Abstract
Stilbenes, particularly resveratrol and resveratrol dimers, could effectively quench singlet oxygen (1O2). It was reported that both resorcinol and carbon-carbon double bond quenching 1O2 can participate in the mechanism. However, it is still not clear which structure plays a dominant role in quenching 1O2. To investigate the characteristic structure in the mechanism of quenching 1O2, the resveratrol, pterostilbene and piceatannol quenching 1O2 abilities were compared by UHPLC-QTOF-MS2 and UHPLC-QQQ-MS2. Results showed that catechol, carbon-carbon double bond and resorcinol participated in the quenching of 1O2. Catechol ring plays a leading role in the mechanism, and the contribution of the structures in quenching 1O2 activity are as follows: catechol ring > carbon-carbon double bond > resorcinol ring, which is supported by the calculation of energy. Our findings will contribute to the future screening of stilbenes with higher activity, and those stilbenes may have great therapeutic potential in 1O2-mediated diseases.
1. Introduction
As mediators of oxidative stress, reactive oxygen species (ROS), including superoxide radical anion (O2•−), hydroxyl radical (·OH), singlet oxygen (1O2) and hydrogen peroxide, have been regarded as the main reason leading to many diseases [1]. As the first excited state 1Δg of molecular oxygen, 1O2 is considered as one of the most active classes involved in chemical and biochemical reactions, since it can easily react with a large number of biological molecules, such as DNA, proteins and lipids [2]. 1O2 mediated DNA damage can lead to neurological disorders like Alzheimer’s and Parkinson’s disease, and even increased risk of cancer [3]. Furthermore, 1O2 ias reported to be closely associated with the occurrence of xeroderma pigmentosum, a rare skin disorder [4,5]. The generation of 1O2 is achieved by endogenous photosensitizers such as chlorophyll or riboflavin absorbing energy from both ultraviolet (UV)-A and UV-B as well as visible light then transferring the energy to the oxygen molecule [6]. Numerous studies have emphasized the hazards caused by 1O2 to human health [7,8].
Stilbenes are a class of natural phytoalexins found in plants (especially grapes) and are well-known for their antioxidant activity, exhibiting various biological activities such as cardioprotection, neuroprotection, anti-diabetic properties, depigmentation, anti-inflammation, and cancer prevention and treatment [9]. Stilbenes have a common backbone stilbene structure but differ in substituents on the ring [10]. Structural complexity and diversity of stilbenes lead to the difference of its biological activity. Ohguchi et al. (2003) suggest that the carbon-carbon double bond in the stilbene skeleton is critical for the inhibitory effects against murine tyrosinase activity [11]. Literature showed that the antioxidant activity was involved in the number and position of hydroxyl groups. Murias found that 3,3′,4,4′,5,5′-hexahydroxystilbene exerted a more than 6600-fold higher antiradical activity than resveratrol and its two other analogues [12]. Similarly, Kotora et al. speculated that releasing of the proton from OH group could be the main mechanism responsible for the antioxidant activity of studied (Hydroxyphenyliminomethyl) phenols [13]. In addition, methoxylation of hydroxyl has been reported to significantly improve the anti-tumor potential of stilbene compounds [14].
The activity of stilbene quenching 1O2 has not caught deserved attention for a long time in the past, until pallidol, a resveratrol dimer, was discovered as a selective 1O2 quencher by He et al. [15]. Additionally, it has been reported that vitisin A from Vitis chunganeniss shows the activity of selective 1O2 quenching [16]. Subsequently, stilbenes like chunganenol, laetevirenols F and laetevirenols G have been determined to be potent 1O2 quenchers by electron paramagnetic resonance experiments [17,18]. Later, scirpusin A, a hydroxystilbene dimer from Xinjiang wine grape, was reported as an effective singlet oxygen quencher [19]. In the further investigation of the activity of stilbenes quenching 1O2, many works so far have focused on its mechanism. Some hold the view that resorcinol ring could be oxidized to quinone when resveratrol quenches 1O2 [20]. Others propose that the mechanism of resveratrol 1O2 quenching is mainly because of the carbon-carbon double bond based on nuclear magnetic resonance (NMR) data [21]. In addition, the mechanism of resveratrol dimers quenching 1O2 has been investigated by UHPLC-QTOF-MS2 in our previous studies, suggesting that resorcinol ring and carbon-carbon double bond both participate in quenching 1O2 [22]. Nevertheless, which structure plays a more important role in the mechanism of quenching 1O2 is still not clear.
Trans-resveratrol (1), trans-pterostilbene (2) and trans-piceatannol (3) all have stilbene skeletons, while their substituents are different. As shown in Figure 1, Trans-resveratrol contains a resorcinol ring and a phenol ring, trans-pterostilbene contains a 3,5-dimethoxybenzene ring and a phenol ring, trans-piceatannol contains a catechol ring and a phenol ring. In order to investigate the characteristic structure and the mechanism of stilbenes quenching 1O2, we performed qualitative and quantitative analysis of reactants and products in the three stilbenes quenching 1O2 by using UHPLC-QTOF-MS2 and UHPLC-QQQ-MS2, and the B3LYP density functional method was used to verify the proposed mechanism.
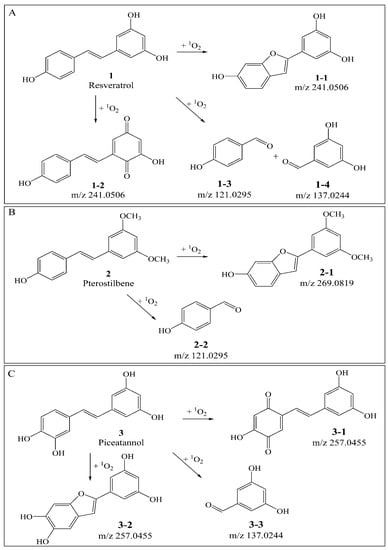
Figure 1.
(A): Proposed mechanism of resveratrol quenching 1O2 (1: resveratrol; 1-1, 1-2, 1-3, 1-4: proposed products of resveratrol against 1O2). (B): Proposed mechanism of pterostilbene quenching 1O2 (2: pterostilbene; 2-1, 2-2: proposed products of pterostilbene against 1O2). (C): Proposed mechanism of piceatannol quenching 1O2 (3: piceatannol; 3-1, 3-2, 3-3: proposed products of piceatannol against 1O2.).
2. Materials and Methods
2.1. Reagents and Sample Preparation
Reagents: Methanol used for UHPLC analysis was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Deionized water was purified with a Milli-Q water system (Millipore, Bedford, MA, USA). Trans-resveratrol (purity > 98%), trans-pterostilbene (purity > 97%) and trans-piceatannol (purity > 98%) were acquired from Sigma-Aldrich (St. Louis, MO, USA). Rose Bengal (RB) was purchased from Beijing Yaanda Biotechnology Co., Ltd. (Beijing, China). As a photosensitizer, RB can absorb energy from light then transfer the energy to molecular oxygen and generating 1O2.
Sample preparation: All experiments involving the application of trans-resveratrol, trans-pterostilbene and trans-piceatannol were carried out in a dimly lit environment to prevent photoisomerisation [23]. The concentration of RB dissolved in deionized water during sample preparation was 18 μmol/L. Both the use and storage of RB need to be performed in a dimly lit environment. The concentration of stilbenes dissolved in methanol was 500 μmol/L. Prepared solutions need to be filtered by 0.22 μm filter. The ultraviolet lamp (UVA, 20 W, λ = 365 W) was turned on for at least 15 min to reach an equilibrium status. Each control sample contained 500 μL ultrapure water and 100 μL trans-stilbenes (1, 2, 3, respectively) without ultraviolet radiation (S1, S2 and S3). Treated samples were composed of 500 μL RB and 100 μL trans-stilbenes (1, 2, 3, respectively); the reaction mixtures were irradiated by the ultraviolet lamp for 2 min at 23 °C ± 2 °C (S4, S5 and S6). All samples were freshly prepared and immediately determined and analyzed after transferring to the auto-sampler vials.
2.2. Density Functional Method
The B3LYP density functional method was employed in this study to carry out the computations. The 6-311g(d,p) basis set was used for all the atoms in geometry optimizations. Vibrational frequency analyses at the same theoretical level were performed on all optimized structures to characterize stationary points as local minima. Only the energy of conformation most stable for compounds was used to compare the stability of possible products. The Gaussian 09 suite of programs was used to compute the energy of possible product (with the aid of literature, compounds 1-1, 1-2 were selected as the possible product of resveratrol quenching 1O2).
2.3. Qualitative Analysis by UHPLC-QTOF-MS2
The preparation of the sample was described in Section 2.2. The UHPLC-QTOF-MS2 experiments were carried out by a Thermo Scientific Dionex UltiMate 3000 system coupled with a Bruker micrOTOF-Q III mass spectrometer (Bruker-Franzen Analytik GmbH, Bremen, Germany). The separation of samples was performed on a Thermo Scientific AcclaimTM RSLC 120 C18 reversed-phase column (3.0 × 100 mm, 2.2 μm, 120 Å), using a gradient elution comprising Ammonium formate in Ultra-pure water (5 mmol/L) and methanol with a flow rate of 0.2 mL/min at 20 °C. The injection volume was 2 μL. The separation was achieved using multi-step gradient using solvent A (water) and solvent B (methanol). The percentage of B (methanol) increased linearly from 5% to 30% in the first 5 min. Afterwards, it increased to 50% during the next 5 min. Then, it increased to 90% in 5 min and held for 5 min. The percentage of B went back to 5% in 7 min, and held for 5 min. The total run-time was 32 min.
The induction of UHPLC effluent introduced into the ESI source by a solvent line (analytical, softron P/N 5040.8117). Software HyStar3.2 (Bruker Hyphenation Star Application, Hamburg, Germany) was used to combine control of UHPLC and MS. Experiments were performed under the negative ion mode of ESI. Nitrogen was used as the nebulizing and drying gas at 1.2 bar and a flow rate of 8.0 L/min, with the dry temperature set as 180 °C. Capillary voltage was 3000 V, and End Plate Offset was 500 V. The scan mode was set to Auto MS/MS with the mass scan range being 50–1000 m/z.
2.4. Quantitative Analysis by UHPLC-QQQ-MS2
The preparation of the sample has been described in Section 2.2. The UHPLC-QQQ-MS2 system from Agilent Technologies (Santa Clara, California) consisted of a 1260-series UHPLC coupled to an Agilent 6460 series triple quadrupole mass spectrometer. Sample separation was performed on an Agilent Poroshell 120 EC-C18 column (3.0 × 100 mm, 2.7 μm), using a gradient elution including the ultra-pure water with 5 mmol/L ammonium formate added (solvent A) and MeOH (solvent B) with a flow rate of 0.2 mL/min at 30 °C and the injection volume was 2 μL. The separation was achieved using multi-step gradient program as follows: 0–2 min, linearly 50% B–60% B; 2–3 min, linearly 60% B–70% B; 3–5 min, linearly 70% B–80% B; 5–12 min, 80% B; 12–14 min, linearly 80% B–90% B; 14–16 min, linearly 90% B; 16–20 min, linearly 90% B–50% B; 20–23 min, 50% B. The total running time was 23 min.
Mass spectrometric detection was performed on an Agilent 6460 QQQ instrument equipped with the Agilent Jet Stream Technologies - Electrospray Ionization (AJS-ESI) source operating in a negative ionization mode, with the conditions as follows: Nitrogen (99.99%) was used as the nebulizing and drying gas at 45 psi and a flow rate of 5.0 L/min, with the drying temperature set as 350 °C; the temperature of sheath gas was 250 °C, and the flow was 11 L/min; the capillary voltage was 3500 V; and the nozzle Voltage was 500 V. Agilent Mass Hunter software was used to control the UHPLC-QQQ-MS2. Multiple Reaction Monitoring (MRM) mode with optimal Fragmentor (V) and Collision Energy (eV) were used to quantify three stilbenes. MRM mode was also used to quantify reactants. The optimal parameters used in MRM mode are shown in Table 1. It is generally accepted that peak area represents the relative content of target compounds. The ratio of the change of reactant content to the initial value of reactant, namely, the relative content change of reactants, was used to evaluate the activity of stilbene quenching 1O2.

Table 1.
The optimal parameters of target compounds in MRM mode.
2.5. Statistical Analysis
Data were analyzed by Microsoft Excel 2016 and Statistical Analysis Software (SPSS18). Results were statistically compared and expressed as means with standard deviations (SD). The data were analyzed by one-way analysis of variance (ANOVA). Comparison of means was made by Duncan’s multiple range tests. Average values and standard errors are shown in the figures. Differences were considered significant when p < 0.05.
3. Results and Discussion
3.1. Qualitative Comparison of the Activity of Double Bond and Resorcinol Ring Quenching 1O2
As compared with sample S1 (resveratrol), the relative abundance of m/z 227.0717 in sample S4 (resveratrol + 1O2) decreased sharply. The product ions of m/z 227.0717 were m/z 185, 159, 143, and its proposed fragmentation pathway is shown in Figure 2A. In the MS/MS spectrum of the [M − H]− ion at m/z 241.0528, it generated losses of 28 Da, 46 Da, 56 Da, 72 Da, 84 Da, 98 Da, corresponding to the product ions at m/z 213, 195, 185, 169, 157, and 143. Its proposed fragmentation pathway is shown in Figure 3A. The precursor ions at m/z 121.0287, m/z 137.0244 and m/z 241.0528 were detected in sample S4, but not in sample S1. It is noteworthy that 241.0528 was 14 Da larger than that of resveratrol (1, m/z 227.0717). The ions at m/z 241.0528 and m/z 227.0717 had similar fragmentation pathways. In other words, they have the same product ions such as m/z 185, 159, 143. Celaje et al. (2011) reported that resveratrol could quench 1O2 and generate p-hydroxybenzaldehyde, 3,5-dihydroxybenzaldehyde and Moracin M, proving that ions at m/z 121.0295 (1-3), m/z 137.0244 (1-4), and m/z 241.0506 (1-1) were the oxide products of resveratrol quenching 1O2 [21]. According to their work, the characteristic structure in the process of resveratrol quenching 1O2 is the carbon-carbon double bond. However, Jiang et al. (2010) reported that resveratrol quinone (1-2) was the main product responsible for quenching 1O2 and suggested that what really matters in the mechanism is the resorcinol moiety [20]. In addition, according to our previous result [22], the carbon-carbon double bond and resorcinol moiety both were verified to be participants in the quenching reaction.
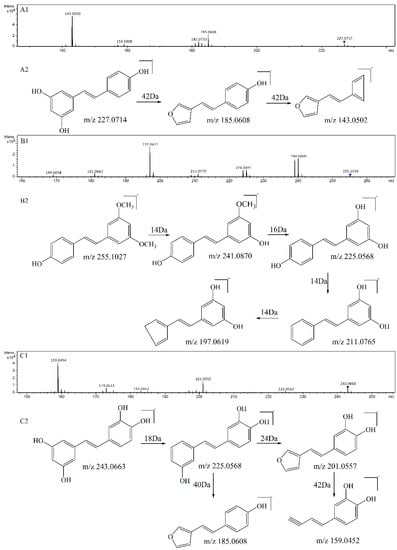
Figure 2.
MS2 spectrum of the precursor ion at m/z 227 (A1) and its proposed fragmentation pathway (A2). MS2 spectrum of the precursor ion at m/z 255 (B1) and its proposed fragmentation pathway (B2). MS2 spectrum of the precursor ion at m/z 243 (C1) and its proposed fragmentation pathway (C2).
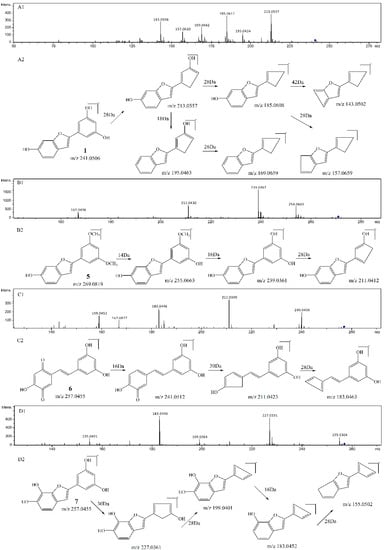
Figure 3.
MS2 spectrum of the precursor ion at m/z 241 (A1) and its proposed fragmentation pathway (A2). MS2 spectrum of the precursor ion at m/z 269 (B1) and its proposed fragmentation pathway (B2). MS2 spectrum of the precursor ion at m/z 257a (C1) and its proposed fragmentation pathway (C2). MS2 spectrum of the precursor ion at m/z 257b (D1) and its proposed fragmentation pathway (D2).
As compared with sample S2 (pterostilbene), the relative abundance of m/z 255.1036 in sample V (pterostilbene + 1O2) decreased significantly. The product ions of m/z 255.1036 were m/z 240, 225, 211, 197, and its proposed fragmentation pathway is shown in Figure 2B. In the MS/MS spectrum of the [M − H]− ion at m/z 269.0815, it generated the losses of 14 Da, 30 Da, 58 Da, corresponding to the product ions at m/z 255, 239, 211. The precursor ions at m/z 121.0290 and m/z 269.0815 were detected in sample S5, but not in sample S2. It is worthwhile mentioning that m/z 269.0815 was14 Da larger than that of pterostilbene (2, m/z 255.1036). The content of pterostilbene decreased (Figure 3B), and ions at m/z 269.0815 and m/z 255.1036 had similar fragmentation pathways. Compounds 2-1, 2-2 could be the oxide products of pterostilbene quenching 1O2.
As compared with sample S3 (piceatannol), the relative abundance of m/z 243.0668 in sample VI (piceatannol + 1O2) decreased sharply. The product ions of m/z 243.0668 were m/z 225, 201, 185, 159, and its proposed fragmentation pathway is shown in Figure 2C. The precursor ions at m/z 137.0256 and m/z 257 were detected in sample S6, but not in sample S3. The precursor ions at m/z 257 were observed at different retention times, corresponding to 3-1 (m/z 257.0477a, T = 13.7 min) and 3-2 (m/z 257.0460b, T = 15.1 min). In the MS/MS spectrum of the [M − H] − ion at m/z 257.0477a (3-1), the mass spectrum generated losses of 16 Da, 46 Da and 74 Da, which correspond to the product ions at m/z 241, 211, 183. The proposed fragmentation pathway of m/z 257.0477a is shown in Figure 3C. In the MS/MS spectrum of the [M − H]− ion at m/z 257.0460b (3-2, T = 15.1 min), the mass spectrum generated losses of 30 Da, 58 Da, 74 Da and 102 Da, which correspond to the product ions at m/z 227, 199, 183, 155. The proposed fragmentation pathway of m/z 257.0460b is shown in Figure 3D. The precursor ions at 257 were only observed in sample S6, and their fragmentation pathways are similar to m/z 243.0668. The precursor ion at 257 is considered a product of piceatannol quenching 1O2.
When 1O2 was added to the reaction, the content of resveratrol, pterostilbene and piceatannol decreased significantly. The identical substructure of the three compounds is the stilbene skeleton, pointing out the carbon-carbon double bond may be the characteristic structure of stilbenes quenching 1O2. We suggest the main mechanism of stilbenes quenching 1O2 could be the [2+2] and [4+2] cycloaddition reaction of the carbon-carbon double bond in stilbenes.
3.2. Quantitative Comparison of the Activity of the Catechol Ring, Carbon-Carbon Double Bond and Resorcinol Ring Quenching 1O2
As shown in Figure 4, the relative content of three stilbenes decreased differently. Piceatannol decreased most significantly, and there was no significant difference between resveratrol and pterostilbene reduction. Quantitative results showed that piceatannol had the strongest activity of quenching 1O2, followed by resveratrol and pterostilbene. Methoxylation of hydroxyl groups did not lead to any significant decrease of the1O2 quenching activity, further proving that cycloaddition reaction of the carbon-carbon double bond plays an important role in quenching 1O2, and it is more effective than the resorcinol ring. Several articles reported that the carotenoids have a strong activity of quenching 1O2, closely related to the existence of conjugated double bonds [24,25]. Pallidol has the strong ability to quench 1O2 [15], but it does not contain any carbon-carbon double bond, only resorcinol rings. We propose that the carbon-carbon double bond is the characteristic functional group in stilbenes quenching 1O2. However, if there is no carbon-carbon double bond in the compound, the resorcinol ring may participate in the reaction of quenching 1O2, generating quinones. Furthermore, the catechol ring has been proposed to be the most vital functional group of quenching 1O2. Our studies showed that piceatannol was the most involved in the reaction. Jung et al. (2010) determined that the total singlet oxygen quenching rate constant for resveratrol in methanol was 2.55 × 107 M−1 s−1 [8]. Choi et al. (2016) determined that the total singlet oxygen quenching rate constant for nordihydroguaiaretic acid (NDGA) in methanol was 9.81 × 107 M−1 s−1 [26]. NDGA contains two catechol groups, while resveratrol contains double bond and resorcinol rings. NDGA has a stronger singlet oxygen quenching activity than resveratrol, indicating that catechol ring was more effective than the carbon-carbon double bond in resorcinol’s quenching of 1O2. Therefore, we conclude that the most important substructure of quenching 1O2 is the catechol ring, then the carbon-carbon double bond, and then the resorcinol ring.
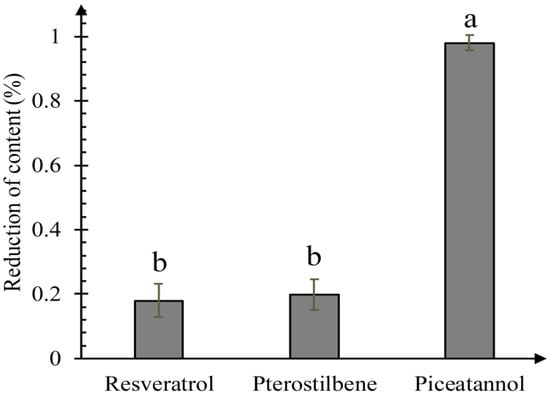
Figure 4.
Changes of three stilbene contents in the prossess of quenching 1O2. The values are means of three replicates and their standard errors. Means with different letters are significantly different according to Duncan’s multiple-range test (p < 0.05).
3.3. Validation of the Mechanism by B3LYP Density Functional Method
According to literature, compounds 1-1 and 1-2 were considered as the possible products of 1 quenching 1O2. In order to determine which product is more effective in resveratrol quenching 1O2, we used the B3LYP density functional method to determine the optimal configurations and computed the energy of two compounds. The parameters of the optimal configurations and energy of compounds 1-1 and 1-2 are shown in Table 2. The energy calculated by the B3LYP density functional method is E1-1(b3lyp/6-311g(d,p)) = −840.6133 Hartree/Particle < E1-2(b3lyp/6-311g(d,p)) = −840.5894 Hartree/Particle. It is widely accepted that compounds with lower energy have higher stability [27], which in our case shows that compound 1-1 is more stable and more likely to generate in theory. Compound 1-1 is the possible product of resveratrol quenching 1O2, pointing to the carbon-carbon double bond as the important structure responsible in resorcinol’s quenching of 1O2. The bond length and bond angle of compound 1-1, 1-2 are shown in Table S1.

Table 2.
The E(b3lyp/6-311g(d,p)) of compound 1-1, 1-2 and parameters of the optimal configurations.
4. Conclusions
The main mechanism of resveratrol quenching 1O2 was the [2+2] and [4+2] cycloaddition reaction of the carbon-carbon double bond. Furthermore, the catechol ring, carbon-carbon double bond and resorcinol ring in stilbenes also participated in quenching 1O2, and the activity of structure responsible for quenching 1O2 decreased in the order: catechol ring > carbon-carbon double bond > resorcinol ring. The mechanism could provide a theoretical basis for future screening and drug design for further investigations of those compounds’ applications in 1O2-mediated diseases.
Supplementary Materials
The following are available online at https://www.mdpi.com/2218-273X/9/7/268/s1, Table S1. The bond length and bong angle of compound 1-1, 1-2 and parameters of the optimal configurations.
Author Contributions
conceptualization, Q.K.; methodology, Q.K., J.Q.; investigation, J.Q., J.Y., X.R.; resources, Q.K.; writing—original draft preparation, J.L., S.W.; writing—review and editing, J.Q., J.Y.; visualization, X.R., S.W.; supervision, J.L.
Funding
This work was supported by the National Natural Sciences Foundation of China (grant numbers 31671904, 31460411, 21362028 and 31260402); the Fundamental Research Funds for the Central Universities (grant numbers 2017CSZ010, 2018CSLY020, GK201603095); Agricultural science and technology innovation and research (grant numbers 2016NY-184, 2016NY-194); One Hundred Person Project of Shaanxi Province (SXBR9197).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Defedericis, H.C.; Patrzyc, H.B.; Rajecki, M.J.; Budzinski, E.E.; Iijima, H.; Dawidzik, J.B.; Evans, M.S.; Greene, K.F.; Box, H.C. Singlet oxygen-induced DNA damage. Radiat. Res. 2006, 165, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Zhang, Y.N.; Wu, Z.Y.; Chen, Y.J.; Yang, X.; Sun, M.T.; Ni, R.Y.; Bian, J.S.; Huang, D.J. A near infrared singlet oxygen probe and its applications in in vivo imaging and measurement of singlet oxygen quenching activity of flavonoids. Sens. Actuator B Chem. 2018, 266, 645–654. [Google Scholar] [CrossRef]
- Lu, W.; Liu, J. Capturing Transient Endoperoxide in the Singlet Oxygen Oxidation of Guanine. Chemistry 2016, 22, 3127–3138. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, S.; Takahashi, Y.; Shimizu, H.; Inoue, M.; Sugiyama, Y.; Inoue, S. Decreased repair of singlet oxygen-induced DNA damage in xeroderma pigmentosum group A cells determined by plasmid host cell reactivation. J. Dermatol. Sci. 2012, 66, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Runger, T.M.; Epe, B.; Moller, K. Repair of ultraviolet B and singlet oxygen-induced DNA damage in xeroderma pigmentosum cells. J. Investig. Dermatol. 1995, 104, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Onyango, A.N. Endogenous Generation of Singlet Oxygen and Ozone in Human and Animal Tissues: Mechanisms, Biological Significance, and Influence of Dietary Components. Oxidative Med. Cell. Longev. 2016, 2016, 22. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli-Neto, O.; Ferreira, A.S.; Martins, W.K.; Pavani, C.; Severino, D.; Faiao-Flores, F.; Maria-Engler, S.S.; Aliprandini, E.; Martinez, G.R.; Di Mascio, P.; et al. Melanin photosensitization and the effect of visible light on epithelial cells. PLoS ONE 2014, 9, e113266. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.Y.; Choi, D.S. Electron spin resonance and luminescence spectroscopic observation and kinetic study of chemical and physical singlet oxygen quenching by resveratrol in methanol. J. Agric. Food Chem. 2010, 58, 11888–11895. [Google Scholar] [CrossRef]
- Akinwumi, B.C.; Bordun, K.M.; Anderson, H.D. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef]
- Rivière, C.; Pawlus, A.D.; Mérillon, J.M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar]
- Ohguchi, K.; Tanaka, T.; Ito, T.; Iinuma, M.; Matsumoto, K.; Akao, Y.; Nozawa, Y. Inhibitory effects of resveratrol derivatives from dipterocarpaceae plants on tyrosinase activity. Biosci. Biotechnol. Biochem. 2003, 67, 1587–1589. [Google Scholar] [CrossRef] [PubMed]
- Murias, M.; Jäger, W.; Handler, N.; Erker, T.; Horvath, Z.; Szekeres, T.; Nohl, H.; Gille, L. Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: Structure–activity relationship. Biochem. Pharmacol. 2005, 69, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Kotora, P.; Sersen, F.; Filo, J.; Loos, D.; Gregan, J.; Gregan, F. The Scavenging of DPPH, Galvinoxyl and ABTS Radicals by Imine Analogs of Resveratrol. Molecules 2016, 21, 127. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, B.; Ammazzalorso, A.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Amoroso, R. Anticancer Activity of Stilbene-Based Derivatives. Chem. Med. Chem. 2017, 12, 558–570. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Jiang, L.; Wu, B.; Pan, Y.; Sun, C. Pallidol, a resveratrol dimer from red wine, is a selective singlet oxygen quencher. Biochem. Biophys. Res. Commun. 2009, 379, 283–287. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Lu, Y.; Jiang, L.; Wu, B.; Zhang, F.; Pan, Y. Preparative isolation and purification of antioxidative stilbene oligomers from Vitis chunganeniss using high-speed counter-current chromatography in stepwise elution mode. J. Sep. Sci. 2009, 32, 2339–2345. [Google Scholar] [CrossRef]
- He, S.; Jiang, L.Y.; Wu, B.; Zhou, J.; Pan, Y.J. Two Novel Antioxidative Stilbene Tetramers from Parthenocissus laetevirens. Helv. Chim. Acta 2009, 92, 1260–1267. [Google Scholar] [CrossRef]
- He, S.; Jiang, L.Y.; Wu, B.; Li, C.; Li, Y.J. Chunganenol: An Unusual Antioxidative Resveratrol Hexamer from Vitis chunganensis. J. Org. Chem. 2009, 74, 7966–7969. [Google Scholar] [CrossRef]
- Kong, Q.; Ren, X.; Jiang, L.; Pan, Y.; Sun, C. Scirpusin A, a hydroxystilbene dimer from Xinjiang wine grape, acts as an effective singlet oxygen quencher and DNA damage protector. J. Sci. Food Agric. 2010, 90, 823–828. [Google Scholar] [CrossRef]
- Jiang, L.Y.; He, S.; Jiang, K.Z.; Jiang, C.R.; Pan, Y.J. Resveratrol and its oligomers from wine grapes are selective 1O2 quenchers: Mechanistic implication by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry and theoretical calculation. J. Agric. Food Chem. 2010, 58, 9020–9027. [Google Scholar] [CrossRef]
- Celaje, J.A.; Zhang, D.; Guerrero, A.M.; Selke, M. Chemistry of trans-Resveratrol with Singlet Oxygen: [2+2] Addition, [4+2] Addition, and Formation of the Phytoalexin Moracin M. Org. Lett. 2011, 13, 4846–4849. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Yu, J.; Kong, Q.; Ren, X. Mechanism of isomers and analogues of resveratrol dimers selectively quenching singlet oxygen by UHPLC-ESI-MS2. Food Chem. 2017, 237, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Choo, Q.Y.; Yeo, S.C.M.; Yeo, P.C.; Tanaka, Y.; Lin, H.S. Pterostilbene surpassed resveratrol for anti-inflammatory application: Potency consideration and pharmacokinetics perspective. J. Funct. Foods 2014, 11, 352–362. [Google Scholar] [CrossRef]
- Hirayama, O.; Nakamura, K.; Hamada, S.; Kobayasi, Y. Singlet oxygen quenching ability of naturally occurring carotenoids. Lipids 1994, 29, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Thomas, H.; Glomb, M.A. Lycopene Inhibits the Isomerization of β-Carotene during Quenching of Singlet Oxygen and Free Radicals. J. Agric. Food Chem. 2015, 63, 3279–3287. [Google Scholar]
- Yun, S.C.; Jung, M.Y. Kinetic study on the singlet oxygen quenching activity of nordihydroguaiaretic acid (NDGA) using methylene blue sensitized photooxidation of α-terpinene. Food Sci. Biotechnol. 2016, 25, 1333–1336. [Google Scholar]
- Liu, C.; Si, H.; Han, P.; Tang, M. Density functional theory study on structure and stability of BeBn+clusters. Rapid Commun. Mass Spectrom. 2017, 31, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).

