Chitosan Film as Eco-Friendly and Recyclable Bio-Adsorbent to Remove/Recover Diclofenac, Ketoprofen, and Their Mixture from Wastewater
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Adsorbent Dosage and DCF Concentration
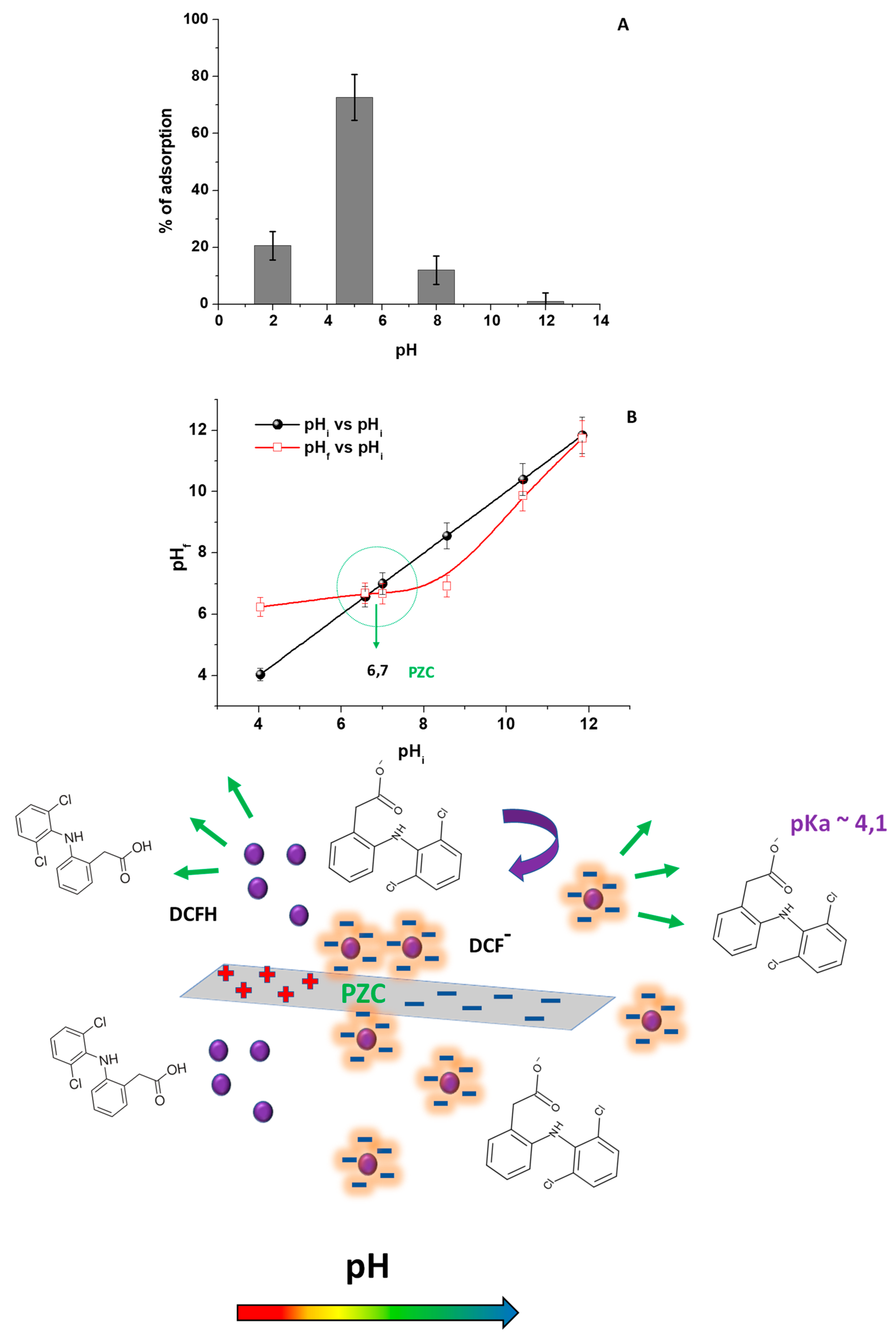
2.2. Effect of pH
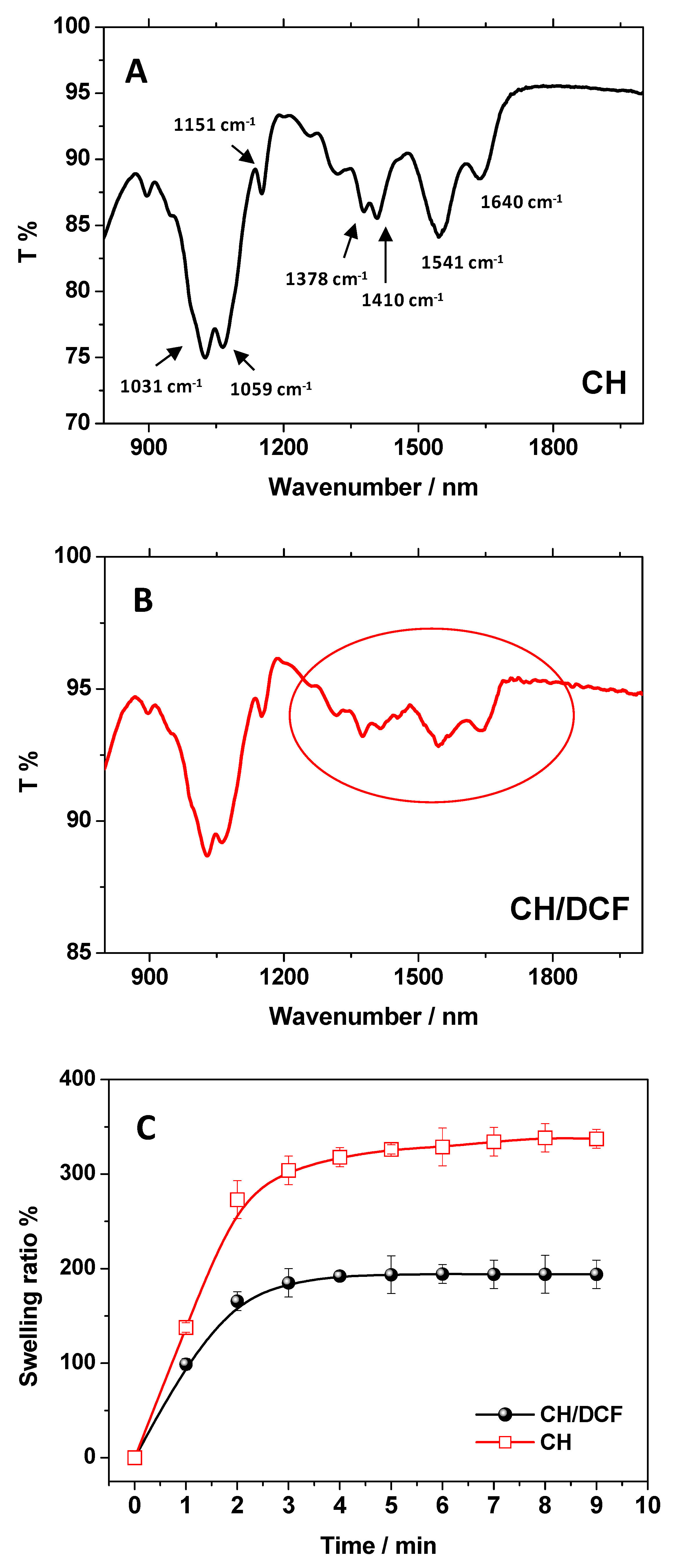
2.3. FTIR-ATR Measurements
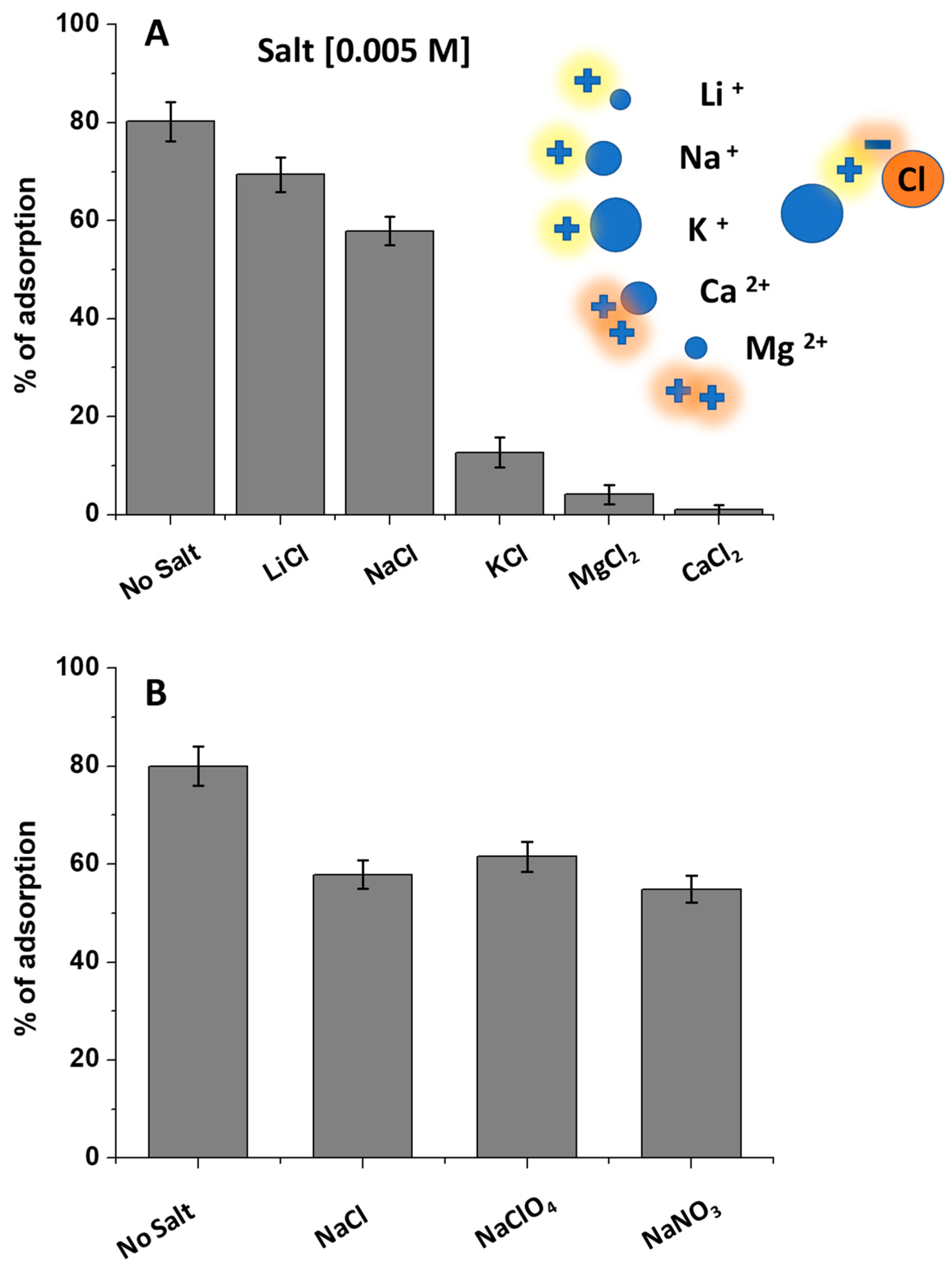
2.4. Effect of Salts in DCF Solutions
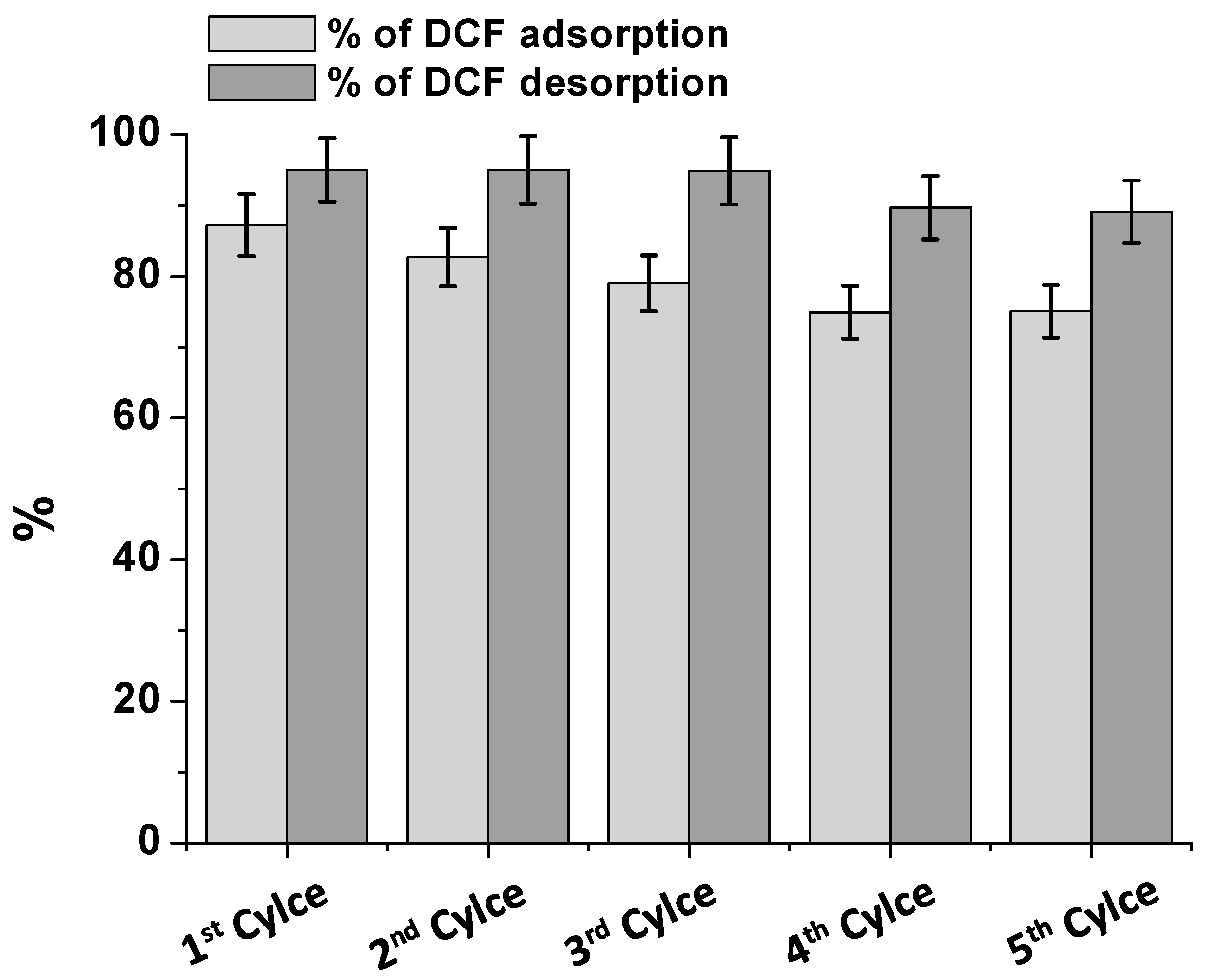
2.5. Consecutive Cycles of Adsorption
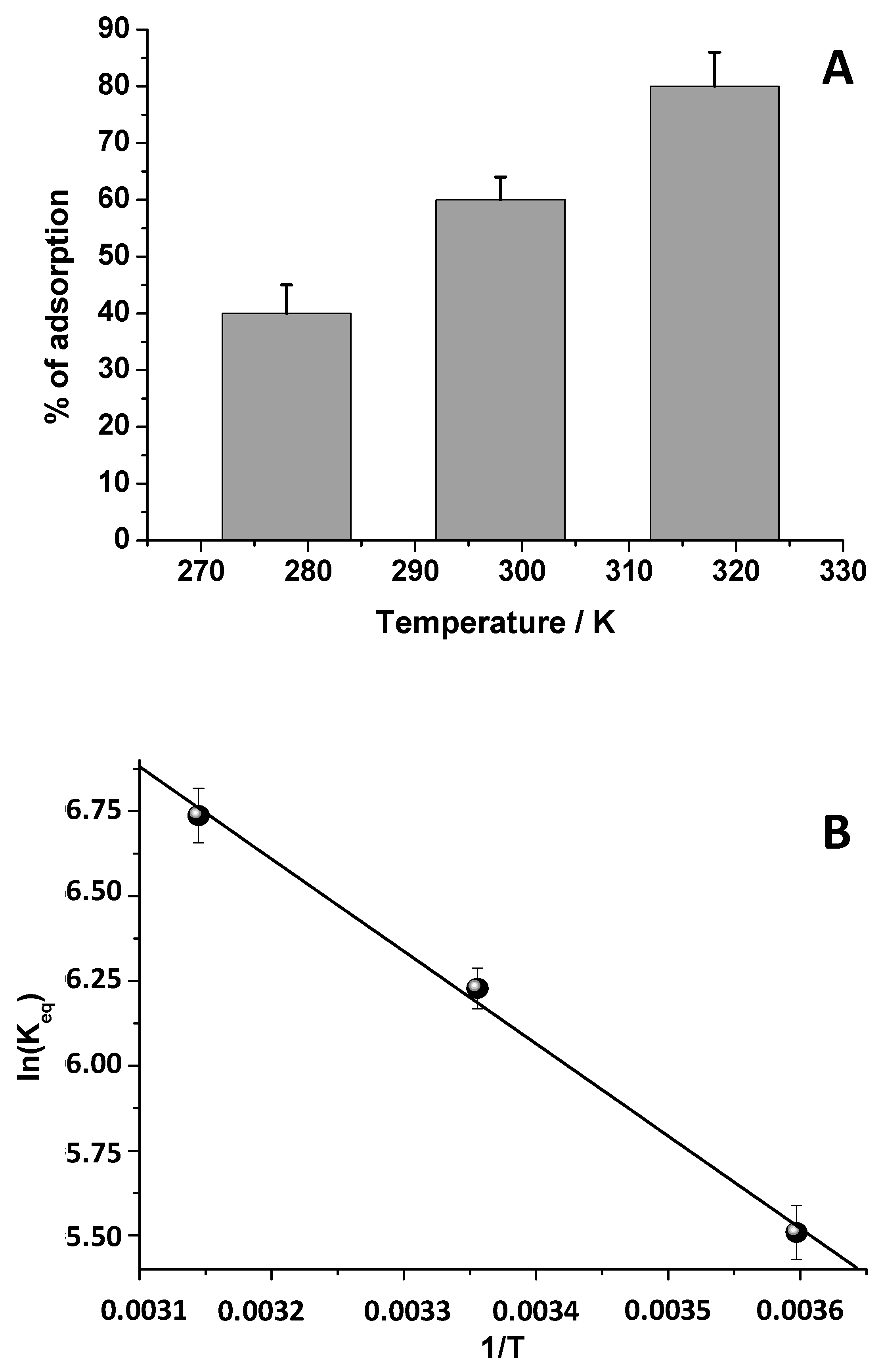
2.6. Thermodynamic Analysis
2.7. Isotherms of Adsorption
2.8. Kinetic Analysis
2.9. Release of DCF and Reuse of The Adsorbent
2.10. Preliminary in Flux Measurements
2.11. Photodegradation of DCF
2.12. Preliminary Experiments about the Removal of Ketoprofen, Diclofenac, and Their Mixture
2.13. Comparison with Other Adsorbents
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Chitosan Films
3.3. In Batch Equilibrium Experiments
3.4. In Batch Desorption Experiments
3.5. In Flow Adsorption/Desorption Experiments
3.6. Determination of Chitosan Film Zero-Point Charge
3.7. Photodegradation of DCF
3.8. UV-Visible Measurements
3.9. FTIR-ATR Spectroscopy Measurements
3.10. Swelling Ratio Measurements of Chitosan Film with and without DCF
3.11. Thermodynamic Studies
3.12. Adsorption Isotherms
3.13. Adsorption Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rizzi, V.; Lacalamita, D.; Gubitosa, J.; Fini, P.; Petrella, A.; Romita, R.; Agostiano, A.; Gabaldón, J.A.; Gorbe, M.I.; Gómez-Morte, T.; et al. Removal of tetracycline from polluted water by chitosan-olive pomace adsorbing films. Sci. Total Environ. 2019, 693, 133620. [Google Scholar] [CrossRef]
- Nisticò, R.; Bianco-Prevot, A.; Magnacca, G.; Canone, L.; García-Ballesteros, S.; Arques, A. Sustainable Magnetic Materials (from Chitosan and Municipal Biowaste) for the Removal of Diclofenac from Water. Nanomaterials 2019, 9, 1091. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.F.; Fernandes, T.; Sacramento, M.; Trindade, T.; Daniel-da-Silva, A.L. Magnetic quaternary chitosan hybrid nanoparticles for the efficient uptake of diclofenac from water. Carbohydr. Polym. 2019, 203, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, C.; Liang, J.; Shang, R.; Zhu, X.; Ding, L.; Deng, H.; Zheng, H.; Strathmann, T.J. Rapid removal of diclofenac in aqueous solution by soluble Mn(III) (aq) generated in a novel Electro-activated carbon fiber-permanganate (E-ACF-PM) process. Water Res. 2019, 165, 114975. [Google Scholar] [CrossRef]
- Lin, K.Y.A.; Yang, H.; Lee, W.D. Enhanced removal of diclofenac from water using a zeolitic imidazole framework functionalized with cetyltrimethylammonium bromide (CTAB). RSC Adv. 2015, 5, 81330–81340. [Google Scholar]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef]
- Desbrières, J.; Guibal, E. Chitosan for wastewater treatment. Polym. Int. 2018, 67, 7–14. [Google Scholar] [CrossRef]
- Rizzi, V.; Longo, A.; Fini, P.; Semeraro, P.; Cosma, P.; Franco, E.; García, R.; Ferrándiz, M.; Núñez, E.; Gabaldón, J.A.; et al. Applicative Study (Part I): The Excellent Conditions to Remove in Batch Direct Textile Dyes (Direct Red, Direct Blue and Direct Yellow) from Aqueous Solutions by Adsorption Processes on Low-Cost Chitosan Films under Different Conditions. Adv. Chem. Eng. Sci. 2014, 4, 454–469. [Google Scholar] [CrossRef]
- Liu, B.; Chen, X.; Zheng, H.; Wang, Y.; Sun, Y.; Zhao, C.; Zhang, S. Rapid and efficient removal of heavy metal and cationic dye by carboxylate rich magnetic chitosan flocculants: Role of ionic groups. Carbohydr. Polym. 2018, 181, 327–336. [Google Scholar] [CrossRef]
- Rizzi, V.; Fini, P.; Fanelli, F.; Placido, T.; Semeraro, P.; Sibillano, T.; Fraix, A.; Sortino, S.; Agostiano, A.; Giannini, C.; et al. Molecular interactions, characterization and photoactivity of Chlorophyll a/chitosan/2-HP-b-cyclodextrin composite films as functional and active surfaces for ROS production. Food Hydrocolloids 2016, 58, 98–112. [Google Scholar] [CrossRef]
- Rizzi, V.; Fini, P.; Semeraro, P.; Cosma, P. Detailed investigation of ROS arisen from chlorophyll a/Chitosanbased-biofilm. Colloids Surf. B 2016, 142, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, V.; Longo, A.; Placido, T.; Fini, P.; Gubitosa, J.; Sibillano, T.; Giannini, C.; Semeraro, P.; Franco, E.; Ferrandiz, M.; et al. A comprehensive investigation of dye–chitosan blended films for green chemistry applications. J. Appl. Polym. Sci. 2018, 135, 45945. [Google Scholar] [CrossRef]
- Lam, B.; Deon, S.; Morin-Crini, N.; Crini, G.; Fieve, P. Polymer-enhanced ultrafiltration for heavy metal removal: Influence of chitosan and carboxymethyl cellulose on filtration performances. J. Cleaner Prod. 2018, 171, 927–933. [Google Scholar] [CrossRef]
- Sutirman, Z.A.; Sanagi, M.M.; Karim, J.A.; Naim, A.A.; Wan Ibrahim, W.A. New crosslinked-chitosan graft poly(N-vinyl-2-pyrrolidone) for theremoval of Cu(II) ions from aqueous solutions. Int. J. Biol. Macromol. 2018, 107, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Odelius, K.; Hakkarainen, M. Tunable chitosan hydrogels for adsorption: Property control by biobased modifiers. Carbohydr. Polym. 2018, 196, 135–145. [Google Scholar] [CrossRef]
- Pereira, K.A.A.; Osório, L.R.; Silva, M.P.; Sousa, K.S.; Da Silva Filho, E.C. Chemical Modification of Chitosan in the Absence of Solvent for Diclofenac Sodium Removal: pH and Kinetics Studies. Mater. Res. 2014, 17, 141–145. [Google Scholar] [CrossRef]
- Zhou, X.; Dong, C.; Yang, Z.; Tian, Z.; Lu, L.; Yang, W.; Wang, Y.; Zhang, L.; Li, A.; Chen, J. Enhanced adsorption of pharmaceuticals onto core-brush shaped aromatic rings-functionalized chitosan magnetic composite particles: Effects of structural characteristics of both pharmaceuticals and brushes. J. Cleaner Prod. 2018, 172, 1025–1034. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Z.; Dai, C.; Zhou, X. Removal of selected pharmaceuticals from aqueous solution using magnetic chitosan: Sorption behavior and mechanism. Environ. Sci. Pollut. Res. 2014, 21, 12780–12789. [Google Scholar] [CrossRef]
- Carvalho, T.O.; Matias, A.E.B.; Braga, L.R.; Evangelista, S.M.; Prado, A.G.S. Calorimetric studies of removal of nonsteroidal anti-inflammatory drugs diclofenac and dipyrone from water. J. Therm. Anal. Calorim. 2011, 106, 475–481. [Google Scholar] [CrossRef]
- Rizzi, V.; D’Agostino, F.; Fini, P.; Semeraro, P.; Cosma, P. An interesting environmental friendly cleanup: The excellent potential of olive pomace for disperse blue adsorption/desorption from wastewater. Dyes Pigment. 2017, 140, 480–490. [Google Scholar] [CrossRef]
- Cuklev, F.; Fick, J.; Cvijovic, M.; Kristiansson, E.; Förlin, L.; Larsson, D.G.J. Does ketoprofen or diclofenac pose the lowest risk to fish? J. Hazard. Mater. 2012, 229–230, 100106. [Google Scholar] [CrossRef] [PubMed]
- Diniz, M.S.; Salgado, R.; Pereira, V.J.; Carvalho, G.; Oehmen, A.; Reis, M.A.M.; Noronha, J.P. Ecotoxicity of ketoprofen, diclofenac, atenolol and their photolysis byproducts in zebrafish (Danio rerio). Sci. Total Environ. 2015, 505, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Hasan, H.A.; Abdullah, S.R.S.; Al-Attabi, A.W.N.; Nash, D.A.H.; Anuar, N.; Rahman, N.A.; Titah, H.S. Removal of ibuprofen, ketoprofen, COD and nitrogen compounds from pharmaceutical wastewater using aerobic suspension-sequencing batch reactor (ASSBR). Sep. Purif. Technol. 2016, 157, 215–221. [Google Scholar] [CrossRef]
- Jankunaite, D.; Tichonovas, M.; Buivydiene, D.; Radziuniene, I.; Racys, V.; Krugly, E. Removal of Diclofenac, Ketoprofen, and Carbamazepine from Simulated Drinking Water by Advanced Oxidation in a Model Reactor. Water Air Soil Pollut. 2017, 228, 1–15. [Google Scholar] [CrossRef]
- Nagy, Z.M.; Molnár, M.; Fekete-Kertész, I.; Molnár-Perl, I.; Fenyvesi, É.; Gruiz, K. Removal of emerging micropollutants from water using cyclodextrin. Sci. Total Environ 2014, 485–486, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Madikizela, L.M.; Zunngu, S.S.; Mlunguza, N.Y.; Tavengwa, N.T.; Mdluli, P.S.; Chimuka, L. Application of molecularly imprinted polymer designed for the selective extraction of ketoprofen from wastewater. Water SA 2018, 44, 406–418. [Google Scholar] [CrossRef]
- Yu, H.; Nie, E.; Xu, J.; Yan, S.; Cooper, W.J.; Song, W. Degradation of Diclofenac by Advanced Oxidation and Reduction Processes: Kinetic Studies, Degradation Pathways and Toxicity Assessments. Water Res. 2013, 47, 1909–1918. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, G.; Liu, H.; Wang, Y.; He, Z.; Wang, G. Diclofenac photodegradation under simulated sunlight: Effect of different forms of nitrogen and Kinetics. J. Hazard. Mater. 2011, 192, 411–418. [Google Scholar] [CrossRef]
- Martinez-Haya, R.; Miranda, M.A.; Marin, M.L. Type I vs Type II photodegradation of pollutants. Catal. Today 2018, 313, 161–166. [Google Scholar] [CrossRef]
- Iovino, P.; Chianese, S.; Canzano, S.; Prisciandaro, M.; Musmarra, D. Photodegradation of diclofenac in wastewaters. Desalin. Water Treat. 2017, 61, 293–297. [Google Scholar]
- Galmier, M.J.; Bouchon, B.; Madelmont, J.C.; Mercier, F.; Pilotaz, F.; Lartigue, C. Identification of degradation products of diclofenac by electrospray ion trap mass spectrometry. J. Pharm. Biomed. Anal. 2005, 38, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Amasha, M.; Baalbaki, A.; Ghauch, A.A. comparative study of the common persulfate activation techniques for the complete degradation of an NSAID: The case of ketoprofen. Chem. Eng. J. 2018, 350, 395–410. [Google Scholar] [CrossRef]
- Rizzi, V.; D′Agostino, F.; Gubitosa, J.; Fini, P.; Petrella, A.; Agostiano, A.; Semeraro, P.; Cosma, P. An Alternative Use of Olive Pomace as a Wide-Ranging Bioremediation Strategy to Adsorb and Recover Disperse Orange and Disperse Red Industrial Dyes from Wastewater. Separations 2017, 4, 29. [Google Scholar] [CrossRef]
- Rizzi, V.; Mongiovì, C.; Fini, P.; Petrella, P.; Semeraro, P.; Cosma, P. Operational parameters affecting the removal and recycling of direct blue industrial dye from wastewater using bleached oil mill waste as alternative adsorbent material. Int. J. Environ. Agric. Biotechnol. 2017, 2, 1560–1572. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Removal of various pollutants from water and wastewater by modified chitosan adsorbents. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2331–2386. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzi, V.; Romanazzi, F.; Gubitosa, J.; Fini, P.; Romita, R.; Agostiano, A.; Petrella, A.; Cosma, P. Chitosan Film as Eco-Friendly and Recyclable Bio-Adsorbent to Remove/Recover Diclofenac, Ketoprofen, and Their Mixture from Wastewater. Biomolecules 2019, 9, 571. https://doi.org/10.3390/biom9100571
Rizzi V, Romanazzi F, Gubitosa J, Fini P, Romita R, Agostiano A, Petrella A, Cosma P. Chitosan Film as Eco-Friendly and Recyclable Bio-Adsorbent to Remove/Recover Diclofenac, Ketoprofen, and Their Mixture from Wastewater. Biomolecules. 2019; 9(10):571. https://doi.org/10.3390/biom9100571
Chicago/Turabian StyleRizzi, Vito, Fabio Romanazzi, Jennifer Gubitosa, Paola Fini, Roberto Romita, Angela Agostiano, Andrea Petrella, and Pinalysa Cosma. 2019. "Chitosan Film as Eco-Friendly and Recyclable Bio-Adsorbent to Remove/Recover Diclofenac, Ketoprofen, and Their Mixture from Wastewater" Biomolecules 9, no. 10: 571. https://doi.org/10.3390/biom9100571
APA StyleRizzi, V., Romanazzi, F., Gubitosa, J., Fini, P., Romita, R., Agostiano, A., Petrella, A., & Cosma, P. (2019). Chitosan Film as Eco-Friendly and Recyclable Bio-Adsorbent to Remove/Recover Diclofenac, Ketoprofen, and Their Mixture from Wastewater. Biomolecules, 9(10), 571. https://doi.org/10.3390/biom9100571