Triterpene Derivatives as Relevant Scaffold for New Antibiofilm Drugs
Abstract
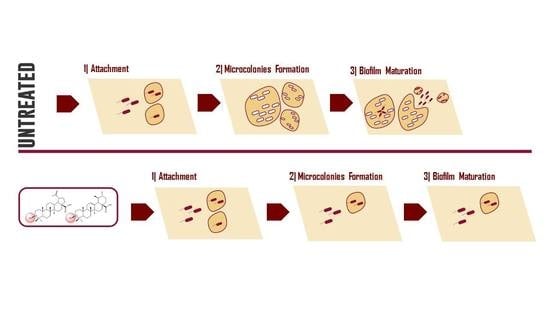
:1. Introduction
2. Materials and Methods
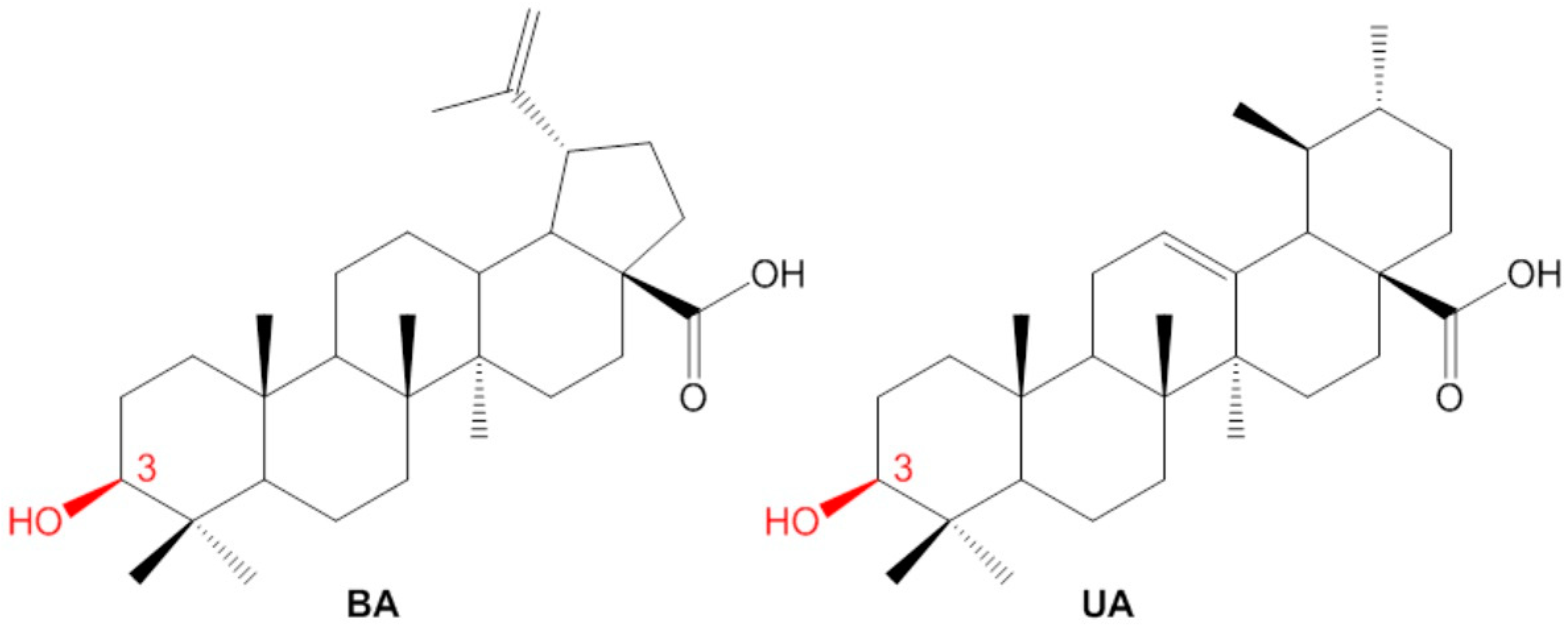
2.1. Isolation of Betulinic Acid and Ursolic Acid
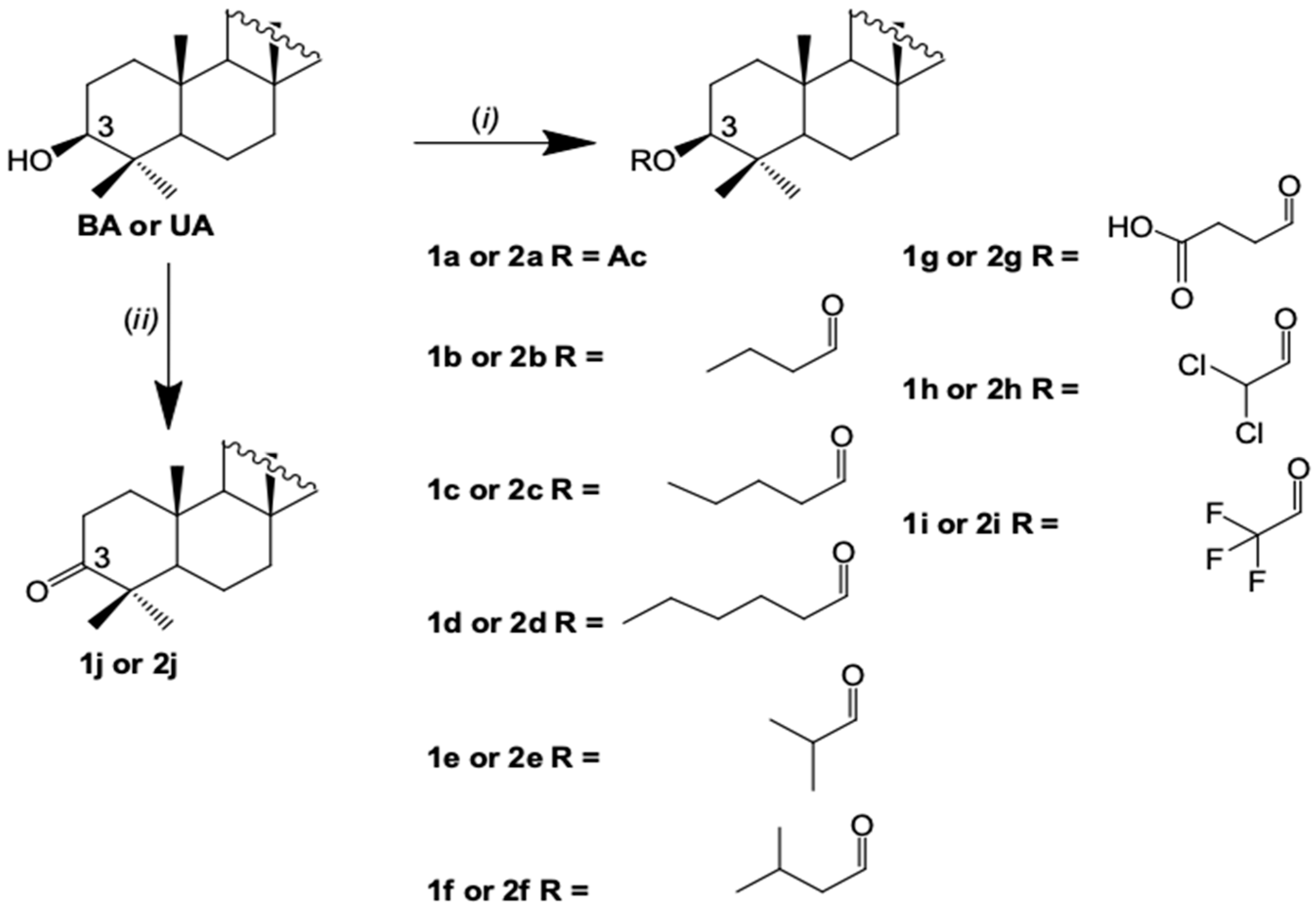
2.2. Semisynthesis of the Betulinic Acid and Ursolic Acid Derivatives
2.3. Bacteria Strain and Culture Condition
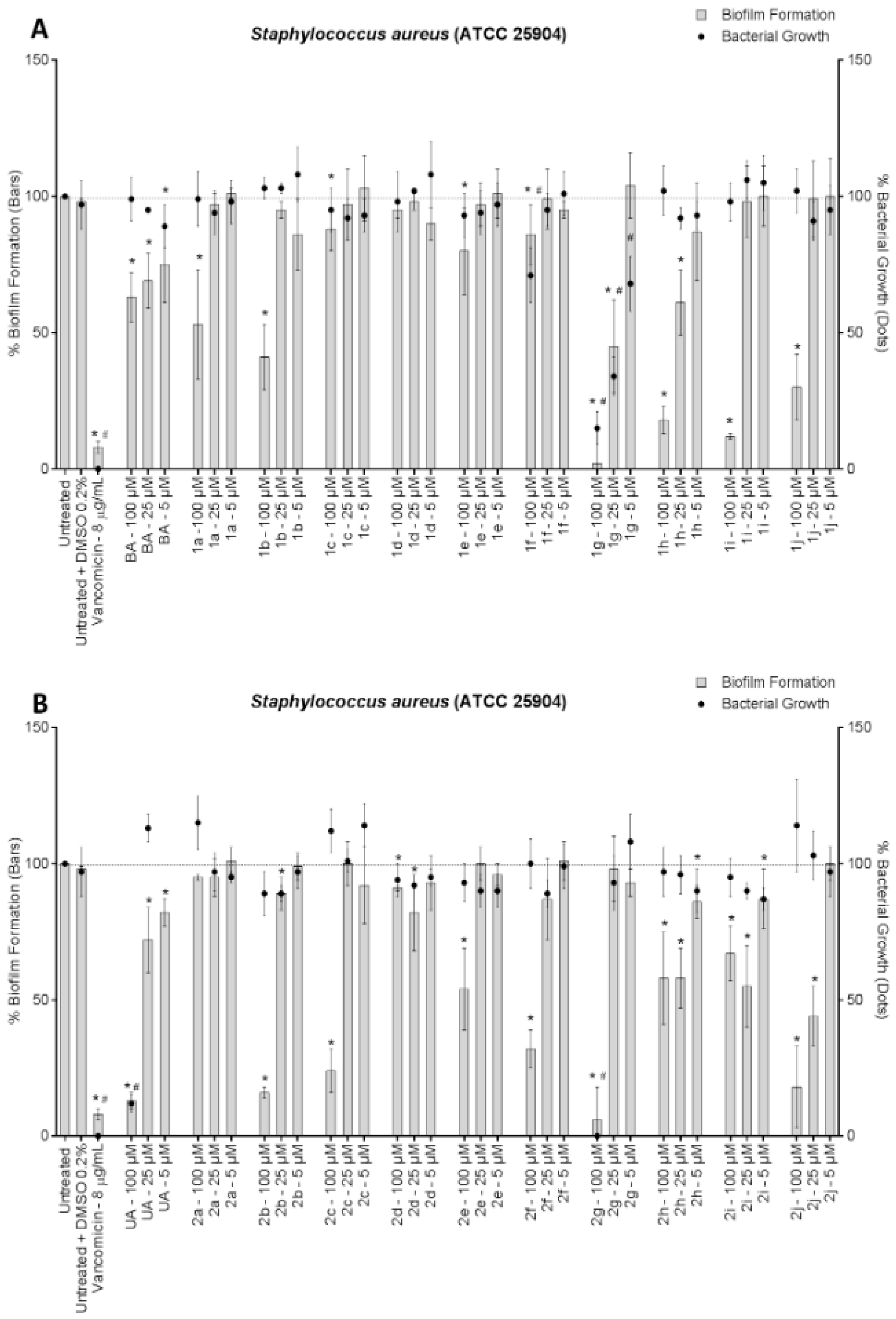
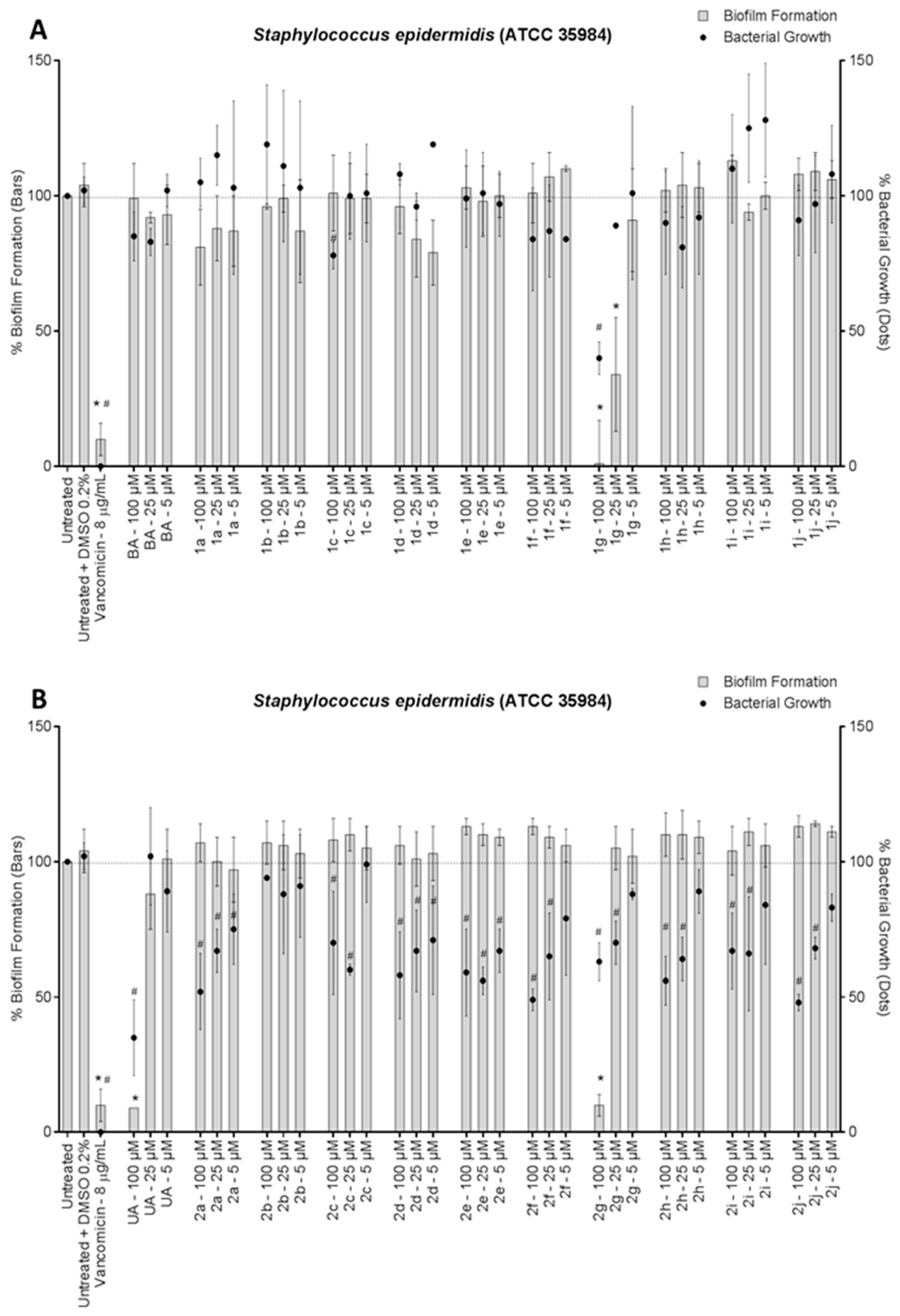
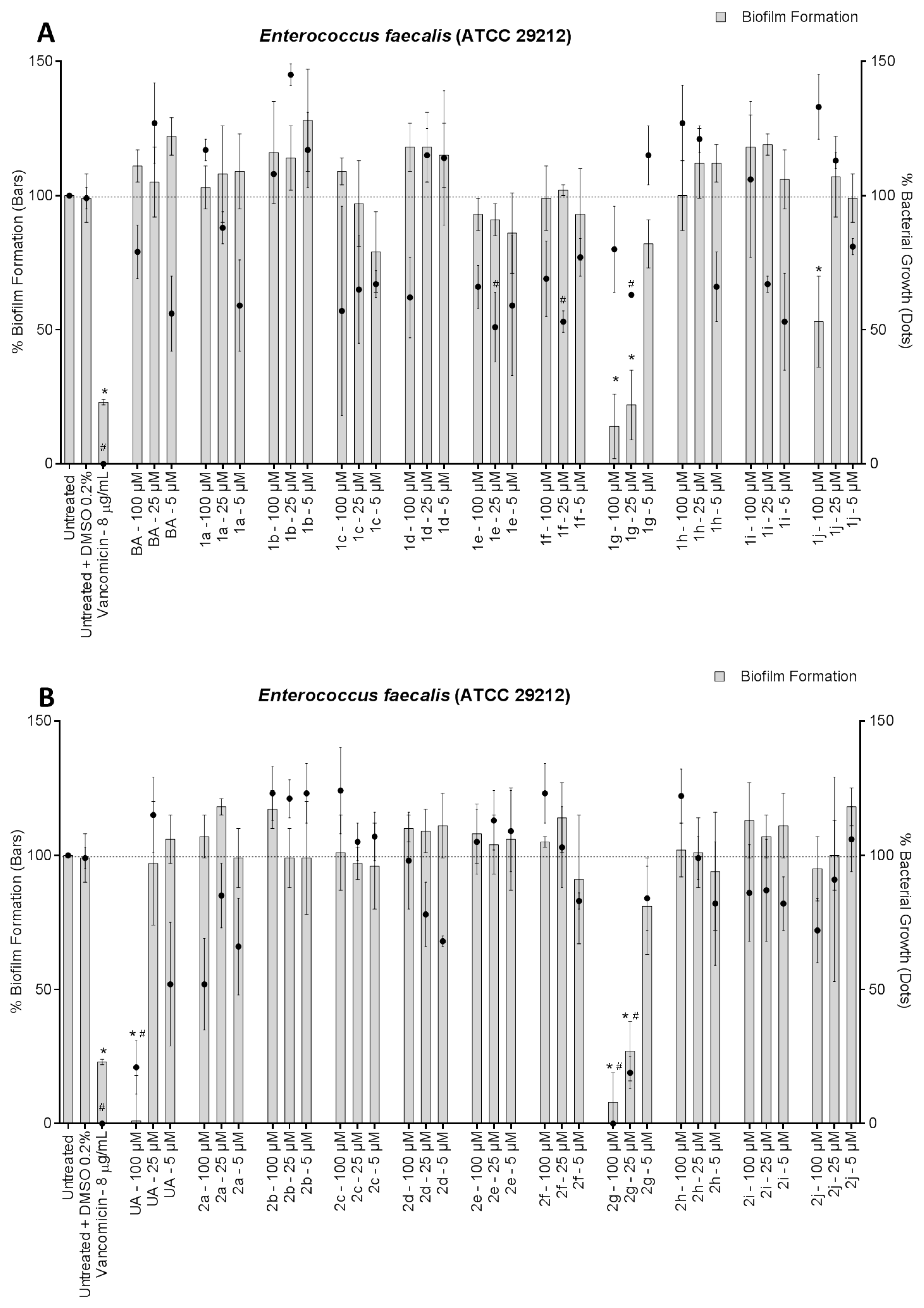
2.4. Antibiofilm and Antibacterial Activity Assays
2.5. Cytotoxicity Assay
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Outterson, K.; Rex, J.H.; Jinks, T.; Jackson, P.; Hallinan, J.; Karp, S.; Hung, D.T.; Franceschi, F.; Merkeley, T.; Houchens, C.; et al. Accelerating global innovation to address antibacterial resistance: Introducing CARB-X. Nat. Rev. Drug Discov. 2016, 15, 589–590. [Google Scholar] [CrossRef]
- Wu, H.; Moser, C.; Wang, H.Z.; Høiby, N.; Song, Z.J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente-Núñez, C.; Reffuveille, F.; Fernández, L.; Hancock, R.E. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Stephens, C. Microbiology: Breaking Down Biofilms. Curr. Biol. 2002, 12, R132–R134. [Google Scholar] [CrossRef]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Lee, J.H. Antibiofilm agents: A new respective for antimicrobial strategy. J. Microbiol. 2017, 35, 753–766. [Google Scholar] [CrossRef]
- Yogeeswari, P.; Sriram, D. Betulinic Acid and Its Derivatives: A Review on their Biological Properties. Curr. Med. Chem. 2005, 12, 657–666. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech. 2015, 5, 129–151. [Google Scholar] [CrossRef]
- Innocente, A.M.; Silva, G.N.S.; Cruz, L.N.; Moraes, M.S.; Nakabashi, M.; Sonnet, P.; Gosmann, G.; Garcia, C.R.; Gnoatto, S.C.B. Synthesis and antiplasmodial activity of betulinic acid and ursolic acid analogues. Molecules 2012, 17, 12003–12014. [Google Scholar] [CrossRef] [PubMed]
- Innocente, A.M.; Casanova, B.B.; Klein, F.; Lana, A.D.; Pereira, D.; Muniz, M.N.; Sonnet, P.; Gosmann, G.; Fuentefria, A.M.; Gnoatto, S.C.B. Synthesis of isosteric triterpenoid derivatives and antifungal activity. Chem. Biol. Drug Des. 2014, 83, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.N.S.; Maria, N.R.; Schuck, D.C.; Cruz, L.N.; Moraes, M.S.; Nakabashi, M.; Graebin, C.; Gosmann, G.; Garcia, C.R.S.; Gnoatto, S.C.B. Two series of new semisynthetic triterpene derivatives: Differences in anti-malarial activity, cytotoxicity and mechanism of action. Malar. J. 2013, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Waechter, F.; Silva, G.N.S.; Willig, J.B.; de Oliveira, C.B.; Vieira, B.D.; Trivella, D.B.B.; Zimmer, A.R.; Buffon, A.; Pilger, D.A.; Gnoatto, S.C.B. Design, Synthesis and Biological Evaluation of Betulinic Acid Derivatives as New Antitumor Agents for Leukemia. Anticancer Agents Med. Chem. 2017, 17, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Cargnin, S.T.; Gnoatto, S.C.B. Ursolic acid from apple pomace and traditional plants: A valuable triterpenoid with functional properties. Food Chem. 2017, 220, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Omori, S. A simple preparation of betulinic acid from sycamore bark. J. Wood Sci. 2012, 58, 169–173. [Google Scholar] [CrossRef]
- Zhou, L.; Ding, Y.; Chen, W.; Zhang, P.; Chen, Y.; Lv, X. The in vitro study of ursolic acid and oleanolic acid inhibiting cariogenic microorganisms as well as biofilm. Oral Dis. 2013, 19, 494–500. [Google Scholar] [CrossRef]
- Fontanay, S.; Grare, M.; Mayer, J.; Finance, C.; Duval, R.E. Ursolic, oleanolic and betulinic acids: Antibacterial spectra and selectivity indexes. J. Ethnopharmacol. 2008, 120, 272–276. [Google Scholar] [CrossRef]
- Gnoatto, S.C.B.; Dassonville-Klimpt, A.; Da Nascimento, S.; Galera, P.; Boumediene, K.; Gosmann, G.; Sonnet, P.; Moslemi, S. Evaluation of ursolic acid isolated from Ilex paraguariensis and derivatives on aromatase inhibition. Eur. J. Med. Chem. 2008, 43, 1865–1877. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Nineteenth Informational Supplement; CLSI: Wayne, PA, USA, 2009. [Google Scholar]
- Trentin, D.S.; Giordani, R.B.; Zimmer, K.R.; Silva, A.G.; Silva, M.V.; Correia, M.T.; Baumvol, I.J.; Macedo, A.J. Potential of medicinal plants from the Brazilian semi-arid region (Caatinga) against Staphylococcus epidermidis planktonic and biofilm lifestyles. J. Ethnopharmacol. 2011, 137, 327–335. [Google Scholar] [CrossRef]
- Antunes, A.L.; Trentin, D.S.; Bonfanti, J.W.; Pinto, C.C.; Perez, L.R.; Macedo, A.J.; Barth, A.L. Application of a feasible method for determination of biofilm antimicrobial susceptibility in staphylococci. APMIS 2010, 118, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Henning, S.M.; Heber, D. Limitations of MTT and MTS-Based Assays for Measurement of Antiproliferative Activity of Green Tea Polyphenols. PLoS ONE 2010, 5, e10202. [Google Scholar] [CrossRef] [PubMed]
- Yuyama, K.T.; Wendt, L.; Surup, F.; Kretz, R.; Chepkirui, C.; Wittstein, K.; Boonlarppradab, C.; Wongkanoun, S.; Luangsa-ard, J.; Stadler, M.; et al. Cytochalasans Act as Inhibitors of Biofilm Formation of Staphylococcus aureus. Biomolecules 2018, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Wojnicz, D.; Tichaczek-Goska, D.; Kicia, M. Pentacyclic triterpenes combined with ciprofloxacin help to eradicate the biofilm formed in vitro by Escherichia coli. Indian J. Med. Res. 2015, 141, 343. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Horiuchi, K.; Shiota, S.; Hatano, T.; Yoshida, T.; Kuroda, T.; Tsuchiya, T. Antimicrobial Activity of Oleanolic Acid from Salvia officinalis and Related Compounds on Vancomycin-Resistant Enterococci (VRE). Biol. Pharm. Bull. 2007, 30, 1147–1149. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, M.H.; Dayer, M.J.; Prendergast, B.; Baddour, L.M.; Jones, S.; Lockhart, P.B. Incidence and nature of adverse reactions to antibiotics used as endocarditis prophylaxis. J. Antimicrob. Chemother. 2015, 70, 2382–2388. [Google Scholar] [CrossRef]
| VERO Cells Viability (%) of Compounds | |||||||||
| 100 µM | 25 µM | 5 µM | 100 µM | 25 µM | 5 µM | ||||
| BA | 25 ± 3 * | 30 ± 3 * | 40 ± 6* | UA | 13 ± 12 * | 32 ± 7 * | 37 ± 4 * | ||
| 1a | 31 ± 8 * | 35 ± 11 * | 47 ± 12 * | 2a | 55 ± 30 * | 74 ± 14 | 73 ± 10 * | ||
| 1b | 30 ± 7 * | 34 ± 11 * | 46 ± 12 * | 2b | 60 ± 15 * | 76 ± 15 | 90 ± 13 | ||
| 1c | 27 ± 10 * | 33 ± 16 * | 50 ± 14 * | 2c | 64 ± 20 * | 92 ± 6 | 68 ± 20 * | ||
| 1d | 41 ± 20 * | 56 ± 17 * | 66 ± 8 * | 2d | 55 ± 4 * | 67 ± 7 * | 65 ± 11 * | ||
| 1e | 29 ± 11 * | 64 ± 13 * | 63 ± 21 * | 2e | 52 ± 18 * | 94 ± 18 | 100 ± 16 | ||
| 1f | 58 ± 9 * | 60 ± 5 * | 63 ± 14* | 2f | 72 ± 26 | 105 ± 10 | 113 ± 32 | ||
| 1g | 67 ± 8* | 82 ± 17 | 56 ± 4 * | 2g | 61 ± 15 * | 46 ± 6 * | 52 ± 3 * | ||
| 1h | 23 ± 5 * | 35 ± 13 * | 92 ± 6 | 2h | 81 ± 11 | 81 ± 35 | 86 ± 21 | ||
| 1i | 10 ± 4 * | 72 ± 10 | 61 ± 7 * | 2i | 4 ± 0 * | 28 ± 5 * | 69 ± 7 * | ||
| 1j | 36 ± 18 * | 57 ± 10 * | 52 ± 3 * | 2j | 45 ± 3 * | 87 ± 8 | 64 ± 16 * | ||
| VERO Cells Viability (%) of Controls | |||||||||
| Negative (Untreated) | Vehicle (DMSO 0.1%) | Positive Control (Triton-X 0.1%) | |||||||
| 100 ± 21 | 91 ± 20 | 3 ± 0 * | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, G.N.S.; Primon-Barros, M.; Macedo, A.J.; Gnoatto, S.C.B. Triterpene Derivatives as Relevant Scaffold for New Antibiofilm Drugs. Biomolecules 2019, 9, 58. https://doi.org/10.3390/biom9020058
da Silva GNS, Primon-Barros M, Macedo AJ, Gnoatto SCB. Triterpene Derivatives as Relevant Scaffold for New Antibiofilm Drugs. Biomolecules. 2019; 9(2):58. https://doi.org/10.3390/biom9020058
Chicago/Turabian Styleda Silva, Gloria Narjara Santos, Muriel Primon-Barros, Alexandre José Macedo, and Simone Cristina Baggio Gnoatto. 2019. "Triterpene Derivatives as Relevant Scaffold for New Antibiofilm Drugs" Biomolecules 9, no. 2: 58. https://doi.org/10.3390/biom9020058
APA Styleda Silva, G. N. S., Primon-Barros, M., Macedo, A. J., & Gnoatto, S. C. B. (2019). Triterpene Derivatives as Relevant Scaffold for New Antibiofilm Drugs. Biomolecules, 9(2), 58. https://doi.org/10.3390/biom9020058