Functionalized Upconversion Nanoparticles for Targeted Labelling of Bladder Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of UCNP
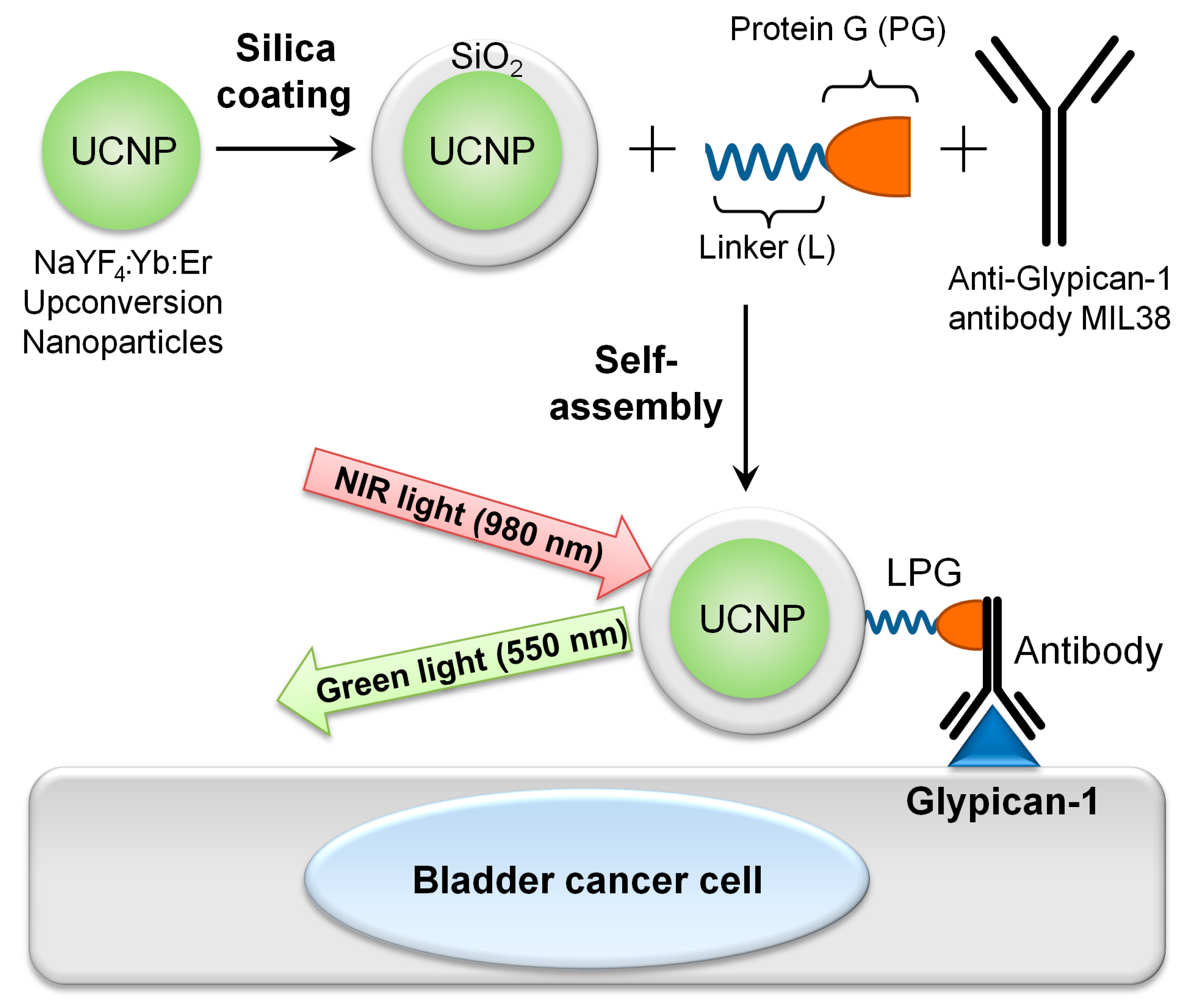
2.2. Production of the UCNP Nanoconjugates
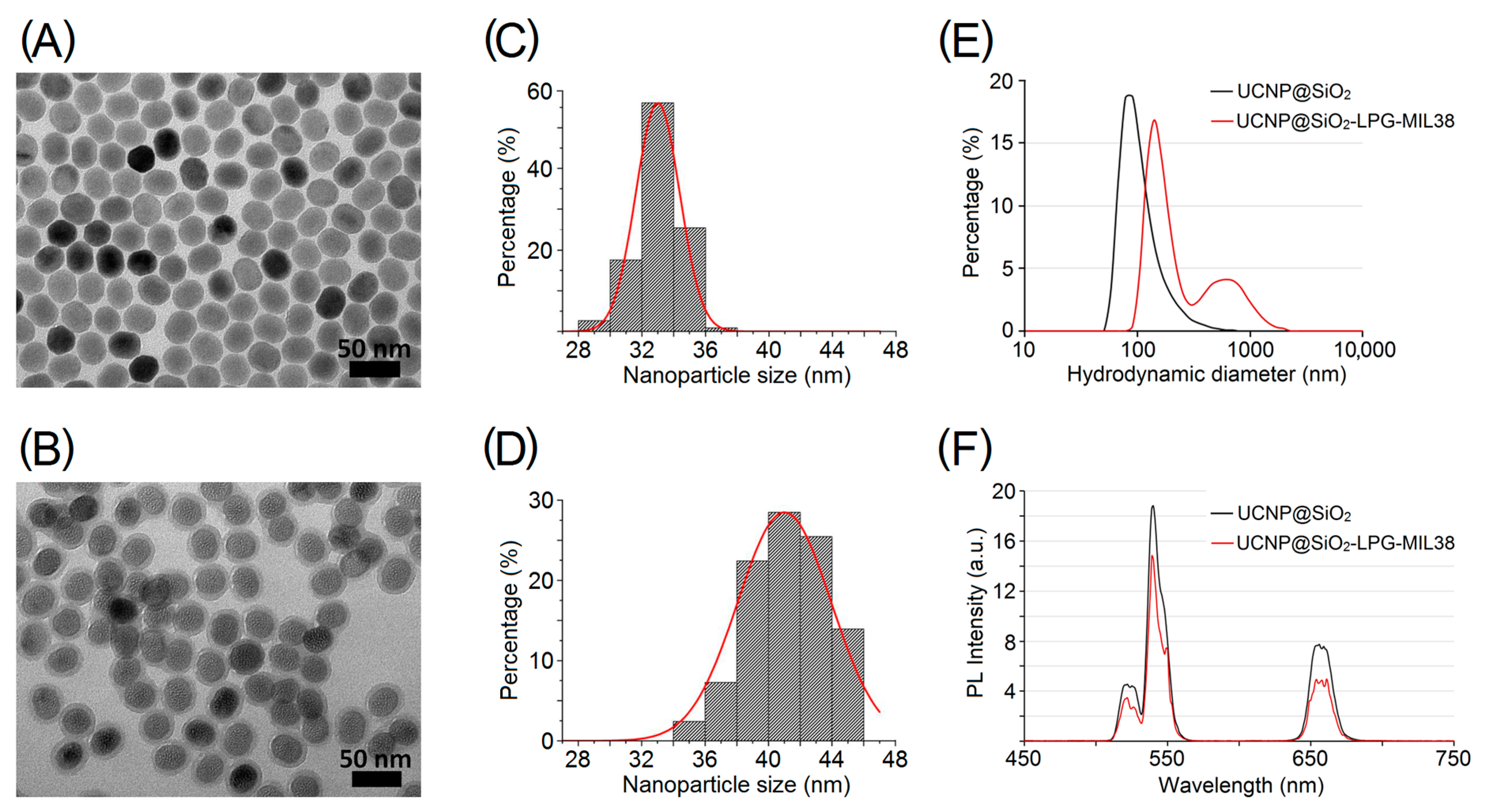
2.3. Characterisation of UCNP/UCNP@SiO2/Nanoconjugates
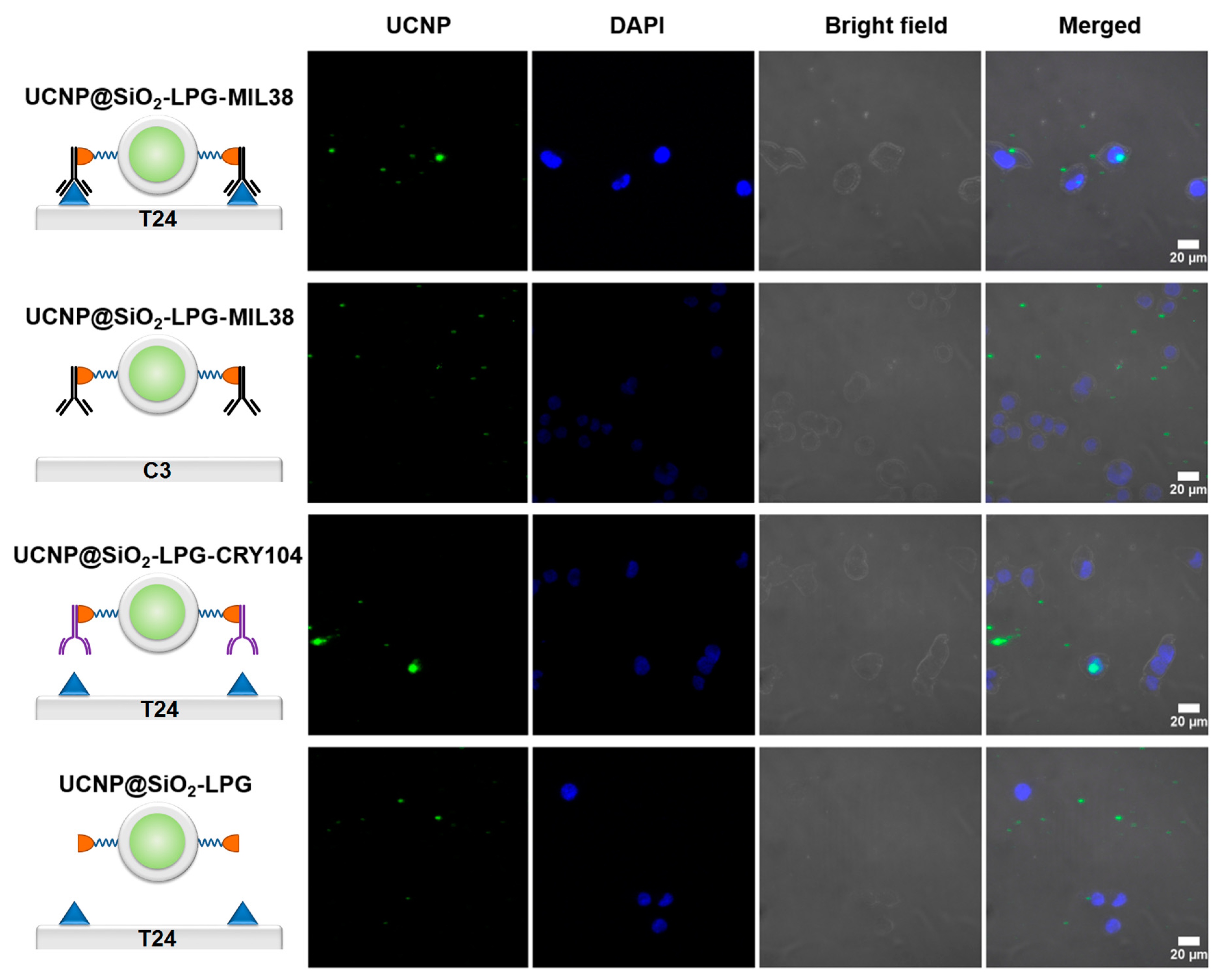
2.4. Cell Labelling
3. Results
3.1. Production and Characterisation of Targeted Nanoconjugates UCNP@SiO2-LPG-MIL38
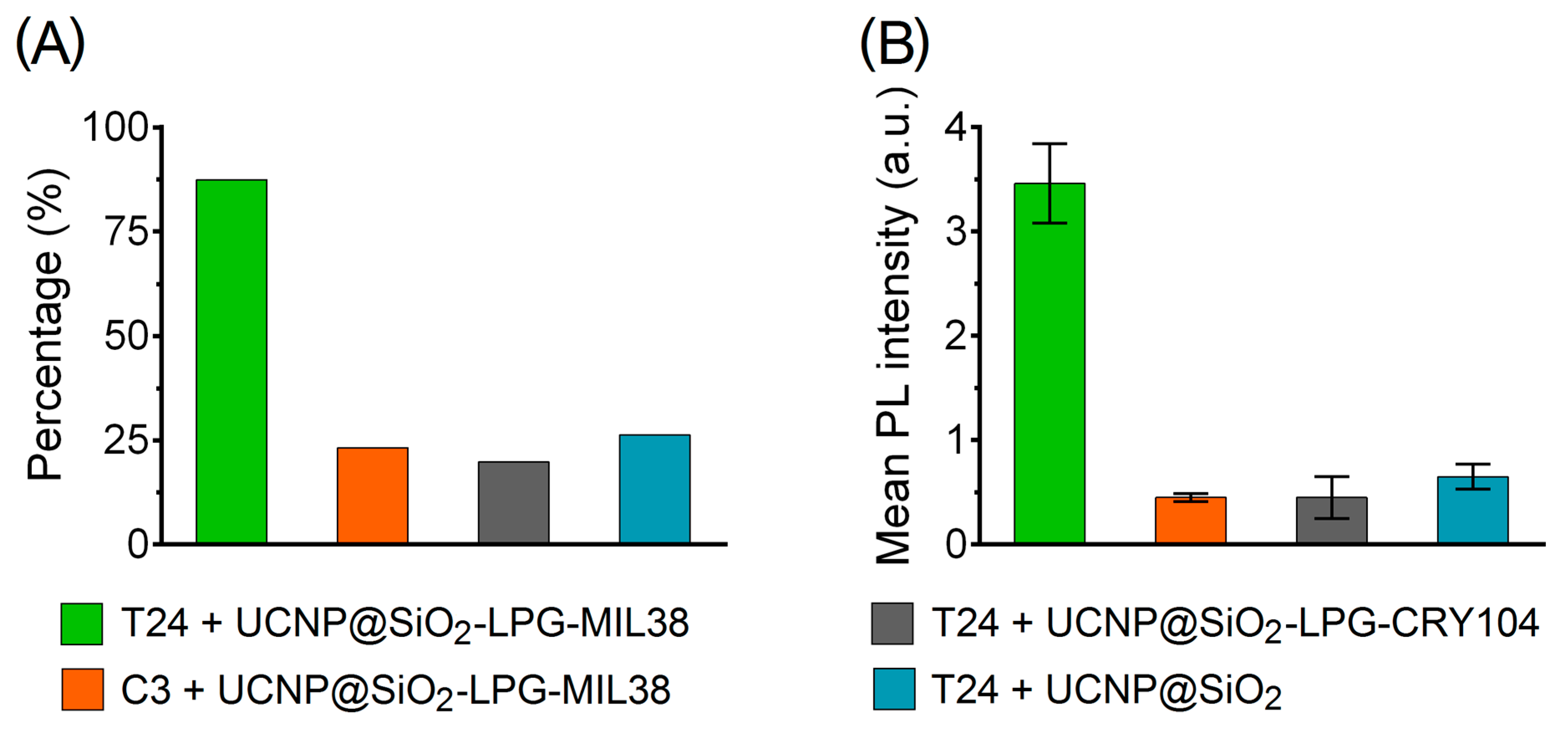
3.2. Interaction of UCNP Nanoconjugates with Urothelial Carcinoma Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.; Dinh, T.; Lee, J. The health economics of bladder cancer: An updated review of the published literature. Pharm. Econ. 2014, 32, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Zigeuner, R.; Shariat, S.F.; van Rhijn, B.W.; Compérat, E.; Sylvester, R.J.; Kaasinen, E.; Böhle, A.; Palou Redorta, J.; et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update 2013. Eur. Urol. 2013, 64, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Brausi, M.; Witjes, J.A.; Lamm, D.; Persad, R.; Palou, J.; Colombel, M.; Buckley, R.; Soloway, M.; Akaza, H.; Böhle, A. A review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the International Bladder Cancer Group. J. Urol. 2011, 186, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Green, D.A.; Rink, M.; Cha, E.K.; Xylinas, E.; Chughtai, B.; Scherr, D.S.; Shariat, S.F.; Lee, R.K. Cost-effective treatment of low-risk carcinoma not invading bladder muscle. BJU Int. 2013, 111, E78–E84. [Google Scholar] [CrossRef] [PubMed]
- Svatek, R.S.; Hollenbeck, B.K.; Holmäng, S.; Lee, R.; Kim, S.P.; Stenzl, A.; Lotan, Y.; Lotan, Y. The economics of bladder cancer: Costs and considerations of caring for this disease. Eur. Urol. 2014, 66, 253–262. [Google Scholar] [CrossRef]
- Sylvester, R.J.; van der Meijden, A.P.M.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.W.; Kurth, K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 2006, 49, 466–477. [Google Scholar] [CrossRef]
- Hemmings, B.A. Akt signaling: Linking membrane events to life and death decisions. Science 1997, 275, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Ellsworth, R.E.; Ellsworth, D.L.; Patney, H.L.; Deyarmin, B.; Love, B.; Hooke, J.A.; Shriver, C.D. Amplification of HER2 is a marker for global genomic instability. BMC Cancer 2008, 8, 297. [Google Scholar] [CrossRef]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Guryev, E.L.; Shilyagina, N.Y.; Kostyuk, A.B.; Sencha, L.M.; Balalaeva, I.V.; Vodeneev, V.A.; Kutova, O.M.; Lyubeshkin, A.V.; Yakubovskaya, R.I.; Pankratov, A.A.; et al. Preclinical Study of Biofunctional Polymer-Coated Upconversion Nanoparticles. Toxicol. Sci. 2019, 170, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Nadort, A.; Sreenivasan, V.K.; Song, Z.; Grebenik, E.A.; Nechaev, A.V.; Semchishen, V.A.; Panchenko, V.Y.; Zvyagin, A.V. Quantitative imaging of single upconversion nanoparticles in biological tissue. PLoS ONE 2013, 8, e63292. [Google Scholar] [CrossRef]
- Liu, Q.; Feng, W.; Yang, T.; Yi, T.; Li, F. Upconversion luminescence imaging of cells and small animals. Nat. Protoc. 2013, 8, 2033–2044. [Google Scholar] [CrossRef]
- Maeda, H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjug. Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef]
- Kutova, O.M.; Guryev, E.L.; Sokolova, E.A.; Alzeibak, R.; Balalaeva, I.V. Targeted Delivery to Tumors: Multidirectional Strategies to Improve Treatment Efficiency. Cancers 2019, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.P.; Shen, B.; Bu, W.; Chen, F.; He, Q.; Zhao, K.; Zhang, S.; Zhou, L.; Peng, W.; Xiao, Q.; et al. A smart upconversion-based mesoporous silica nanotheranostic system for synergetic chemo-/radio-/photodynamic therapy and simultaneous MR/UCL imaging. Biomaterials 2014, 35, 8992–9002. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.H.; Hu, Y.; Ma, X.; Wang, Y.; Fan, J.; Hu, X. A novel template-free and one-step method to fabricate hollow mesoporous structured upconversion luminescent NaYF4:Yb3+, Er3+ nanoparticles. Mater. Lett. 2014, 129, 107–110. [Google Scholar] [CrossRef]
- Zhou, A.G.; Wei, Y.C.; Wu, B.Y.; Chen, Q.; Xing, D. Pyropheophorbide A and c(RGDyK) Comodified Chitosan-Wrapped Upconversion Nanoparticle for Targeted Near-Infrared Photodynamic Therapy. Mol. Pharm. 2012, 9, 1580–1589. [Google Scholar] [CrossRef]
- Guryev, E.L.; Volodina, N.O.; Shilyagina, N.Y.; Gudkov, S.V.; Balalaeva, I.V.; Volovetskiy, A.B.; Lyubeshkin, A.V.; Sen’, A.V.; Ermilov, S.A.; Vodeneev, V.A.; et al. Radioactive (90Y) upconversion nanoparticles conjugated with recombinant targeted toxin for synergistic nanotheranostics of cancer. PNAS 2018, 115, 9690–9695. [Google Scholar] [CrossRef]
- Zeng, L.Y.; Luo, L.; Pan, Y.; Luo, S.; Lu, G.; Wu, A. In vivo targeted magnetic resonance imaging and visualized photodynamic therapy in deep-tissue cancers using folic acid-functionalized superparamagnetic-upconversion nanocomposites. Nanoscale 2015, 7, 8946–8954. [Google Scholar] [CrossRef]
- Zhang, P.; Steelant, W.; Kumar, M.; Scholfield, M. Versatile photosensitisers for photodynamic therapy at infrared excitation. J. Am. Chem Soc. 2007, 129, 4526–4527. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.Z.; Russell, P.J.; Kingsley, E.A.; Philips, J.; Raghavan, D. Detection of Malignant-Cells in Voided Urine from Patients with Bladder-Cancer, a Novel Monoclonal Assay. J. Urol. 1989, 142, 1578–1583. [Google Scholar] [CrossRef]
- Filmus, J.; Selleck, S.B. Glypicans: Proteoglycans with a surprise. J. Clin. Invest. 2001, 108, 497–501. [Google Scholar] [CrossRef]
- Truong, Q.; Justiniano, I.O.; Nocon, A.L.; Soon, J.T.; Wissmueller, S.; Campbell, D.H.; Walsh, B.J. Glypican-1 as a Biomarker for Prostate Cancer: Isolation and Characterization. J. Cancer 2016, 7, 1002–1009. [Google Scholar] [CrossRef][Green Version]
- Diamandis, E.P.; Plebani, M. Glypican-1 as a highly sensitive and specific pancreatic cancer biomarker. Clin. Chem Lab. Med. 2016, 54, e1–e2. [Google Scholar] [CrossRef]
- Hara, H.; Takahashi, T.; Serada, S.; Fujimoto, M.; Ohkawara, T.; Nakatsuka, R.; Harada, E.; Nishigaki, T.; Takahashi, Y.; Nojima, S.; et al. Overexpression of glypican-1 implicates poor prognosis and their chemoresistance in oesophageal squamous cell carcinoma. Br. J. Cancer 2016, 115, 66–75. [Google Scholar] [CrossRef]
- Matsuda, K.; Maruyama, H.; Guo, F.; Kleeff, J.; Itakura, J.; Matsumoto, Y.; Lander, A.D.; Korc, M. Glypican-1 is overexpressed in human breast cancer and modulates the mitogenic effects of multiple heparin-binding growth factors in breast cancer cells. Cancer Res. 2001, 61, 5562–5569. [Google Scholar]
- Su, G.; Meyer, K.; Nandini, C.D.; Qiao, D.; Salamat, S.; Friedl, A. Glypican-1 is frequently overexpressed in human gliomas and enhances FGF-2 signaling in glioma cells. Am. J. Pathol. 2006, 168, 2014–2026. [Google Scholar] [CrossRef]
- Care, A.; Bergquist, P.L.; Sunna, A. Solid-binding peptides: Smart tools for nanobiotechnology. Trends Biotechnol. 2015, 33, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Avvakumova, S.; Colombo, M.; Tortora, P.; Prosperi, D. Biotechnological approaches toward nanoparticle biofunctionalization. Trends Biotechnol. 2014, 32, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sunna, A.; Chi, F.; Bergquist, P.L. A linker peptide with high affinity towards silica-containing materials. New Biotechnol. 2013, 30, 485–492. [Google Scholar] [CrossRef]
- Shipunova, V.O.; Zelepukin, I.V.; Stremovskiy, O.A.; Nikitin, M.P.; Care, A.; Sunna, A.; Zvyagin, A.V.; Deyev, S.M. Versatile Platform for Nanoparticle Surface Bioengineering Based on SiO2-Binding Peptide and Proteinaceous Barnase*Barstar Interface. ACS Appl. Mater. Interfaces 2018, 10, 17437–17447. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zhang, Y.; Jiang, S. Multicolor Core/Shell-Structured Upconversion Fluorescent Nanoparticles. Adv. Mater. 2009, 21, 4768. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Xing, H.; Bu, W.; Zhang, S.; Zheng, X.; Li, M.; Chen, F.; He, Q.; Zhou, L.; Peng, W.; Hua, Y.; et al. Multifunctional nanoprobes for upconversion fluorescence, MR and CT trimodal imaging. Biomaterials 2012, 33, 1079–1089. [Google Scholar] [CrossRef]
- Russell, P.J.; Ow, K.T.; Tam, P.N.; Juarez, J.; Kingsley, E.A.; Qu, C.F.; Li, Y.; Cozzi, P.J.; Martiniello-Wilks, R. Immunohistochemical characterisation of the monoclonal antibody BLCA-38 for the detection of prostate cancer. Cancer Immunol. Immun. 2004, 53, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Ding, L.; Tao, W.; Yang, X.; Qian, H.; Yao, R. Mesoporous-silica-coated upconversion nanoparticles loaded with vitamin B-12 for near-infrared-light mediated photodynamic therapy. Mater. Lett. 2016, 167, 205–208. [Google Scholar] [CrossRef]
- Liu, J.N.; Bu, W.B.; Shi, J.L. Silica Coated Upconversion Nanoparticles: A Versatile Platform for the Development of Efficient Theranostics. Account. Chem. Res. 2015, 48, 1797–1805. [Google Scholar] [CrossRef]
- Liang, L.; Care, A.; Qian, Y.; Zhang, R.; Packer, N.H.; Sunna, A.; Lu, Y.; Zvyagin, A.V. Facile Assembly of Functional Upconversion Nanoparticles for Targeted Cancer Imaging and Photodynamic Therapy. Acs Appl. Mater. Inter. 2016, 8, 11945–11953. [Google Scholar] [CrossRef]
- Care, A.; Chi, F.; Bergquist, P.L.; Sunna, A. Biofunctionalization of silica-coated magnetic particles mediated by a peptide. J. Nanopart. Res. 2014, 16, 2543. [Google Scholar] [CrossRef]
- Jacobs, M.; Selvam, A.P.; Craven, J.E.; Prasad, S. Antibody-Conjugated Gold Nanoparticle Based Immunosensor for Ultra-Sensitive Detection of Troponin-T. J. Lab. Autom. 2014, 19, 546–554. [Google Scholar] [CrossRef]
- Mironova, K.E.; Khochenkov, D.A.; Generalova, A.N.; Rocheva, V.V.; Sholina, N.V.; Nechaev, A.V.; Semchishen, V.A.; Deyev, S.M.; Zvyagin, A.V.; Khaydukov, E.V. Ultraviolet phototoxicity of upconversion nanoparticles illuminated with near-infrared light. Nanoscale 2017, 9, 14921. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer active targeting by nanoparticles: A comprehensive review of literature. J. Cancer Res. Clin. Oncol. 2015, 141, 769–784. [Google Scholar] [CrossRef]
- Steinhauser, I.; Spankuch, B.; Strebhardt, K.; Langer, K. Trastuzumab-modified nanoparticles: Optimisation of preparation and uptake in cancer cells. Biomaterials 2006, 27, 4975–4983. [Google Scholar] [CrossRef]
| UCNP/Nanoconjugate | Zeta Potential, mV |
|---|---|
| UCNP@SiO2 | −16.6 |
| UCNP@SiO2-LPG | −9.65 |
| UCNP@SiO2-LPG-MIL-38 | −5.75 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polikarpov, D.; Liang, L.; Care, A.; Sunna, A.; Campbell, D.; Walsh, B.; Balalaeva, I.; Zvyagin, A.; Gillatt, D.; Guryev, E. Functionalized Upconversion Nanoparticles for Targeted Labelling of Bladder Cancer Cells. Biomolecules 2019, 9, 820. https://doi.org/10.3390/biom9120820
Polikarpov D, Liang L, Care A, Sunna A, Campbell D, Walsh B, Balalaeva I, Zvyagin A, Gillatt D, Guryev E. Functionalized Upconversion Nanoparticles for Targeted Labelling of Bladder Cancer Cells. Biomolecules. 2019; 9(12):820. https://doi.org/10.3390/biom9120820
Chicago/Turabian StylePolikarpov, Dmitry, Liuen Liang, Andrew Care, Anwar Sunna, Douglas Campbell, Bradley Walsh, Irina Balalaeva, Andrei Zvyagin, David Gillatt, and Evgenii Guryev. 2019. "Functionalized Upconversion Nanoparticles for Targeted Labelling of Bladder Cancer Cells" Biomolecules 9, no. 12: 820. https://doi.org/10.3390/biom9120820
APA StylePolikarpov, D., Liang, L., Care, A., Sunna, A., Campbell, D., Walsh, B., Balalaeva, I., Zvyagin, A., Gillatt, D., & Guryev, E. (2019). Functionalized Upconversion Nanoparticles for Targeted Labelling of Bladder Cancer Cells. Biomolecules, 9(12), 820. https://doi.org/10.3390/biom9120820










