Retinol Saturase Knock-Out Mice are Characterized by Impaired Clearance of Apoptotic Cells and Develop Mild Autoimmunity
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Animals
2.3. Flow Cytometry Analysis of Freshly Isolated Thymocytes
2.4. Retinoid Measurement by High-performance Liquid Chromatography Mass Spectrometry (HPLC/MS/MS)
2.5. Thymocyte Apoptosis in Vitro
2.6. Bone-Marrow-Derived (BMDM), Peritoneal, or Thioglycolate-elicited Macrophage Generation, Cell Culture, and Treatment
2.7. In Vitro Apoptotic Cell Phagocytosis
2.8. Confocal Microscopy
2.9. mRNA Sequencing
2.10. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis of mRNA Expression
2.11. Anti-Nuclear Antibody Detection by Indirect Immunofluorescence Assay
2.12. Anti-dsDNA Antibody ELISA
2.13. Caspase-3 Immunohistochemistry
2.14. Detection of IgM-Containing Immune Complexes
2.15. Determination of Serum Urea Concentration
2.16. Statistical Analysis
3. Results
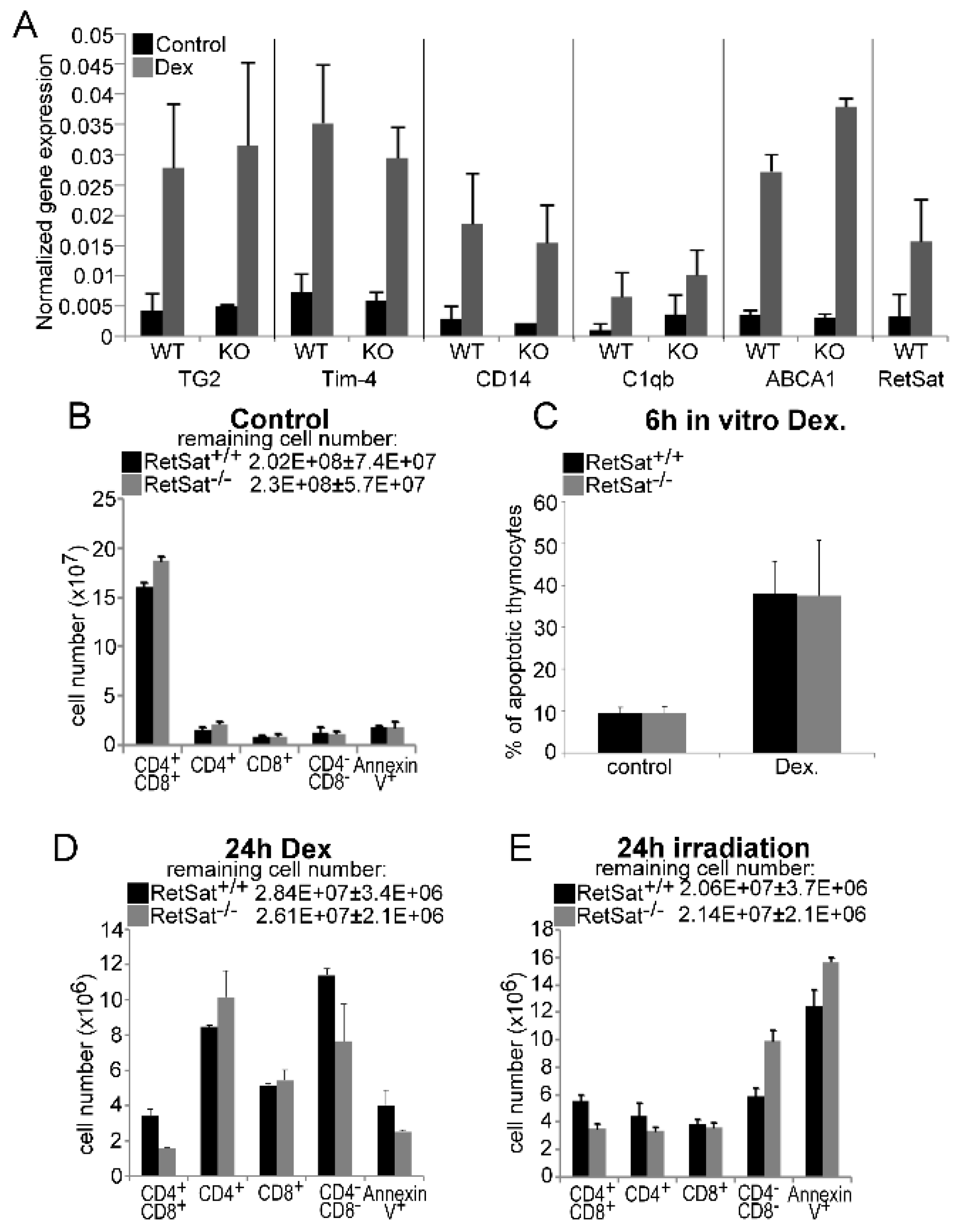
3.1. Loss of Retinol Saturase Does Not Affect the Induction of Retinoid-Regulated Genes or the Thymic Apopto-Phagocytosis Program
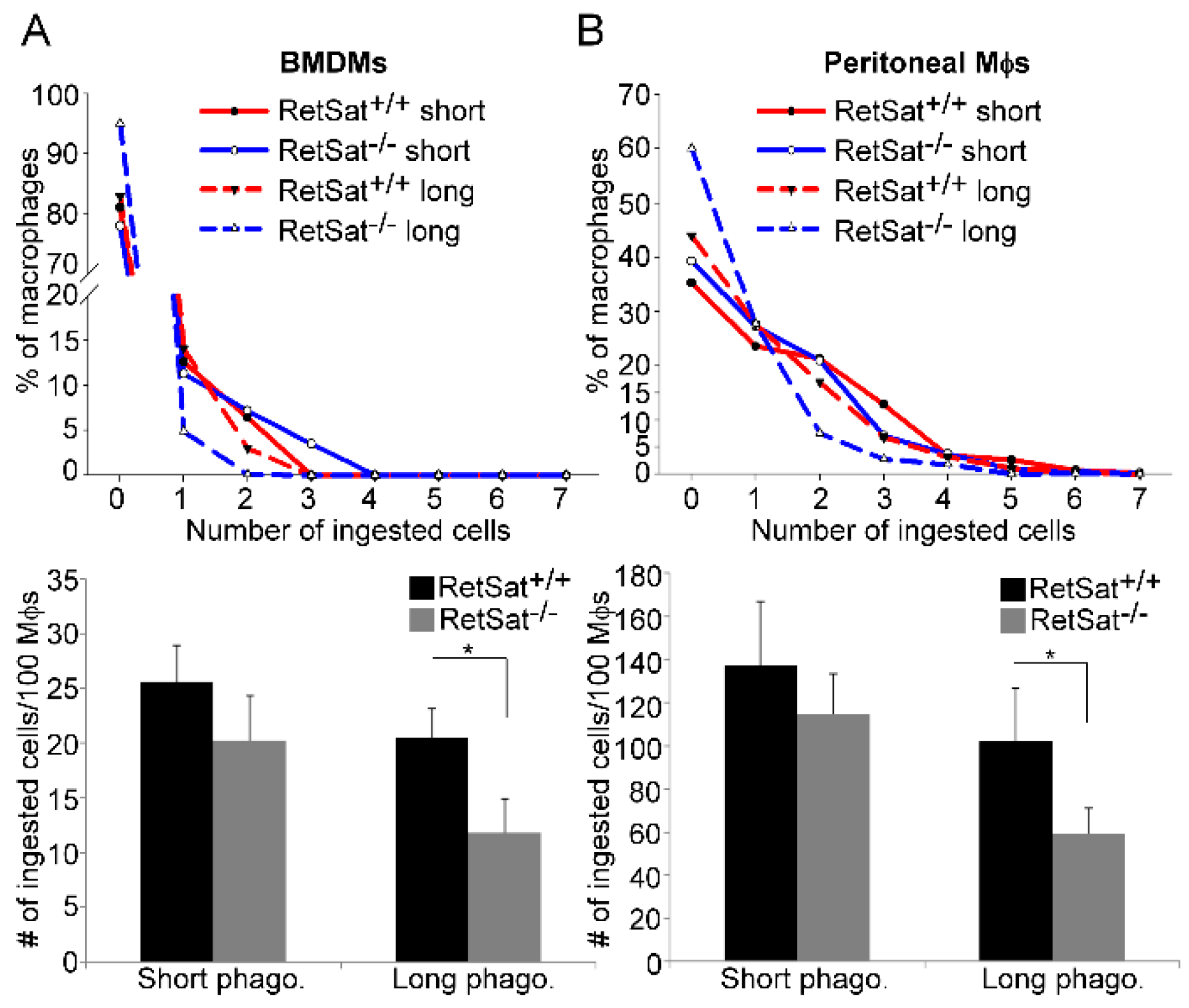
3.2. RetSat-Null Macrophages Are Characterized by Impaired Long-Term Phagocytosis
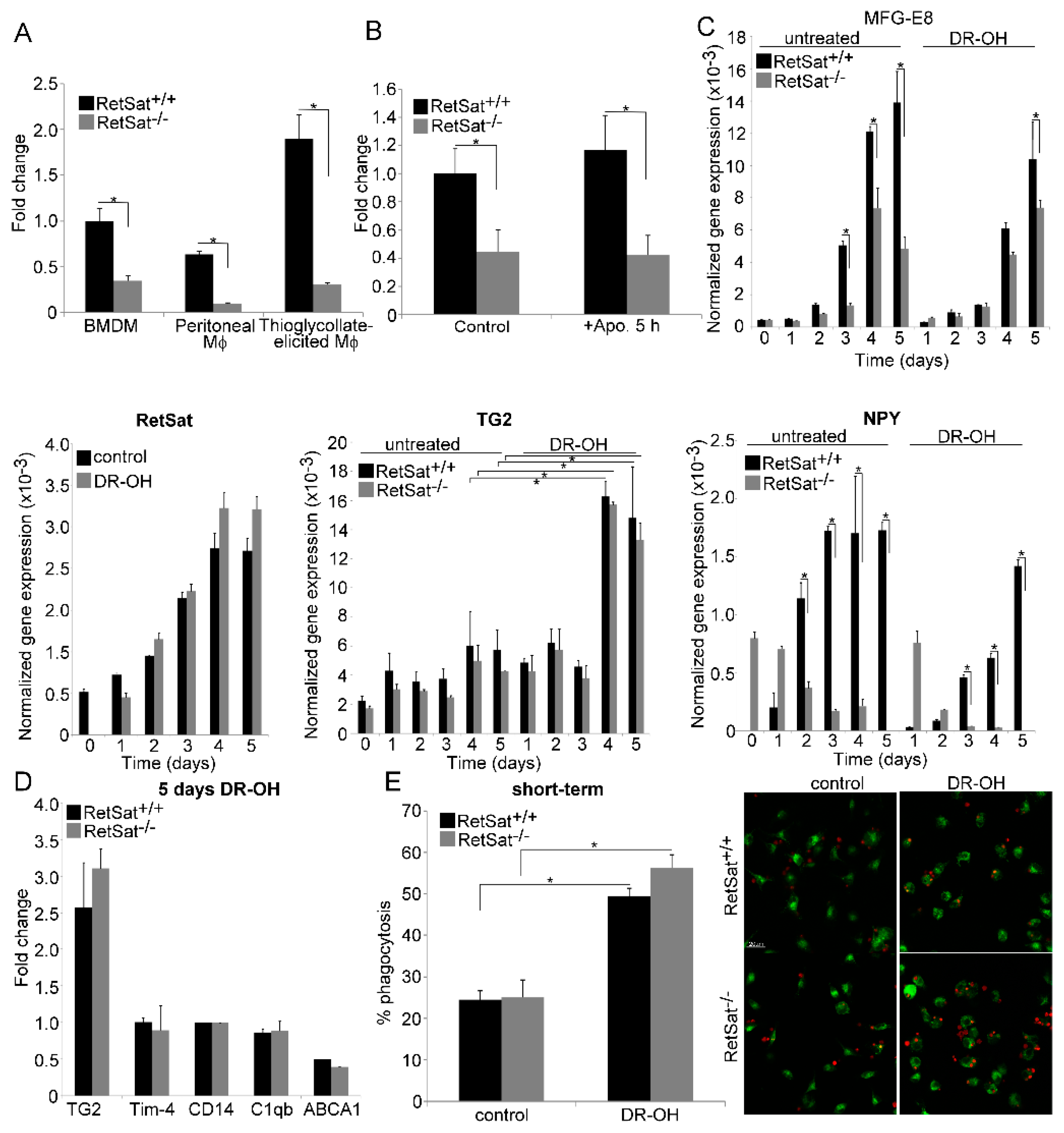
3.3. RetSat-Null Macrophages Express Less MFG-E8
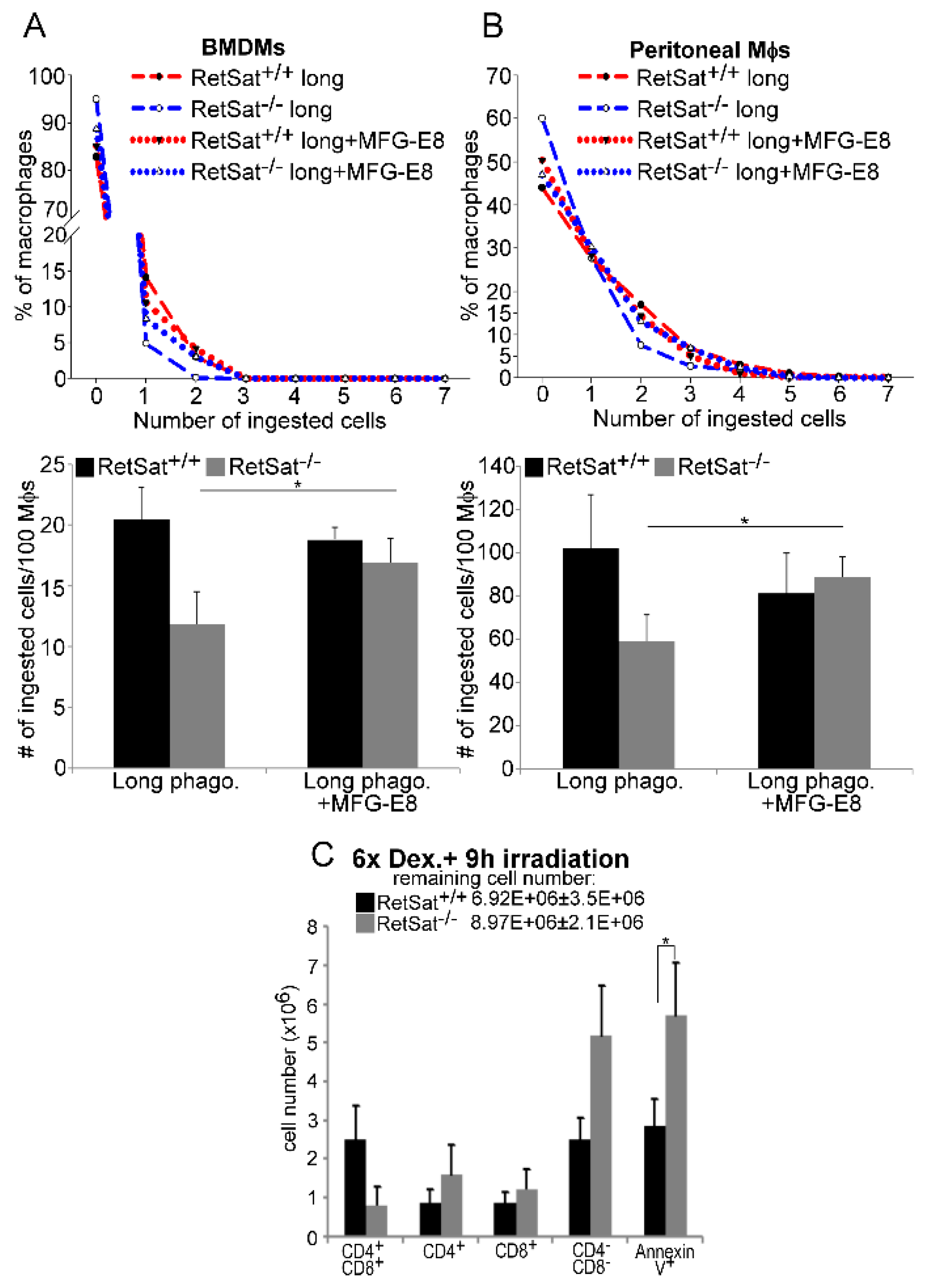
3.4. Lower MFG-E8 Production Is Responsible for the Defect in Long-Term Phagocytosis of RetSat-Null Macrophages
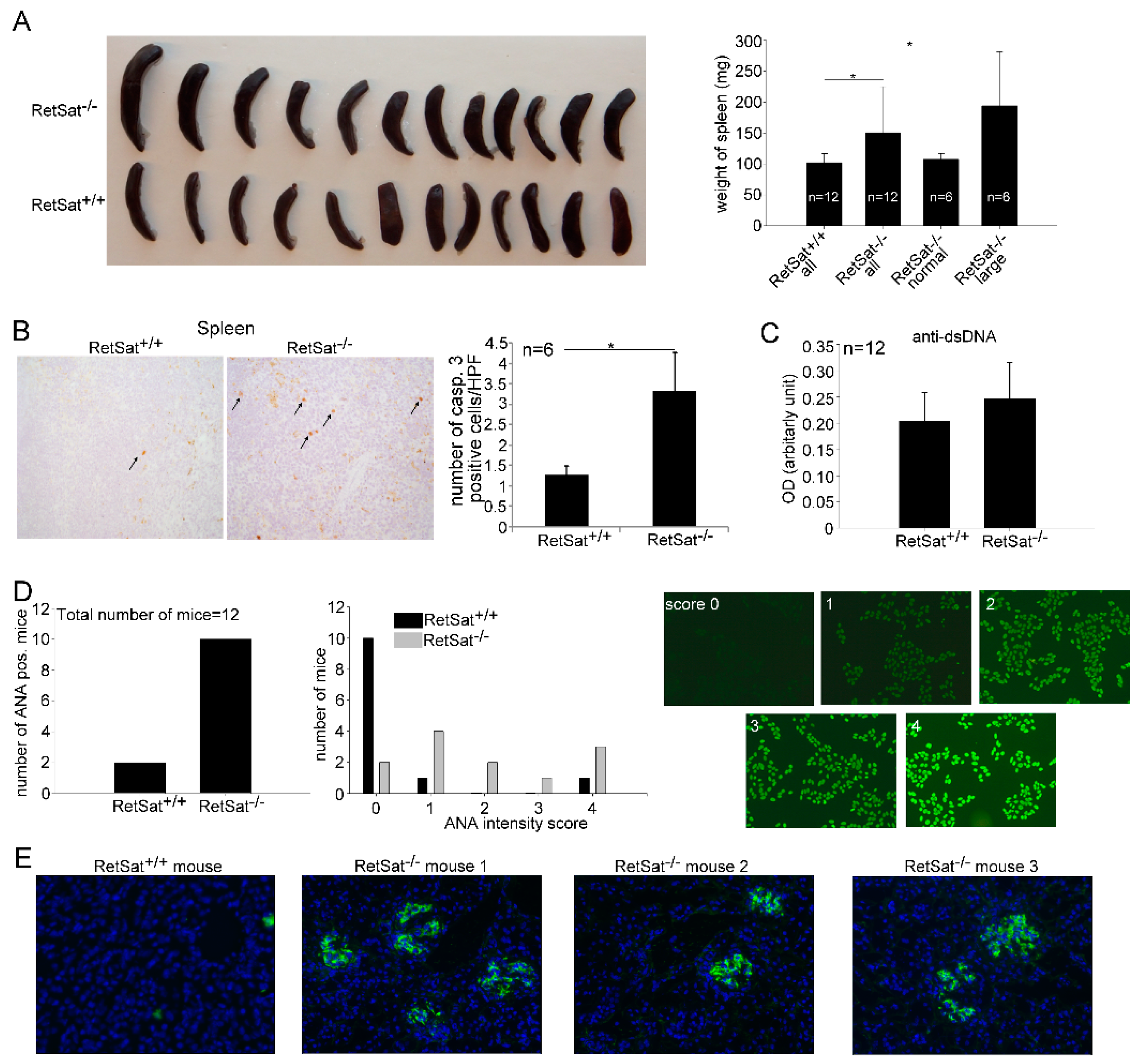
3.5. Female RetSat-Null Mice Are Prone To Develop Mild Systemic Lupus Erythematosus (SLE)-Like Autoimmunity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arandjelovic, S.; Ravichandran, K.S. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 2015, 16, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Henson, P.M. Apoptosis: Giving phosphatidylserine recognition an assist--with a twist. Curr. Biol. 2003, 13, R655–R657. [Google Scholar] [CrossRef]
- Park, D.; Tosello-Trampont, A.C.; Elliott, M.R.; Lu, M.; Haney, L.B.; Ma, Z.; Klibanov, A.L.; Mandell, J.W.; Ravichandran, K.S. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 2007, 450, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jung, M.Y.; Kim, H.J.; Lee, S.J.; Kim, S.Y.; Lee, B.H.; Kwon, T.H.; Park, R.W.; Kim, I.S. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008, 15, 192–201. [Google Scholar] [CrossRef]
- Miyanishi, M.; Tada, K.; Koike, M.; Uchiyama, Y.; Kitamura, T.; Nagata, S. Identification of Tim4 as a phosphatidylserine receptor. Nature 2007, 450, 435–439. [Google Scholar] [CrossRef]
- Hanayama, R.; Tanaka, M.; Miwa, K.; Shinohara, A.; Iwamatsu, A.; Nagata, S. Identification of a factor that links apoptotic cells to phagocytes. Nature 2002, 417, 182–187. [Google Scholar] [CrossRef]
- Savill, J.S.; Hogg, N.; Ren, Y.; Haslett, C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J. Clin. Invest. 1992, 90, 1513–1522. [Google Scholar] [CrossRef]
- Stitt, T.N.; Conn, G.; Gore, M.; Lai, C.; Bruno, J.; Radziejewski, C.; Mattsson, K.; Fisher, J.; Gies, D.R.; Jones, P.F.; et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell 1995, 80, 661–670. [Google Scholar] [CrossRef]
- Botto, M.; Dell’Agnola, C.; Bygrave, A.E.; Thompson, E.M.; Cook, H.T.; Petry, F.; Loos, M.; Pandolfi, P.P.; Walport, M.J. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 1998, 19, 56–59. [Google Scholar] [CrossRef]
- Park, D.; Hochreiter-Hufford, A.; Ravichandran, K.S. The phosphatidylserine receptor TIM-4 does not mediate direct signaling. Curr. Biol. 2009, 19, 346–351. [Google Scholar] [CrossRef]
- Devitt, A.; Parker, K.G.; Ogden, C.A.; Oldreive, C.; Clay, M.F.; Melville, L.A.; Bellamy, C.O.; Lacy-Hulbert, A.; Gangloff, S.C.; Goyert, S.M.; et al. Persistence of apoptotic cells without autoimmune disease or inflammation in CD14−/− mice. J. Cell. Biol. 2004, 167, 1161–1170. [Google Scholar] [CrossRef]
- Greenberg, M.E.; Sun, M.; Zhang, R.; Febbraio, M.; Silverstein, R.; Hazen, S.L. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J. Exp. Med. 2006, 203, 2613–2625. [Google Scholar] [CrossRef]
- Cohen, P.L.; Caricchio, R.; Abraham, V.; Camenisch, T.D.; Jennette, J.C.; Roubey, R.A.; Earp, H.S.; Matsushima, G.; Reap, E.A. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-Mertk membrane tyrosine kinase. J. Exp. Med. 2002, 196, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Tóth, B.; Garabuczi, E.; Sarang, Z.; Vereb, G.; Vámosi, G.; Aeschlimann, D.; Blaskó, B.; Bécsi, B.; Erdődi, F.; Lacy-Hulbert, A.; et al. Transglutaminase 2 is needed for the formation of an efficient phagocyte portal in macrophages engulfing apoptotic cells. J. Immunol. 2009, 182, 2084–2092. [Google Scholar] [CrossRef] [PubMed]
- Kinchen, J.M.; Cabello, J.; Klingle, D.; Wong, K.; Freichtinger, R.; Schnabel, H.; Schnabel, R.; Hengartner, M.O. Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans, Nature 2005, 43, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Miksa, M.; Amin, D.; Wu, R.; Jacob, A.; Zhou, M.; Dong, W.; Yang, W.L.; Ravikumar, T.S.; Wang, P. Maturation-induced down-regulation of MFG-E8 impairs apoptotic cell clearance and enhances endotoxin response. Int. J. Mol. Med. 2008, 22, 743–748. [Google Scholar] [PubMed]
- Noelia, A.; Bensinger, S.J.; Hong, C.; Beceiro, S.; Bradley, M.N.; Zelcer, N.; Deniz, J.; Ramirez, C.; Díaz, M.; Gallardo, G.; et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity 2009, 31, 245–258. [Google Scholar] [CrossRef]
- Roszer, T.; Menéndez-Gutiérrez, M.P.; Lefterova, M.I.; Alameda, D.; Núñez, V.; Lazar, M.A.; Fischer, T.; Ricote, M. Autoimmune kidney disease and impaired engulfment of apoptotic cells in mice with macrophage peroxisome proliferator-activated receptor gamma or retinoid X receptor alpha deficiency. J. Immunol. 2011, 186, 621–631. [Google Scholar] [CrossRef]
- Mukundan, L.; Odegaard, J.I.; Morel, C.R.; Heredia, J.E.; Mwangi, J.W.; Ricardo-Gonzalez, R.R.; Goh, Y.P.; Eagle, A.R.; Dunn, S.E.; Awakuni, J.U.; et al. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat. Med. 2009, 15, 1266–1272. [Google Scholar] [CrossRef]
- Garabuczi, É.; Kiss, B.; Felszeghy, S.; Tsay, G.J.; Fésüs, L.; Szondy, Z. Retinoids produced by macrophages engulfing apoptotic cells contribute to the appearance of transglutaminase 2 in apoptotic thymocytes. Amino Acids 2013, 44, 235–244. [Google Scholar] [CrossRef]
- Sarang, Z.; Joós, G.; Garabuczi, É.; Rühl, R.; Gregory, C.D.; Szondy, Z. Macrophages engulfing apoptotic cells produce nonclassical retinoids to enhance their phagocytic capacity. J. Immunol. 2014, 192, 5730–5738. [Google Scholar] [CrossRef] [PubMed]
- Moise, A.R.; Domínguez, M.; Alvarez, S.; Alvarez, R.; Schupp, M.; Cristancho, A.G.; Kiser, P.D.; de Lera, A.R.; Lazar, M.A.; Palczewski, K. Stereospecificity of retinol saturase: Absolute configuration, synthesis, and biological evaluation of dihydroretinoids. J. Am. Chem. Soc. 2008, 130, 1154–1155. [Google Scholar] [CrossRef] [PubMed]
- Moise, A.R.; Alvarez, S.; Domínguez, M.; Alvarez, R.; Golczak, M.; Lobo, G.P.; von Lintig, J.; de Lera, A.R.; Palczewski, K. Activation of retinoic acid receptors by dihydroretinoids. Mol. Pharmacol. 2009, 76, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Krężel, W.; Rühl, R.; de Lera, A.R. Alternative retinoid X receptor (RXR) ligands. Mol. Cell. Endocrinol. 2019, 491, 110436. [Google Scholar] [CrossRef] [PubMed]
- Schupp, M.; Lefterova, M.; Janke, J.; Leitner, K.; Cristancho, A.G.; Mullican, S.E.; Qatanani, M.; Szwergold, N.; Steger, D.J.; Curtin, J.C.; et al. Retinol saturase promotes adipogenesis and is downregulated in obesity. Proc. Natl Acad. Sci. USA. 2009, 106, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.Y.; Wang, S.; Jurczak, M.J.; Shulman, G.I.; Moise, A.R. Retinolsaturase modulates lipid metabolism and the production of reactive oxygen species. Arch. Biochem. Biophys. 2017, 633, 93–102. [Google Scholar] [CrossRef]
- Heidenreich, S.; Witte, N.; Weber, P.; Goehring, I.; Tolkachov, A.; von Loeffelholz, C.; Döcke, S.; Bauer, M.; Stockmann, M.; Pfeiffer, A.F.H.; et al. Retinolsaturase coordinates liver metabolism by regulating ChREBP activity. Nat. Commun. 2017, 8, 384. [Google Scholar] [CrossRef]
- Moise, A.R.; Lobo, G.P.; Erokwu, B.; Wilson, D.L.; Peck, D.; Alvarez, S.; Domínguez, M.; Alvarez, R.; Flask, C.A.; de Lera, A.R.; et al. Increased adiposity in the retinol saturase-knockout mouse. FASEB J. 2010, 24, 1261–1270. [Google Scholar] [CrossRef]
- Lauber, K.; Keppeler, H.; Munoz, L.E.; Koppe, U.; Schröder, K.; Yamaguchi, H.; Krönke, G.; Uderhardt, S.; Wesselborg, S.; Belka, C.; et al. Milk fat globule-EGF factor 8 mediates the enhancement of apoptotic cell clearance by glucocorticoids. Cell Death Differ. 2013, 20, 1230–1240. [Google Scholar] [CrossRef]
- Rühl, R. Method to determine 4-oxo-retinoic acids, retinoic acids and retinol in serum and cell extracts by liquid chromatography/diode-array detection atmospheric pressure chemical ionisation tandem mass spectrometry. Rapid. Commun. Mass Spectrum 2006, 20, 2497–2504. [Google Scholar] [CrossRef]
- Rahmatullah, M.; Boyde, T.R. Improvements in the determination of urea using diacetyl monoxime; methods with and without deproteinisation. Clin. Chim. Acta. 1980, 107, 3–9. [Google Scholar] [CrossRef]
- Starr, T.K.; Jameson, S.C.; Hogquist, K.A. Positive and negative selection of T Cells. Ann. Rev. Immunol. 2003, 21, 139–176. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Konkel, J.E. Development of thymic Foxp3(+) regulatory T cells: TGF-β matters. Eur. J. Immunol. 2015, 45, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Sarang, Z.; Garabuczi, É.; Joós, G.; Kiss, B.; Tóth, K.; Rühl, R.; Szondy, Z. Macrophages engulfing apoptotic thymocytes produce retinoids to promote selection, differentiation, removal and replacement of double positive thymocytes. Immunobiology 2013, 218, 1354–1360. [Google Scholar] [CrossRef]
- Buttari, B.; Profumo, E.; Darcangelo, D.; Di Raimo, T.; Businaro, R.; Capoano, R.; Salvati, B.; Saso, L.; Elenkov, I. Neuropeptide Y as regulator of macrophage phenotype and functions: A neuroimmune CUE in atherosclerosis regression? Atherosclerosis 2017, 263, e2. [Google Scholar] [CrossRef]
- Zizzo, G.; Hilliard, B.A.; Monestier, M.; Cohen, P.L. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J. Immunol. 2012, 189, 3508–3520. [Google Scholar] [CrossRef]
- Pedragosa-Badia, X.; Stichel, J.; Beck-Sickinger, A.G. Neuropeptide Y receptors: How to get subtype selectivity. Front. Endocrinol. 2013, 4, 5. [Google Scholar] [CrossRef]
- Hanayama, R.; Tanaka, M.; Miyasak, K.; Aozasa, K.; Koike, M.; Uchiyama, Y.; Nagata, S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 2004, 304, 1147–1150. [Google Scholar] [CrossRef]
- Abdolmaleki, F.; Farahani, N.; Gheibi Hayat, S.M.; Pirro, M.; Bianconi, V.; Barreto, G.E.; Sahebkar, A. The Role of Efferocytosis in Autoimmune Diseases. Front. Immunol. 2018, 9, 1645. [Google Scholar] [CrossRef]
- Mevorach, D.; Zhou, J.L.; Song, X.; Elkon, K.B. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J. Exp. Med. 1998, 188, 387–392. [Google Scholar] [CrossRef]
- Weckerle, C.E.; Niewold, T.B. The Unexplained Female Predominance of Systemic Lupus Erythematosus: Clues from Genetic and Cytokine Studies. Clin. Rev. Allergy Immunol. 2011, 40, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Szondy, Z.; Garabuczi, E.; Joós, G.; Tsay, G.J.; Sarang, Z. Impaired clearance of apoptotic cells in chronic inflammatory diseases: Therapeutic implications. Front. Immunol. 2014, 5, 354. [Google Scholar] [CrossRef] [PubMed]
- GeneAtlas MOE430, gcrma. Available online: http://ds.biogps.org/?dataset=GSE10246&gene=67442 (accessed on 14 October 2019).
- Matsuda, A.; Jacob, A.; Wu, R.; Zhou, M.; Nicastro, J.M.; Coppa, G.F.; Wang, P. Milk fat globule-EGF factor VIII in sepsis and ischemia-reperfusion injury. Mol. Med. 2011, 17, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Michalski, M.N.; Seydel, A.L.; Siismets, E.M.; Zweifler, L.E.; Koh, A.J.; Sinder, B.P.; Aguirre, J.I.; Atabai, K.; Roca, H.; McCauley, L.K. Inflammatory bone loss associated with MFG-E8 deficiency is rescued by teriparatide. FASEB J. 2018, 32, 3730–3741. [Google Scholar] [CrossRef]
- Szondy, Z.; Sarang, Z.; Molnar, P.; Nemeth, T.; Piacentini, M.; Mastroberardino, P.G.; Falasca, L.; Aeschlimann, D.; Kovacs, J.; Kiss, I.; et al. Transglutaminase 2−/− mice reveal a phagocytosis-associated crosstalk between macrophages and apoptotic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 7812–7817. [Google Scholar] [CrossRef]
- Dimitrijević, M.; Stanojević, S.; Mitić, K.; Kustrimović, N.; Vujić, V.; Miletić, T.; Kovacević-Jovanović, V. The anti-inflammatory effect of neuropeptide Y (NPY) in rats is dependent on dipeptidyl peptidase 4 (DP4) activity and age. Peptides 2008, 29, 2179–2187. [Google Scholar]
- Soki, F.N.; Koh, A.J.; Jones, J.D.; Kim, Y.W.; Dai, J.; Keller, E.T.; Pienta, K.J.; Atabai, K.; Roca, H.; McCauley, L.K. Polarization of prostate cancer-associated macrophages is induced by milk fat globule-EGF factor 8 (MFG-E8)-mediated efferocytosis. J. Biol. Chem. 2014, 289, 24560–24572. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef]
- Jinushi, M.; Nakazaki, Y.; Dougan, M.; Carrasco, D.R.; Mihm, M.; Dranoff, G. MFG-E8-mediated uptake of apoptotic cells by APCs links the pro- and antiinflammatory activities of GM-CSF. J. Clin. Invest. 2007, 117, 1902–1913. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar] [CrossRef]
- Yan, L.; Singh, L.S.; Zhang, L.; Xu, Y. Role of OGR1 in myeloid-derived cells in prostate cancer. Oncogene 2014, 33, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Khalifeh-Soltani, A.; McKleroy, W.; Sakuma, S.; Cheung, Y.Y.; Tharp, K.; Qiu, Y.; Turner, S.M.; Chawla, A.; Stahl, A.; Atabai, K. Mfge8 promotes obesity by mediating the uptake of dietary fats and serum fatty acids. Nat. Med. 2014, 20, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Khalifeh-Soltani, A.; Gupta, D.; Ha, A.; Iqbal, J.; Hussain, M.; Podolsky, M.J.; Atabai, K. Mfge8 regulates enterocyte lipid storage by promoting enterocyte triglyceride hydrolase activity. JCI Insight. 2016, 1, e87418. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, S.E.; Grijalva, A.; Xu, X.; Ables, E.; Nomani, A.; Ferrante, A.W. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science 2019, 363, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell. Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.P.; Boyd, A.S.; Fugazza, C.; May, G.E.; Guo, Y.; Tipping, A.J.; Scadden, D.T.; Vyas, P.; Enver, T. GATA-2 regulates granulocyte-macrophage progenitor cell function. Blood 2008, 112, 4862–4873. [Google Scholar] [CrossRef] [PubMed]
- Rui, W.; Jin, Z.; Zhe, G.; Song, H. The methylation of C/EBP β gene promoter and regulated by GATA-2 protein. Mol. Biol. Rep. 2013, 40, 797–801. [Google Scholar] [CrossRef]
- Aziz, M.M.; Ishihara, S.; Rumi, M.A.; Mishima, Y.; Oshima, N.; Kadota, C.; Moriyama, I.; Li, Y.Y.; Rahman, F.B.; Otani, A.; et al. Prolactin induces MFG-E8 production in macrophages via transcription factor C/EBPbeta-dependent pathway. Apoptosis. 2008, 13, 609–620. [Google Scholar] [CrossRef]
- Sawatani, Y.; Miyamoto, T.; Nagai, S.; Maruya, M.; Imai, J.; Miyamoto, K.; Fujita, N.; Ninomiya, K.; Suzuki, T.; Iwasaki, R.; et al. The role of DC-STAMP in maintenance of immune tolerance through regulation of dendritic cell function. Int. Immunol. 2008, 20, 1259–1268. [Google Scholar] [CrossRef]
| Gene Symbol | FC | p (Corr) | Gene Symbol | FC | p (Corr) |
|---|---|---|---|---|---|
| Npy | −742.1 | 1.6 × 10−05 | Dgat2 | 19.0 | 1.3 × 10−04 |
| 1700112E06Rik | −9.2 | 1.4 × 10−04 | Nbea | 8.2 | 6.9 × 10−04 |
| Rtn4rl2 | −5.4 | 2.6 × 10−02 | Morn4 | 7.8 | 3.8 × 10−06 |
| Dcstamp | −4.7 | 1.4 × 10−02 | 2410066E13Rik | 7.4 | 1.3 × 10−06 |
| Itgb7 | −4.7 | 2.0 × 10−02 | Fah | 6.7 | 7.2 × 10−08 |
| Gata2 | −4.5 | 1.5 × 10−02 | Hddc3 | 5.9 | 7.1 × 10−07 |
| Wdr54 | −3.8 | 1.4× 10−02 | Wdfy1 | 5.8 | 2.2 × 10−07 |
| Elmod3 | −3.2 | 3.2 × 10−04 | Dkk2 | 4.9 | 2.7 × 10−05 |
| Gm15446 | −2.9 | 5.3 × 10−03 | Cdo1 | 4.4 | 1.9 × 10−04 |
| Gm7799 | −2.9 | 5.0 × 10−02 | Sh3d21 | 4.4 | 6.8 × 10−06 |
| Gpr68 | −2.9 | 4.8 × 10−02 | Slco3a1 | 4.3 | 8.4 × 10−09 |
| Krt80 | −2.9 | 2.5 × 10−02 | AC166773.1 | 3.9 | 4.1 × 10−06 |
| Mfge8 | −2.7 | 2.4 × 10−02 | Gbp10 | 3.6 | 1.7 × 10−03 |
| Pde1c | −2.6 | 7.1 × 10−03 | Gpx3 | 3.5 | 2.6 × 10−04 |
| Galnt9 | −2.5 | 3.5 × 10−02 | Gm13014 | 3.5 | 3.0 × 10−04 |
| Gm12895 | −2.5 | 2.9 × 10−02 | Camk2b | 3.5 | 1.6 × 10−03 |
| Gm14109 | −2.5 | 2.6 × 10−03 | Pde2a | 3.2 | 4.4 × 10−07 |
| Gm9746 | −2.4 | 2.5 × 10−04 | Lrrc9 | 3.2 | 7.3 × 10−04 |
| Kalrn | −2.4 | 4.0 × 10−02 | Itm2a | 3.0 | 2.9 × 10−04 |
| Il1rn | −2.4 | 1.8 × 10−02 | Xkr6 | 2.9 | 7.7 × 10−04 |
| Itpka | −2.3 | 5.4 × 10−05 | Rab4a | 2.7 | 5.3 × 10−07 |
| Dnajb13 | −2.3 | 3.5 × 10−02 | Pyroxd2 | 2.7 | 8.0 × 10−04 |
| Tnip3 | −2.2 | 1.0 × 10−02 | AC123679.1 | 2.6 | 8.2 × 10−06 |
| Gpc1 | −2.2 | 2.0 × 10−02 | Efcab7 | 2.4 | 3.6 × 10−04 |
| RP24-281K23.1 | −2.1 | 7.1 × 10−03 | Prdm5 | 2.3 | 1.7 × 10−04 |
| Gm3788 | −2.1 | 1.0 × 10−02 | Mageh1 | 2.2 | 9.8 × 10−04 |
| Gm9844 | −2.1 | 1.0 × 10−02 | Mlph | 2.2 | 1.5 × 10−03 |
| Tmsb10 | −2.1 | 1.9 × 10−02 | Gm4772 | 2.2 | 3.8 × 10−04 |
| 4931413K12Rik | −2.0 | 1.4 × 10−02 | Nnt | 2.1 | 7.3 × 10−07 |
| D14Ertd449e | −2.0 | 1.8 × 10−03 | Dhcr24 | 2.1 | 5.0 × 10−05 |
| RetSat | −2.0 | 2.9 × 10−04 | Syp | 2.1 | 1.6 × 10−03 |
| RP23-291E6.5 | −2.0 | 2.9 × 10−02 | Klra3 | 2.1 | 1.4 × 10−05 |
| Itgax | −1.9 | 4.0 × 10−02 | B130055M24Rik | 2.0 | 6.3 × 10−04 |
| Ak4 | −1.9 | 3.0 × 10−03 | C1qtnf6 | 2.0 | 6.1 × 10−05 |
| Csf1 | −1.9 | 1.4 × 10−02 | Pdgfc | 2.0 | 7.6 × 10−05 |
| Tiam1 | −1.9 | 3.3 × 10−02 | AL627077.2 | 2.0 | 1.0 × 10−03 |
| Maff | −1.9 | 1.0 × 10−02 | Gm13228 | 2.0 | 9.8 × 10−09 |
| Cd52 | −1.9 | 2.9 × 10−03 | Spr-ps1 | 2.0 | 5.8 × 10−04 |
| Zfp932 | −1.8 | 1.6 × 10−02 | Neo1 | 1.9 | 9.7 × 10−04 |
| Gm11625 | −1.8 | 3.4 × 10−02 | Tmem231 | 1.9 | 4.7 × 10−04 |
| Ide | −1.7 | 7.2 × 10−04 | Fam115a | 1.9 | 6.3 × 10−04 |
| Dfna5 | −1.7 | 1.4 × 10−02 | Usp11 | 1.8 | 9.5 × 10−04 |
| Sema4a | −1.7 | 1.2 × 10−02 | Aldh1l1 | 1.8 | 4.6 × 10−04 |
| Cldn26 | −1.7 | 1.7 × 10−02 | Zcchc14 | 1.8 | 1.2 × 10−03 |
| Lancl2 | −1.7 | 7.1 × 10−04 | Dynlt1b | 1.8 | 6.8 × 10−04 |
| Blm | −1.7 | 4.3 × 10−02 | Dynlt1a | 1.8 | 8.3 × 10−04 |
| Oit3 | −1.7 | 4.0 × 10−02 | Ric3 | 1.7 | 6.4 × 10−04 |
| Cebpb | −1.7 | 3.1 × 10−02 | Slc40a1 | 1.7 | 7.9 × 10−05 |
| Hilpda | −1.6 | 1.3 × 10−02 | Ppp1r9a | 1.7 | 2.6 × 10−04 |
| S100a8 | −1.6 | 2.5 × 10−03 | Pbxip1 | 1.7 | 8.5 × 10−04 |
| Padi4 | −1.6 | 1.9 × 10−02 | Dynlt1-ps1 | 1.6 | 1.5 × 10−04 |
| Ephx1 | −1.6 | 1.3 × 10−02 | Cd59a | 1.6 | 1.2 × 10−05 |
| Gm7665 | −1.5 | 4.5 × 10−02 | Ms4a6b | 1.6 | 4.9 × 10−05 |
| Entpd4 | −1.5 | 2.9 × 10−04 | Sass6 | 1.6 | 1.3 × 10−04 |
| Gm11810 | −1.5 | 4.2 × 10−02 | Plekha8 | 1.6 | 3.9 × 10−04 |
| Napsa | −1.5 | 3.1 × 10−02 | Cadm1 | 1.6 | 1.4 × 10−03 |
| Ifi30 | −1.5 | 1.1 × 10−02 | Nradd | 1.6 | 4.4 × 10−04 |
| Gm12854 | −1.5 | 1.5 × 10−02 | AC113059.1 | 1.5 | 1.1 × 10−03 |
| Gm10108 | −1.5 | 1.9 × 10−02 |
| GO-Term | Description | Count in Gene Set | FDR |
|---|---|---|---|
| GO:0061003 | positive regulation of dendritic spine morphogenesis | 4 of 23 | 0.0107 |
| GO:0036006 | cellular response to macrophage colony-stimulating factor stimulus | 3 of 8 | 0.0126 |
| GO:0045655 | regulation of monocyte differentiation | 3 of 17 | 0.0294 |
| GO:0032502 | developmental process | 40 of 5213 | 0.0294 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarang, Z.; Sághy, T.; Budai, Z.; Ujlaky-Nagy, L.; Bedekovics, J.; Beke, L.; Méhes, G.; Nagy, G.; Rühl, R.; Moise, A.R.; et al. Retinol Saturase Knock-Out Mice are Characterized by Impaired Clearance of Apoptotic Cells and Develop Mild Autoimmunity. Biomolecules 2019, 9, 737. https://doi.org/10.3390/biom9110737
Sarang Z, Sághy T, Budai Z, Ujlaky-Nagy L, Bedekovics J, Beke L, Méhes G, Nagy G, Rühl R, Moise AR, et al. Retinol Saturase Knock-Out Mice are Characterized by Impaired Clearance of Apoptotic Cells and Develop Mild Autoimmunity. Biomolecules. 2019; 9(11):737. https://doi.org/10.3390/biom9110737
Chicago/Turabian StyleSarang, Zsolt, Tibor Sághy, Zsófia Budai, László Ujlaky-Nagy, Judit Bedekovics, Lívia Beke, Gábor Méhes, Gábor Nagy, Ralph Rühl, Alexander R. Moise, and et al. 2019. "Retinol Saturase Knock-Out Mice are Characterized by Impaired Clearance of Apoptotic Cells and Develop Mild Autoimmunity" Biomolecules 9, no. 11: 737. https://doi.org/10.3390/biom9110737
APA StyleSarang, Z., Sághy, T., Budai, Z., Ujlaky-Nagy, L., Bedekovics, J., Beke, L., Méhes, G., Nagy, G., Rühl, R., Moise, A. R., Palczewski, K., & Szondy, Z. (2019). Retinol Saturase Knock-Out Mice are Characterized by Impaired Clearance of Apoptotic Cells and Develop Mild Autoimmunity. Biomolecules, 9(11), 737. https://doi.org/10.3390/biom9110737






