Natural Products and Synthetic Analogs as a Source of Antitumor Drugs
Abstract
1. Introduction
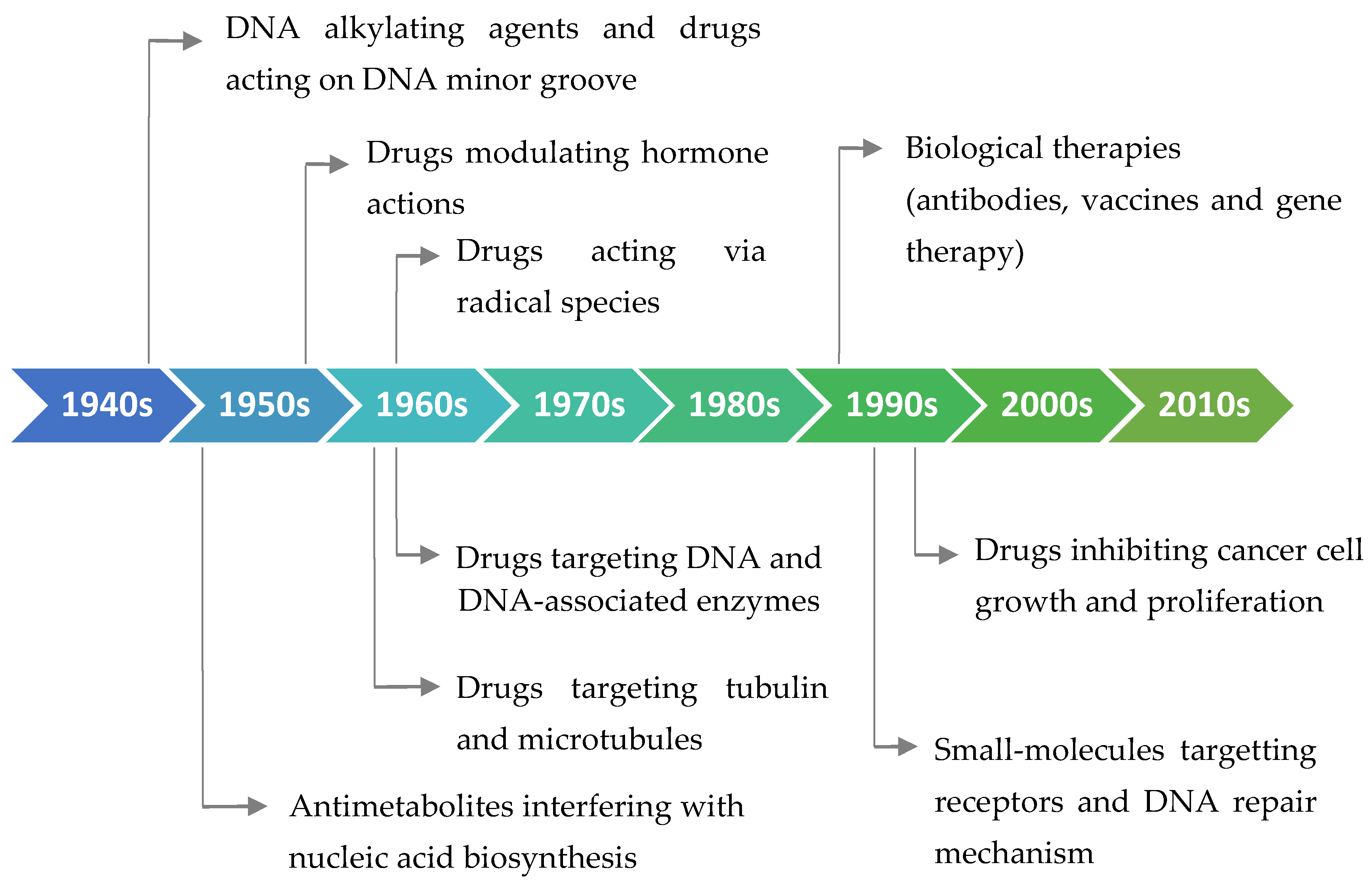
2. Antitumor Drugs: A Brief Medical History, Different Origins, and General Bioactivity
2.1. FDA-Approved Small Molecules as Antitumor Drugs in the Last 10 Years
2.2. FDA-Approved Protein-Based Therapeutics in the Last 10 Years
2.3. FDA-Approved Antibody–Drug Conjugates in the Last 10 Years
3. Plants and Related Bioactive Compounds as New Drug Sources for Different Cancers
3.1. Oral, Gastrointestinal, and Pancreatic Cancers
3.2. Skin Cancer
3.3. Brain Cancer
3.4. Breast Cancer
4. Conclusions and Future Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Cancer Institute. Cancer Statistics. Available online: https://www.cancer.gov/about-cancer/understanding/ (accessed on 31 December 2017).
- World Health Organization (WHO). Cancer: Fact Sheet. Available online: http://www.who.int/mediacentre/factsheets/fs297/en (accessed on 31 December 2017).
- Cho, W.C. Molecular connections of aging and cancer. Aging Dis. 2017, 8, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part B: biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Soares, J.P.; Cortinhas, A.; Bento, T.; Leitao, J.C.; Collins, A.R.; Gaivao, I.; Mota, M.P. Aging and DNA damage in humans: a meta-analysis study. Aging 2014, 6, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Lombard, D.B.; Chua, K.F.; Mostoslavsky, R.; Franco, S.; Gostissa, M.; Alt, F.W. DNA repair, genome stability, and aging. Cell 2005, 120, 497–512. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Z. Anticancer drug discovery in the future: An evolutionary perspective. Drug Discov. Today 2009, 14, 1136–1142. [Google Scholar] [CrossRef]
- Widmer, N.; Bardin, C.; Chatelut, E.; Paci, A.; Beijnen, J.; Leveque, D.; Veal, G.; Astier, A. Review of therapeutic drug monitoring of anticancer drugs part two—targeted therapies. Eur. J. Cancer 2014, 50, 2020–2036. [Google Scholar] [CrossRef]
- Kuczynski, E.A.; Sargent, D.J.; Grothey, A.; Kerbel, R.S. Drug rechallenge and treatment beyond progression—implications for drug resistance. Nat. Rev. Clin. Oncol. 2013, 10, 571–587. [Google Scholar] [CrossRef]
- Check Hayden, E. Cancer complexity slows quest for cure. Nature 2008, 455, 148. [Google Scholar] [CrossRef]
- Jung, K.H.; Noh, J.H.; Kim, J.K.; Eun, J.W.; Bae, H.J.; Chang, Y.G.; Kim, M.G.; Park, W.S.; Lee, J.Y.; Lee, S.Y.; et al. Histone deacetylase 6 functions as a tumor suppressor by activating c-Jun NH2-terminal kinase-mediated beclin 1-dependent autophagic cell death in liver cancer. Hepatology 2012, 56, 644–657. [Google Scholar] [CrossRef]
- Aggarwal, B.; Prasad, S.; Sung, B.; Krishnan, S.; Guha, S. Prevention and Treatment of Colorectal Cancer by Natural Agents From Mother Nature. Curr. Colorectal Cancer Rep. 2013, 9, 37–56. [Google Scholar] [CrossRef]
- Pike, M.C.; Spicer, D.V.; Dahmoush, L.; Press, M.F. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol. Rev. 1993, 15, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Yamane, K.; Tateishi, K.; Klose, R.J.; Fang, J.; Fabrizio, L.A.; Erdjument-Bromage, H.; Taylor-Papadimitriou, J.; Tempst, P.; Zhang, Y. PLU-1 is an H3K4 demethylase involved in transcriptional repression and breast cancer cell proliferation. Mol. Cell 2007, 25, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Shoeb, M. Anticancer agents from medicinal plants. Bangladesh J. Pharmacol. 2008, 1, 35–41. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Drugs and Drug Candidates from marine sources: An assessment of the current “state of play”. Planta Med. 2016, 82, 775–789. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef]
- Mans, D.R.; da Rocha, A.B.; Schwartsmann, G. Anti-cancer drug discovery and development in Brazil: Targeted plant collection as a rational strategy to acquire candidate anti-cancer compounds. Oncologist 2000, 5, 185–198. [Google Scholar] [CrossRef]
- Somasundaram, S.; Edmund, N.A.; Moore, D.T.; Small, G.W.; Shi, Y.Y.; Orlowski, R.Z. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res. 2002, 62, 3868–3875. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Grothaus, P.G.; Cragg, G.M.; Newman, D.J. Plant natural products in anticancer drug discovery. Curr. Org. Chem. 2010, 14, 1781–1791. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef]
- Basmadjian, C.; Zhao, Q.; Bentouhami, E.; Djehal, A.; Nebigil, C.G.; Johnson, R.A.; Serova, M.; de Gramont, A.; Faivre, S.; Raymond, E.; et al. Cancer wars: Natural products strike back. Front. Chem. 2014, 2, 20. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Nature: A vital source of leads for anticancer drug development. Phytochem. Rev. 2009, 8, 313–331. [Google Scholar] [CrossRef]
- Sithranga Boopathy, N.; Kathiresan, K. Anticancer drugs from marine flora: An overview. J. Oncol. 2010, 2010, 214186. [Google Scholar] [CrossRef]
- Kingston, D.G.I. Modern natural products drug discovery and its relevance to biodiversity conservation. J. Nat. Prod. 2011, 74, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.J.; Murphy, L.L.; Venema, R.C.; Singletary, K.W.; Young, A.J. Curcumin binds tubulin, induces mitotic catastrophe, and impedes normal endothelial cell proliferation. Food Chem. Toxicol. 2013, 60, 431–438. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Sharad, S.; Maheshwari, R.K. Skin regenerative potentials of curcumin. Biofactors 2013, 39, 141–149. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.J.; Pandey, S. Chemo-resistant melanoma sensitized by tamoxifen to low dose curcumin treatment through induction of apoptosis and autophagy. Cancer Biol. 2011, 11, 216–228. [Google Scholar] [CrossRef]
- Malik, S.; Cusidó, R.M.; Mirjalili, M.H.; Moyano, E.; Palazón, J.; Bonfill, M. Production of the anticancer drug taxol in Taxus baccata suspension cultures: A review. Process Biochem. 2011, 46, 23–34. [Google Scholar] [CrossRef]
- Prota, A.E.; Bargsten, K.; Zurwerra, D.; Field, J.J.; Diaz, J.F.; Altmann, K.H.; Steinmetz, M.O. Molecular mechanism of action of microtubule-stabilizing anticancer agents. Science 2013, 339, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Yang, H.; Cabral, F. Paclitaxel-dependent cell lines reveal a novel drug activity. Mol. Cancer Ther. 2010, 9, 2914–2923. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, K.; Aparajitha, U.K. Paclitaxel against cancer: A short review. Med. Chem. 2012, 2, 139–141. [Google Scholar] [CrossRef]
- Morales-Cano, D.; Calvino, E.; Rubio, V.; Herraez, A.; Sancho, P.; Tejedor, M.C.; Diez, J.C. Apoptosis induced by paclitaxel via Bcl-2, Bax and caspases 3 and 9 activation in NB4 human leukaemia cells is not modulated by ERK inhibition. Exp. Toxicol. Pathol. 2013, 65, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Harsha, C.; Banik, K.; Vikkurthi, R.; Sailo, B.L.; Bordoloi, D.; Gupta, S.C.; Aggarwal, B.B. Is curcumin bioavailability a problem in humans: Lessons from clinical trials. Expert Opin. Drug Metab. Toxicol. 2019, 15, 705–733. [Google Scholar] [CrossRef]
- Costa-Lotufo, L.V.; Wilke, D.V.; Jimenez, P.C.; Epifanio, R.d.A. Organismos marinhos como fonte de novos fármacos: Histórico & perspectivas. Química Nova 2009, 32, 703–716. [Google Scholar]
- La Clair, J.J. Natural product mode of action (MOA) studies: A link between natural and synthetic worlds. Nat. Prod. Rep. 2010, 27, 969–995. [Google Scholar] [CrossRef]
- Carlson, E.E. Natural products as chemical probes. Acs Chem. Biol. 2010, 5, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Valli, M.; dos Santos, R.N.; Figueira, L.D.; Nakajima, C.H.; Castro-Gamboa, I.; Andricopulo, A.D.; Bolzani, V.S. Development of a natural products database from the biodiversity of Brazil. J. Nat. Prod. 2013, 76, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. New horizons for old drugs and drug leads. J. Nat. Prod. 2014, 77, 703–723. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, J.F.; Labes, A.; Wiese, J. Bio-mining the microbial treasures of the ocean: New natural products. Biotechnol. Adv. 2011, 29, 468–482. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Latest global cancer data: Cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018. Int. Agency Res. Cancer 2018, 263. [Google Scholar]
- Yan, S.-H. An early history of human breast cancer: West meets East. Chin. J. Cancer 2013, 32, 475. [Google Scholar] [CrossRef]
- Sudhakar, A. History of cancer, ancient and modern treatment methods. J. Cancer Sci. 2009, 1, 1. [Google Scholar] [CrossRef]
- Ghosh, S.K. Giovanni Battista Morgagni (1682–1771): Father of pathologic anatomy and pioneer of modern medicine. Anat. Sci. Int. 2017, 92, 305–312. [Google Scholar] [CrossRef]
- Dobson, J. John Hunter’s views on cancer. Ann. R. Coll. Surg. Engl. 1959, 25, 176. [Google Scholar] [PubMed]
- Walter, E.; Scott, M. The life and work of Rudolf Virchow 1821–1902: “Cell theory, thrombosis and the sausage duel”. J. Intensive Care Soc. 2017, 18, 234–235. [Google Scholar] [CrossRef] [PubMed]
- Papac, R.J. Origins of cancer therapy. Yale J. Biol. Med. 2001, 74, 391. [Google Scholar] [PubMed]
- Kaufmann, S.H. Paul Ehrlich: Founder of chemotherapy. Nat. Rev. Drug Discov. 2008, 7, 373. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.T.; Chu, E. A history of cancer chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef] [PubMed]
- Avendaño, C.; Menendez, J.C. Medicinal chemistry of anticancer drugs; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Kinch, M.S. An analysis of FDA-approved drugs for oncology. Drug Discov. Today 2014, 19, 1831–1835. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.G.; Ferreira, L.L.; Andricopulo, A.D. Recent advances and perspectives in cancer drug design. Da Acad. Bras. De Ciências 2018, 90, 1233–1250. [Google Scholar]
- Williams, C.T. Food and Drug Administration drug approval process: a history and overview. Nurs. Clin. 2016, 51, 1–11. [Google Scholar] [CrossRef]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016, 40–41, 1–3. [Google Scholar] [CrossRef]
- Nightingale, G.; Ryu, J. Cabazitaxel (jevtana): A novel agent for metastatic castration-resistant prostate cancer. Pharm. Ther. 2012, 37, 440. [Google Scholar]
- Pal, S.K.; Twardowski, P.; Sartor, O. Critical appraisal of cabazitaxel in the management of advanced prostate cancer. Clin. Interv. Aging 2010, 5, 395. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirata, Y.; Uemura, D. Halichondrins-antitumor polyether macrolides from a marine sponge. Pure Appl. Chem. 1986, 58, 701–710. [Google Scholar] [CrossRef]
- Towle, M.J.; Salvato, K.A.; Budrow, J.; Wels, B.F.; Kuznetsov, G.; Aalfs, K.K.; Welsh, S.; Zheng, W.; Seletsky, B.M.; Palme, M.H. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 2001, 61, 1013–1021. [Google Scholar] [PubMed]
- Aicher, T.D.; Buszek, K.R.; Fang, F.G.; Forsyth, C.J.; Jung, S.H.; Kishi, Y.; Matelich, M.C.; Scola, P.M.; Spero, D.M.; Yoon, S.K. Total synthesis of halichondrin B and norhalichondrin B. J. Am. Chem. Soc. 1992, 114, 3162–3164. [Google Scholar] [CrossRef]
- Wang, Y.; Habgood, G.J.; Christ, W.J.; Kishi, Y.; Littlefield, B.A.; Melvin, J.Y. Structure–activity relationships of halichondrin B analogues: modifications at C. 30–C. 38. Bioorg. Med. Chem. Lett. 2000, 10, 1029–2032. [Google Scholar] [CrossRef]
- Bai, R.; Paull, K.D.; Herald, C.L.; Malspeis, L.; Pettit, G.R.; Hamel, E. Halichondrin B and homohalichondrin B, marine natural products binding in the vinca domain of tubulin. Discovery of tubulin-based mechanism of action by analysis of differential cytotoxicity data. J. Biol. Chem. 1991, 266, 15882–15889. [Google Scholar]
- Stamos, D.P.; Chen, S.S.; Kishi, Y. New Synthetic Route to the C. 14− C. 38 Segment of Halichondrins. J. Org. Chem. 1997, 62, 7552–7553. [Google Scholar] [CrossRef]
- Jordan, M.A.; Kamath, K.; Manna, T.; Okouneva, T.; Miller, H.P.; Davis, C.; Littlefield, B.A.; Wilson, L. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol. Cancer. Ther. 2005, 4, 1086–1095. [Google Scholar] [CrossRef]
- Dabydeen, D.A.; Burnett, J.C.; Bai, R.; Verdier-Pinard, P.; Hickford, S.J.; Pettit, G.R.; Blunt, J.W.; Munro, M.H.; Gussio, R.; Hamel, E. Comparison of the activities of the truncated halichondrin B analog NSC 707389 (E7389) with those of the parent compound and a proposed binding site on tubulin. Mol. Pharm. 2006, 70, 1866–1875. [Google Scholar] [CrossRef]
- Alday, P.H.; Correia, J.J. Macromolecular interaction of halichondrin B analogues eribulin (E7389) and ER-076349 with tubulin by analytical ultracentrifugation. Biochemistry 2009, 48, 7927–7938. [Google Scholar] [CrossRef]
- Okouneva, T.; Azarenko, O.; Wilson, L.; Littlefield, B.A.; Jordan, M.A. Inhibition of centromere dynamics by eribulin (E7389) during mitotic metaphase. Mol. Cancer Ther. 2008, 7, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, G.; Towle, M.J.; Cheng, H.; Kawamura, T.; TenDyke, K.; Liu, D.; Kishi, Y.; Melvin, J.Y.; Littlefield, B.A. Induction of morphological and biochemical apoptosis following prolonged mitotic blockage by halichondrin B macrocyclic ketone analog E7389. Cancer Res. 2004, 64, 5760–5766. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I.S.; Armstrong, J.G.; Gorman, M.; Burnett, J.P. The vinca alkaloids: A new class of oncolytic agents. Cancer Res. 1963, 23. [Google Scholar]
- Noble, R.L. The discovery of the vinca alkaloids—chemotherapeutic agents against cancer. Biochem. Cell Biol. 1990, 68, 1344–1351. [Google Scholar] [CrossRef]
- Noble, R.; Beer, C.; Cutts, J. Role of chance observations in chemotherapy: Vinca rosea. Ann. N. Y. Acad. Sci. 1958, 76, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.A.; Deitcher, S.R. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharm. 2013, 71, 555–564. [Google Scholar] [CrossRef]
- Morales, J.; Li, L.; Fattah, F.J.; Dong, Y.; Bey, E.A.; Patel, M.; Gao, J.; Boothman, D.A. Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases. Crit. Rev. TM Eukaryot. Gene Expr. 2014, 24. [Google Scholar] [CrossRef]
- Jenner, Z.B.; Sood, A.K.; Coleman, R.L. Evaluation of rucaparib and companion diagnostics in the PARP inhibitor landscape for recurrent ovarian cancer therapy. Future Oncol. 2016, 12, 1439–1456. [Google Scholar] [CrossRef]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef]
- McCann, K.E. Advances in the use of PARP inhibitors for BRCA1/2-associated breast cancer: Talazoparib. Future Oncol. 2019, 15, 1707–1715. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860. [Google Scholar] [CrossRef] [PubMed]
- Murata, M. Inflammation and cancer. Environ. Health Prev. Med. 2018, 23, 50. [Google Scholar] [CrossRef] [PubMed]
- Kapche, D.W.; Lekane, N.M.; Kulabas, S.S.; Ipek, H.; Tok, T.T.; Ngadjui, B.T.; Demirtas, I.; Tumer, T.B. Aryl benzofuran derivatives from the stem bark of Calpocalyx dinklagei attenuate inflammation. Phytochemistry 2017, 141, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Tumer, T.B.; Yılmaz, B.; Ozleyen, A.; Kurt, B.; Tok, T.T.; Taskin, K.M.; Kulabas, S.S. GR24, a synthetic analog of Strigolactones, alleviates inflammation and promotes Nrf2 cytoprotective response: In vitro and in silico evidences. Comput. Biol. Chem. 2018, 76, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584. [Google Scholar] [CrossRef]
- Kulabas, S.; Ipek, H.; Tufekci, A.; Arslan, S.; Demirtas, I.; Ekren, R.; Sezerman, U.; Tumer, T. Ameliorative potential of Lavandula stoechas in metabolic syndrome via multitarget interactions. J. Ethnopharmacol. 2018, 223, 88–98. [Google Scholar] [CrossRef]
- McCurdy, A.R.; Lacy, M.Q. Pomalidomide and its clinical potential for relapsed or refractory multiple myeloma: An update for the hematologist. Ther. Adv. Hematol. 2013, 4, 211–216. [Google Scholar] [CrossRef]
- Morabito, F.; Skafi, M.; Recchia, A.G.; Kashkeesh, A.; Hindiyeh, M.; Sabatleen, A.; Morabito, L.; Alijanazreh, H.; Hamamreh, Y.; Gentile, M. Lenalidomide for the treatment of mantle cell lymphoma. Expert Opin. Pharmacother. 2019, 1–8. [Google Scholar] [CrossRef]
- Hideshima, T.; Raje, N.; Richardson, P.G.; Anderson, K.C. A review of lenalidomide in combination with dexamethasone for the treatment of multiple myeloma. Ther. Clin. Risk Manag. 2008, 4, 129. [Google Scholar] [CrossRef]
- Butti, R.; Das, S.; Gunasekaran, V.P.; Yadav, A.S.; Kumar, D.; Kundu, G.C. Receptor tyrosine kinases (RTKs) in breast cancer: Signaling, therapeutic implications and challenges. Mol. Cancer 2018, 17, 34. [Google Scholar] [CrossRef]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Vlahovic, G.; Crawford, J. Activation of tyrosine kinases in cancer. Oncologist 2003, 8, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Ito, F. Receptor tyrosine kinases and targeted cancer therapeutics. Biol. Pharm. Bull. 2011, 34, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Nishina, T.; Takahashi, S.; Iwasawa, R.; Noguchi, H.; Aoki, M.; Doi, T. Safety, pharmacokinetic, and pharmacodynamics of erdafitinib, a pan-fibroblast growth factor receptor (FGFR) tyrosine kinase inhibitor, in patients with advanced or refractory solid tumors. Investig. New Drugs 2018, 36, 424–434. [Google Scholar] [CrossRef]
- Haugsten, E.M.; Wiedlocha, A.; Olsnes, S.; Wesche, J. Roles of fibroblast growth factor receptors in carcinogenesis. Mol. Cancer Res. 2010, 8, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Lenvatinib: First global approval. Drugs 2015, 75, 553–560. [Google Scholar] [CrossRef]
- Verheul, H.M.; Pinedo, H.M. The role of vascular endothelial growth factor (VEGF) in tumor angiogenesis and early clinical development of VEGFReceptor kinase inhibitors. Clin. Breast Cancer 2000, 1, S80–S84. [Google Scholar] [CrossRef]
- Kelly, R.J.; Rixe, O. Axitinib—a selective inhibitor of the vascular endothelial growth factor (VEGF) receptor. Target. Oncol. 2009, 4, 297–305. [Google Scholar] [CrossRef]
- DrugBank. The Drug Database. Available online: https://www.drugbank.ca/ (accessed on 31 October 2019).
- Dimitrov, D.S. Therapeutic proteins. In Therapeutic Proteins; Springer: Totowa, NJ, USA, 2012; pp. 1–26. [Google Scholar]
- Lagassé, H.D.; Alexaki, A.; Simhadri, V.L.; Katagiri, N.H.; Jankowski, W.; Sauna, Z.E.; Kimchi-Sarfaty, C. Recent advances in (therapeutic protein) drug development. F1000Research 2017, 6, 113. [Google Scholar] [CrossRef]
- Zhu, J. Mammalian cell protein expression for biopharmaceutical production. Biotechnol. Adv. 2012, 30, 1158–1170. [Google Scholar] [CrossRef]
- Tai, W.; Mahato, R.; Cheng, K. The role of HER2 in cancer therapy and targeted drug delivery. J. Control. Release 2010, 146, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Gunturu, K.S.; Woo, Y.; Beaubier, N.; Remotti, H.E.; Saif, M.W. Gastric cancer and trastuzumab: First biologic therapy in gastric cancer. Ther. Adv. Med Oncol. 2013, 5, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Casak, S.J.; Fashoyin-Aje, I.; Lemery, S.J.; Zhang, L.; Jin, R.; Li, H.; Zhao, L.; Zhao, H.; Zhang, H.; Chen, H. FDA approval summary: Ramucirumab for gastric cancer. Clin. Cancer Res. 2015, 21, 3372–3376. [Google Scholar] [CrossRef] [PubMed]
- Vora, C.; Gupta, S. Targeted therapy in cervical cancer. Esmo Open 2019, 3, e000462. [Google Scholar] [CrossRef] [PubMed]
- The Nobel Prize in Physiology or Medicine. 2018. Available online: https://www.nobelprize.org/prizes/medicine/2018/summary/ (accessed on 31 October 2019).
- Hammerich, L.; Binder, A.; Brody, J.D. In situ vaccination: Cancer immunotherapy both personalized and off-the-shelf. Mol. Oncol. 2015, 9, 1966–1981. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.D.; Hurwitz, A.A.; Foster, B.A.; Madias, C.; Feldhaus, A.L.; Greenberg, N.M.; Burg, M.B.; Allison, J.P. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc. Natl. Acad. Sci. USA 1997, 94, 8099–8103. [Google Scholar] [CrossRef]
- Hodi, F.S.; Mihm, M.C.; Soiffer, R.J.; Haluska, F.G.; Butler, M.; Seiden, M.V.; Davis, T.; Henry-Spires, R.; MacRae, S.; Willman, A. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc. Natl. Acad. Sci. USA 2003, 100, 4712–4717. [Google Scholar] [CrossRef]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science (N. Y.) 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Johnson, D.B.; Peng, C.; Abramson, R.G.; Ye, F.; Zhao, S.; Wolchok, J.D.; Sosman, J.A.; Carvajal, R.D.; Ariyan, C.E. Clinical activity of ipilimumab in acral melanoma: A retrospective review. Oncologist 2015, 20, 648–652. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.M. Pembrolizumab: First global approval. Drugs 2014, 74, 1973–1981. [Google Scholar] [CrossRef] [PubMed]
- FDA Approval. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-ipilimumab-msi-h-or-dmmr-metastatic-colorectal-cancer (accessed on 31 October 2019).
- Massard, C.; Gordon, M.S.; Sharma, S.; Rafii, S.; Wainberg, Z.A.; Luke, J.; Curiel, T.J.; Colon-Otero, G.; Hamid, O.; Sanborn, R.E. Safety and efficacy of durvalumab (MEDI4736), an anti–programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J. Clin. Oncol. 2016, 34, 3119. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Atezolizumab: first global approval. Drugs 2016, 76, 1227–1232. [Google Scholar] [CrossRef]
- Diamantis, N.; Banerji, U. Antibody-drug conjugates—an emerging class of cancer treatment. Br. J. Cancer 2016, 114, 362. [Google Scholar] [CrossRef]
- Tsuchikama, K.; An, Z. Antibody-drug conjugates: Recent advances in conjugation and linker chemistries. Protein Cell 2018, 9, 33–46. [Google Scholar] [CrossRef]
- FDA Approval History. Available online: https://www.centerwatch.com/drug-information/fda-approved-drugs/drug/1254/kadcyla-ado-trastuzumab-emtansine (accessed on 31 October 2019).
- Phillips, G.D.L.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.; Lutz, R.J. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody–cytotoxic drug conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef]
- Asparlas Approval History. Available online: https://www.drugs.com/history/asparlas.html (accessed on 31 October 2019).
- Angiolillo, A.L.; Schore, R.J.; Devidas, M.; Borowitz, M.J.; Carroll, A.J.; Gastier-Foster, J.M.; Heerema, N.A.; Keilani, T.; Lane, A.R.; Loh, M.L. Pharmacokinetic and pharmacodynamic properties of calaspargase pegol Escherichia coli L-asparaginase in the treatment of patients with acute lymphoblastic leukemia: Results from Children’s Oncology Group Study AALL07P4. J. Clin. Oncol. 2014, 32, 3874. [Google Scholar] [CrossRef]
- Mehta, R.G.; Murillo, G.; Naithani, R.; Peng, X. Cancer chemoprevention by natural products: How far have we come? Pharm. Res. 2010, 27, 950–961. [Google Scholar] [CrossRef]
- Huang, W.Y.; Cai, Y.Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2010, 62, 1–20. [Google Scholar] [CrossRef]
- Desai, A.G.; Qazi, G.N.; Ganju, R.K.; El-Tamer, M.; Singh, J.; Saxena, A.K.; Bedi, Y.S.; Taneja, S.C.; Bhat, H.K. Medicinal plants and cancer chemoprevention. Curr. Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Nagler, R.M. Saliva as a tool for oral cancer diagnosis and prognosis. Oral Oncol. 2009, 45, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Amin, A.R.; Shin, D.M. Chemopreventive potential of natural compounds in head and neck cancer. Nutr. Cancer 2010, 62, 973–987. [Google Scholar] [CrossRef]
- Nam, W.; Tak, J.; Ryu, J.K.; Jung, M.; Yook, J.I.; Kim, H.J.; Cha, I.H. Effects of artemisinin and its derivatives on growth inhibition and apoptosis of oral cancer cells. Head Neck 2007, 29, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Kojima, H.; Yoshida, K.; Kufe, D. Caspase-3-mediated cleavage of protein kinase C theta in induction of apoptosis. J. Biol. Chem. 1997, 272, 20317–20320. [Google Scholar] [CrossRef]
- D’Amours, D.; Germain, M.; Orth, K.; Dixit, V.M.; Poirier, G.G. Proteolysis of poly(ADP-ribose) polymerase by caspase 3: Kinetics of cleavage of mono(ADP-ribosyl)ated and DNA-bound substrates. Radiat. Res. 1998, 150, 3–10. [Google Scholar] [CrossRef]
- Fujisawa, S.; Atsumi, T.; Satoh, K.; Kadoma, Y.; Ishihara, M.; Okada, N.; Nagasaki, M.; Yokoe, I.; Sakagami, H. Radical generation, radical-scavenging activity, and cytotoxicity of eugenol-related compounds. Vitr. Mol. Toxicol. 2000, 13, 269–280. [Google Scholar]
- Carrasco, A.H.; Espinoza, C.L.; Cardile, V.; Gallardo, C.; Cardona, W.; Lombardo, L.; Catalán, M.K.; Cuellar, F.M.; Russo, A. Eugenol and its synthetic analogues inhibit cell growth of human cancer cells (Part I). J. Braz. Chem. Soc. 2008, 19, 543–548. [Google Scholar] [CrossRef]
- Jeng, J.H.; Hahn, L.J.; Lu, F.J.; Wang, Y.J.; Kuo, M.Y. Eugenol triggers different pathobiological effects on human oral mucosal fibroblasts. J. Dent. Res. 1994, 73, 1050–1055. [Google Scholar] [CrossRef]
- Ziech, D.; Anestopoulos, I.; Hanafi, R.; Voulgaridou, G.P.; Franco, R.; Georgakilas, A.G.; Pappa, A.; Panayiotidis, M.I. Pleiotrophic effects of natural products in ROS-induced carcinogenesis: The role of plant-derived natural products in oral cancer chemoprevention. Cancer Lett. 2012, 327, 16–25. [Google Scholar] [CrossRef]
- Ni, W.F.; Tsai, C.H.; Yang, S.F.; Chang, Y.C. Elevated expression of NF-kappaB in oral submucous fibrosis--evidence for NF-kappaB induction by safrole in human buccal mucosal fibroblasts. Oral Oncol. 2007, 43, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, A.; Tony Kong, A.N. Anticarcinogenesis by dietary phytochemicals: Cytoprotection by Nrf2 in normal cells and cytotoxicity by modulation of transcription factors NF-kappa B and AP-1 in abnormal cancer cells. Food Chem. Toxicol. 2008, 46, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.L.; Liu, T.Y.; Wu, C.W.; Chi, C.W. Berberine modulates expression of mdr1 gene product and the responses of digestive track cancer cells to Paclitaxel. Br. J. Cancer 1999, 81, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Yang, J.S.; Chen, J.T.; Fan, S.; Yu, F.S.; Yang, J.L.; Lu, C.C.; Kao, M.C.; Huang, A.C.; Lu, H.F.; et al. Berberine induces apoptosis in human HSC-3 oral cancer cells via simultaneous activation of the death receptor-mediated and mitochondrial pathway. Anticancer Res. 2007, 27, 3371–3378. [Google Scholar] [PubMed]
- Liang, W.Z.; Chou, C.T.; Lu, T.; Chi, C.C.; Tseng, L.L.; Pan, C.C.; Lin, K.L.; Kuo, C.C.; Jan, C.R. The mechanism of carvacrol-evoked [Ca2+]i rises and non-Ca2+-triggered cell death in OC2 human oral cancer cells. Toxicology 2013, 303, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Sertel, S.; Eichhorn, T.; Plinkert, P.K.; Efferth, T. Cytotoxicity of Thymus vulgaris essential oil towards human oral cavity squamous cell carcinoma. Anticancer Res. 2011, 31, 81–87. [Google Scholar]
- Sertel, S.; Eichhorn, T.; Plinkert, P.K.; Efferth, T. [Anticancer activity of Salvia officinalis essential oil against HNSCC cell line (UMSCC1)]. Hno 2011, 59, 1203–1208. [Google Scholar] [CrossRef]
- Sertel, S.; Eichhorn, T.; Plinkert, P.K.; Efferth, T. Chemical Composition and antiproliferative activity of essential oil from the leaves of a medicinal herb, Levisticum officinale, against UMSCC1 head and neck squamous carcinoma cells. Anticancer Res. 2011, 31, 185–191. [Google Scholar]
- Manosroi, J.; Dhumtanom, P.; Manosroi, A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett. 2006, 235, 114–120. [Google Scholar] [CrossRef]
- Cha, J.D.; Jeong, M.R.; Kim, H.Y.; Lee, J.C.; Lee, K.Y. MAPK activation is necessary to the apoptotic death of KB cells induced by the essential oil isolated from Artemisia iwayomogi. J. Ethnopharmacol. 2009, 123, 308–314. [Google Scholar] [CrossRef]
- Cha, J.D.; Moon, S.E.; Kim, H.Y.; Cha, I.H.; Lee, K.Y. Essential oil of Artemisia capillaris induces apoptosis in KB cells via mitochondrial stress and caspase activation mediated by MAPK-stimulated signaling pathway. J. Food Sci. 2009, 74, T75–T81. [Google Scholar] [CrossRef] [PubMed]
- Keawsa-ard, S.; Liawruangrath, B.; Liawruangrath, S.; Teerawutgulrag, A.; Pyne, S.G. Chemical constituents and antioxidant and biological activities of the essential oil from leaves of Solanum spirale. Nat. Prod. Commun. 2012, 7, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.E. Anti-cancer properties of Nigella spp. essential oils and their major constituents, thymoquinone and beta-elemene. Curr. Clin. Pharm. 2009, 4, 43–46. [Google Scholar] [CrossRef]
- Pisseri, F.; Bertoli, A.; Pistelli, L. Essential oils in medicine: principles of therapy. Parassitologia 2008, 50, 89–91. [Google Scholar]
- Seifried, H.E.; Anderson, D.E.; Fisher, E.I.; Milner, J.A. A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 2007, 18, 567–579. [Google Scholar] [CrossRef]
- Ziech, D.; Franco, R.; Georgakilas, A.G.; Georgakila, S.; Malamou-Mitsi, V.; Schoneveld, O.; Pappa, A.; Panayiotidis, M.I. The role of reactive oxygen species and oxidative stress in environmental carcinogenesis and biomarker development. Chem.-Biol. Interact. 2010, 188, 334–339. [Google Scholar] [CrossRef]
- Harish Kumar, G.; Vidya Priyadarsini, R.; Vinothini, G.; Vidjaya Letchoumy, P.; Nagini, S. The neem limonoids azadirachtin and nimbolide inhibit cell proliferation and induce apoptosis in an animal model of oral oncogenesis. Investig. New Drugs 2010, 28, 392–401. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Lee, M.S.; Wu, C.R.; Cho, H.J.; Lin, K.Y.; Lai, G.H.; Wang, S.Y.; Kuo, Y.H.; Kumar, K.J.; Yang, H.L. The chalcone flavokawain B induces G2/M cell-cycle arrest and apoptosis in human oral carcinoma HSC-3 cells through the intracellular ROS generation and downregulation of the Akt/p38 MAPK signaling pathway. J. Agric. Food Chem. 2012, 60, 2385–2397. [Google Scholar] [CrossRef]
- Su, M.; Chung, H.Y.; Li, Y. Deoxyelephantopin from Elephantopus scaber L. induces cell-cycle arrest and apoptosis in the human nasopharyngeal cancer CNE cells. Biochem. Biophys. Res. Commun. 2011, 411, 342–347. [Google Scholar] [CrossRef]
- Liang, Z.; Guo, Y.T.; Yi, Y.J.; Wang, R.C.; Hu, Q.L.; Xiong, X.Y. Ganoderma lucidum polysaccharides target a Fas/caspase dependent pathway to induce apoptosis in human colon cancer cells. Asian Pac. J. Cancer Prev. 2014, 15, 3981–3986. [Google Scholar] [CrossRef]
- Thyagarajan, A.; Jedinak, A.; Nguyen, H.; Terry, C.; Baldridge, L.A.; Jiang, J.; Sliva, D. Triterpenes from Ganoderma Lucidum induce autophagy in colon cancer through the inhibition of p38 mitogen-activated kinase (p38 MAPK). Nutr. Cancer 2010, 62, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Romano, B.; Borrelli, F.; Pagano, E.; Cascio, M.G.; Pertwee, R.G.; Izzo, A.A. Inhibition of colon carcinogenesis by a standardized Cannabis sativa extract with high content of cannabidiol. Phytomedicine 2014, 21, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, W.; Liu, F. Inhibition of proliferation and induction of apoptosis by genistein in colon cancer HT-29 cells. Cancer Lett. 2004, 215, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, S.X.; Zhou, Z.Q.; Wang, Z.; Zhang, Y.G.; Zhang, Y.; Zhao, P. Apoptotic effect of genistein on human colon cancer cells via inhibiting the nuclear factor-kappa B (NF-kappaB) pathway. Tumour Biol. 2014, 35, 11483–11488. [Google Scholar] [CrossRef]
- Cha, J.H.; Kim, W.K.; Ha, A.W.; Kim, M.H.; Chang, M.J. Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr. Res. Pract. 2017, 11, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Miao, Y.X.; Wang, X.J.; Yu, Z.; Geng, M.Y.; Han, Y.T.; Wang, L.X. Effects of Ginkgo biloba extract EGb761 on human colon adenocarcinoma cells. Cell. Physiol. Biochem. 2011, 27, 227–232. [Google Scholar] [CrossRef]
- Lefort, E.C.; Blay, J. The dietary flavonoid apigenin enhances the activities of the anti-metastatic protein CD26 on human colon carcinoma cells. Clin. Exp. Metastasis 2011, 28, 337–349. [Google Scholar] [CrossRef]
- Psahoulia, F.H.; Drosopoulos, K.G.; Doubravska, L.; Andera, L.; Pintzas, A. Quercetin enhances TRAIL-mediated apoptosis in colon cancer cells by inducing the accumulation of death receptors in lipid rafts. Mol. Cancer Ther. 2007, 6, 2591–2599. [Google Scholar] [CrossRef]
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Statistics on Brain Cancer. Available online: http://Www.Who.Int/Cancer/En/ (accessed on 31 December 2018).
- Salehi, B.; Fokou, P.V.T.; Yamthe, L.R.T.; Tali, B.T.; Adetunji, C.O.; Rahavian, A.; Mudau, F.N.; Martorell, M.; Setzer, W.N.; Rodrigues, C.F.; et al. Phytochemicals in Prostate Cancer: From Bioactive Molecules to Upcoming Therapeutic Agents. Nutrients 2019, 11, 1483. [Google Scholar] [CrossRef]
- Berkovich, L.; Earon, G.; Ron, I.; Rimmon, A.; Vexler, A.; Lev-Ari, S. Moringa Oleifera aqueous leaf extract down-regulates nuclear factor-kappaB and increases cytotoxic effect of chemotherapy in pancreatic cancer cells. BMC Complement. Altern. Med. 2013, 13, 212. [Google Scholar] [CrossRef] [PubMed]
- Win, N.N.; Awale, S.; Esumi, H.; Tezuka, Y.; Kadota, S. Bioactive secondary metabolites from Boesenbergia pandurata of Myanmar and their preferential cytotoxicity against human pancreatic cancer PANC-1 cell line in nutrient-deprived medium. J. Nat. Prod. 2007, 70, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, B.B.; Jamal, M.S.; Fischer, J.W.; Mustafa, A.; Verma, A.K. Plumbagin, a plant derived natural agent inhibits the growth of pancreatic cancer cells in in vitro and in vivo via targeting EGFR, Stat3 and NF-kappaB signaling pathways. Int. J. Cancer 2012, 131, 2175–2186. [Google Scholar] [CrossRef] [PubMed]
- Zalatnai, A.; Bocsi, J. Mimosine, a plant-derived amino acid induces apoptosis in human pancreatic cancer xenografts. Anticancer Res. 2003, 23, 4007–4009. [Google Scholar]
- Shankar, S.; Ganapathy, S.; Hingorani, S.R.; Srivastava, R.K. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front. Biosci. 2008, 13, 440–452. [Google Scholar] [CrossRef]
- Chan, L.L.; George, S.; Ahmad, I.; Gosangari, S.L.; Abbasi, A.; Cunningham, B.T.; Watkin, K.L. Cytotoxicity effects of amoora rohituka and chittagonga on breast and pancreatic cancer cells. Evid. -Based Complement. Altern. Med. 2011, 2011, 86060. [Google Scholar] [CrossRef]
- Awale, S.; Nakashima, E.M.; Kalauni, S.K.; Tezuka, Y.; Kurashima, Y.; Lu, J.; Esumi, H.; Kadota, S. Angelmarin, a novel anti-cancer agent able to eliminate the tolerance of cancer cells to nutrient starvation. Bioorg. Med. Chem. Lett. 2006, 16, 581–583. [Google Scholar] [CrossRef]
- Rifai, Y.; Arai, M.A.; Koyano, T.; Kowithayakorn, T.; Ishibashi, M. Terpenoids and a flavonoid glycoside from Acacia pennata leaves as hedgehog/GLI-mediated transcriptional inhibitors. J. Nat. Prod. 2010, 73, 995–997. [Google Scholar] [CrossRef]
- Drag, M.; Surowiak, P.; Drag-Zalesinska, M.; Dietel, M.; Lage, H.; Oleksyszyn, J. Comparision of the cytotoxic effects of birch bark extract, betulin and betulinic acid towards human gastric carcinoma and pancreatic carcinoma drug-sensitive and drug-resistant cell lines. Molecules 2009, 14, 1639–1651. [Google Scholar] [CrossRef]
- Iwanski, G.B.; Lee, D.H.; En-Gal, S.; Doan, N.B.; Castor, B.; Vogt, M.; Toh, M.; Bokemeyer, C.; Said, J.W.; Thoennissen, N.H.; et al. Cucurbitacin B, a novel in vivo potentiator of gemcitabine with low toxicity in the treatment of pancreatic cancer. Br. J. Pharm. 2010, 160, 998–1007. [Google Scholar] [CrossRef]
- Rausch, V.; Liu, L.; Kallifatidis, G.; Baumann, B.; Mattern, J.; Gladkich, J.; Wirth, T.; Schemmer, P.; Buchler, M.W.; Zoller, M.; et al. Synergistic activity of sorafenib and sulforaphane abolishes pancreatic cancer stem cell characteristics. Cancer Res. 2010, 70, 5004–5013. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.X.; Ding, X.L.; Huang, J.F.; Zhang, H.; Wu, S.B.; Cheng, J.P.; Wei, Q. Apoptosis of human pancreatic cancer cells induced by Triptolide. World J. Gastroenterol. 2008, 14, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Anastyuk, S.D.; Shevchenko, N.M.; Ermakova, S.P.; Vishchuk, O.S.; Nazarenko, E.L.; Dmitrenok, P.S.; Zvyagintseva, T.N. Anticancer activity in vitro of a fucoidan from the brown alga Fucus evanescens and its low-molecular fragments, structurally characterized by tandem mass-spectrometry. Carbohydr. Polym. 2012, 87, 186–194. [Google Scholar] [CrossRef]
- Brohem, C.A.; Sawada, T.C.; Massaro, R.R.; Almeida, R.L.; Rivelli, D.P.; Ropke, C.D.; da Silva, V.V.; de Lima, T.M.; Curi, R.; Barros, S.B.; et al. Apoptosis induction by 4-nerolidylcatechol in melanoma cell lines. Toxicol. Vitr. 2009, 23, 111–119. [Google Scholar] [CrossRef]
- Zhang, W.; Lan, Y.; Huang, Q.; Hua, Z. Galangin induces B16F10 melanoma cell apoptosis via mitochondrial pathway and sustained activation of p38 MAPK. Cytotechnology 2013, 65, 447–455. [Google Scholar] [CrossRef]
- Teh, S.S.; Ee, G.C.; Mah, S.H.; Yong, Y.K.; Lim, Y.M.; Rahmani, M.; Ahmad, Z. In vitro cytotoxic, antioxidant, and antimicrobial activities of Mesua beccariana (Baill.) Kosterm., Mesua ferrea Linn., and Mesua congestiflora extracts. Biomed Res. Int. 2013, 2013, 517072. [Google Scholar] [CrossRef]
- Elmasri, W.A.; Hegazy, M.-E.F.; Mechref, Y.; Paré, P.W. Cytotoxic saponin poliusaposide from Teucrium polium. Rsc Adv. 2015, 5, 27126–27133. [Google Scholar] [CrossRef]
- Nayak, D.; Pradhan, S.; Ashe, S.; Rauta, P.R.; Nayak, B. Biologically synthesised silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J. Colloid Interface Sci. 2015, 457, 329–338. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. A case study–Regulation and functional mechanisms of cancer cells and control its activity using plants and their derivatives. J. Pharm. Res. 2013, 6, 884–892. [Google Scholar] [CrossRef][Green Version]
- Balasubramanian, R.; Narayanan, M.; Kedalgovindaram, L.; Devarakonda Rama, K. Cytotoxic activity of flavone glycoside from the stem of Indigofera aspalathoides Vahl. J. Nat. Med. 2006, 61, 80–83. [Google Scholar] [CrossRef]
- Tourino, S.; Selga, A.; Jimenez, A.; Julia, L.; Lozano, C.; Lizarraga, D.; Cascante, M.; Torres, J.L. Procyanidin fractions from pine (Pinus pinaster) bark: Radical scavenging power in solution, antioxidant activity in emulsion, and antiproliferative effect in melanoma cells. J. Agric. Food Chem. 2005, 53, 4728–4735. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.L.; Tezuka, Y.; Banskota, A.H.; Tran, Q.K.; Saiki, I.; Kadota, S. New spirostanol steroids and steroidal saponins from roots and rhizomes of Dracaena angustifolia and their antiproliferative activity. J. Nat. Prod. 2001, 64, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Huang, Z.; Kim, D.J.; Kim, S.H.; Kim, M.O.; Lee, S.Y.; Xie, H.; Park, S.J.; Kim, J.Y.; Kundu, J.K.; et al. Direct targeting of MEK1/2 and RSK2 by silybin induces cell-cycle arrest and inhibits melanoma cell growth. Cancer Prev. Res. 2013, 6, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, K.; Agarwal, R. Multitargeted therapy of cancer by silymarin. Cancer Lett. 2008, 269, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, J.; Paul, A.; Samadder, A.; Khuda-Bukhsh, A.R. Apigenin, a bioactive flavonoid from Lycopodium clavatum, stimulates nucleotide excision repair genes to protect skin keratinocytes from ultraviolet B-induced reactive oxygen species and DNA damage. J. Acupunct. Meridian Stud. 2013, 6, 252–262. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Saab, A.M.; Statti, G.A.; Menichini, F. Cytotoxic activity of essential oils from labiatae and lauraceae families against in vitro human tumor models. Anticancer Res. 2007, 27, 3293–3299. [Google Scholar]
- Fouche, G.; Cragg, G.M.; Pillay, P.; Kolesnikova, N.; Maharaj, V.J.; Senabe, J. In vitro anticancer screening of South African plants. J. Ethnopharmacol. 2008, 119, 455–461. [Google Scholar] [CrossRef]
- Darmanin, S.; Wismayer, P.S.; Camilleri Podesta, M.T.; Micallef, M.J.; Buhagiar, J.A. An extract from Ricinus communis L. leaves possesses cytotoxic properties and induces apoptosis in SK-MEL-28 human melanoma cells. Nat. Prod. Res. 2009, 23, 561–571. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar]
- Niles, R.M.; McFarland, M.; Weimer, M.B.; Redkar, A.; Fu, Y.M.; Meadows, G.G. Resveratrol is a potent inducer of apoptosis in human melanoma cells. Cancer Lett. 2003, 190, 157–163. [Google Scholar] [CrossRef]
- World Cancer Research Fund International. Available online: https://Www.Wcrf.Org/ (accessed on 31 December 2018).
- Globocan. Worldwide Incidence and Mortality of Cancer, 2002 [computer program]. Version 2.0; IARC CancerBase: Lyon, France, 2002. [Google Scholar]
- Bezerra, D.P.; Marinho Filho, J.D.; Alves, A.P.; Pessoa, C.; de Moraes, M.O.; Pessoa, O.D.; Torres, M.C.; Silveira, E.R.; Viana, F.A.; Costa-Lotufo, L.V. Antitumor activity of the essential oil from the leaves of Croton regelianus and its component ascaridole. Chem. Biodivers. 2009, 6, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- el-Sawi, S.A.; Motawae, H.M.; Ali, A.M. Chemical composition, cytotoxic activity and antimicrobial activity of essential oils of leaves and berries of Juniperus phoenicea L. grown in Egypt. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Scheck, A.C.; Perry, K.; Hank, N.C.; Clark, W.D. Anticancer activity of extracts derived from the mature roots of Scutellaria baicalensis on human malignant brain tumor cells. BMC Complement. Altern. Med. 2006, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Tsai, N.M.; Lin, S.Z.; Lee, C.C.; Chen, S.P.; Su, H.C.; Chang, W.L.; Harn, H.J. The antitumor effects of Angelica sinensis on malignant brain tumors in vitro and in vivo. Clin. Cancer Res. 2005, 11, 3475–3484. [Google Scholar] [CrossRef] [PubMed]
- Okokon, J.E.; Dar, A.; Choudhary, M.I. Immunomodulatory, cytotoxic and antileishmanial activity of phytoconstituents of Croton zambesicus. Phytopharm. J. 2013, 4, 31–40. [Google Scholar]
- Wu, X.S.; Xie, T.; Lin, J.; Fan, H.Z.; Huang-Fu, H.J.; Ni, L.F.; Yan, H.F. An investigation of the ability of elemene to pass through the blood-brain barrier and its effect on brain carcinomas. J. Pharm. Pharmacol. 2009, 61, 1653–1656. [Google Scholar] [CrossRef]
- Ferriola, P.C.; Cody, V.; Middleton, E., Jr. Protein kinase C inhibition by plant flavonoids. Kinetic mechanisms and structure-activity relationships. Biochem. Pharm. 1989, 38, 1617–1624. [Google Scholar] [CrossRef]
- Dimitric Markovic, J.M.; Pejin, B.; Milenkovic, D.; Amic, D.; Begovic, N.; Mojovic, M.; Markovic, Z.S. Antiradical activity of delphinidin, pelargonidin and malvin towards hydroxyl and nitric oxide radicals: The energy requirements calculations as a prediction of the possible antiradical mechanisms. Food Chem. 2017, 218, 440–446. [Google Scholar] [CrossRef]
- Puli, S.; Jain, A.; Lai, J.C.; Bhushan, A. Effect of combination treatment of rapamycin and isoflavones on mTOR pathway in human glioblastoma (U87) cells. Neurochem. Res. 2010, 35, 986–993. [Google Scholar] [CrossRef]
- Tsai, N.M.; Chen, Y.L.; Lee, C.C.; Lin, P.C.; Cheng, Y.L.; Chang, W.L.; Lin, S.Z.; Harn, H.J. The natural compound n-butylidenephthalide derived from Angelica sinensis inhibits malignant brain tumor growth in vitro and in vivo. J. Neurochem. 2006, 99, 1251–1262. [Google Scholar] [CrossRef]
- Jeon, H.Y.; Park, C.G.; Ham, S.W.; Choi, S.H.; Lee, S.Y.; Kim, J.Y.; Seo, S.; Jin, X.; Kim, J.K.; Eun, K.; et al. BRM270, a Compound from Natural Plant Extracts, Inhibits Glioblastoma Stem Cell Properties and Glioblastoma Recurrence. J. Med. Food 2017, 20, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Banik, N.L.; Ray, S.K. N-(4-Hydroxyphenyl) retinamide induced both differentiation and apoptosis in human glioblastoma T98G and U87MG cells. Brain Res. 2008, 1227, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.X.; Ma, J.W.; Li, H.Y.; Ye, J.C.; Xie, S.M.; Du, B.; Zhong, X.Y. EGCG inhibits properties of glioma stem-like cells and synergizes with temozolomide through downregulation of P-glycoprotein inhibition. J. Neuro-Oncol. 2015, 121, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Galanti, G.; Fisher, T.; Kventsel, I.; Shoham, J.; Gallily, R.; Mechoulam, R.; Lavie, G.; Amariglio, N.; Rechavi, G.; Toren, A. Delta 9-tetrahydrocannabinol inhibits cell cycle progression by downregulation of E2F1 in human glioblastoma multiforme cells. Acta Oncol. 2008, 47, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Marcu, J.P.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Horowitz, M.P.; Lee, J.; Pakdel, A.; Allison, J.; Limbad, C.; Moore, D.H.; et al. Cannabidiol enhances the inhibitory effects of delta9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol. Cancer Ther. 2010, 9, 180–189. [Google Scholar] [CrossRef]
- Liao, C.H.; Pan, S.L.; Guh, J.H.; Chang, Y.L.; Pai, H.C.; Lin, C.H.; Teng, C.M. Antitumor mechanism of evodiamine, a constituent from Chinese herb Evodiae fructus, in human multiple-drug resistant breast cancer NCI/ADR-RES cells in vitro and in vivo. Carcinogenesis 2005, 26, 968–975. [Google Scholar] [CrossRef]
- Kim, S.; Lee, T.J.; Leem, J.; Choi, K.S.; Park, J.W.; Kwon, T.K. Sanguinarine-induced apoptosis: Generation of ROS, down-regulation of Bcl-2, c-FLIP, and synergy with TRAIL. J. Cell. Biochem. 2008, 104, 895–907. [Google Scholar] [CrossRef]
- Gautam, N.; Mantha, A.K.; Mittal, S. Essential oils and their constituents as anticancer agents: A mechanistic view. Biomed Res. Int. 2014, 2014, 154106. [Google Scholar] [CrossRef]
- Li, H.; Tan, G.; Jiang, X.; Qiao, H.; Pan, S.; Jiang, H.; Kanwar, J.R.; Sun, X. Therapeutic effects of matrine on primary and metastatic breast cancer. Am. J. Chin. Med. 2010, 38, 1115–1130. [Google Scholar] [CrossRef]
- Lai, L.H.; Fu, Q.H.; Liu, Y.; Jiang, K.; Guo, Q.M.; Chen, Q.Y.; Yan, B.; Wang, Q.Q.; Shen, J.G. Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacol. Sin. 2012, 33, 523–530. [Google Scholar] [CrossRef]
- Zu, Y.; Yu, H.; Liang, L.; Fu, Y.; Efferth, T.; Liu, X.; Wu, N. Activities of ten essential oils towards Propionibacterium acnes and PC-3, A-549 and MCF-7 cancer cells. Molecules 2010, 15, 3200–3210. [Google Scholar] [CrossRef] [PubMed]
- Suhail, M.M.; Wu, W.; Cao, A.; Mondalek, F.G.; Fung, K.M.; Shih, P.T.; Fang, Y.T.; Woolley, C.; Young, G.; Lin, H.K. Boswellia sacra essential oil induces tumor cell-specific apoptosis and suppresses tumor aggressiveness in cultured human breast cancer cells. BMC Complement. Altern. Med. 2011, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, G.A.; Al Ati, H.Y.; El Gamal, A.A. Chemical composition and biological evaluation of essential oils of Pulicaria jaubertii. Pharmacogn. Mag. 2013, 9, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Owolabi, M.S.; Ogundajo, A.L.; Dosoky, N.S.; Setzer, W.N. The cytotoxic activity of Annona muricata leaf oil from Badagary, Nigeria. AJEONP 2013, 1, 1–13. [Google Scholar]
- Afoulous, S.; Ferhout, H.; Raoelison, E.G.; Valentin, A.; Moukarzel, B.; Couderc, F.; Bouajila, J. Chemical composition and anticancer, antiinflammatory, antioxidant and antimalarial activities of leaves essential oil of Cedrelopsis grevei. Food Chem. Toxicol. 2013, 56, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Shahabipour, S.; Firuzi, O.; Asadollahi, M.; Miri, M.; Javidnia, K. Essential Oil Composition and Cytotoxic Activity of Libanotis transcaucasica Schischk. from Iran. Nat. Prod. Chem. Res. 2013, 1, 1–3. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Wink, M.; Khalifaev, D.R.; Zhang, H.; Dosoky, N.S.; Setzer, W.N. Composition and bioactivity of the essential oil of Melissa officinalis L. growing wild in Tajikistan. Int. J. Tradit. Nat. Med. 2013, 2, 86–96. [Google Scholar]
- El Hadri, A.; del Rio, M.G.; Sanz, J.; Coloma, A.G.; Idaomar, M.; Ozonas, B.R.; González, J.B.; Reus, M.I.S. Cytotoxic activity of α-humulene and transcaryophyllene from Salvia officinalis in animal and human tumor cells. R. Acad. Nac. Farm. 2010, 76, 343–356. [Google Scholar]
- Chen, Y.; Zhou, C.; Ge, Z.; Liu, Y.; Liu, Y.; Feng, W.; Li, S.; Chen, G.; Wei, T. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol. Lett. 2013, 6, 1140–1146. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Chatha, S.A.S.; Latif, S.; Sherazi, S.T.H.; Ahmad, A.; Worthington, J.; Sarker, S.D. Chemical composition and bioactivity studies of the essential oils from two Thymus species from the Pakistani flora. Lwt Food Sci. Technol. 2013, 50, 185–192. [Google Scholar] [CrossRef]
- da Silva, E.B.; Matsuo, A.L.; Figueiredo, C.R.; Chaves, M.H.; Sartorelli, P.; Lago, J.H. Chemical constituents and cytotoxic evaluation of essential oils from leaves of Porcelia macrocarpa (Annonaceae). Nat. Prod. Commun. 2013, 8, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Arunasree, K.M. Anti-proliferative effects of carvacrol on a human metastatic breast cancer cell line, MDA-MB 231. Phytomedicine 2010, 17, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Chaouki, W.; Leger, D.Y.; Liagre, B.; Beneytout, J.L.; Hmamouchi, M. Citral inhibits cell proliferation and induces apoptosis and cell cycle arrest in MCF-7 cells. Fundam. Clin. Pharmacol. 2009, 23, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Santha, S.; Bommareddy, A.; Rule, B.; Guillermo, R.; Kaushik, R.S.; Young, A.; Dwivedi, C. Antineoplastic effects of alpha-santalol on estrogen receptor-positive and estrogen receptor-negative breast cancer cells through cell cycle arrest at G2/M phase and induction of apoptosis. PLoS ONE 2013, 8, e56982. [Google Scholar] [CrossRef]
- Patel, P.B.; Thakkar, V.R.; Patel, J.S. Cellular effect of curcumin and citral combination on breast cancer cells: Induction of apoptosis and cell cycle arrest. J. Breast Cancer 2015, 18, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, B.; Brooks, K.; Pavey, S. Defective cell cycle checkpoints as targets for anti-cancer therapies. Front. Pharmacol. 2012, 3, 9. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Chatha, S.A.; Jabbar, A.; Mahboob, S.; Nigam, P.S. Rosmarinus officinalis essential oil: Antiproliferative, antioxidant and antibacterial activities. Braz. J. Microbiol. 2010, 41, 1070–1078. [Google Scholar] [CrossRef]
- Li, Y.L.; Yeung, C.M.; Chiu, L.C.; Cen, Y.Z.; Ooi, V.E. Chemical composition and antiproliferative activity of essential oil from the leaves of a medicinal herb, Schefflera heptaphylla. Phytother. Res. 2009, 23, 140–142. [Google Scholar] [CrossRef]
- Ashour, H.M. Antibacterial, antifungal, and anticancer activities of volatile oils and extracts from stems, leaves, and flowers of Eucalyptus sideroxylon and Eucalyptus torquata. Cancer Biol. Ther. 2008, 7, 399–403. [Google Scholar] [CrossRef]
- Sireesha, D.; Reddy, B.S.; Reginald, B.A.; Samatha, M.; Kamal, F. Effect of amygdalin on oral cancer cell line: An in vitro study. J. Oral Maxillofac. Pathol. 2019, 23, 104–107. [Google Scholar] [CrossRef]
- Jo, J.R.; Park, J.S.; Park, Y.K.; Chae, Y.Z.; Lee, G.H.; Park, G.Y.; Jang, B.C. Pinus densiflora leaf essential oil induces apoptosis via ROS generation and activation of caspases in YD-8 human oral cancer cells. Int. J. Oncol. 2012, 40, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.L.; Cheng, F.C.; Wang, S.P.; Chou, S.T.; Shih, Y. Cinnamomum cassia essential oil and its major constituent cinnamaldehyde induced cell cycle arrest and apoptosis in human oral squamous cell carcinoma HSC-3 cells. Environ. Toxicol. 2017, 32, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.D.; Kim, J.Y. Essential oil from Cryptomeria japonica induces apoptosis in human oral epidermoid carcinoma cells via mitochondrial stress and activation of caspases. Molecules 2012, 17, 3890–3901. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.I.; Mohamed, I.; Mohamed, A.A.; Abdelkader, M.; Yalcin, H.C.; Aboulkassim, T.; Batist, G.; Yasmeen, A.; Moustafa, A.A. Elaeagnus angustifolia Plant Extract Inhibits Angiogenesis and Downgrades Cell Invasion of Human Oral Cancer Cells via Erk1/Erk2 Inactivation. Nutr. Cancer 2018, 70, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Hsu, K.P.; Wang, E.I.; Ho, C.L. Composition and in vitro anticancer activities of the leaf essential oil of Neolitsea variabillima from Taiwan. Nat. Prod. Commun. 2013, 8, 531–532. [Google Scholar] [CrossRef]
- Li, S.; Cha, I.H.; Nam, W. Chios mastic gum extracts as a potent antitumor agent that inhibits growth and induces apoptosis of oral cancer cells. Asian Pac. J. Cancer Prev. 2011, 12, 1877–1880. [Google Scholar]
- Moon, S.M.; Yun, S.J.; Kook, J.K.; Kim, H.J.; Choi, M.S.; Park, B.R.; Kim, S.G.; Kim, B.O.; Lee, S.Y.; Ahn, H.; et al. Anticancer activity of Saussurea lappa extract by apoptotic pathway in KB human oral cancer cells. Pharm. Biol. 2013, 51, 1372–1377. [Google Scholar] [CrossRef]
- Fekrazad, R.; Afzali, M.; Pasban-Aliabadi, H.; Esmaeili-Mahani, S.; Aminizadeh, M.; Mostafavi, A. Cytotoxic Effect of Thymus caramanicus Jalas on Human Oral Epidermoid Carcinoma KB Cells. Braz. Dent. J. 2017, 28, 72–77. [Google Scholar] [CrossRef]
- Nedel, F.; Begnini, K.; Carvalho, P.H.; Lund, R.G.; Beira, F.T.; Del Pino, F.A. Antiproliferative activity of flower hexane extract obtained from Mentha spicata associated with Mentha rotundifolia against the MCF7, KB, and NIH/3T3 cell lines. J. Med. Food 2012, 15, 955–958. [Google Scholar] [CrossRef]
- Fathilah, A.R.; Sujata, R.; Norhanom, A.W.; Adenan, M.I. Antiproliferative activity of aqueous extract of Piper betle L. and Psidium guajava L. on KB and HeLa cell lines. J. Med. Plants Res. 2010, 4, 987–990. [Google Scholar]
- Kim, L.-H.; Khadka, S.; Shin, J.-A.; Jung, J.-Y.; Ryu, M.-H.; Yu, H.-J.; Lee, H.N.; Jang, B.; Yang, I.-H.; Won, D.-H.; et al. Nitidine chloride acts as an apoptosis inducer in human oral cancer cells and a nude mouse xenograft model via inhibition of STAT3. Oncotarget 2017, 8, 91306–91315. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Dong, W. Aloe-Emodin Induces Endoplasmic Reticulum Stress-Dependent Apoptosis in Colorectal Cancer Cells. Med. Sci. Monit. 2018, 24, 6331–6339. [Google Scholar] [CrossRef] [PubMed]
- Pejin, B.; Iodice, C.; Bogdanović, G.; Kojić, V.; Tešević, V. Stictic acid inhibits cell growth of human colon adenocarcinoma HT-29 cells. Arab. J. Chem. 2017, 10, S1240–S1242. [Google Scholar] [CrossRef]
- Carnesecchi, S.; Bras-Goncalves, R.; Bradaia, A.; Zeisel, M.; Gosse, F.; Poupon, M.F.; Raul, F. Geraniol, a component of plant essential oils, modulates DNA synthesis and potentiates 5-fluorouracil efficacy on human colon tumor xenografts. Cancer Lett. 2004, 215, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Sawada, T.; Osawa, T.; Adachi, M.; Kubota, K. MK615 inhibits pancreatic cancer cell growth by dual inhibition of Aurora A and B kinases. World J. Gastroenterol. 2008, 14, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Melstrom, L.G.; Salabat, M.R.; Ding, X.Z.; Milam, B.M.; Strouch, M.; Pelling, J.C.; Bentrem, D.J. Apigenin inhibits the GLUT-1 glucose transporter and the phosphoinositide 3-kinase/Akt pathway in human pancreatic cancer cells. Pancreas 2008, 37, 426–431. [Google Scholar] [CrossRef]
- Wicker, C.A.; Sahu, R.P.; Kulkarni-Datar, K.; Srivastava, S.K.; Brown, T.L. BITC Sensitizes Pancreatic Adenocarcinomas to TRAIL-induced Apoptosis. Cancer Growth Metastasis 2010, 2009, 45–55. [Google Scholar] [CrossRef]
- Bence, A.K.; Adams, V.R.; Crooks, P.A. L-Canavanine as a radiosensitization agent for human pancreatic cancer cells. Mol. Cell. Biochem. 2003, 244, 37–43. [Google Scholar] [CrossRef]
- Lee, S.H.; Ryu, J.K.; Lee, K.Y.; Woo, S.M.; Park, J.K.; Yoo, J.W.; Kim, Y.T.; Yoon, Y.B. Enhanced anti-tumor effect of combination therapy with gemcitabine and apigenin in pancreatic cancer. Cancer Lett. 2008, 259, 39–49. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Chen, L.; Li, H. The dietary compound luteolin inhibits pancreatic cancer growth by targeting BCL-2. Food Funct. 2018, 9, 3018–3027. [Google Scholar] [CrossRef]
- Jia, S.; Xu, X.; Zhou, S.; Chen, Y.; Ding, G.; Cao, L. Fisetin induces autophagy in pancreatic cancer cells via endoplasmic reticulum stress- and mitochondrial stress-dependent pathways. Cell Death Dis. 2019, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Hoca, M.; Becer, E.; Kabadayi, H.; Yucecan, S.; Vatansever, H.S. The Effect of Resveratrol and Quercetin on Epithelial-Mesenchymal Transition in Pancreatic Cancer Stem Cell. Nutr. Cancer 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tourino, S.; Lizarraga, D.; Carreras, A.; Lorenzo, S.; Ugartondo, V.; Mitjans, M.; Vinardell, M.P.; Julia, L.; Cascante, M.; Torres, J.L. Highly galloylated tannin fractions from witch hazel (Hamamelis virginiana) bark: Electron transfer capacity, in vitro antioxidant activity, and effects on skin-related cells. Chem. Res. Toxicol. 2008, 21, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Cowan, J.; Shadab, M.; Nadkarni, D.H.; Kc, K.; Velu, S.E. A novel marine natural product derived pyrroloiminoquinone with potent activity against skin cancer cells. Mar. drugs 2019, 17, 443. [Google Scholar] [CrossRef]
- Kremer, J.L.; Melo, G.P.; Marinello, P.C.; Bordini, H.P.; Rossaneis, A.C.; Sabio, L.R.; Cecchini, R.; Cecchini, A.L.; Verri, W.A., Jr.; Luiz, R.C. Citral prevents UVB-induced skin carcinogenesis in hairless mice. J. Photochem. Photobiol. B Biol. 2019, 198, 111565. [Google Scholar] [CrossRef]
- Arcella, A.; Oliva, M.A.; Sanchez, M.; Staffieri, S.; Esposito, V.; Giangaspero, F.; Cantore, G. Effects of hispolon on glioblastoma cell growth. Environ. Toxicol. 2017, 32, 2113–2123. [Google Scholar] [CrossRef]
- Arcella, A.; Oliva, M.A.; Staffieri, S.; Sanchez, M.; Madonna, M.; Riozzi, B.; Esposito, V.; Giangaspero, F.; Frati, L. Effects of aloe emodin on U87MG glioblastoma cell growth: In vitro and in vivo study. Environ. Toxicol. 2018, 33, 1160–1167. [Google Scholar] [CrossRef]
- Jin, J.; Qiu, S.; Wang, P.; Liang, X.; Huang, F.; Wu, H.; Zhang, B.; Zhang, W.; Tian, X.; Xu, R.; et al. Cardamonin inhibits breast cancer growth by repressing HIF-1alpha-dependent metabolic reprogramming. J. Exp. Clin. Cancer Res. 2019, 38, 377. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Z.; Wu, M.; Zhang, L.; Wang, J.; Fu, W.; Xu, N.; Zhao, Z.; Lao, Y.; Xu, H. The natural compound neobractatin inhibits tumor metastasis by upregulating the RNA-binding-protein MBNL2. Cell Death Dis. 2019, 10, 554. [Google Scholar] [CrossRef]
- Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Anticancer activity of an ultrasonic nanoemulsion formulation of Nigella sativa L. essential oil on human breast cancer cells. Ultrason. Sonochem. 2016, 31, 449–455. [Google Scholar] [CrossRef]
- Stone, S.C.; Vasconcellos, F.A.; Lenardão, E.J.; do Amaral, R.C.; Jacob, R.G.; Leite, F.L. Evaluation of potential use of Cymbopogon sp. essential oils,(R)-citronellal and N-citronellylamine in cancer chemotherapy. Int. J. Appl. Res. Nat. Prod. 2013, 6, 11–15. [Google Scholar]
- Yu, J.Q.; Lei, J.C.; Zhang, X.Q.; Yu, H.D.; Tian, D.Z.; Liao, Z.X.; Zou, G.L. Anticancer, antioxidant and antimicrobial activities of the essential oil of Lycopus lucidus Turcz. var. hirtus Regel. Food Chem. 2011, 126, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, V.; Shrivastava, R.; Hussain, S.; Ganguly, C.; Bharadwaj, M. Comparative anticancer potential of clove (Syzygium aromaticum)—An Indian spice—Against cancer cell lines of various anatomical origin. Asian Pac. J. Cancer Prev. 2011, 12, 1989–1993. [Google Scholar] [PubMed]
- Al-Kalaldeh, J.Z.; Abu-Dahab, R.; Afifi, F.U. Volatile oil composition and antiproliferative activity of Laurus nobilis, Origanum syriacum, Origanum vulgare, and Salvia triloba against human breast adenocarcinoma cells. Nutr. Res. 2010, 30, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Bendaoud, H.; Romdhane, M.; Souchard, J.P.; Cazaux, S.; Bouajila, J. Chemical composition and anticancer and antioxidant activities of Schinus molle L. and Schinus terebinthifolius Raddi berries essential oils. J. Food Sci. 2010, 75, C466–C472. [Google Scholar] [CrossRef]
- Diaz, C.; Quesada, S.; Brenes, O.; Aguilar, G.; Ciccio, J.F. Chemical composition of Schinus molle essential oil and its cytotoxic activity on tumour cell lines. Nat. Prod. Res. 2008, 22, 1521–1534. [Google Scholar] [CrossRef]
- Lei, J.; Yu, J.; Yu, H.; Liao, Z. Composition, cytotoxicity and antimicrobial activity of essential oil from Dictamnus dasycarpus. Food Chem. 2008, 107, 1205–1209. [Google Scholar] [CrossRef]
- Apel, M.A.; Lima, M.E.L.; Souza, A.; Cordeiro, I.; Young, M.C.M.; Sobral, M.E.; Suffredini, I.B.; Moreno, P.R.H. Screening of the biological activity from essential oils of native species from the Atlantic rain forest (São Paulo–Brazil). Pharmacologyonline 2006, 3, 376–383. [Google Scholar]
- Monajemi, R.; Oryan, S.; Haeri-Roohani, A.; Ghannadi, A.; Jafarian, A. Cytotoxic Effects of Essential Oils of Some Iranian Citrus Peels. Iran. J. Pharm. Res. 2010, 4, 183–187. [Google Scholar] [CrossRef]
- Goren, A.; Topcu, G.; Bilsel, G.; Bilsel, M.; Aydogmus, Z.; Pezzuto, J.M. The chemical constituents and biological activity of essential oil of Lavandula stoechas ssp. stoechas. Z. Fur Nat. 2002, 57, 797–800. [Google Scholar] [CrossRef]
- Manikandan, P.; Vinothini, G.; Vidya Priyadarsini, R.; Prathiba, D.; Nagini, S. Eugenol inhibits cell proliferation via NF-kappaB suppression in a rat model of gastric carcinogenesis induced by MNNG. Investig. New Drugs 2011, 29, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, M.; Karimi, E. Galbanic acid: Induced antiproliferation in estrogen receptor-negative breast cancer cells and enhanced cellular redox state in the human dermal fibroblasts. J. Biochem. Mol. Toxicol. 2019, e22402. [Google Scholar] [CrossRef] [PubMed]
- Kakarala, M.; Brenner, D.E.; Korkaya, H.; Cheng, C.; Tazi, K.; Ginestier, C.; Liu, S.; Dontu, G.; Wicha, M.S. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res. Treat. 2010, 122, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Pejin, B.; Kojic, V.; Bogdanovic, G. An insight into the cytotoxic activity of phytol at in vitro conditions. Nat. Prod. Res. 2014, 28, 2053–2056. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Liu, Q.; Liu, K.; Yagasaki, K.; Wu, E.; Zhang, G. Matrine suppresses breast cancer cell proliferation and invasion via VEGF-Akt-NF-kappaB signaling. Cytotechnology 2009, 59, 219–229. [Google Scholar] [CrossRef]
- Choi, Y.H.; Choi, W.Y.; Hong, S.H.; Kim, S.O.; Kim, G.Y.; Lee, W.H.; Yoo, Y.H. Anti-invasive activity of sanguinarine through modulation of tight junctions and matrix metalloproteinase activities in MDA-MB-231 human breast carcinoma cells. Chem.-Biol. Interact. 2009, 179, 185–191. [Google Scholar] [CrossRef]
- Fu, L.W.; Zhang, Y.M.; Liang, Y.J.; Yang, X.P.; Pan, Q.C. The multidrug resistance of tumour cells was reversed by tetrandrine in vitro and in xenografts derived from human breast adenocarcinoma MCF-7/adr cells. Eur. J. Cancer 2002, 38, 418–426. [Google Scholar] [CrossRef]
- Li, X.; Xie, W.; Xie, C.; Huang, C.; Zhu, J.; Liang, Z.; Deng, F.; Zhu, M.; Zhu, W.; Wu, R.; et al. Curcumin modulates miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7 breast cancer cell proliferation. Phytother. Res. 2014, 28, 1553–1560. [Google Scholar] [CrossRef]
- Bimonte, S.; Barbieri, A.; Palma, G.; Rea, D.; Luciano, A.; D’Aiuto, M.; Arra, C.; Izzo, F. Dissecting the role of curcumin in tumour growth and angiogenesis in mouse model of human breast cancer. Biomed Res. Int. 2015, 2015, 878134. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S.; Takada, Y.; Banerjee, S.; Newman, R.A.; Bueso-Ramos, C.E.; Price, J.E. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin. Cancer Res. 2005, 11, 7490–7498. [Google Scholar] [CrossRef]
- Lv, Z.D.; Liu, X.P.; Zhao, W.J.; Dong, Q.; Li, F.N.; Wang, H.B.; Kong, B. Curcumin induces apoptosis in breast cancer cells and inhibits tumor growth in vitro and in vivo. Int. J. Clin. Exp. Pathol. 2014, 7, 2818–2824. [Google Scholar] [PubMed]
- Khan, H.; Ullah, H.; Martorell, M.; Esteban Valdes, S.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Pezzani, R.; Salehi, B.; Vitalini, S.; Iriti, M.; Zuniga, F.A.; Sharifi-Rad, J.; Martorell, M.; Martins, N. Synergistic effects of plant derivatives and conventional chemotherapeutic agents: An update on the cancer perspective. Medicina 2019, 55, 110. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.K.; Agrahari, V.; Mandal, A.; Cholkar, K.; Natarajan, C.; Shah, S.; Joseph, M.; Trinh, H.M.; Vaishya, R.; Yang, X.; et al. Novel delivery approaches for cancer therapeutics. J. Control. Release 2015, 219, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Sailo, B.L.; Roy, N.K.; Thakur, K.K.; Banik, K.; Shakibaei, M.; Gupta, S.C.; Aggarwal, B.B. Cancer drug development: The missing links. Exp. Biol. Med. 2019, 244, 663–689. [Google Scholar] [CrossRef]
- Hoonjan, M.; Jadhav, V.; Bhatt, P. Arsenic trioxide: insights into its evolution to an anticancer agent. J. Biol. Inorg. Chem. 2018, 23, 313–329. [Google Scholar] [CrossRef]
- Slezakova, S.; Ruda-Kucerova, J. Anticancer activity of artemisinin and its derivatives. Anticancer Res. 2017, 37, 5995–6003. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp. Biol. Med. 2015, 240, 760–773. [Google Scholar] [CrossRef]
| Antitumor Drug | Chemical Structure | Natural Source/Analog |
|---|---|---|
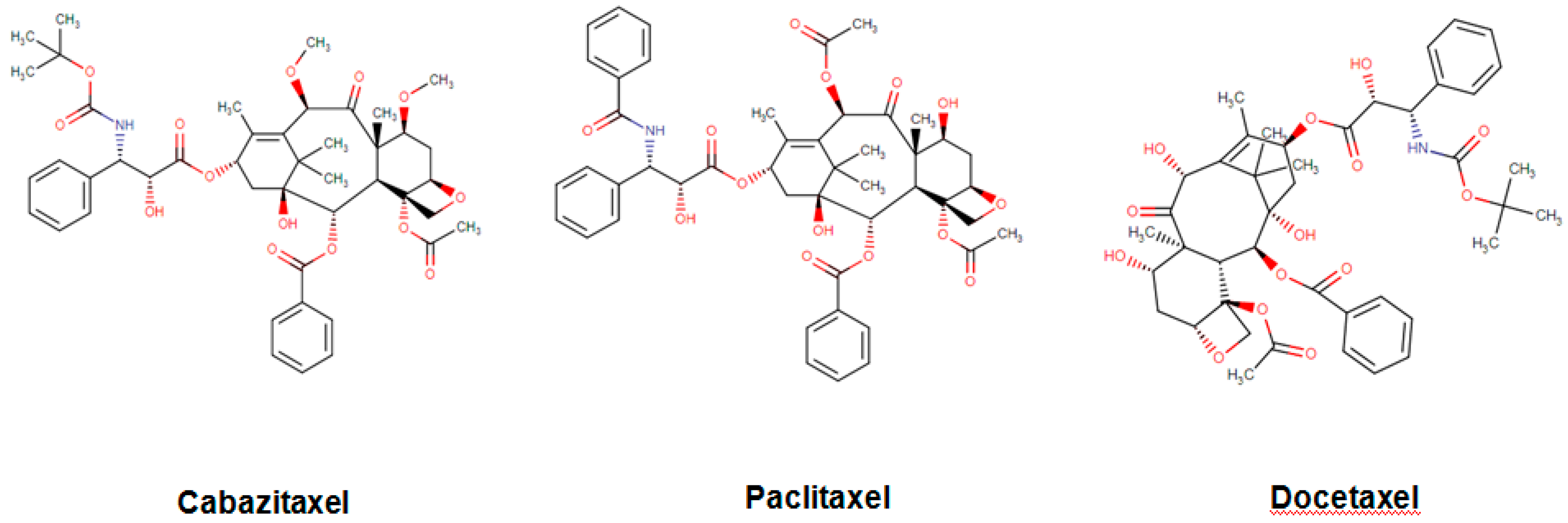
| Paclitaxel | 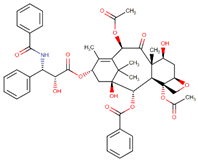 | Taxus brevifolia Nutt. |
| Docetaxel | 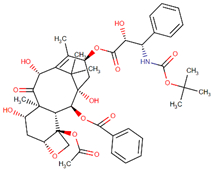 | Paclitaxel analog |
| Cabazitaxel | 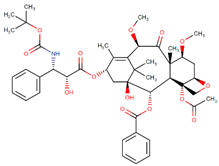 | Paclitaxel analog |
| Camptothecin | 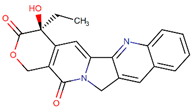 | Camptotheca acuminata Decne. |
| Belotecan | 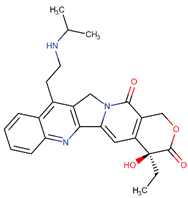 | Camptothecin analog |
| Topotecan | 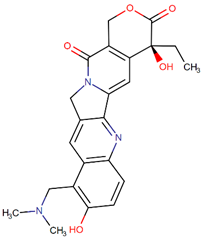 | Camptothecin analog |
| Irinotecan |  | Camptothecin analog |
| Vinblastine | 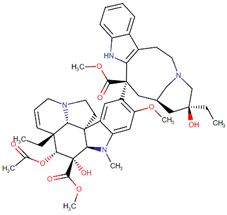 | Vinca rosea L. |
| Vincristine | 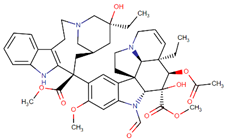 | Vinca rosea L. |
| Vindesine | 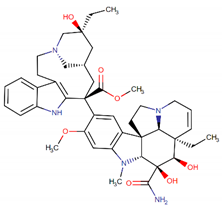 | Vincristine analog |
| Vinorelbine | 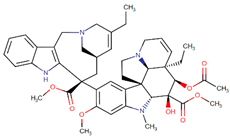 | Vincristine analog |
| Podophyllotoxin | 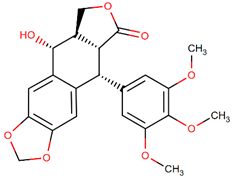 | Podophyllum |
| Etoposide | 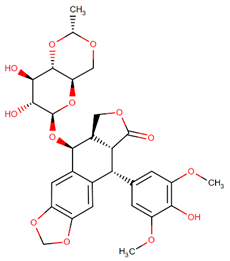 | Podophyllotoxin analog |
| Teniposide | 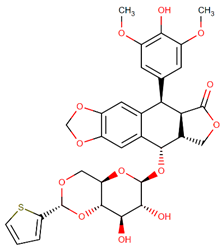 | Podophyllotoxin analog |
| Bleomycin | 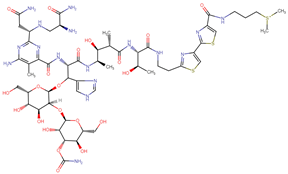 | Streptomyces verticillus |
| Dactinomycin | 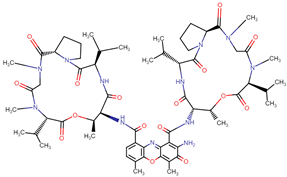 | Streptomyces |
| Doxorubicin | 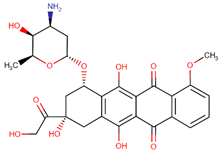 | Streptomyces peucetius var. caesius |
| Daunorubicin | 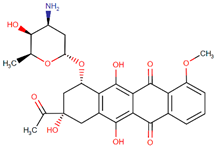 | Streptomyces |
| Epirubicin |  | Doxorubicin analog |
| Ingredient Name Product Name | Chemical Classification | Associated Conditions | Mechanism of Action | Target(s) | FDA Approval |
|---|---|---|---|---|---|
| Cabazitaxel Jevtana | Organic compound Taxanes and derivatives Diterpenoids | Refractory, metastatic prostate cancer Effective against docetaxel-sensitive and insensitive tumors | Tubulin-based antimitotic | Tubulin alpha-4A chain Tubulin beta-1 chain | June 2010 |
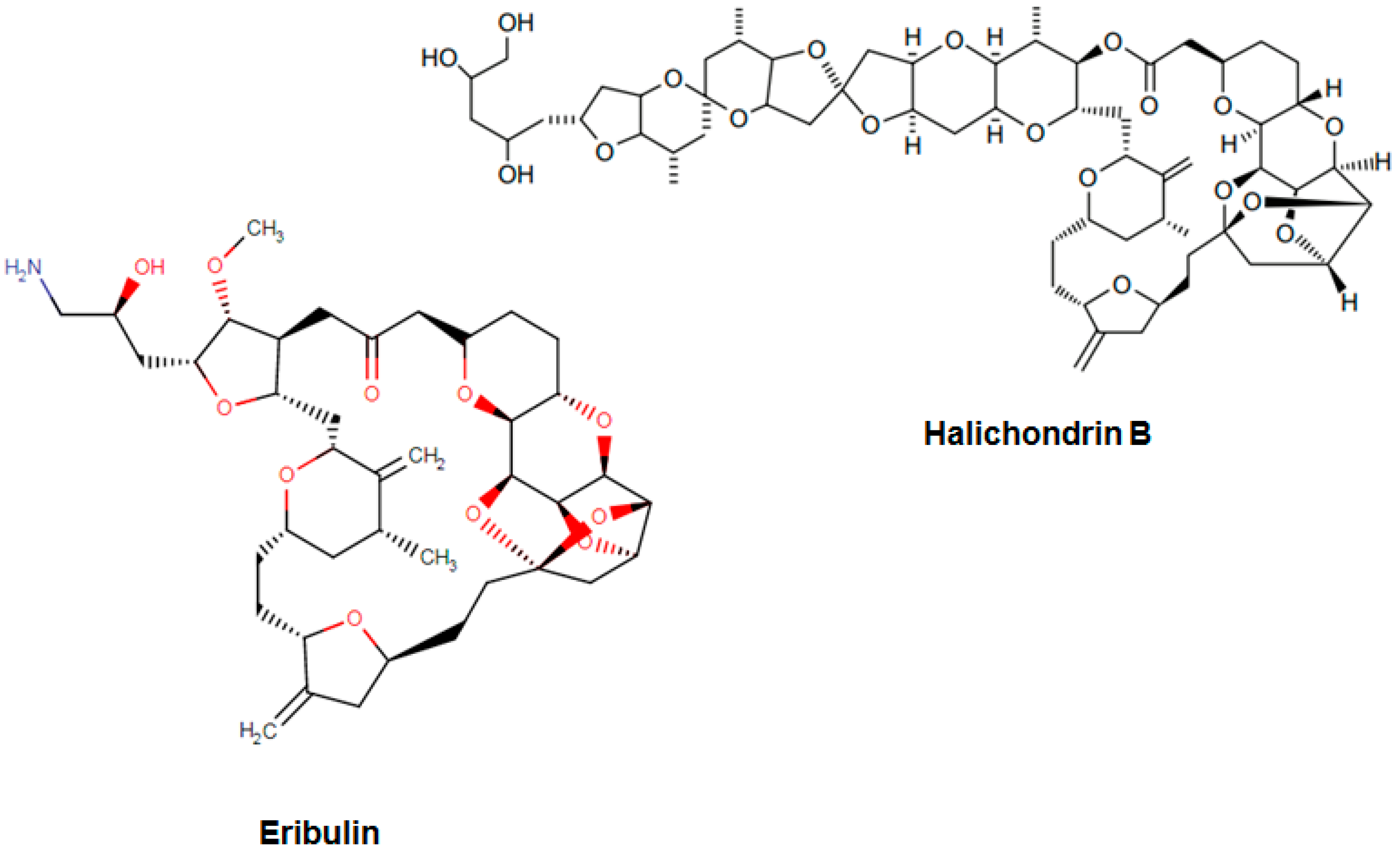
| Eribulin Halaven | Organic compound Furopyrans | Metastatic liposarcoma Refractory, metastatic breast cancer Unresectable liposarcoma | Tubulin-based antimitotic | Apoptosis regulator Bcl-2 Tubulin beta-1 chain | November 2010 |
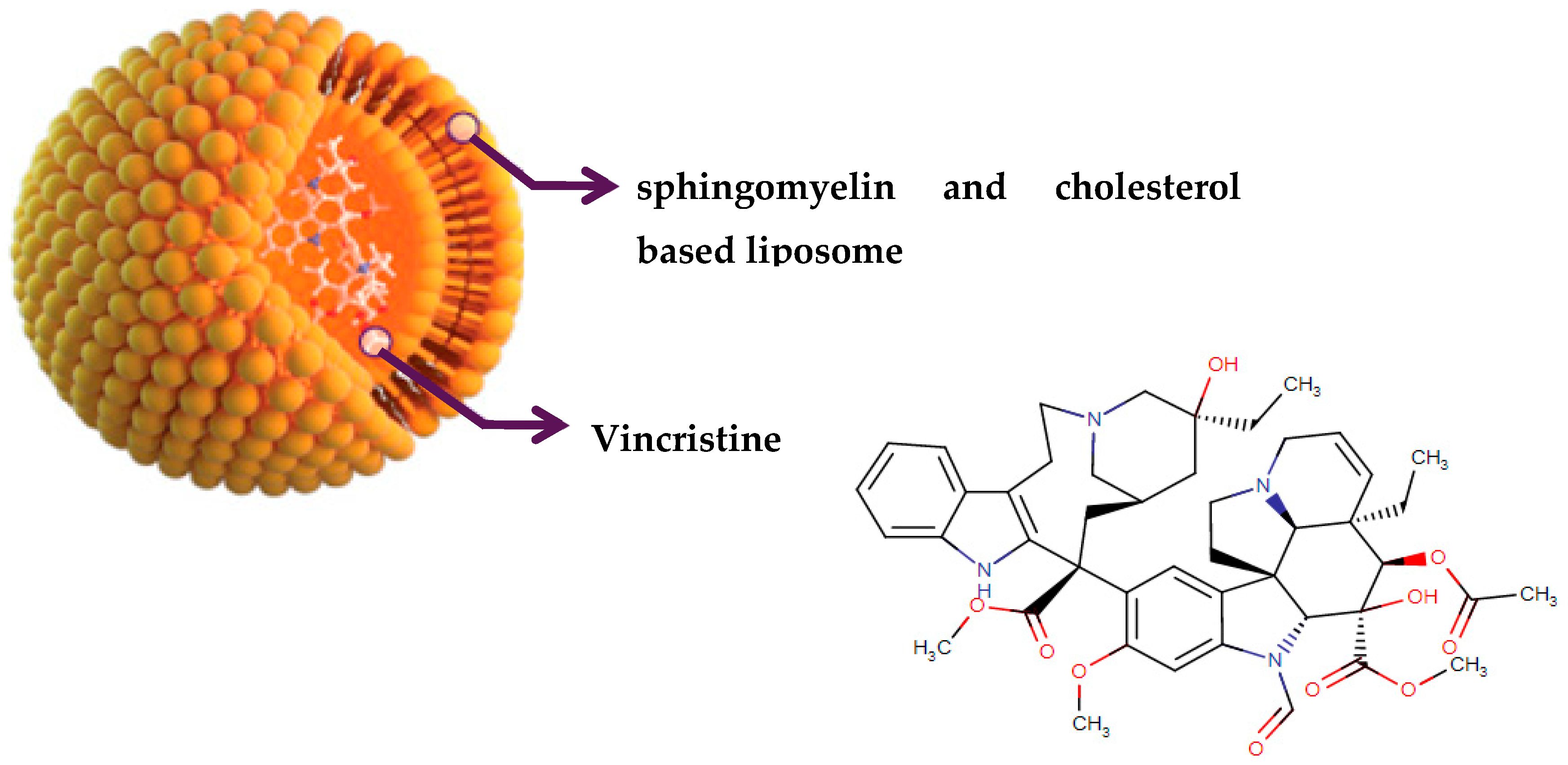
| VinCRIStine sulfate LIPOSOME Marqibo | Vinca alkaloids formulated in liposomes (sphingomyelin and cholesterol based) | Relapsed Philadelphia chromosome-negative (Ph-) acute lymphoblastic leukemia Malignant lymphoma Hodgkin’s disease | Tubulin-based antimitotic | Tubulin beta chain Tubulin alpha-4A chain | August 2012 |
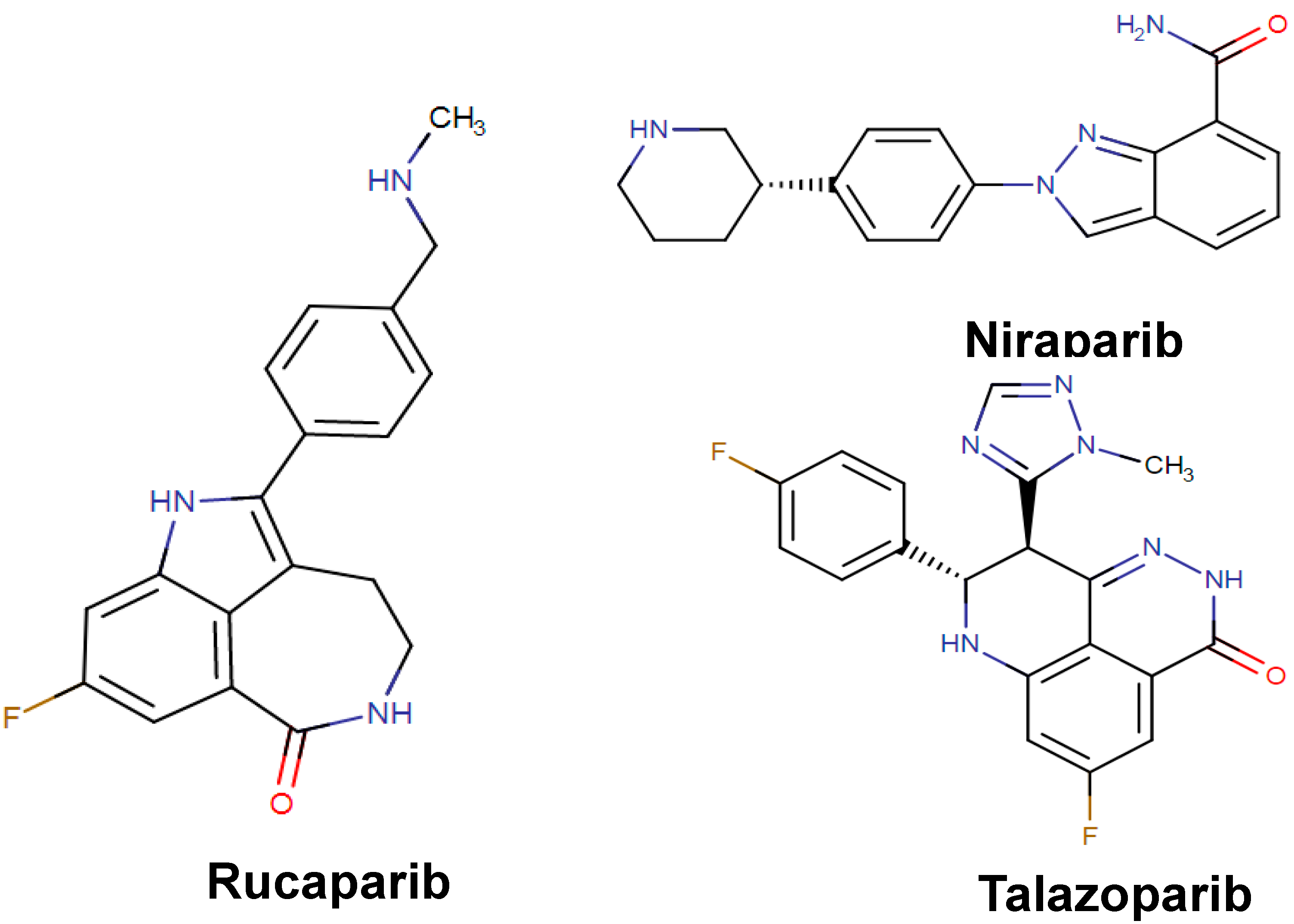
| Rucaparib Rubraca | Organic compound Indoles and derivatives Indoles | Advanced ovarian cancer | PARPs inhibitor leading to DNA damage, apoptosis, and cell death | Poly(ADP-ribose) polymerase 1 Poly(ADP-ribose) polymerase 2 Poly(ADP-ribose) polymerase 3 | December 2016 |
| Niraparib Zejula | Organic compound Indoles and derivatives Indoles | Ovarian epithelial cancer Fallopian Tube Cancer Primary Peritoneal Cancer | PARPs inhibitor leading to DNA damage, apoptosis, and cell death | Poly(ADP-ribose) polymerase 1 Poly(ADP-ribose) polymerase 2 | March 2017 |
| Talazoparib Talzenna | Organic compound Quinolines and derivatives Phenylquinolines | Locally advanced breast cancer Metastatic breast cancer | PARPs inhibitor leading to DNA damage, apoptosis, and cell death | Poly(ADP-ribose) polymerase 1 Poly(ADP-ribose) polymerase 2 | October 2018 |
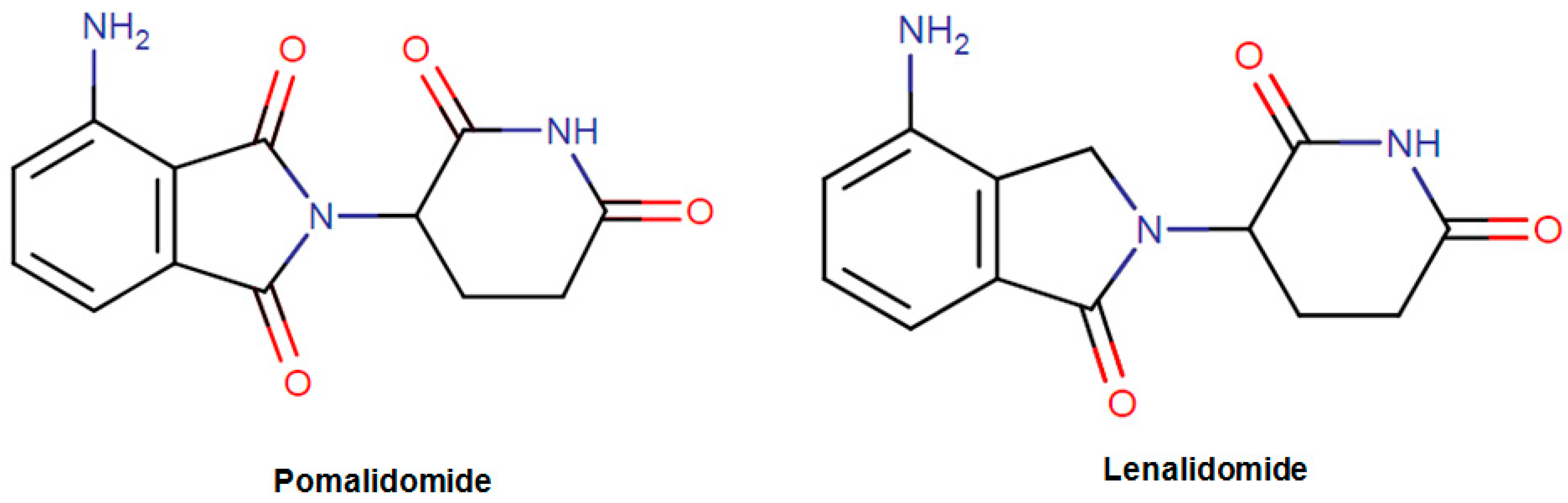
| Pomalidomide Pomalyst | Organic compound Isoindoles and derivatives Isoindolines | Refractory multiple myeloma | İnhibition of the proliferation and stimulation of apoptosis. Inhibition of the production of proinflammatory cytokines. | Protein cereblon Tumor necrosis factor Prostaglandin G/H synthase 2 | February 2013 |
| Lenalidomide Revlimid | Organic compound Soindoles and derivatives Isoindolines | Chronic Lymphocytic Leukemia Mantle Cell Lymphoma Multiple Myeloma | Inhibition of the release of proinflammatory cytokines and increasing the secretion of anti-inflammatory cytokines | Protein cereblon Tumor necrosis factor ligand superfamily member 11 Prostaglandin G/H synthase 2 | June 2013 |
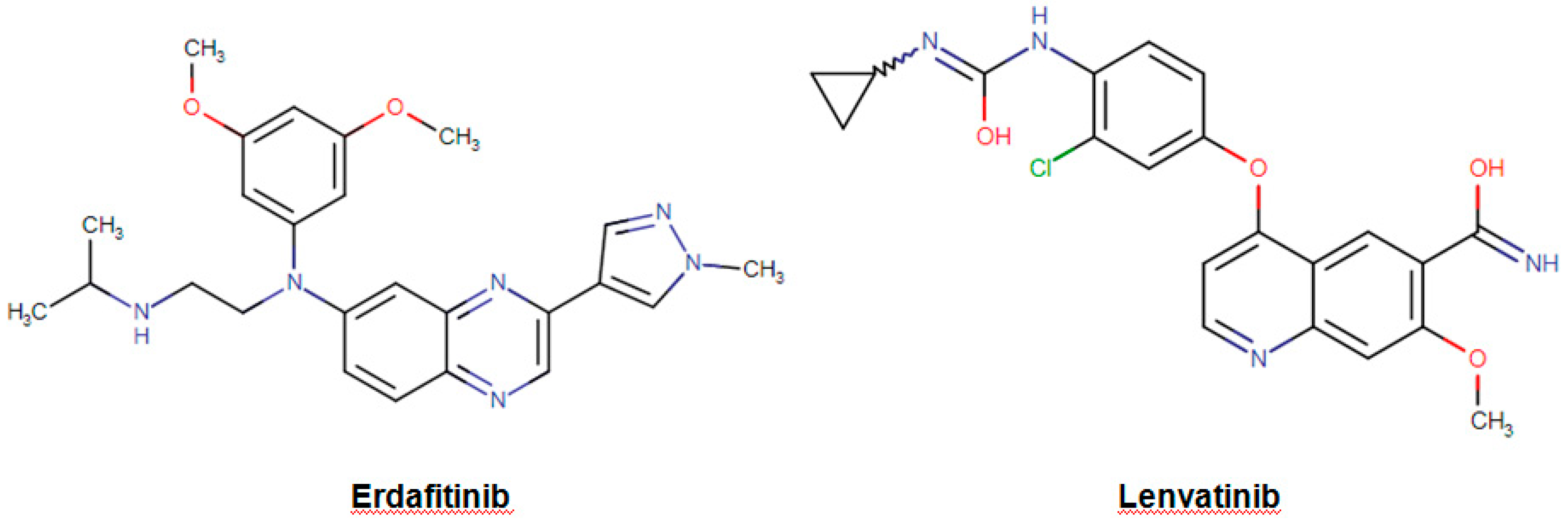
| Erdafitinib Balversa | Organic compound Organic nitrogen compounds Amines | Locally advanced urothelial carcinoma Metastatic urothelial carcinoma | Inhibition of the enzymatic activity of expressed FGFR1, FGFR2, FGFR3, and FGFR4. | Fibroblast growth factor receptor 1 Fibroblast growth factor receptor 2 Fibroblast growth factor receptor 3 Fibroblast growth factor receptor 4 | April 2019 |
| Lenvatinib Lenvima | Organic compounds Quinolines and derivatives Quinoline carboxamides | Advanced renal cell carcinoma Locally recurrent radioactive iodine-refractory thyroid cancer Metastatic radioactive iodine-refractory thyroid cancer | İnhibition of the receptor tyrosine kinases leading to suppression of angiogenesis, tumor growth, and cancer progression. | Vascular endothelial growth factor receptor 1/2/3 Fibroblast growth factor receptor 1/2/3/4 Platelet derived growth factor receptor alpha | August 2015 |
| Plants | Extracts/Molecules | Biological Approach (In Vitro/In Vivo) | Cell Lines Tested | Key Finding/Mechanisms | References |
|---|---|---|---|---|---|
| - | Artemisinin and its derivatives | In vitro | YD-10B cell line | Induction of apoptosis | [133] |
| Amonds | Amygdalin | In vitro | KB cells | cytotoxic and antiproliferative activity | [242] |
| - | Eugenol | In vitro | HSC-2 cells | Cytotoxic effect | [136] |
| Cloves oil | Eugenol | In vitro | KB cells | Cytotoxic effect | [137] |
| - | Eugenol | In vitro | Human oral mucosal fibroblasts | Cytotoxic effect | [138] |
| Piper betle L. | Safrole | In vitro | Human buccal mucosal fibroblasts (BMFs) | Cytotoxic effect | [140] |
| - | Berberine | In vitro | OC2 and KB cells |
| [143] |
| Thymus vulgaris L. | Essential oil | In vitro | UMSCC1 cells | Cytotoxic effect | [145] |
| Azadirachta indica A.Juss. | Limonoids | In vivo | Hamster buccal pouch (HBP) carcinogenesis | Inhibition of cell proliferation and induction of apoptosis | [156] |
| Alpinia pricei Hayata | Chalcone | In vitro | HSC-3 cells |
| [157] |
| Pinus densiflora Siebold & Zucc. | Essential oil | In vitro | YD-8 cells |
| [243] |
| Artemisia capillaris Thunb. | Essential oil | In vitro | KB cells | Induction of apoptosis | [150] |
| Cinnamomum cassia (L.) J.Presl | Cinnamaldehyde Essential oil | In vitro | HSC-3 cells | Cell cycle arrest and apoptosis | [244] |
| Cryptomeria japonica (Thunb. ex L.f.) D.Don | Essential oil | In vitro | KB cells |
| [245] |
| Solanum spirale Roxb. | Essential oil | In vitro | KB cells | Cytotoxic effect | [151] |
| Salvia officinalis L. | Essential oil | In vitro | UMSCC1 cells | Cytotoxic effect | [146] |
| Levisticum officinale W.D.J.Koch | Essential oil | In vitro | UMSCC1 cells | Cytotoxic effect | [147] |
| Elaeagnus angustifolia L. | Aqueous extract | In vitro | SCC25 cells |
| [246] |
| Neolitsea variabillima (Hayata) Kaneh. & Sasaki | Essential oil | In vitro | OEC-M1 cells | Cytotoxic effect | [247] |
| - | Carvacrol | In vitro | OC2 cells |
| [144] |
| - | Chios mastic gum extract | In vitro | YD-10B cells |
| [248] |
| Saussurea costus (Falc.) Lipsch. | Methanol extract | In vitro | KB cells | Induction of apoptosis | [249] |
| Thymus caramanicus Jalas | Hydro-ethanolic extractEssential oil | In vitro | KB cells | Cytotoxic effect | [250] |
| Artemisia gmelinii Weber ex Stechm. | Essential oil | In vitro | KB cells | Cytotoxic effect | [149] |
| Mentha spicata L. Associated with Mentha × rotundifolia (L.) Huds. | Hexane Extract | In vitro | KB cells | Anti-neoplastic activity | [251] |
| Piper betle L. and Psidium guajava L. | Aqueous extract | In vitro | KB cells | Cytotoxic effect | [252] |
| Zanthoxylum nitidum (Roxb.) DC. | Nitidine chloride | In vitro and in vivo | HSC-3 and HSC-4 cells | Decreased cell viability via apoptosis | [253] |
| Molecules | Biological Approach (in vitro/in vivo) | Cell lines tested | Key Finding/mechanisms | References |
|---|---|---|---|---|
| Ganoderma lucidum | In vitro | HCT116 | Induced apoptosis in HCT116 and increased caspase-8, caspase-3, and Fas | [159] |
| Aloe-Emodin | In vitro | SW-620 and HT-29 | Suppress cell proliferation in a dose-dependent manner and induced ROS production | [254] |
| Triterpenes | In vitro | HT-29 | Suppresses the proliferation, inhibits tumor growth in the colon carcinoma xenograft model | [160] |
| Cannabidiol | In vivo | HCT116 mice xenograft | Reduced pre-neoplastic lesions and azoxymethane-induced polyps | [161] |
| Genistein | In vitro | HT-29 | Proapoptotic effect: increases expression of Bax or p21 proteins; inhibits NF-kB and topoisomerase II, in combination with cisplatin inhibits cell growth; and induces apoptosis | [162,163] |
| Stictic acid | In vitro | HT-29 | Moderate anticancer activity and low growth inhibition on nonmalignant cells (MRC-5) | [255] |
| Apigenin | In vitro | HT-29 and HRT-18 | Increases activity of CD26, more in combination with irinotecan | [166] |
| Quercetin | In vitro | SW-620, HT-29, Caco-2 | Sensitizes cells against TRAIL, causing apoptosis, generating of ROS | [167] |
| Geraniol | In vitro | Caco-2 | Increased apoptosis combined with 5-FU | [256] |
| Lycopene | In vitro | SW480 | Acts anti-inflammatory suppresses the expression of PCNA and b-catechins | [164] |
| Ginkgo biloba L. extract | In vitro | HT29 | Inhibits progression of the tumor, increases caspase-3 activity, elevates p53 expression, and decreases expression of Bcl-2 | [165] |
| Plants | Extracts/Molecules | Biological Approach (In Vitro/In Vivo) | Cell Lines Tested | Key Finding/Mechanisms | References |
|---|---|---|---|---|---|
| Plumbago zeylanica L. | Plumbagin | In vitro | Panc-1, BxPC3, and ASPC1 | Induced apoptosis and inhibited the cell viability of PC cells Inhibited the cell invasion of PC cells | [173] |
| Plumbago zeylanica L. | Plumbagin | In vivo | Inhibition of both tumor weight and volume | [173] | |
| Moringa oleifera Lam. | In vitro | Panc-1 | Inhibited the growth of all pancreatic cell lines Enhanced the cytotoxic effect of cisplatin on Panc-1 cells | [171] | |
| - | Mimosine | In vitro | Xenografts | Inhibited of the cell cycle giving rise to growth arrest in G1-phase | [174] |
| - | Epigallocatechin-3-gallate | In vitro | Cells in a xenograft model system. | Inhibited the cell growth and induced apoptosis in human pancreatic cancer cells | [175] |
| - | MK615 | In vitro | Panc-1, PK-1, and PK45H | Increased the population of cells in G2/M phase Inhibited the expression of Aurora A and B kinases | [257] |
| Acacia pennata (L.) Willd. | In vitro | Panc-1 | Induced a cytotoxic effect | [178] | |
| - | Chittagonga CH2Cl2 | In vitro | Panc-1, Mia-PaCa2, and Capan1 | Cytotoxic activity | [176] |
| Angelica pubescens Maxim. | Angelmarin | In vitro | Panc-1 | Cytotoxic effect | [177] |
| Betulinic | In vitro | EPP85-181P | Cytotoxic effect | [179] | |
| Boesenbergia rotunda (L.) Mansf | Chloroform | In vitro | Panc-1 | Cytotoxic effect | [172] |
| - | Cucurbitacin B | In vivo | Inhibited significantly the tumor growth of pancreatic cancer xenografts | [180] | |
| - | Inositol hexaphosphate (IP6) | In vitro | Mia-PaCa et Panc-1 | Decreased the cellular growth and increased apoptosis | [22] |
| - | Apigenin | In vitro | CD18 et S2-013 | Decreased glucose uptake and downregulated the GLUT-1 glucose transporter in human pancreatic cancer cells. | [258] |
| Cruciferous vegetables | Sorafenib | In vivo | Inhibited of angiogenesis Induced of apoptosis | [181] | |
| - | Benzyl isothiocyanate | In vitro | BxPC3, Mia-PaCa2 and Panc-1 | Inhibited of cell cycle Activated of apoptotic pathways | [259] |
| - | L-canavanine | In vitro | Panc-1 and Mia-PaCa2 | Synergistic effect with radiation may have clinical potential in the treatment of pancreatic cancer | [260] |
| - | Apigenin | In vitro | MiaPaca-2, AsPC-1 | Induced of apoptosis | [261] |
| Tripterygium wilfordii Hook. f. | Triptolide | In vitro | SW1990 | Inhibited the growth of human pancreatic cancer Apoptotic activity | [182] |
| - | Luteolin | In vitro | SW1990 | Induced apoptosis by targeting Bcl-2 | [262] |
| - | Fisetetin | In vitro | Panc-1 | Increased autophagy via endoplasmic reticulum stress- and mitochondrial stress-dependent pathways | [263] |
| - | Resveratrol and quercetin | In vitro | Panc-1 | Resveratrol and quercetin affected metastasis in pancreatic cells | [264] |
| Plants | Extracts/Molecules | Biological Approach (In Vitro/In Vivo) | Cell Lines Tested | Key Finding/Mechanisms | References |
|---|---|---|---|---|---|
| Piper umbellatum L. | 4-nerolidylcatechol | In vitro | SK-MEL-2, SK-MEL-103, SK-MEL-147 | G-1 phase arrest, inhibit the effect of matrix metalloproteinase MMP-2 and MMP-9 activity, loss of membrane integrity | [184] |
| Indigofera aspalathoides DC. | Flavone glycoside | In vitro | Melanoma (LOX IMVI, MALME-3M, SK-MEL-2, SK-MEL-28, SK-MEL-5, UACC-257, UACC-62) | Important cytotoxic effect | [190] |
| Fucus evanescens C.Agardh | Fucoidans | In vitro | SK-MEL-28 and SK-MEL-5 | Inhibited colony formation and cell proliferation | [183] |
| Alpinia officinarum Hance | Galangin | In vitro | B16F10 cell line (murine melanoma cell) | Reduced the mitochondrial membrane potential | [185] |
| Hamamelis virginiana L. | Polyphenols | In vitro | SK-MEL-28 | Pro-oxidant effects | [265] |
| Satureja thymbra L. | Linalool | In vitro | Amelanotic melanoma C32 | Inhibited tumor cell growth (Mechanism Still unknown) | [196] |
| Mesua ferrea L. | Non-polar extract | In vitro | Melanoma SK-MEL-28 | Cytotoxicity | [186] |
| Euphorbia lagascae Spreng. | Polyphenol | In vitro | Melanoma SK-MEL-28 | Initiated G2/M arrest by downregulating expression of cyclins A, E, and B1. | [187] |
| Pinus pinaster Aiton | Procyanidins | In vitro | SK-MEL-28 | Cytotoxicity | [191] |
| Vitis vinifera L. | Resveratrol | In vitro | Melanoma (A-375, A-431, SK-MEL-28) | Enhanced the phosphorylation of ERK1/2. Induced an arresting of the cell cycle at G1-phase. Induced the downregulating of the protein expression of Cyclin D1, D2, and E and also cdk2, cdk4, and cdk6. | [199,200] |
| Dracaena angustifolia (Medik.) Roxb. | Saponins | In vitro | B-16 melanoma cells | Cytotoxicity | [192] |
| Schkuhria pinnata (Lam.) Kuntze ex Thell. | Sesquiterpene lactones | In vitro | Melanoma UACC-62 | Cytotoxicity | [197] |
| Silybum marianum (L.) Gaertn. | Silybin | In vitro | Human melanoma SK-MEL-5, SK-MEL-28 | Inhibited the expression of Cyclin D1 and caused the cell cycle arrest at G-1 phase. Downregulated the expression of NF-kB, Ap-1 and STAT3. Inhibited the phosphorylation of ERK1/2 and RSK2. Inhibited the activity of MEK1 and MEK2. | [193] |
| Silybum marianum (L.) Gaertn. | Silymarin | In vitro | Human malignant melanoma A375-S2 cells | Increased the expression of cell surface ligand death receptors such as Fas and Fas ligand helped the activation and cleavage of procaspase-8 that cause cell death by apoptosis. | [194] |
| Ricinus communis L. | Terpenoids (monoterpenoids: 1,8-cineole, camphor and pinene, and sesquiterpenoid: caryophyllene) | In vitro | SK-MEL-28 cells | Induced apoptosis | [198] |
| Nigella sativa L. | Thymoquinone | In vitro | Skin cancer | Cytotoxicity | [189] |
| Moringa oleifera Lam. | Extract (silver nanoparticle) | In vitro | A-431 epedermoid carcinoma cell lines | Cytotoxicity | [188] |
| Lycopodium clavatum L. | Apigenin (flavonoid) | In vitro | Human keratinocyte cell line HaCaT | Inhibited the formation of ROS, interfered with NF-kB and p38MAPK signaling pathways | [195] |
| - | Pyrroloiminoquinone compounds | In vitro | SCC13 | Inhibited cancer cell migration and invasion | [266] |
| - | Citral | In vivo | Inhibited UVB-induced skin carcinogenesis by reducing levels of oxidative stress and proinflammatory cytokines, increasing apoptotic rate in the skin | [267] |
| Plants | Extracts/Molecules | Biological Approach (In Vitro/In Vivo) | Cell Lines Tested | Key Finding/Mechanisms | References |
|---|---|---|---|---|---|
| Croton regelianus Müll.Arg. | Essential oil | In vitro | SF-295 | Cytotoxic effects | [203] |
| Juniperus phoenicea L. | Essential oil | In vitro | U251 | Cytotoxic effects | [204] |
| - | Carvone | In vitro | Primary rat neuron and neuroblastoma (N2a) cells | Increased in antioxidant level in primary cells with little potential in treatment of brain tumor | [207] |
| - | β-Elemene | In vitro | G-422 tumor cells in mice | Inhibited brain carcinomas | [208] |
| - | Flavonoid | In vitro | Rat brain PKC. | Inhibited the kinase | [209] |
| Angelica sinensis (Oliv.) Diels | Chloroform | In vitro | Glioblastoma multiforme (GBM) | Changed the cell cycle distribution, and induced apoptosis | [206] |
| Angelica sinensis (Oliv.) Diels | Chloroform | In vivo | Reduced the volume of tumor | [206] | |
| - | BRM270 | In vitro | GBM | Induced of apoptosis and inhibited cell growth | [213] |
| - | BRM270 | In vivo | Induced of apoptosis and inhibited cell growth | [213] | |
| - | Isoflavones | In vitro | GBM | By its combination with rapamycin, its isoflavones decreased the phosphorylation of Akt and eIF4E proteins, and rendered U87 cells more sensitive to rapamycin treatment | [211] |
| - | Retinoids | In vitro | T98G | Induced of apoptosis with activation of caspase-8 and cleavage of Bid to truncated Bid (tBid) | [214] |
| - | Retinoids | In vitro | U87MG | Induced apoptosis with activation of caspase-8 and cleavage of Bid to truncated Bid (tBid) | [214] |
| - | Epigallocatechin gallate | In vitro | U87 | Induced apoptosis via downregulation of p-Akt and Bcl-2 | [215] |
| - | delta-9-Tetrahydrocannabinol (Δ9-THC) | In vitro | GBM | Decreased cell viability | [216] |
| - | delta-9-tetrahydrocannabinol (Δ9-THC) | In vitro | U251 and SF126 | Acted synergistically to inhibit cell proliferation | [217] |
| - | n-Butylidenephthalide | In vitro | GBM | Decreased the cell proliferation, and induced apoptotic pathways | [212] |
| - | n-Butylidenephthalide | In vivo | Suppressed the growth of malignant brain tumor cells without inducing cytotoxicity on fibroblast | [212] | |
| Scutellaria baicalensis Georgi | Ethanolic extract | In vitro | GBM | Inhibited the cellular growth | [205] |
| Phellinus linteus | Hispolon | In vitro | U87MG | Inhibits cell viability, induced G2/M cell cycle arrest and apoptosis | [268] |
| - | Aloe-Emodin | In vitro and in vivo | U87MG | Inhibited the cellular growth | [269] |
| Plants | Extracts/Molecules | Biological Approach (In Vitro/In Vivo) | Cell Lines Tested | Key Finding/Mechanisms | References |
|---|---|---|---|---|---|
| Alpiniae katsumadai | Cardamonin | In vitro | MDA-MB-231 cells | Inhibition of the HIF-1α pathway and modulated cancer cell metabolism | [270] |
| Garcinia bracteata C.Y.Wu ex Y.H.Li | Neobractatin | In vitro | MDA-MB-231 and MCF-7 cells | Prevention of metastasis | [271] |
| Tetradium ruticarpum (A.Juss.) T.G.Hartley | Evodiamine | In vitro and in vivo | NCI/ADR-RES cells | Inhibition of the proliferation of NCI / ADR-RES cells of human breast cancer resistant to adriamycin | [218] |
| Sanguinaria canadensis L. | Sanguinarine | In vivo |
| Analyzing changes in expression levels with various antiapoptotic proteins. | [219] |
| Sophora flavescens Aiton | Matrine | In vitro and in vivo | MCF-7 cells and mouse 4T1 breast cancer cell lines | Reduced viability of both types of cells and induction of apoptosis in MCF-7 cells | [221] |
| Piper nigrum L. | Piperine | In vitro and in vivo | 4T1 cells |
| [222] |
| Curcuma longa L. | Curcumin | In vivo | MCF-7, MDA-MB-231, BT-474 | Inhibition of tumor regression induced by cyclophosphamide. | [22] |
| - | Carvacrol | In vitro | MDA-MB-231 | Induction of apoptosis in cells | [234] |
| Boswellia sacra Flueck. | Essential oil | In vitro | T47D, MCF-7, MDA-MB-231 | Suppression of cellular network formation and disruption of spheroid development of breast cancer cells. | [224] |
| Nigella sativa L. | Essential oil | In vitro | MCF-7 cells | Induction of apoptosis in cancer cells. | [272] |
| Pulicaria jaubertii E.Gamal-Eldin | Essential oil | In vitro | MCF-7 cells | Cytotoxic effect | [225] |
| Commiphora pyracanthoides Engl. Boswellia carterii Balf.f. | Essential oil | In vitro | MCF-7 cells | Induction of apoptosis in cells | [231] |
| Cymbopogon citratus (DC.) Stapf Cymbopogon nardus (L.) Rendle | In vitro | MCF-7 cells | Cytotoxic effect | [273] | |
| Thymus linearis Benth. Thymus serpyllum L. | Essential oil | In vitro | MCF-7 cells | Antiproliferative activity | [232] |
| Porcelia macrocarpa R.E.Fr. | Essential oil | In vitro | SKBr cells | Cytotoxic effect | [233] |
| Annona muricata L. | Essential oil | In vitro | MCF-7 cells | Cytotoxic effect | [226] |
| Cedrelopsis grevei Baill. & Courchet | Essential oil | In vitro | MCF-7 cells | Cytotoxic effect | [227] |
| Seseli transcaucasicum Pimenov & Sdobnina | Essential oil | In vitro | MCF-7 cells | Cytotoxic effect | [228] |
| Melissa officinalis L. | Essential oil | In vitro | MCF-7 cells | Cytotoxic effect | [229] |
| Lycopus lucidus Turcz. var. hirtus Regel | Essential oil | In vitro | MDA-MB-435S and ZR-75-30 cell lines | Cytotoxic effect | [274] |
| Syzygium aromaticum (L.) Merr. & L.M.Perry | Aqueous extract, ethanolic extract, oil extract | In vitro | MCF-7 and MDA-MB-231 | Cytotoxic effect | [275] |
| Salvia officinalis L. | Essential oil | In vitro | MCF-7 cells | Cytotoxic effect | [230] |
| Mentha spicata L. Zingiber officinale Roscoe Citrus limon (L.) Osbeck Jasminum grandiflorum L. Matricaria chamomilla L. Thymus vulgaris L. Rosa × damascena Herrm. | Essential oil | In vitro | MCF-7 cells | Cytotoxic effect | [223] |
| Laurus nobilis L. Origanum syriacum L. Origanum vulgare L. Salvia fruticosa Mill. | Volatile oil | In vitro | MCF-7 cells | Cytotoxic effect | [276] |
| Schinus molle L. Schinus terebinthifolia Raddi | Essential oil | In vitro | MCF-7 cells | Cytotoxic effect | [277] |
| Rosmarinus officinalis L. | Essential oil | In vitro | MCF-7 cells | Antiproliferative activity | [239] |
| Schefflera heptaphylla (L.) Frodin | Essential oil | In vitro | MCF-7 cells | Antiproliferative activity | [240] |
| Eucalyptus sideroxylon A.Cunn. ex Woolls Eucalyptus torquata Luehm. | Essential oil, methanolic extract, aqueous extract | In vitro | MCF-7 cells | Antiproliferative activity | [241] |
| Schinus molle L. | Essential oil | In vitro | EMT6 cell lines | Cytotoxic effect | [278] |
| Dictamnus albus L. | Essential oil | In vitro | MCF-7, ZR-75-30 and MDA-MB-435S | Antiproliferative activity | [279] |
| Salvia officinalis L. Sideritis perfoliata L. Satureja thymbra L. Laurus nobilis L. | Essential oil | In vitro | MCF-7 cells | Cytotoxic effect | [196] |
| Juniperus phoenicea L. | Essential oil | In vitro | MCF-7 cells | Cytotoxic effect | [204] |
| Magnolia ovata (A.St.-Hil.) Spreng. Symphyopappus itatiayensis (Hieron.) R.M.King & H.Rob. Myrciaria floribunda (H.West ex Willd.) O.Berg Psidium cattleianum Afzel. ex Sabine Nectandra megapotamica (Spreng.) Mez | Essential oil | In vitro | MCF-7 cells | Cytotoxic effect | [280] |
| Citrus limon (L.) Osbeck | Essential oil | In vitro | MCF-7 cells | Cytotoxic effect | [281] |
| Lavandula stoechas L. | Essential oil | In vitro | BC1 cells | Cytotoxic effect | [282] |
| - | Citral | In vitro | MCF-7 cells | Cycle arrest in G2/M phase and apoptosis induction | [235] |
| - | Eugenol | In vivo | MCF-7 cells | Growth inhibition and apoptosis induction | [283] |
| - | Galbanic acid | In vitro | MCF-7 and MDA-MB-231 cells | Inhibited proliferation and induced apoptosis | [284] |
| - | α-Santalol | In vitro | MCF-7 and MDA-MB-231 cells | G2/M phase cell cycle arrest and apoptosis | [236] |
| - | Piperine | In vivo | MCF-7 cells | Inhibition of self-renewal of breast stem cells | [285] |
| - | Phytol | in vitro | MCF-7 cells | Cytotoxic activity in a dose-dependent manner | [286] |
| - | Matrine | In vitro | MDA-MB-231 cell line | Inhibition of proliferation and invasion of cancer cells via the EGF/VEGF-VEGFR1-Akt-NF-κB signaling pathway. | [287] |
| - | Sanguinarine | In vitro | MDA-MB-231 cells | Inhibition of cell growth and migration of MDA-MB-231 cells | [288] |
| - | Tetrandrine | In vivo | MCF-7/adr cell lines | inhibition of cell growth in MCF-7 and MCF-7/adr cells | [289] |
| - | Curcumin | In vitro | MCF-7 cells | Inhibition of proliferative effects of bisphenol A on MCF-7 cells. | [290] |
| - | Curcumin and citral, | In vitro | MCF-7 and MDA-MB-231 cell lines | Induction of apoptosis and cell cycle arrest at G0/G1 phase in breast cancer cells | [237] |
| - | Curcumin | In vitro and in vivo | MDA-MB-231 cells |
| [291] |
| - | Curcumin | In vivo | MDA-MB-435 cells |
| [292] |
| - | Curcumin | In vitro and in vivo | MCF-7 and MDA-MB-231 |
| [293] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharifi-Rad, J.; Ozleyen, A.; Boyunegmez Tumer, T.; Oluwaseun Adetunji, C.; El Omari, N.; Balahbib, A.; Taheri, Y.; Bouyahya, A.; Martorell, M.; Martins, N.; et al. Natural Products and Synthetic Analogs as a Source of Antitumor Drugs. Biomolecules 2019, 9, 679. https://doi.org/10.3390/biom9110679
Sharifi-Rad J, Ozleyen A, Boyunegmez Tumer T, Oluwaseun Adetunji C, El Omari N, Balahbib A, Taheri Y, Bouyahya A, Martorell M, Martins N, et al. Natural Products and Synthetic Analogs as a Source of Antitumor Drugs. Biomolecules. 2019; 9(11):679. https://doi.org/10.3390/biom9110679
Chicago/Turabian StyleSharifi-Rad, Javad, Adem Ozleyen, Tugba Boyunegmez Tumer, Charles Oluwaseun Adetunji, Nasreddine El Omari, Abdelaali Balahbib, Yasaman Taheri, Abdelhakim Bouyahya, Miquel Martorell, Natália Martins, and et al. 2019. "Natural Products and Synthetic Analogs as a Source of Antitumor Drugs" Biomolecules 9, no. 11: 679. https://doi.org/10.3390/biom9110679
APA StyleSharifi-Rad, J., Ozleyen, A., Boyunegmez Tumer, T., Oluwaseun Adetunji, C., El Omari, N., Balahbib, A., Taheri, Y., Bouyahya, A., Martorell, M., Martins, N., & C. Cho, W. (2019). Natural Products and Synthetic Analogs as a Source of Antitumor Drugs. Biomolecules, 9(11), 679. https://doi.org/10.3390/biom9110679











