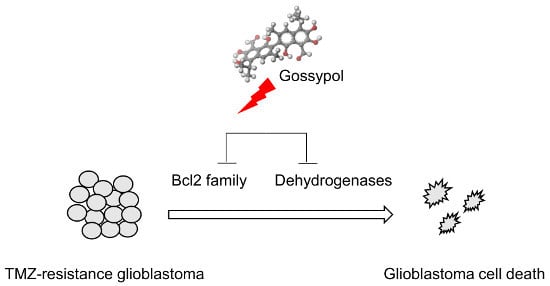
Gossypol Suppresses Growth of Temozolomide-Resistant Glioblastoma Tumor Spheres
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
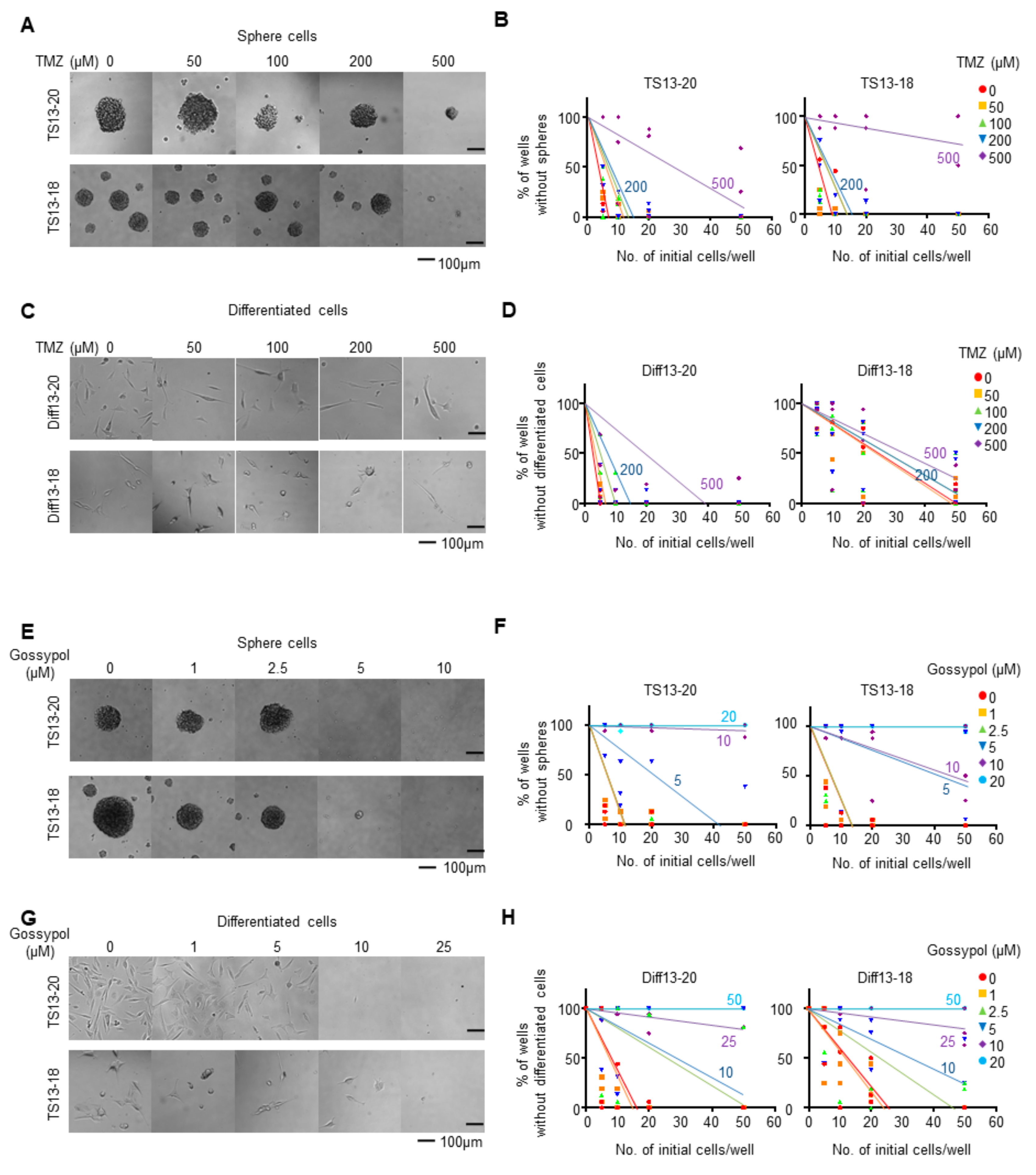
2.2. Limiting Dilution Assays
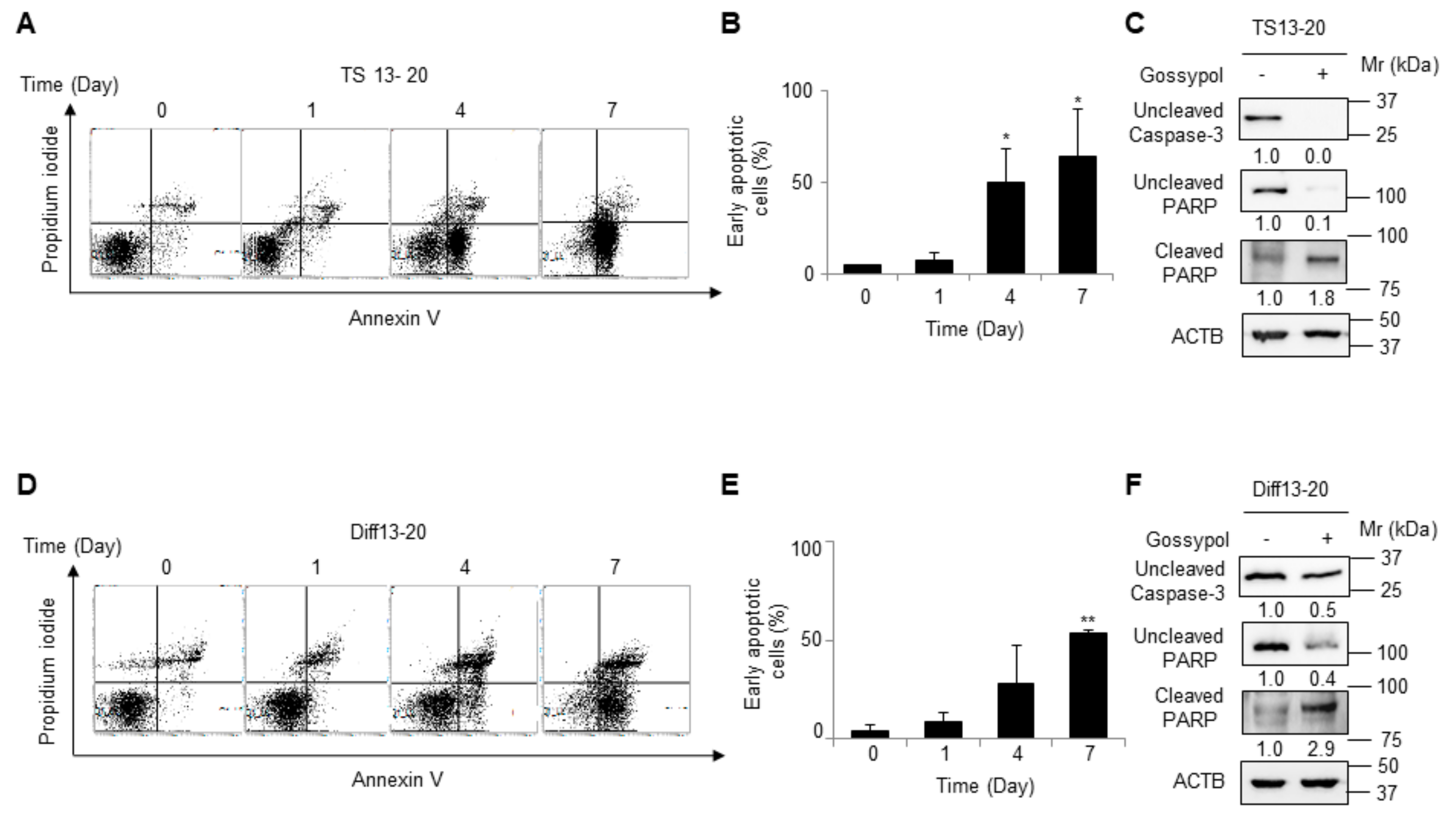
2.3. Detection of Apoptosis
2.4. Western Blot
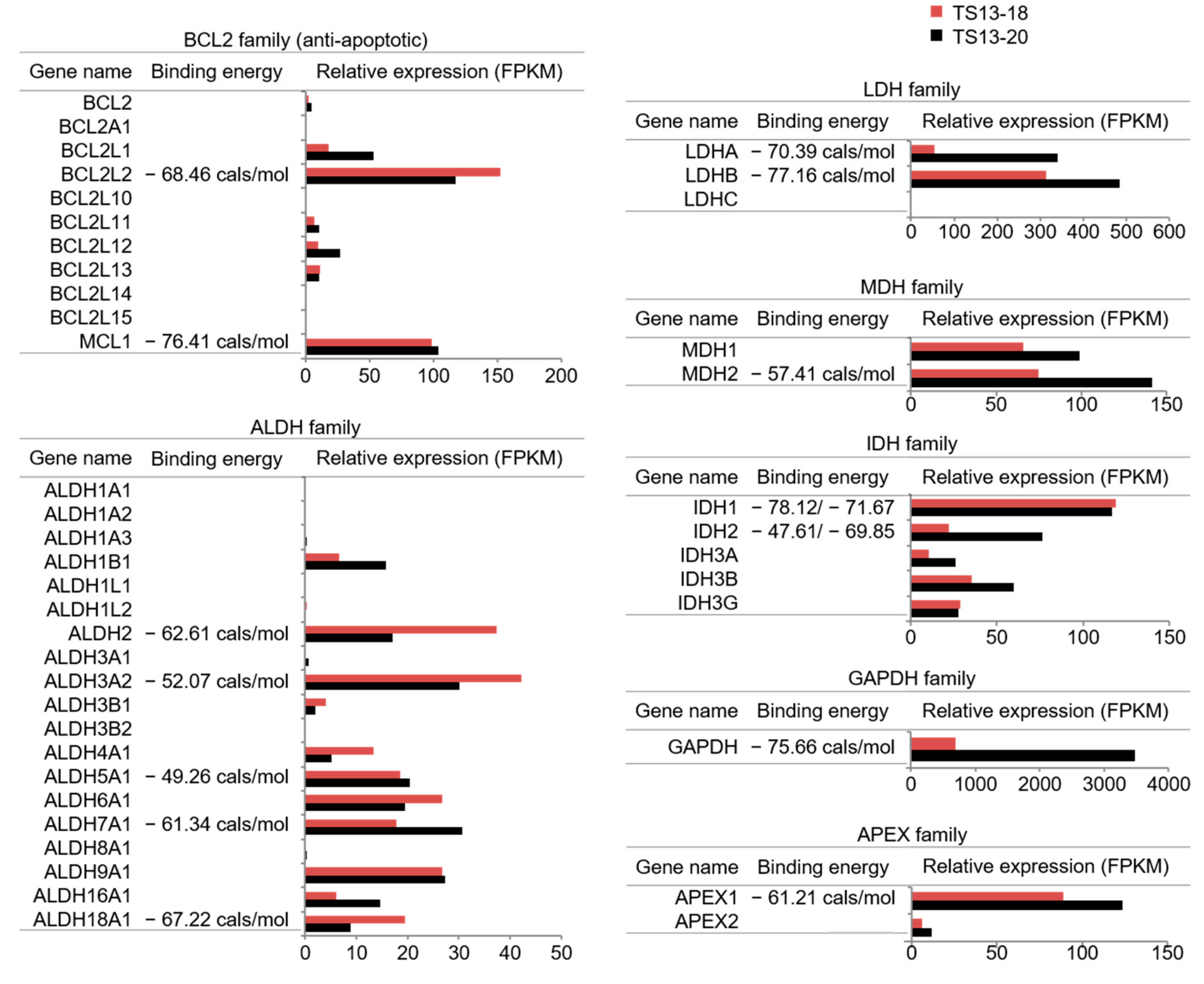
2.5. Binding Energy Calculation
2.6. Image-Based Quantification of Sphere and Cell Numbers
2.7. Measurement of Mitochondrial Membrane Potential
2.8. Measurement of ATP Level
2.9. Cell Viability Assay
2.10. Statistical Analysis
3. Results
3.1. Gossypol Suppresses Growth of Temozolomide-Resistant Glioblastoma Cells
3.2. Gossypol Induces Apoptosis in Both Glioblastoma Tumor Spheres and Differentiated Cells
3.3. The Mode of Action of Gossypol in Glioblastoma
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stupp, R.; Brada, M.; Van Den Bent, M.J.; Tonn, J.C.; Pentheroudakis, and Esmo Guidelines Working Group. High-grade glioma: Esmo Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25, iii93–iii101. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Hau, P.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the eortc-ncic trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Batash, R.; Asna, N.; Schaffer, P.; Francis, N.; Schaffer, M. Glioblastoma multiforme, diagnosis and treatment; recent literature review. Curr. Med. Chem. 2017, 24, 3002–3009. [Google Scholar] [CrossRef]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Ma, J.; Xu, L.; Wu, D. Natural product gossypol and its derivatives in precision cancer medicine. Curr. Med. Chem. 2019, 26, 1849–1873. [Google Scholar] [CrossRef]
- Wang, G.; Nikolovska-Coleska, Z.; Yang, C.Y.; Wang, R.; Tang, G.; Guo, J.; Meagher, J.; Roller, P.R.; Tomita, Y.; Jiang, S.; et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J. Med. Chem. 2006, 49, 6139–6142. [Google Scholar] [CrossRef] [PubMed]
- Masood, A.; Chitta, K.; Paulus, A.; Khan, A.N.H.; Sher, T.; Ersing, N.; Lee, K.P.; Miller, K.C.; Manfredi, D.; Ailawadhi, S.; et al. Downregulation of Bcl2 by at-101 Enhances the Antileukaemic Effect of Lenalidomide Both by an Immune Dependant and Independent Manner. Br. J. Haematol. 2012, 157, 59–66. [Google Scholar] [CrossRef]
- Van Poznak, C.; Seidman, A.D.; Reidenberg, M.M.; Moasser, M.M.; Sklarin, N.; Van Zee, K.; Bloch, R.; Sonenberg, M.; Norton, L.; Hudis, C.; et al. Oral gossypol in the treatment of patients with refractory metastatic breast cancer: A phase I/II clinical trial. Breast Cancer Res. Treat. 2001, 66, 239–248. [Google Scholar] [CrossRef]
- Xiong, J.; Li, J.; Yang, Q.; Wang, J.; Su, T.; Zhou, S. Gossypol has anti-cancer effects by dual-targeting Mdm2 and Vegf in human breast cancer. Breast Cancer Res. 2017, 19, 27. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.S.; Seo, J.; Lee, S.H.; Kang, J.H.; Song, J.; Kim, S.Y. Targeting Mitochondrial Oxidative Phosphorylation Abrogated Irinotecan Resistance in Nsclc. Sci. Rep. 2018, 8, 15707. [Google Scholar] [CrossRef]
- Kang, J.H.; Lee, S.H.; Hong, D.; Lee, J.S.; Ahn, H.S.; Ahn, J.H.; Lee, C.; Lee, J.H.; Kim, S.Y.; Hong, K.M.; et al. Aldehyde dehydrogenase is used by cancer cells for energy metabolism. Exp. Mol. Med. 2016, 48, e272. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, C.; Yan, X.; Lan, B.; Wang, J.; Wei, C.; Cao, X.; Wang, R.; Yao, J.; Zhou, T.; et al. A novel bioavailable Bh3 mimetic efficiently inhibits colon cancer via cascade effects of mitochondria. Clin. Cancer Res. 2016, 22, 1445–1458. [Google Scholar] [CrossRef] [PubMed]
- Mani, J.; Vallo, S.; Rakel, S.; Antonietti, P.; Gessler, F.; Blaheta, R.; Kögel, D.G.; Bartsch, M.; Michaelis, J.; Cinatl, A.; et al. Chemoresistance Is Associated with Increased Cytoprotective Autophagy and Diminished Apoptosis in Bladder Cancer Cells Treated with the Bh3 Mimetic (-)-Gossypol (at-101). BMC Cancer 2015, 15, 224. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Tang, A.J.; Castoreno, A.B.; Kuo, S.Y.; Wang, Q.; Kuballa, P.; Wagner, B.K.; Xavier, R.; Shamji, A.F.; Schreiber, S.L. Gossypol and an Hmt G9a inhibitor act in synergy to induce cell death in pancreatic cancer cells. Cell Death Dis. 2013, 4, e690. [Google Scholar] [CrossRef] [PubMed]
- Voss, V.; Senft, C.; Lang, V.; Ronellenfitsch, M.W.; Steinbach, J.P.; Seifert, V.; Kögel, D. The pan-Bcl-2 inhibitor (-)-gossypol triggers autophagic cell death in malignant glioma. Mol. Cancer Res. 2010, 8, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Warnsmann, V.; Meyer, N.; Hamann, A.; Kögel, D.; Osiewacz, H.D. A novel role of the mitochondrial permeability transition pore in (-)-gossypol-induced mitochondrial dysfunction. Mech. Ageing Dev. 2018, 170, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Bushunow, P.; Reidenberg, M.M.; Wasenko, J.; Winfield, J.; Lorenzo, B.; Lemke, S.; Coyle, T.; Himpler, B.; Corona, R. Gossypol treatment of recurrent adult malignant gliomas. J. Neurooncol. 1999, 43, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Jarzabek, M.A.; Amberger-Murphy, V.; Callanan, J.J.; Gao, C.; Zagozdzon, A.M.; Shiels, L.; Gallagher, W.M.; Wang, J.; Ligon, K.L.; Rich, B.E.; et al. Interrogation of gossypol therapy in glioblastoma implementing cell line and patient-derived tumour models. Br. J. Cancer 2014, 111, 2275–2286. [Google Scholar] [CrossRef]
- Adamski, V.; Hempelmann, A.; Flüh, C.; Lucius, R.; Synowitz, M.; Hattermann, K.; Held-Feindt, J. Dormant glioblastoma cells acquire stem cell characteristics and are differentially affected by temozolomide and At101 treatment. Oncotarget 2017, 8, 108064–108078. [Google Scholar] [CrossRef]
- Adamski, V.; Schmitt, C.; Ceynowa, F.; Adelung, R.; Lucius, R.; Synowitz, M.; Held-Feindt, J.; Hattermann, K. Effects of sequentially applied single and combined temozolomide, hydroxychloroquine and At101 treatment in a long-term stimulation glioblastoma in vitro model. J. Cancer Res. Clin. Oncol. 2018, 144, 1475–1485. [Google Scholar] [CrossRef]
- Park, J.; Shim, J.K.; Kang, J.H.; Choi, J.; Chang, J.H.; Kim, S.Y.; Kang, S.G. Regulation of bioenergetics through dual inhibition of aldehyde dehydrogenase and mitochondrial complex I suppresses glioblastoma tumorspheres. Neuro-Oncology 2018, 20, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Linder, B.; Wehle, A.; Hehlgans, S.; Bonn, F.; Dikic, I.; Rödel, F.; Kögel, D.; Seifert, V. Arsenic Trioxide and (-)-Gossypol Synergistically Target Glioma Stem-Like Cells Via Inhibition of Hedgehog and Notch Signaling. Cancers 2019, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Kitada, S.; Leone, M.; Sareth, S.; Zhai, D.; Reed, J.C.; Pellecchia, M. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-Cell lymphocyte/leukemia-2 proteins. J. Med. Chem. 2003, 46, 4259–4264. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C.L.; Bauer, J.A.; Wolter, K.G.; Ubell, M.L.; Narayan, A.; O’Connell, K.M.; Carey, T.E.; Fisher, S.G.; Wang, S.; Wu, X.; et al. In vitro effects of the BH3 mimetic, (-)-gossypol, on head and neck squamous cell carcinoma cells. Clin. Cancer Res. 2004, 10, 7757–7763. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Moon, Y.S.; Yuan, J.H.; Chen, A.F. Enzyme inactivation and inhibition by gossypol. Mol. Cell. Biochem. 1982, 47, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M. Inhibition kinetics of NAD-linked enzymes by gossypol acetic acid. Andrologia 1990, 22, 409–416. [Google Scholar] [CrossRef]
- Messiha, F.S. Effect of gossypol on kinetics of mouse liver alcohol and aldehyde dehydrogenase. Gen. Pharmacol. 1991, 22, 573–576. [Google Scholar] [CrossRef]
- Rekha, G.K.; Rekha, G.K.; Sladek, N.E. Inhibition of human class 3 aldehyde dehydrogenase, and sensitization of tumor cells that express significant amounts of this enzyme to oxazaphosphorines, by the naturally occurring compound gossypol. Adv. Exp. Med. Biol. 1997, 414, 133–146. [Google Scholar]
- Qian, C.; Li, M.; Sui, J.; Ren, T.; Li, Z.; Zhang, L.; Wang, D.; Zhou, L.; Cheng, Y. Identification of a novel potential antitumor activity of gossypol as an Ape1/Ref-1 inhibitor. Drug Des. Dev. Ther. 2014, 8, 485–496. [Google Scholar]
- Kim, H.Y.; Kim, D.K.; Bae, S.H.; Gwak, H.; Jeon, J.H.; Kim, J.K.; Kim, S.Y.; Lee, B.I.; You, H.J.; Shin, D.H.; et al. Farnesyl diphosphate synthase is important for the maintenance of glioblastoma stemness. Exp. Mol. Med. 2018, 50, 137. [Google Scholar] [CrossRef]
- Hu, Y.; Smyth, G.K. Elda: Extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods 2009, 347, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, H.K.; Kim, H.Y.; Gawk, H.; Bae, S.H.; Sim, H.W.; Hong, K.M.; Kang, E.K.; Seoh, J.Y.; Jang, H. Fak-Copy-Gain Is a Predictive Marker for Sensitivity to Fak Inhibition in Breast Cancer. Cancers 2019, 11, 1288. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Kim, D.K.; Shin, Y.; Kim, H.Y.; Song, B.; Lee, E.Y.; Kim, S.T.; Kim, J.K.; You, H.J.; Cheong, H.; et al. Migration and invasion of drug-resistant lung adenocarcinoma cells are dependent on mitochondrial activity. Exp. Mol. Med. 2016, 48, e277. [Google Scholar] [CrossRef] [PubMed]
- Ulasov, I.V.; Mijanovic, O.; Savchuk, S.; Gonzalez-Buendia, E.; Sonabend, A.; Xiao, T.; Lesniak, M.S.; Timashev, P. Tmz regulates GBM stemness via MMP14-Dll4-Notch3 pathway. Int. J. Cancer 2019. [Google Scholar] [CrossRef] [PubMed]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; De Tribolet, N.; Weller, M.; Bromberg, J.E.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; et al. Mgmt Gene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yuan, X.; Zeng, Z.; Tunici, P.; Ng, H.; Abdulkadir, I.R.; John, S.Y.; Lu, L.; Irvin, D.; Black, K.L. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol. Cancer 2006, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Antonietti, P.; Linder, B.; Hehlgans, S.; Mildenberger, I.C.; Burger, M.C.; Fulda, S.; Kögel, D.; Steinbach, J.P.; Gessler, F.; Rodel, F.; et al. Interference with the Hsf1/Hsp70/Bag3 Pathway Primes Glioma Cells to Matrix Detachment and Bh3 Mimetic-Induced Apoptosis. Mol. Cancer Ther. 2017, 16, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Zielke, S.; Michaelis, J.B.; Linder, B.; Warnsmann, V.; Rakel, S.; Behrends, C.; Osiewacz, H.D.; Fulda, S.; Mittelbronn, M.; et al. At 101 induces ezrly mitochondrial dysfunction and hmox1 (heme oxygenase 1) to trigger mitophagic cell death in glioma cells. Autophagy 2018, 14, 1693–1709. [Google Scholar] [CrossRef] [PubMed]
- Keshmiri-Neghab, H.; Goliaei, B.; Nikoofar, A. Gossypol enhances radiation induced autophagy in glioblastoma multiforme. Gen. Physiol. Biophys. 2014, 33, 433–442. [Google Scholar] [CrossRef]
- Ryskalin, L.; Gaglione, A.; Limanaqi, F.; Biagioni, F.; Familiari, P.; Frati, A.; Fornai, F.; Espostio, V. The autophagy status of cancer stem cells in glioblastoma multiforme: From cancer promotion to therapeutic strategies. Int. J. Mol. Sci. 2019, 20, 3824. [Google Scholar] [CrossRef]
- Vitale, I.; Manic, G.; Dandrea, V.; De Maria, R. Role of autophagy in the maintenance and function of cancer stem cells. Int. J. Dev. Biol. 2015, 59, 95–108. [Google Scholar] [CrossRef] [PubMed]

| Status | Study Title | Phase | Main Targets | Tumor Type |
|---|---|---|---|---|
| Completed | Gossypol in Treating Patients with Progressive or Recurrent Glioblastoma Multiforme | Phase 2 | Bcl-2 family | Glioblastoma |
| Completed | Gossypol (AT-101) and Temozolomide With or Without Radiation Therapy in Treating Patients with Newly Diagnosed Glioblastoma Multiforme | Phase 1 | Bcl-2 family | Glioblastoma |
| Completed | Gossypol Acetic Acid in Treating Patients with Recurrent, Metastatic, or Primary Adrenocortical Cancer that Cannot be Removed by Surgery | Phase 2 | unclear | Adrenocortical carcinoma |
| Completed | R-(−)-Gossypol Acetic Acid, Cisplatin, and Etoposide in Treating Patients with Advanced Solid Tumors or Extensive-Stage Small-Cell Lung Cancer | Phase 1 | Bcl-2 family | Small-cell lung cancer, advanced solid tumor |
| Unknown | Gossypol Combined with Docetaxel and Cisplatin Scheme in Advanced Non-Small-Cell Lung Cancers with APE1 High-Expression | Phase 3 | APE1 | Non-small-cell lung cancer |
| Withdrawn | Tarceva and AT-101 for Patients with Advanced Non-Small-Cell Lung Cancer | Phase 1 | Bcl-2 family | Non-small-cell lung cancer |
| Suspended | R-(−)-Gossypol Acetic Acid with Lenalidomide and Dexamethasone in Treating Patients with Relapsed Symptomatic Multiple Myeloma | Phase1/2 | Bcl-2 family | Recurrent plasma cell myeloma |
| Completed | R-(−)-Gossypol Acetic Acid in Treating Patients with Recurrent Extensive-Stage Small-Cell Lung Cancer | Phase 2 | Bcl-2 family | Small-cell lung cancer |
| Terminated | Erlotinib and AT-101 in Advanced Non-Small Cell Lung Cancer (NSCLC) Patients with Epidermal Growth Factor Receptor (EGFR) Activating Mutations | Phase 2 | Bcl-2 family | Non-small-cell lung cancer |
| Active, not recruiting | Lenalidomide and AT-101 in Treating Patients with Relapsed B-Cell Chronic Lymphocytic Leukemia | Phase1/2 | Bcl-2 family | Chronic lymphocytic leukemia |
| Completed | Phase 2 Safety and Efficacy Study of AT-101 in Combination with Rituximab in Patients with Chronic Lymphocytic Leukemia | Phase 2 | Bcl-2 family | Chronic lymphocytic leukemia |
| Completed | R-(−)-Gossypol and Androgen Ablation Therapy in Treating Patients with Newly Diagnosed Metastatic Prostate Cancer | Phase 2 | Bcl-2 family | Prostate cancer |
| Completed | Safety and Efficacy Study of AT-101 in Combination with Docetaxel and Prednisone in Men With HRPC | Phase1/2 | Bcl-2 family | Prostate cancer |
| Terminated | An Open-Label, Single-Center, Phase 1/ 2 Study of Chemoradiotherapy and AT-101 in Patients with Locally Advanced Esophageal or Gastroesophageal Junction Cancer | Phase1/2 | Bcl-2 family | Esophageal or Gastroesophageal junction cancer |
| Completed | Gossypol, Paclitaxel, and Carboplatin in Treating Patients with Solid Tumors That Are Metastatic or Cannot Be Removed by Surgery | Phase 1 | unclear | Lymphoma |
| Completed | A Randomized Phase 2 Study of AT-101 in Combination with Docetaxel in Relapsed/Refractory Non-Small-Cell Lung Cancer | Phase 2 | Bcl-2 family | Non-small-cell lung cancer |
| Completed | A Study Comparing AT-101 in Combination with Docetaxel and Prednisone Versus Docetaxel and Prednisone in Men with Chemotherapy-Naive Metastatic Hormone Refractory Prostate Cancer (HRPC) | Phase 2 | Bcl-2 family | Hormone refractory prostate cancer |
| Completed | Study of AT-101 in Combination with Topotecan in Relapsed/Refractory Small-Cell Lung Cancer | Phase1/2 | Bcl-2 family | Small-cell lung cancer |
| Terminated | A Study of AT-101 in Combination with Docetaxel in Squamous Cell Carcinoma of the Head and Neck | Phase 2 | Bcl-2 family | Head and neck Squamous cell carcinoma |
| Completed | Safety & Efficacy Study of AT-101 in Combination w/ Rituximab in Previously Untreated Grade I–II Follicular Non-Hodgkin’s Lymphoma | Phase 2 | Bcl-2 family | Follicular lymphoma |
| Completed | Phase II Safety and Efficacy Study of Single-agent AT-101 in Patients with Relapsed or Refractory B-cell Malignancies | Phase 2 | Bcl-2 family | Lymphoma |
| Active, not recruiting | Chemotherapy and Bcl-xL Inhibitor (AT-101) for Organ Preservation in Adults with Advanced Laryngeal Cancer | Phase 2 | Bcl-2 family | Laryngeal cancer |
| Completed | A Study of Single-Agent AT-101 in Men with Hormone Refractory Prostate Cancer | Phase1/2 | Bcl-2 family | Prostate cancer |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.Y.; Lee, B.I.; Jeon, J.H.; Kim, D.K.; Kang, S.-G.; Shim, J.-K.; Kim, S.Y.; Kang, S.W.; Jang, H. Gossypol Suppresses Growth of Temozolomide-Resistant Glioblastoma Tumor Spheres. Biomolecules 2019, 9, 595. https://doi.org/10.3390/biom9100595
Kim HY, Lee BI, Jeon JH, Kim DK, Kang S-G, Shim J-K, Kim SY, Kang SW, Jang H. Gossypol Suppresses Growth of Temozolomide-Resistant Glioblastoma Tumor Spheres. Biomolecules. 2019; 9(10):595. https://doi.org/10.3390/biom9100595
Chicago/Turabian StyleKim, Hee Yeon, Byung Il Lee, Ji Hoon Jeon, Dong Keon Kim, Seok-Gu Kang, Jin-Kyoung Shim, Soo Youl Kim, Sang Won Kang, and Hyonchol Jang. 2019. "Gossypol Suppresses Growth of Temozolomide-Resistant Glioblastoma Tumor Spheres" Biomolecules 9, no. 10: 595. https://doi.org/10.3390/biom9100595
APA StyleKim, H. Y., Lee, B. I., Jeon, J. H., Kim, D. K., Kang, S.-G., Shim, J.-K., Kim, S. Y., Kang, S. W., & Jang, H. (2019). Gossypol Suppresses Growth of Temozolomide-Resistant Glioblastoma Tumor Spheres. Biomolecules, 9(10), 595. https://doi.org/10.3390/biom9100595