Laboratory-Scale Isolation of Insect Antifreeze Protein for Cryobiology
Abstract
1. Introduction
2. Materials and Methods
2.1. Beetle Cultivation
2.2. Tenebrio Molitor Antifreeze Protein (TmAFP) Extraction
2.3. Rotary Ice-Affinity Purification
2.4. Concentration of Final Ice Fraction
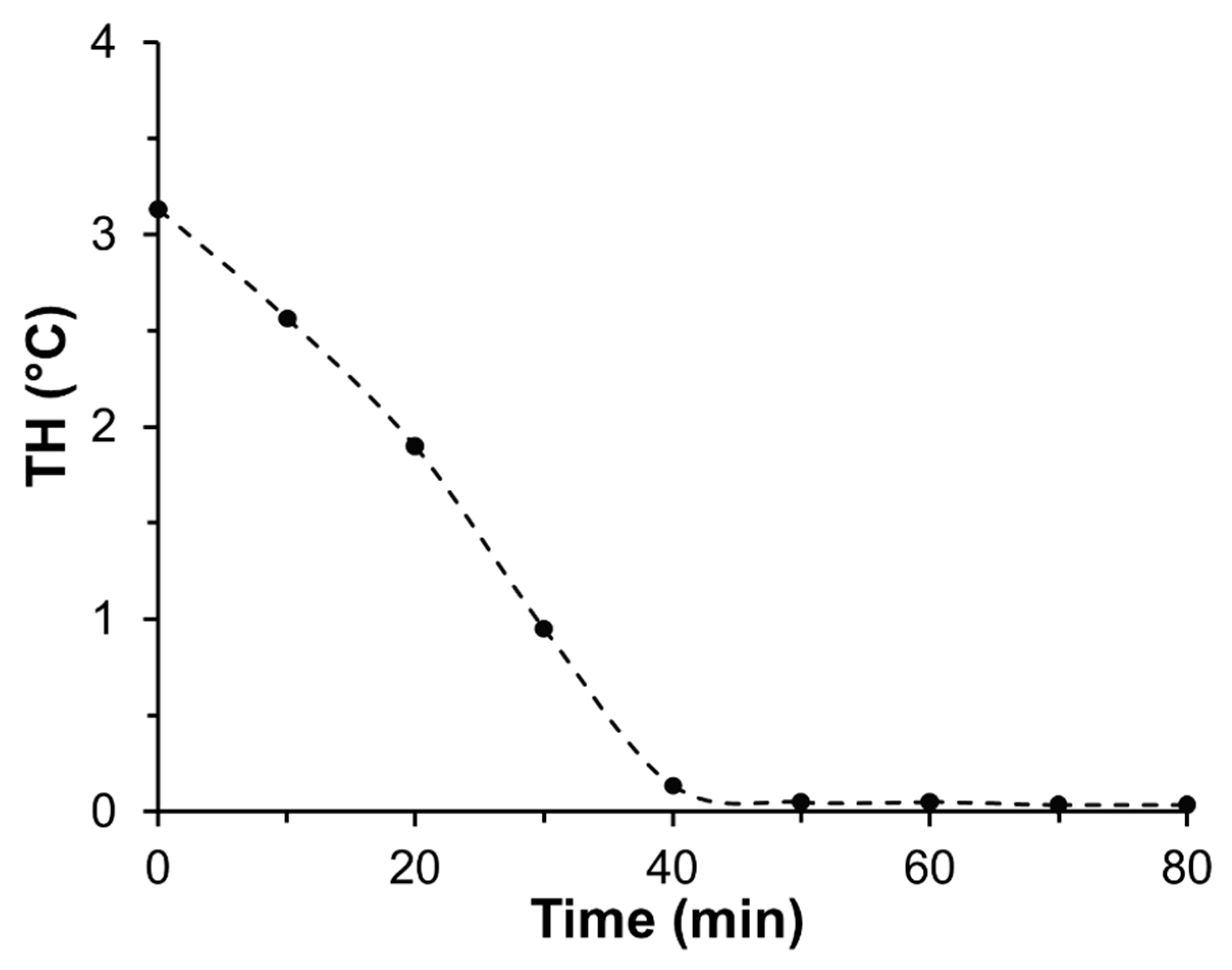
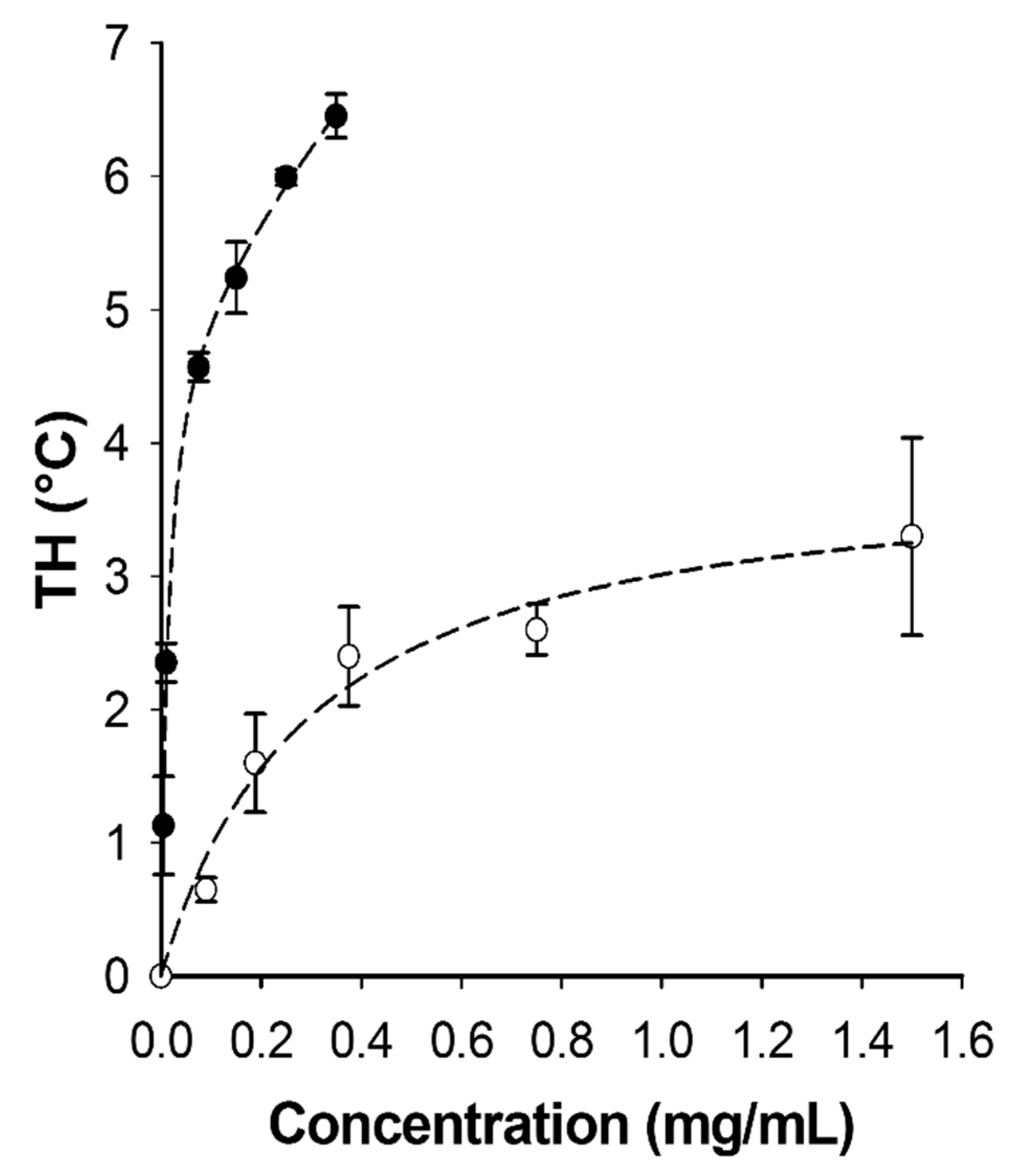
2.5. Thermal Hysteresis (TH)
2.6. Amino Acid Analysis
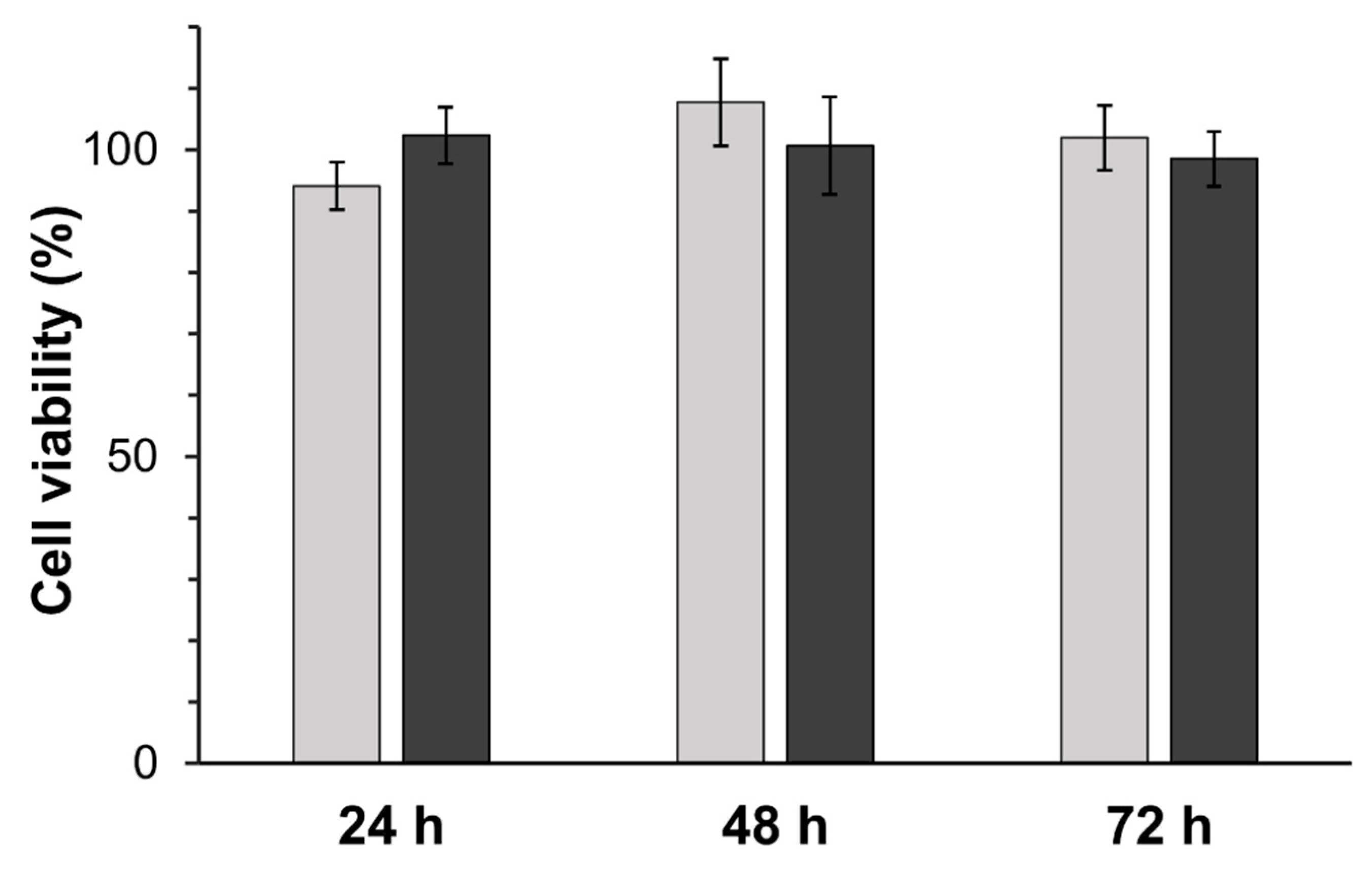
2.7. Toxicity Assays in HEK 293 T Cell Line
2.8. Recycling of Used TmAFP
3. Results
3.1. Maintaining the Yield of Larvae
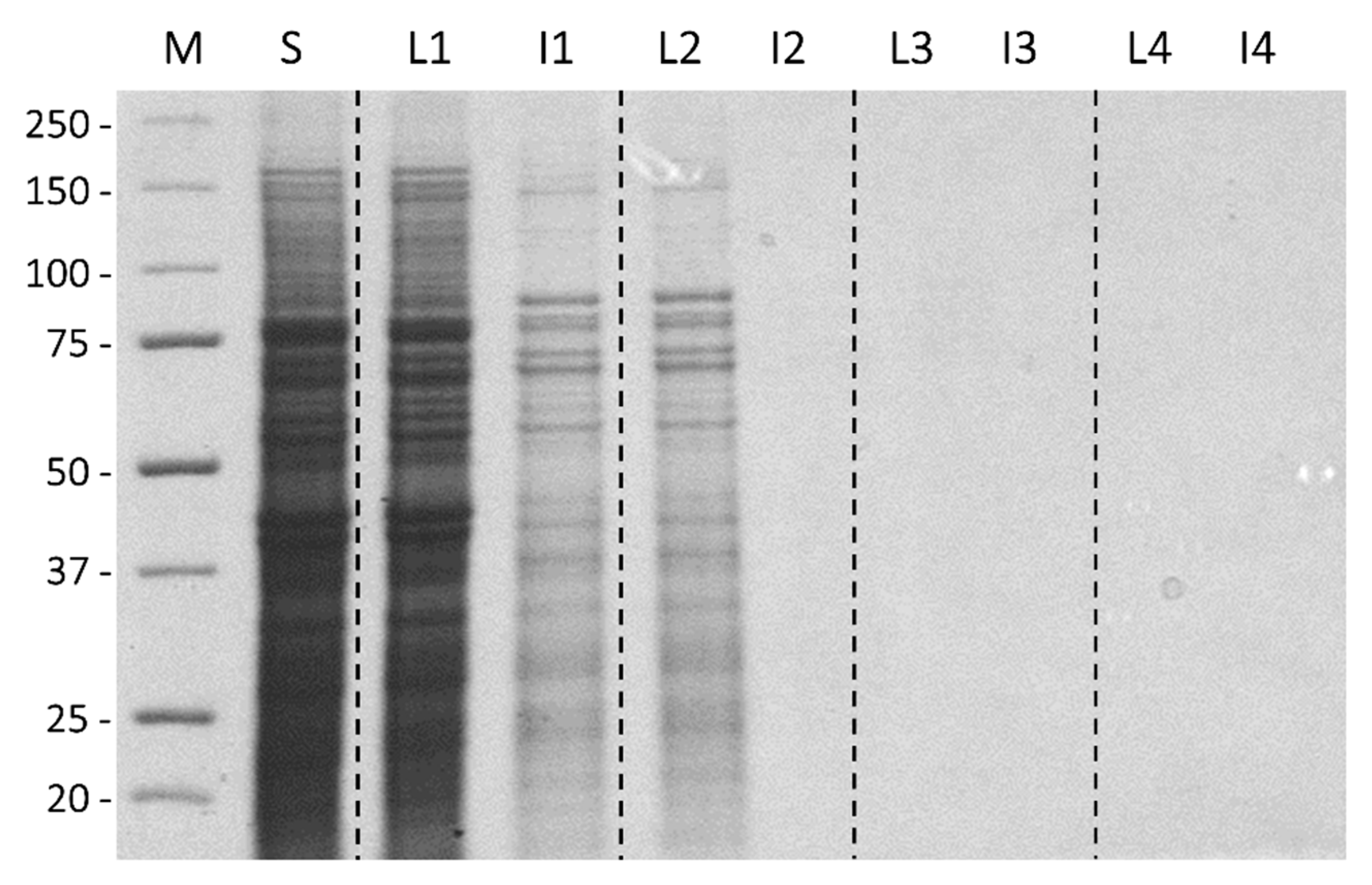
3.2. Ice-Affinity as the Sole Purification Procedure
3.3. Phenoloxidase Inhibition
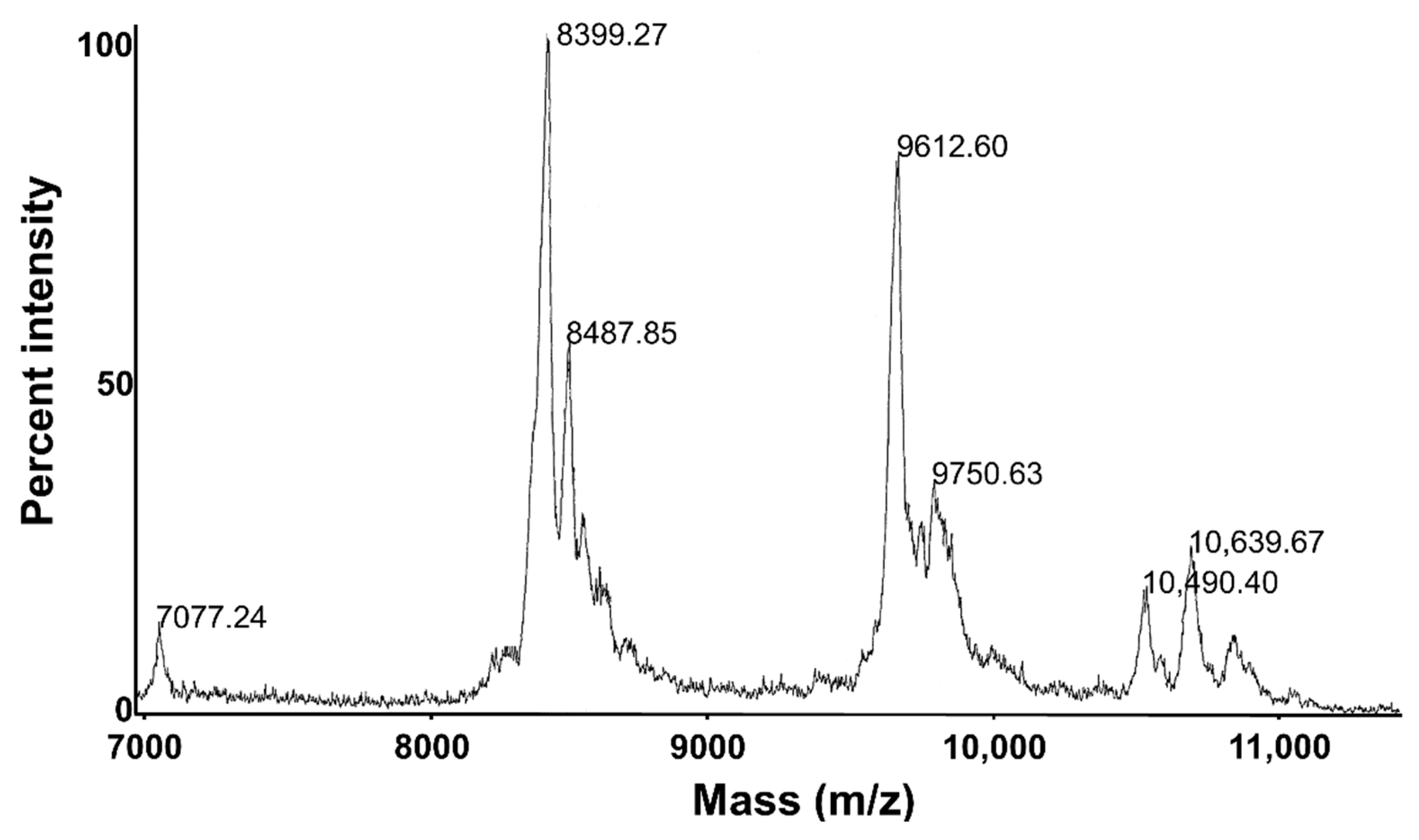
3.4. TmAFP Recovery and Yields
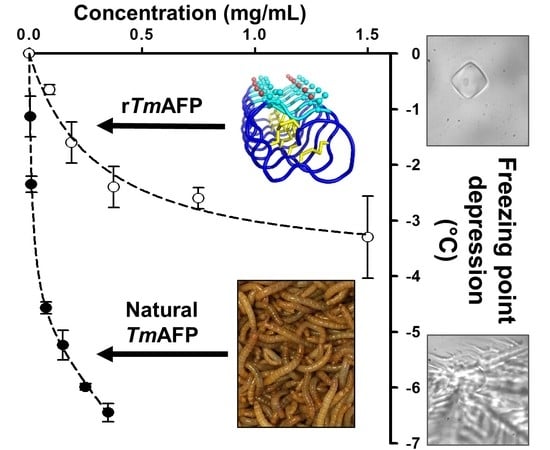
3.5. TmAFP Isoform Synergy
3.6. Toxicity Testing
3.7. Recycling of Used TmAFP
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DeVries, A.L.; Wohlschlag, D.E. Freezing resistance in some Antarctic fishes. Science 1969, 163, 1073–1075. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.A.; DeVries, A.L. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc. Natl. Acad. Sci. USA 1977, 74, 2589–2593. [Google Scholar] [CrossRef] [PubMed]
- Hew, C.; Poon, R.; Xiong, F.; Gauthier, S.; Shears, M.; King, M.; Davies, P.; Fletcher, G. Liver-specific and seasonal expression of transgenic Atlantic salmon harboring the winter flounder antifreeze protein gene. Transgenic Res. 1999, 8, 405–414. [Google Scholar] [CrossRef]
- Duman, J.G. Antifreeze and Ice Nucleator Proteins in Terrestrial Arthropods. Annu. Rev. Physiol. 2001, 63, 327–357. [Google Scholar] [CrossRef]
- Graham, L.A.; Liou, Y.-C.; Walker, V.K.; Davies, P.L. Hyperactive antifreeze protein from beetles. Nature 1997, 388, 727. [Google Scholar] [CrossRef]
- Graether, S.P.; Kuiper, M.J.; Gagné, S.M.; Walker, V.K.; Jia, Z.; Sykes, B.D.; Davies, P.L. β-Helix structure and ice-binding properties of a hyperactive antifreeze protein from an insect. Nature 2000, 406, 325. [Google Scholar] [CrossRef] [PubMed]
- Pertaya, N.; Marshall, C.B.; Celik, Y.; Davies, P.L.; Braslavsky, I. Direct Visualization of Spruce Budworm Antifreeze Protein Interacting with Ice Crystals: Basal Plane Affinity Confers Hyperactivity. Biophys. J. 2008, 95, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Middleton, A.J.; Marshall, C.B.; Faucher, F.; Bar-Dolev, M.; Braslavsky, I.; Campbell, R.L.; Walker, V.K.; Davies, P.L. Antifreeze Protein from Freeze-Tolerant Grass Has a Beta-Roll Fold with an Irregularly Structured Ice-Binding Site. J. Mol. Biol. 2012, 416, 713–724. [Google Scholar] [CrossRef]
- Davies, P.L. Ice-binding proteins: A remarkable diversity of structures for stopping and starting ice growth. Trends Biochem. Sci. 2014, 39, 548–555. [Google Scholar] [CrossRef]
- Brockbank, K.G.M.; Campbell, L.H.; Greene, E.D.; Brockbank, M.C.G.; Duman, J.G. Lessons from nature for preservation of mammalian cells, tissues, and organs. In Vitro Cell. Dev. Biol. Anim. 2011, 47, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Halwani, D.O.; Brockbank, K.G.M.; Duman, J.G.; Campbell, L.H. Recombinant Dendroides canadensis antifreeze proteins as potential ingredients in cryopreservation solutions. Cryobiology 2014, 68, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Tomalty, H.E.; Hamilton, E.F.; Hamilton, A.; Kukal, O.; Allen, T.; Walker, V.K. Kidney preservation at subzero temperatures using a novel storage solution and insect ice-binding proteins. CryoLetters 2017, 38, 100–107. [Google Scholar]
- Liou, Y.-C.; Daley, M.E.; Graham, L.A.; Kay, C.M.; Walker, V.K.; Sykes, B.D.; Davies, P.L. Folding and Structural Characterization of Highly Disulfide-Bonded Beetle Antifreeze Protein Produced in Bacteria. Protein Expr. Purif. 2000, 19, 148–157. [Google Scholar] [CrossRef]
- Bar, M.; Bar-Ziv, R.; Scherf, T.; Fass, D. Efficient production of a folded and functional, highly disulfide-bonded β-helix antifreeze protein in bacteria. Protein Expr. Purif. 2006, 48, 243–252. [Google Scholar] [CrossRef]
- Graham, L.A.; Walker, V.K.; Davies, P.L. Developmental and environmental regulation of antifreeze proteins in the mealworm beetle Tenebrio molitor. Eur. J. Biochem. 2000, 267, 6452–6458. [Google Scholar] [CrossRef] [PubMed]
- Liou, Y.-C.; Thibault, P.; Walker, V.K.; Davies, P.L.; Graham, L.A. A Complex Family of Highly Heterogeneous and Internally Repetitive Hyperactive Antifreeze Proteins from the Beetle Tenebrio molitor. Biochemistry 1999, 38, 11415–11424. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Duman, J.G. Antifreeze Proteins of the Beetle Dendroides canadensis Enhance One Another’s Activities †. Biochemistry 2005, 44, 10305–10312. [Google Scholar] [CrossRef]
- Kuiper, M.J.; Lankin, C.; Gauthier, S.Y.; Walker, V.K.; Davies, P.L. Purification of antifreeze proteins by adsorption to ice. Biochem. Biophys. Res. Commun. 2003, 300, 645–648. [Google Scholar] [CrossRef]
- Marshall, C.J.; Basu, K.; Davies, P.L. Ice-shell purification of ice-binding proteins. Cryobiology 2016, 72, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Vance, T.D.R.; Graham, L.A.; Davies, P.L. An ice-binding and tandem beta-sandwich domain-containing protein in Shewanella frigidimarina is a potential new type of ice adhesin. FEBS J. 2018, 285, 1511–1527. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Revenis, C. Role and Importance of Phenoloxidase in Insect Hemostasis. J. Innate Immun. 2011, 3, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.B.; Daley, M.E.; Graham, L.A.; Sykes, B.D.; Davies, P.L. Identification of the ice-binding face of antifreeze protein from Tenebrio molitor. FEBS Lett. 2002, 529, 261–267. [Google Scholar] [CrossRef]
- Tyshenko, M.G.; d’Anjou, M.; Davies, P.L.; Daugulis, A.J.; Walker, V.K. Challenges in the expression of disulfide bonded, threonine-rich antifreeze proteins in bacteria and yeast. Protein Expr. Purif. 2006, 47, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Adar, C.; Sirotinskaya, V.; Dolev, M.B.; Friehmann, T.; Braslavsky, I. Falling water ice affinity purification of ice-binding proteins. Sci. Rep. 2018, 8, 11046. [Google Scholar] [CrossRef] [PubMed]
- Grau, T.; Vilcinskas, A.; Joop, G. Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed. Z. Für Naturforschung C 2017, 72, 337–349. [Google Scholar] [CrossRef]

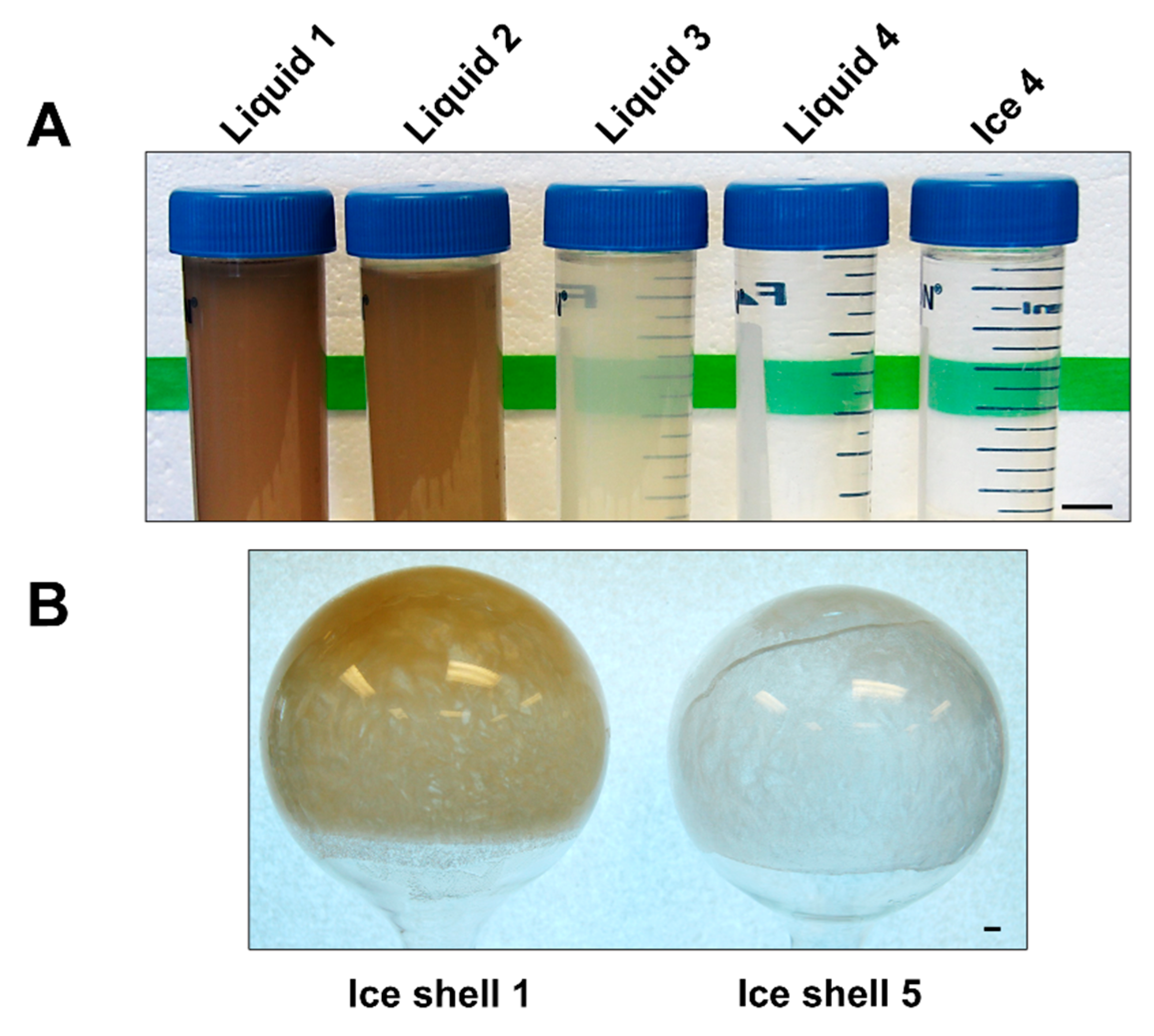
| Sample | Total Protein (mg) | Total Protein (%) | TH (° C) |
|---|---|---|---|
| Supernatant | 44,301 (± 1206) | 100 | 3.51 ± 0.43 °C |
| Liquid 1 | 41,160 (± 133) | 92.9 | 0.24 ± 0.31 °C |
| Ice 1 | 3889 (± 28) | 8.7 | 3.45 ± 0.58 °C |
| Liquid 2 | 2208 (± 24) | 4.9 | 0.16 ± 0.43 °C |
| Ice 2 | 506 (± 16) | 1.1 | 3.22 ± 0.88 °C |
| Liquid 3 | 493 (± 15) | 1.1 | 0.09 ± 0.14 °C |
| Ice 3 | 34 (± 1.6) | 0.07 | 3.47 ± 0.68 °C |
| Liquid 4 | 29 (± 2) | 0.06 | 0.03 ± 0.01 °C |
| Ice 4 | n.d. | N/A | 3.55 ± 0.36 °C |
| Liquid 5 | n.d. | N/A | 0.06 ± 0.03 °C |
| Ice 5 | n.d. | N/A | 3.73 ± 0.59 °C |
| A.A. | Predicted (%) | TmAFP #1 (%) | TmAFP #2 (%) | TmAFP #3 (%) | TmAFP #4a (%) | TmAFP #4b (%) |
|---|---|---|---|---|---|---|
| Asx | 13.0 | 13.4 | 13.8 | 12.8 | 13.1 | 14.7 |
| Glx | 3.9 | 4.0 | 3.9 | 4.3 | 3.7 | 4.6 |
| Ser | 7.6 | 7.3 | 7.6 | 6.6 | 7.4 | 7.8 |
| Gly | 8.2 | 8.3 | 8.4 | 8.5 | 8.4 | 9.4 |
| His | 3.2 | 2.2 | 2.4 | 2.4 | 2.3 | 1.9 |
| Arg | 0.7 | 1.2 | 1.0 | 1.0 | 1.2 | 1.2 |
| Thr | 21.9 | 20.9 | 22.2 | 21.8 | 21.4 | 18.8 |
| Ala | 11.0 | 10.1 | 10.4 | 10.7 | 10.2 | 11.1 |
| Pro | 2.4 | 2.4 | 2.5 | 2.5 | 2.5 | 2.9 |
| Tyr | 1.2 | 1.5 | 1.3 | 1.6 | 1.5 | 0.5 |
| Val | 2.5 | 2.7 | 2.4 | 2.8 | 2.6 | 2.7 |
| Met | 0.0 | 0.3 | 0.2 | 0.3 | 0.4 | 0.3 |
| Cys | 19.0 | n.d. | n.d. | n.d. | n.d. | 17.2 |
| Ile | 0.0 | 0.4 | 0.3 | 0.5 | 0.5 | 0.4 |
| Leu | 0.0 | 0.8 | 0.4 | 0.9 | 0.8 | 0.8 |
| Phe | 1.2 | 1.5 | 1.0 | 1.3 | 1.3 | 1.6 |
| Lys | 4.3 | 4.1 | 3.0 | 3.0 | 3.9 | 4.1 |
| Total | 100.0 | 81.0 | 81.0 | 81.0 | 81.0 | 100.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomalty, H.E.; Graham, L.A.; Eves, R.; Gruneberg, A.K.; Davies, P.L. Laboratory-Scale Isolation of Insect Antifreeze Protein for Cryobiology. Biomolecules 2019, 9, 180. https://doi.org/10.3390/biom9050180
Tomalty HE, Graham LA, Eves R, Gruneberg AK, Davies PL. Laboratory-Scale Isolation of Insect Antifreeze Protein for Cryobiology. Biomolecules. 2019; 9(5):180. https://doi.org/10.3390/biom9050180
Chicago/Turabian StyleTomalty, Heather E., Laurie A. Graham, Robert Eves, Audrey K. Gruneberg, and Peter L. Davies. 2019. "Laboratory-Scale Isolation of Insect Antifreeze Protein for Cryobiology" Biomolecules 9, no. 5: 180. https://doi.org/10.3390/biom9050180
APA StyleTomalty, H. E., Graham, L. A., Eves, R., Gruneberg, A. K., & Davies, P. L. (2019). Laboratory-Scale Isolation of Insect Antifreeze Protein for Cryobiology. Biomolecules, 9(5), 180. https://doi.org/10.3390/biom9050180