Comparison of the Neuroprotective Effects of Aspirin, Atorvastatin, Captopril and Metformin in Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Drugs
2.2. Streptozotocin-Induced Diabetes
2.3. Animals
2.4. Experimental Groups
2.5. Measurement of Total Thiol
2.6. Measurement of Malondialdehyde
2.7. Determination of Superoxide Dismutase (SOD) Activity
2.8. Measurement of Catalase Activity
2.9. Lipid Profile Assessment
2.10. Data Analysis
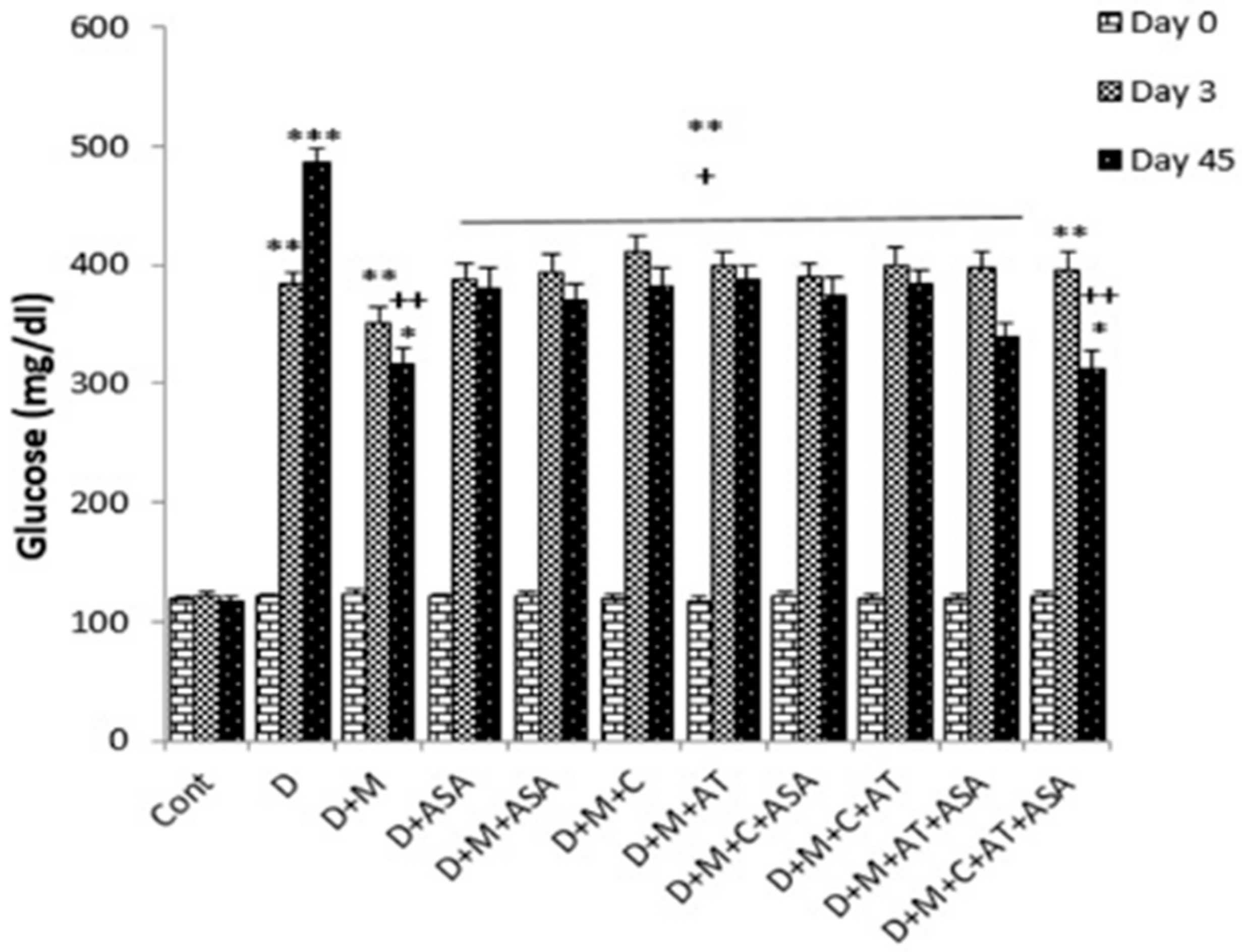
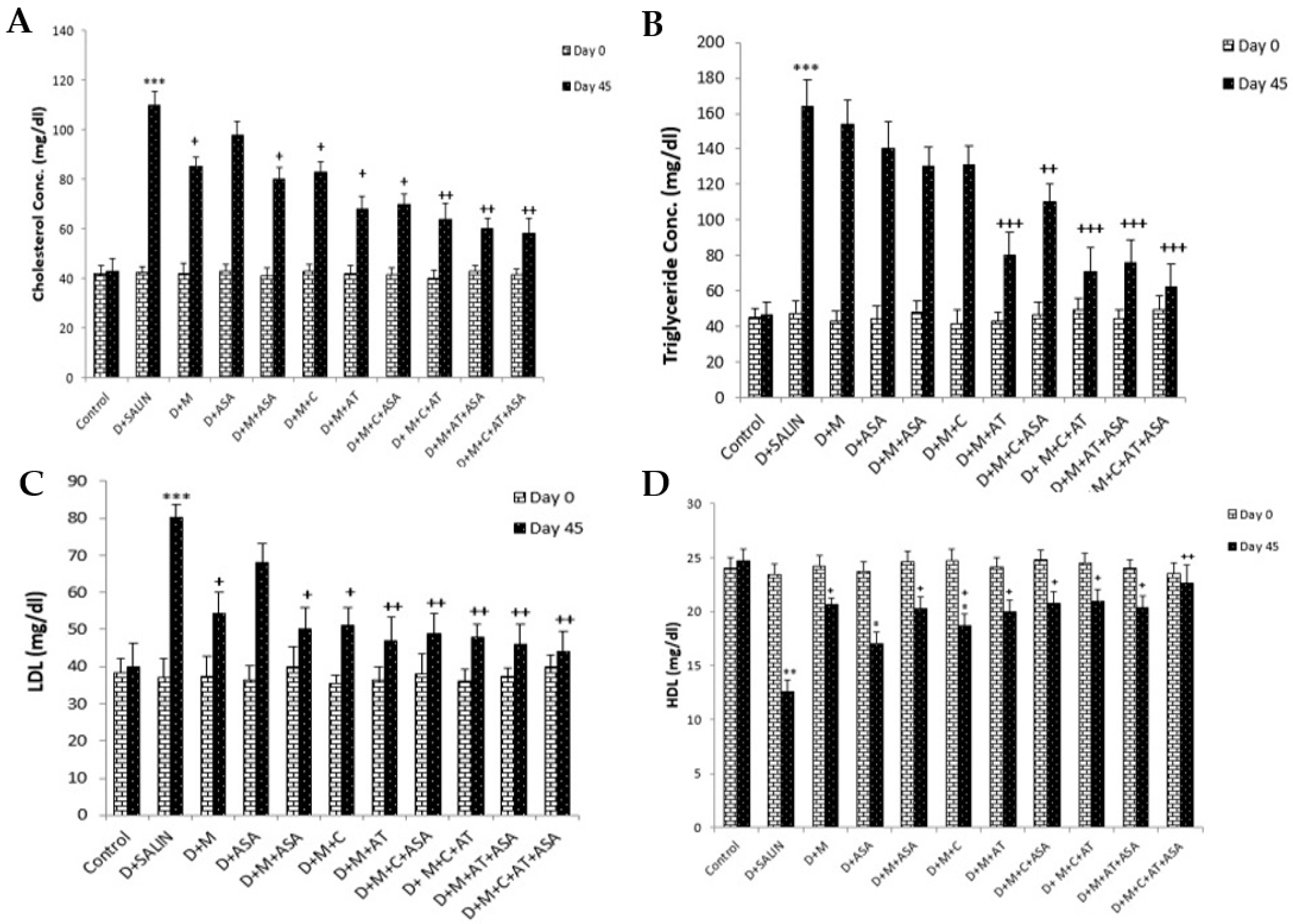
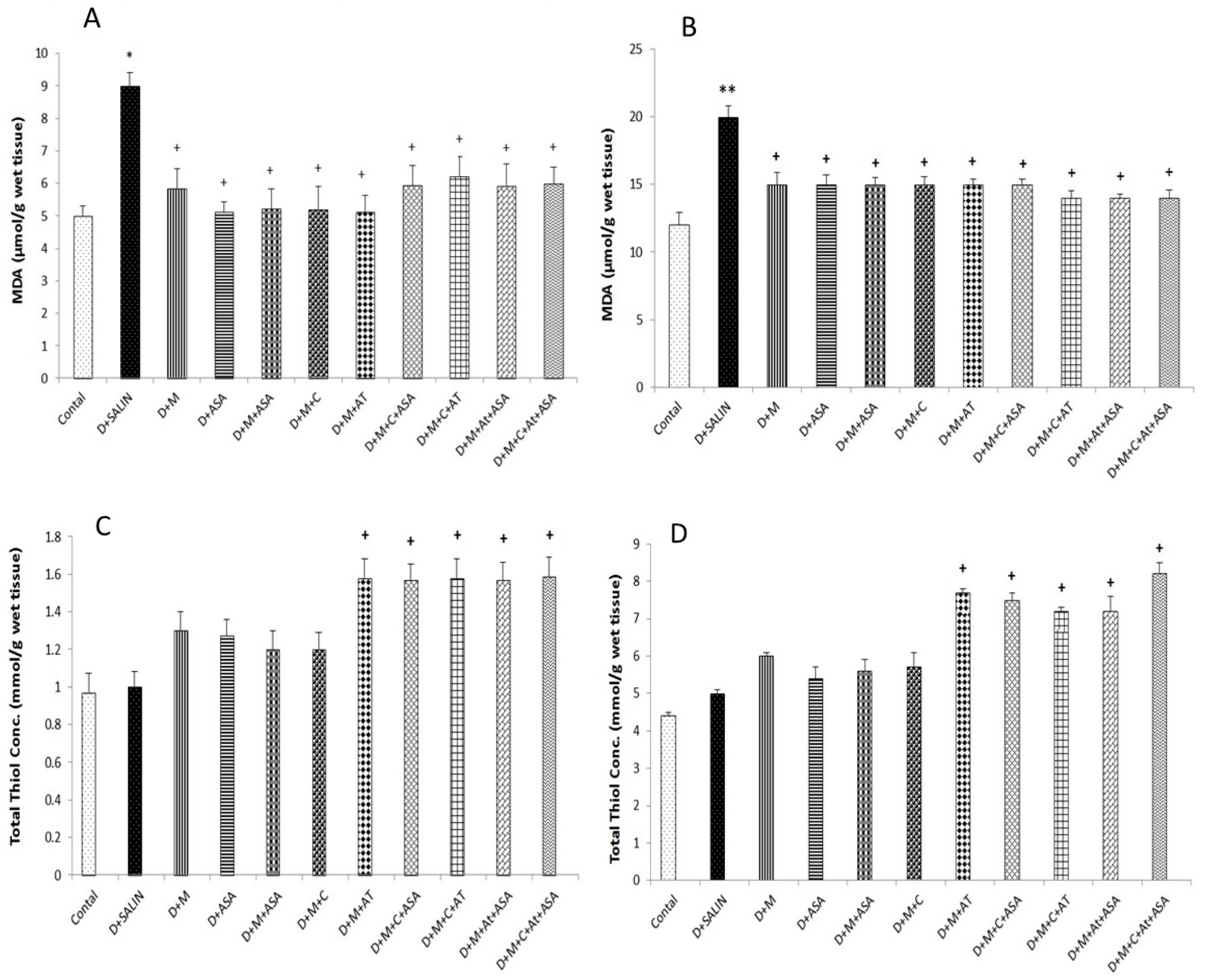
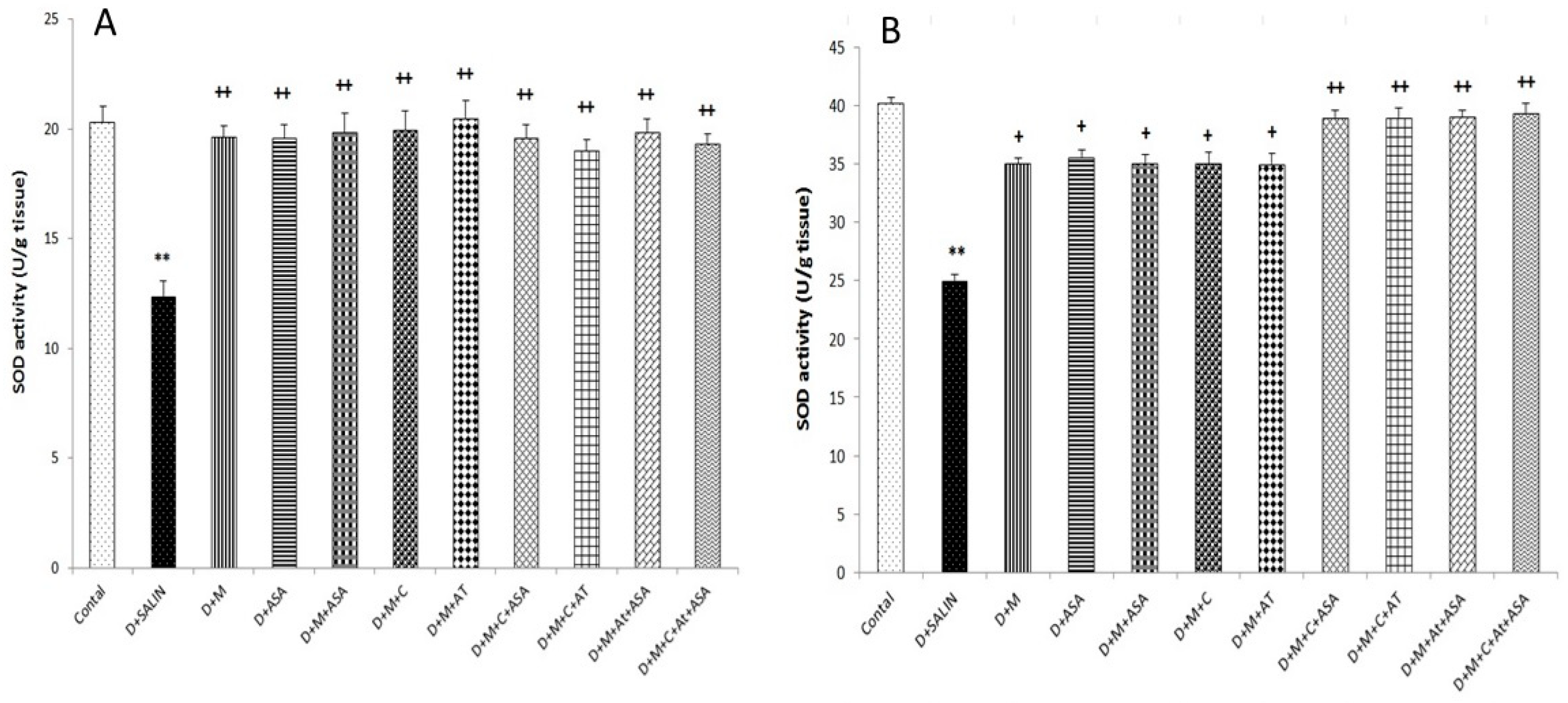
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ruggenenti, P.; Porrini, E.L.; Gaspari, F.; Motterlini, N.; Cannata, A.; Carrara, F.; Cella, C.; Ferrari, S.; Stucchi, N.; Parvanova, A.; et al. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care 2012, 35, 2061–2068. [Google Scholar] [CrossRef]
- Wulsin, L.R.; Horn, P.S.; Perry, J.L.; Massaro, J.M.; D’agostino, R.B. Autonomic imbalance as a predictor of metabolic risks, cardiovascular disease, diabetes, and mortality. J. Clin. Endocrinol. Metab. 2015, 100, 2443–2448. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B. Pathophysiology of oxidative stress in diabetes mellitus. J. Diabetes Its Complicat. 2001, 15, 203–210. [Google Scholar] [CrossRef]
- Petrie, J.R.; Chaturvedi, N.; Ford, I.; Brouwers, M.C.; Greenlaw, N.; Tillin, T.; Hramiak, I.; Hughes, A.D.; Jenkins, A.J.; Klein, B.E.; et al. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): A double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 597–609. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Niazmand, S.; Hosseini, M.; Hassanzadeh, Z.; Sadeghnia, H.R.; Vafaee, F.; Keshavarzi, Z. Beneficial effects of Teucrium polium and metformin on diabetes-induced memory impairments and brain tissue oxidative damage in rats. Int. J. Alzheimers Dis. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Wu, L.; Parhofer, K.G. Diabetic dyslipidemia. Metabol.-Clin. Exp. 2014, 63, 1469–1479. [Google Scholar] [CrossRef]
- Chehade, J.M.; Gladysz, M.; Mooradian, A.D. Dyslipidemia in type 2 diabetes: Prevalence, pathophysiology, and management. Drugs 2013, 73, 327–339. [Google Scholar] [CrossRef]
- Chruściel, P.; Sahebkar, A.; Rembek-Wieliczko, M.; Serban, M.C.; Ursoniu, S.; Mikhailidis, D.P.; Jones, S.R.; Mosteoru, S.; Blaha, M.J.; Martin, S.S.; et al. Impact of statin therapy on plasma adiponectin concentrations: A systematic review and meta-analysis of 43 randomized controlled trial arms. Atherosclerosis 2016, 253, 194–208. [Google Scholar] [CrossRef]
- Sahebkar, A.; Kotani, K.; Serban, C.; Ursoniu, S.; Mikhailidis, D.P.; Jones, S.R.; Ray, K.K.; Blaha, M.J.; Rysz, J.; Toth, P.P.; et al. Statin therapy reduces plasma endothelin-1 concentrations: A meta-analysis of 15 randomized controlled trials. Atherosclerosis 2015, 241, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Serban, C.; Mikhailidis, D.P.; Undas, A.; Lip, G.Y.; Muntner, P.; Bittner, V.; Ray, K.K.; Watts, G.F.; Hovingh, G.K.; et al. Association between statin use and plasma d-dimer levels: A systematic review and meta-analysis of randomised controlled trials. Thromb. Haemost. 2015, 114, 546–557. [Google Scholar] [PubMed]
- Sahebkar, A.; Serban, C.; Ursoniu, S.; Mikhailidis, D.P.; Undas, A.; Lip, G.Y.; Bittner, V.; Ray, K.K.; Watts, G.F.; Hovingh, G.K.; et al. The impact of statin therapy on plasma levels of von Willebrand factor antigen: Systematic review and meta-analysis of Randomised placebo-controlled trials. Thromb. Haemost. 2016, 115, 520–532. [Google Scholar] [CrossRef]
- Serban, C.; Sahebkar, A.; Ursoniu, S.; Mikhailidis, D.P.; Rizzo, M.; Lip, G.Y.; Hovingh, G.K.; Kastelein, J.J.; Kalinowski, L.; Rysz, J.; et al. A systematic review and meta-analysis of the effect of statins on plasma asymmetric dimethylarginine concentrations. Sci. Rep. 2015, 5, 9902. [Google Scholar] [CrossRef]
- Inoguchi, T.; Sonta, T.; Tsubouchi, H.; Etoh, T.; Kakimoto, M.; Sonoda, N.; Sato, N.; Sekiguchi, N.; Kobayashi, K.; Sumimoto, H.; et al. Protein kinase C–dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: Role of vascular NAD (P) H oxidase. J. Am. Soc. Nephrol. 2003, 14 (Suppl. 3), S227–S232. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, S.; Laufs, U.; Bäumer, A.T.; Müller, K.; Ahlbory, K.; Linz, W.; Itter, G.; Rösen, R.; Böhm, M.; Nickenig, G. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension 2001, 37, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Parizadeh, S.M.; Azarpazhooh, M.R.; Moohebati, M.; Nematy, M.; Ghayour-Mobarhan, M.; Tavallaie, S.; Rahsepar, A.A.; Amini, M.; Sahebkar, A.; Mohammadi, M.; et al. Simvastatin therapy reduces prooxidant-antioxidant balance: Results of a placebo-controlled cross-over trial. Lipids 2011, 46, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Calvin, A.D.; Aggarwal, N.R.; Murad, M.H.; Shi, Q.; Elamin, M.B.; Geske, J.B.; Fernandez-Balsells, M.M.; Albuquerque, F.N.; Lampropulos, J.F.; Erwin, P.J.; et al. Aspirin for the primary prevention of cardiovascular events: A systematic review and meta-analysis comparing patients with and without diabetes. Diabetes Care 2009, 32, 2300–2306. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.-C.; Lee, W.-J.; Wu, C.-M.; Chen, J.F.-M.; Sheu, W.H.-H. Aspirin prevents resistin-induced endothelial dysfunction by modulating AMPK, ROS, and Akt/eNOS signaling. J. Vasc. Surg. 2012, 55, 1104–1115. [Google Scholar] [CrossRef]
- Bolterman, R.J.; Manriquez, M.C.; Ruiz, M.C.O.; Juncos, L.A.; Romero, J.C. Effects of captopril on the renin angiotensin system, oxidative stress, and endothelin in normal and hypertensive rats. Hypertension 2005, 46, 943–947. [Google Scholar] [CrossRef]
- Hundal, R.S.; Petersen, K.F.; Mayerson, A.B.; Randhawa, P.S.; Inzucchi, S.; Shoelson, S.E.; Shulman, G.I. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J. Clin. Investig. 2002, 109, 1321–1326. [Google Scholar] [CrossRef]
- Heeba, G.H.; Hassan, M.K.; Amin, R.S. Gastroprotective effect of simvastatin against indomethacin-induced gastric ulcer in rats: Role of nitric oxide and prostaglandins. Eur. J. Pharmacol. 2009, 607, 188–193. [Google Scholar] [CrossRef]
- Abbasnezhad, A.; Niazmand, S.; Mahmoudabady, M.; Soukhtanloo, M.; Rezaee, S.A.; Mousavi, S.M. Nigella sativa seed decreases endothelial dysfunction in streptozotocin-induced diabetic rat aorta. Avicenna J. Phytomed. 2016, 6, 67. [Google Scholar]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar]
- Madesh, M.; Balasubramanian, K. Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J. Biochem. Biophys. 1998, 35, 184–188. [Google Scholar]
- Zini, A.; Lamirande, E.; Gagnon, C. Reactive oxygen species in semen of infertile patients: Levels of superoxide dismutase-and catalase-like activities in seminal plasma and spermatozoa. Int. J. Androl. 1993, 16, 183–188. [Google Scholar] [CrossRef]
- Dervisevik, M.; Dinevska-Kovkarovska, S.; Dimitrovska, M.; Cipanovska, N.; Miova, B. High dose of aspirin moderates diabetes-induced changes of heart glycogen/glucose metabolism in rats. J. Physiol. Sci. 2014, 64, 411–420. [Google Scholar] [CrossRef]
- Fullerton, M.D.; Ford, R.J.; McGregor, C.P.; LeBlond, N.D.; Snider, S.A.; Stypa, S.A.; Day, E.A.; Lhoták, Š.; Schertzer, J.D.; Austin, R.C.; et al. Salicylate improves macrophage cholesterol homeostasis via activation of Ampk. J. Lipid Res. 2015, 56, 1025–1033. [Google Scholar] [CrossRef]
- Wulffelé, E.M.; Kooy, A.; De Zeeuw, D.; Stehouwer, C.; Gansevoort, R. The effect of metformin on blood pressure, plasma cholesterol and triglycerides in type 2 diabetes mellitus: A systematic review. J. Intern. Med. 2004, 256, 1–14. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Malmqvist, K.; Kahan, T.; Isaksson, H.; Östergren, J. Regression of left ventricular mass with captopril and metoprolol, and the effects on glucose and lipid metabolism. Blood Press. 2001, 10, 101–110. [Google Scholar] [CrossRef]
- Kawamoto, S.; Kawamura, T.; Miyazaki, Y.; Hosoya, T. Effects of atorvastatin on hyperlipidemia in kidney disease patients. Nihon Jinzo Gakkai Shi 2007, 49, 41–48. [Google Scholar]
- Vandresen-Filho, S.; Martins, W.C.; Bertoldo, D.B.; Mancini, G.; Herculano, B.A.; Andreza, F.; Tasca, C.I. Atorvastatin prevents cell damage via modulation of oxidative stress, glutamate uptake and glutamine synthetase activity in hippocampal slices subjected to oxygen/glucose deprivation. Neurochem. Int. 2013, 62, 948–955. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, A.; Watkins, J.B., III. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Cetin, F.; Yazihan, N.; Dincer, S.; Akbulut, G. The effect of intracerebroventricular injection of beta amyloid peptide (1–42) on caspase-3 activity, lipid peroxidation, nitric oxide and NOS expression in young adult and aged rat brain. Turk Neurosurg. 2013, 23, 144–150. [Google Scholar] [CrossRef]
- Thöne-Reineke, C.; Zimmermann, M.; Neumann, C.; Krikov, M.; Li, J.; Gerova, N.; Unger, T. Are angiotensin receptor blockers neuroprotective? Curr. Hypertens. Rep. 2004, 6, 257–266. [Google Scholar] [CrossRef]
- Mogi, M.; Li, J.M.; Tsukuda, K.; Iwanami, J.; Min, L.J.; Sakata, A.; Fujita, T.; Iwai, M.; Horiuchi, M. Telmisartan prevented cognitive decline partly due to PPAR-γ activation. Biochem. Biophys. Res. Commun. 2008, 375, 446–449. [Google Scholar] [CrossRef]
- Abbassi, Y.A.; Mohammadi, M.T.; Foroshani, M.S.; Sarshoori, J.R. Captopril and valsartan may improve cognitive function through potentiation of the brain antioxidant defense system and attenuation of oxidative/nitrosative damage in STZ-induced dementia in rat. Adv. Pharm. Bull. 2016, 6, 531. [Google Scholar] [CrossRef]
- Alhaider, A.A.; Korashy, H.M.; Sayed-Ahmed, M.M.; Mobark, M.; Kfoury, H.; Mansour, M.A. Metformin attenuates streptozotocin-induced diabetic nephropathy in rats through modulation of oxidative stress genes expression. Chem.-Biol. Interact. 2011, 192, 233–242. [Google Scholar] [CrossRef]
- Cahova, M.; Palenickova, E.; Dankova, H.; Sticova, E.; Burian, M.; Drahota, Z.; Cervinkova, Z.; Kucera, O.; Gladkova, C.; Stopka, P.; et al. Metformin prevents ischemia reperfusion-induced oxidative stress in the fatty liver by attenuation of reactive oxygen species formation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2015, 309, G100–G111. [Google Scholar] [CrossRef]
- Correia, S.; Carvalho, C.; Santos, M.S.; Proenca, T.; Nunes, E.; Duarte, A.I.; Monteiro, P.; Seica, R.; Oliveira, C.R.; Moreira, P.I. Metformin protects the brain against the oxidative imbalance promoted by type 2 diabetes. Med. Chem. 2008, 4, 358–364. [Google Scholar] [CrossRef]
- De Cristóbal, J.; Madrigal, J.L.M.; Lizasoain, I.; Lorenzo, P.; Leza, J.C.; Moro, M.A. Aspirin inhibits stress-induced increase in plasma glutamate, brain oxidative damage and ATP fall in rats. Neuroreport 2002, 13, 217–221. [Google Scholar] [CrossRef]
- Castillo, J.; Leira, R.; Moro, M.Á.; Lizasoain, I.; Serena, J.; Dávalos, A. Neuroprotective effects of aspirin in patients with acute cerebral infarction. Neurosci. Lett. 2003, 339, 248–250. [Google Scholar] [CrossRef]
- Kopp, E.; Ghosh, S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science 1994, 265, 956–959. [Google Scholar] [CrossRef]
- Schreck, R.; Meier, B.; Männel, D.N.; Dröge, W.; Baeuerle, P.A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J. Exp. Med. 1992, 175, 1181–1194. [Google Scholar] [CrossRef]
- Grilli, M.; Pizzi, M.; Memo, M.; Spano, P. Neuroprotection by aspirin and sodium salicylate through blockade of NF-κB activation. Science 1996, 274, 1383–1385. [Google Scholar] [CrossRef]
- Bösel, J.; Gandor, F.; Harms, C.; Synowitz, M.; Harms, U.; Djoufack, P.C.; Megow, D.; Dirnagl, U.; Hörtnagl, H.; Fink, K.B.; et al. Neuroprotective effects of atorvastatin against glutamate-induced excitotoxicity in primary cortical neurones. J. Neurochem. 2005, 92, 1386–1398. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, Y.-H.; Kim, Y.-J.; Yoon, B.-W. Atorvastatin enhances hypothermia-induced neuroprotection after stroke. J. Neurol. Sci. 2008, 275, 64–68. [Google Scholar] [CrossRef]
- Vaughan, C.J.; Delanty, N.; Basson, C.T. Do statins afford neuroprotection in patients with cerebral ischaemia and stroke? Cns Drugs 2001, 15, 589–596. [Google Scholar] [CrossRef]
- Barone, E.; Cenini, G.; Di Domenico, F.; Martin, S.; Sultana, R.; Mancuso, C.; Murphy, M.P.; Head, E.; Butterfield, D.A. Long-term high-dose atorvastatin decreases brain oxidative and nitrosative stress in a preclinical model of Alzheimer disease: A novel mechanism of action. Pharmacol. Res. 2011, 63, 172–180. [Google Scholar] [CrossRef]
- Van der Most, P.J.; Dolga, A.M.; Nijholt, I.M.; Luiten, P.G.; Eisel, U.L. Statins: Mechanisms of neuroprotection. Prog. Neurobiol. 2009, 88, 64–75. [Google Scholar] [CrossRef]
- Moro, M.A.; De Alba, J.; Cárdenas, A.; De Cristóbal, J.; Leza, J.C.; Lizasoain, I.; Dıaz-Guerra, M.J.; Boscá, L.; Lorenzo, P. Mechanisms of the neuroprotective effect of aspirin after oxygen and glucose deprivation in rat forebrain slices. Neuropharmacology 2000, 39, 1309–1318. [Google Scholar] [CrossRef]
- El-Mir, M.Y.; Detaille, D.; Gloria, R.; Delgado-Esteban, M.; Guigas, B.; Attia, S.; Fontaine, E.; Almeida, A.; Leverve, X. Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons. J. Mol. Neurosci. 2008, 34, 77–87. [Google Scholar] [CrossRef]
- Koh, K.K.; Son, J.W.; Ahn, J.Y.; Kim, D.S.; Jin, D.K.; Kim, H.S.; Han, S.H.; Seo, Y.H.; Chung, W.J.; Kang, W.C.; et al. Simvastatin combined with ramipril treatment in hypercholesterolemic patients. Hypertension 2004, 44, 180–185. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, Z.; Liu, J.; Sun, L.; Hu, L.; Cooper, M.E.; Cao, Z. Effects of the combination of an angiotensin II antagonist with an HMG-CoA reductase inhibitor in experimental diabetes. Kidney Int. 2003, 64, 565–571. [Google Scholar] [CrossRef]
- De Berardis, G.; Sacco, M.; Evangelista, V.; Filippi, A.; Giorda, C.B.; Tognoni, G.; Valentini, U.; Nicolucci, A. Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trial in Diabetes (ACCEPT-D): Design of a randomized study of the efficacy of low-dose aspirin in the prevention of cardiovascular events in subjects with diabetes mellitus treated with statins. Trials 2007, 8, 21. [Google Scholar]
- Yaribeygi, H.; Butler, A.E.; Barreto, G.E.; Sahebkar, A. Antioxidative potential of antidiabetic agents: A possible protective mechanism against vascular complications in diabetic patients. J. Cell. Physiol. 2019, 234, 2436–2446. [Google Scholar] [CrossRef]
- Sahebkar, A.; Watts, G.F. New therapies targeting apoB metabolism for high-risk patients with inherited dyslipidaemias: what can the clinician expect? Cardiovasc. Drugs Ther. 2013, 27, 559–567. [Google Scholar] [CrossRef]
- McFadyen, J.D.; Peter, K. Novel Antithrombotic Drugs on the Horizon: The Ultimate Promise to Prevent Clotting While Avoiding Bleeding. Circ. Res. 2017, 121, 1133–1135. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paseban, M.; Mohebbati, R.; Niazmand, S.; Sathyapalan, T.; Sahebkar, A. Comparison of the Neuroprotective Effects of Aspirin, Atorvastatin, Captopril and Metformin in Diabetes Mellitus. Biomolecules 2019, 9, 118. https://doi.org/10.3390/biom9040118
Paseban M, Mohebbati R, Niazmand S, Sathyapalan T, Sahebkar A. Comparison of the Neuroprotective Effects of Aspirin, Atorvastatin, Captopril and Metformin in Diabetes Mellitus. Biomolecules. 2019; 9(4):118. https://doi.org/10.3390/biom9040118
Chicago/Turabian StylePaseban, Maryam, Reza Mohebbati, Saeed Niazmand, Thozhukat Sathyapalan, and Amirhossein Sahebkar. 2019. "Comparison of the Neuroprotective Effects of Aspirin, Atorvastatin, Captopril and Metformin in Diabetes Mellitus" Biomolecules 9, no. 4: 118. https://doi.org/10.3390/biom9040118
APA StylePaseban, M., Mohebbati, R., Niazmand, S., Sathyapalan, T., & Sahebkar, A. (2019). Comparison of the Neuroprotective Effects of Aspirin, Atorvastatin, Captopril and Metformin in Diabetes Mellitus. Biomolecules, 9(4), 118. https://doi.org/10.3390/biom9040118






