Lignin Accumulation in Three Pumelo Cultivars in Association with Sucrose and Energy Depletion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
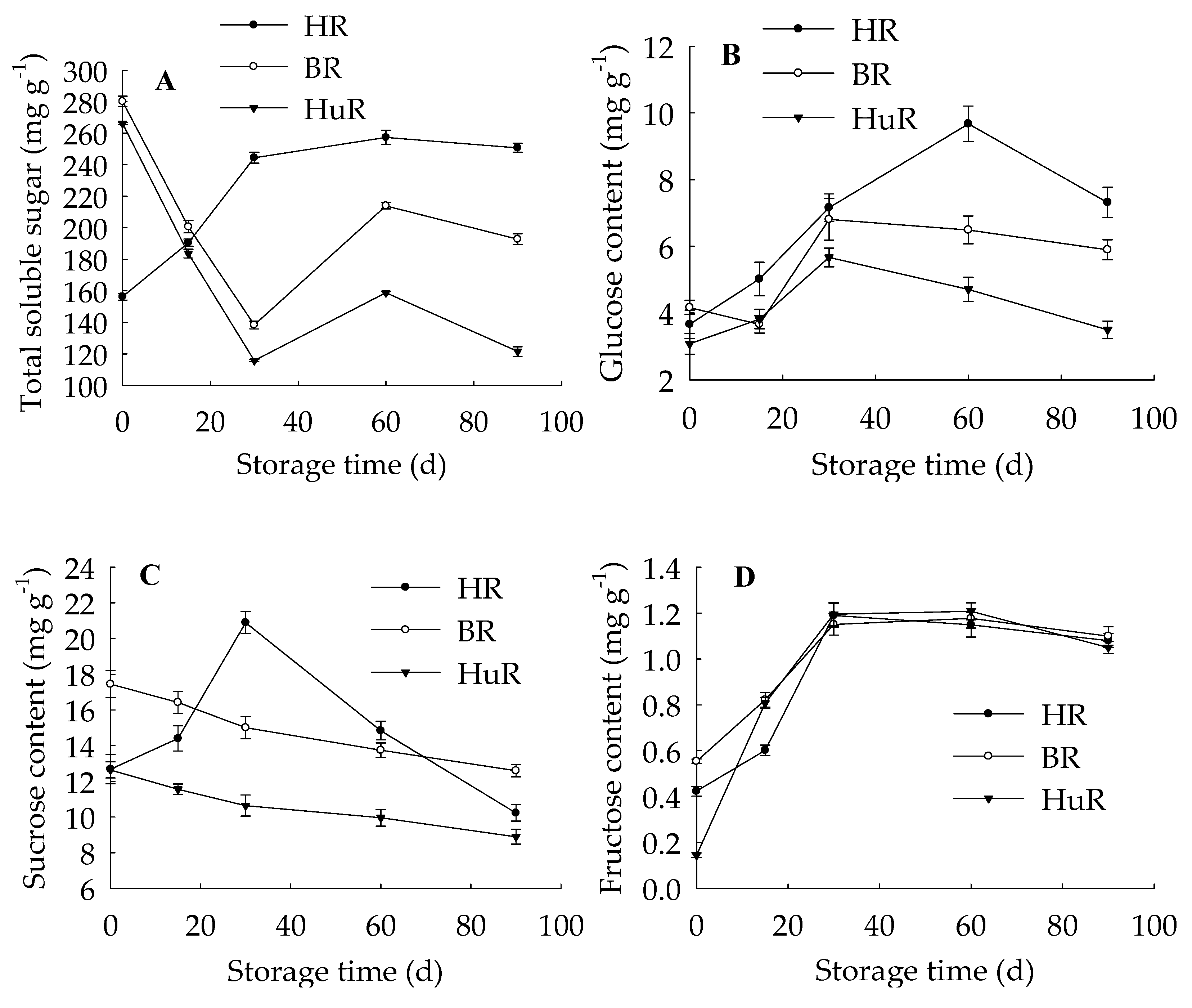
2.2. Determination of Sugar
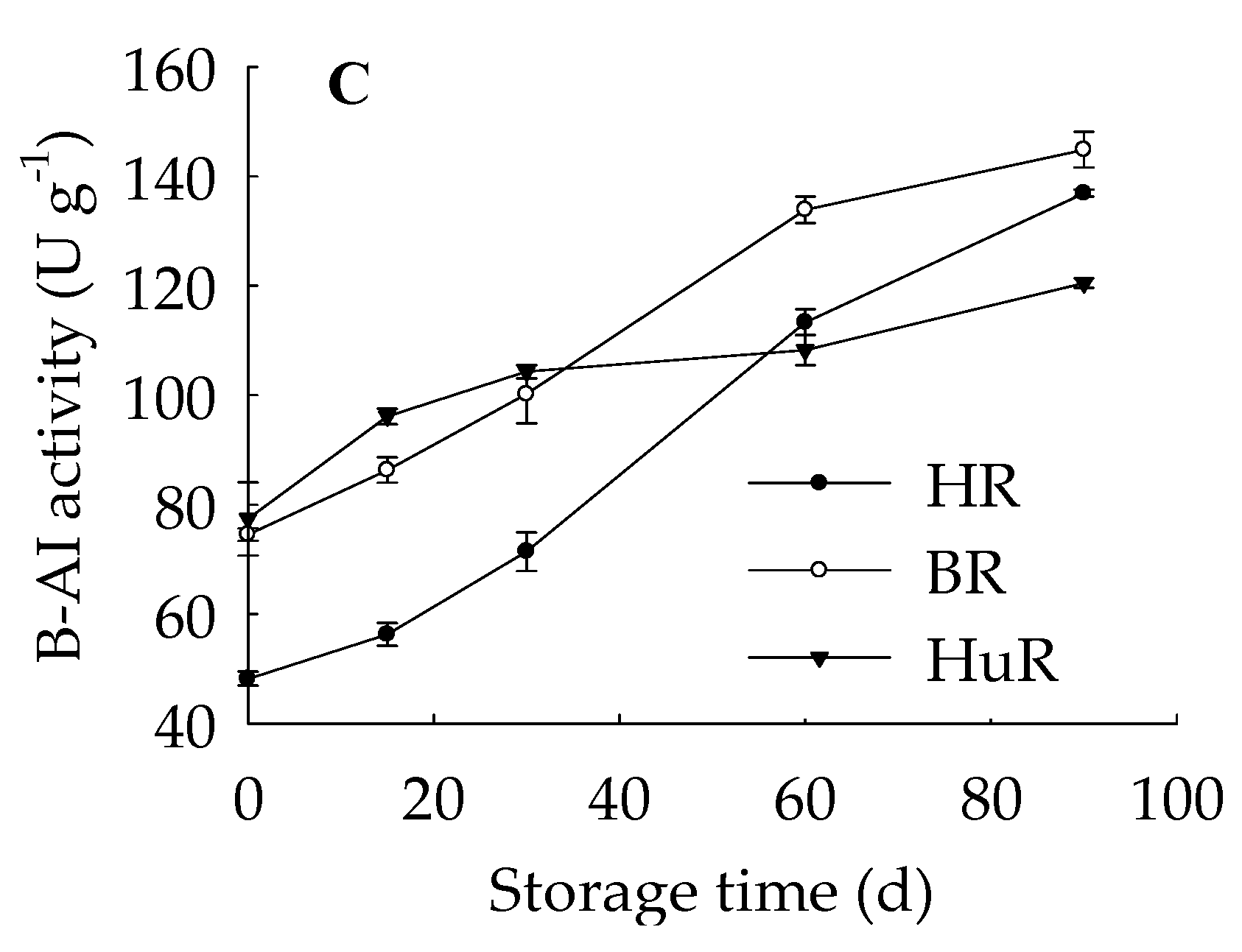
2.3. Assays of Enzyme Activities
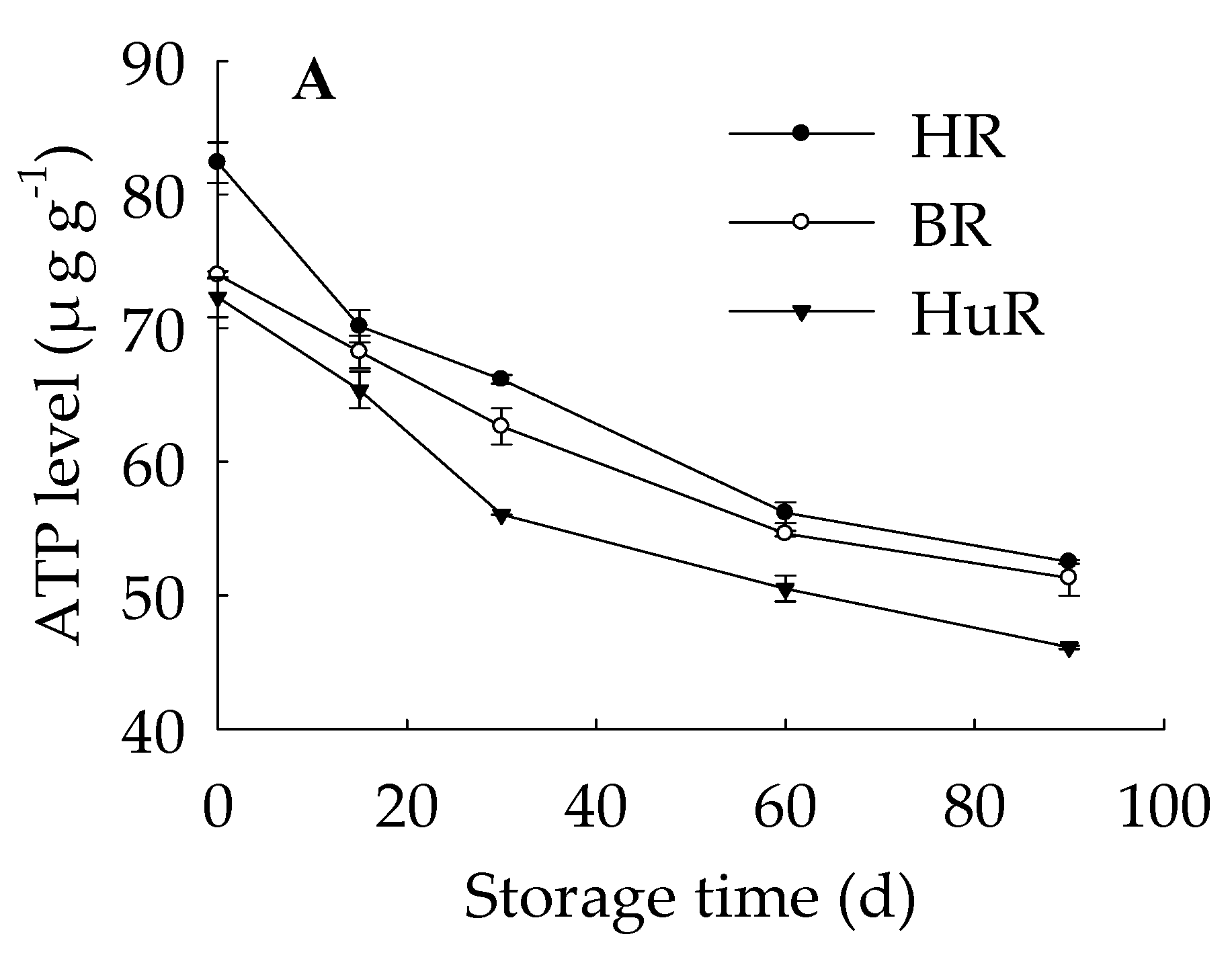
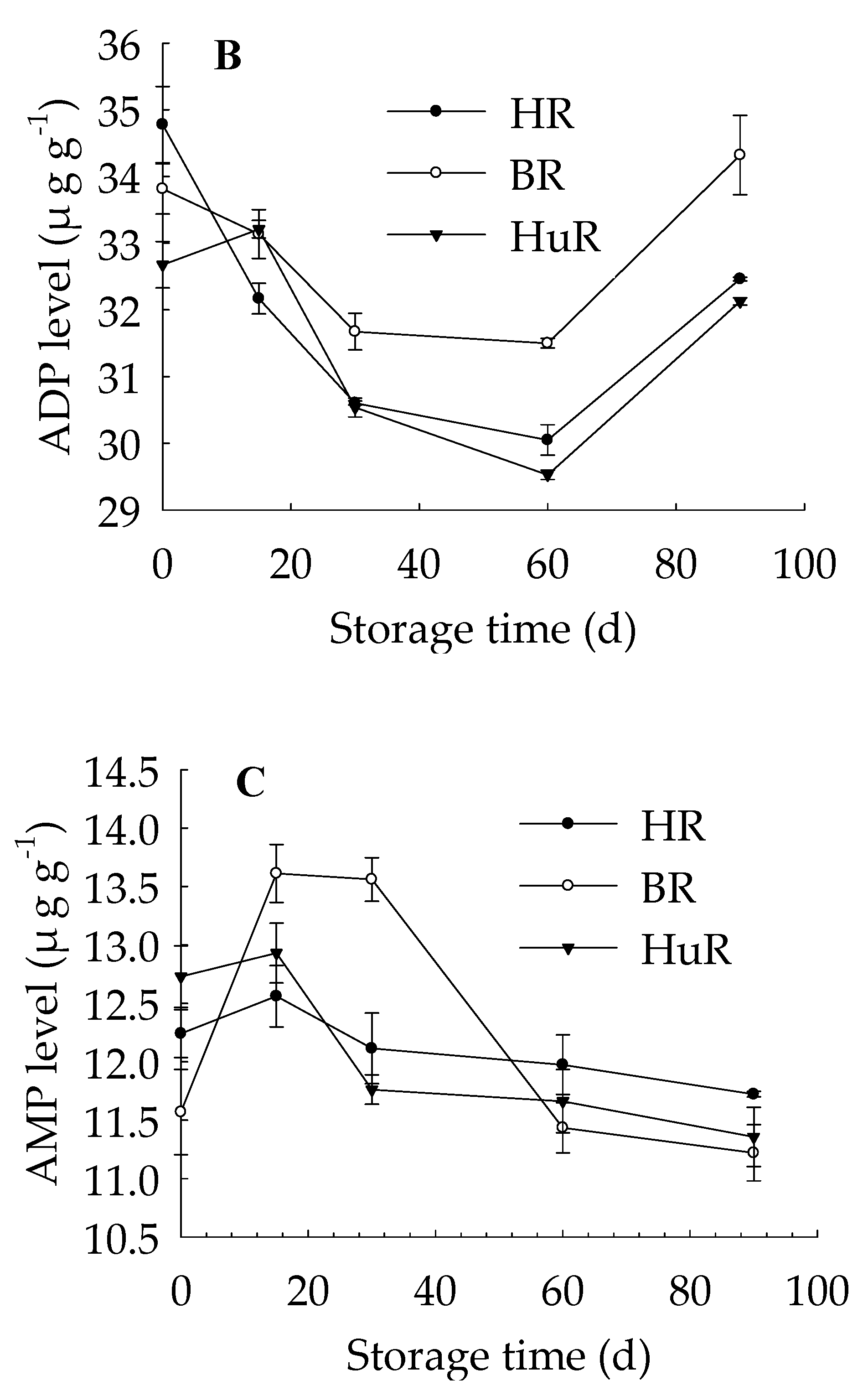
2.4. Determination of ATP, ADP, and AMP
2.5. Assays of PAL, POD, and PPO Activities
2.6. Measurement of Lignin
2.7. Statistical Analysis
3. Results
3.1. Changes in Sugar Content in Juice Sacs of Three Pummelo Cultivars
3.2. Changes in NI, S-AI, B-AI Activities in Juice Sacs of Three Pummelo Cultivars
3.3. Changes in ATP, ADP, and AMP Levels in Juice Sacs of Three PUMMELO Cultivars
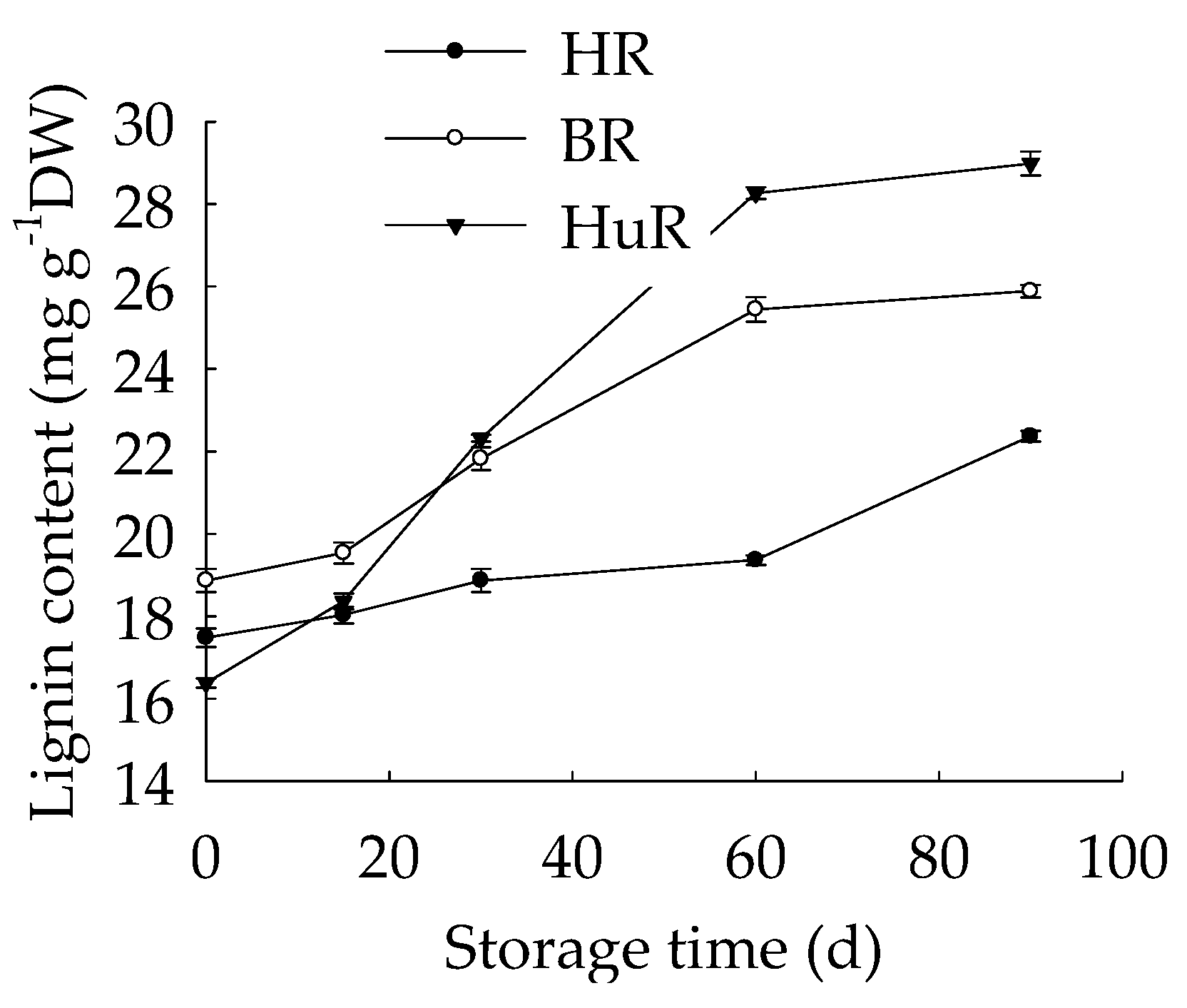
3.4. Changes in Lignin Content in Juice Sacs of Three Pummelo Cultivars
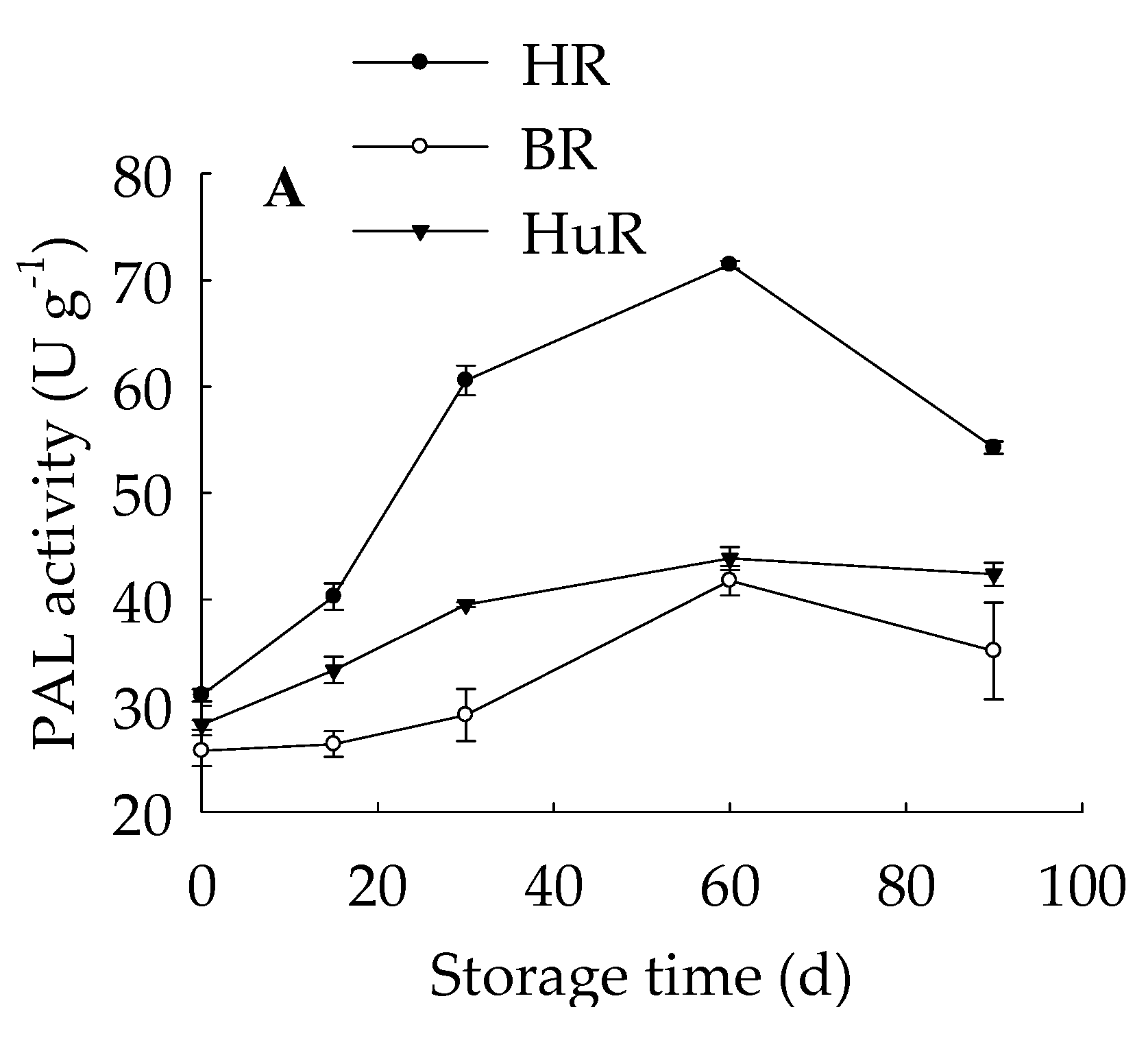
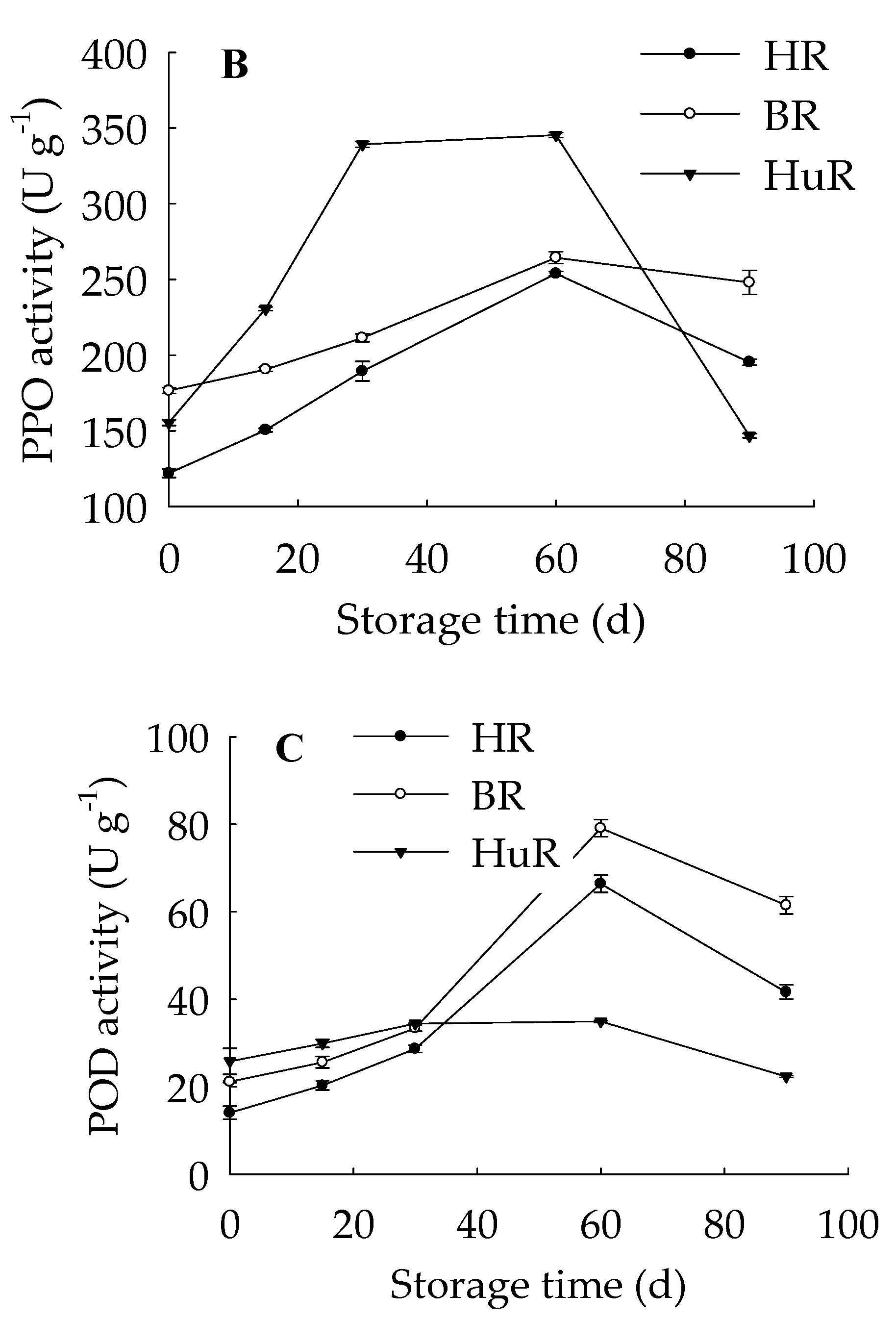
3.5. Changes in PAL, PPO, and POD Activities in Juice Sacs of Three Pummelo Cultivars
4. Discussion
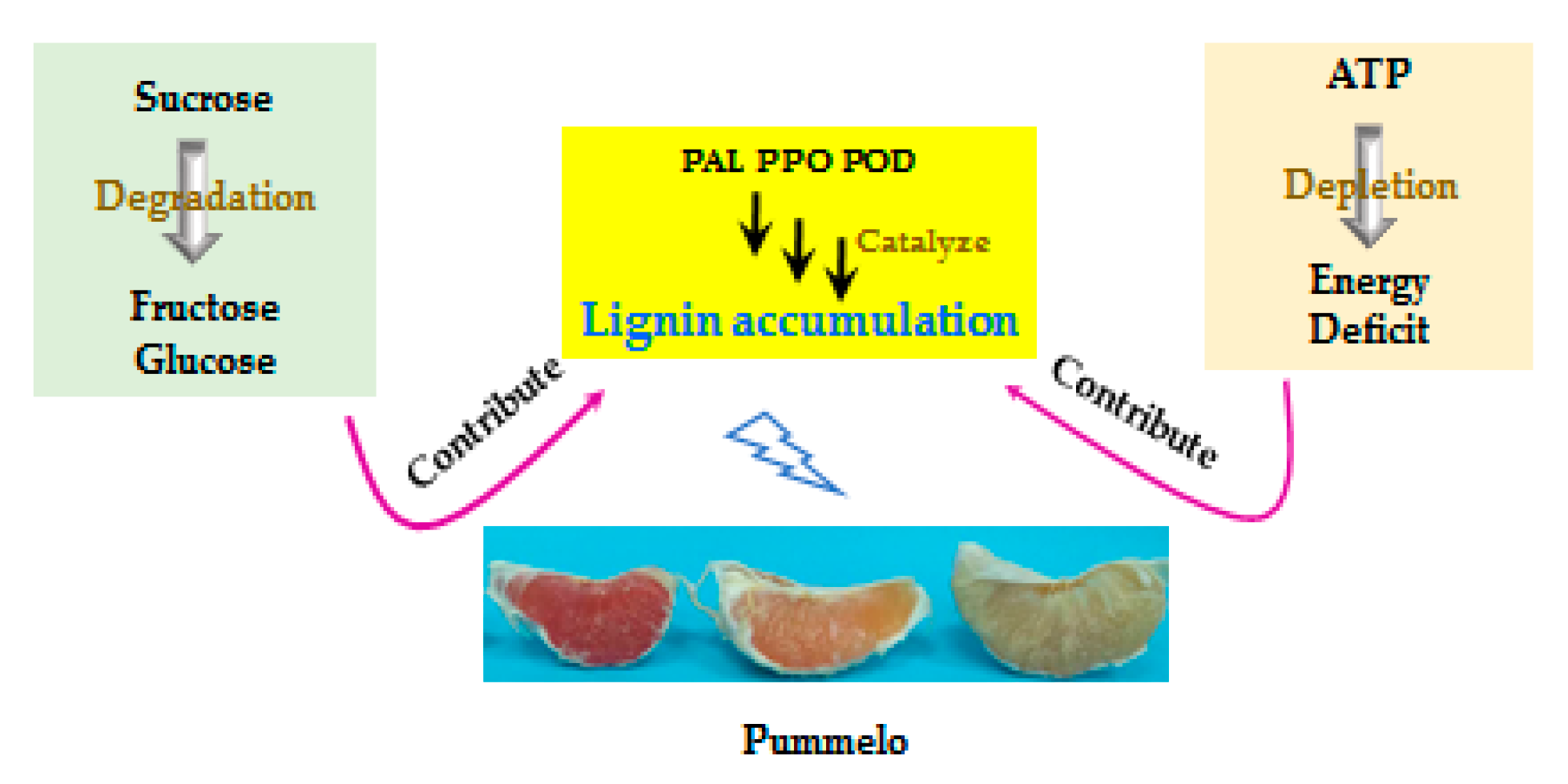
4.1. Lignin Accumulation Was Correlated with Sugar Metabolism
4.2. Lignin Accumulation Was Correlated with Energy Metabolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pan, D.M.; Zheng, G.H.; Chen, G.X.; She, W.Q.; Guo, Z.X.; Shi, M.T.; Lin, H.Y. Analysis of the reasons caused granulation of juice sacs in Guanximiyou pomelo variety. J. Fruit Sci. 1999, 16, 202–209. [Google Scholar]
- She, W.Q. An Analysis on Physiological Changes and Gene Differential Expressions in the Process of Pummelo Fruit [Citrus grandis (L.) osbeck] Juice sac Granulation. Ph.D. Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2009. [Google Scholar]
- Shomer, I.; Chalutz, E.; Vasiliver, R.; Lomaniec, E.; Berman, M. Scierification of juice sacs in pummelo (Citrus grandis) fruit. Can. J. Bot. 1989, 67, 625–632. [Google Scholar] [CrossRef]
- Wu, J.L.; Pan, T.F.; Guo, Z.X.; Pan, D.M. Specific lignin accumulation in granulated juice sacs of citrus maxima. J. Agric. Food Chem. 2014, 62, 12082–12089. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Hang, G.; Chen, Z.; Xian, L.; Donald, G.; Chen, K.S.; Yin, X.R. Ejodo1, a MYB transcription factor, regulating lignin biosynthesis in developing loquat (Eriobotrya japonica) fruit. Front. Plant. Sci. 2016, 7, 1360. [Google Scholar]
- Kamdee, C.; Imsabai, W.; Kirk, R.; Allan, A.C.; Ferguson, I.B.; Ketsa, S. Regulation of lignin biosynthesis in fruit pericarp hardening of mangosteen (Garcinia mangostana L.) after impact. Postharvest Biol. Technol. 2014, 97, 68–76. [Google Scholar] [CrossRef]
- Li, H.; Suo, J.; Han, Y.; Liang, C.; Jin, M.; Zhang, Z.; Rao, J. The effect of 1-methylcyclopropene, methyl jasmonate and methyl salicylate on lignin accumulation and gene expression in postharvest ‘xuxiang’ kiwifruit during cold storage. Postharvest Biol. Technol. 2017, 124, 107–118. [Google Scholar] [CrossRef]
- Cai, C.; Xu, C.J.; Li, X.; Ferguson, I.; Chen, K.S. Accumulation of lignin in relation to change in activities of lignification enzymes in loquat fruit flesh after harvest. Postharvest Biol. Technol. 2006, 40, 163–169. [Google Scholar] [CrossRef]
- Ranadive, A.S.; Haard, N.F. Peroxidase localization and lignin formation in developing pear fruit. J. Fruit Sci. 2010, 37, 381–383. [Google Scholar] [CrossRef]
- Boudet, A.M.; Kajita, S.; Grima-Pettenati, J.; Goffner, D. Lignins and lignocellulosics: A better control of synthesis for new and improved uses. Trends Plant Sci. 2003, 8, 576–581. [Google Scholar] [CrossRef]
- Donaldson, L.A. Lignification and lignin topochemistry—An ultrastructural view. Phytochemistry 2001, 57, 859–873. [Google Scholar] [CrossRef]
- Ipelcl, Z.; Ogras, T.; Altinkut, A. Reduced leaf peroxidase activity is associated with reduced lignin content in transgenic poplar. Plant Biotechnol. 1999, 16, 381–387. [Google Scholar]
- Sabella, E.; Luvisi, A.; Aprile, A.; Negro, C.; Vergine, M.; Nicolì, F.; Miceli, A.; Bellis, L.D. Xylella fastidiosa induces differential expression of lignification related-genes and lignin accumulation in tolerant olive trees cv. leccino. J. Plant Physiol. 2018, 220, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Chen, F.; Wu, G.; Gao, W. Impact of postharvest nitric oxide treatment on lignin biosynthesis-related genes in wax apple (Syzygium samarangense) fruit. J. Agric. Food Chem. 2016, 64, 8483–8490. [Google Scholar] [CrossRef]
- Herrmann, K.M.; Weaver, L.M. The shikimate pathway. Annu. Rev. Plant Phys. 1999, 50, 473–503. [Google Scholar] [CrossRef] [PubMed]
- Kocal, N.; Sonnewald, U.; Sonnewald, S. Cell wall-bound invertase limits sucrose export and is involved in symptom development and inhibition of photosynthesis during compatible interaction between tomato and Xanthomonas camtpestris pv vesicatoria. Plant Physiol. 2016, 148, 1523–1536. [Google Scholar] [CrossRef]
- Schaewen, A.V.; Stitt, M.; Schmidt, R.; Sonnewald, U.; Willmitzer, L. Expression of a yeast-derived invertase in the cell wall of tobacco and Arabidopsis plants leads to accumulation of carbohydrate and inhibition of photosynthesis and strongly influences growth and phenotype of transgenic tobacco plants. EMBO J. 1990, 9, 3033–3044. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, M.; Wu, G. Study of lignification’s delaying and its relationship with energy metabolism in loquat fruits after nitric oxide fumigation. Sci. Agric. Sin. 2014, 47, 2425–2434. [Google Scholar]
- Li, L.; Kitazawa, H.; Wang, X.; Sun, H. Regulation of respiratory pathway and electron transport chain in relation to senescence of postharvest white mushroom (Agaricus bisporus) under high O2/CO2 controlled atmospheres. J. Agric. Food Chem. 2017, 65, 3351–3359. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.J.; Li, T.T.; Yun, Z.; Duan, X.W.; Jiang, Y.M. Fibroin treatment inhibits chilling injury of banana fruit via energy regulation. Sci. Hortic. 2019, 248, 8–13. [Google Scholar] [CrossRef]
- Song, H.; Wang, X.; Hu, W.; Yang, X.; Diao, E.; Shen, T.; Qiang, Q. Acold-induced phytosulfokine peptide is related to the improvement of loquat fruit chilling tolerance. Food Chem. 2017, 232, 434–442. [Google Scholar] [CrossRef]
- Eyéghé-Bickong, H.A.; Alexandersson, E.O.; Gouws, L.M.; Young, P.R.; Vivier, M.A. Optimization of an HPLC method for the simultaneous quantification of the major sugars and organic acids in grapevine berries. J. Chromatogr. 2012, 885, 43–49. [Google Scholar]
- Huang, H.; Jing, G.; Wang, H.; Duan, X.; Qu, H.; Jiang, Y. The combined effects of phenylurea and gibberellins on quality maintenance and shelf life extension of banana fruit during storage. Sci. Hortic. 2014, 167, 36–42. [Google Scholar] [CrossRef]
- Jiang, Y.; Joyce, D.C. ABA effects on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Growth Regul. 2003, 39, 171–174. [Google Scholar] [CrossRef]
- Huang, H.; Jian, Q.J.; Jiang, Y.M.; Duan, X.W.; Qu, H.X. Enhanced chilling tolerance of banana fruit treated with malic acid prior to low-temperature storage. Postharvest Biol. Technol. 2016, 111, 209–213. [Google Scholar] [CrossRef]
- Yang, A.; Zhang, Z.; Cao, A.; Hua, B. Studies of changes in sugar accumulation and lignin deposition during peach fruit endocarp development. Acta Hortic. Sin. 2009, 36, 1113–1119. [Google Scholar]
- Liu, X.; Zhang, J.W.; Guo, L.X.; Liu, Y.Z.; Jin, L.F.; Hussain, S.B.; Du, W.; Zhao, D.; Peng, S.A. Transcriptome changes associated with boron deficiency in leaves of two citrus scion-rootstock combinations. Front. Plant Sci. 2017, 8, 317. [Google Scholar] [CrossRef]
- Verbančič, J.; Lunn, J.E.; Stitt, M.; Persson, S. Carbon supply and the regulation of cell wall synthesis. Mol. Plant 2018, 11, 75–94. [Google Scholar] [CrossRef]
- Chen, X.; Alonso, A.P.; Shacharhill, Y. Dynamic metabolic flux analysis of plant cell wall synthesis. Metab. Eng. 2013, 18, 78–85. [Google Scholar] [CrossRef]
- Shumei, L.; Su, X.; Muhammad, A.; Sun, Y.; Li, G.; Cheng, X.; Lin, Y.; Cai, Y.; Jin, Q. Effects of different pollens on primary metabolism and lignin biosynthesis in pear. Int. J. Mol. Sci. 2018, 19, 2273. [Google Scholar]
- Schäfer, J.; Wagner, S.; Trierweiler, B.; Bunzel, M. Characterization of cell wall components and their modifications during post-harvest storage of Asparagus officinalis L.: Storage-related changes in dietary fiber composition. J. Agric. Food Chem. 2016, 64, 478–486. [Google Scholar]
- Song, L.; Chen, H.; Gao, H.; Fang, X.; Mu, H.; Yuan, Y.; Yang, Q.; Jiang, Y. Combined modified atmosphere packaging and low temperature storage delay lignification and improve the defense response of minimally processed water bamboo shoot. Chem. Cent. J. 2013, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Ring, L.; Yeh, S.Y.; Hucherig, S.; Hoffmann, T.; Blanco-Portales, R.; Fouche, M.; Villatoro, C.; Denoyes, B.; Monfort, A.; Caballero, J.L.; et al. Metabolic interaction between anthocyanin and lignin biosynthesis is associated with peroxidase FaPRX27 in strawberry fruit. Plant Physiol. 2013, 163, 43–60. [Google Scholar] [CrossRef]
- Yi, C.; Jiang, Y.M.; Shi, J.; Qu, H.X.; Duan, X.W.; Yang, B.; Prasad, N.K.; Liu, T. Effect of adenosine triphosphate on changes of fatty acids in harvested litchi fruit infected by Peronophythora litchii. Postharvest Biol. Technol. 2009, 54, 159–164. [Google Scholar] [CrossRef]
- Chumyam, A.; Shank, L.; Uthaibutra, J.; Saengnil, K. Effects of chlorine dioxide on mitochondrial energy levels and redox status of ‘Daw’ longan pericarp during storage. Postharvest Biol. Technol. 2016, 116, 26–35. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, C.; Cheng, S.; Wei, B.; Liu, X.; Ji, S. Changes in energy metabolism accompanying pitting in blueberries stored at low temperature. Food Chem. 2014, 164, 493–501. [Google Scholar] [CrossRef]
- Pan, Y.G.; Yuan, M.Q.; Zhang, W.M.; Zhang, Z.K. Effect of low temperatures on chilling injury in relation to energy status in papaya fruit during storage. Postharvest Biol. Technol. 2017, 125, 181–187. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, Y.; Lin, H.; Ritenour, M.A.; Shi, J.; Zhang, S.; Chen, Y. Hydrogen peroxide-induced pericarp browning of harvested longan fruit in association with energy metabolism. Food Chem. 2017, 225, 31–36. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Huang, Q.; Kang, P.; Liang, L.; Chen, J. Lignin Accumulation in Three Pumelo Cultivars in Association with Sucrose and Energy Depletion. Biomolecules 2019, 9, 701. https://doi.org/10.3390/biom9110701
Liu J, Huang Q, Kang P, Liang L, Chen J. Lignin Accumulation in Three Pumelo Cultivars in Association with Sucrose and Energy Depletion. Biomolecules. 2019; 9(11):701. https://doi.org/10.3390/biom9110701
Chicago/Turabian StyleLiu, Juan, Qinghua Huang, Peizi Kang, Lei Liang, and Junjia Chen. 2019. "Lignin Accumulation in Three Pumelo Cultivars in Association with Sucrose and Energy Depletion" Biomolecules 9, no. 11: 701. https://doi.org/10.3390/biom9110701
APA StyleLiu, J., Huang, Q., Kang, P., Liang, L., & Chen, J. (2019). Lignin Accumulation in Three Pumelo Cultivars in Association with Sucrose and Energy Depletion. Biomolecules, 9(11), 701. https://doi.org/10.3390/biom9110701




