Comparison Study of Different Extracts of Plectranthus madagascariensis, P. neochilus and the Rare P. porcatus (Lamiaceae): Chemical Characterization, Antioxidant, Antimicrobial and Cytotoxic Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines, Chemicals and Biochemicals
2.2. Plant Material
2.3. Extract Preparation
2.4. DPPH Radical Scavenging Assay
2.5. Antimicrobial Activity Assays
2.6. Cytotoxicity Evaluation
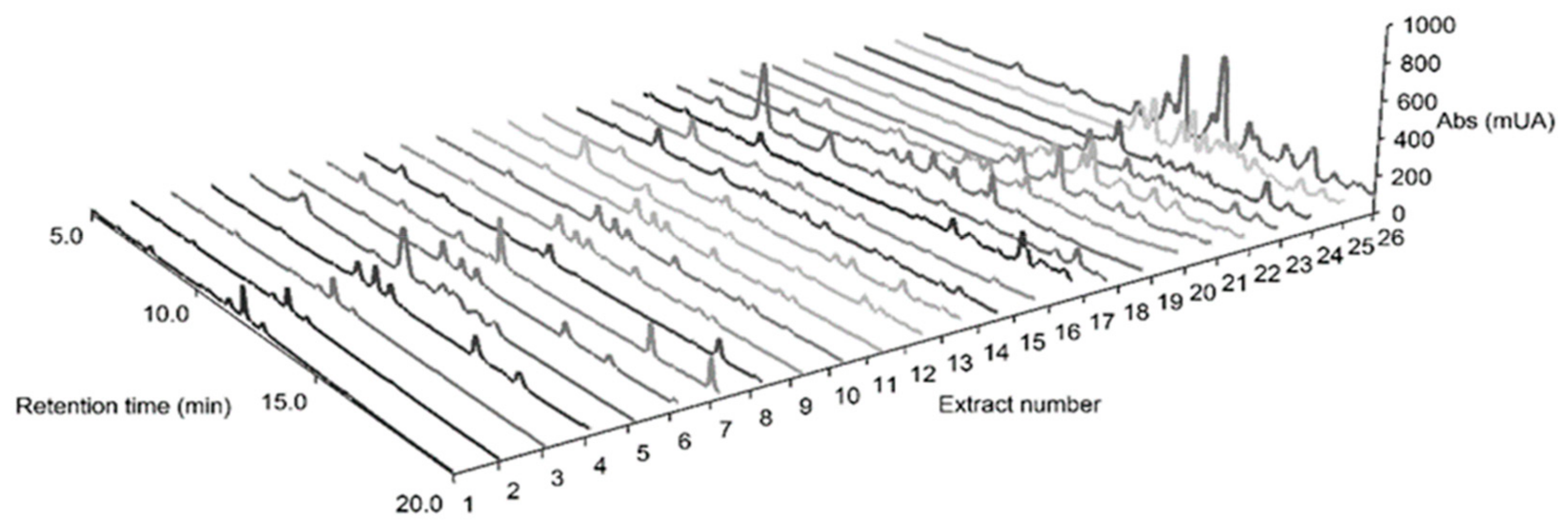
2.7. HPLC-DAD Fingerprinting
2.8. Statistical Analysis and Software Editing
3. Results and Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Traditional Medicine Strategy 2014–2023; WHO Press: Geneva, Switzerland, 2013; pp. 1–78. [Google Scholar]
- Lukhoba, C.W.; Simmonds, M.S.J.; Paton, A.J. Plectranthus: A review of ethnobotanical uses. J. Ethnopharmacol. 2006, 103, 1–24. [Google Scholar] [PubMed]
- Abdel-Mogib, M.; Albar, H.A.; Batterjee, S.M. Chemistry of the genus Plectranthus. Molecules 2002, 7, 271–301. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hedge, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Van Jaarsveld, E.; Thomas, V. The Southern African Plectranthus: And the Art of Turning Shade to Glade; Fernwood Press: Pretoria, South Africa, 2006. [Google Scholar]
- Ascensão, L.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Schripsema, J.; Deans, S.G.; Scheffer, J.J.C. Plectranthus madagascariensis: Morphology of the glandular trichomes, essential oil composition, and its biological activity. Int. J. Plant. Sci. 1998, 159, 31–38. [Google Scholar] [CrossRef]
- Gazim, Z.C.; Rodrigues, F.; Amorin, A.C.L.; de Rezende, C.M.; Sokovic, M.; Teševic, V.; Vuckovic, I.; Krstic, G.; Cortez, L.E.R.; Colauto, N.B.; et al. New natural diterpene-type abietane from Tetradenia riparia essential oil with cytotoxic and antioxidant activities. Molecules 2014, 19, 514–524. [Google Scholar] [CrossRef]
- Wellsow, J.; Grayer, R.J.; Veitch, N.C.; Kokubun, T.; Lelli, R.; Kite, G.C.; Simmonds, M.S.J. Insect-antifeedant and antibacterial activity of diterpenoids from species of Plectranthus. Phytochemistry 2006, 67, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Rijo, P.; Matias, D.; Fernandes, A.S.; Simões, M.F.; Nicolai, M.; Reis, C.P. Antimicrobial plant extracts encapsulated into polymeric beads for potential application on the skin. Polymers 2014, 6, 479–490. [Google Scholar] [CrossRef]
- Kubínová, R.; Pořízková, R.; Navrátilová, A.; Farsa, O.; Hanáková, Z.; Bačinská, A.; Cížek, A.; Valentová, M. Antimicrobial and enzyme inhibitory activities of the constituents of Plectranthus madagascariensis (Pers.) Benth. J. Enzym. Inhib. Med. Chem. 2014, 6366, 1–4. [Google Scholar]
- Duarte, M.R.; Lopes, J.F. Stem and leaf anatomy of Plectranthus neochilus Schltr., Lamiaceae. Rev. Bras. Farmacogn. 2007, 17, 549–556. [Google Scholar] [CrossRef]
- York, T.; de Wet, H.; van Vuuren, S.F. Plants used for treating respiratory infections in rural Maputaland, KwaZulu-Natal, South Africa. J. Ethnopharmacol. 2011, 135, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Mota, L.; Figueiredo, A.C.; Pedro, L.G.; Barroso, J.G.; Miguel, M.G.; Faleiro, M.L.; Ascensão, L. Volatile-oils composition, and bioactivity of the essential oils of Plectranthus barbatus, P. neochilus, and P. ornatus grown in Portugal. Chem. Biodivers. 2014, 11, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Caixeta, S.C.; Magalhães, L.G.; de Melo, N.I.; Wakabayashi, K.A.L.; Aguiar, G.P.; Aguiar, D.P.; Mantovani, A.L.L.; Alves, J.M.; Oliveira, P.F.; Tavares, D.C.; et al. Chemical composition and in vitro schistosomicidal activity of the essential oil of Plectranthus neochilus grown in Southeast Brazil. Chem. Biodivers. 2011, 8, 2149–2157. [Google Scholar] [CrossRef]
- Lawal, O.A.; Hutchings, A.H.; Oyedeji, O. Chemical composition of the leaf oil of Plectranthus neochilus Schltr. J. Essent. Oil Res. 2010, 22, 546–547. [Google Scholar] [CrossRef]
- Crevelin, E.J.; Caixeta, S.C.; Dias, H.J.; Groppo, M.; Cunha, W.R.; Martins, C.H.G.; Crotti, A.E.M. Antimicrobial activity of the essential oil of Plectranthus neochilus against cariogenic bacteria. Evid. Based Complement. Alternat. Med. 2015, 2015, 102317. [Google Scholar] [CrossRef]
- Baldin, E.L.L.; Crotti, A.E.M.; Wakabayashi, K.A.L.; Silva, J.P.G.F.; Aguiar, G.P.; Souza, E.S.; Veneziani, R.C.S.; Groppo, M. Plant-derived essential oils affecting settlement and oviposition of Bemisia tabaci (Genn.) biotype B on tomato. J. Pest Sci. 2013, 86, 301–308. [Google Scholar] [CrossRef]
- Tempone, A.G.; Sartorelli, P.; Teixeira, D.; Prado, F.O.; Calixto, I.A.R.L.; Lorenzi, H.; Melhem, M.S.C. Brazilian flora extracts as source of novel antileishmanial and antifungal compounds. Mem. Inst. Oswaldo Cruz 2008, 103, 443–449. [Google Scholar] [CrossRef]
- Arcanjo, D.D.R.; Albuquerque, A.C.M.; Melo-Neto, B.; Santana, L.C.L.R.; Medeiros, M.G.F.; Citó, A.M.G.L. Bioactivity evaluation against Artemia salina Leach of medicinal plants used in Brazilian Northeastern folk medicine. Braz. J. Biol. 2012, 72, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Winter, P.J.D.; van Jaarsveld, E. Plectranthus porcatus, a new species endemic to the Sekhukhuneland Centre of Plant Endemism, Limpopo Province, South Africa. Bothalia 2005, 35, 169–173. [Google Scholar]
- Simões, M.F.; Rijo, P.; Duarte, A.; Barbosa, D.; Matias, D.; Delgado, J.; Cirilo, N.; Rodríguez, B. Two new diterpenoids from Plectranthus species. Phytochem. Lett. 2010, 3, 221–225. [Google Scholar] [CrossRef]
- Rijo, P.; Falé, P.L.; Serralheiro, M.L.; Simões, M.F.; Gomes, A.; Reis, C. Optimization of medicinal plant extraction methods and their encapsulation through extrusion technology. Measurement 2014, 58, 249–255. [Google Scholar] [CrossRef]
- Gaspar-Marques, C.; Rijo, P.; Simões, M.F.; Duarte, M.A.; Rodriguez, B. Abietanes from Plectranthus grandidentatus and P. hereroensis against methicillin- and vancomycin-resistant bacteria. Phytomedicine 2006, 13, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Bernardo-Gil, M.G.; Cebola, M.J.; Maurício, E.; Romano, A. Supercritical fluid extracts with antioxidant and animicrobial activities from myrtle (Myrtus communis L.) leaves. Response surface optimization. J. Supercrit. Fluids 2013, 83, 57–64. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Serejo, J.; Gaspar, J.; Cabral, F.; Bettencourt, A.F.; Rueff, J.; Castro, M.; Costa, J.; Oliveira, N.G. Oxidative injury in V79 Chinese hamster cells: Protective role of the superoxide dismutase mimetic MnTM-4-PyP. Cell Biol. Toxicol. 2010, 26, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Falé, P.L.; Borges, C.; Madeira, P.J.A.; Ascensão, L.; Araújo, M.E.M.; Florêncio, M.H.; Serralheiro, M.L.M. Rosmarinic acid, scutellarein 4′-methyl ether 7-O-glucuronide and (16S)-coleon E are the main compounds responsible for the antiacetylcholinesterase and antioxidant activity in herbal tea of Plectranthus barbatus (“Falso boldo”). Food Chem. 2009, 114, 798–805. [Google Scholar] [CrossRef]
- Kubínová, R.; Švajdlenka, E.; Schneiderová, K.; Hanáková, Z.; Dall’Acqua, S.; Farsa, O. Polyphenols and diterpenoids from Plectranthus forsteri “Marginatus”. Biochem. Syst. Ecol. 2013, 49, 39–42. [Google Scholar] [CrossRef]
- Rijo, P.; Simões, M.F.; Francisco, A.P.; Rojas, R.; Gilman, R.H.; Vaisberg, A.J.; Rodríguez, B.; Moiteiro, C. Antimycobacterial metabolites from Plectranthus: Royleanone derivatives against Mycobacterium tuberculosis strains. Chem. Biodivers. 2010, 7, 922–932. [Google Scholar] [CrossRef]
- Marques, C.G.; Pedro, M.; Simões, M.F.A.; Nascimento, M.S.J.; Pinto, M.M.M.; Rodriguez, B. Effect of abietane diterpenes from Plectranthus grandidentatus on the growth of human cancer cell lines. Planta Med. 2002, 68, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Burmistrova, O.; Simões, M.F.; Rijo, P.; Quintana, J.; Bermejo, J.; Estévez, F. Antiproliferative activity of abietane diterpenoids against human tumor cells. J. Nat. Prod. 2013, 76, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.-X.; Jiang, B.; Niu, X.-M.; Li, M.-L.; Yang, H.; Na, Z.; Lin, Z.-W.; Li, C.-M.; Sun, H.-D.; Jones, N.D.; et al. Abietane diterpenoids from Coleus xanthanthus. J. Nat. Prod. 2002, 65, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, I.; Pereira, G.; Simões, M.F.; Côrte-Real, M.; Gonçalves, J.; Saraiva, L. Selective activation of protein kinase C-δ and -ε by 6,11,12,14-tetrahydroxy-abieta-5,8,11,13-tetraene-7-one (coleon U). Biochem. Pharmacol. 2009, 78, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Rijo, P.; Faustino, C.; Simões, M.F. Antimicrobial natural products from Plectranthus plants. In Microbial Pathogens and Strategies for Combating them: Science, Technology and Education; Méndez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2013; pp. 922–931. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
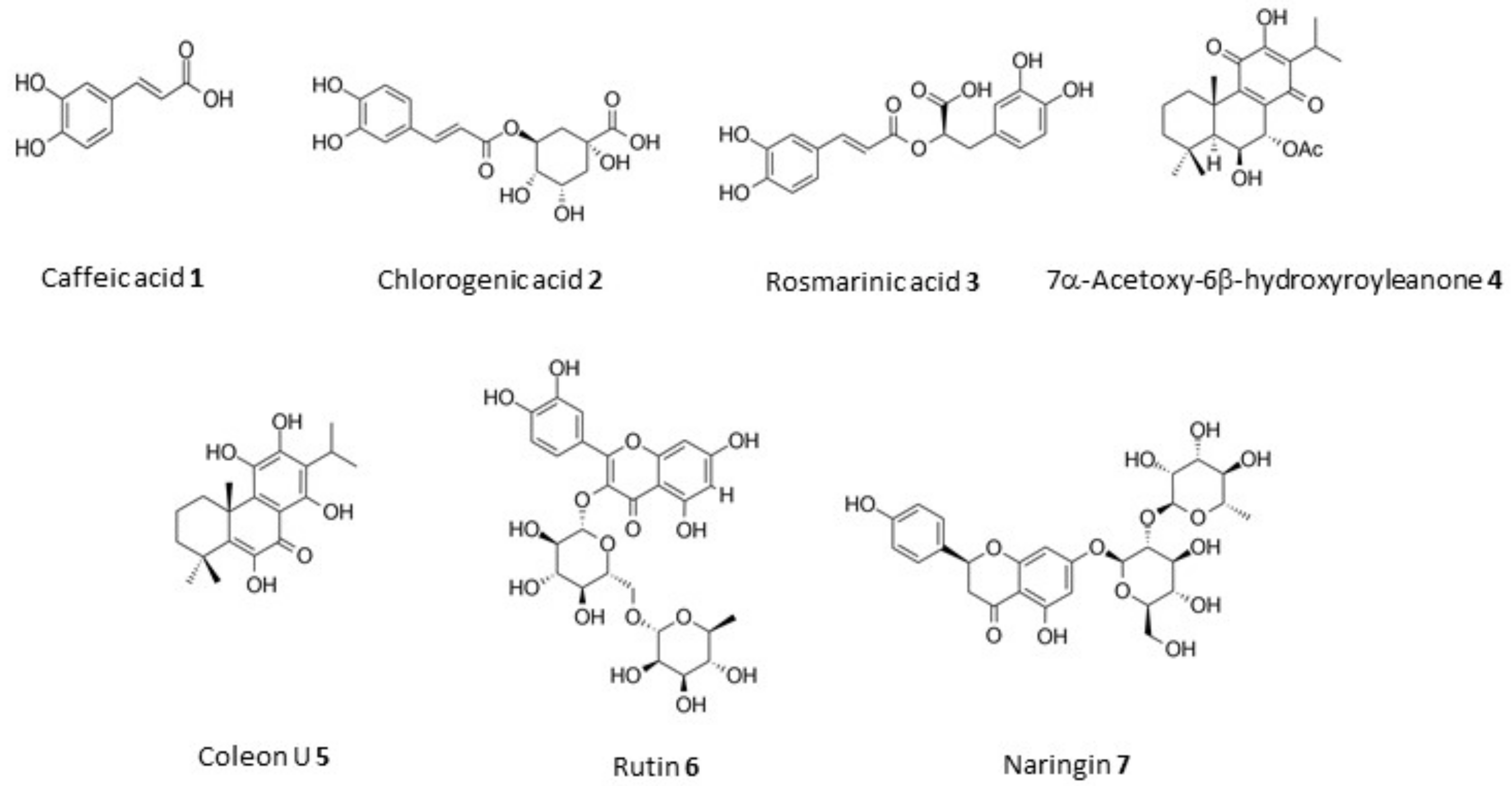
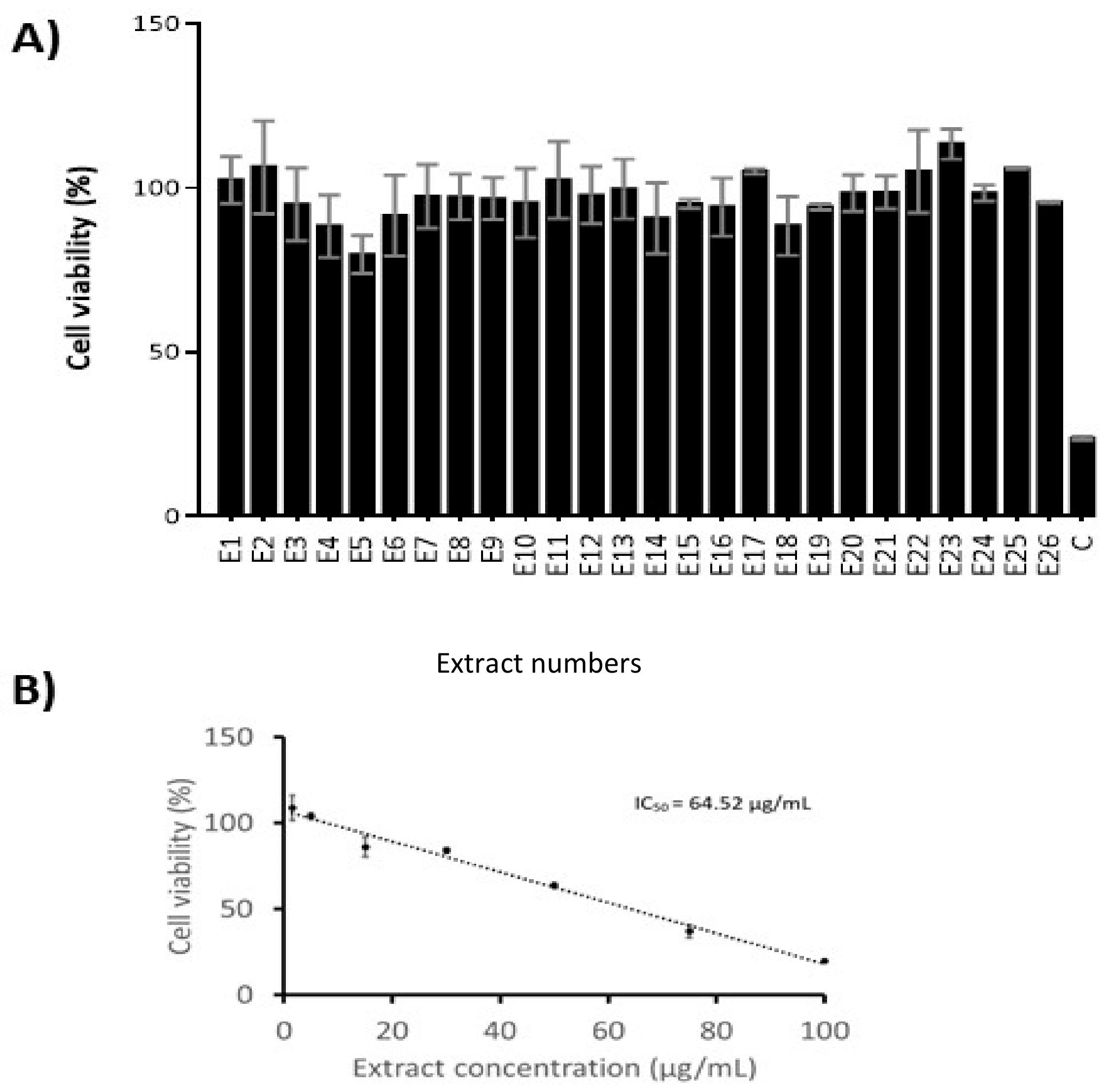
| Plant | Solvent | Extraction Method a | Dry Residue (g) | Yield (mg/g) | Extract | RSA b (%) |
|---|---|---|---|---|---|---|
| Plectranthus madagascariensis | Water | INF | 0.23 ± 0.10 | 2.3 | E1 | <50 |
| MW | 0.11 ± 0.04 | 1.1 | E2 | <50 | ||
| DEC | 0.22 ± 0.02 | 2.2 | E3 | <50 | ||
| Acetone | US | 0.151 | 1.5 | E4 | <50 | |
| MA | 0.377 | 3.8 | E5 | <50 | ||
| Methanol | US | 0.656 | 6.6 | E6 | 89.0 | |
| MA | 1.146 | 11.5 | E7 | 64.8 | ||
| scCO2 | SCFE | 0.394 | 0.01 | E8 | <50 | |
| Acetone | R-SCFE | 0.885 | 0.03 | E9 | <50 | |
| Plectranthus neochilus | Water | INF | 0.26 ± 0.04 | 2.6 | E10 | <50 |
| MW | 0.15 ± 0.04 | 1.5 | E11 | <50 | ||
| DEC | 0.22 ± 0.01 | 2.2 | E12 | <50 | ||
| Acetone | US | 0.180 | 1.8 | E13 | <50 | |
| MA | 0.125 | 1.3 | E14 | <50 | ||
| Methanol | US | 0.702 | 7.0 | E15 | 64.9 | |
| MA | 0.600 | 6.0 | E16 | 62.3 | ||
| scCO2 | SCFE | 0.251 | 0.8 | E17 | <50 | |
| Acetone | R-SCFE | 0.417 | 1.4 | E18 | <50 | |
| Plectranthus porcatus | Water | INF | n/d | n/d | E19 | <50 |
| MW | n/d | n/d | E20 | <50 | ||
| Acetone | US | 0.865 | 8.7 | E21 | <50 | |
| MA | 0.872 | 8.7 | E22 | <50 | ||
| Methanol | US | 1.566 | 15.7 | E23 | 60.8 | |
| MA | 2.237 | 22.4 | E24 | 65.9 | ||
| scCO2 | SCFE | 0.191 | 0.64 | E25 | <50 | |
| Acetone | R-SCFE | 0.868 | 2.9 | E26 | <50 |
| Microbial Strains | Extract | Positive Control a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| E4 | E5 | E6 | E7 | E13 | E14 | E15 | E16 | ||
| Gram-positive | |||||||||
| Bacillus subtilis ATCC 6633 | 24 | 20 | 12 | 11 | 15 | 14 | 11 | 10 | 31 (VAN) |
| Staphylococcus. aureus ATCC 25923 | 24 | 20 | 7 | 8 | 8 | 8 | 8 | 8 | 24 (VAN) |
| Staphylococcus. epidermidis ATCC 12228 | 10 | 25 | nt | 13 | 15 | 11 | nt | 5 | 20 (VAN) |
| Mycobacterium smegmatis ATCC 607 | 26 | 26 | 23 | 17 | 20 | 15 | 15 | 20 | 33 (RIF) |
| Gram-negative | |||||||||
| Klebsiella. pneumoniae ATCC 9997 | 25 | 22 | 5 | 5 | 5 | 5 | 5 | 5 | 25 (NOR) |
| Microbial Strains | Extract | Positive Control a | ||
|---|---|---|---|---|
| E4 | E5 | E13 | ||
| Gram-positive | ||||
| B. subtilis ATCC 6633 | 3.91 | 62.5 | 125 | <0.48 (VAN) |
| S. aureus ATCC 25923 | 3.91 | 250 | 250 | 7.81 (VAN) |
| S. aureus CIP 106760 | 1.95 | 15.62 | 31.25 | <0.98 (VAN) |
| S. epidermidis ATCC 12228 | 7.81 | 62.5 | 62.5 | 7.81 (VAN) |
| M. smegmatis ATCC 607 | 31.25 | 62.5 | 15.62 | <0.48 (RIF) |
| Gram-negative | ||||
| Klebsiella pneumoniae ATCC 9997 | <0.48 | 3.91 | 0.98 | 15.62 (NOR) |
| Extract n | RT (min) | ||||||
|---|---|---|---|---|---|---|---|
| Clo 6.54 | Caf 8.32 | Nar 10.14 | Rut 11.67 | Ros 12.43 | Roy 14.75 | ColU15.44 | |
| E1 | + | + | − | + | + | − | − |
| E2 | + | + | − | + | + | − | − |
| E3 | + | + | − | + | + | − | − |
| E4 | + | + | − | + | + | + | − |
| E5 | + | + | − | − | + | + | + |
| E6 | + | + | − | − | + | − | − |
| E7 | + | + | − | − | + | − | − |
| E8 | + | + | − | − | + | + | − |
| E9 | + | + | − | + | + | + | − |
| E10 | + | + | − | + | + | − | − |
| E11 | + | + | − | + | + | − | − |
| E12 | + | + | − | + | + | − | − |
| E13 | + | + | − | + | + | − | − |
| E14 | + | + | − | − | + | − | − |
| E15 | + | + | − | + | + | − | − |
| E16 | + | + | − | − | + | − | − |
| E17 | + | − | − | − | − | − | − |
| E18 | + | + | − | − | + | − | − |
| E19 | − | + | + | + | + | − | − |
| E20 | + | + | + | + | + | − | − |
| E21 | − | + | + | − | − | − | − |
| E22 | + | + | + | − | + | − | − |
| E23 | − | − | − | − | − | − | − |
| E24 | − | + | + | − | − | − | − |
| E25 | + | − | − | − | − | − | − |
| E26 | + | + | + | − | + | − | − |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matias, D.; Nicolai, M.; Fernandes, A.S.; Saraiva, N.; Almeida, J.; Saraiva, L.; Faustino, C.; Díaz-Lanza, A.M.; Reis, C.P.; Rijo, P. Comparison Study of Different Extracts of Plectranthus madagascariensis, P. neochilus and the Rare P. porcatus (Lamiaceae): Chemical Characterization, Antioxidant, Antimicrobial and Cytotoxic Activities. Biomolecules 2019, 9, 179. https://doi.org/10.3390/biom9050179
Matias D, Nicolai M, Fernandes AS, Saraiva N, Almeida J, Saraiva L, Faustino C, Díaz-Lanza AM, Reis CP, Rijo P. Comparison Study of Different Extracts of Plectranthus madagascariensis, P. neochilus and the Rare P. porcatus (Lamiaceae): Chemical Characterization, Antioxidant, Antimicrobial and Cytotoxic Activities. Biomolecules. 2019; 9(5):179. https://doi.org/10.3390/biom9050179
Chicago/Turabian StyleMatias, Diogo, Marisa Nicolai, Ana Sofia Fernandes, Nuno Saraiva, Joana Almeida, Lucília Saraiva, Célia Faustino, Ana María Díaz-Lanza, Catarina P. Reis, and Patrícia Rijo. 2019. "Comparison Study of Different Extracts of Plectranthus madagascariensis, P. neochilus and the Rare P. porcatus (Lamiaceae): Chemical Characterization, Antioxidant, Antimicrobial and Cytotoxic Activities" Biomolecules 9, no. 5: 179. https://doi.org/10.3390/biom9050179
APA StyleMatias, D., Nicolai, M., Fernandes, A. S., Saraiva, N., Almeida, J., Saraiva, L., Faustino, C., Díaz-Lanza, A. M., Reis, C. P., & Rijo, P. (2019). Comparison Study of Different Extracts of Plectranthus madagascariensis, P. neochilus and the Rare P. porcatus (Lamiaceae): Chemical Characterization, Antioxidant, Antimicrobial and Cytotoxic Activities. Biomolecules, 9(5), 179. https://doi.org/10.3390/biom9050179













