The Relationship between Epicardial Adipose Tissue Thickness and Serum Interleukin-17a Level in Patients with Isolated Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
- High Triglyceride level: ≥ 150 mg/dL (1.7 mmol/L).
- Low HDL cholesterol: < 40 mg/dL (1.03 mmol/L) in men and < 50mg/dL (1.29 mmol/L) in women.
- High blood pressure: Systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥ 85 mmHg.
- Increased fasting plasma glucose: Fasting plasma glucose ≥ 100 mg/dL (5.6 mmol/L).
2.2. Measurement of Epicardial Adipose Tissue Thickness by Echocardiography
2.3. Laboratory Parameters
2.4. Determination of Serum IL-17A Level
2.5. Other Parameters
2.6. Statistical Analysis
3. Results
The Predictive Values of Epicardial Adipose Tissue Thickness and Interleukin 17A Level for Metabolic Syndrome
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- El Brini, O.; Akhouayri, O.; Gamal, A.; Mesfioui, A.; Benazzouz, B. Prevalence of metabolic syndrome and its components based on a harmonious definition among adults in Morocco. Diabetes Metab. Syndr. Obes. 2014, 7, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.A.; Lee, J.H.; Lim, S.Y.; Ha, H.S.; Kwon, H.S.; Park, Y.M.; Lee, W.C.; Kang, M.I.; Yim, H.W.; Yoon, K.H.; et al. Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J. Diabetes Investig. 2013, 4, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 2014, 943162. [Google Scholar] [CrossRef] [PubMed]
- Voils, S.A.; Cooper-DeHoff, R.M. Association between high sensitivity C-reactive protein and metabolic syndrome in subjects completing the National Health and Nutrition Examination Survey (NHANES) 2009–2010. Diabetes Metab. Syndr. 2014, 8, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.Z.; Chou, K.J.; Huang, Y.L.; Wu, M.T. The relation of location-specific epicardial adipose tissue thickness and obstructive coronary artery disease: Systemic review and meta-analysis of observational studies. BMC Cardiovasc. Disord. 2014, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Balcioğlu, A.S.; Durakoğlugil, M.E.; Ciçek, D.; Bal, U.A.; Boyaci, B.; Müderrisoğlu, H. Epicardial adipose tissue thickness and plasma homocysteine in patients with metabolic syndrome and normal coronary arteries. Diabetol. Metab. Syndr. 2014, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Tok, D.; Kadife, I.; Turak, O.; Ozcan, F.; Başar, N.; Cağlı, K.; Aras, D.; Topaloğlu, S.; Aydoğdu, S. Increased epicardial adipose thickness is associated with low grade systemic inflammation in metabolic syndrome. Turk Kardiyol. Dern. Ars. 2012, 40, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lee, J.; Zhang, Y.; Wang, H.; Liu, X.; Shang, F.; Zheng, Q. Increased Th17 cells in coronary artery disease are associated with neutrophilic inflammation. Scand. Cardiovasc. J. 2011, 45, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Wang, Q.; Guo, C.; Wang, X.; Cao, X.; Shi, Y.; Gao, F.; Ma, C.; Zhang, L. IL-17 induces apoptosis of vascular endothelial cells: A potential mechanism for human acute coronary syndrome. Clin. Immunol. 2011, 141, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, G.; Miossec, P. Interleukin 17 contributes to the chronicity of inflammatory diseases such as rheumatoid arthritis. Eur. J. Immunol. 2014, 44, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Li, C.R.; Mueller, E.E.; Bradley, L.M. Islet antigen-specific Th17 cells can induce TNF-α-dependent autoimmune diabetes. J. Immunol. 2014, 192, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Cleeman, J.I.; Grundy, S.M.; Becker, D.; Clark, L. Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults; Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar]
- International Diabetes Fedaration. The IDF Consensus Worldwide Defination of the Metabolic Syndrome. 2005. Available online: http:/www.idf.org/webdata/docs/metac_syndrome_def.pdf (accessed on 24 September 2005).
- Bertaso, A.G.; Bertol, D.; Duncan, B.B.; Foppa, M. Epicardial adipose: Definition, measurements and systematic review of main outcomes. Arq. Bras. Cardiol. 2013, 101, e18–e28. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Ahn, S.G.; Hwang, J.W.; Lim, H.S.; Choi, B.J.; Choi, S.Y.; Yoon, M.H.; Hwang, G.S.; Tahk, S.J.; Shin, J.H. Impact of body mass index on the relationship of epicardial adipose tissue to metabolic syndrome and coronaryartery disease in an Asian population. Cardiovasc. Diabetol. 2010, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Lima-Martínez, M.M.; Paoli, M.; Donis, J.H.; Odreman, R.; Torres, C.; Iacobellis, G. Cut-off point of epicardial adipose tissue thickness for predicting metabolic syndrome in Venezuelan population. Endocrinol. Nutr. 2013, 60, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.P.; Hsu, H.L.; Hung, W.C.; Yu, T.H.; Chen, Y.H.; Chiu, C.A.; Lu, L.F.; Chung, F.M.; Shin, S.J.; Lee, Y.J. Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin. Endocrinol. 2009, 70, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Ribaudo, M.C.; Assael, F.; Vecci, E.; Tiberti, C.; Zappaterreno, A.; Di Mario, U.; Leonetti, F. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: A new indicator of cardiovascular risk. J. Clin. Endocrinol. Metab. 2003, 88, 5163–5168. [Google Scholar] [CrossRef] [PubMed]
- Yorgun, H.; Canpolat, U.; Hazırolan, T.; Ateş, A.H.; Sunman, H.; Dural, M.; Şahiner, L.; Kaya, E.B.; Aytemir, K.; Tokgözoğlu, L.; et al. Increased epicardial adipose tissue is a marker of metabolic syndrome in adult patients. Int. J. Cardiol. 2013, 165, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Pistilli, D.; Gucciardo, M.; Leonetti, F.; Miraldi, F.; Brancaccio, G.; Gallo, P.; Di Gioia, C.R. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine 2005, 29, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Tabata, M.; Kurobe, H.; Motoki, T.; Akaike, M.; Nishio, C.; Higashida, M.; Mikasa, H.; Nakaya, Y.; Takanashi, S.; et al. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J. Am. Coll. Cardiol. 2011, 58, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Gorter, P.M.; van Lindert, A.S.; de Vos, A.M.; Meijs, M.F.; van der Graaf, Y.; Doevendans, P.A.; Prokop, M.; Visseren, F.L. Quantification of epicardial and peri-coronary adipose using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis 2008, 197, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Willens, H.J.; Barbaro, G.; Sharma, A.M. Threshold values of high-risk echocardiographic epicardial adipose thickness. Obesity 2008, 16, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Willens, H.J.; Byers, P.; Chirinos, J.A.; Labrador, E.; Hare, J.M.; de Marchena, E. Effects of weight loss after bariatric surgery on epicardial adipose measured using echocardiography. Am. J. Cardiol. 2007, 99, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Barbaro, G.; Gerstein, H.C. Relationship of epicardial adipose thickness and fasting glucose. Int. J. Cardiol. 2008, 128, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Pierdomenico, S.D.; Pierdomenico, A.M.; Cuccurullo, F.; Iacobellis, G. Meta-analysis of the relation of echocardiographic epicardial adipose tissue thickness and the metabolic syndrome. Am. J. Cardiol. 2013, 111, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.J.; Acevedo, L.B.; Pérez, N.C.; Martínez, A.L.; Gutiérrez, N.T.; García, S.V.; Cruz, A.R.; Martínez, A.D.; García, R.S.; Treviño, A.Z.; et al. Epicardial adipose tissue is associated with visceral adipose, metabolic syndrome, and insulin resistance in menopausal women. Revista Española Cardiología 2014, 67, 436–441. [Google Scholar] [CrossRef]
- Surendar, J.; Aravindhan, V.; Rao, M.M.; Ganesan, A.; Mohan, V. Decreased serum interleukin17 and increased transforming growth factorβ levels in subjects with metabolic syndrome (Chennai Urban Rural Epidemiology Study-95). Metabolism 2011, 60, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, K.; Mogi, M.; Jing, F.; Iwanami, J.; Tsukuda, K.; Min, L.J.; Higaki, J.; Horiuchi, M. Roles of interleukin 17 in angiotensin II type 1 receptor-mediated insulin resistance. Hypertension 2012, 59, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga, L.A.; Shen, W.J.; Joyce-Shaikh, B.; Pyatnova, E.A.; Richards, A.G.; Thom, C.; Andrade, S.M.; Cua, D.J.; Kraemer, F.B.; Butcher, E.C. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J. Immunol. 2010, 185, 6947–6959. [Google Scholar] [CrossRef] [PubMed]
- Eid, R.E.; Rao, D.A.; Zhou, J.; Lo, S.F.; Ranjbaran, H.; Gallo, A.; Sokol, S.I.; Pfau, S.; Pober, J.S.; Tellides, G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary arteryinfiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation 2009, 119, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- De Boer, O.J.; van der Meer, J.J.; Teeling, P.; van der Loos, C.M.; Idu, M.M.; van Maldegem, F.; Aten, J.; van der Wal, A.C. Differential expression of interleukin-17 family cytokines in intact and complicated human atherosclerotic plaques. J. Pathol. 2010, 220, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, S.; Zeng, Q.T. Role of interleukin-17 and interleukin-17-induced cytokines interleukin-6 and interleukin-8 in unstable coronary artery disease. Coron. Artery Dis. 2006, 17, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Nordlohne, J.; von Vietinghoff, S. Interleukin 17A in atherosclerosis—Regulation and pathophysiologic effector function. Cytokine 2017. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Liu, Z.; Liu, J.; Zhou, P.; Liu, Y.; Lu, X. The paradoxical role of IL-17 in atherosclerosis. Cell. Immunol. 2015, 297, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Butcher, M.J.; Waseem, T.C.; Galkina, E.V. Smooth Muscle Cell-Derived Interleukin-17C Plays an Atherogenic Role via the Recruitment of Proinflammatory Interleukin-17A + T Cells to the Aorta. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
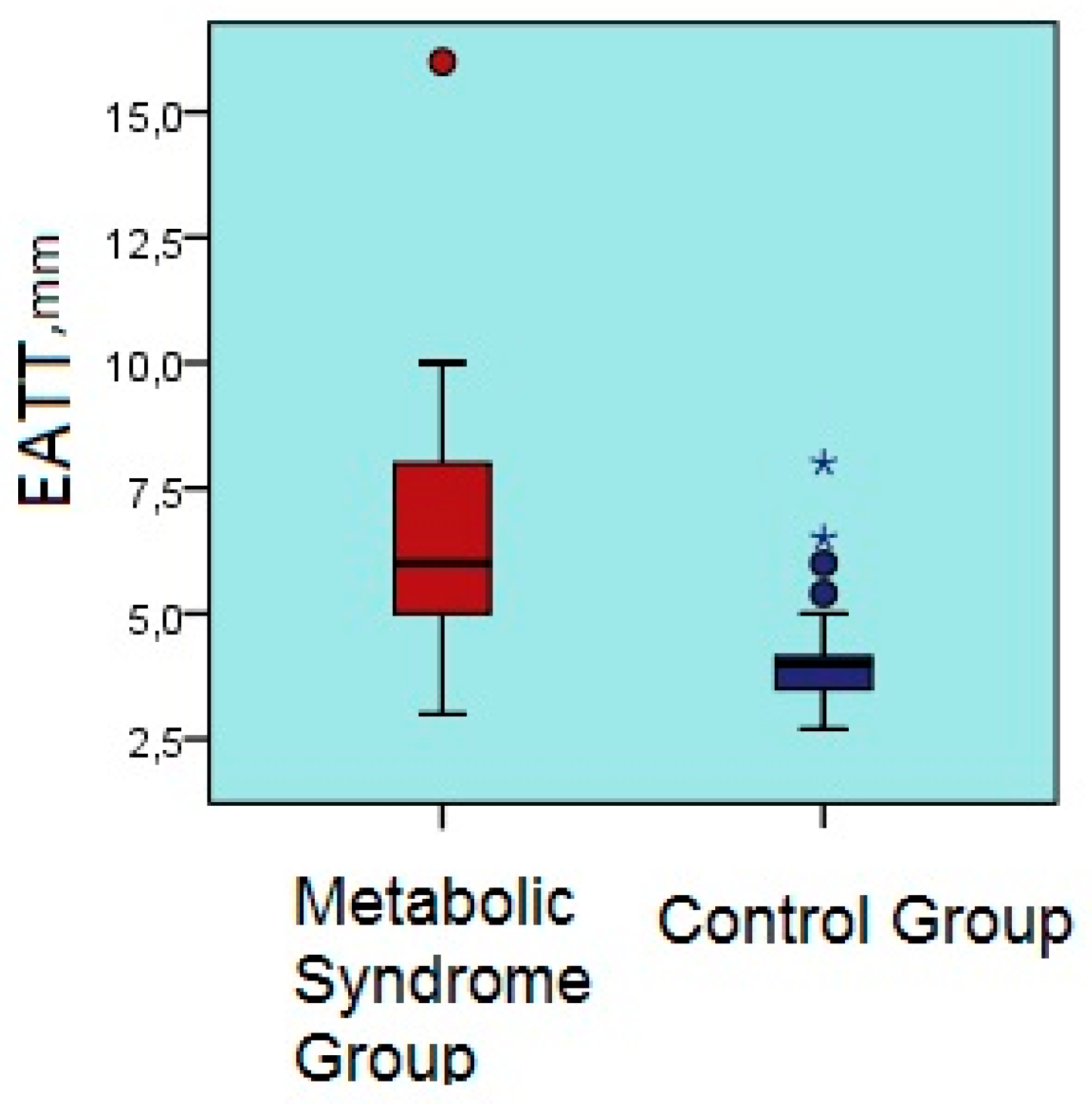
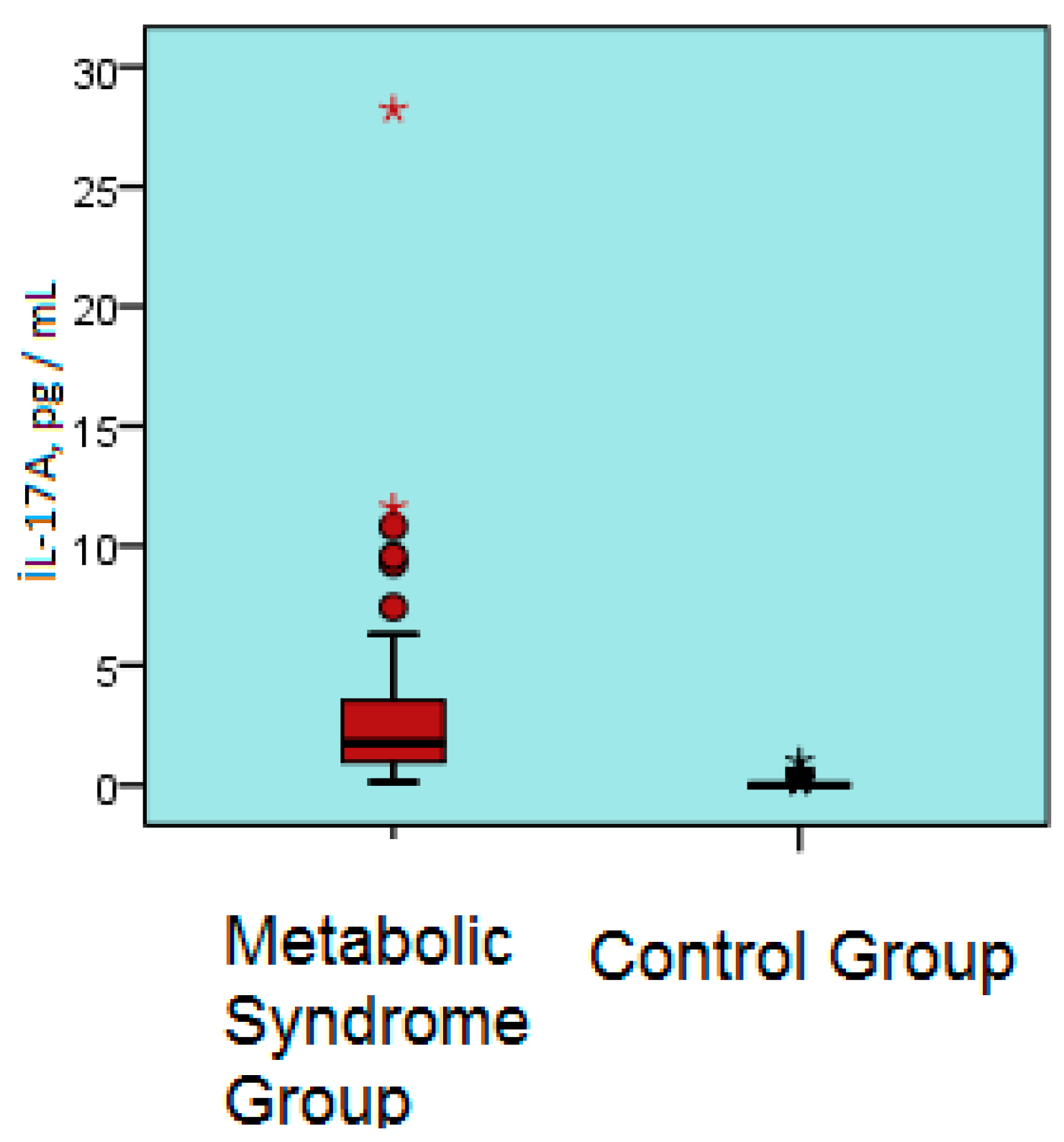
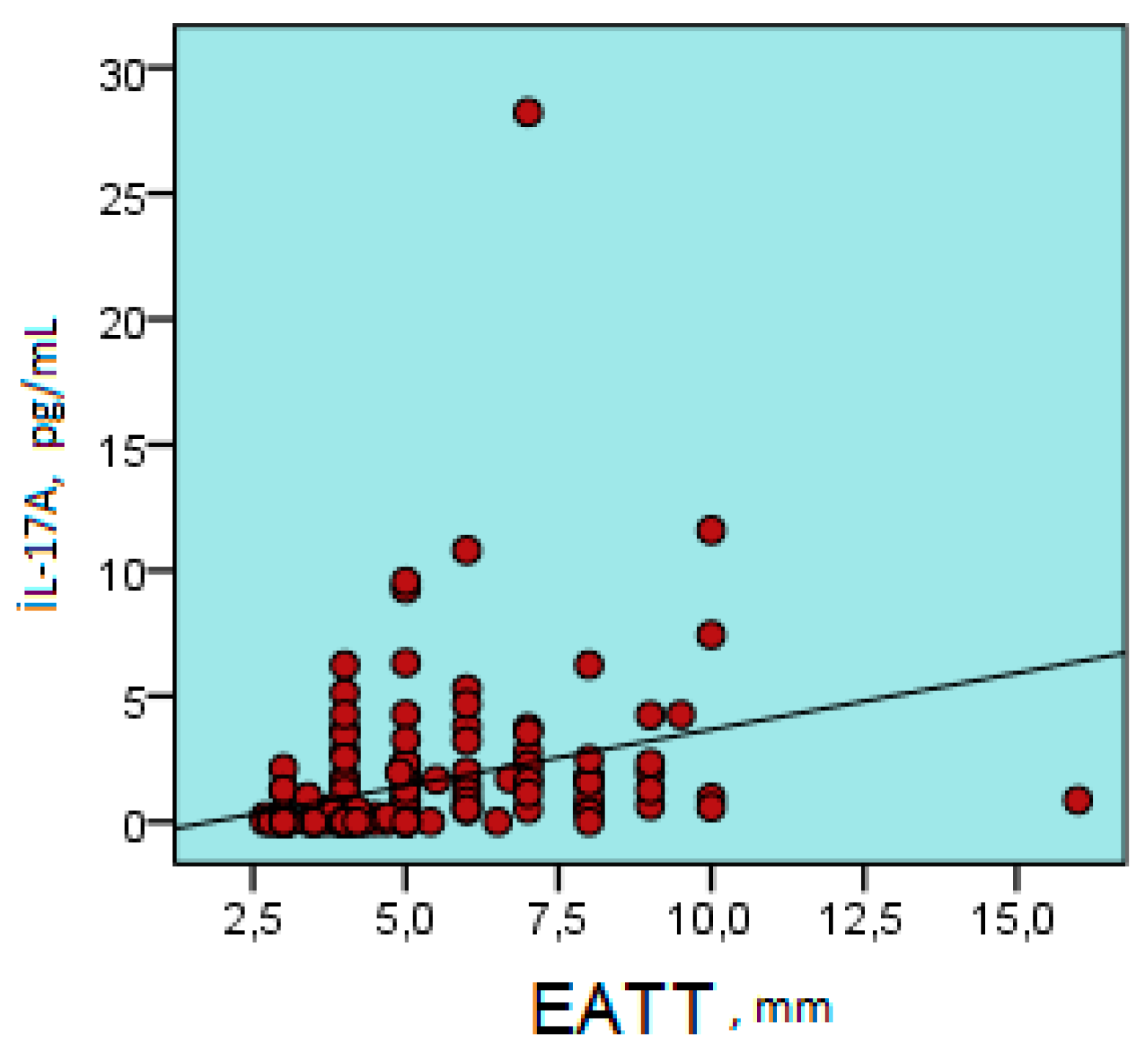
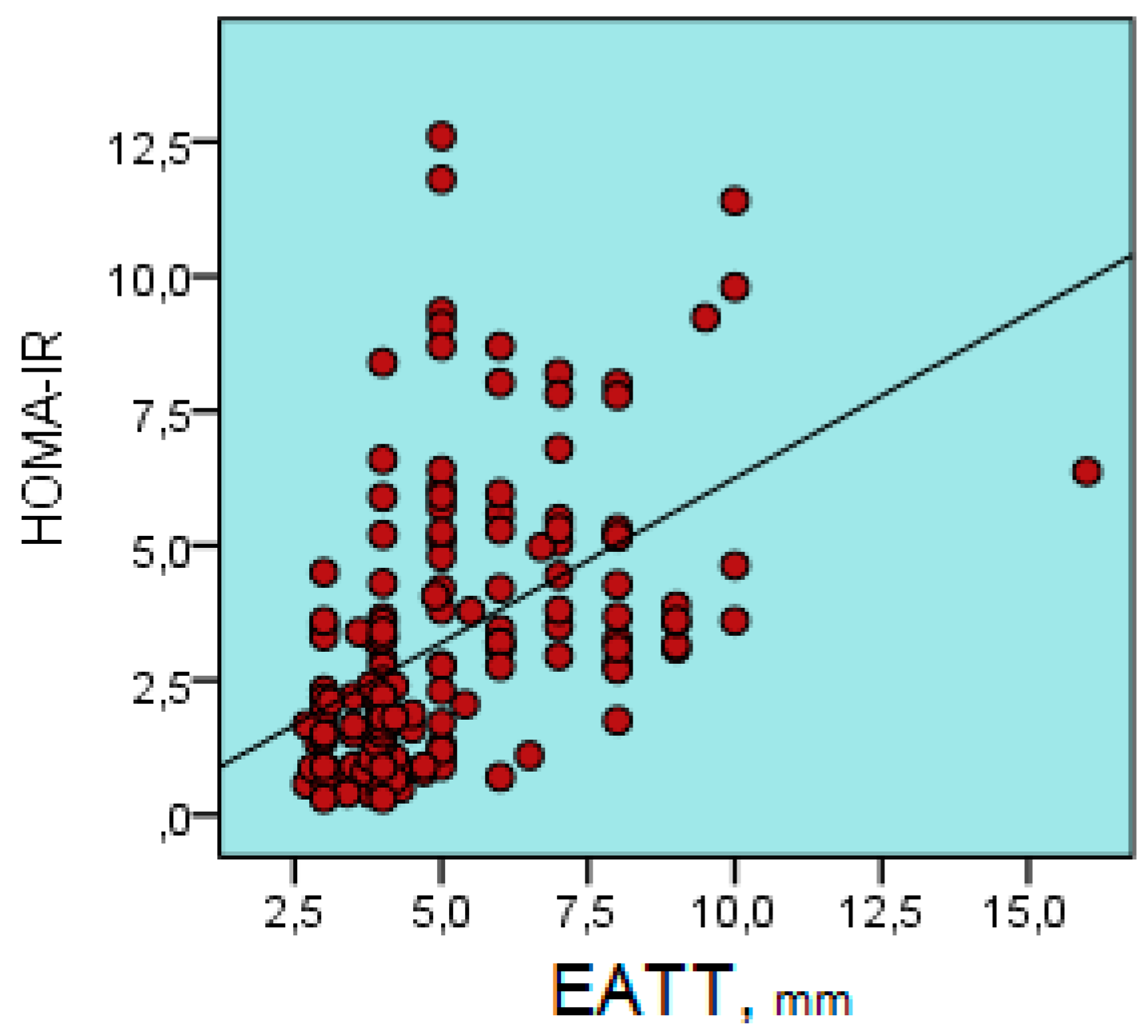

| Parameter | Metabolic Syndrome Group | Control Group | p Value |
|---|---|---|---|
| Mean ± Standard Deviation | Mean ± Standard Deviation | ||
| Age, years | 39.0 ± 11.5 | 39.6 ± 9.4 | 0.747 |
| Sex (Male/Female) | 23/57 | 16/64 | 0.197 |
| BMI, kg/m2 | 34.8 ± 6.7 | 25.9 ± 4.9 | <0.001 |
| Smoking, n | 30 | 24 | 0.316 |
| Hypertension, n | 50 | 4 | <0.001 |
| Hyperlipidemia, n | 11 | 4 | 0.058 |
| Family history, n | 50 | 25 | <0.001 |
| Systolic blood presure, mmHg | 133.8 ± 22.4 | 111.3 ± 13.4 | <0.001 |
| Diastolic blood pressure, mmHg | 83.8 ± 12.4 | 71.3 ± 9.6 | <0.001 |
| Glucose, mg/dL | 96.4 ± 11.7 | 84.9 ±10.3 | <0.001 |
| Urea, mg/dL | 26.8 ± 10.4 | 25.1 ± 6.8 | 0.246 |
| Creatinine, mg/dL | 0.7 ± 0.2 | 0.7 ± 0.4 | 0.536 |
| T. Chol, mg/dL | 189.7 ± 38.6 | 183.1 ± 39.5 | 0.077 |
| LDL, mg/dL | 110.4 ± 29.8 | 106.3 ± 33.9 | 0.102 |
| HDL, mg/dL | 44.7 ± 11.2 | 51.9 ± 11.9 | <0.001 |
| TG, mg/dL | 183.3 ± 91.0 | 108.7 ± 67.6 | <0.001 |
| GGT, U/L | 28.9 ± 19.3 | 20.5 ± 17.0 | <0.001 |
| AST, U/L | 27.5 ± 17.1 | 23.8. ± 18.7 | 0.007 |
| ALT, U/L | 33.0 ± 28.1 | 23.8 ± 33.5 | <0.001 |
| Insulin, mIU/ mL | 22.0 ± 9.6 | 18.5 ± 10.3 | <0.001 |
| HOMA-IR | 5.2 ± 2.3 | 1.4 ± 0.6 | <0.001 |
| IL-17A, pg /mL | 2.9 ± 3.7 | 0.1 ± 0.2 | <0.001 |
| hs-CRP, mg/L | 3.8 ± 0.6 | 0.8 ± 0.8 | <0.001 |
| EATT, mm | 6.2 ± 2.2 | 4.0 ± 0.8 | <0.001 |
| Hb, g/dL | 13.1 ± 1.8 | 12.7 ± 1.6 | 0.169 |
| Thrombocyte count (x109) | 286.9 ± 110.9 | 257.1 ± 64.1 | 0.069 |
| Parameter | Age | BMI | Glucose | İnsulin | LDL | TG | HDL | GGT | HOMA-IR | hs-CRP | İL-17A | Urea | SBP | DBP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EATT | r | 0.180 | 0.539 | 0.194 | 0.556 | 0.092 | 0.279 | -0.142 | 0.281 | 0.567 | 0.666 | 0.308 | 0.181 | 0.421 | 0.394 |
| p | 0.023 | 0.000 | 0.014 | 0.000 | 0.248 | 0.000 | 0.074 | 0.000 | 0.000 | 0.000 | 0.000 | 0.022 | 0.000 | 0.000 | |
| Multivariable Model | B | Standard Error | Beta | t | p |
|---|---|---|---|---|---|
| Age | 0.00 | 0.00 | 0.18 | 2.70 | 0.008 |
| Diastolic blood pressure | 0.00 | 0.00 | 0.22 | 3.06 | 0.003 |
| HOMA-IR | 0.03 | 0.01 | 0.40 | 5.65 | 0.000 |
| urea | 0.00 | 0.00 | 0.17 | 2.58 | 0.011 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demir, E.; Harmankaya, N.Ö.; Kıraç Utku, İ.; Açıksarı, G.; Uygun, T.; Özkan, H.; Demir, B. The Relationship between Epicardial Adipose Tissue Thickness and Serum Interleukin-17a Level in Patients with Isolated Metabolic Syndrome. Biomolecules 2019, 9, 97. https://doi.org/10.3390/biom9030097
Demir E, Harmankaya NÖ, Kıraç Utku İ, Açıksarı G, Uygun T, Özkan H, Demir B. The Relationship between Epicardial Adipose Tissue Thickness and Serum Interleukin-17a Level in Patients with Isolated Metabolic Syndrome. Biomolecules. 2019; 9(3):97. https://doi.org/10.3390/biom9030097
Chicago/Turabian StyleDemir, Esra, Nazmiye Özlem Harmankaya, İrem Kıraç Utku, Gönül Açıksarı, Turgut Uygun, Hanise Özkan, and Bülent Demir. 2019. "The Relationship between Epicardial Adipose Tissue Thickness and Serum Interleukin-17a Level in Patients with Isolated Metabolic Syndrome" Biomolecules 9, no. 3: 97. https://doi.org/10.3390/biom9030097
APA StyleDemir, E., Harmankaya, N. Ö., Kıraç Utku, İ., Açıksarı, G., Uygun, T., Özkan, H., & Demir, B. (2019). The Relationship between Epicardial Adipose Tissue Thickness and Serum Interleukin-17a Level in Patients with Isolated Metabolic Syndrome. Biomolecules, 9(3), 97. https://doi.org/10.3390/biom9030097





