Uncoupling of Carbonic Anhydrase from Na-H exchanger-1 in Experimental Colitis: A Possible Mechanistic Link with Na-H Exchanger
Abstract
1. Introduction
2. Materials and Methods
2.1. Induction of Colitis
2.2. Characterization of Colitis
2.3. Western Blot Analysis
2.3.1. Preparation of Tissue Lysates and Crude Microsomes
2.3.2. Protein Concentration
2.3.3. Protein Separation
2.4. Co-Immunoprecipitation
2.5. Confocal Immunofluorescence Localization
2.6. Effect of TNF-α on the Expression of CA Isoforms
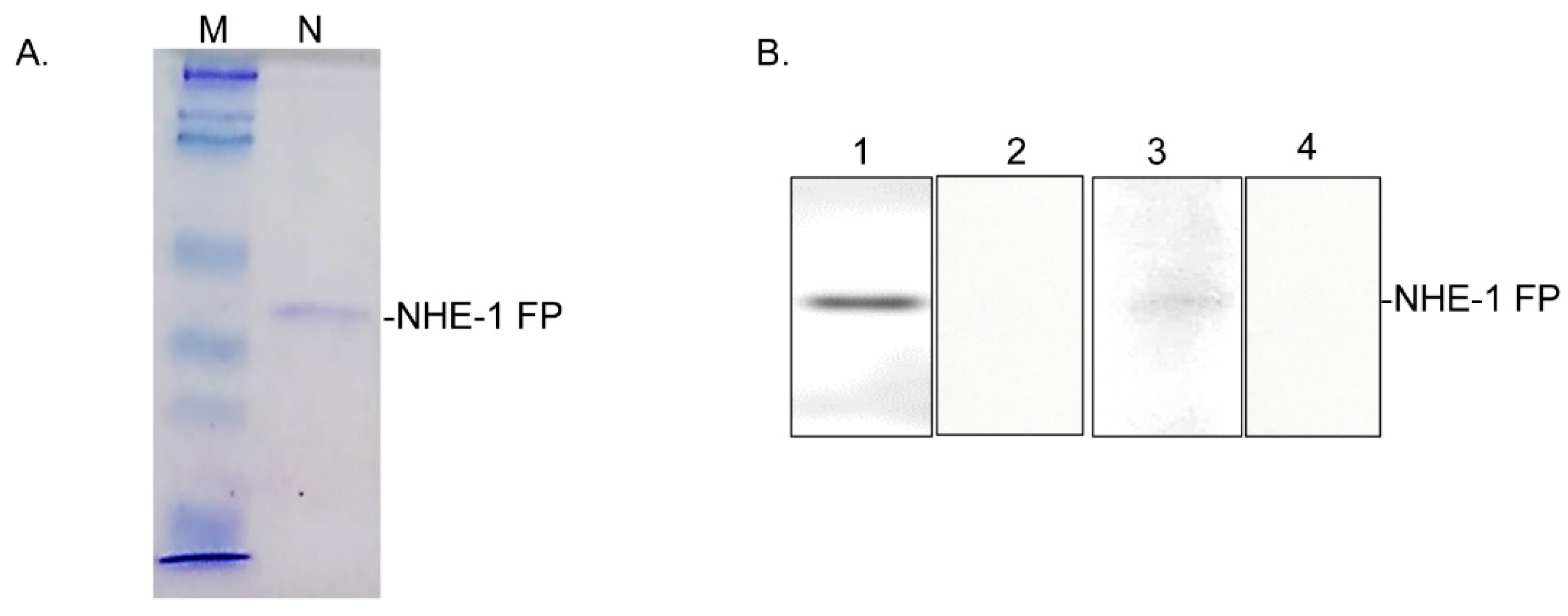
2.7. CA-Binding Site in the NHE-1 C-Terminal Peptide (CP)
2.8. Statistical Analysis
3. Results
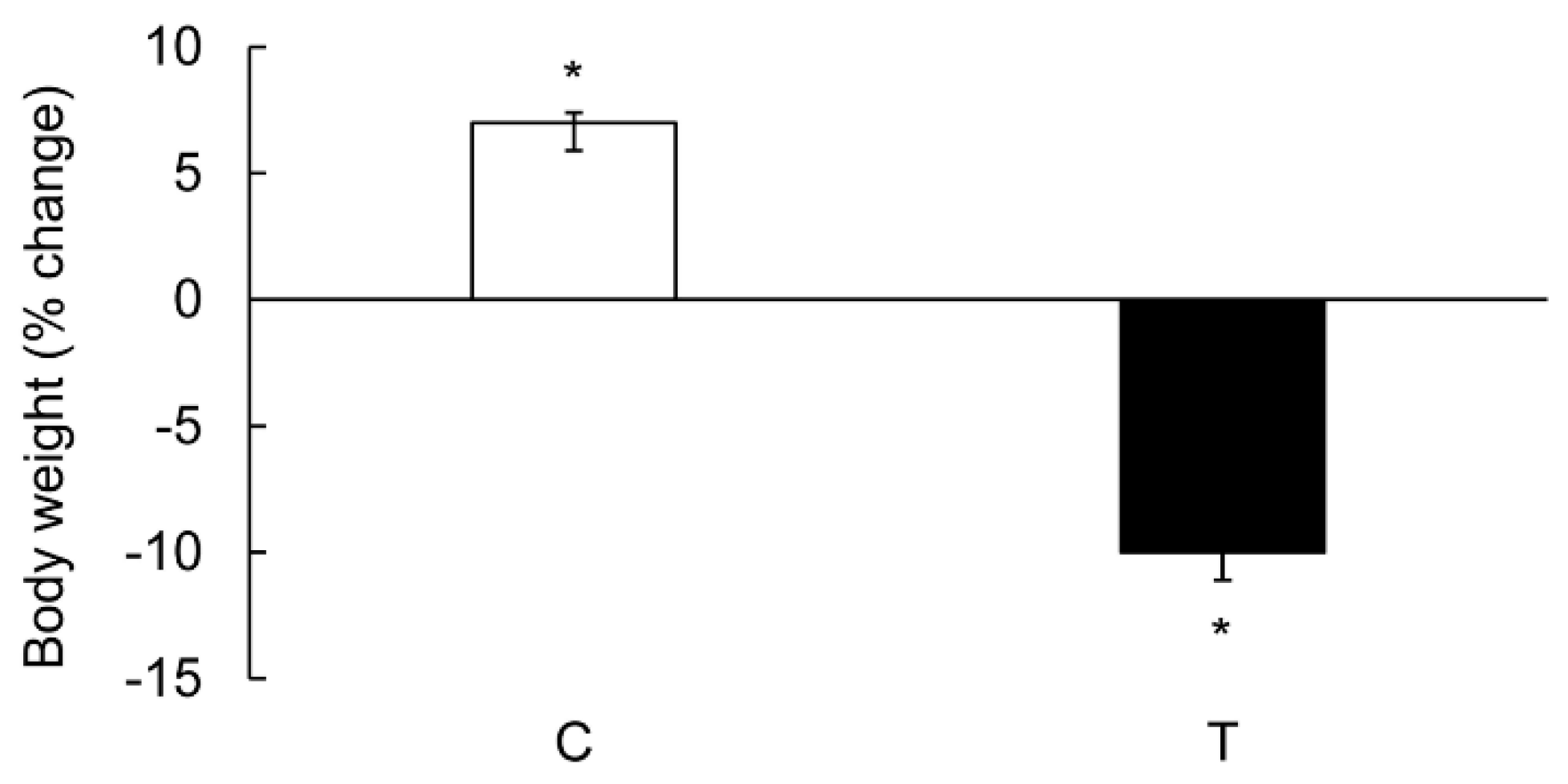
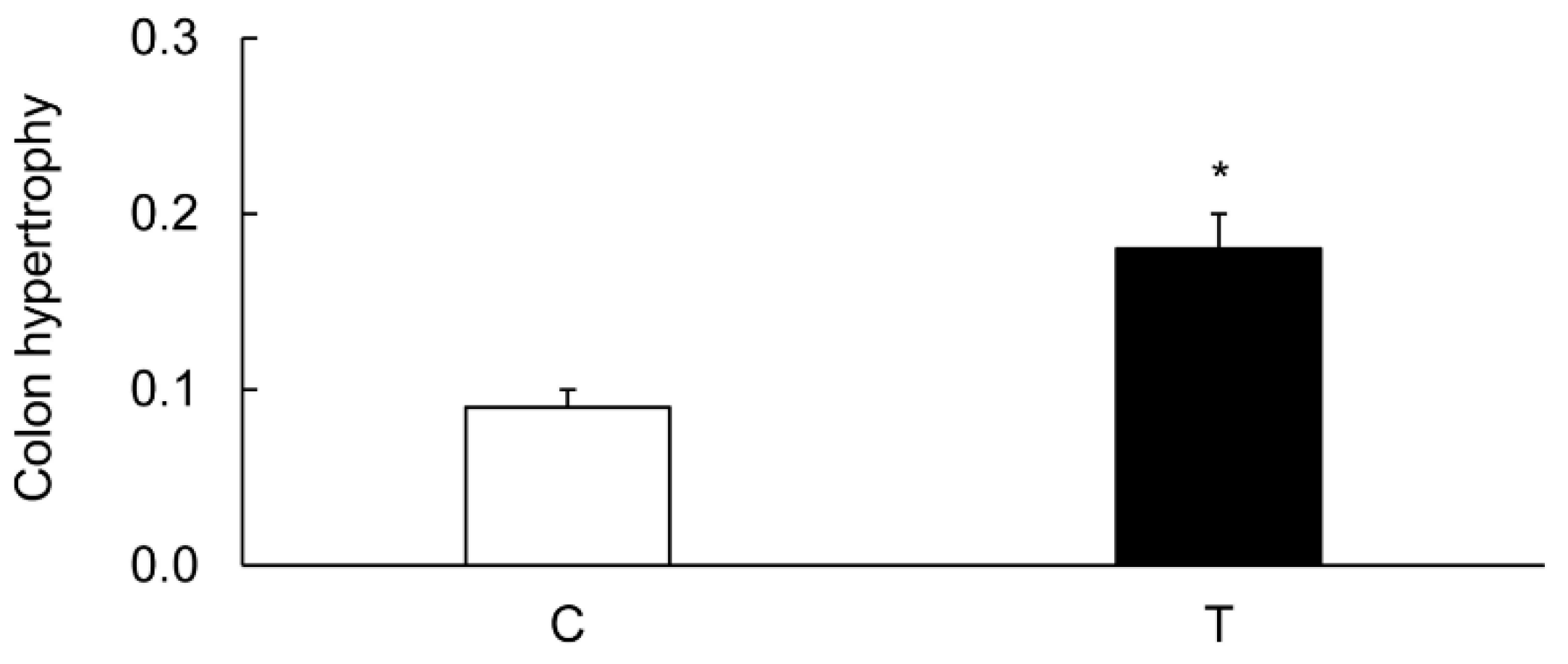
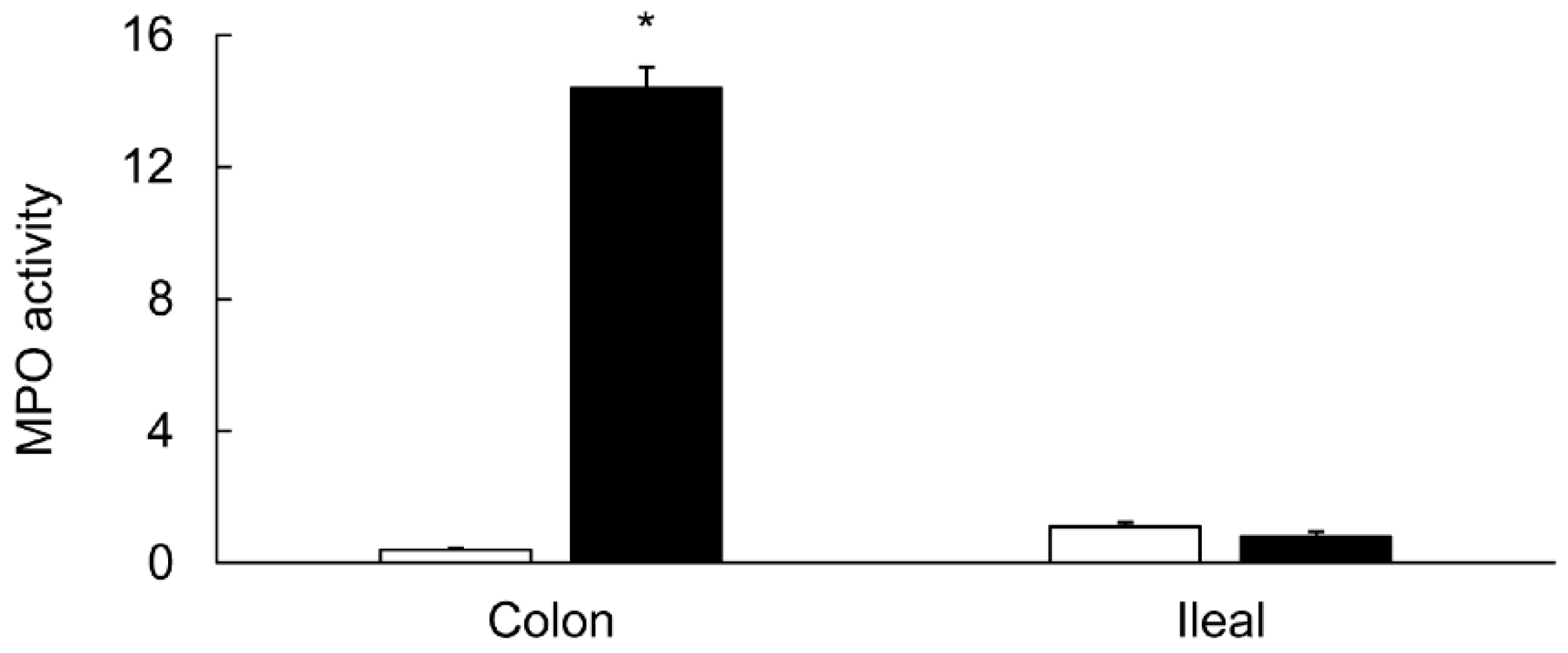
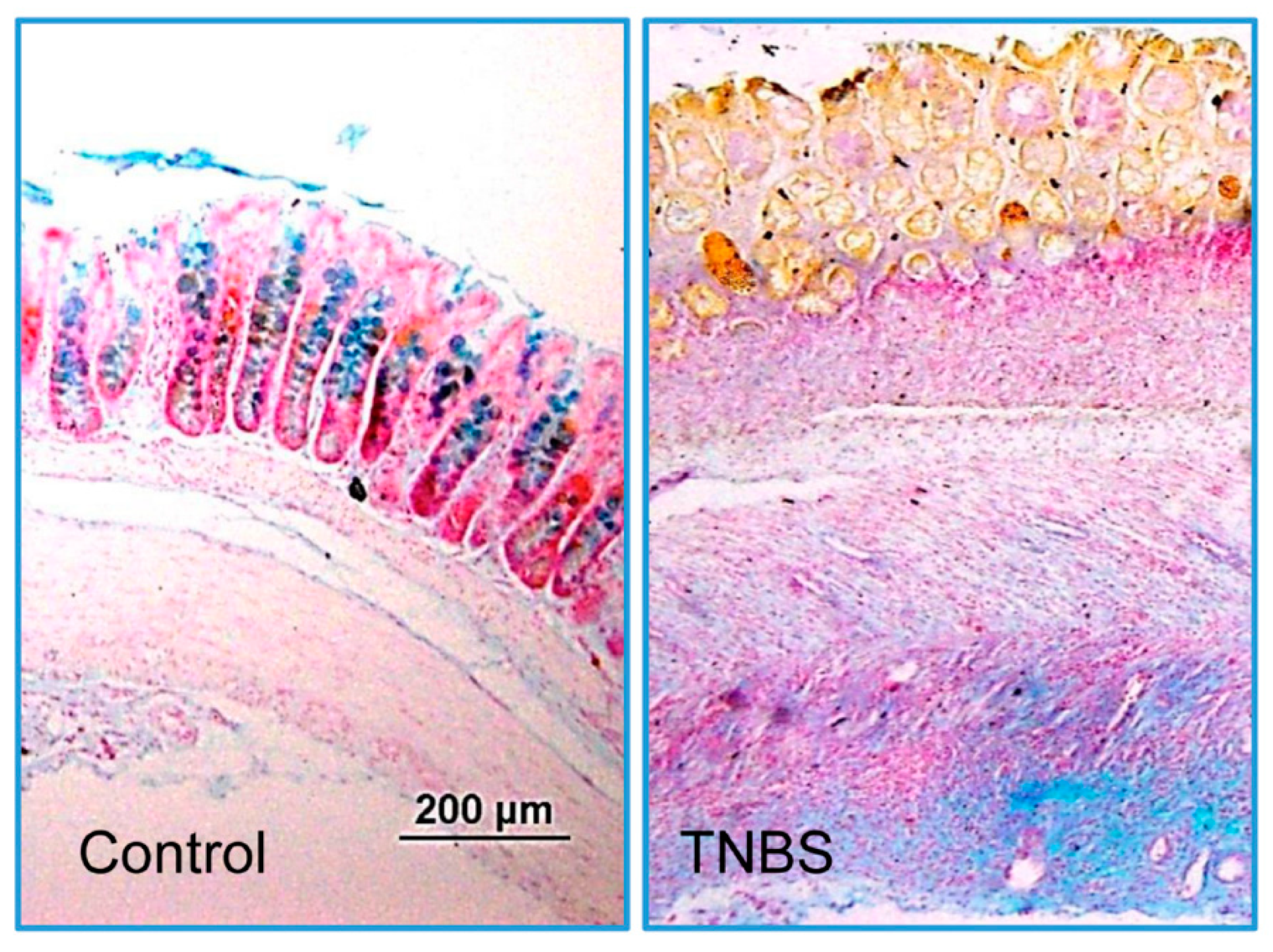
3.1. Characterization of Colitis
3.2. Protein Yield
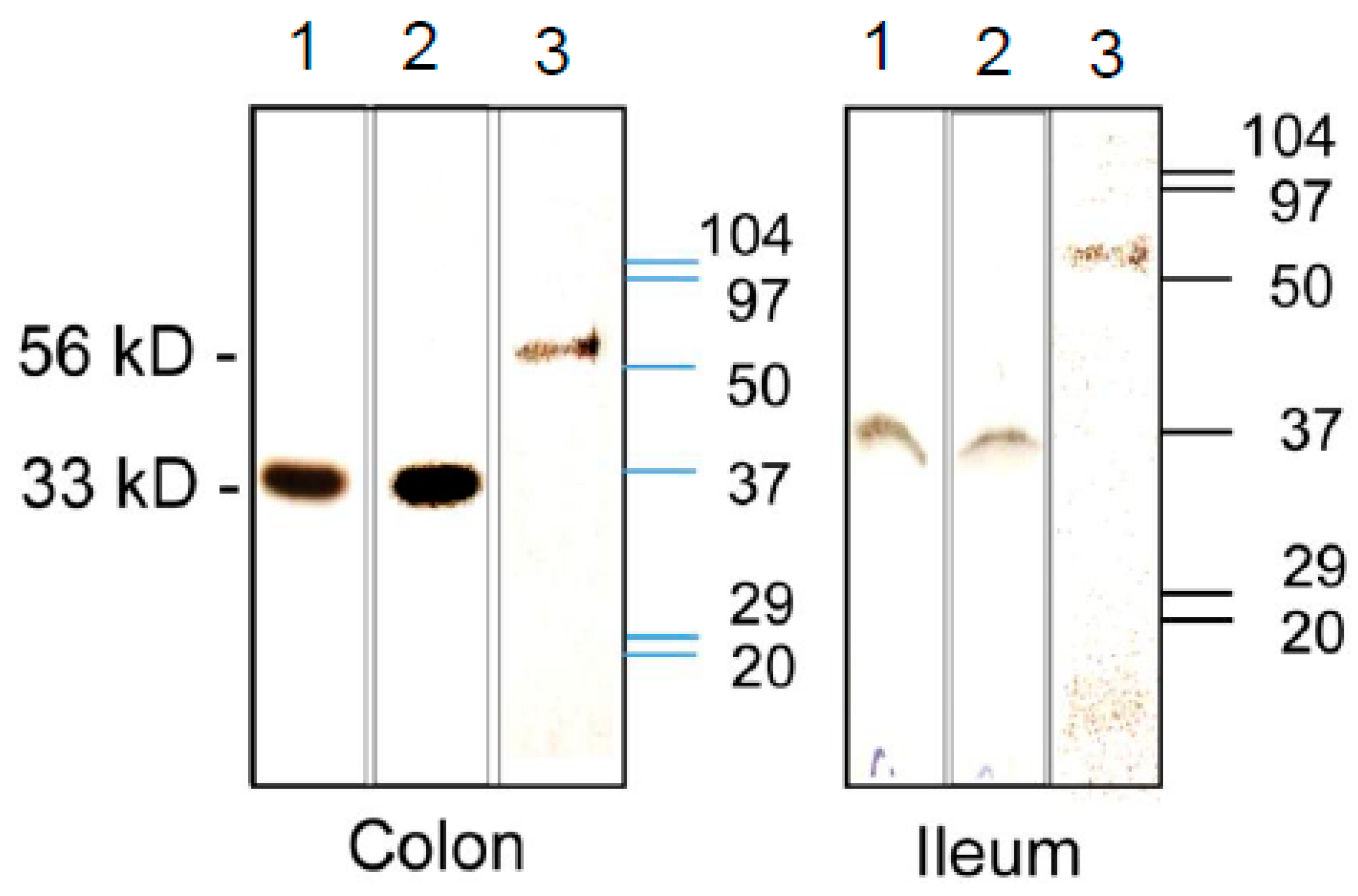
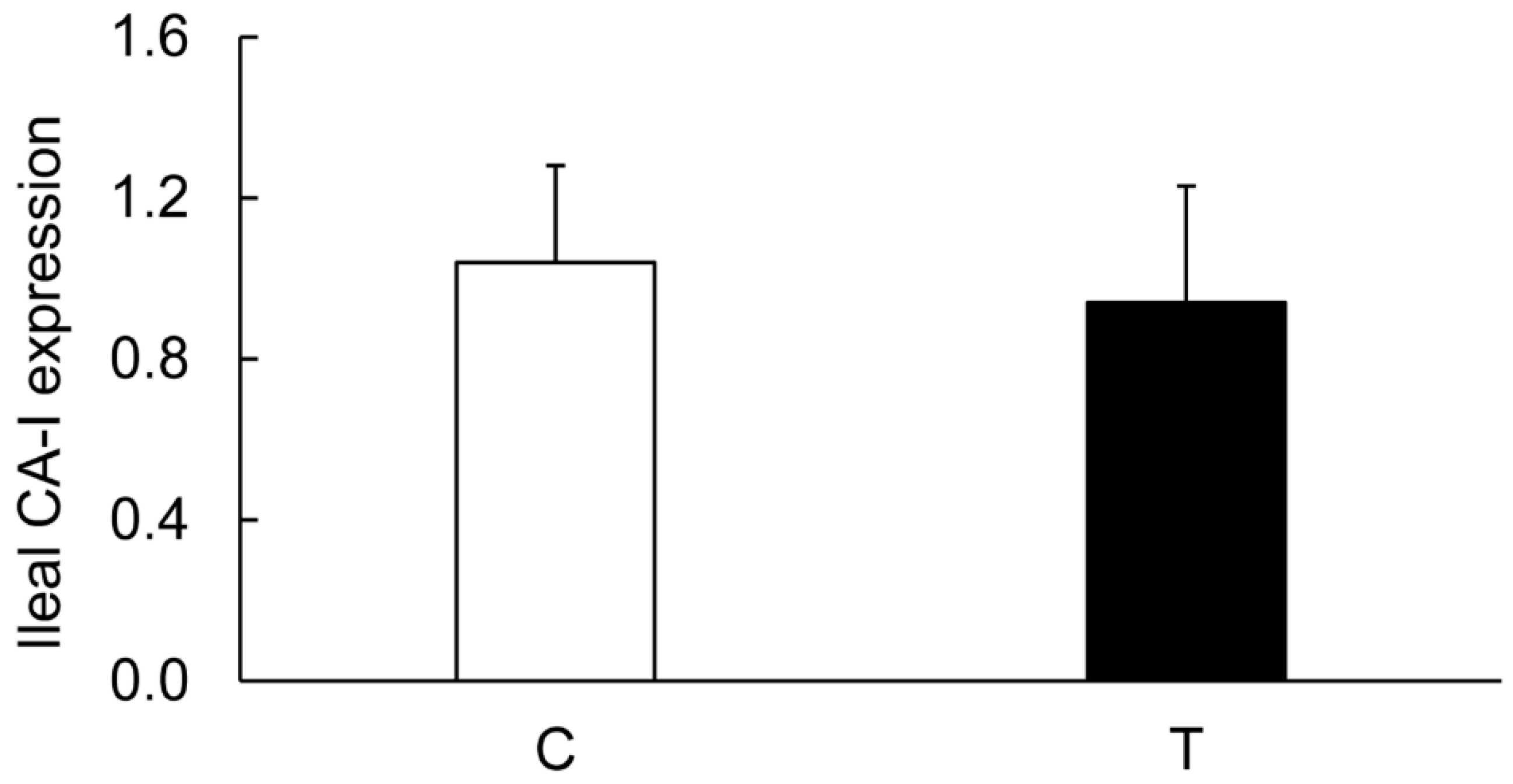
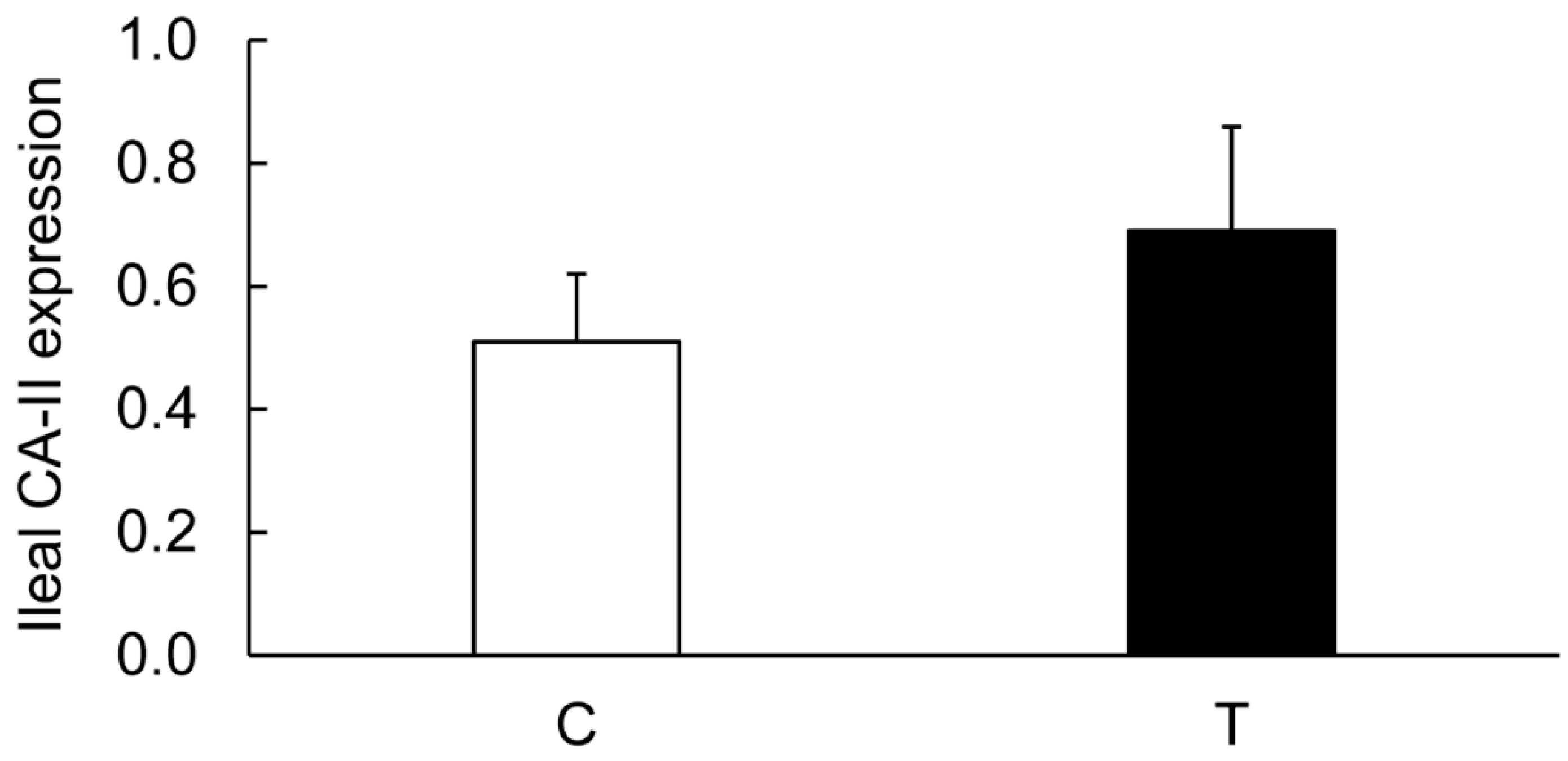
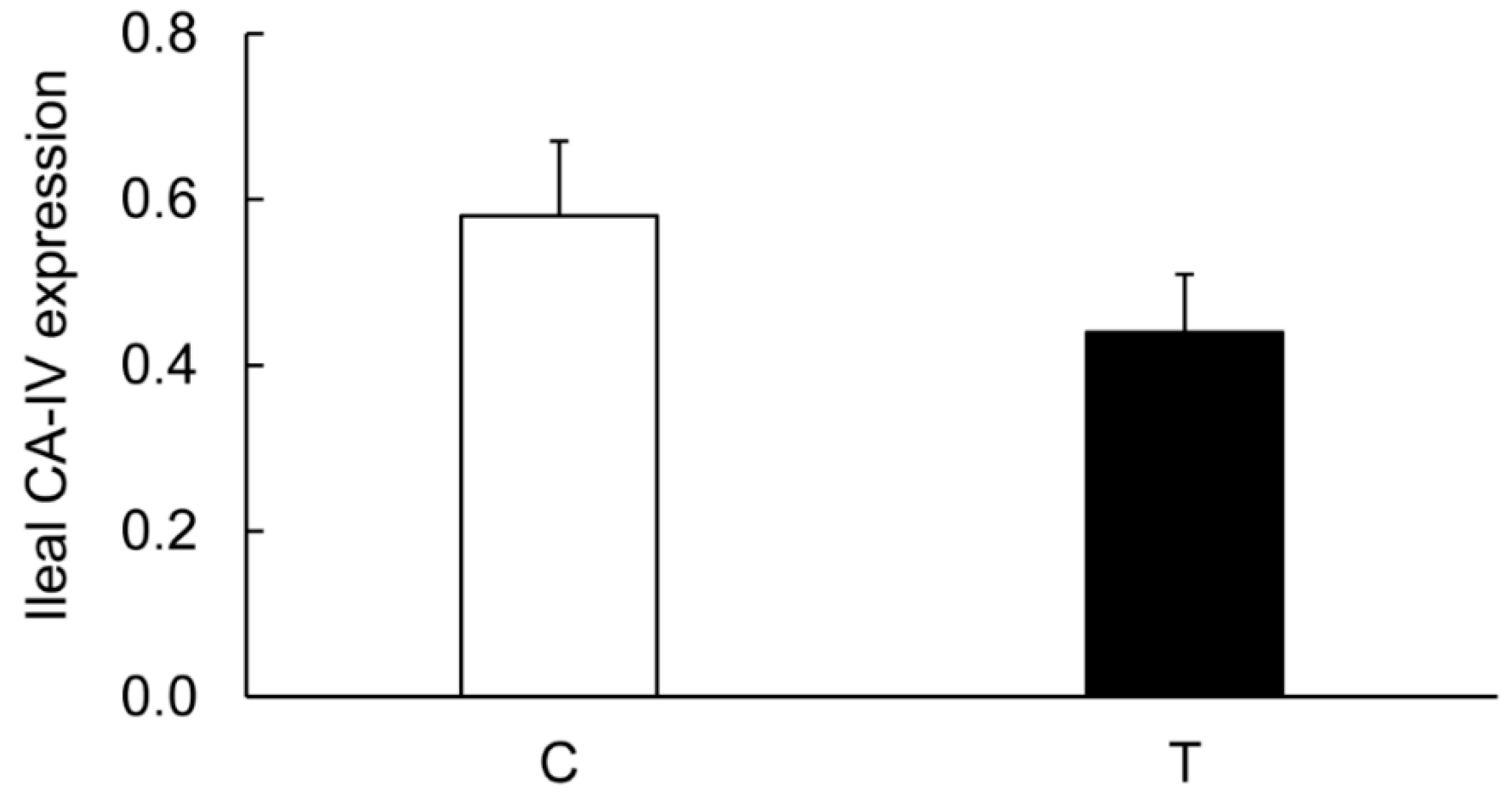
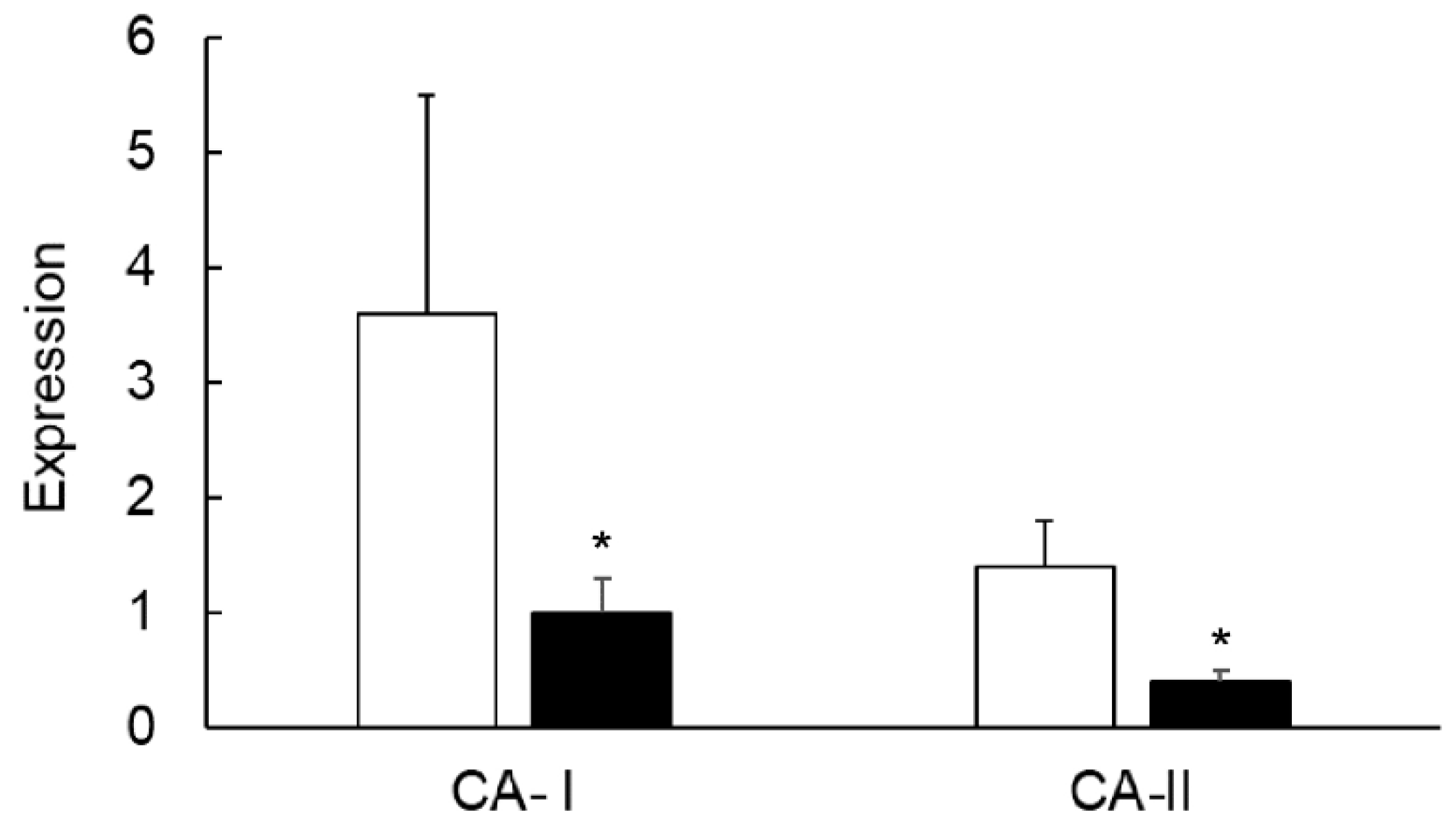
3.3. Expression of CA Isozymes
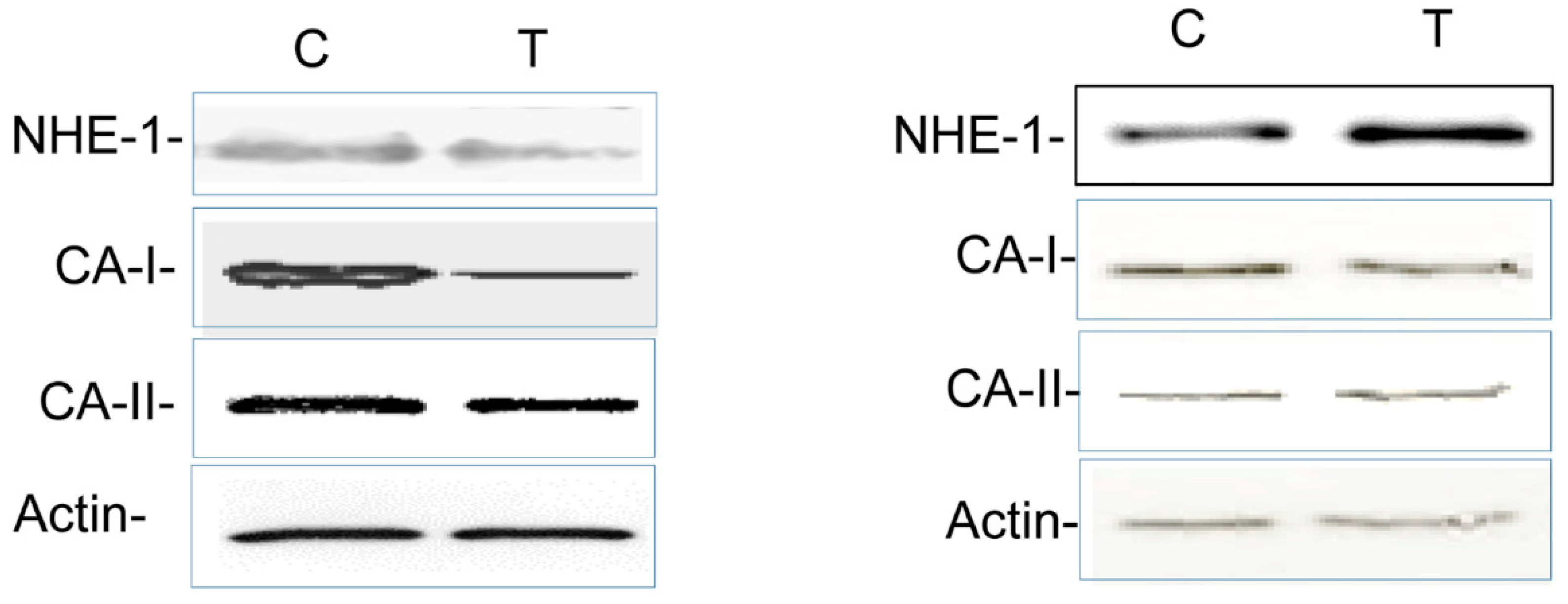
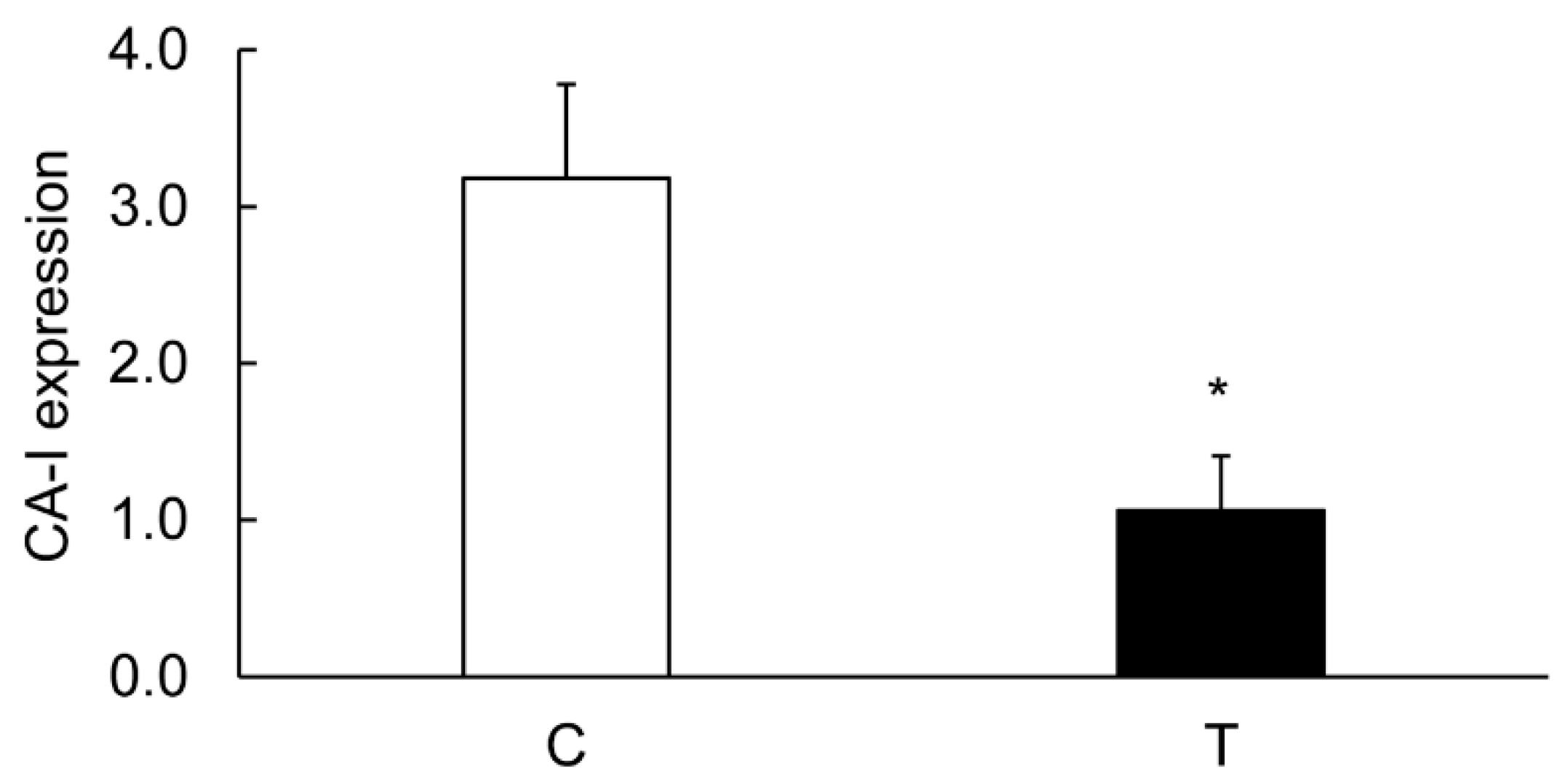
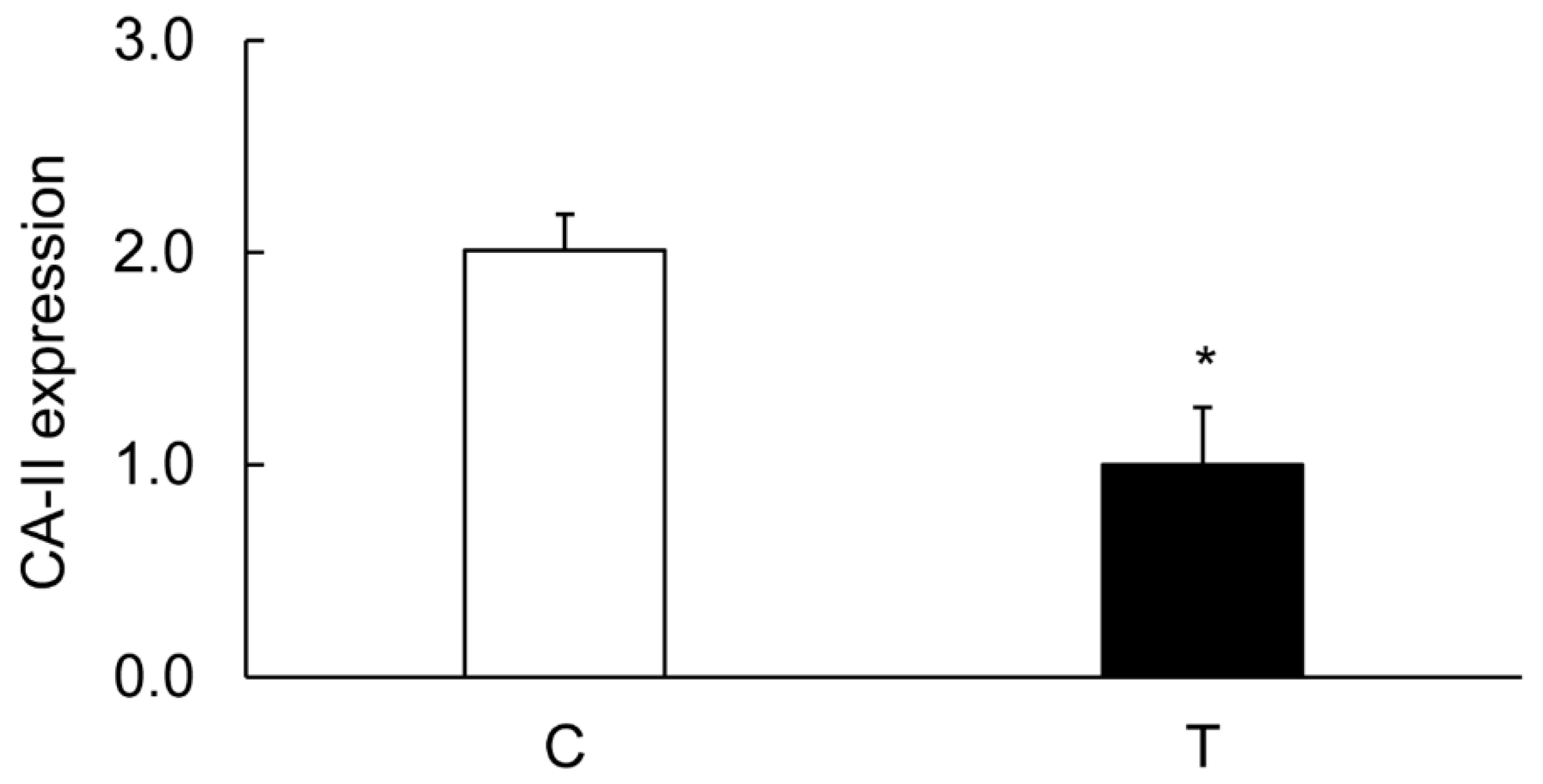
3.4. Colitis-Induced Expression of CA Isozyme
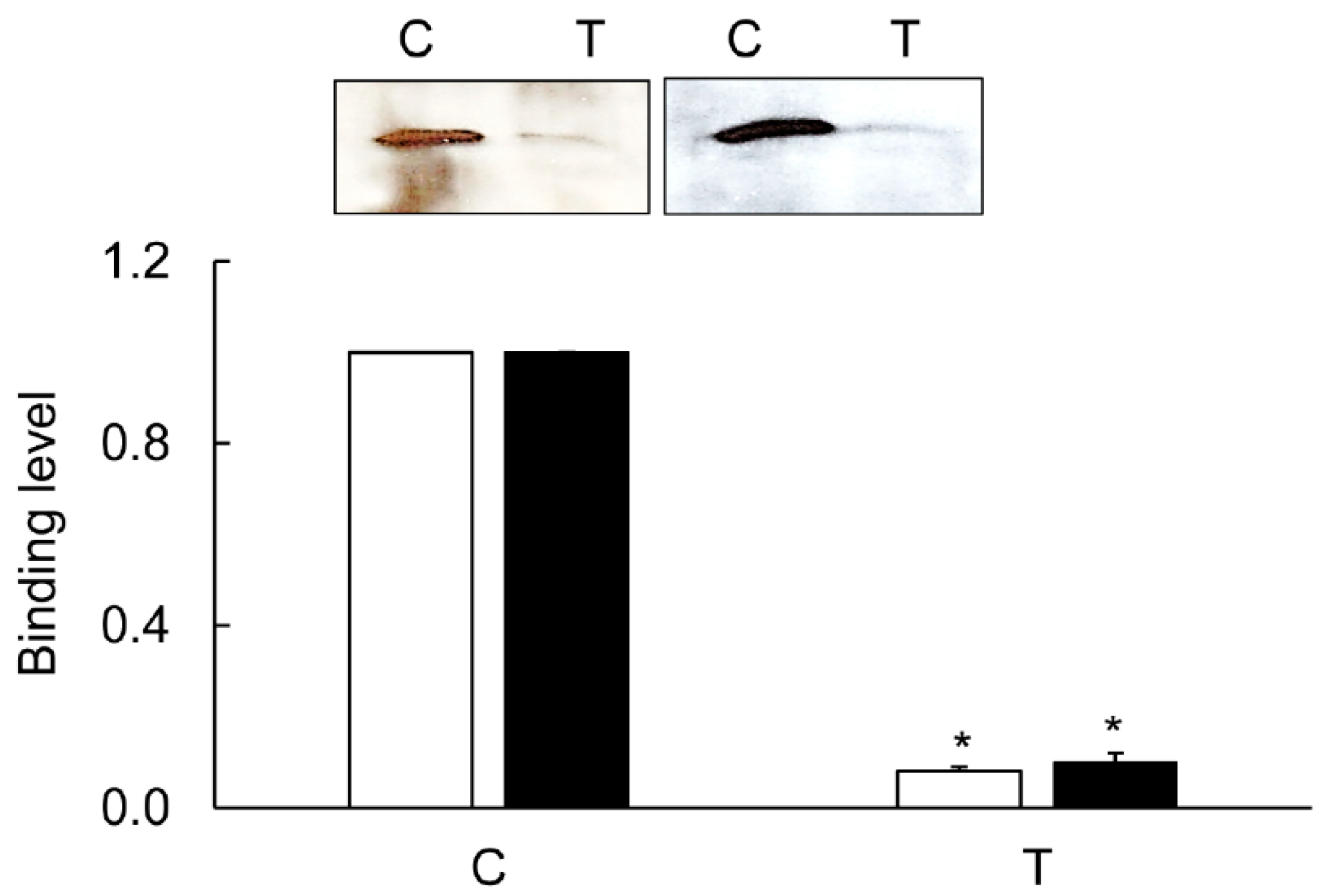
3.5. Immunoprecipitation
3.6. Mapping of the CA-Binding Site
3.7. Effects of TNF-α on the Colonic Expression of CA-I And CA-II Ex Vivo
4. Discussion
5. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tu, C.; Foster, L.; Alvarado, A.; McKenna, R.; Silverman, D.N.; Frost, S.C. Role of zinc in catalytic activity of carbonic anhydrase IX. Arch Biochem Biophys. 2012, 521, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Leppilampi, M.; Pastorekova, S.; Pastorek, J.; Waheed, A.; Sly, W.S.; Parkkila, S. Carbonic anhydrase gene expression in CA II-deficient (Car2-/-) and CA IX-deficient (Car9-/-) mice. J. Physiol. 2006, 571, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, A.J.; Kivelä, J.; Saarnio, J.; Parkkila, S. Carbonic anhydrases in normal gastrointestinal tract and gastrointestinal tumours. World J. Gastroenterol. 2005, 11, 155–163. [Google Scholar] [CrossRef]
- Carter, M.J. Carbonic anhydrase: Isoenzymes, properties, distribution, and functional significance. Biol. Rev. Camb. Philos. Soc. 1972, 47, 465–513. [Google Scholar] [CrossRef]
- Lönnerholm, G.; Selking, O.; Wistrand, P.J. Amount and distribution of carbonic anhydrases CA I and CA II in the gastrointestinal tract. Gastroenterology 1985, 88, 1151–1161. [Google Scholar] [CrossRef]
- Fleming, R.E.; Parkkila, S.; Parkkila, A.K.; Rajaniemi, H.; Waheed, A.; Sly, W.S. Carbonic anhydrase IV expression in rat and human gastrointestinal tract regional, cellular, and subcellular localization. J. Clin. Invest. 1996, 96, 2907–2913. [Google Scholar] [CrossRef]
- Supuran, C.T.; Anna Di Fiore, A.D.; Alterio, V.; Monti, S.M.; Giuseppina De Simone, G.D. Recent Advances in Structural Studies of the Carbonic Anhydrase Family: The Crystal Structure of Human CA IX and CA XIII. Curr. Pharm. Des. 2010, 16. [Google Scholar] [CrossRef]
- Endeward, V.; Kleinke, T.; Gros, G. Carbonic anhydrase in the gastrointestinal mucus of mammals--possible protective role against carbon dioxide. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2003, 136, 281–287. [Google Scholar] [CrossRef]
- Andoh, A.; Fujiyama, Y.; Yoshioka, U.; Sasaki, M.; Araki, Y.; Tsujikawa, T.; Bamba, T. Elevated serum anti-carbonic anhydrase II antibodies in patients with ulcerative colitis. Int. J. Mol. Med. 2002, 9, 499–502. [Google Scholar] [CrossRef]
- Collins, S.M. The immunomodulation of enteric neuromuscular function, implications for motility and inflammatory disorders. Gastroenterology 1996, 111, 1683–1699. [Google Scholar] [CrossRef]
- Satokari, R. Contentious host-microbiota relationship in inflammatory bowel disease - can foes become friends again? Scand. J. Gastroenterol. 2015, 50, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Bashashati, M.; Habibi, H.R.; Keshavarzian, A.; Schmulson, M.; Sharkey, K.A. Intestinal Microbiota, a Regulator of Intestinal Inflammation and Cardiac Ischemia? Curr. Drug Targets 2015, 16, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Collins, S.M. Altered expression of sodium pump isoforms in the inflamed intestine of Trichinella spiralis-infected rats. Am. J. Physiol. 1993, 264, G1160–G1168. [Google Scholar] [CrossRef]
- Siddique, I.; Khan, I. Mechanism of regulation of Na-H exchanger in inflammatory bowel disease: Role of TLR-4 signaling mechanism. Dig. Dis. Sci. 2011, 56, 1656–1662. [Google Scholar] [CrossRef]
- Farkas, K.; Yeruva, S.; Rakonczay, Z., Jr.; Ludolph, L.; Molnár, T.; Nagy, F.; Szepes, Z.; Schnúr, A.; Wittmann, T.; Hubricht, J.; et al. New therapeutic targets in ulcerative colitis: The importance of ion transporters in the human colon. Inflamm. Bowel Dis. 2011, 17, 884–898. [Google Scholar] [CrossRef]
- Soleiman, A.A.; Thameem, F.; Khan, I. Mechanism of down regulation of Na-H exchanger-2 in experimental colitis. PLoS ONE 2017, 12, e0176767. [Google Scholar] [CrossRef]
- Caprilli, R.; Vernia, P.; Colaneri, O.; Torsoli, A. Blood pH: A test for assessment of severity in proctocolitis. Gut 1976, 17, 763–769. [Google Scholar] [CrossRef][Green Version]
- Caprilli, R.; Frieri, G.; Latella, G.; Vernia, P.; Santoro, M.L. Faecal excretion of bicarbonate in ulcerative colitis. Digestion 1986, 35, 136–142. [Google Scholar] [CrossRef]
- Scheppach, W.; Christl, S.U.; Bartram, H.P.; Richter, F.; Kasper, H. Effects of short-chain fatty acids on the inflamed colonic mucosa. Scand. J. Gastroenterol. Suppl. 1997, 222, 53–57. [Google Scholar] [CrossRef]
- Thibault, R.; Blachier, F.; Darcy-Vrillon, B.; de Coppet, P.; Bourreille, A.; Segain, J.P. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: A transport deficiency. Inflamm. Bowel. Dis. 2010, 16, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, L.; Zhang, W.; Xu, H.; Chang, X. Transgenic mice over-expressing carbonic anhydrase I showed aggravated joint inflammation and tissue destruction. BMC Musculoskelet. Disord. 2012, 13, 256. [Google Scholar] [CrossRef] [PubMed]
- Borenshtein, D.; Schlieper, K.A.; Rickman, B.H.; Chapman, J.M.; Schweinfest, C.W.; Fox, J.G.; Schauer, D.B. Decreased expression of colonic Slc26a3 and carbonic anhydrase IV as a cause of fatal infectious diarrhea in mice. Infect Immun. 2009, 77, 3639–3650. [Google Scholar] [CrossRef] [PubMed]
- Alver, A.; Menteşe, A.; Karahan, S.C.; Erem, C.; Keha, E.E.; Arikan, M.K.; Eminağaoğlu, M.S.; Deger, O. Increased serum anti-carbonic anhydrase II antibodies in patients with Graves’ disease. Exp. Clin. Endocrinol. Diabetes 2007, 115, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Fonti, R.; Latella, G.; Caprilli, R.; Frieri, G.; Marcheggiano, A.; Sambuy, Y. Carbonic anhydrase I reduction in colonic mucosa of patients with active ulcerative colitis. Dig. Dis. Sci. 1998, 43, 2086–2092. [Google Scholar] [PubMed]
- Li, X.; Alvarez, B.; Casey, J.R.; Reithmeier, R.A.; Fliegel, L. Carbonic anhydrase II binds to and enhances activity of the Na+/H+ exchanger. J. Biol. Chem. 2002, 277, 36085–36091. [Google Scholar] [CrossRef] [PubMed]
- Vince, J.W.; Carlsson, U.; Reithmeier, R.A. Localization of the Cl−/HCO3− anion exchanger binding site to the amino-terminal region of carbonic anhydrase II. Biochemistry 2000, 39, 13344–13349. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Thomas, N.; Haridas, S. Expression and sub cellular localization of the sodium hydrogen exchanger isoform-1 in rat tissues: A possible functional relevance. Mol. Cell. Biochem. 2001, 219, 153–161. [Google Scholar] [CrossRef]
- Al-Shamali, A.; Khan, I. Expression of Na-H exchanger-8 isoform is suppressed in experimental colitis in adult rat: Lack of reversibility by dexamethasone. Scand. J. Gastroenterol. 2011, 46, 20–29. [Google Scholar] [CrossRef]
- Bradley, P.P.; Priebat, D.A.; Christensen, R.D.; Rothstein, G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Algayer, H.; Kruis, W.; Paumgartner, G.; Wiebecke, B.; Brown, L.; Erdmann, E. Inverse relationship between colonic (Na+ + K+)-ATPase activity and degree of mucosal inflammation in inflammatory bowel disease. Dig. Dis. Sci. 1988, 33, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Alka, K.; Casey, J.R. Bicarbonate Transport in Health and Disease. Int. Union Biochem. Mol. Biol. Life. 2014, 66, 596–615. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, I.; Khan, K. Uncoupling of Carbonic Anhydrase from Na-H exchanger-1 in Experimental Colitis: A Possible Mechanistic Link with Na-H Exchanger. Biomolecules 2019, 9, 700. https://doi.org/10.3390/biom9110700
Khan I, Khan K. Uncoupling of Carbonic Anhydrase from Na-H exchanger-1 in Experimental Colitis: A Possible Mechanistic Link with Na-H Exchanger. Biomolecules. 2019; 9(11):700. https://doi.org/10.3390/biom9110700
Chicago/Turabian StyleKhan, Islam, and Khalid Khan. 2019. "Uncoupling of Carbonic Anhydrase from Na-H exchanger-1 in Experimental Colitis: A Possible Mechanistic Link with Na-H Exchanger" Biomolecules 9, no. 11: 700. https://doi.org/10.3390/biom9110700
APA StyleKhan, I., & Khan, K. (2019). Uncoupling of Carbonic Anhydrase from Na-H exchanger-1 in Experimental Colitis: A Possible Mechanistic Link with Na-H Exchanger. Biomolecules, 9(11), 700. https://doi.org/10.3390/biom9110700



