A Novel Approach for Investigating Upper Airway Hyperresponsiveness Using Micro-CT in Eosinophilic Upper Airway Inflammation such as Allergic Rhinitis Model
Abstract
1. Introduction
2. Methods
2.1. Mice
2.2. Ovalbumin (OVA)-Induced AR Model
2.3. Evaluation of Nasal Mucosa by Micro-Computed Tomography
2.4. Statistical Analysis
3. Results
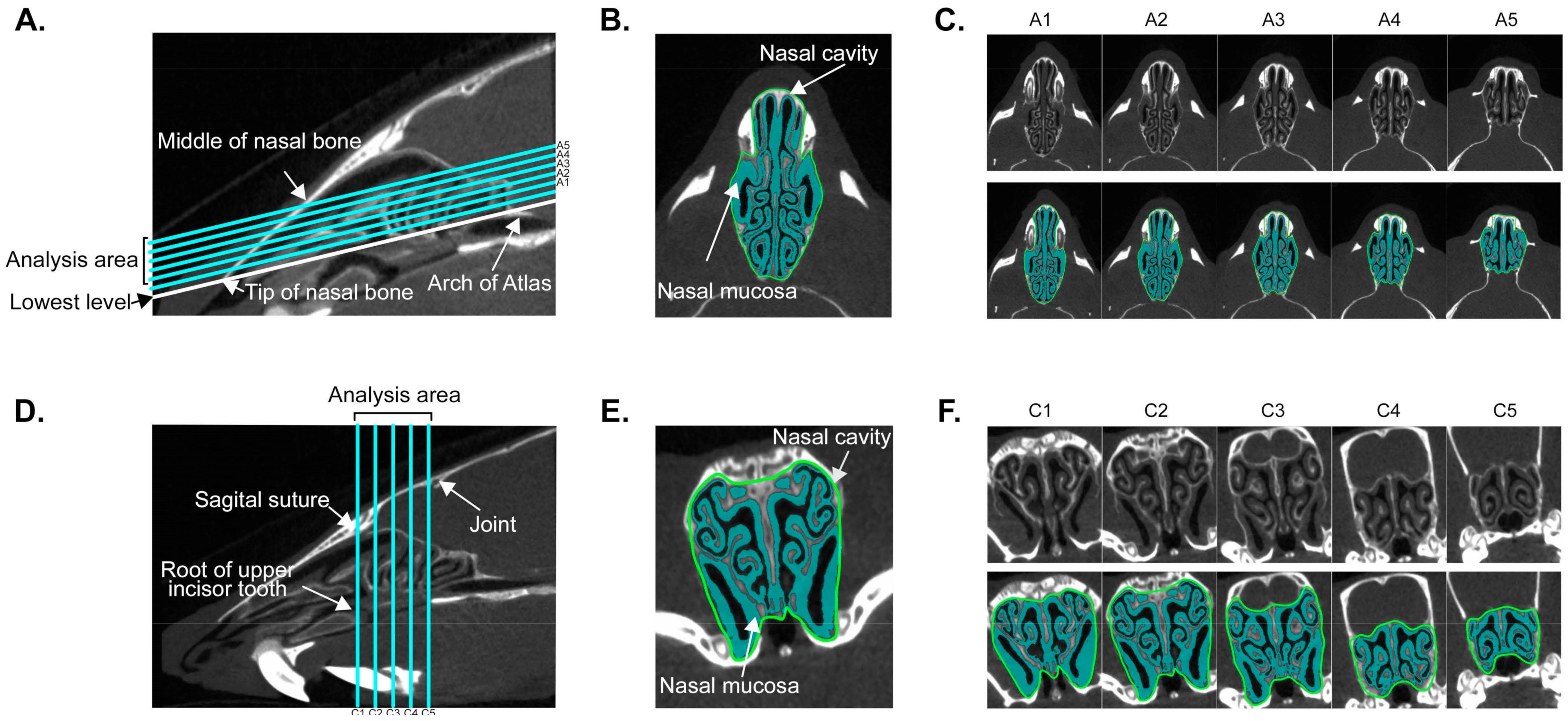
3.1. Establishment of Quantitative Analysis for Nasal Mucosa Using Micro-Computed Tomography
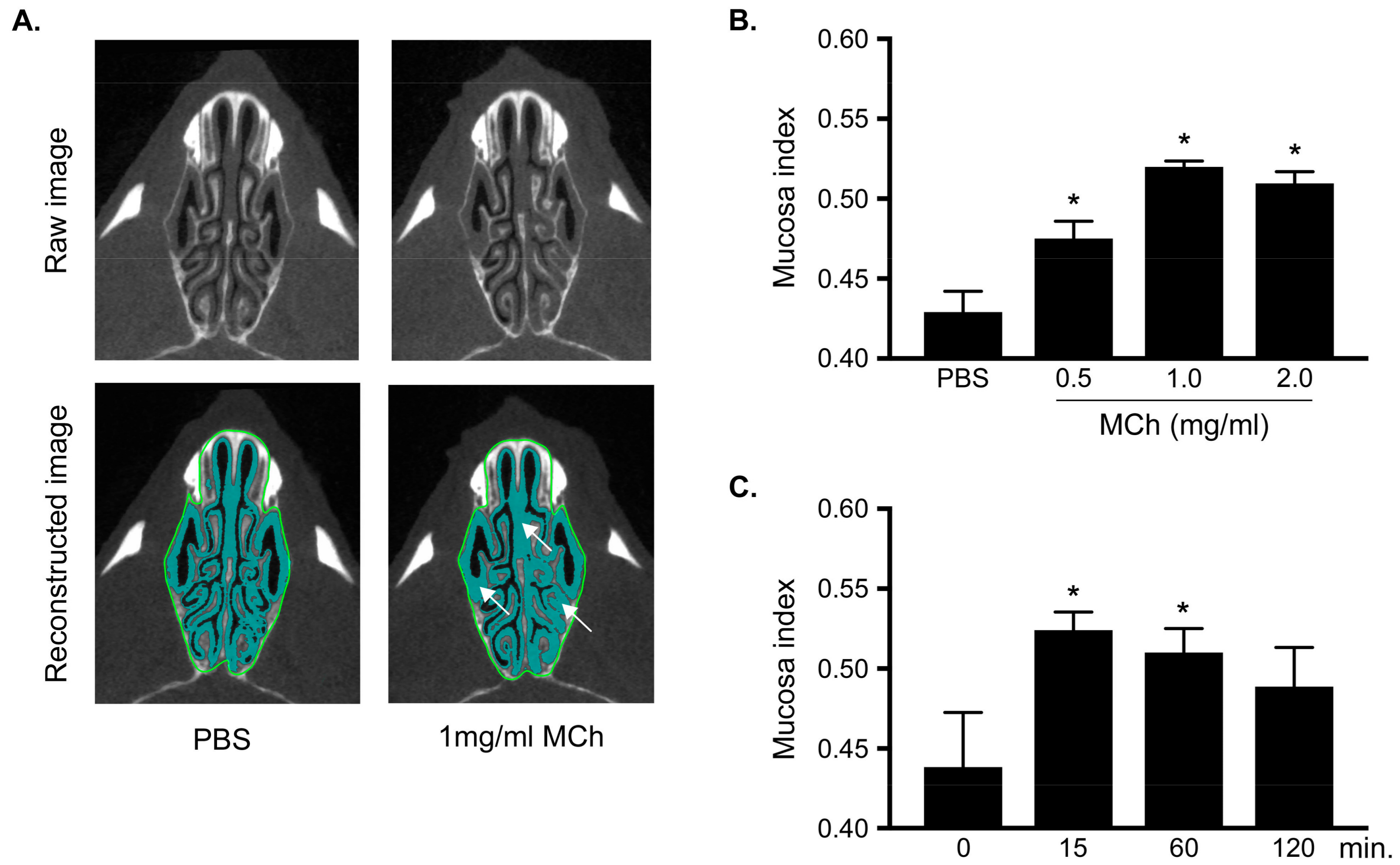
3.2. Measurement of MCh-Induced Swelling of the Nasal Mucosa
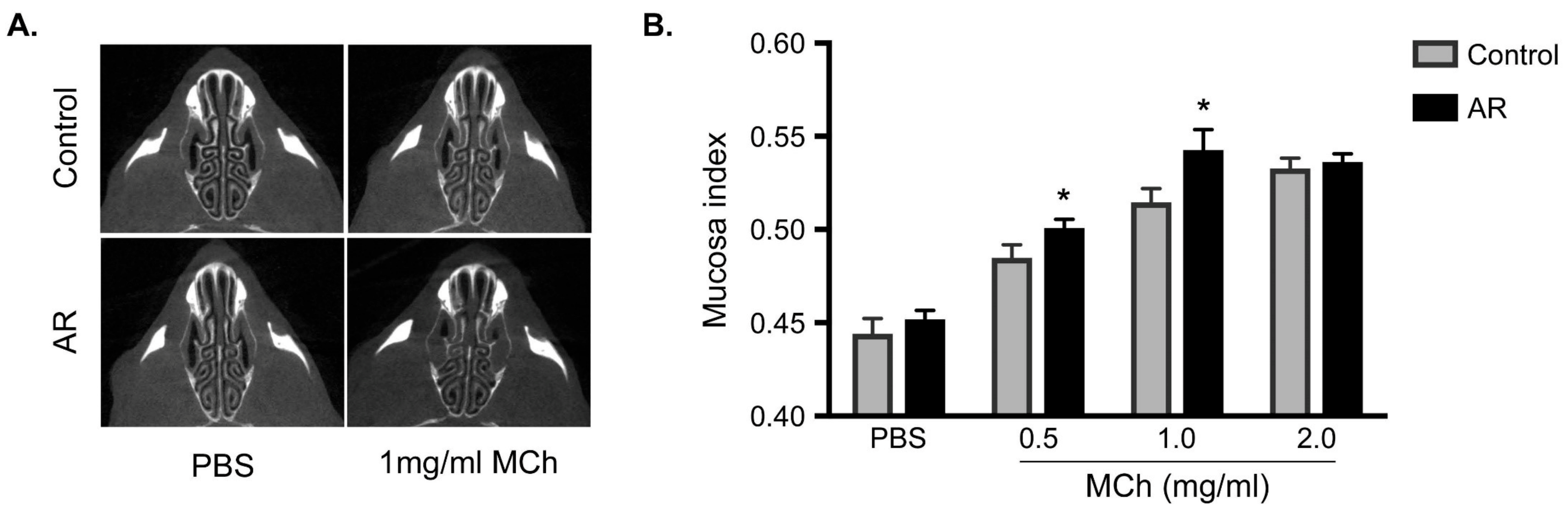
3.3. Increasing in the Mucosa Index in Allergic Rhinitis
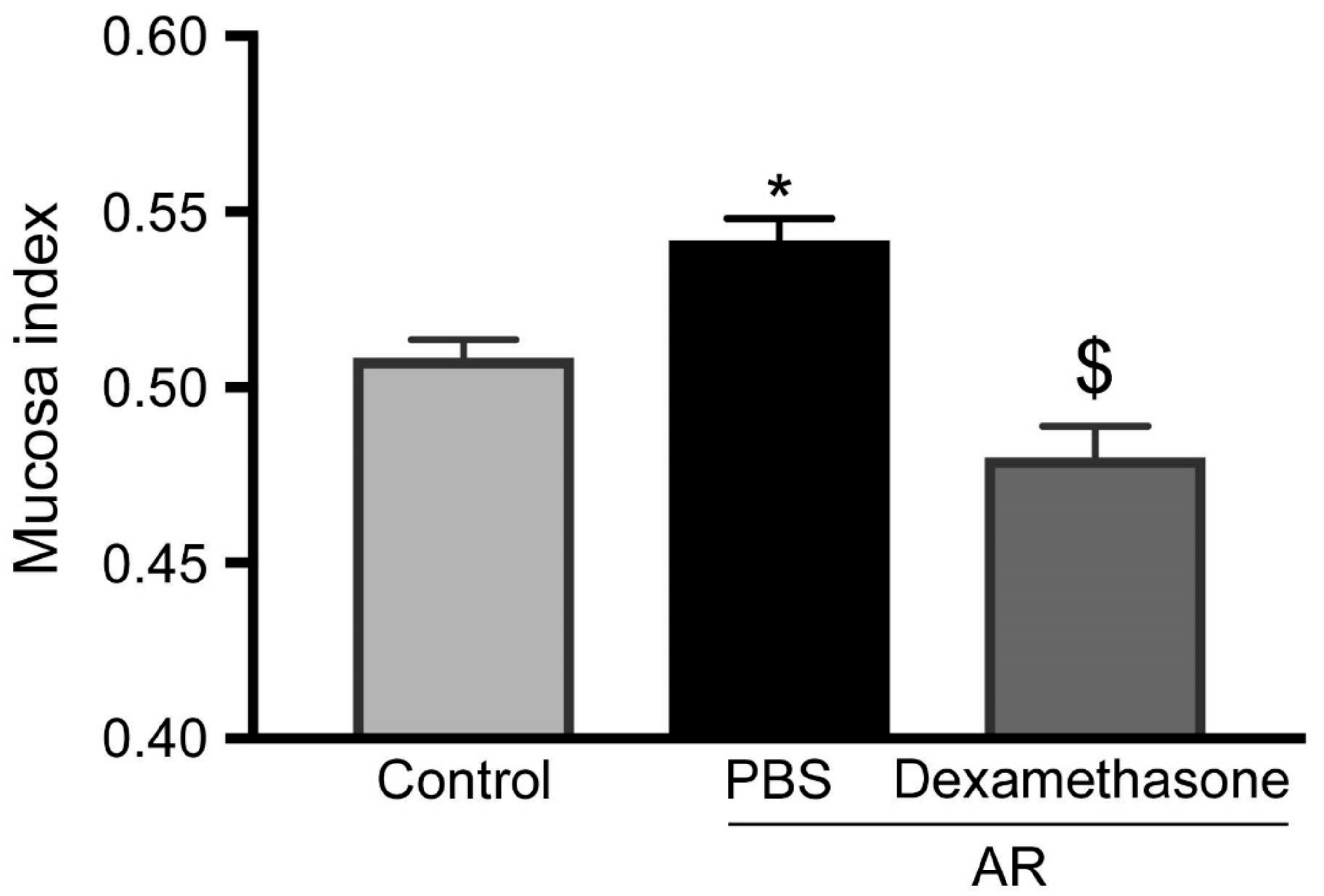
3.4. Steroids Reduced Increasing of Upper Airway Hyperresponsiveness in Allergic Rhinitis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; van Weel, C.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008, 63, 8–160. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Kurono, Y.; Ichimura, K.; Enomoto, T.; Okamoto, Y.; Kawauchi, H.; Suzaki, H.; Fujieda, S.; Masuyama, K. Japanese guidelines for allergic rhinitis 2017. Allergol. Int. 2017, 66, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, M.; Sugiura, H.; Nagase, H.; Yamaguchi, M.; Inoue, H.; Sagara, H.; Tamaoki, J.; Tohda, Y.; Munakata, M.; Yamauchi, K.; et al. Japanese guidelines for adult asthma 2017. Allergol. Int. 2017, 66, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Segboer, C.L.; Holland, C.T.; Reinartz, S.M.; Terreehorst, I.; Gevorgyan, A.; Hellings, P.W.; Van Drunen, C.M.; Fokkens, W.J. Nasal hyper-reactivity is a common feature in both allergic and nonallergic rhinitis. Allergy 2013, 68, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Postma, D.S.; Kerstjens, H.A.M. Characteristics of Airway Hyperresponsiveness in Asthma and Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1998, 158, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, M.; Okubo, K.; Okuda, M. Repeated antigen challenge in patients with perennial allergic rhinitis to house dust mites. Allergol. Int. 2003, 52, 207–212. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Davis, B.E. Mechanisms of airway hyperresponsiveness. J. Allergy Clin. Immunol. 2006, 118, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Asthma as an axon reflex. Lancet 1986, 327, 242–245. [Google Scholar] [CrossRef]
- Sin, B.; Togias, A. Pathophysiology of Allergic and Nonallergic Rhinitis. Proc. Am. Thorac. Soc. 2011, 8, 106–114. [Google Scholar] [CrossRef]
- Naclerio, R.M.; Baroody, F.M. Response of nasal mucosa to histamine or methacholine challenge: Use of a quantitative method to examine the modulatory effects of atropine and ipratropium bromide. J. Allergy Clin. Immunol. 1992, 90, 1051–1054. [Google Scholar] [CrossRef]
- Hamelmann, E.; Schwarze, J.; Takeda, K.; Oshiba, A.; Larsen, G.L.; Irvin, C.G.; Gelfand, E.W. Noninvasive Measurement of Airway Responsiveness in Allergic Mice Using Barometric Plethysmography. Am. J. Respir. Crit. Care Med. 1997, 156, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.R.; Gerard, N.P.; Galli, S.J.; Drazen, J.M. Pulmonary responses to bronchoconstrictor agonists in the mouse. J. Appl. Physiol. 1988, 64, 2318–2323. [Google Scholar] [CrossRef]
- Tomkinson, A.; Cieslewicz, G.; Duez, C.; Larson, K.A.; Lee, J.J.; Gelfand, E.W. Temporal Association between Airway Hyperresponsiveness and Airway Eosinophilia in Ovalbumin-Sensitized Mice. Am. J. Respir. Crit. Care Med. 2001, 163, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Eum, S.-Y.; Maghni, K.; Hamid, Q.; Eidelman, D.H.; Campbell, H.; Isogai, S.; Martin, J.G. Inhibition of allergic airways inflammation and airway hyperresponsiveness in mice by dexamethasone: Role of eosinophils, IL-5, eotaxin, and IL-13. J. Allergy Clin. Immunol. 2003, 111, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.D.; Winkelmann, C.T.; Dogdas, B.; Bagchi, A. Micro-computed tomography imaging and analysis in developmental biology and toxicology. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 71–82. [Google Scholar] [CrossRef]
- Clark, D.P.; Badea, C.T.; Badea, C. Micro-CT of rodents: state-of-the-art and future perspectives. Phys. Medica 2014, 30, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Murase, K. Ex vivo imaging of mouse brain using micro-CT with non-ionic iodinated contrast agent: a comparison with myelin staining. Br. J. Radiol. 2012, 85, e973–e978. [Google Scholar] [CrossRef]
- Jacob, A.; Chole, R.A. Survey Anatomy of the Paranasal Sinuses in the Normal Mouse. Laryngoscope 2006, 116, 558–563. [Google Scholar] [CrossRef]
- Phillips, J.E.; Ji, L.; Rivelli, M.A.; Chapman, R.W.; Corboz, M.R. Three-dimensional analysis of rodent paranasal sinus cavities from X-ray computed tomography (CT) scans. Can. J. Vet. Res. 2009, 73, 205–211. [Google Scholar]
- Kanthawatana, S.; Maturim, W.; Fooanant, S.; Manorot, M.; Trakultivakorn, M. Evaluation of threshold criteria for the nasal histamine challenge test in perennial allergic rhinitis. Asian Pac. J. Allergy Immunol. 1997, 15, 65–69. [Google Scholar]
- Yamamoto, K.; Kawamura, I.; Tominaga, T.; Nomura, T.; Ito, J.; Mitsuyama, M. Listeriolysin O derived from Listeria monocytogenes inhibits the effector phase of an experimental allergic rhinitis induced by ovalbumin in mice. Clin. Exp. Immunol. 2006, 144, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Howie, K.; Wattie, J.; Denburg, A.; Ellis, R.; Inman, M.D.; Denburg, J.A. Allergen-induced murine upper airway inflammation: Local and systemic changes in murine experimental allergic rhinitis. Immunol. 2001, 104, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, S.; Miyahara, N.; Lucas, J.J.; Joetham, A.; Matsubara, S.; Ohnishi, H.; Dakhama, A.; Gelfand, E.W. Contribution of allergen-specific and nonspecific nasal responses to early-phase and late-phase nasal responses. J. Allergy Clin. Immunol. 2008, 121, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Burggraaf, J.; Schoemaker, R.C.; Sterk, P.J.; Cohen, A.F.; Diamant, Z. Relationship between airway responsiveness to neurokinin A and methacholine in asthma. Pulm. Pharmacol. Ther. 2005, 18, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.; Undem, B.; Sanico, A.; Togias, A. The role of the nervous system in rhinitis. J. Allergy Clin. Immunol. 2006, 118, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Glaab, T.; Taube, C.; Braun, A.; Mitzner, W. Invasive and noninvasive methods for studying pulmonary function in mice. Respir. Res. 2007, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, S.; Miyahara, N.; Takeda, K.; Joetham, A.; Gelfand, E.W. Physiologic assessment of allergic rhinitis in mice: role of the high-affinity IgE receptor (FcepsilonRI). J. Allergy Clin. Immunol. 2005, 116, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, N.; Nabe, T.; Sasaki, K.; Takenaka, H.; Kohno, S. Nasal hyperresponsiveness to histamine induced by repetitive exposure to cedar pollen in guinea-pigs. Eur. Respir. J. 1999, 14, 1363–1367. [Google Scholar] [CrossRef]
- Giavina-Bianchi, P.; Aun, M.V.; Takejima, P.; Kalil, J.; Agondi, R.C. United airway disease: current perspectives. J. Asthma Allergy 2016, 9, 93–100. [Google Scholar] [CrossRef]
- Miyahara, S.; Miyahara, N.; Matsubara, S. Measurement of early-phase, late-phase, and nonspecific nasal responses in allergic rhinitis mouse by whole body plethysmography. Folia Pharmacol. Jpn. 2011, 137, 141–145. [Google Scholar] [CrossRef][Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, D.V.; Kanda, A.; Kobayashi, Y.; Sakata, Y.; Kono, Y.; Kamakura, Y.; Jinno, T.; Yun, Y.; Suzuki, K.; Sawada, S.; et al. A Novel Approach for Investigating Upper Airway Hyperresponsiveness Using Micro-CT in Eosinophilic Upper Airway Inflammation such as Allergic Rhinitis Model. Biomolecules 2019, 9, 252. https://doi.org/10.3390/biom9070252
Bui DV, Kanda A, Kobayashi Y, Sakata Y, Kono Y, Kamakura Y, Jinno T, Yun Y, Suzuki K, Sawada S, et al. A Novel Approach for Investigating Upper Airway Hyperresponsiveness Using Micro-CT in Eosinophilic Upper Airway Inflammation such as Allergic Rhinitis Model. Biomolecules. 2019; 9(7):252. https://doi.org/10.3390/biom9070252
Chicago/Turabian StyleBui, Dan Van, Akira Kanda, Yoshiki Kobayashi, Yoshiko Sakata, Yumiko Kono, Yoshiyuki Kamakura, Takao Jinno, Yasutaka Yun, Kensuke Suzuki, Shunsuke Sawada, and et al. 2019. "A Novel Approach for Investigating Upper Airway Hyperresponsiveness Using Micro-CT in Eosinophilic Upper Airway Inflammation such as Allergic Rhinitis Model" Biomolecules 9, no. 7: 252. https://doi.org/10.3390/biom9070252
APA StyleBui, D. V., Kanda, A., Kobayashi, Y., Sakata, Y., Kono, Y., Kamakura, Y., Jinno, T., Yun, Y., Suzuki, K., Sawada, S., Asako, M., Nakamura, A., Dombrowicz, D., Utsunomiya, K., Noboru, T., Tomoda, K., & Iwai, H. (2019). A Novel Approach for Investigating Upper Airway Hyperresponsiveness Using Micro-CT in Eosinophilic Upper Airway Inflammation such as Allergic Rhinitis Model. Biomolecules, 9(7), 252. https://doi.org/10.3390/biom9070252





