholar] [CrossRef]
- Tseng, W.; Hsu, Y.; Pan, T. Neuroprotective effects of dimerumic acid and deferricoprogen from Monascus purpureus NTU 568-fermented rice against 6-hydroxydopamine-induced oxidative stress and apoptosis in differentiated pheochromocytoma PC-12 cells. Pharm. Biol. 2016, 54, 1434–1444. [Google Scholar] [CrossRef]
- Freyssin, A.; Page, G.; Fauconneau, B.; Bilan, A.R. Natural polyphenols effects on protein aggregates in Alzheimer’s and Parkinson’s prion-like diseases. Neural Regen. Res. 2018, 13, 955–961. [Google Scholar] [PubMed]
- Kujawska, M.; Jodynis-Liebert, J. Polyphenols in Parkinson’s Disease: A Systematic Review of In Vivo Studies. Nutrients 2018, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, M.; Rajasankar, S.; Gobi, V.V.; Dhanalakshmi, C. Neuroprotective effect of Demethoxycurcumin, a natural derivative of Curcumin on rotenone induced neurotoxicity in SH-SY 5Y Neuroblastoma cells. Complement. Altern. Med. 2017, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Karthivashan, G.; Ko, H.M.; Cho, D.; Kim, J.; Cho, D.J.; Ganesan, P.; Su-kim, I.; Choi, D. Aqueous Extract of Dendropanax morbiferus Leaves Effectively Alleviated Neuroinflammation and Behavioral Impediments in MPTP-Induced Parkinson’s Mouse Model. Oxid. Med. Cell. Longev. 2018, 2018, 3175214. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; De Jesus-Cortes, H.; Huntington, P.; Estill, S.; Morlock, L.K.; Starwalt, R.; Mangano, T.J.; Williams, N.S.; Pieper, A.A.; Ready, J.M. Discovery of a Neuroprotective Chemical, (S)-N-(3-(3,6-Dibromo-9H-carbazol-9-yl)-2-fluoropropyl)-6-methoxypyridin-2-amine[(−)-P7C3-S243], with Improved Druglike Properties. J. Med. Chem. 2014, 57, 3746–3754. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Qiao, J.B.; Hu, Z.X. Caffeoylquinic Acid Derivatives Protect SH-SY5Y Neuroblastoma Cells from Hydrogen Peroxide-Induced Injury Through Modulating Oxidative Status. Cell. Mol. Neurobiol. 2017, 37, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Zhao, Y.; Cao, T.; Zhen, X. Dihydromyricetin protects neurons in an MPTP-induced model of Parkinson’s disease by suppressing glycogen synthase kinase-3 beta activity. Acta Pharmacol. Sin. 2016, 37, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Fonseca, L.A.; Nuñez-Figueredo, Y.; Sánchez, J.R.; Guerra, M.W.; Ochoa-Rodríguez, E.; Verdecia-Reyes, Y.; Hernádez, R.D.; Menezes-Filho, N.J.; Cristina, T.; Costa, S.; et al. KM-34, a Novel Antioxidant Compound, Protects against 6-Hydroxydopamine-Induced Mitochondrial Damage and Neurotoxicity. In Neurotoxicity Research; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Kim, N.; Yoo, H.; Ju, Y.; Oh, M.S.; Lee, K.; Inn, K.; Kim, N.; Lee, J.K. Synthetic 3′,4′-Dihydroxyflavone Exerts Anti-Neuroinflammatory Effects in BV2 Microglia and a Mouse Model Namkwon. Biomol. Ther. 2018, 26, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.K.; Sun, K.; Sun, M.K.; Lee, M.; Tan, Y.; Chen, G.Q. 7,8-Dihydroxyflavone protects nigrostriatal dopaminergic neurons from rotenone-induced neurotoxicity in rodents. Park. Dis. 2019, 2019, 9193534. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xiang, Z.; Zhu, X.; Ai, Z.; Shen, J. Neuroprotective Effects of 7,8-dihydroxyflavone on Midbrain Dopaminergic Neurons in MPP+-treated Monkeys. Sci. Rep. 2016, 6, 34339. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Joo, Y.; Shin, M.; Kim, H.; Jae, M.; Hun, S.; Pil, S.; Kwon, S. Acacetin inhibits neuronal cell death induced by 6-hydroxydopamine in cellular Parkinson’s disease model. Bioorg. Med. Chem. Lett. 2017, 27, 5207–5212. [Google Scholar]
- Ning, X.; Yuan, M.; Guo, Y.; Tian, C.; Wang, X.; Ning, X.; Yuan, M.; Guo, Y.; Tian, C.; Wang, X. Neuroprotective effects of (E)-3, 4-diacetoxystyryl sulfone and sulfoxide derivatives in vitro models of Parkinson’s disease. J. Enzym. Inhib. Med. Chem. 2016, 31, 464–469. [Google Scholar]
- Lee, D.; Lee, M.; Hyun, S.; Saeng, G. Involvement of heme oxygenase-1 induction in the cytoprotective and neuroinflammatory activities of Siegesbeckia Pubescens isolated from 5,3′-dihydroxy-3,7,4′-trimethoxyflavone in HT22 cells and BV2 cells. Int. Immunopharmacol. 2016, 40, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, F.; Zhang, T.; Gu, J.; Li, C. Tetramethylpyrazine Nitrone Improves Neurobehavioral Functions and Confers Neuroprotection on Rats with Traumatic Brain Injury. Neurochem. Res. 2016, 41, 2948–2957. [Google Scholar] [CrossRef] [PubMed]
- Kunisawa, N.; Shimizu, S.; Kato, M.; Iha, H.A.; Iwai, C.; Hashimura, M.; Ogawa, M.; Kawaji, S.; Kawakita, K.; Abe, K.; et al. Pharmacological characterization of nicotine-induced tremor: Responses to anti-tremor and anti-epileptic agents. J. Pharmacol. Sci. 2018, 137, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, B.M.; Martini, A.; Aversa, D.; Piccinino, D.; Botta, L.; Berretta, N. Tyrosinase mediated oxidative functionalization in the synthesis of DOPA-derived peptidomimetics with anti-Parkinson activity. RSC Adv. 2017, 7, 20502–20509. [Google Scholar] [CrossRef]
- Malmlöf, T.; Feltmann, K.; Konradsson-Geuken, Å.; Schneider, F.; Alken, R.G.; Svensson, T.H.; Schilström, B. Deuterium-substituted L-DOPA displays increased behavioral potency and dopamine output in an animal model of Parkinson’s disease: Comparison with the effects produced by L-DOPA and an MAO-B inhibitor. J. Neural Transm. 2015, 122, 259–272. [Google Scholar] [CrossRef] [PubMed]
- González-lizárraga, F.; Socías, S.B.; Ávila, C.L.; Torres-Bugeau, C.M.; Barbosa, L.R.S.; Binolfi, A.; Sepúlveda-Díaz, J.E.; Del-Bel, E.; Fernandez, C.O.; Papy-Garcia, D.; et al. Repurposing doxycycline for synucleinopathies: Remodelling of α-synuclein oligomers towards non-toxic parallel beta-sheet structured species. Sci. Rep. 2017, 7, 41755. [Google Scholar] [CrossRef]
- Santa-Cecılia, F.V.; Socia, B.; Ouidja, M.O.; Sepulveda-Diaz, J.E.; Silva, R.L.; Michel, P.P.; Del-Bal, E.; Cunha, T.M.; Raisman-Vozari, R. Doxycycline Suppresses Microglial Activation by Inhibiting the p38 MAPK and NF-kB Signaling Pathways. Neurotox. Res. 2016, 29, 447–459. [Google Scholar] [CrossRef]
- Chen, C.; Xia, B.; Tang, L.; Wu, W.; Tang, J.; Liang, Y.; Yang, H.; Zhang, Z. Echinacoside protects against MPTP/MPP+ -induced neurotoxicity via regulating autophagy pathway mediated by Sirt1. Metab. Brain Dis. 2019, 34, 203–212. [Google Scholar] [CrossRef]
- Bello, M.; Morales-González, J.A. Molecular recognition between potential natural inhibitors of the Keap1-Nrf2 complex. Int. J. Biol. Macromol. 2017, 105, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Azab, S.M.; Ave, P. Glycine and Glod nanoparticles for the electrochemical determination of an anti-Parkinson’s drug in a tertiary mixture. Int. J. Pharm. Sci. Res. 2017, 8, 4839–4847. [Google Scholar]
- Vadlamudi, H.C.; Yalavarthi, P.R.; Rao, V.M.B.; Thanniru, J.; Vandana, K.R.; Sundaresan, C.R. Potential of microemulsified entacapone drug delivery systems in the management of acute Parkinson’s disease. J. Acute Dis. 2016, 5, 315–325. [Google Scholar] [CrossRef]
- Renaud, J.; Nabavi, S.F.; Daglia, M.; Nabavi, S.M.; Martinoli, M.G. Epigallocatechin-3-gallate, a promising molecule for Parkinson’s disease? Rejuvenation Res. 2015, 18, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, L.; Hu, X.; Cao, S.; Yang, J. Effects and mechanism of epigallocatechin-3-gallate on apoptosis and mTOR/AKT/GSK-3 β pathway in substantia nigra neurons in Parkinson rats. Eur. J. Med. Chem. 2019, 30, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hui, Y.C.; Guogang, W.; Zhuo, R. Neuroprotective Effects of Etidronate and 2, 3, 3-Trisphosphonate Against Glutamate-Induced Toxicity in PC12 Cells. Neurochem. Res. 2016, 41, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.T.; Varney, M.A.; McCreary, A.C. Effects of the Serotonin 5-HT 1A Receptor Biased Agonists, F13714 and F15599, on Striatal Neurotransmitter Levels Following l -DOPA Administration in Hemi-Parkinsonian Rats. Neurochem. Res. 2018, 43, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Huot, P.; Johnston, T.H.; Fox, S.H.; Newman-tancredi, A.; Brotchie, J.M. The highly-selective 5-HT 1A agonist F15599 reduces L -DOPA-induced dyskinesia without compromising anti-parkinsonian bene fi ts in the MPTP-lesioned macaque. Neuropharmacology 2015, 97, 306–311. [Google Scholar] [CrossRef]
- Cui, B.; Guo, X.; You, Y.; Fu, R. Farrerol attenuates MPP+-induced inflammatory response by TLR4 signaling in a microglia cell line. Phyther. Res. 2019, 33, 1134–1141. [Google Scholar] [CrossRef]
- Watanabe, R.; Kurose, T.; Morishige, Y.; Fujimori, K. Protective Effects of Fisetin Against 6-OHDA-Induced Apoptosis by Activation of PI3K-Akt Signaling in Human Neuroblastoma SH-SY5Y. Neurochem. Res. 2018, 43, 488–499. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Habtemariam, S.; Sureda, S.; Manayu, A. Neuroprotective effects of fisetin in Alzheimer’s and Parkinson’s Diseases: From chemistry to medicine. Curr. Top. Med. Chem. 2016, 16, 1910–1915. [Google Scholar] [CrossRef] [PubMed]
- Prakash, D.; Sudhandiran, G. Dietary flavonoid fisetin regulates Aluminium chloride induced neuronal apoptosis in cortex and hippocampus of mice brain. J. Nutr. Biochem. 2015, 26, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, P.; Na, J.; Jung, I.; Cho, J.; Lee, J. Anti-neuroinflammatory effects of galangin in LPS-stimulated BV-2 microglia through regulation of IL-1 production and the NF-B signaling pathways. Mol. Cell. Biochem. 2019, 451, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Phani, Y.C.G.; Ramya, K.E.M. Gallic Acid Protects 6-OHDA Induced Neurotoxicity by Attenuating Oxidative Stress in Human Dopaminergic Cell Line. Neurochem. Res. 2018, 43, 1150–1160. [Google Scholar]
- Minhas, S.T.; Al-tel, T.H.; Al-hayani, A.A.; Haque, M.E.; Eliezer, D.; El-agnaf, O.M.A. Structure activity relationship of phenolic acid inhibitors of α-synuclein fibril formation and toxicity. Front. Aging Neurosci. 2014, 6, 197. [Google Scholar]
- Khairujjaman, M.; Nivedita, M.; Anupom, B. Garcinol prevents hyperhomocysteinemia and enhances bioavailability of L-DOPA by inhibiting catechol-O-methyltransferase: An in silico approach. Med. Chem. Res. 2015, 25, 116–122. [Google Scholar]
- Wu, H.; Hu, Q.; Zhang, S.; Wang, Y.; Jin, Z.; Lv, L.; Zhang, S.; Liu, Z. Neuroprotective effects of genistein on SH-SY5Y cells overexpressing A53T mutant α-synuclein. Neural 2018, 13, 1375–1383. [Google Scholar]
- Zarmouh, N.O.; Messeha, S.S.; Elshami, F.M.; Soliman, K.F.A. Evaluation of the Isoflavone Genistein as Reversible Human Monoamine Oxidase-A and -B Inhibitor. Evid. Based Complement. Altern. Med. 2016, 2016, 1423052. [Google Scholar] [CrossRef]
- Wang, H.; Tang, C.; Jiang, Z.; Zhou, X.; Chen, J.; Na, M.; Shen, H.; Lin, Z. Glutamine promotes Hsp70 and inhibits α -Synuclein accumulation in pheochromocytoma PC12 cells. Exp. Ther. Med. 2017, 14, 1253–1259. [Google Scholar] [CrossRef][Green Version]
- Cacciatore, I.; Cornacchia, C.; Fornasari, E.; Baldassarre, L.; Pinnen, F.; Sozio, P.; di Stefano, A.; Marinelli, L.; Dean, A.; Fulle, S.; et al. A Glutathione Derivative with Chelating and in vitro Neuroprotective Activities: Synthesis, Physicochemical Properties, and Biological Evaluation. ChemMedChem 2013, 8, 1818–1829. [Google Scholar] [CrossRef]
- Cacciatore, I.; Marinelli, L.; di Stefano, A.; di Marco, V.; Orlando, G.; Gabriele, M.; Gatta, D.M.P.; Ferrone, A.; Franceschelli, S.; Spenaza, L.; et al. Chelating and antioxidant properties of L-Dopa containing tetrapeptide for the treatment of neurodegenerative diseases. Neuropeptides 2018, 71, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Aimé, P.; Dai, D.; Ramalingam, N.; Crary, J.F.; Burke, R.E.; Greene, L.A.; Levy, O.A. Guanabenz promotes neuronal survival via enhancement of ATF4 and parkin expression in models of Parkinson disease. Exp. Neurol. 2018, 303, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Hajialyani, M.; Hosein Farzaei, M.; Echeverría, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef] [PubMed]
- Varier, K.M.; Sumathi, T. Hinokitiol Offers Neuroprotection Against 6-OHDA-Induced Toxicity in SH-SY5Y Neuroblastoma Cells by Downregulating mRNA Expression of MAO/α -Synuclein/LRRK2/PARK7/PINK1/PTEN Genes. Neurotox. Res. 2019, 35, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Niveditha, S.; Shivanandappa, T. Neuroprotective action of 4-Hydroxyisophthalic acid against paraquat-induced motor impairment involves amelioration of mitochondrial damage and neurodegeneration in Drosophila. Neurotoxicology 2018, 66, 160–169. [Google Scholar]
- Workman, D.G.; Tsatsanis, A.; Lewis, F.W.; Boyle, J.P.; Mousadoust, M.; Hettiarachchi, N.T.; Hunter, M.; Peers, C.S.; Te, D.; Duce, J.A. Protection from neurodegeneration in the 6-hydroxydopamine model of Parkinson’s with novel 1-hydroxypyridin-2-one metal chelators. Metallomics 2015, 7, 867–876. [Google Scholar] [CrossRef]
- Athauda, D.; Foltynie, T. The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat. Rev. Neurol. 2014, 11, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, V.; Sgarlata, C.; Vecchio, G. Cyclodextrins 3-Functionalized with 8-Hydroxyquinolines: Copper- Binding Ability and Inhibition of Synuclein Aggregation. Chem. Asian J. 2016, 11, 2436–2442. [Google Scholar] [CrossRef]
- Cukierman, D.S.; Pinheiro, A.B.; Castiñeiras-Filho, S.L.; da Silva, A.S.; Miotto, M.C.; de Falco, A.; de Ribeiro, P.T.; Maisonette, S.; de Cunha, A.L.; Hauser-Davis, R.A.; et al. A moderate metal-binding hydrazone meets the criteria for a bioinorganic approach towards Parkinson’s disease: Therapeutic potential, blood-brain barrier crossing evaluation and preliminary toxicological studies. J. Inorg. Biochem. 2017, 170, 160–168. [Google Scholar] [CrossRef]
- Funakohi-Tago, M.; Sakata, T.; Fujiwara, S.; Sakakura, A.; Sugai, T. Hydroxytyrosol butyrate inhibits 6-OHDA-induced apoptosis through activation of the Nrf2/HO-1 axis in SH-SY5Y cells. Eur. J. Pharmacol. 2018, 834, 246–256. [Google Scholar] [CrossRef]
- Jin, J.; Wang, H.; Hua, X.; Chen, D.; Huang, C.; Chen, Z. An outline for the pharmacological effect of icariin in the nervous system. Eur. J. Pharmacol. 2019, 842, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Deng, Y.; Li, F.; Yin, C.; Shi, J.; Gong, Q. Icariside II attenuates lipopolysaccharide-induced neuroinflammation through inhibiting TLR4/MyD88/NF- κB pathway in rats. Biomed. Pharmacother. 2019, 111, 315–324. [Google Scholar] [CrossRef]
- Kumari, N.; Agrawal, S.; Kumari, R.; Sharma, D.; Luthra, P.M. Neuroprotective effect of IDPU (1-(7-imino-3-propyl-2,3-dihydrothiazolo [4,5-d]pyrimidin-6(7H)-yl)urea) in 6-OHDA induced Rodent model of hemiparkinson’s disease. Neurosci. Lett. 2018, 675, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Kandil, E.A.; Abdelkader, N.F.; El-sayeh, B.M.; Saleh, S. Imipramine and amitriptyline ameliorate the rotenone model of parkinson’s disease in rats. Neuroscience 2016, 332, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Wang, S.; Wang, M.; Fu, W.; Zhang, C.; Xu, D. Isobavachalcone Attenuates MPTP-Induced Parkinson’s Disease in Mice by Inhibition of Microglial Activation through NF-κ B Pathway. PLoS ONE 2017, 12, e0169560. [Google Scholar] [CrossRef] [PubMed]
- Magalingam, K.B.; Radhakrishnan, A. Protective effects of quercetin glycosides, rutin, and isoquercetrin against neurotoxicity in rat pheochromocytoma. Int. J. Immunopathol. Pharmacol. 2016, 29, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Yu, C.; Lee, J.; Moon, K.; Kim, E.; Yoo, S.; Koo, T. Subacute toxicity evaluation of KR-33493, FAF1 inhibitor for a new anti-parkinson’s disease agent, after oral administration in rats and dogs. Regul. Toxicol. Pharmacol. 2016, 81, 387–396. [Google Scholar] [CrossRef]
- Hu, X.; Niu, Y.; Zhang, Q.; Tian, X.; Gao, L.; Guo, L. Neuroprotective effects of Kukoamine B against hydrogen peroxide-induced apoptosis and potential mechanisms in SH-SY5Y cells. Environ. Toxicol. Pharmacol. 2015, 40, 230–240. [Google Scholar] [CrossRef]
- Seifar, F.; Khalili, M.; Khaledyan, H.; Amiri Moghadam, S.; Izadi, A.; Seifar, F.; Shakouri, S.K. α-Lipoic acid, functional fatty acid, as a novel therapeutic alternative for central nervous system diseases: A review. Nutr. Neurosci. 2019, 22, 306–316. [Google Scholar] [CrossRef]
- Kulikova, O.I.; Berezhnoy, D.S.; Stvolinsky, S.L.; Lopachev, A.V.; Orlova, V.S.; Fedorova, T.N. Neuroprotective effect of the carnosine—α-lipoic acid nanomicellar complex in a model of early-stage Parkinson’s disease. Regul. Toxicol. Pharmacol. 2018, 95, 254–259. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, C.; Lin, J.; Wang, M.; Wang, X. Lipoic acid alleviates L-DOPA-induced dyskinesia in 6-OHDA parkinsonian rats via anti-oxidative stress. Mol. Med. Rep. 2018, 17, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Amit, T.; Bar-Am, O.; Mechlovich, D.; Kupershmidt, L.; Youdim, M.B.H.; Weinreb, O. The novel multitarget iron chelating and propargylamine drug M30 affects APP regulation and processing activities in Alzheimer’s disease models. Neuropharmacology 2017, 123, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, G.; Li, P.; Wang, Y.; Si, C.; He, J.; Long, W.; Bai, Y.; Feng, Z.; Wang, X. Neuroprotective effects of macranthoin G from Eucommia ulmoides against hydrogen peroxide-induced apoptosis in PC12 cells via inhibiting NF-κB activation. Chem. Biol. Interact. 2014, 224, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Qiu, Y.; Bao, Z. Magnesium Lithospermate B Suppresses Lipopolysaccharide-Induced Neuroinflammation in BV2 Microglial Cells and Attenuates Neurodegeneration in Lipopolysaccharide-Injected Mice. J. Mol. Neurosci. 2018, 64, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Janhom, P.; Dharmasaroja, P. Neuroprotective Effects of Alpha-Mangostin on MPP(+)-Induced Apoptotic Cell Death in Neuroblastoma SH-SY5Y Cells. J. Toxicol. 2015, 2015, 919058. [Google Scholar] [CrossRef] [PubMed]
- Jaisin, Y.; Ratanachamnong, P.; Kuanpradit, C.; Khumpum, W.; Suksamrarn, S. Protective effects of γ-Mangostin on 6-OHDA-Induced Toxicity in SH-SY5Y Cells. Neurosci. Lett. 2018, 665, 229–235. [Google Scholar] [CrossRef]
- Paz, R.M.; Tubert, C.; Stahl, A.; Díaz, A.L.; Etchenique, R.; Murera, M.G.; Rela, L. Inhibition of striatal cholinergic interneuron activity by the Kv7 opener retigabine and the nonsteroidal anti-inflammatory drug diclofenac. Neuropharmacology 2018, 137, 309–321. [Google Scholar] [CrossRef]
- Ryu, Y.; Park, H.; Go, J.; Choi, D.; Kim, Y.; Hwang, J.H.; Noh, J.; Lee, T.G.; Lee, C.; Kim, K. Metformin Inhibits the Development of L-DOPA-Induced Dyskinesia in a Murine Model of Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 5715–5726. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Huttunen, K.M.; Sikora, J. Metformin—A Future Therapy for Neurodegenerative Diseases. Pharm. Res. 2017, 34, 2614–2627. [Google Scholar] [CrossRef]
- Agrawal, N.; Mishra, P. Synthesis, monoamine oxidase inhibitory activity and computational study of novel isoxazole derivatives as potential antiparkinson agents. Comput. Biol. Chem. 2019, 79, 63–72. [Google Scholar] [CrossRef]
- Beitnere, U.; van Groen, T.; Kumar, A.; Jansone, B.; Klusa, V.; Kadish, I. Mildronate Improves Cognition and Reduces Amyloid-β Pathology in Transgenic Alzheimer’s Disease Mice. J. Neurosci. Res. 2014, 92, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.K.; Singh, D.K.; Gupta, S.; Gupta, P.; Singh, A.; Biswas, J.; Singh, S. Minocycline diminishes the rotenone induced neurotoxicity and glial activation via suppression of apoptosis, nitrite levels and oxidative stress. Neurotoxicology 2018, 65, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Acquarone, M.; De Melo, T.M.; Meireles, F.; Brito-Moreira, J.; Houzel, J.; Rehen, S.K.; Meraz-Ríos, M.A. Mitomycin-treated undifferentiated embryonic stem cells as a safe and effective therapeutic strategy in a mouse model of Parkinson’s disease. Front. Cell. Neurosci. 2015, 9, 97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choudhury, A.; Chakraborty, I.; Banerjee, T.S.; Vana, D.R.; Adapa, D. Efficacy of Morin as a Potential Therapeutic Phytocomponent: Insights into the Mechanism of Action. Int. J. Med. Res. Health Sci. 2017, 6, 175–194. [Google Scholar]
- Cerri, S.; Levandis, G.; Ambrosi, G.; Montepeloso, E.; Lanciego, L.; Baqi, Y.; Antoninetti, G.F.; Franco, R.; Mu, C.E.; Pinna, A.; et al. Neuroprotective Potential of Adenosine A2A and Cannabinoid CB1 Receptor Antagonists in an Animal Model of Parkinson Disease. J. Neuropathol. Exp. Neurol. 2014, 73, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Ara, G.; Afzal, M.; Jyoti, S.; Rahul, F.N.; Siddique, Y.H. Effect of Myricetin on the Loss of Dopaminergic Neurons in the Transgenic Drosophila Model of Parkinson’s Disease. Curr. Drug Ther. 2019, 14, 58–64. [Google Scholar] [CrossRef]
- Das, S.; Pukala, T.L.; Smid, S.D. Exploring the Structural Diversity in Inhibitors of α-Synuclein Amyloidogenic Folding, Aggregation and Neurotoxicity. Front. Chem. 2018, 6, 181. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Pu, X. Myricitrin Alleviates Methylglyoxal-Induced Mitochondrial Dysfunction and AGEs/RAGE/NF-κB Pathway Activation in SH-SY5Y Cells. Mol. Neurosci. 2014, 53, 562–570. [Google Scholar] [CrossRef]
- Wu, L.; Lin, C.; Lin, H.; Liu, Y.; Wu, C.Y.; Tsai, C. Naringenin Suppresses Neuroinflammatory Responses Through Inducing Suppressor of Cytokine Signaling 3 Expression. Mol. Neurob. 2015, 53, 1080–1091. [Google Scholar] [CrossRef]
- Jung, U.J.; Kim, S.R. Effects of naringin, a flavanone glycoside in grapefruits and citrus fruits, on the nigrostriatal dopaminergic projection in the adult brain. Neural Regen. Res. 2014, 9, 7–10. [Google Scholar]
- Leem, E.; Han, J.; Jeon, M.; Shin, W.; Won, S.; Park, S. Naringin protects the nigrostriatal dopaminergic projection through induction of GDNF in a neurotoxin model of Parkinson’s disease. J. Nutr. Biochem. 2014, 25, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhu, Z.; Wu, F.; Zhou, Y.; Sheng, R.; Wu, J.; Qin, Z. NADPH ameliorates MPTP-induced dopaminergic neurodegeneration through inhibiting p38MAPK activation. Acta Pharmacol. Sin. 2018, 40, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, J.; Sheng, R.; Li, M.; Wang, Y.; Han, R.; Han, F. Reduced nicotinamide adenine dinucleotide phosphate inhibits MPTP-induced neuroinflammation and neurotoxicity. Neuroscience 2018, 391, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.E.I.; Tang, L.E.; Wei, W.; Hong, Y.; Chen, H.; Ying, W.; Chen, S. Nicotinamide mononucleotide improves energy activity and survival rate in an in vitro model of Parkinson’s disease. Exp. Ther. Med. 2014, 8, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Dahodwala, M.; Willis, A.W.; Li, P.; Doshi, J.A. Prevalence and Correlates of Anti-Parkinson Drug Use in a Nationally Representative. Mov. Disord. Clin. Pract. 2016, 22, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Min, J.; Lee, J.Y.; Chae, U.; Yang, E.J.; Song, K.S.; Lee, H.S.; Lee, H.J.; Lee, S.R.; Lee, D.S. Oleuropein isolated from Fraxinus rhynchophylla inhibits glutamate-induced neuronal cell death by attenuating mitochondrial dysfunction. Nutr. Neurosci. 2018, 21, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Hu, Q.; Wu, J.; Mu, C.; Ren, H. P7C3 Inhibits LPS-Induced Microglial Activation to Protect Dopaminergic Neurons Against Inflammatory Factor-Induced Cell Death in vitro and in vivo. Front. Cell. Neurosci. 2018, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- De Jesús-Cortés, H.; Miller, A.D.; Britt, J.K.; DeMarco, A.J.; De Jesús-Cortés, M.; Stuebing, E.; Naidoo, J.; Vázquez-Rosa, E.; Morlock, L.; Williams, N.S.; et al. Protective efficacy of P7C3-S243 in the 6-hydroxydopamine model of Parkinson’s disease. Front. Cell. Neurosci. 2015, 12, 3–8. [Google Scholar] [CrossRef]
- Pinna, A. Adenosine A 2A Receptor Antagonists in Parkinson’s Disease: Progress in Clinical Trials from the Newly Approved Istradefylline to Drugs in Early Development and Those Already Discontinued. CNS Drugs 2014, 28, 455–474. [Google Scholar] [CrossRef]
- Adlard, P.A.; Cherny, R.A.; Finkelstein, D.I.; Gautier, E.; Robb, E.; Cortes, M.; Volitakis, I.; Liu, X.; Smith, J.P.; Perez, K.; et al. Rapid restoration of cognition in alzheimer’s transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial abeta. Neuron 2008, 59, 43–55. [Google Scholar] [CrossRef]
- Finkelstein, D.I.; Billings, J.L.; Adlard, P.A.; Ayton, S.; Sedjahtera, A.; Masters, C.L.; Wilkins, S.; Shackleford, D.M.; Charman, S.A.; Bal, W.; et al. The novel compound PBT434 prevents iron mediated neurodegeneration and alpha-synuclein toxicity in multiple models of Parkinson’s disease. Acta Neuropathol. Commun. 2017, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Uliassi, E.; Pena-Altamira, L.; Morales, A.; Massenzio, F.; Petralla, S.; Rossi, M.; Roberti, M.; Gonzalez, L.; Martinez, A.; Monti, B. A focused library of psychotropic analogs with neuroprotective and neuroregenerative potential. ACS Chem. Neurosci. 2018, 10, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Gaisina, I.N.; Lee, S.H.; Kaidery, N.A.; Aissa, M.B.; Ahuja, M.; Smirnova, N.N.; Wakade, S.; Gaisin, A.; Bourassa, M.W.; Ratan, R.R.; et al. Activation of Nrf2 and Hypoxic Adaptive Response Contribute to Neuroprotection Elicited by Phenylhydroxamic Acid Selective HDAC6 Inhibitors. ACS Chem. Neurosci. 2018, 9, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tang, B.; Feng, Y.; Tang, F.; Hoi, M.P.; Su, Z.; Lee, S.M. Pinostrobin Exerts Neuroprotective Actions in Neurotoxin-Induced Parkinson’s Disease Models through Nrf2 Induction. J. Agric. Food Chem. 2018, 66, 8307–8318. [Google Scholar] [CrossRef] [PubMed]
- Kin, W.; Ko, D.; Li, Q.; Yun, L.; Morelli, M.; Carta, M.; Bezard, E. A preclinical study on the combined effects of repeated eltoprazine and preladenant treatment for alleviating L-DOPA-induced dyskinesia in Parkinson’s disease. Eur. J. Pharmacol. 2017, 813, 10–16. [Google Scholar]
- Nusrat, S.; Zaman, M.; Masroor, A.; Khursheed, M. Deciphering the enhanced inhibitory, disaggregating and cytoprotective potential of promethazine towards amyloid fibrillation. Int. J. Biol. Macromol. 2018, 106, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Li-Chao, W.; Li-Xi, L.; Ming-bo, Z.; Xin, D. Protosappanin A exerts anti-neuroinflammatory effect by inhibiting JAK2-STAT3 pathway in lipopolysaccharide-induced BV2 microglia. Chin. J. Nat. Med. 2017, 15, 674–679. [Google Scholar]
- Chu, J.F.; Han, W. Punicalagin Exerts Beneficial Functions in 6-Hydroxydopamine-Treated SH-SY5Y Cells by Attenuating Mitochondrial Dysfunction and Inflammatory Responses. Med. Sci. Monit. 2018, 24, 5905. [Google Scholar] [CrossRef]
- Gardiner, J.M.; Iqbal, J. Pyrazolobenzothiazine-based carbothioamides as new structural leads for the inhibition of monoamine oxidases: Design, synthesis, in vitro bioevaluation and molecular docking studies. Medchemcomm 2017, 8, 452–464. [Google Scholar]
- Awale, M.; Reymond, J.; Hediger, M.A. Discovery and characterization of a novel non-competitive inhibitor of the divalent metal transporter DMT1/SLC11A2. Biochem. Pharmacol. 2015, 96, 216–224. [Google Scholar]
- Singh, S.; Kumar, P. Piperine in combination with quercetin halt 6-OHDA induced neurodegeneration in experimental rats: Biochemical and neurochemical evidences. Neurosci. Res. 2018, 133, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Ay, M.; Luo, J.; Langley, M.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, G.A. Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunction and progressive dopaminergic neurodegeneration in cell cul. J. Neurochem. 2017, 141, 766–782. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Park, Y.J.; Yun, S.P.; Kwon, S.; Kim, D.; Kim, D.Y.; Shin, J.S.; Cho, D.J.; Lee, G.Y.; et al. The c-Abl inhibitor, Radotinib HCl, is neuroprotective in a preclinical Parkinson’s disease mouse model. Hum. Mol. Genet. 2018, 27, 2344–2356. [Google Scholar] [CrossRef] [PubMed]
- Saedisomeolia, A.; Ashoori, M. Riboflavin in Human Health: A Review of Current Evidences. Adv. Food Nutr. Res. 2018, 83, 57–81. [Google Scholar] [PubMed]
- Marashly, E.T.; Bohlega, S.A. Riboflavin Has Neuroprotective Potential: Focus on Parkinson’s Disease and Migraine. Front. Neurol. 2017, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Xu, H.; Jiang, H.; Xu, H.; Jiang, H. Rosmarinic acid protects against MPTP-induced toxicity and inhibits iron-induced α-synuclein aggregation. Neuropharmacology 2018, 144, 291–300. [Google Scholar] [CrossRef]
- Seeman, P. Parkinson’s Disease Treatment May Cause Impulse—Control Disorder Via Dopamine D3 Receptors. Synapse 2015, 69, 183–189. [Google Scholar] [CrossRef]
- Thakur, P.; Nehru, B. Modulatory effects of sodium salicylate on the factors affecting protein aggregation during rotenone induced Parkinson’s disease pathology. Neurochem. Int. 2014, 75, 1–10. [Google Scholar] [CrossRef]
- Michel, A.; Downey, P.; Nicolas, J.; Scheller, D. Unprecedented Therapeutic Potential with a Combination of A 2A/NR2B Receptor Antagonists as Observed in the 6-OHDA Lesioned Rat Model of Parkinson’s Disease. PLoS ONE 2014, 9, 1–25. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, S.; Du, N.; Liu, J.; Huang, Y.; Han, M. Neuroprotective effects of stemazole in the MPTP-induced acute model of Parkinson’s disease: Involvement of the dopamine system. Neurosci. Lett. 2016, 616, 152–159. [Google Scholar] [CrossRef]
- Kwon, S.; Ma, S.; Lee, S.; Jang, C. Sulfuretin inhibits 6-hydroxydopamine-induced neuronal cell death via reactive oxygen species-dependent mechanisms in human neuroblastoma SH-SY5Y cells. Neurochem. Int. 2014, 74, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Che, Y.; Sun, F.; Wang, Q. Taurine protects noradrenergic locus coeruleus neurons in a mouse Parkinson’s disease model by inhibiting microglial M1 polarization. Amino Acids 2018, 50, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Kim, Y.J.; Kim, B.; Park, G.; Jeong, S. The Anti-neuroinflammatory Activity of Tectorigenin Pretreatment via Downregulated NF-κB and ERK/JNK Pathways in BV-2 Microglial and Microglia Inactivation in Mice With Lipopolysaccharide. Front. Pharmacol. 2018, 9, 462. [Google Scholar] [CrossRef] [PubMed]
- Bortolanza, M.; Nascimento, G.C.; Socias, S.B.; Ploper, D.; Chehín, R.N.; Raisman-Vozari, R.; Del-Bel, E. Tetracycline repurposing in neurodegeneration: Focus on Parkinson’s disease. J. Neural Transm. 2018, 125, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Kheradmand, A.; Nayebi, A.M.; Jorjani, M.; Haddadi, R. Effect of WR-1065 on 6-hydroxydopamine-induced catalepsy and IL-6 level in rats. Iran. J. Basic Med. Sci. 2016, 19, 490–496. [Google Scholar] [PubMed]
- Hossain, M.M.; Weig, B.; Reuhl, K.; Gearing, M.; Wu, L.J. The anti-parkinsonian drug zonisamide reduces neuro inflammation: Role of microglial Nav 1.6. Exp. Neurol. 2018, 308, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Hershey, L.; Irwin, D. Zonisamide for DLB parkinsonism: An old drug used in a new context. Neurology 2018, 90, 349–350. [Google Scholar] [CrossRef]
- Uemura, M.T.; Asano, T.; Hikawa, R.; Yamakado, H.; Takahashi, R. Zonisamide inhibits monoamine oxidase and enhances motor performance and social activity. Neurosci. Res. 2017, 124, 25–32. [Google Scholar] [CrossRef]
- Ohman, L.; Sjijberg, S. The experimental determination of thermodynamic properties for aqueous aluminium complexes. Coord. Chem. Rev. 1996, 149, 33–57. [Google Scholar] [CrossRef]
- Kozlowski, H.; Remelli, M. Prion proteins and copper ions. Biological and chemical controversies. Dalton Trans. 2010, 39, 6371–6385. [Google Scholar] [CrossRef]
- Zhou, T.; Kong, X.; Hider, R.C. Synthesis and iron chelating properties of hydroxypyridinone and hydroxypyranone hexadentate ligands. Dalton Trans. 2019, 48, 3459–3466. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yang, L.; He, L. Overview of the detection methods for equilibrium dissociation constant K D of drug-receptor interaction. J. Pharm. Anal. 2018, 8, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Faller, P.; Hureau, C. Bioinorganic chemistry of copper and zinc ions coordinated to amyloid-β peptide. Dalton Trans. 2009, 21, 1080–1094. [Google Scholar] [CrossRef] [PubMed]
- Dalvit, C.; Parent, A.; Mathieu, M.; Vallèe, F.; Rak, A. Fast NMR methods for measuring in direct and/or competition mode the dissociation binding constants of chemical fragments interacting with a receptor. ChemMedChem 2019, 14, 1115–1127. [Google Scholar] [CrossRef]
- Neumaier, F.; Alpdogan, S.; Hescheler, J.; Schneider, T. A practical guide to the preparation and use of metal ion-buffered systems for physiological research. Acta Physiol. 2018, 222, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pedro, L.; van Voorhis, W.C.; Quinn, R.J. Optimization of Electrospray Ionization by Statistical Design of Experiments and Response Surface Methodology: Protein–Ligand Equilibrium Dissociation Constant Determinations. J. Am. Soc. Mass Spectrom. 2016, 27, 1520–1530. [Google Scholar] [CrossRef]
- ScQuery. The IUPAC Stability Constant Database; vers. 5.84; Academic Software: Yorks, UK, 2006. [Google Scholar]
- Ma, J.; Ni, X.; Gao, Y.; Huang, K.; Liu, J.; Wang, Y.; Chen, R.; Wang, C. Identification and biological evaluation of novel benzothiazole derivatives bearing a pyridine-semicarbazone moiety as apoptosis inducers via activation of procaspase-3 to caspase-3. Medchemcomm 2019, 10, 465–477. [Google Scholar] [CrossRef]
- Kalinowski, D.S.; Stefani, C.; Toyokuni, S.; Ganz, T.; Anderson, G.J.; Subramaniam, N.V.; Trinder, D.; Olynyk, J.K.; Chua, A.; Jansson, P.J.; et al. Redox cycling metals: Pedaling their roles in metabolism and their use in the development of novel therapeutics. BBA Mol. Cell Res. 2016, 1863, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.; Ferlin, M.G.; Cvijovic, M.; Djurdjevic, P.; Dotto, F.; Badocco, D.; Pastore, P.; Venzo, A.; Di Marco, V.B. Evaluation of 1,2-dimethyl-3-hydroxy-4-pyridinecarboxylic acid and of other 3-hydroxy-4-pyridinecarboxylic acid derivatives for possible application in iron and aluminium chelation therapy. Polyhedron 2014, 67, 520–528. [Google Scholar] [CrossRef]
- Merkofer, M.; Kissner, R.; Hider, R.C.; Brunk, U.T.; Koppenol, W.H. Fenton Chemistry and Iron Chelation under Physiologically Relevant Conditions: Electrochemistry and Kinetics. Chem. Res. Toxicol. 2006, 19, 1263–1269. [Google Scholar] [CrossRef]
- Koppenol, W.; Hider, R. Iron and redox cycling. Do’s and don’ts. Free Radic. Biol. Med. 2019, 133, 3–10. [Google Scholar] [CrossRef]
- Kiss, T.; Enyedy, E.; Jakusch, T. Development of the application of speciation in chemistry. Coord. Chem. Rev. 2017, 352, 401–423. [Google Scholar] [CrossRef]
- Kiss, T.; Enyedy, E.; Jakusch, T.; Domotor, O. Speciation of Metal Complexes of Medicinal Interest: Relationship between Solution Equilibria and Pharmaceutical Properties. Curr. Med. Chem. 2019, 26, 580–606. [Google Scholar] [CrossRef]
- Melchior, A.; Peralta, E.; Valiente, M.; Tavagnacco, C.; Endrizzi, F.; Tolazzi, M. Interaction of d10 metal ions with thioether ligands: A thermodynamic and theoretical study. Dalton Trans. 2013, 42, 6074–6082. [Google Scholar] [CrossRef]
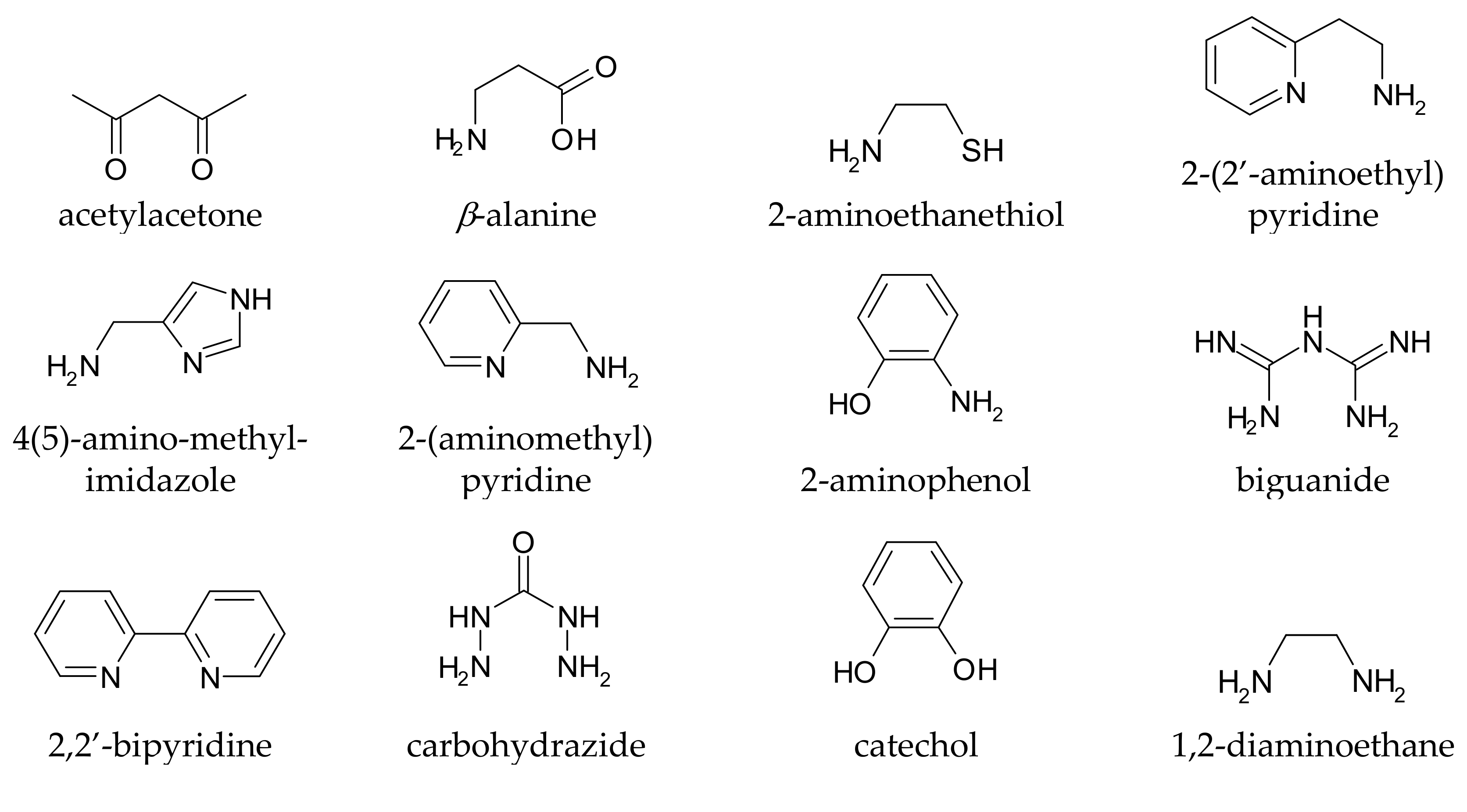

| Metal Ion | Kd (nmol/L) | References |
|---|---|---|
| Cu(II) | 102 | [12,61] |
| Cu(I) | 104–103 | [61] |
| Fe(III) | 10−4 | [12,60] |
| Fe(II) | 106–5 × 104 | [12,60] |
| Mn(II) | 106 | [60] |
| Zn(II) | >106 | [61] |
| Compound Name(s) | References |
|---|---|
| 7DH | [112] |
| 7MH | [112] |
| 8A | [89] |
| 8B | [89] |
| 8C | [89] |
| 8E | [89] |
| 8F | [89] |
| N-Acetylcysteine | [113,114] |
| ACPT-I | [115] |
| ADX88178 | [116] |
| Alaternin | [117] |
| Alvespimycin | [118] |
| AM-251 | [119,120] |
| Ambroxol | [121,122] |
| 3-(7-Amino-5-(cyclohexyl-amino) -[1,2,4]triazolo[1,5-a] [1,3,5]triazin-2-yl)-2 -cyanoacrylamide | [123] |
| Aminothiazoles derivatives as SUMOylation activators | [124] |
| AMN082 | [115] |
| Amodiaquine | [125,126] |
| Antagonist of the A(2A) adenosine receptor-derivative 49 | [127] |
| Apigenin | [128,129,130,131] |
| Apomorphine | [132,133] |
| l-Arginine | [134] |
| Aromadendrin | [128] |
| Ascorbic acid | [135,136] |
| ASI-1 | [12] |
| ASI-5 | [12] |
| Astilbin | [137] |
| Azilsartan | [138] |
| Baicalein | [139,140,141] |
| Benserazide | [142,143] |
| 7H-Benzo, perimidin-7-one derivatives (R6 = OH) | [144] |
| 4H-1-Benzopyran-4-one | [145] |
| 8-Benzyl-tetrahydropyrazino, purinedione derivatives (derivative n.57) | [146] |
| Bikaverin | [147] |
| (−)-N6-(2-(4-(Biphenyl-4-yl) piperazin-1-yl)-ethyl)-N6- propyl-4,5,6,7-tetrahydro- benzo, thiazole-2,6-diamine derivatives | [148] |
| 2.2’-Bipyridyl (2,2’-bipyridine) | [112] |
| 4-((5-Bromo-3-chloro-2- hydroxybenzyl) amino)-2- hydroxybenzoic acid (LX007, ZL006) | [149,150] |
| C-3 (α carboxyfullerene) | [151] |
| Caffeic acid amide analogues | [152,153,154,155] |
| Carbazole-derived compounds | [156] |
| Carbidopa | [135,157] |
| Carnosic acid | [154,158] |
| Catechin | [24,128] |
| Ceftriaxone | [12,159,160,161] |
| Celastrol | [162,163] |
| CEP-1347 | [164,165] |
| Chebulagic acid | [166] |
| Chlorogenic acid | [167] |
| 3′-O-(3-Chloropivaloyl) quercetin | [168] |
| Chlorpromazine | [108] |
| Chrysin | [128,169,170] |
| Clioquinol | [89,91,171,172] |
| Clioquinol-selegiline hybrid | [79] |
| Clovamide analogues (R1 and R2 = OH, and/or R3 and R4 = OH) | [173] |
| “Compound 1” | [174] |
| “Compound (−)-8a” | [175] |
| “Compound 8” | [176] |
| “Compound 21”, derivative of 3-methyl-1-(2,4,6-trihydroxy phenyl) butan-1-one | [177] |
| “Compound (−)-21a” , derivative of N-6-(2-(4-(1H-indol-5-yl) piperazin-1-yl)ethyl)-N-6- propyl-4,5,6,7-tetrahydro- benzo[d]thiazole-2,6-diamine | [178] |
| Creatine | [179,180] |
| Cudraflavone B | [181] |
| Curcumin | [89,117,182,183,184] |
| Cyanidin | [185,186] |
| D-512 | [187] |
| D-607 (bipyridyl-D2R/D3R agonist hybrid) | [12,188,189] |
| DA-2 (8D) | [12,89] |
| DA-3 | [12] |
| DA-4 | [12] |
| Dabigatran etexilate | [190] |
| Dabrafenib | [191] |
| (S)-3,4-DCPG | [115] |
| Deferasirox | [24] |
| Deferricoprogen | [192] |
| Delphinidin | [160,185,193,194] |
| Demethoxycurcumin | [195] |
| Dendropanax morbifera active compound | [196] |
| Desferrioxamine (Desferoxamine, Desferal, DFO) | [112] |
| (S)-N-(3-(3,6-Dibromo-9H -carbazol-9-yl)-2-fluoropropyl)-6-methoxypyridin-2-amine | [197] |
| 4,5-O-Dicaffeoyl-1-O-(malic acid methyl ester)-quinic acid derivatives (R1, R2, R3, R4, or R5 = caffeoyl) | [198] |
| Dihydromyricetin | [199] |
| 5-(3,4-Dihydroxybenzylidene) -2,2-dimethyl-1,3-dioxane-4,6-dione | [200] |
| 7,8-Dihydroxycoumarin derivative DHC12 | [79] |
| 3′,4′-Dihydroxyflavone | [201] |
| 7,8-Dihydroxyflavone | [202,203] |
| 5,7-Dihydroxy-4′-methoxy flavone | [204] |
| (E)-3,4-Dihydroxystyryl aralkyl sulfones | [205] |
| (E)-3,4-Dihydroxystyryl aralkyl sulfoxides | [205] |
| 5,3’-Dihydroxy-3,7,4’ -trimethoxyflavone | [206] |
| 2-[[(1,1-Dimethylethyl) oxidoimino]-methyl]-3,5,6- trimethylpyrazine | [207] |
| DKP | [85] |
| L-DOPA (levodopa, CVT-301) | [132,135,208] |
| DOPA-derived peptido-mimetics (deprotected) | [209] |
| DOPA-derived peptido-mimetics (protected) | [209] |
| L-DOPA deuterated (D3-L-DOPA) | [210] |
| Doxycycline | [211,212] |
| Droxidopa | [110] |
| Echinacoside | [213] |
| Ellagic acid | [214] |
| Entacapone (Comtan, ASI-6) | [12,215,216] |
| Enzastaurin | [164] |
| Epicatechin | [128,160,193,194] |
| Epigallocatechin-3-gallate | [117,217,218] |
| Etidronate (HEDPA) | [219] |
| Exifone | [12] |
| F13714 | [220] |
| F15599 | [220,221] |
| Farrerol | [222] |
| Fisetin (3,3′,4′,7-Tetrahydroxy flavone) | [223,224,225] |
| Fraxetin | [117] |
| Galangin | [226] |
| Gallic acid and derivatives | [214,227,228] |
| Gallocatechin | [128] |
| Garcinol | [229] |
| Genistein | [117,128,230,231] |
| Glutamine | [232] |
| Glutathione derivatives | [63] |
| Glutathione -hydroxyquinoline compound | [233] |
| Glutathione-l-DOPA compound | [234] |
| Gly-N-C-DOPA | [209] |
| GSK2795039 | [108] |
| Guanabenz | [235] |
| Hesperidin | [128,236] |
| Hinokitiol | [237] |
| 8-HQ-MC-5 (VK-28) | [12,24,89,92,93] |
| 4-Hydroxyisophthalic acid | [238] |
| 1-Hydroxy-2-pyridinone derivatives | [89,239] |
| 3-Hydroxy-4(1H)-pyridinone (Deferiprone) | [112,239,240] |
| 8-Hydroxyquinoline | [241] |
| 8-Hydroxyquinoline-2- carboxaldehyde isonicotinoyl hydrazone | [242] |
| Hydroxyquinoline-propargyl hybrid (HLA 20) | [12,79,89] |
| Hydroxytyrosol butyrate | [243] |
| Hyperoside | [117] |
| IC87201 | [150] |
| Icariin | [244] |
| Icariside II | [245] |
| l-(7-imino-3-propyl-2,3- dihydrothiazolo [4,5-d]pyrimidin-6(7H)-yl)urea | [246] |
| Imipramine | [247] |
| Isobavachalcone | [248] |
| Isochlorogenic acid | [167] |
| Isoquercetin (Isoquercitrin) | [249] |
| Kaempferol | [128,160,193,194] |
| Kaempferol, 3-O-a-l arabinofuranoside-7-O-a-l-rhamnopyranoside | [214] |
| KR33493 | [250] |
| Kukoamine | [251] |
| Lestaurtinib | [164] |
| Lipoic acid | [252,253,254] |
| Luteolin | [128] |
| LY354740 | [115] |
| M10 | [24] |
| M30 (VAR10303) | [112,255] |
| M99 | [24] |
| Macranthoin G | [256] |
| Magnesium lithospermate B | [257] |
| α-Mangostin | [258] |
| γ-Mangostin | [259] |
| MAOI-1 | [12] |
| MAOI-2 | [12] |
| MAOI-4 | [12] |
| MAOI-8 | [12] |
| Meclofenamic acid | [260] |
| Metformin (Met) | [261,262] |
| Methoxy-6-acetyl-7- methylijuglone | [117] |
| N’-(4-Methylbenzylidene)-5-phenylisoxazole-3-carbohydrazide | [263] |
| Mildronate | [264] |
| Minocycline | [12,160,265] |
| Mitomycin C | [266] |
| MitoQ | [108] |
| Morin (3,5,7,29,49-pentahydroxy flavone) | [160,193,194,267] |
| [18F]MPPF | [107] |
| MSX-3 | [268] |
| Myricetin | [128,269,270] |
| Myricitrin | [271] |
| Naringenin | [128,272] |
| Naringin | [117,273,274] |
| Nicotinamide adenine dinucleotide phosphate (NADPH) | [275,276] |
| Nicotinamide mononucleotide | [277] |
| Nitecapone | [278] |
| Nordihydroguaiaretic | [160,193,194] |
| Oleuropein | [279] |
| Opicapone | [278] |
| P7C3 | [280,281] |
| PBF-509 | [282] |
| PBT2 | [89,283] |
| PBT434 | [284] |
| Petunidin | [185] |
| Phenothiazine 2Bc (n = 0 and n = 1) | [285] |
| Phenylhydroxamates | [286] |
| Piceatannol | [160,193,194] |
| Pinostrobin | [287] |
| Piperazine-8-OH-quinolone hybrid | [79] |
| Preladenant | [282,288] |
| Promethazine | [289] |
| Protocatechuic acid | [170] |
| Protosappanin A | [290] |
| Punicalangin | [270,291] |
| Pyrazolobenzothiazine-based carbothioamides | [292] |
| Pyridoxal isonicotinoyl hydrazone (PIH) and related compounds | [24,89] |
| Pyrimidinone 8 | [293] |
| Q1 | [89] |
| Q4 | [89] |
| Quercetin | [117,294,295] |
| Quinolines Derivatives as SUMOylation activators | [124] |
| Radotinib | [296] |
| Riboflavin | [297,298] |
| Rifampicin (ASI-3) | [12,160] |
| Rimonabant | [119,120,282] |
| Rosmarinic acid | [154,299] |
| Rotigotine | [105,133,278,300] |
| Rutin | [128,249] |
| Salicylate, sodium salt | [301] |
| Salvianolic Acid B | [117] |
| SCH-58261 | [105,302] |
| SCH412348 | [105] |
| Silibinin (silybin) A, B | [89] |
| Silydianin | [24] |
| ST1535 | [282] |
| ST4206 | [282] |
| Staurosporine | [164] |
| Stemazole | [303] |
| Sulfuretin | [304] |
| Tannic acid | [160,193,194] |
| Tanshinol | [117] |
| Taurine | [305] |
| Taxifolin | [128] |
| Tectorigenin | [306] |
| Tetracycline | [307] |
| Tolcapone (ASI-7) | [12,105] |
| Tozadenant | [282,302] |
| Transilitin | [270] |
| O-Trensox | [24] |
| 2′,3′,4′-Trihydroxyflavone | [270] |
| 2,3,3-Trisphosphonate | [219] |
| V81444 | [282] |
| VAS3947 | [108] |
| VAS2870 | [108] |
| Verbascoside | [160,193,194] |
| WIN 55,212-2 | [119,120] |
| WR-1065 | [308] |
| Zonisamide | [309,310,311] |
| Compound Name(s) | pCu(II) | Kd (nmol/L) | Most Abundant Complex |
|---|---|---|---|
| 7DH 7MH | 14.2 | 5.91 × 10−5 | CuL2 |
| 8A 8B 8C | 14.2 | 5.91 × 10−5 | CuL2 |
| 8E 8F | 10.6 | 2.35 × 10−1 | CuL2 |
| N-Acetyl cysteine | 6.2 | 3.82 × 104 | CuL |
| ACPT-I | 7.3 | 5.93 × 102 | CuL |
| ADX88178 | 7.4 | 5.15 × 102 | CuL |
| Alaternin | 16.5 | 3.39 × 10−7 | CuL2 |
| Alvespimycin | 10.3 | 5.35 × 10−1 | CuL22+ |
| AM-251 | 6.3 | 1.47 × 104 | CuL |
| Ambroxol | 9.2 | 7.26 | CuL+ |
| 3-(7-Amino-5-(cyclohexylamino)-[1,2,4]triazolo[1,5-a][1,3,5] triazin-2-yl)-2-cyanoacrylamide | 9.1 | 7.58 | CuL2 |
| Aminothiazoles derivatives as SUMOylation activators | 9.7 | 1.86 | CuL2 |
| AMN082 | 10.3 | 5.35 × 10−1 | CuL22+ |
| Amodiaquine | 6.1 | 1.90 × 107 | CuHL |
| Antagonist of the A(2A) adenosine receptor-derivative 49 | 6.4 | 9.65 × 103 | CuL |
| Apigenin | 6.8 | 2.39 × 103 | CuH2L+ |
| Apomorphine | 7.4 | 5.06 × 102 | CuL |
| l-Arginine | 7.1 | 9.57 × 102 | CuL2+ |
| Aromadendrin | 11.1 | 8.29 × 10−2 | CuL |
| Ascorbic acid | 6.1 | 2.43 × 105 | Cu2H–2L2 |
| ASI-1 | 7.9 | 1.21 × 102 | CuL2 |
| ASI-5 | 6.1 | 2.23 × 106 | CuL |
| Astilbin | 7.4 | 5.06 × 102 | CuL |
| Azilsartan | 6.1 | 4.53 × 105 | CuL |
| Baicalein | 9.3 | 5.41 | CuL22− |
| Benserazide | 9.3 | 5.30 | CuL2 |
| 7H-benzo[e] perimidin-7-one derivatives | 14 | 1.05 × 10−4 | CuL2 |
| 8-Benzyl-tetrahydropyrazino[2,1-f]purinedione (derivative n. 57) | 6.1 | 3.36 × 106 | CuL |
| Bikaverin | 14 | 1.05 × 10−4 | CuL2 |
| (−)-N6-(2-(4-(Biphenyl-4-yl)piperazin-1-yl)-ethyl)-N6-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine derivatives | 10.3 | 5.35 × 10−1 | CuL22+ |
| 2,2′-bipyridyl | 10.6 | 2.35 × 10−1 | CuL22+ |
| 4-((5-bromo-3-chloro-2-hydroxybenzyl) amino)-2-hydroxybenzoic acid (LX007, ZL006) | 6.2 | 6.51 × 104 | CuL |
| C-3 (α-carboxyfullerene) | 6.9 | 1.96 × 103 | CuL |
| Caffeic acid amide analogues | 7.3 | 6.02 × 102 | CuH−1L |
| Carbazole-derived compounds | 10.3 | 5.35 × 10−1 | CuL22+ |
| Carbidopa | 15.1 | 8.11 × 10−6 | CuH−2L |
| Carnosic acid | 7.4 | 5.06 × 102 | CuL |
| Catechin | 7.9 | 1.50 × 102 | CuH2L |
| Ceftriaxone | 6.1 | 4.04 × 106 | CuL |
| Celastrol | 6.5 | 5.27 × 103 | CuL |
| Chebulagic acid | 6.3 | 1.51 × 104 | CuHL |
| Chlorogenic acid | 8.3 | 5.04 × 101 | CuL− |
| 3′-O-(3-chloropivaloyl) quercetin | 11.1 | 8.29 × 10−2 | CuL |
| Chlorpromazine | 6.3 | 1.47 × 104 | CuL2+ |
| Chrysin | 10.6 | 2.97 × 10−1 | CuHL+ |
| Clioquinol | 14.2 | 5.91 × 10−5 | CuL2 |
| Clovamide analogues (R1 and R2 = OH, and/or R3 and R4 = OH) | 7.4 | 5.06 × 102 | CuL |
| “Compound 1” | 10.3 | 5.35 × 10−1 | CuL22+ |
| “Compound 8” | 6.1 | 4.37 × 105 | CuL2 |
| “Compound 21”, derivative of 3-methyl-1-(2,4,6-trihydroxyphenyl) butan-1-one | 7.3 | 5.93 × 102 | CuL+ |
| “Compound (−)-21a”, derivative of N-6-(2-(4-(1H-indol-5-yl) piperazin-1-yl)ethyl)-N-6-propyl-4,5,6,7-tetrahydrobenzo[d] thiazole-2,6-diamine | 10.3 | 5.35 × 10−1 | CuL23+ |
| Creatine | 6.8 | 2.26 × 103 | CuH−1L |
| Cudraflavone B | 11.1 | 8.29 × 10−2 | CuL |
| Curcumin | 7.9 | 1.21 × 102 | CuL2 |
| Cyanidin | 7.4 | 5.06 × 102 | CuL |
| D512 | 10.3 | 5.35 × 10−1 | CuL22+ |
| D607 (bipyridyl-D2R/D3R agonist hybrid) | 10.6 | 2.35 × 10−1 | CuL2 |
| DA-2 (8D) | 14.2 | 5.91 × 10−5 | CuL2 |
| DA-3 | 10.3 | 5.35 × 10−1 | CuL2 |
| DA-4 | 10.3 | 5.35 × 10−1 | CuL2 |
| Dabigatran etexilate | 10.3 | 5.35 × 10−1 | CuL2 |
| Dabrafenib | 6.1 | 3.36 × 106 | CuL |
| (S)-3-4-DCPG | 6.1 | 4.54 × 105 | CuL |
| Deferricoprogen | 12.6 | 3.17 × 10−3 | CuHL |
| Delphinidin | 9.3 | 5.30 | CuL2 |
| Demethoxycurcumin | 7.9 | 1.21 × 102 | CuL2 |
| Dendropanax morbifera active compound | 7.4 | 5.06 × 102 | CuL |
| Desferrioxamine (Deferoxamine, Desferal, DFO) | 11.4 | 4.98 × 10−2 | CuH2L+ |
| (S)-N-(3-(3-6-dibromo-9H-carbazol-9-yl)-2-fluoropropyl)-6- methoxypyridin-2-amine | 6.3 | 1.47 × 104 | CuL |
| 4, 5-O-Dicaffeoyl-1-O-(malic acid methyl ester)-quinic acid (R1, R2, R3, R4, or R5 = caffeoyl) | 7.4 | 5.06 × 102 | CuL |
| Dihydromyricetin | 9.3 | 5.30 | CuL2 |
| 5-(3,4-Dihydroxybenzylidene)-2,2-dimethyl-1,3-dioxane-4,6-dione | 7.4 | 5.06 × 102 | CuL |
| 7,8-Dihydroxycoumarin derivative DHC12 | 7.4 | 5.06 × 102 | CuL |
| 3′,4′-Dihydroxyflavone | 7.4 | 5.06 × 102 | CuL |
| 7,8-Dihydroxyflavone | 7.4 | 5.06 × 102 | CuL |
| 5,7-Dihydroxy-4′-methoxyflavone | 6.6 | 4.58 × 103 | CuL |
| (E)-3, 4-Dihydroxystyryl aralkyl sulfones | 7.4 | 5.06 × 102 | CuL |
| (E)-3, 4-Dihydroxystyryl aralkyl sulfoxides | 7.4 | 5.06 × 102 | CuL |
| 5,3′-Dihydroxy-3,7,4′-trimethoxyflavone | 6.6 | 4.58 × 103 | CuL |
| 2-[[(1,1-Dimethylethyl) oxidoimino]-methyl]-3,5,6-trimethylpyrazine | 9.6 | 2.98 | CuL |
| DKP | 8.1 | 8.43 × 101 | CuL |
| l-DOPA (levodopa, CVT-301) | 15.2 | 7.95 × 10−6 | CuH−2L3− |
| DOPA-derived peptido-mimetics (deprotected) | 15.2 | 7.95 × 10−6 | CuH−2L2 |
| DOPA-derived peptido-mimetics (protected) | 7.4 | 5.06 × 102 | CuL |
| l-DOPA deuterated | 15.2 | 7.95 × 10−6 | CuH−2L3− |
| Doxycycline | 8.9 | 1.28 × 101 | CuL |
| Droxidopa | 15.2 | 7.95 × 10−6 | CuH−2L3− |
| Echinacoside | 7.4 | 5.06 × 102 | CuL |
| Ellagic acid | 7.4 | 5.06 × 102 | CuL |
| Entacapone (comtan, ASI-6) | 10.1 | 8.00 × 10−1 | CuL22− |
| Enzastaurin | 10.3 | 5.35 × 10−1 | CuL2 |
| Epigallocatechin-3-gallate | 6.1 | 1.33 × 105 | CuH2L2 |
| Etidronate (HEDPA) | 9 | 1.11 × 101 | CuL2− |
| Exifone | 6.3 | 1.51 × 104 | CuHL |
| F13714, F15599 | 9.7 | 1.86 | CuL2 |
| Farrerol | 11.1 | 8.29 × 10−2 | CuL |
| Fisetin (3,3′,4′,7-tetra-hydroxy-flavone) | 7.4 | 5.06 × 102 | CuL |
| Fraxetin | 7.4 | 5.06 × 102 | CuL |
| Galangin | 9.1 | 8.20 | CuL |
| Gallic acid derivatives | 6.3 | 1.51 × 104 | CuHL− |
| Gallocatechin | 9.3 | 5.30 | CuL2 |
| Garcinol | 7.4 | 5.06 × 102 | CuL |
| Genistein | 11.1 | 8.29 × 10−2 | CuL |
| Glutamine | 7.3 | 5.38 × 102 | CuL+ |
| Glutathione derivatives | 6.2 | 7.23 × 104 | CuL− |
| Glutathione-hydroxy-quinoline compound | 9.4 | 5.00 | CuH−1L+ |
| Glutathione-l-DOPA compound | 13.5 | 3.98 × 10−4 | CuH−1L |
| Gly-N-C-DOPA | 15.2 | 7.95 × 10−6 | CuH−2L3− |
| GSK2795039 | 12 | 1.02 × 10−2 | CuL2 |
| Guanabenz | 10.3 | 5.35 × 10−1 | CuL2 |
| Hesperidin | 11.1 | 8.29 × 10−2 | CuL |
| Hinokitiol | 7.3 | 6.69 × 102 | CuL+ |
| 8-HQ-MC-5 (VK-28) | 14.2 | 5.91 × 10−5 | CuL2 |
| 4-Hydroxyisophthalic acid | 6.3 | 2.06 × 104 | CuL |
| 1-Hydroxy-2-pyridinone derivatives | 8.4 | 4.26 × 101 | CuL2 |
| 3-Hydroxy-4(1H)pyridinone (Deferiprone) | 10.2 | 6.60 × 10−1 | CuL2 |
| 3-Hydroxy-4(1H)pyridinone derivatives (R = H) | 10.2 | 6.60 × 10−1 | CuL2 |
| 8-Hydroxyquinoline | 14.2 | 5.91 × 10−5 | CuL2 |
| 8-Hydroxyquinoline-2-carboxaldehyde isonicotinoyl hydrazone | 14.2 | 5.91 × 10−5 | CuL2 |
| Hydroxy-quinoline-propargyl hybrids (HLA20) | 14.2 | 5.91 × 10−5 | CuL2 |
| Hydroxytyrosol butyrate | 7.4 | 5.06 × 102 | CuL |
| Hyperoside | 9.1 | 8.20 | CuL |
| IC87201 | 6.1 | 1.90 × 107 | CuHL |
| Icariin | 6.6 | 4.58 × 103 | CuL |
| Icariside II | 6.6 | 4.58 × 103 | CuL |
| l-(7-Imino-3-propyl-2,3-dihydrothiazolo[4,5-d]pyrimidin -6(7H)-yl)urea | 6.1 | 4.37 × 105 | CuL2 |
| Imipramine | 6.3 | 1.47 × 104 | CuL2+ |
| Isobavachalcone | 6.2 | 8.13 × 104 | CuL+ |
| Isochlorogenic acid | 8.3 | 5.04 × 101 | CuL− |
| Isoquercetin (isoquercitrin) | 9.1 | 8.20 | CuL |
| Kaempferol | 9.1 | 8.20 | CuL |
| KR33493 | 7.3 | 5.93 × 102 | CuL |
| Kukoamine | 7.4 | 5.06 × 102 | CuL |
| Lestaurtinib | 10.3 | 5.35 × 10−1 | CuL2 |
| Lipoic acid | 6.1 | 4.41 × 105 | CuL+ |
| Luteolin | 7.4 | 5.06 × 102 | CuL |
| M10 M30 (VAR10303) M99 | 14.2 | 5.91 × 10−5 | CuL2 |
| Macranthoin G | 7.4 | 5.06 × 102 | CuL |
| Magnesium lithospermate B | 7.4 | 5.06 × 102 | CuL |
| α-mangostin | 7.4 | 5.06 × 102 | CuL |
| γ-Mangostin | 7.4 | 5.06 × 102 | CuL |
| MAOI-1 | 9.3 | 5.30 | CuL2 |
| MAOI-2 | 10.3 | 5.35 × 10−1 | CuL2 |
| MAOI-4 | 6.3 | 1.47 × 104 | CuL |
| MAOI-8 | 6.1 | 1.90 × 107 | CuHL |
| Metformin (met) | 6.3 | 1.61 × 104 | CuL+ |
| Methoxy-6-acetyl-7-methylijuglone | 14 | 1.05 × 10−4 | CuL2 |
| N′-(4-methylbenzylidene)-5-phenylisoxazole-3-carbohydrazide | 6.3 | 1.38 × 104 | CuL |
| Minocycline | 11.6 | 2.83 × 10−2 | CuL |
| Mitomycin C | 7.1 | 9.14 × 102 | CuL |
| Morin | 6.1 | 2.27 × 1015 | CuH3L |
| [18F]MPPF | 10.3 | 5.35 × 10−1 | CuL2 |
| MSX-3 | 6.1 | 2.01 × 106 | CuL |
| Myricetin Myricitrin | 9.1 | 8.20 | CuL |
| Naringin | 6.9 | 1.58 × 103 | CuHL |
| Naringenin | 6.9 | 1.58 × 103 | CuHL |
| Nicotinamide adenine dinucleotide phosphate (NADPH) | 6.4 | 8.91 × 103 | CuL |
| Nicotinamide mononucleotide | 6.1 | 2.01 × 106 | CuL |
| Nitecapone | 10.1 | 8.00 × 10−1 | CuL22− |
| Nordihydroguaiaretic acid | 7.4 | 5.06 × 102 | CuL |
| Oleuropein | 7.4 | 5.06 × 102 | CuL |
| Opicapone | 10.1 | 8.00 × 10−1 | CuL2 |
| P7C3 | 7.8 | 3.76 × 102 | Cu2H−2L2+ |
| PBT2 | 12.4 | 4.04 × 10−3 | CuL+ |
| PBT434 | 11.2 | 7.21 × 10−2 | CuL+ |
| Petunidin | 7.4 | 5.06 × 102 | CuL |
| Phenothiazine 2Bc (n=0) | 10.3 | 5.35 × 10−1 | CuL22+ |
| Phenothiazine 2Bc (n=1) | 6.3 | 1.47 × 104 | CuL2+ |
| Phenylhydroxamates | 7 | 1.46 × 103 | CuL |
| Piceatannol | 7.4 | 5.06 × 102 | CuL |
| Pinostrobin (5-hydroxy-7-methoxy-flavone) | 6.6 | 4.58 × 103 | CuL+ |
| Piperazine-8-OH-quinolone hybrid | 14.2 | 5.91 × 10−5 | CuL2 |
| Preladenant | 10.3 | 5.35 × 10−1 | CuL2 |
| Promethazine | 8.2 | 7.99 × 101 | CuL2+ |
| Protocatechuic acid | 8.1 | 8.13 × 101 | CuL− |
| Protosappanin A | 7.4 | 5.06 × 102 | CuL |
| Punicalangin | 8.2 | 6.89 × 101 | CuL |
| Pyrazolobenzothiazine-based carbothioamides | 6.1 | 4.66 × 105 | CuL |
| Pyrimidinone 8 | 10.3 | 5.35 × 10−1 | CuL2 |
| Q1 Q4 | 14.2 | 5.91 × 10−5 | CuL2 |
| Quercetin | 9.1 | 8.20 | CuL3− |
| Quinoline derivatives SUMOylation activators | 7.2 | 7.26 × 102 | CuL2+ |
| Radotinib | 10.6 | 2.35 × 10−1 | CuL2 |
| Riboflavin | 6.1 | 1.65 × 105 | CuHL3+ |
| Rifampicin (ASI-3) | 6.5 | 6.97 × 103 | CuL |
| Rimonabant | 8.1 | 8.43 × 101 | CuL |
| Rosmarinic acid | 7.4 | 5.06 × 102 | CuL |
| Rotigotine | 6.1 | 1.45 × 108 | CuL2 |
| Rutin | 9.1 | 8.20 | CuL |
| Salicylate, sodium salt | 6.3 | 2.06 × 104 | CuL |
| Salvianolic acid B | 7.4 | 5.06 × 102 | CuL |
| SCH58261 SCH412348 | 9.1 | 7.58 | CuL2 |
| ST1535 ST4206 | 9.1 | 7.58 | CuL2 |
| Staurosporine | 10.3 | 5.35 × 10−1 | CuL2 |
| Stemazole | 6.1 | 4.66 × 105 | CuL |
| Sulfuretin | 7.4 | 5.06 × 102 | CuL |
| Tannic acid | 6.1 | 1.84 × 105 | CuL |
| Tanshinol | 7.4 | 5.06 × 102 | CuL |
| Taurine | 6.1 | 1.23 × 107 | CuL+ |
| Taxifolin | 10.4 | 4.55 × 10−1 | CuL2− |
| Tectorigenin | 11.1 | 8.29 × 10−2 | CuL |
| Tetracycline | 6.4 | 1.08 × 104 | CuL |
| Tolcapone (ASI-7) | 10.1 | 8.00 × 10−1 | CuL2 |
| Transilitin | 7.4 | 5.06 × 102 | CuL |
| o-Trensox | 22.9 | 1.51 × 10−13 | CuL4− |
| 2′, 3′, 4′-Trihydroxyflavone | 9.3 | 5.30 | CuL22− |
| 2,3,3-Trisphosphonate | 14 | 9.98 × 10−5 | CuL2 |
| V81444 | 9.7 | 1.86 | CuL2 |
| VAS3947 VAS2870 | 6.1 | 4.37 × 105 | CuL2 |
| Verbascoside | 7.3 | 6.02 × 102 | CuH−1L |
| WIN 55, 212-2 | 10.3 | 5.35 × 10−1 | CuL22+ |
| WR-1065 | 6.6 | 3.67 × 103 | CuL2+ |
| Zonisamide | 7.4 | 4.62 × 102 | CuL |
| Compound Name(s) | pCu(I) | Kd (nmol/L) | Most Abundant Complex |
|---|---|---|---|
| 7DH 7MH | 6.3 | 8.38 × 103 | CuL2 |
| 8A 8B 8C | 6.3 | 8.38 × 103 | CuL2 |
| 8E 8F | 6.2 | 1.87 × 104 | CuL |
| ACPT-I | 6 | 2.54 × 108 | CuL2 |
| ADX88178 | 7.7 | 1.77 × 102 | CuL |
| Alvespimycin | 6 | 7.98 × 107 | CuL2+ |
| 3-(7-Amino-5-(cyclohexylamino)-[1,2,4]triazolo[1,5-a] [1,3,5]triazin-2-yl)-2-cyanoacrylamide | 6 | 7.98 × 107 | CuL2 |
| Aminothiazoles derivatives as SUMOylation activators | 6 | 2.66 × 106 | CuL2 |
| AMN082 | 6 | 7.98 × 107 | CuL2 |
| Antagonist of the A(2A) adenosine receptor (derivative 49) | 6 | 3.06 × 107 | CuL2 |
| 8-Benzyl-tetrahydropyrazino[2,1-f]purinedione (derivative 57) | 7.6 | 2.17 × 102 | CuL |
| (−)-N6-(2-(4-(Biphenyl-4-yl)piperazin-1-yl)-ethyl)-N6-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine derivatives | 6 | 7.98 × 107 | CuL2+ |
| 2,2′-bipyridyl | 6.2 | 1.87 × 104 | CuL+ |
| Carbazole-derived compounds | 6 | 7.98 × 107 | CuL2+ |
| Ceftriaxone | 6 | 2.54 × 108 | CuL2 |
| Clioquinol | 7.2 | 5.79 × 102 | CuL2− |
| “Compound 1” | 6 | 7.98 × 107 | CuL2+ |
| “Compound 8” | 7.6 | 2.17 × 102 | CuL |
| “Compound 21”, derivative of 3-methyl-1-(2,4,6-trihydroxy phenyl) butan-1-one | 6 | 2.54 × 108 | CuL2− |
| “Compound (−)-21a”, derivative of N-6-(2-(4-(1H-indol-5-yl) piperazin-1-yl)ethyl)-N-6-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine | 6 | 7.98 × 107 | CuL2+ |
| Creatine | 6 | 2.54 × 108 | CuL2− |
| D512 | 6 | 7.98 × 107 | CuL2+ |
| D607(bipyridyl-D2R/D3R agonist hybrid) | 6.2 | 1.87 × 104 | CuL |
| DA-2 (8D) | 6.3 | 8.38 × 103 | CuL2 |
| DA-3 | 6 | 7.98 × 107 | CuL2 |
| DA-4 | 6 | 7.98 × 107 | CuL2 |
| Dabigatran etexilate | 6 | 7.98 × 107 | CuL2 |
| Dabrafenib | 7.7 | 1.77 × 102 | CuL |
| 2-[[(1,1-Dimethylethyl) oxidoimino]-methyl]-3,5,6-trimethylpyrazine | 6.9 | 1.07 × 103 | CuH2L2 |
| DKP | 7.4 | 3.64 × 102 | CuL |
| Doxycycline | 9.2 | 1.26 × 101 | Cu2L |
| Enzastaurin | 6 | 7.98 × 107 | CuL2 |
| F13714 F15599 | 6 | 7.83 × 105 | Cu2L |
| Glutathione-hydroxy-quinoline compound | 6.3 | 8.38 × 103 | CuL2− |
| Glutathione derivatives | 15.2 | 6.21 × 10−6 | CuHL− |
| Guanabenz | 6 | 7.98 × 107 | CuL2 |
| 8-HQ-MC-5 (VK-28) | 6.3 | 8.38 × 103 | CuL2 |
| 8-hydroxyquinoline | 6.3 | 8.38 × 103 | CuL2− |
| 8-hydroxyquinoline-2-carboxaldehyde isonicotinoyl hydrazone | 6.3 | 8.38 × 103 | CuL2 |
| Hydroxy-quinoline-propargyl hybrids (HLA20) | 6.3 | 8.38 × 103 | CuL2 |
| l-(7-Imino-3-propyl-2,3-dihydrothiazolo [4,5-d] pyrimidin-6(7H)-yl)urea | 7.6 | 2.17 × 102 | CuL |
| KR33493 | 6 | 2.54 × 108 | CuL2 |
| Lestaurtinib | 6 | 7.98 × 107 | CuL2 |
| M10 M30 (VAR10303) M99 | 6.3 | 8.38 × 103 | CuL2 |
| MAOI-2 | 6 | 7.98 × 107 | CuL2 |
| [18F]MPPF | 6 | 7.98 × 107 | CuL2 |
| PBF-509 | 6 | 7.98 × 107 | CuL2 |
| PBT2 | 6.3 | 8.38 × 103 | CuL2 |
| Phenothiazine 2Bc (n=0) | 6 | 7.98 × 107 | CuL2+ |
| Piperazine-8-OH-quinolone hybrid | 6.3 | 8.38 × 103 | CuL2 |
| Preladenant | 6 | 7.98 × 107 | CuL2 |
| Promethazine | 6 | 7.98 × 107 | CuL2+ |
| Pyrimidinone 8 | 6 | 7.98 × 107 | CuL2 |
| Q1 Q4 | 6.3 | 8.38 × 103 | CuL2 |
| Radotinib | 6.2 | 1.87 × 104 | CuL |
| Rifampicin (ASI-3) | 9.2 | 1.26 × 101 | Cu2L |
| Rimonabant | 7.4 | 3.64 × 102 | CuL |
| Rotigotine | 7.7 | 1.77 × 102 | CuL |
| SCH58261 SCH412348 | 6 | 7.98 × 107 | CuL2 |
| ST1535 ST4206 | 6 | 7.98 × 107 | CuL2 |
| Staurosporine | 6 | 7.98 × 107 | CuL2 |
| V81444 | 6 | 2.66 × 106 | CuL2 |
| VAS3947 VAS2870 | 7.6 | 2.17 × 102 | CuL |
| WIN 55, 212-2 | 6 | 7.98 × 107 | CuL2+ |
| WR-1065 | 7.6 | 2.17 × 102 | CuL |
| Compound Name(s) | pFe(III) | Kd (nmol/L) | Most Abundant Complex |
|---|---|---|---|
| 7DH 7MH | 20.6 | 2.15 × 10−1 | FeL3 |
| 8A 8B 8C | 20.6 | 2.15 × 10−1 | FeL3 |
| 8E 8F | 21.5 | 3.01 × 10−2 | FeH–2L2 |
| N-Acetyl cysteine | 16.1 | 4.59 × 109 | FeL2− |
| ACPT-I | 16.1 | 9.59 × 107 | FeL2 |
| Ambroxol | 16.3 | 1.70 × 104 | FeL2+ |
| Apigenin | 16.1 | 7.55 × 108 | FeL2+ |
| Apomorphine | 16.3 | 1.35 × 104 | FeL2 |
| l-Arginine | 16.1 | 1.18 × 1012 | FeL3+ |
| Aromadendrin | 16.1 | 7.55 × 108 | FeL |
| Ascorbic acid | 16.1 | 4.99 × 1017 | FeL2+ |
| ASI-1 | 16.8 | 2.28 × 103 | FeL |
| ASI-5 | 18 | 1.06 × 102 | FeL |
| Astilbin | 16.3 | 1.35 × 104 | FeL2 |
| Baicalein | 16.1 | 7.55 × 108 | FeL2+ |
| 4H-1-benzopyran-4-one | 18.1 | 8.14 × 101 | FeL2 |
| 2,2′-bipyridyl | 21.5 | 3.01 × 10−2 | FeH−2L2+ |
| 4-((5-bromo-3-chloro-2-hydroxybenzyl) amino)-2-hydroxybenzoic acid (LX007, ZL006) | 16.1 | 4.46 × 107 | FeL2 |
| C-3 (α carboxyfullerene) | 16.1 | 2.54 × 1010 | FeL2 |
| Caffeic acid amide analogues | 16.3 | 1.35 × 104 | FeL2 |
| Carbidopa | 16.2 | 2.96 × 104 | FeL |
| Carnosic acid | 16.3 | 1.35 × 104 | FeL2 |
| Catechin | 16.1 | 4.49 × 1017 | FeHL |
| Ceftriaxone | 16.1 | 9.59 × 107 | FeL2 |
| Celastrol | 19.2 | 6.33 | FeL2 |
| Chebulagic acid | 16.1 | 7.45 × 105 | FeHL |
| Chlorogenic acid | 16.1 | 1.07 × 107 | FeL |
| 3′-O-(3-Chloropivaloyl) quercetin | 16.1 | 7.55 × 108 | FeL |
| Chrysin | 16.1 | 7.55 × 108 | FeL+ |
| Clioquinol | 20.6 | 2.15 × 10−1 | FeL3 |
| Clioquinol-selegiline hybrid | 22.9 | 1.07 × 10−3 | FeL2 |
| Clovamide analogues (R1 and R2 = OH, and/or R3 and R4 = OH) | 16.3 | 1.35 × 104 | FeL2 |
| “Compound (−)-8a” | 16.3 | 1.35 × 104 | FeL2 |
| “Compound 21”, derivative of 3-methyl-1-(2,4,6-trihydroxyphenyl) butan-1-one | 16.1 | 9.59 × 107 | FeL2+ |
| Creatine | 16.1 | 9.59 × 107 | FeL2+ |
| Cudraflavone B | 16.1 | 7.55 × 108 | FeL |
| Curcumin | 16.6 | 4.09 × 103 | FeL |
| Cyanidin | 16.3 | 1.35 × 104 | FeL2 |
| D607 (bipyridyl-D2R/D3R agonist hybrid) | 21.5 | 3.01 × 10−2 | FeH−2L2 |
| DA-2 (8D) | 20.6 | 2.15 × 10−1 | FeL3 |
| DA-3 | 17.2 | 5.61 × 102 | FeL2 |
| DA-4 | 17.2 | 5.61 × 102 | FeL2 |
| Deferasirox | 23.5 | 3.22 × 10−4 | FeL23− |
| Delphinidin | 16.3 | 1.35 × 104 | FeL2 |
| Demethoxycurcumin | 16.8 | 2.28 × 103 | FeL |
| Dendropanax morbifera active compound | 16.3 | 1.35 × 104 | FeL2 |
| Desferrioxamine (Deferoxamine, Desferal, DFO) | 26.8 | 1.81 × 10−7 | FeHL+ |
| 4,5-O-Dicaffeoyl-1-O-(malic acid methyl ester)-quinic acid derivatives (R1, R2, R3, R4, or R5 = caffeoyl) | 16.3 | 1.35 × 104 | FeL2 |
| Dihydromyricetin | 16.3 | 1.35 × 104 | FeL2 |
| 5-(3,4-dihydroxybenzylidene)-2,2-dimethyl-1,3-dioxane-4,6-dione | 16.3 | 1.35 × 104 | FeL2− |
| 7,8-dihydroxycoumarin derivative DHC12 | 16.3 | 1.35 × 104 | FeL2 |
| 3′,4′-dihydroxyflavone | 16.3 | 1.35 × 104 | FeL2− |
| 7,8-dihydroxyflavone | 16.3 | 1.35 × 104 | FeL2− |
| 5,7-dihydroxy-4′-methoxyflavone | 18.1 | 8.14 × 101 | FeL2 |
| (E)-3,4-dihydroxystyryl aralkyl sulfones | 16.3 | 1.35 × 104 | FeL2− |
| (E)-3,4-dihydroxystyryl aralkyl sulfoxides | 16.3 | 1.35 × 104 | FeL2− |
| 5,3′-dihydroxy-3,7,4′-trimethoxyflavone | 18.1 | 8.14 × 101 | FeL2 |
| 2-[[(1,1-Dimethylethyl) oxidoimino]-methyl]-3,5,6-trimethylpyrazine | 16.1 | 1.86 × 1010 | FeL |
| DKP | 16.1 | 7.43 × 1021 | FeL2 |
| l-DOPA (levodopa, CVT-301) | 16.2 | 2.96 × 104 | FeL |
| DOPA-derived peptido-mimetics (deprotected) | 16.2 | 2.96 × 104 | FeL |
| DOPA-derived peptido-mimetics (protected) | 16.3 | 1.35 × 104 | FeL2 |
| l-dopa deuterated | 16.2 | 2.96 × 104 | FeL |
| Doxycycline | 18.1 | 7.22 × 101 | FeL2 |
| Droxidopa | 16.2 | 2.96 × 104 | FeL |
| Echinacoside | 16.3 | 1.35 × 104 | FeL2 |
| Ellagic acid | 16.3 | 1.35 × 104 | FeL2 |
| Entacapone (comtan, ASI-6) | 19.3 | 3.99 | FeL33− |
| Epicatechin | 16.3 | 1.35 × 104 | FeL2 |
| Epigallocatechin-3-gallate | 16.1 | 7.45 × 105 | FeHL |
| Etidronate (HEDPA) | 23.5 | 3.22 × 10−4 | FeH−1L |
| Exifone | 16.1 | 7.45 × 105 | FeHL |
| Farrerol | 16.1 | 7.55 × 108 | FeL |
| Fiset (3,3′,4′,7-tetra-hydroxy-flavone) | 16.3 | 1.35 × 104 | FeL2 |
| Fraxetin | 16.3 | 1.35 × 104 | FeL2− |
| Galangin | 27.0 | 9.36 × 10−8 | FeH−1L |
| Gallic acid derivatives | 16.1 | 7.45 × 105 | FeHL |
| Garcinol | 16.3 | 1.35 × 104 | FeL2 |
| Genistein | 16.1 | 7.55 × 108 | FeL |
| Glutathione-hydroxy-quinoline compound | 18 | 1.04 × 102 | FeH−2L+ |
| Glutathione-l-DOPA compound | 16.3 | 1.35 × 104 | FeL2 |
| Gly-N-C-DOPA | 16.2 | 2.96 × 104 | FeL |
| Hesperidin | 16.1 | 7.55 × 108 | FeL |
| Hinokitiol | 16.1 | 4.30 × 108 | FeL2+ |
| 8-HQ-MC-5 (VK-28) | 20.6 | 2.15 × 10−1 | FeL3 |
| 4-Hydroxyisophthalic acid | 16.1 | 1.64 × 106 | FeL3 |
| 1-Hydroxy-2-pyridinone derivatives | 17.7 | 1.50 × 102 | FeL3 |
| 3-Hydroxy-4(1H)pyridinone (Deferiprone) | 19.3 | 3.92 | FeL3 |
| 3-Hydroxy-4(1H)pyridinone derivatives (R = H) | 19.3 | 3.92 | FeL3 |
| 8-Hydroxyquinoline | 20.6 | 2.15 × 10−1 | FeL3 |
| 8-Hydroxyquinoline-2-carboxaldehyde isonicotinoyl hydrazone | 20.6 | 2.15 × 10−1 | FeL3 |
| Hydroxy-quinoline-propargyl hybrids (HLA20) | 20.6 | 2.15 × 10−1 | FeL3 |
| Hydroxytyrosol butyrate | 16.3 | 1.35 × 104 | FeL2− |
| Hyperoside | 27.0 | 9.36 × 10−8 | FeH−1L |
| Icariin | 18.1 | 8.14 × 101 | FeL2 |
| Icariside II | 18.1 | 8.14 × 101 | FeL2 |
| Isobavachalcone | 16.1 | 3.23 × 108 | FeL2+ |
| Isochlorogenic acid | 16.1 | 1.07 × 107 | FeL |
| Isoquercetin (isoquercitrin) | 27.0 | 9.36 × 10−8 | FeH−1L |
| Kaempferol | 27.0 | 9.36 × 10−8 | FeH−1L |
| Kaempferol, 3-O-a-L arabino-furanoside-7-O-a-L- rhamno-pyranoside | 18.1 | 8.14 × 101 | FeL2 |
| KR33493 | 16.3 | 1.06 × 104 | FeL2 |
| Kukoamine | 16.3 | 1.35 × 104 | FeL2 |
| Luteolin | 16.3 | 1.35 × 104 | FeL2 |
| M10 M30 (VAR10303) M99 | 20.6 | 2.15 × 10−1 | FeL3 |
| Macranthoin G | 16.3 | 1.35 × 104 | FeL2 |
| Magnesium lithospermate B | 16.3 | 1.35 × 104 | FeL2 |
| α-mangostin | 16.3 | 1.35 × 104 | FeL2 |
| γ-mangostin | 16.3 | 1.35 × 104 | FeL2 |
| Metformin (Met) | 16.1 | 1.36 × 109 | FeL2+ |
| MitoQ | 16.1 | 5.72 × 1010 | FeL2 |
| Morin | 18.1 | 8.14 × 101 | FeL2 |
| Myricetin Myricitrin | 27.0 | 9.36 × 10−8 | FeH−1L |
| Naringenin | 16.1 | 7.55 × 108 | FeL |
| Naringin | 16.1 | 7.55 × 108 | FeL |
| Nicotinamide adenine dinucleotide phosphate (NADPH) | 16.1 | 6.01 × 1010 | FeL2 |
| Nitecapone | 16.8 | 2.04 × 103 | FeL2− |
| Nordihydroguaiaretic acid | 16.3 | 1.35 × 104 | FeL2 |
| Oleuropein | 16.3 | 1.35 × 104 | FeL2− |
| Opicapone | 16.8 | 2.04 × 103 | FeL2 |
| PBT2 | 20.6 | 2.15 × 10−1 | FeL3 |
| PBT434 | 16.1 | 3.22 × 109 | FeL2+ |
| Petunidin | 16.3 | 1.35 × 104 | FeL2 |
| Phenylhydroxamates | 16.1 | 9.46 × 104 | FeL2 |
| Piceatannol | 16.3 | 1.35 × 104 | FeL2 |
| Pinostrobin (5-hydroxy-7-methoxy-flavone) | 18.1 | 8.14 × 101 | FeL2 |
| Piperazine-8-OH-quinolone hybrid | 20.6 | 2.15 × 10−1 | FeL3 |
| Protocatechuic acid | 22.2 | 6.16 × 10−3 | FeL23− |
| Protosappanin A | 16.3 | 1.35 × 104 | FeL2 |
| Punicalangin | 16.1 | 1.14 × 1012 | FeL |
| Pyridoxal isonicotinoyl hydrazone (PIH) | 22.9 | 1.07 × 10−3 | FeL2 |
| Pyridoxal isonicotinoyl hydrazone derivatives: PCIH PCTH H2NPH H2PPH | 22.9 | 1.07 × 10−3 | FeL2 |
| Q1 Q4 | 20.6 | 2.15 × 10−1 | FeL3 |
| Quercetin | 27.0 | 9.36 × 10−8 | FeH–1L3− |
| Quinoline derivatives as SUMOylation activators | 16.1 | 1.14 × 1016 | FeL2+ |
| Radotinib | 21.5 | 3.01 × 10−2 | FeH−2L2 |
| Rimonabant | 16.1 | 7.43 × 1021 | FeL2 |
| Rosmarinic acid | 16.3 | 1.35 × 104 | FeL2 |
| Rutin | 27.0 | 9.36 × 10−8 | FeH−1L |
| Salicylate, sodium salt | 16.1 | 1.64 × 106 | FeL33− |
| Salvianolic acid B | 16.3 | 1.35 × 104 | FeL2 |
| Silibinin (silybin) A, B | 16.1 | 1.99 × 1019 | FeH3L3+ |
| Silydianin | 16.1 | 1.99 × 1019 | FeH3L3+ |
| Sulfuretin | 16.3 | 1.35 × 104 | FeL2 |
| Tanshinol | 16.3 | 1.35 × 104 | FeL2 |
| Tannic acid | 16.1 | 6.05 × 1049 | Fe4L |
| Taxifolin | 16.1 | 7.55 × 108 | FeL |
| Tectorigenin | 16.1 | 7.55 × 108 | FeL |
| Tetracycline | 16.1 | 8.35 × 1010 | FeL22− |
| Tolcapone (ASI-7) | 16.8 | 2.04 × 103 | FeL2− |
| Transilitin | 16.3 | 1.35 × 104 | FeL2 |
| o-Trensox | 29.5 | 3.36 × 10−10 | FeL3− |
| 2,3,3-Trisphosphonate | 18 | 1.06 × 102 | FeL |
| Verbascoside | 16.3 | 1.35 × 104 | FeL2 |
| Zonisamide | 16.1 | 1.86 × 1011 | FeL2 |
| Compound Name(s) | pFe(II) | Kd (nmol/L) | Most Abundant Complex |
|---|---|---|---|
| 7DH 7MH | 6.9 | 1.35 × 103 | FeL |
| 8A 8B 8C | 6.9 | 1.35 × 103 | FeL |
| 8E 8F | 6.1 | 4.62 × 104 | FeL2 |
| ACPT-I | 6 | 1.20 × 107 | FeL |
| Alvespimycin | 6 | 2.07 × 107 | FeL2+ |
| Aminothiazoles derivatives as SUMOylation activators | 6 | 2.77 × 106 | FeL |
| AMN082 | 6 | 2.07 × 107 | FeL2+ |
| Apomorphine | 6 | 1.53 × 107 | FeHL |
| l-Arginine | 6 | 3.30 × 107 | FeL2+ |
| Ascorbic acid | 6 | 1.89 × 109 | FeL+ |
| ASI-1 | 6 | 1.78 × 105 | FeL+ |
| ASI-5 | 10.7 | 1.95 × 10−1 | FeL |
| Astilbin | 6 | 1.53 × 107 | FeHL |
| Azilsartan | 6 | 2.53 × 1015 | FeL2 |
| Baicalein | 9.9 | 1.09 | FeL22− |
| Benserazide | 9.9 | 1.09 | FeL2 |
| (−)-N6-(2-(4-(Biphenyl-4-yl)piperazin-1-yl)-ethyl)-N6-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine derivatives | 6 | 2.07 × 107 | FeL2+ |
| 2,2′-Bipyridyl | 6.1 | 4.62 × 104 | FeL22+ |
| 4-((5-bromo-3-chloro-2-hydroxybenzyl) amino)-2-hydroxybenzoic acid (LX007, ZL006) | 6 | 3.13 × 108 | FeL |
| C-3 (α carboxyfullerene) | 6 | 6.90 × 106 | FeL |
| Caffeic acid amide analogues | 6.7 | 2.02 × 103 | FeH−1L |
| Carbazole-derived compounds | 6 | 2.07 × 107 | FeL2+ |
| Carbidopa | 6.3 | 1.07 × 104 | FeL |
| Carnosic acid | 6 | 1.53 × 107 | FeHL |
| Catechin | 6 | 1.53 × 107 | FeHL |
| Ceftriaxone | 6 | 1.20 × 107 | FeL |
| Celastrol | 6 | 1.53 × 107 | FeHL |
| CEP-1347 | 6 | 1.53 × 107 | FeHL |
| Chebulagic acid | 6 | 5.66 × 105 | FeL |
| Chlorogenic acid | 6 | 2.93 × 106 | FeHL |
| Clioquinol | 7.9 | 1.01 × 102 | FeL2 |
| Clioquinol-selegiline hybrid | 7.1 | 6.37 × 102 | FeH2L2 |
| Clovamide analogues (R1 and R2 = OH, and/or R3 and R4 = OH) | 6 | 1.53 × 107 | FeHL |
| “Compound 1” | 6 | 2.07 × 107 | FeL2+ |
| “Compound (−)-8a” | 6 | 1.53 × 107 | FeHL |
| “Compound 21”, derivative of 3-methyl-1-(2,4,6-trihydroxyphenyl) butan-1-one | 6 | 1.20 × 107 | FeL+ |
| “Compound (−)-21a”, derivative of N-6-(2-(4-(1H-indol-5-yl) piperazin-1-yl)ethyl)-N-6-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine | 6 | 2.07 × 107 | FeL2+ |
| Creatine | 6 | 1.20 × 107 | FeL+ |
| Curcumin | 6 | 4.16 × 109 | FeH2L+ |
| Cyanidin | 6 | 1.53 × 107 | FeHL |
| D512 | 6 | 2.07 × 107 | FeL2+ |
| D607 (bipyridyl-D2R/D3R agonist hybrid) | 6.1 | 4.63 × 104 | FeL2 |
| DA-2 (8D) | 6.9 | 1.35 × 103 | FeL |
| DA-3 | 6 | 2.07 × 107 | FeL |
| DA-4 | 6 | 2.07 × 107 | FeL |
| Dabigatran etexilate | 6 | 2.07 × 107 | FeL |
| Delphinidin | 9.9 | 1.09 | FeL2 |
| Demethoxycurcumin | 6 | 1.78 × 105 | FeL |
| Dendropanax morbifera | 6 | 1.53 × 107 | FeHL |
| Desferrioxamine (Deferoxamine, Desferal, DFO) | 6.2 | 2.24 × 104 | FeH2L+ |
| 4,5-O-Dicaffeoyl-1-O-(malic acid methyl ester)-quinic acid derivatives (R1, R2, R3, R4, or R5 = caffeoyl) | 6 | 1.53 × 107 | FeHL |
| Dihydromyricetin | 9.9 | 1.09 | FeL2 |
| 5-(3,4-Dihydroxybenzylidene)-2,2-dimethyl-1,3-dioxane-4,6-dione | 6 | 1.53 × 107 | FeHL+ |
| 7, 8-Dihydroxycoumarin derivative DHC12 | 6 | 1.53 × 107 | FeHL |
| 3′,4′-Dihydroxyflavone | 14.8 | 1.58 × 10−5 | FeL+ |
| 7,8-Dihydroxyflavone | 6 | 1.53 × 107 | FeHL+ |
| (E)-3,4-Dihydroxystyryl aralkyl sulfones | 6 | 1.53 × 107 | FeHL+ |
| (E)-3,4-Dihydroxystyryl aralkyl sulfoxides | 6 | 1.53 × 107 | FeHL+ |
| 2-[[(1,1-Dimethylethyl) oxidoimino]-methyl]-3,5,6-trimethylpyrazine | 8.2 | 5.40 × 101 | FeL2 |
| DKP | 6 | 4.33 × 105 | FeL |
| l-DOPA (levodopa, CVT-301) | 6.3 | 1.07 × 104 | FeL− |
| DOPA-derived peptido-mimetics (deprotected) | 10.5 | 2.82 × 10−1 | FeHL |
| DOPA-derived peptido-mimetics (protected) | 6 | 1.53 × 107 | FeHL |
| l-DOPA deuterated | 6.3 | 1.07 × 104 | FeL− |
| Doxycycline | 6 | 4.07 × 108 | FeL |
| Droxidopa | 6.3 | 1.07 × 104 | FeL− |
| Echinacoside | 6 | 1.53 × 107 | FeHL |
| Ellagic acid | 6 | 1.53 × 107 | FeHL |
| Entacapone (comtan, ASI-6) | 12.7 | 1.65 × 10−3 | FeL22− |
| Enzastaurin | 6 | 2.07 × 107 | FeL |
| Epicatechin | 6 | 1.53 × 107 | FeHL |
| Etidronate (HEDPA) | 9.9 | 1.14 | FeL2− |
| F13714 F15599 | 6 | 2.77 × 106 | FeL |
| FIsetin (3,3′,4′,7-tetra-hydroxy-flavone) | 6 | 1.53 × 107 | FeHL |
| Fraxetin | 6 | 1.53 × 107 | FeHL+ |
| Gallocatechin | 9.9 | 1.09 | FeL2 |
| Garcinol | 6 | 1.53 × 107 | FeHL |
| Glutamine | 6 | 7.54 × 105 | FeL+ |
| Glutathione-hydroxy-quinoline compound | 6.9 | 1.35 × 103 | FeL+ |
| Glutathione-l-DOPA compound | 6 | 1.53 × 107 | FeHL |
| Gly-N-C-DOPA | 6.3 | 1.07 × 104 | FeL− |
| Guanabenz | 6 | 2.07 × 107 | FeL |
| 8-HQ-MC-5 (VK28) | 6.9 | 1.35 × 103 | FeL |
| 4-Hydroxyisophthalic acid | 6 | 3.13 × 108 | FeL |
| 8-hydroxyquinoline | 6.9 | 1.35 × 103 | FeL+ |
| 8-hydroxyquinoline-2-carboxaldehyde isonicotinoyl hydrazone | 6.9 | 1.35 × 103 | FeL |
| Hydroxy-quinoline-propargyl hybrids (HLA20) | 6.9 | 1.35 × 103 | FeL |
| Hydroxytyrosol butyrate | 6 | 1.53 × 107 | FeHL+ |
| Isochlorogenic acid | 6 | 2.93 × 106 | FeHL |
| KR33493 | 6 | 1.20 × 107 | FeL |
| Kukoamine | 6 | 1.53 × 107 | FeHL |
| Lestaurtinib | 6 | 2.07 × 107 | FeL |
| M10 M30 (VAR10303) M99 | 6.9 | 1.35 × 103 | FeL |
| Macranthoin G | 6 | 1.53 × 107 | FeHL |
| Magnesium lithospermate B | 6 | 1.53 × 107 | FeHL |
| α-mangostin | 6 | 1.53 × 107 | FeHL |
| γ-mangostin | 6 | 1.53 × 107 | FeHL |
| MAOI-1 | 9.9 | 1.09 | FeL2 |
| MAOI-2 | 6 | 2.07 × 107 | FeL |
| Meclofenamic acid | 6 | 2.53 × 1015 | FeL2 |
| Mildronate | 6 | 2.53 × 1015 | FeL2 |
| Mitomycin C | 6 | 6.57 × 107 | FeL |
| [18F]MPPF | 6 | 2.07 × 107 | FeL |
| Nitecapone | 12.7 | 1.65 × 10−3 | FeL22− |
| Nordihydroguaiaretic acid | 6 | 1.53 × 107 | FeHL |
| Oleuropein | 6 | 1.53 × 107 | FeHL+ |
| Opicapone | 12.7 | 1.65 × 10−3 | FeL2 |
| PBF-509 | 6 | 2.07 × 107 | FeL |
| PBT2 | 6.9 | 1.35 × 103 | FeL |
| PBT434 | 6.2 | 1.80 × 104 | FeL+ |
| Petunidin | 6 | 1.53 × 107 | FeHL |
| Phenothiazine 2Bc (n=0) | 6 | 2.07 × 107 | FeL2+ |
| Phenylhydroxamates | 6 | 5.96 × 107 | FeL2 |
| Piceatannol | 6 | 1.53 × 107 | FeHL |
| Piperazine-8-OH-quinolone hybrid | 6.9 | 1.35 × 103 | FeL |
| Preladenant | 6 | 2.07 × 107 | FeL |
| Promethazine | 6 | 2.07 × 107 | FeL2+ |
| Protosappanin A | 6 | 1.53 × 107 | FeHL |
| Pyridoxal isonicotinoyl hydrazone (PIH) | 7.1 | 6.37 × 102 | FeH2L2 |
| Pyridoxal isonicotinoyl hydrazone derivatives: PCIH PCTH H2NPH H2PPH | 7.1 | 6.37 × 102 | FeH2L22+ |
| Pyrimidinone 8 | 6 | 2.07 × 107 | FeL |
| Q1 Q4 | 6.9 | 1.35 × 103 | FeL |
| Radotinib | 6.1 | 4.62 × 104 | FeL2 |
| Riboflavin | 6 | 1.22 × 105 | FeL2+ |
| Rifampicin (ASI-3) | 6 | 4.07 × 108 | FeL |
| Rimonabant | 6 | 4.85 × 1011 | FeL |
| Rosmarinic acid | 6 | 1.53 × 107 | FeLH |
| Salicylate, sodium salt | 6 | 3.13 × 108 | FeL |
| Salvianolic acid B | 6 | 1.53 × 107 | FeHL |
| SCH58261SCH412348 | 6 | 2.07 × 107 | FeL |
| ST1535 ST4206 | 6 | 2.07 × 107 | FeL |
| Staurosporine | 6 | 2.07 × 107 | FeL |
| Sulfuretin | 6 | 1.53 × 107 | FeHL |
| Tanshinol | 6 | 1.53 × 107 | FeHL |
| Tetracycline | 6 | 2.73 × 106 | FeL |
| Tolcapone (ASI-7) | 12.7 | 1.65 × 10−3 | FeL22− |
| Transilitin | 6 | 1.53 × 107 | FeHL |
| 2′,3′,4′-trihydroxyflavone | 9.9 | 1.09 | FeL22− |
| 2,3,3-trisphosphonate | 10.7 | 1.95 × 10−1 | FeL |
| V81444 | 6 | 2.77 × 106 | FeL |
| Verbascoside | 6 | 2.02 × 106 | FeL |
| WIN 55, 212-2 | 6 | 2.07 × 107 | FeL2+ |
| Compound Name(s) | pMn(II) | Kd (nmol/L) | Most Abundant Complex |
|---|---|---|---|
| 7DH 7MH | 6.7 | 2.35 × 103 | MnL+ |
| 8A 8B 8C | 6.7 | 2.35 × 103 | MnL |
| 8E 8F | 6 | 2.40 × 106 | MnL |
| N-Acetyl cysteine | 6 | 8.97 × 105 | MnHL+ |
| ACPT-I | 6 | 2.25 × 108 | MnL |
| Alvespimycin | 6 | 7.61 × 108 | MnL2+ |
| Ambroxol | 6.7 | 2.47 × 103 | MnL+ |
| 3-(7-amino-5-(cyclohexylamino)-[1,2,4]triazolo[1,5-a][1,3,5]triazin-2-yl)-2-cyanoacrylamide | 6 | 7.61 × 108 | MnL |
| Aminothiazoles derivatives as SUMOylation activators | 6 | 7.64 × 107 | MnL |
| AMN082 | 6 | 7.61 × 108 | MnL2+ |
| Apomorphine | 6 | 7.16 × 108 | MnL |
| l-Arginine | 6 | 1.45 × 108 | MnL2+ |
| ASI-1 | 6 | 3.44 × 106 | MnL+ |
| ASI-5 | 6 | 7.81 × 106 | MnL |
| Astilbin | 6 | 7.16 × 108 | MnL |
| Azilsartan | 6 | 3.71 × 109 | MnL |
| Baicalein | 6 | 2.62 × 107 | MnL |
| Benserazide | 6 | 2.62 × 107 | MnL |
| (−)-N6-(2-(4-(Biphenyl-4-yl)piperazin-1-yl)-ethyl)-N6-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine derivatives | 6 | 7.61 × 108 | MnL2+ |
| 2,2′-bipyridyl | 6 | 2.40 × 106 | MnL2+ |
| 4-((5-bromo-3-chloro-2-hydroxybenzyl) amino)-2-hydroxybenzoic acid (LX007, ZL006) | 6 | 8.72 × 108 | MnL |
| C-3 (α carboxyfullerene) | 6 | 5.06 × 106 | MnL |
| Caffeic acid amide analogues | 6 | 6.62 × 107 | MnH−1L |
| Carbazole-derived compounds | 6 | 7.61 × 108 | MnL2+ |
| Carbidopa | 7.6 | 2.33 × 102 | MnHL |
| Carnosic acid | 6 | 7.16 × 108 | MnL |
| Catechin | 6 | 7.16 × 108 | MnL |
| Ceftriaxone | 6 | 2.25 × 108 | MnL |
| Celastrol | 6 | 7.16 × 108 | MnL |
| CEP1347 | 6 | 3.71 × 109 | MnL |
| Chebulagic acid | 6 | 2.62 × 107 | MnL |
| Chlorogenic acid | 6 | 3.91 × 107 | MnL− |
| Clioquinol | 6.7 | 2.35 × 103 | MnL+ |
| Clovamide analogues (R1 and R2 = OH, and/or R3 and R4 = OH) | 6 | 7.16 × 108 | MnL |
| “Compound 1” | 6 | 7.61 × 108 | MnL++ |
| “Compound (−)-8a” | 6 | 7.16 × 108 | MnL |
| “Compound 21”, derivative of 3-methyl-1-(2,4,6-trihydroxyphenyl) butan-1-one | 6 | 2.25 × 108 | MnL+ |
| “Compound (−)-21a”, derivative of N-6-(2-(4-(1H -indol-5-yl)piperazin-1-yl)ethyl)-N-6-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine | 6 | 7.61 × 108 | MnL++ |
| Creatine | 6 | 2.25 × 108 | MnL+ |
| Curcumin | 6 | 3.44 × 106 | MnL |
| Cyanidin | 6 | 7.16 × 108 | MnL |
| D512 | 6 | 7.61 × 108 | MnL2+ |
| D607 (bipyridyl-D2R/D3R agonist hybrid) | 6 | 2.40 × 106 | MnL |
| DA-2 (8D) | 6.7 | 2.35 × 103 | MnL |
| DA-3 | 6 | 7.61 × 108 | MnL |
| DA-4 | 6 | 7.61 × 108 | MnL |
| Dabigatran etexilate | 6 | 7.61 × 108 | MnL |
| (S)-3,4-DCPG | 6 | 5.91 × 106 | MnL |
| Delphinidin | 6 | 2.62 × 107 | MnL |
| Demethoxycurcumin | 6 | 3.44 × 106 | MnL |
| Dendropanax morbifera active compound | 6 | 7.16 × 108 | MnL |
| 4,5-O-Dicaffeoyl-1-O-(malic acid methyl ester)-quinic acid derivatives (R1, R2, R3, R4, or R5 = caffeoyl) | 6 | 7.16 × 108 | MnL |
| Dihydromyricetin | 6 | 2.62 × 107 | MnL |
| 5-(3,4-Dihydroxybenzylidene) -2,2-dimethyl-1,3-dioxane-4,6-dione | 6 | 7.16 × 108 | MnL |
| 7,8-Dihydroxycoumarin derivative DHC12 | 6 | 7.16 × 108 | MnL |
| 3′,4′-Dihydroxyflavone | 6 | 7.16 × 108 | MnL |
| 7,8-dihydroxyflavone | 6 | 7.16 × 108 | MnL |
| (E)-3,4-Dihydroxystyryl aralkyl sulfones | 6 | 7.16 × 108 | MnL |
| (E)-3,4-Dihydroxystyryl aralkyl sulfoxides | 6 | 7.16 × 108 | MnL |
| 2-[[(1,1-Dimethylethyl) oxidoimino]-methyl]-3,5,6-trimethylpyrazine | 6 | 2.58 × 106 | MnL |
| DKP | 6 | 1.00 × 106 | MnHL |
| l-DOPA (levodopa, CVT-301) | 7.6 | 2.26 × 102 | MnHL |
| DOPA-derived peptido-mimetics (deprotected) | 7.6 | 2.33 × 102 | MnHL |
| DOPA-derived peptido-mimetics (protected) | 6 | 7.16 × 108 | MnL |
| l-dopa deuterated | 7.6 | 2.26 × 102 | MnHL |
| Droxidopa | 7.6 | 2.33 × 102 | MnHL |
| Echinacoside | 6 | 7.16 × 108 | MnL |
| Ellagic acid | 6 | 7.16 × 108 | MnL |
| Entacapone (comtan, ASI-6) | 6 | 6.74 × 105 | MnL |
| Enzastaurin | 6 | 7.61 × 108 | MnL |
| Epicatechin | 6 | 7.16 × 108 | MnL |
| Etidronate (HEDPA) | 6 | 1.03 × 106 | MnL2− |
| F13714 F15599 | 6 | 7.64 × 107 | MnL |
| Fisetin (3,3′,4′,7-tetra-hydroxy-flavone) | 6 | 7.16 × 108 | MnL |
| Fraxetin | 6 | 7.16 × 108 | MnL |
| Gallocatechin | 6 | 2.62 × 107 | MnL |
| Garcinol | 6 | 7.16 × 108 | MnL |
| Glutamine | 6 | 2.30 × 107 | MnL+ |
| Glutathione derivatives | 6 | 4.07 × 105 | MnL− |
| Glutathione-hydroxy-quinoline compound | 6.7 | 2.35 × 103 | MnL+ |
| Glutathione-l-DOPA compound | 6 | 7.16 × 108 | MnL |
| Gly-N-C-DOPA | 7.6 | 2.33 × 102 | MnHL |
| Guanabenz | 6 | 7.61 × 108 | MnL |
| Hinokitiol | 6.1 | 2.99 × 104 | MnL+ |
| 8-HQ-MC-5 (VK-28) | 6.7 | 2.35 × 103 | MnL |
| 4-Hydroxyisophthalic acid | 6 | 8.72 × 108 | MnL |
| 8-hydroxyquinoline | 6.7 | 2.35 × 103 | MnL+ |
| 8-Hydroxyquinoline-2-carboxaldehyde isonicotinoyl hydrazone | 6.7 | 2.35 × 103 | MnL |
| Hydroxy-quinoline-propargyl hybrids (HLA20) | 6.7 | 2.35 × 103 | MnL |
| Hydroxytyrosol butyrate | 6 | 7.16 × 108 | MnL |
| Isobavachalcone | 6.2 | 1.55 × 104 | MnL+ |
| Isochlorogenic acid | 6 | 3.91 × 107 | MnL− |
| KR33493 | 6 | 2.25 × 108 | MnL |
| Kukoamine | 6 | 7.16 × 108 | MnL |
| Lestaurtinib | 6 | 7.61 × 108 | MnL |
| Lipoic acid | 6 | 9.53 × 106 | MnL+ |
| Luteolin | 6 | 7.16 × 108 | MnL |
| M10 M30 (VAR10303) M99 | 6.7 | 2.35 × 103 | MnL |
| Macranthoin G | 6 | 7.16 × 108 | MnL |
| Magnesium lithospermate B | 6 | 7.16 × 108 | MnL |
| α-mangostin | 6 | 7.16 × 108 | MnL |
| γ-mangostin | 6 | 7.16 × 108 | MnL |
| MAOI-1 | 6 | 2.62 × 107 | MnL |
| MAOI-2 | 6 | 7.61 × 108 | MnL |
| Meclofenamic acid | 6 | 3.71 × 109 | MnL |
| Mildronate | 6 | 3.71 × 109 | MnL |
| Mitomycin C | 6 | 7.46 × 107 | MnL |
| MitoQ | 6 | 1.29 × 105 | MnL |
| [18F]MPPF | 6 | 7.61 × 108 | MnL |
| Nicotinamide adenine dinucleotide phosphate (NADPH) | 6 | 2.01 × 107 | MnL |
| Nicotinamide mononucleotide | 6 | 6.59 × 106 | MnL |
| Nitecapone | 6 | 6.74 × 105 | MnL |
| Nordihydroguaiaretic acid | 6 | 7.16 × 108 | MnL |
| Oleuropein | 6 | 7.16 × 108 | MnL |
| Opicapone | 6 | 6.74 × 105 | MnL |
| PBF-509 | 6 | 2.41 × 109 | MnL |
| PBT2 | 6 | 5.22 × 105 | MnL+ |
| Petunidin | 6 | 7.16 × 108 | MnL |
| Phenothiazine 2Bc (n=0) | 6 | 7.61 × 108 | MnL2+ |
| Phenylhydroxamates | 6 | 5.81 × 106 | MnL |
| Piceatannol | 6 | 7.16 × 108 | MnL |
| Piperazine-8-OH-quinolone hybrid | 6.7 | 2.35 × 103 | MnL |
| Preladenant | 6 | 7.61 × 108 | MnL |
| Promethazine | 6 | 7.61 × 108 | MnL2+ |
| Protocatechuic acid | 6 | 3.66 × 108 | MnL− |
| Protosappanin A | 6 | 7.16 × 108 | MnL |
| Pyrimidinone 8 | 6 | 7.61 × 108 | MnL |
| Q1 Q4 | 6.7 | 2.35 × 103 | MnL |
| Radotinib | 6 | 2.40 × 106 | MnL |
| Riboflavin | 6 | 5.75 × 105 | MnHL3+ |
| Rifampicin (ASI-3) | 6 | 1.03 × 106 | MnL |
| Rimonabant | 6 | 1.00 × 106 | MnHL |
| Rosmarinic acid | 6 | 7.16 × 108 | MnL |
| Salicylate, sodium salt | 6 | 8.72 × 108 | MnL |
| Salvianolic acid B | 6 | 7.16 × 108 | MnL |
| SCH58261 SCH412348 | 6 | 7.61 × 108 | MnL |
| ST1535 ST4206 | 6 | 7.61 × 108 | MnL |
| Staurosporine | 6 | 7.61 × 108 | MnL |
| Sulfuretin | 6 | 7.16 × 108 | MnL |
| Tanshinol | 6 | 7.16 × 108 | MnL |
| Taurine | 6 | 6.39 × 1011 | MnL2 |
| Tetracycline | 6 | 2.14 × 107 | MnL |
| Tolcapone (ASI-7) | 6 | 6.74 × 105 | MnL |
| Transilitin | 6 | 7.16 × 108 | MnL |
| 2′,3′,4′-Trihydroxyflavone | 6 | 2.62 × 107 | MnL |
| V81444 | 6 | 7.64 × 107 | MnL |
| Verbascoside | 6 | 6.62 × 107 | MnH−1L |
| WIN 55,212-2 | 6 | 7.61 × 108 | MnL2+ |
| Compound Name(s) | pZn(II) | Kd (nmol/L) | Most Abundant Complex |
|---|---|---|---|
| 7DH 7MH | 7.5 | 3.16 × 102 | ZnL |
| 8A 8B 8C | 7.5 | 3.16 × 102 | ZnL |
| 8E 8F | 6.4 | 6.31 × 103 | ZnL |
| N-acetyl cystein | 6 | 1.66 × 106 | ZnL |
| ACPT-I | 6 | 1.46 × 107 | ZnL |
| Alaternin | 6.8 | 1.83 × 103 | ZnL |
| Alvespimycin | 6 | 7.85 × 105 | ZnL2+ |
| AM-251 | 6 | 1.95 × 106 | ZnHL |
| Ambroxol | 7.7 | 1.90 × 102 | ZnL2 |
| 3-(7-amino-5-(cyclohexylamino)-[1,2,4]triazolo[1,5-a][1,3,5]triazin-2-yl)-2-cyanoacrylamide | 6 | 7.85 × 105 | ZnL |
| Aminothiazoles derivatives as SUMOylation activators | 6 | 2.28 × 105 | ZnL |
| AMN082 | 6 | 7.85 × 105 | ZnL2+ |
| Antagonist of the A(2A) adenosine receptor - derivative 49 | 6 | 1.95 × 106 | ZnHL |
| Apigenin | 6 | 1.23 × 1029 | ZnH3L |
| Apomorphine | 6 | 7.76 × 106 | ZnL |
| l-Arginine | 6 | 4.14 × 106 | ZnL2+ |
| Ascorbic acid | 6.1 | 4.67 × 104 | ZnL+ |
| ASI-1 | 6 | 5.76 × 105 | ZnL+ |
| ASI-5 | 6 | 9.06 × 106 | ZnL |
| Astilbin | 6 | 7.76 × 106 | ZnL |
| Azilsartan | 6 | 5.22 × 105 | ZnL |
| Baicalein | 6 | 2.71 × 105 | ZnL |
| Benserazide | 6 | 2.71 × 105 | ZnL |
| 7H-Benzo[e] perimidin-7-one derivatives (R6 = OH) | 6.1 | 4.82 × 104 | ZnL2 |
| 4H-1-benzopyran-4-one | 6 | 3.56 × 1020 | ZnH3L |
| 8-Benzyl-tetrahydropyrazino[2,1-f]purinedione (derivative n. 57) | 6 | 9.67 × 1011 | ZnL |
| Bikaverin | 6.3 | 1.07 × 104 | ZnH−2L2− |
| (−)-N6-(2-(4-(Biphenyl-4-yl)piperazin-1-yl)-ethyl)-N6-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine derivatives | 6 | 7.85 × 105 | ZnL2+ |
| 2,2′-bipyridyl | 6.4 | 6.31 × 103 | ZnL2+ |
| 4-((5-Bromo-3-chloro-2-hydroxybenzyl) amino)-2-hydroxybenzoic acid (LX007, ZL006) | 6 | 8.04 × 108 | ZnH−1L |
| C-3 (α carboxyfullerene) | 6 | 2.08 × 106 | ZnL |
| Caffeic acid amide analogues | 6 | 9.29 × 105 | ZnH−1L |
| Carbazole-derived compounds | 6 | 7.85 × 105 | ZnL2+ |
| Carbidopa | 6 | 2.96 × 106 | ZnHL |
| Carnosic acid | 6 | 7.76 × 106 | ZnL |
| Cathechin | 6 | 7.76 × 106 | ZnL |
| Ceftriaxone | 6.1 | 3.68 × 104 | ZnL |
| Celastrol | 6 | 7.76 × 106 | ZnL |
| Chebulagic acid | 6 | 1.81 × 1013 | Zn2L |
| Chlorogenic acid | 6 | 6.86 × 105 | ZnL− |
| 3′-O-(3-Chloropivaloyl) quercetin | 6 | 5.69 × 1016 | ZnH3L |
| Chlorpromazine | 6 | 1.95 × 106 | ZnHL3+ |
| Chrysin | 6 | 5.69 × 1016 | ZnH3L |
| Clioquinol | 7.5 | 3.16 × 102 | ZnL+ |
| Clovamide analogues (R1 and R2 = OH, and/or R3 and R4 = OH) | 6 | 7.76 × 106 | ZnL |
| “Compound 1” | 6 | 7.85 × 105 | ZnL2+ |
| “Compound (−)-8a” | 6 | 7.76 × 106 | ZnL |
| “Compound 8” | 6.4 | 6.47 × 103 | ZnH−1L |
| “Compound 21”, derivative of 3-methyl-1-(2,4,6-trihydroxyphenyl) butan-1-one | 6 | 1.54 × 106 | ZnL+ |
| “Compound (−)-21a”, derivative of N-6-(2-(4-(1H -indol-5-yl)piperazin-1-yl)ethyl)-N-6-propyl-4,5,6,7-tetrahydrobenzo [d]thiazole-2,6-diamine | 6 | 7.85 × 105 | ZnL2+ |
| Creatine | 6 | 1.54 × 106 | ZnL+ |
| Cudraflavone B | 6 | 5.69 × 1016 | ZnH3L |
| Curcumin | 6 | 5.76 × 105 | ZnL |
| Cyanidin | 6 | 7.76 × 106 | ZnL |
| D512 | 6 | 7.85 × 105 | ZnL2+ |
| D607 (bipyridyl-D2R/D3R agonist hybrid) | 6.4 | 6.31 × 103 | ZnL |
| DA-2 (8D) | 7.5 | 2.80 × 102 | ZnL |
| DA-3 | 6 | 7.85 × 105 | ZnL |
| DA-4 | 6 | 7.85 × 105 | ZnL |
| Dabigatran etexilate | 6 | 7.85 × 105 | ZnL |
| Dabrafenib | 6 | 8.23 × 107 | ZnL |
| (S)-3,4-DCPG | 6 | 6.84 × 106 | ZnL |
| Deferricoprogen | 8.3 | 4.35 × 101 | ZnHL |
| Delphinidin | 6 | 2.71 × 105 | ZnL |
| Demethoxycurcumin | 6 | 5.76 × 105 | ZnL |
| Dendropanax morbifera active compound | 6 | 7.76 × 106 | ZnL |
| Desferrioxamine (Deferoxamine, Desferal, DFO) | 7.4 | 3.97 × 102 | ZnH2L+ |
| (S)-N-(3-(3,6-Dibromo-9H-carbazol-9-yl)-2-fluoropropyl) -6-methoxypyridin-2-amine | 6 | 1.95 × 106 | ZnHL |
| 4,5-O-Dicaffeoyl-1-O-(malic acid methyl ester)-quinic acid derivatives (R1, R2, R3, R4, or R5 = caffeoyl) | 6 | 7.76 × 106 | ZnL |
| Dihydromyricetin | 6 | 2.71 × 105 | ZnL |
| 5-(3,4-Dihydroxybenzylidene)-2,2-dimethyl-1,3-dioxane-4,6-dione | 6 | 7.76 × 106 | ZnL |
| 7,8-Dihydroxycoumarin derivative DHC12 | 6 | 7.76 × 106 | ZnL |
| 3′,4′-Dihydroxyflavone | 6 | 7.76 × 106 | ZnL |
| 7,8-Dihydroxyflavone | 6 | 7.76 × 106 | ZnL |
| (E)-3,4-Dihydroxystyryl aralkyl sulfones | 6 | 7.76 × 106 | ZnL |
| (E)-3,4-Dihydroxystyryl aralkyl sulfoxides | 6 | 7.76 × 106 | ZnL |
| 2-[[(1,1-Dimethylethyl) oxidoimino]-methyl]-3,5,6-trimethylpyrazine | 6 | 2.20 × 106 | ZnL |
| DKP | 6 | 1.52 × 106 | ZnL |
| l-DOPA (levodopa, CVT-301) | 6 | 2.96 × 106 | ZnHL |
| DOPA-derived peptido-mimetics (deprotected) | 6 | 2.96 × 106 | ZnHL |
| DOPA-derived peptido-mimetics (protected) | 6 | 7.76 × 106 | ZnL |
| l-dopa deuterated | 6 | 2.96 × 106 | ZnHL |
| Doxycycline | 6.1 | 3.88 × 104 | ZnL |
| Droxidopa | 10.9 | 1.18 × 10−1 | ZnHL |
| Echinacoside | 6 | 7.76 × 106 | ZnL |
| Ellagic acid | 6 | 7.76 × 106 | ZnL |
| Entacapone (comtan, ASI-6) | 6.2 | 2.62 × 104 | ZnL |
| Enzastaurin | 6 | 7.85 × 105 | ZnL |
| Epicatechin | 6 | 7.76 × 106 | ZnL |
| Epigallocatechin-3-gallate | 6 | 1.81 × 1013 | Zn2L |
| Etidronate (HEDPA) | 7.4 | 4.32 × 102 | ZnL2− |
| Exifone | 6 | 1.81 × 1013 | Zn2L |
| F13714, F15599 | 6 | 2.28 × 105 | ZnL |
| Fisetin (3,3′,4′,7-tetra-hydroxy-flavone) | 6 | 7.76 × 106 | ZnL |
| Fraxetin | 6 | 7.76 × 106 | ZnL |
| Gallic acid derivatives | 6 | 1.81 × 1013 | Zn2L |
| Gallocatechin | 6 | 2.71 × 105 | ZnL |
| Garcinol | 6 | 7.76 × 106 | ZnL |
| Glutamine | 6 | 8.61 × 105 | ZnL+ |
| Glutathione derivatives | 14.8 | 1.20 × 10−5 | ZnH−2L22− |
| Glutathione-hydroxy-quinoline compound | 7.8 | 1.37 × 102 | ZnH−1L+ |
| Glutathione-l-DOPA compound | 6.3 | 1.20 × 104 | ZnH−1L |
| Gly-N-C-DOPA | 6 | 2.96 × 106 | ZnHL |
| GSK2795039 | 9.7 | 1.64 | ZnL2 |
| Guanabenz | 6 | 7.85 × 105 | ZnL |
| Hinokitiol | 6.2 | 2.06 × 104 | ZnL+ |
| 8-HQ-MC-5 (VK-28) | 7.5 | 3.16 × 102 | ZnL |
| 4-Hydroxyisophthalic acid | 6 | 9.02 × 107 | ZnL |
| 1-Hydroxy-2-pyridinone derivatives | 6.3 | 1.01 × 104 | ZnL |
| 3-Hydroxy-4(1H)pyridinone (Deferiprone) | 6.2 | 1.45 × 104 | ZnL+ |
| 3-Hydroxy-4(1H)pyridinone derivatives (R = H) | 6.2 | 1.45 × 104 | ZnL |
| 8-hydroxyquinoline-2-carboxaldehyde isonicotinoyl hydrazone | 7.5 | 3.16 × 102 | ZnL |
| Hydroxy-quinoline-propargyl hybrids (HLA20) | 7.5 | 3.16 × 102 | ZnL |
| Hydroxytyrosol butyrate | 6 | 7.76 × 106 | ZnL |
| l-(7-Imino-3-propyl-2,3-dihydrothiazolo [4,5-d] pyrimidin-6(7H)-yl)urea | 6.5 | 5.10 × 103 | ZnH−1L |
| Imipramine | 6 | 1.95 × 106 | ZnHL3+ |
| Isobavachalcone | 6 | 2.12 × 105 | ZnL+ |
| Isochlorogenic acid | 6 | 6.86 × 105 | ZnL− |
| Isoquercetin (isoquercitrin) | 6 | 3.94 × 1024 | ZnH4L |
| Kaempferol | 6 | 3.94 × 1024 | ZnH4L+ |
| Kaempferol, 3-O-a-L arabino-furanoside-7-O-a- L-rhamno-pyranoside | 6 | 3.56 × 1020 | ZnH3L |
| KR33493 | 6 | 1.54 × 106 | ZnL |
| Kukoamine | 6 | 7.76 × 106 | ZnL |
| Lestaurtinib | 6 | 7.85 × 105 | ZnL |
| Lipoic acid | 6 | 3.84 × 106 | ZnL+ |
| LY354740 | 6 | 1.46 × 107 | ZnL |
| M10 M30 (VAR10303) M99 | 7.5 | 3.16 × 102 | ZnL |
| Macranthoin G | 6 | 7.76 × 106 | ZnL |
| Magnesium lithospermate B | 6 | 7.76 × 106 | ZnL |
| α-mangostin | 6 | 7.76 × 106 | ZnL |
| γ- mangostin | 6 | 7.76 × 106 | ZnL |
| MAOI-1 | 6 | 2.71 × 105 | ZnL |
| MAOI-2 | 6 | 7.85 × 105 | ZnL |
| MAOI-4 | 6 | 5.88 × 105 | ZnHL |
| Metformin (Met) | 6 | 1.87 × 106 | ZnL+ |
| Methoxy-6-acetyl-7-methylijuglone | 6.1 | 4.82 × 104 | ZnL2 |
| N′-(4-methylbenzylidene)-5-phenylisoxazole-3-carbohydrazide | 6 | 1.76 × 106 | ZnL |
| Minocycline | 6.4 | 7.52 × 103 | ZnHL |
| Mitomycin C | 6 | 3.49 × 106 | ZnL |
| MitoQ | 7.1 | 8.74 × 102 | ZnL |
| Morin | 6 | 5.69 × 1016 | ZnH3L |
| [18F]MPPF | 6 | 7.85 × 105 | ZnL |
| MSX-3 | 6 | 8.19 × 106 | ZnL |
| Nicotinamide adenine dinucleotide phosphate (NADPH) | 6.1 | 3.72 × 104 | ZnL |
| Nicotinamide mononucleotide | 6 | 8.19 × 106 | ZnL |
| Nitecapone | 6.2 | 2.62 × 104 | ZnL |
| Nordihydroguaiaretic acid | 6 | 7.76 × 106 | ZnL |
| Oleuropein | 6 | 7.76 × 106 | ZnL |
| Opicapone | 6.2 | 2.62 × 104 | ZnL |
| P7C3 | 6 | 1.96 × 107 | ZnL2+ |
| PBF-509 | 6 | 7.85 × 105 | ZnL |
| PBT2 | 7.5 | 3.16 × 102 | ZnL |
| PBT434 | 7.9 | 1.19 × 102 | ZnL2 |
| Petunidin | 6 | 7.76 × 106 | ZnL |
| Phenothiazine 2Bc (n=0) | 6 | 7.85 × 105 | ZnL2+ |
| Phenothiazine 2Bc (n=1) | 6 | 1.95 × 106 | ZnHL3+ |
| Phenylhydroxamates | 6 | 2.07 × 105 | ZnL |
| Piceatannol | 6 | 7.76 × 106 | ZnL |
| Piperazine-8-OH-quinolone hybrid | 7.5 | 3.16 × 102 | ZnL |
| Preladenant | 6 | 7.85 × 105 | ZnL |
| Promethazine | 6 | 7.85 × 105 | ZnL2+ |
| Protocatechuic acid | 6 | 1.25 × 107 | ZnL− |
| Protosappanin A | 6 | 7.76 × 106 | ZnL |
| Pyrazolobenzothiazine-based carbothioamides | 6 | 4.73 × 107 | ZnL |
| Pyrimidinone 8 | 6 | 7.85 × 105 | ZnL |
| Q1 Q4 | 7.5 | 3.16 × 102 | ZnL |
| Quercetin | 6 | 3.94 × 1024 | ZnH4L+ |
| Quinoline derivatives as SUMOylation activators | 6 | 3.94 × 106 | ZnL2+ |
| Radotinib | 6.4 | 6.31 × 103 | ZnL |
| Riboflavin | 6 | 2.16 × 105 | ZnHL3+ |
| Rifampicin (ASI-3) | 6.1 | 7.04 × 104 | ZnL |
| Rimonabant | 6 | 1.52 × 106 | ZnL |
| Rosmarinic acid | 6 | 7.76 × 106 | ZnL |
| Rutin | 6 | 3.94 × 1024 | ZnH4L+ |
| Salicylate, sodium salt | 6 | 9.02 × 107 | ZnL |
| Salvianolic acid B | 6 | 7.76 × 106 | ZnL |
| SCH58261 SCH412348 | 6 | 7.85 × 105 | ZnL |
| ST1535 ST4206 | 6 | 7.85 × 105 | ZnL |
| Staurosporine | 6 | 7.85 × 105 | ZnL |
| Stemazole | 6 | 4.73 × 107 | ZnL |
| Sulfuretin | 6 | 7.76 × 106 | ZnL |
| Tannic acid | 6 | 1.22 × 105 | ZnL |
| Tanshinol | 6 | 7.76 × 106 | ZnL |
| Taurine | 6 | 1.26 × 1012 | ZnL2 |
| Tetracycline | 6 | 7.01 × 106 | ZnL |
| Tolcapone (ASI-7) | 6.2 | 2.62 × 104 | ZnL |
| Tozadenant | 8.7 | 1.62 × 101 | ZnL2 |
| Transilitin | 6.1 | 8.21 × 104 | ZnL |
| o-Trensox | 21.7 | 1.95 × 10−12 | ZnL4− |
| 2′, 3′, 4′-Trihydroxyflavone | 6 | 2.71 × 105 | ZnL |
| 2,3,3-Trisphosphonate | 12.1 | 7.93 × 10−3 | ZnL |
| V81444 | 6 | 2.28 × 105 | ZnL |
| VAS3947 VAS2870 | 6.5 | 5.10 × 103 | ZnH−1L |
| Verbascoside | 6 | 9.29 × 105 | ZnH−1L |
| WIN 55,212-2 | 6 | 7.85 × 105 | ZnL2+ |
| WR-1065 | 6 | 1.95 × 106 | ZnHL3+ |
| Zonisamide | 8 | 8.81 × 101 | ZnL |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tosato, M.; Di Marco, V. Metal Chelation Therapy and Parkinson’s Disease: A Critical Review on the Thermodynamics of Complex Formation between Relevant Metal Ions and Promising or Established Drugs. Biomolecules 2019, 9, 269. https://doi.org/10.3390/biom9070269
Tosato M, Di Marco V. Metal Chelation Therapy and Parkinson’s Disease: A Critical Review on the Thermodynamics of Complex Formation between Relevant Metal Ions and Promising or Established Drugs. Biomolecules. 2019; 9(7):269. https://doi.org/10.3390/biom9070269
Chicago/Turabian StyleTosato, Marianna, and Valerio Di Marco. 2019. "Metal Chelation Therapy and Parkinson’s Disease: A Critical Review on the Thermodynamics of Complex Formation between Relevant Metal Ions and Promising or Established Drugs" Biomolecules 9, no. 7: 269. https://doi.org/10.3390/biom9070269
APA StyleTosato, M., & Di Marco, V. (2019). Metal Chelation Therapy and Parkinson’s Disease: A Critical Review on the Thermodynamics of Complex Formation between Relevant Metal Ions and Promising or Established Drugs. Biomolecules, 9(7), 269. https://doi.org/10.3390/biom9070269