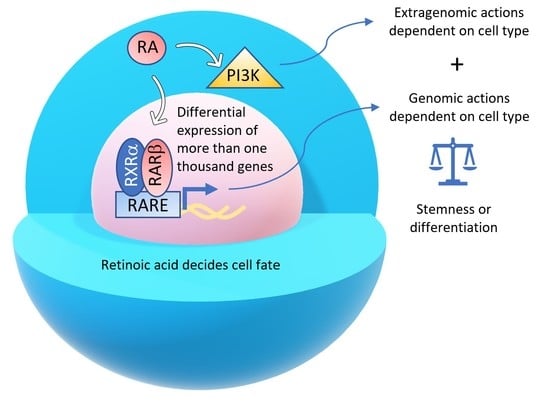
Two Opposing Faces of Retinoic Acid: Induction of Stemness or Induction of Differentiation Depending on Cell-Type
Abstract
:1. Introduction
2. Retinoic Acid Induces Stemness or Differentiation in the Mammary Gland and Breast Cancer Cells
2.1. Growth-Promoting and Growth-Inhibiting Actions of RA in Breast Cancer Depend on the Cell Context-Specific Balance of Activation of Transcriptional and Nontranscriptional Pathways
2.2. Retinoic Acid Induces Tumor-Promoting or Tumor-Suppressive Actions in Triple-Negative Breast Cancer Cells Due to Variable Gene Expression in Cell Lines with Differences in DNA Methylation
2.3. Retinoic Acid Upregulates the Signaling Pathway Src-YAP-IL6 Involved in Stemness in Triple-Negative MDA-MB-231 Breast Cancer Cells and Downregulates the Same Pathway in Triple-Negative MDA-MB-468 Breast Cancer Cell Line
2.4. Retinoic Acid Conferred Stemness Properties to Breast Cancer MCF-7 Cells
2.5. RARβ Expression in the Mammary Gland Stroma Shapes the Tumor Microenvironment Favoring Breast Tumor Growth and Invasion
2.6. Retinoic Acid Induces Cell Differentiation and Downregulates Stemness in a Nontumorigenic Immortalized Mammary Epithelial Cell Line and a Non-Invasive Breast Cancer Cell line but Does Not Perform These Actions in Aggressive Breast Cancers
2.7. Retinoic Acid Blocks the Progesterone Induction of Cytokeratin-5 Expressing Breast Cancer Stem Cells
3. Janus Faces of RA in Other Tissues
3.1. Retinoic Acid Sustains Pluripotency and Suppresses Differentiation of Human Induced Pluripotent Stem Cells
3.2. Retinoic Acid Induces Stemness or Differentiation in the Neural System
3.2.1. Retinoic Acid Induces Proliferation in Cerebral Cortex Early Neurogenesis
3.2.2. Retinoic Acid Induces Proliferation in Adult Neurogenesis in the Hippocampus
3.2.3. Retinoic Acid Induces Stemness Rather Than Differentiation in Stem-Like Glioma Cells
3.3. Retinoic Acid Induces Stemness or Differentiation in the Hematopoietic System
3.3.1. Retinoic Acid Prevents Differentiation of Dormant Primitive Hematopoietic Stem Cells and Induces Differentiation of More Mature Blood Cells
3.3.2. Variable Effects of RA on Tumor Immunosuppression
3.4. RARγ Inhibits Colorectal Cancer Tumorigenesis and Metastasis, Restricting the YAP Signaling Pathway
3.5. Cytoplasmic Accumulation of RARγ in Hepatocellular Carcinoma Cells Plays an Oncogenic Role Via Nongenomic Activation of Akt-NFκB Signaling
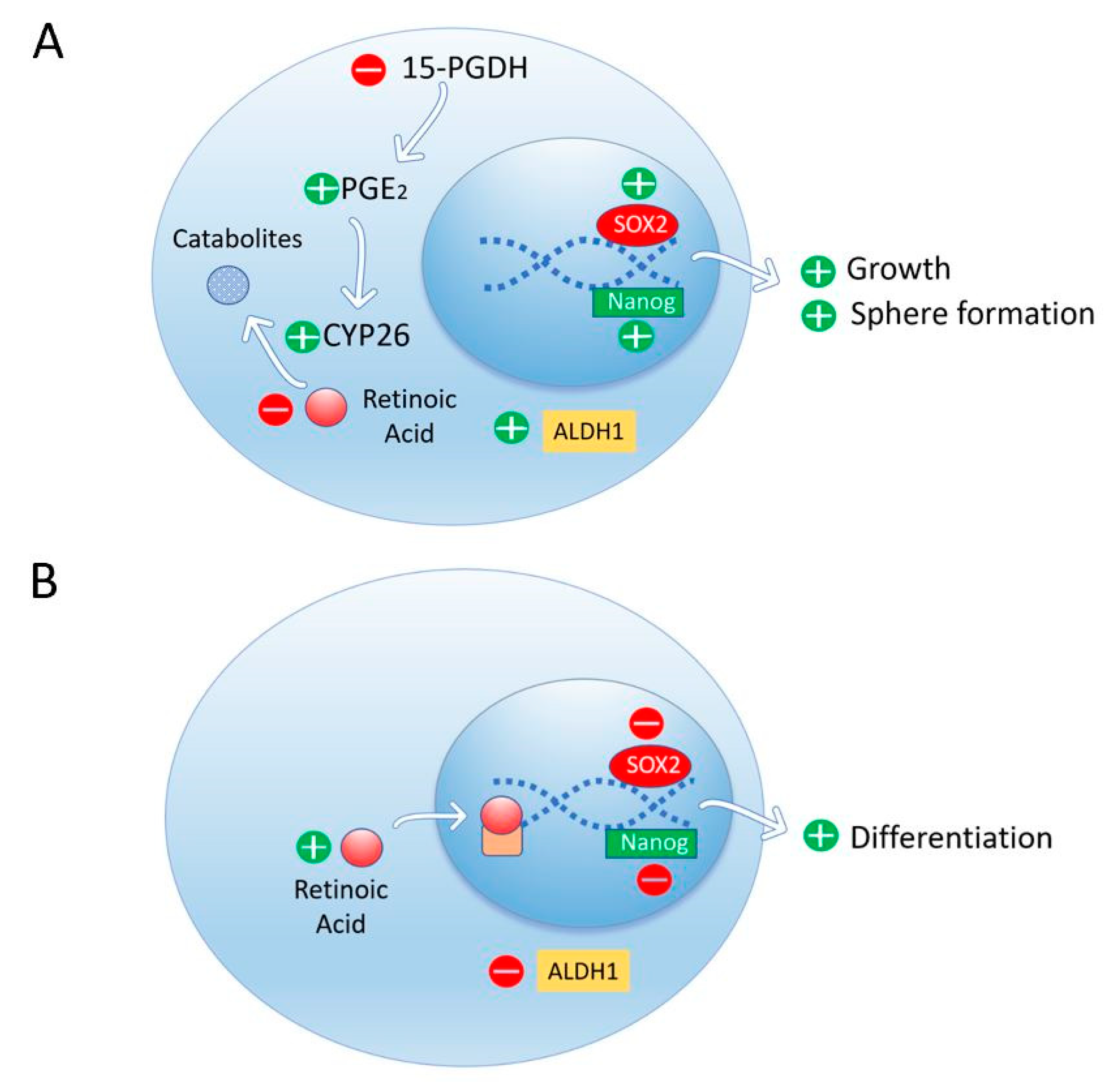
3.6. Retinoic Acid Induces Cell Differentiation and Reduces Stem Cell Markers in Pancreatic Cancer Cells
3.7. Retinoic Acid Downregulates ALDH1-Mediated Stemness and Inhibits Tumor Formation in Ovarian Cancer Cells
3.8. Retinoic Acid Induces Cell Differentiation and Proliferation During Spermatogenesis
3.9. Retinoic Acid Controls the Regeneration of Tissues in the Adult Organism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghyselinck, N.B.; Duester, G. Retinoic acid signaling pathways. Development 2019, 146, dev167502. [Google Scholar] [CrossRef] [PubMed]
- Vassalli, G. Aldehyde Dehydrogenases: Not Just Markers, but Functional Regulators of Stem Cells. Stem Cells Int. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, Y. Retinoic acid signaling in heart development. Genes 2019, 57, e23300. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.J.; Duester, G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat. Rev. Mol. Cell Boil. 2015, 16, 110–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samarut, E.; Fraher, D.; Laudet, V.; Gibert, Y. ZebRA: An overview of retinoic acid signaling during zebrafish development. Biochim. et Biophys. Acta (BBA) Bioenerg. 2015, 1849, 73–83. [Google Scholar] [CrossRef]
- Gutierrez-Mazariegos, J.; Schubert, M.; Laudet, V. Evolution of Retinoic Acid Receptors and Retinoic Acid Signaling. Subcell. Biochem. 2014, 70, 55–73. [Google Scholar] [PubMed]
- Das, B.C.; Thapa, P.; Karki, R.; Das, S.; Mahapatra, S.; Liu, T.C.; Torregroza, I.; Wallace, D.P.; Kambhampati, S.; Van Veldhuizen, P.; et al. Retinoic acid signaling pathways in development and diseases. Bioorg. Med. Chem. 2014, 22, 673–683. [Google Scholar] [CrossRef]
- Tang, X.-H.; Gudas, L.J. Retinoids, Retinoic Acid Receptors, and Cancer. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 345–364. [Google Scholar] [CrossRef]
- Theodosiou, M.; Laudet, V.; Schubert, M. From carrot to clinic: an overview of the retinoic acid signaling pathway. Cell. Mol. Life Sci. 2010, 67, 1423–1445. [Google Scholar] [CrossRef]
- Black, W.J.; Stagos, D.; Marchitti, S.A.; Nebert, D.W.; Tipton, K.F.; Bairoch, A.; Vasiliou, V. Human aldehyde dehydrogenase genes: alternatively spliced transcriptional variants and their suggested nomenclature. Pharm. Genom. 2009, 19, 893–902. [Google Scholar] [CrossRef] [Green Version]
- Thatcher, J.E.; Isoherranen, N. The role of CYP26 enzymes in retinoic acid clearance. Expert Opin. Drug Metab. Toxicol. 2009, 5, 875–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevison, F.; Jing, J.; Tripathy, S.; Isoherranen, N. Role of Retinoic Acid-Metabolizing Cytochrome P450s, CYP26, in Inflammation and Cancer. HIV-1: Mol. Boil. Pathog. 2015, 74, 373–412. [Google Scholar] [Green Version]
- Alonso, S.; Jones, R.J.; Ghiaur, G. Retinoic acid, CYP26, and drug resistance in the stem cell niche. Exp. Hematol. 2017, 54, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrane, M.M. Vitamin A regulation of gene expression: molecular mechanism of a prototype gene. J. Nutr. Biochem. 2007, 18, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Gilardi, F.; Desvergne, B. RXRs: Collegial Partners. Subcell. Biochem. 2014, 70, 75–102. [Google Scholar] [PubMed]
- Balmer, J.E.; Blomhoff, R. Gene expression regulation by retinoic acid. J. Lipid Res. 2002, 43, 1773–1808. [Google Scholar] [CrossRef] [Green Version]
- Schug, T.T.; Berry, D.C.; Shaw, N.S.; Travis, S.N.; Noy, N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell 2007, 129, 723–733. [Google Scholar] [CrossRef]
- Shaw, N.; Elholm, M.; Noy, N. Retinoic Acid Is a High Affinity Selective Ligand for the Peroxisome Proliferator-activated Receptor β/δ. J. Boil. Chem. 2003, 278, 41589–41592. [Google Scholar] [CrossRef]
- Ochoa, W.F.; Torrecillas, A.; Fita, I.; Verdaguer, N.; Corbalán-García, S.; Gómez-Fernández, J.C.; Torrecillas-Sanchez, A. Retinoic Acid Binds to the C2-Domain of Protein Kinase Cα†. Biochemistry 2003, 42, 8774–8779. [Google Scholar] [CrossRef]
- Radominska-Pandya, A.; Chen, G.; Czernik, P.J.; Little, J.M.; Samokyszyn, V.M.; Carter, C.A.; Nowak, G. Direct Interaction of All-trans-retinoic Acid with Protein Kinase C (PKC). J. Boil. Chem. 2000, 275, 22324–22330. [Google Scholar] [CrossRef] [Green Version]
- Masiá, S.; Alvarez, S.; De Lera, A.R.; Barettino, D. Rapid, Nongenomic Actions of Retinoic Acid on Phosphatidylinositol-3-Kinase Signaling Pathway Mediated by the Retinoic Acid Receptor. Mol. Endocrinol. 2007, 21, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.T.; Parrotta, E.I.; Santamaria, G.; Cuda, G. Short-term retinoic acid treatment sustains pluripotency and suppresses differentiation of human induced pluripotent stem cells. Cell Death Dis. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, S.; Ren, M.; Visconti, N.; Corlazzoli, F.; Gagliostro, V.; Somenzi, G.; Yao, J.; Sun, Y.; Sacchi, N. Tracing anti-cancer and cancer-promoting actions of all-trans retinoic acid in breast cancer to a RARa epigenetic mechanism of mammary epithelial cell fate. Oncotarget 2016, 7, 87064–87080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcato, P.; Dean, C.A.; Liu, R.Z.; Coyle, K.M.; Bydoun, M.; Wallace, M.; Clements, D.; Turner, C.; Mathenge, E.G.; Gujar, S.A.; et al. Aldehyde dehydrogenase 1A3 influences breast cancer progression via differential retinoic acid signaling. Mol. Oncol. 2015, 9, 17–31. [Google Scholar] [CrossRef]
- Mezquita, B.; Mezquita, P.; Pau, M.; Gasa, L.; Navarro, L.; Samitier, M.; Pons, M.; Mezquita, C. All-trans-retinoic acid activates the pro-invasive Src-YAP-Interleukin 6 axis in triple-negative MDA-MB-231 breast cancer cells while cerivastatin reverses this action. Sci. Rep. 2018, 8, 7047. [Google Scholar] [CrossRef]
- Ciccone, V.; Terzuoli, E.; Donnini, S.; Giachetti, A.; Morbidelli, L.; Ziche, M. Stemness marker ALDH1A1 promotes tumor angiogenesis via retinoic acid/HIF-1α/VEGF signalling in MCF-7 breast cancer cells. J. Exp. Clin. Cancer Res. 2018, 37, 311. [Google Scholar] [CrossRef]
- Wu, M.-J.; Kim, M.R.; Chen, Y.-S.; Yang, J.-Y.; Chang, C.-J. Retinoic acid directs breast cancer cell state changes through regulation of TET2-PKCζ pathway. Oncogene 2017, 36, 3193–3206. [Google Scholar] [CrossRef]
- Mishra, S.; Kelly, K.K.; Rumian, N.L.; Siegenthaler, J.A. Retinoic Acid Is Required for Neural Stem and Progenitor Cell Proliferation in the Adult Hippocampus. Stem Cell Rep. 2018, 10, 1705–1720. [Google Scholar] [CrossRef] [Green Version]
- Choschzick, I.; Hirseland, E.; Cramer, H.; Schultz, S.; Leppert, J.; Tronnier, V.; Zechel, C. Responsiveness of stem-like human glioma cells to all-trans retinoic acid and requirement of retinoic acid receptor isotypes α, β and γ. Neuroscience 2014, 279, 44–64. [Google Scholar] [CrossRef]
- Cabezas-Wallscheid, N.; Buettner, F.; Sommerkamp, P.; Klimmeck, D.; Ladel, L.; Thalheimer, F.B.; Pastor-Flores, D.; Roma, L.P.; Renders, S.; Zeisberger, P.; et al. Vitamin A-Retinoic Acid Signaling Regulates Hematopoietic Stem Cell Dormancy. Cell 2017, 169, 807–823.e19. [Google Scholar] [CrossRef] [Green Version]
- Purton, L.E.; Dworkin, S.; Olsen, G.H.; Walkley, C.R.; Fabb, S.A.; Collins, S.J.; Chambon, P. RARγ is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation. J. Exp. Med. 2006, 203, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.-D.; Lu, X.-X.; Gan, W.-J.; Li, X.-M.; He, X.-S.; Zhang, S.; Ji, Q.-H.; Zhou, F.; Cao, Y.; Wang, J.-R.; et al. RARγ Downregulation Contributes to Colorectal Tumorigenesis and Metastasis by Derepressing the Hippo–Yap Pathway. Cancer Res. 2016, 76, 3813–3825. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.-D.; Wu, H.; Zhang, H.-P.; Lu, N.; Ye, P.; Yu, F.-H.; Zhou, H.; Li, W.-G.; Cao, X.; Lin, Y.-Y.; et al. Oncogenic Potential of Retinoic Acid Receptor- in Hepatocellular Carcinoma. Cancer Res. 2010, 70, 2285–2295. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Arima, K.; Uchihara, T.; Miyake, K.; Yonemura, A.; Yasuda, T.; Itoyama, R.; Iwatsuki, M.; Baba, Y.; Yoshida, N.; et al. Abstract 4677: Inhibition of 15-PGDH causes Kras-driven tumor expansion through prostaglandin E2-ALDH1 signaling in the pancreas. Tumor Biol. 2019, 79, 4677. [Google Scholar]
- Wang, S.; Wang, X.; Ma, L.; Lin, X.; Zhang, D.; Li, Z.; Wu, Y.; Zheng, C.; Feng, X.; Liao, S.; et al. Retinoic Acid Is Sufficient for the In Vitro Induction of Mouse Spermatocytes. Stem Cell Rep. 2016, 7, 80–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Chen, R.; Sheu, M.; Kim, N.; Kim, S.; Islam, N.; Wier, E.M.; Wang, G.; Li, A.; Park, A.; et al. Noncoding dsRNA induces retinoic acid synthesis to stimulate hair follicle regeneration via TLR3. Nat. Commun. 2019, 10, 2811. [Google Scholar] [CrossRef]
- Cho, K.-W.; Kwon, H.-J.; Shin, J.-O.; Lee, J.-M.; Cho, S.-W.; Tickle, C.; Jung, H.-S. Retinoic acid signaling and the initiation of mammary gland development. Dev. Boil. 2012, 365, 259–266. [Google Scholar] [CrossRef]
- Gudas, L.J.; Wagner, J.A. Retinoids regulate stem cell differentiation. J. Cell. Physiol. 2011, 226, 322–330. [Google Scholar] [CrossRef]
- Chung, J.-H.; Lee, H.J.; Kim, B.-H.; Cho, N.-Y.; Kang, G.H. DNA methylation profile during multistage progression of pulmonary adenocarcinomas. Virchows Archiv. 2011, 459, 201–211. [Google Scholar] [CrossRef]
- Pilato, B.; Pinto, R.; De Summa, S.; Lambo, R.; Paradiso, A.; Tommasi, S. HOX gene methylation status analysis in patients with hereditary breast cancer. J. Hum. Genet. 2013, 58, 51–53. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Singh, A.P.; Batra, S.K. Structure, evolution, and biology of the MUC4 mucin. FASEB J. 2008, 22, 966–981. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Lakshmanan, I.; Ponnusamy, M.P.; Chakraborty, S.; Jain, M.; Pai, P.; Smith, L.M.; Lele, S.M.; Batra, S.K. MUC4 Overexpression Augments Cell Migration and Metastasis through EGFR Family Proteins in Triple Negative Breast Cancer Cells. PLOS ONE 2013, 8, e54455. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, S.; Goto, M.; Yamada, N.; Higashi, M.; Nomoto, M. Expression profiles of MUC1, MUC2, and MUC4 mucins in human neoplasms and their relationship with biological behavior. Proteomics 2008, 8, 3329–3341. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, S.; Higashi, M.; Yamada, N.; Yokoyama, S.; Kitamoto, S.; Kitajima, S.; Goto, M. Mucins in human neoplasms: Clinical pathology, gene expression and diagnostic application. Pathol. Int. 2011, 61, 697–716. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, J.J.; Zhu, R.; Liang, W.B.; Gao, W.T.; Yu, J.B.; Xu, Z.K.; Miao, Y. The increase in the expression and hypomethylation of MUC4 gene with the progression of pancreatic ductal adenocarcinoma. Med. Oncol. 2011, 28, S175–S184. [Google Scholar] [CrossRef]
- Kim, T.; Lim, D.-S. The SRF-YAP-IL6 axis promotes breast cancer stemness. Cell Cycle 2016, 15, 1311–1312. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, W.; Mossmann, D.; Kleemann, J.; Mock, K.; Meisinger, C.; Brummer, T.; Herr, R.; Brabletz, S.; Stemmler, M.P.; Brabletz, T. ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nat. Commun. 2016, 7, 10498. [Google Scholar] [CrossRef]
- Kim, T.; Yang, S.-J.; Hwang, D.; Song, J.; Kim, M.; Kim, S.K.; Kang, K.; Ahn, J.; Lee, D.; Kim, M.-Y.; et al. A basal-like breast cancer-specific role for SRF–IL6 in YAP-induced cancer stemness. Nat. Commun. 2015, 6, 10186. [Google Scholar] [CrossRef]
- Bharti, R.; Dey, G.; Mandal, M. Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells: A snapshot of IL-6 mediated involvement. Cancer Lett. 2016, 375, 51–61. [Google Scholar] [CrossRef]
- Sansone, P.; Storci, G.; Tavolari, S.; Guarnieri, T.; Giovannini, C.; Taffurelli, M.; Ceccarelli, C.; Santini, D.; Paterini, P.; Marcu, K.B.; et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J. Clin. Investig. 2007, 117, 3988–4002. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Soleymani Abyaneh, H.; Gupta, N.; Alshareef, A.; Gopal, K.; Lavasanifar, A.; Lai, R. Hypoxia Induces the Acquisition of Cancer Stem-like Phenotype Via Upregulation and Activation of Signal Transducer and Activator of Transcription-3 (STAT3) in MDA-MB-231, a Triple Negative Breast Cancer Cell Line. Cancer Microenviron. 2018, 11, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liao, W.; Jian, Y.; Peng, Y.; Zhang, X.; Ye, L.; Cui, Y.; Wang, B.; Wu, X.; Xiong, Z.; et al. CGI-99 promotes breast cancer metastasis via autocrine interleukin-6 signaling. Oncogene 2017, 36, 3695–3705. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Resat, H. Constitutive activation of STAT3 in breast cancer cells: A review. Int. J. Cancer 2016, 138, 2570–2578. [Google Scholar] [CrossRef]
- Yu, F.-X.; Guan, K.-L. The Hippo pathway: regulators and regulations. Genes Dev. 2013, 27, 355–371. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, K.; Wu, L.-W.; Grivennikov, S.I.; De Jong, P.R.; Lian, I.; Yu, F.-X.; Wang, K.; Ho, S.B.; Boland, B.S.; Chang, J.T.; et al. A gp130–Src–YAP module links inflammation to epithelial regeneration. Nature 2015, 519, 57–62. [Google Scholar] [CrossRef]
- Taniguchi, K.; Moroishi, T.; De Jong, P.R.; Krawczyk, M.; Grebbin, B.M.; Luo, H.; Xu, R.-H.; Golob-Schwarzl, N.; Schweiger, C.; Wang, K.; et al. YAP–IL-6ST autoregulatory loop activated on APC loss controls colonic tumorigenesis. Proc. Natl. Acad. Sci. USA 2017, 114, 1643–1648. [Google Scholar] [CrossRef]
- Rosenbluh, J.; Nijhawan, D.; Cox, A.G.; Li, X.; Neal, J.T.; Schafer, E.J.; Zack, T.I.; Wang, X.; Tsherniak, A.; Schinzel, A.C.; et al. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell 2012, 151, 1457–1473. [Google Scholar] [CrossRef]
- Zhi, X.; Tao, J.; Xie, K.; Zhu, Y.; Li, Z.; Tang, J.; Wang, W.; Xu, H.; Zhang, J.; Xu, Z. MUC4-induced nuclear translocation of β-catenin: A novel mechanism for growth, metastasis and angiogenesis in pancreatic cancer. Cancer Lett. 2014, 346, 104–113. [Google Scholar] [CrossRef]
- Mejías-Luque, R.; Peiró, S.; Vincent, A.; Van Seuningen, I.; De Bolós, C. IL-6 induces MUC4 expression through gp130/STAT3 pathway in gastric cancer cell lines. Biochim. et Biophys. Acta (BBA) Bioenerg. 2008, 1783, 1728–1736. [Google Scholar] [CrossRef] [Green Version]
- Hindley, C.J.; Condurat, A.L.; Menon, V.; Thomas, R.; Azmitia, L.M.; Davis, J.A.; Pruszak, J. The Hippo pathway member YAP enhances human neural crest cell fate and migration. Sci. Rep. 2016, 6, 23208. [Google Scholar] [CrossRef] [PubMed]
- Camargo, F.D.; Gokhale, S.; Johnnidis, J.B.; Fu, D.; Bell, G.W.; Jaenisch, R.; Brummelkamp, T.R. YAP1 Increases Organ Size and Expands Undifferentiated Progenitor Cells. Curr. Boil. 2007, 17, 2054–2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlegelmilch, K.; Mohseni, M.; Kirak, O.; Pruszak, J.; Rodriguez, J.R.; Zhou, D.; Kreger, B.T.; Vasioukhin, V.; Avruch, J.; Brummelkamp, T.R.; et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 2011, 144, 782–795. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, Y.; Wu, H.; Barry, E.; Yin, Y.; Lawrence, E.; Dawson, D.; Willis, J.E.; Markowitz, S.D.; Camargo, F.D.; et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc. Natl. Acad. Sci. USA 2011, 108, E1312–E1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP and TAZ: a signalling hub of the tumour microenvironment. Nat. Rev. Cancer 2019, 19, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.M.; Beloqui, I.; Garcia-Garcia, F.; Leis, O.; Vázquez-Martín, A.; Eguiara, A.; Cufí, S.; Pavon, A.; Menendez, J.A.; Dopazo, J.; et al. Mammosphere Formation in Breast Carcinoma Cell Lines Depends upon Expression of E-cadherin. PLOS ONE 2013, 8, e77281. [Google Scholar]
- Ginestier, C.; Wicinski, J.; Cervera, N.; Monville, F.; Finetti, P.; Bertucci, F.; Wicha, M.S.; Birnbaum, D.; Charafe-Jauffret, E. Retinoid signaling regulates breast cancer stem cell differentiation. Cell Cycle 2009, 8, 3297–3302. [Google Scholar] [CrossRef]
- Zhao, D.; Pan, C.; Sun, J.; Gilbert, C.; Drews-Elger, K.; Azzam, D.J.; Picon-Ruiz, M.; Kim, M.; Ullmer, W.; El-Ashry, D.; et al. VEGF drives cancer-initiating stem cells through VEGFR-2/Stat3 signaling to upregulate Myc and Sox2. Oncogene 2015, 34, 3107–3119. [Google Scholar] [CrossRef]
- Kim, M.; Jang, K.; Miller, P.; Picon-Ruiz, M.; Yeasky, T.M.; El-Ashry, D.; Slingerland, J.M. VEGFA links self-renewal and metastasis by inducing Sox2 to repress miR-452, driving Slug. Oncogene 2017, 36, 5199–5211. [Google Scholar] [CrossRef]
- Elaimy, A.L.; Guru, S.; Chang, C.; Ou, J.; Amante, J.J.; Zhu, L.J.; Goel, H.L.; Mercurio, A.M. VEGF–Neuropilin-2 signaling promotes stem-like traits in breast cancer cells by TAZ-mediated repression of the Rac GAP β2-chimaerin. Sci. Signal. 2018, 11, eaao6897. [Google Scholar] [CrossRef]
- Mercurio, A.M. VEGF/Neuropilin Signaling in Cancer Stem Cells. Int. J. Mol. Sci. 2019, 20, 490. [Google Scholar] [CrossRef]
- Lee, T.; Seng, S.; Sekine, M.; Hinton, C.; Fu, Y.; Avraham, H.; Avraham, S. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007, 4, e186. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Fan, F.; Wang, R.; Ye, X.; Xia, L.; Boulbes, D.; Ellis, L.M. Intracrine VEGF signalling mediates colorectal cancer cell migration and invasion. Br. J. Cancer 2017, 117, 848–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Nugoli, M.; Laferrière, J.; Saleh, S.M.; Rodrigue-Gervais, I.G.; Saleh, M.; Park, M.; Hallett, M.T.; Muller, W.J.; Giguère, V. Stromal retinoic acid receptor beta promotes mammary gland tumorigenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Martin-Belmonte, F.; Perez-Moreno, M. Epithelial cell polarity, stem cells and cancer. Nat. Rev. Cancer 2011, 12, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Knoblich, J.A. Asymmetric cell division: recent developments and their implications for tumour biology. Nat. Rev. Mol. Cell Boil. 2010, 11, 849–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.A.; Lau, K.M.; Rahmani, Z.; Dho, S.E.; Brothers, G.; She, Y.M.; Berry, D.M.; Bonneil, E.; Thibault, P.; Schweisguth, F.; et al. aPKC-mediated phosphorylation regulates asymmetric membrane localization of the cell fate determinant Numb. EMBO J. 2007, 26, 468–480. [Google Scholar] [CrossRef] [Green Version]
- Urtreger, A.J.; Kazanietz, M.G.; Bal de Kier Joffé, E.D. Contribution of individual PKC isoforms to breast cancer progression. IUBMB Life 2012, 64, 18–26. [Google Scholar] [CrossRef]
- Song, S.J.; Poliseno, L.; Song, M.S.; Ala, U.; Webster, K.; Ng, C.; Beringer, G.; Brikbak, N.J.; Yuan, X.; Cantley, L.C.; et al. MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell 2013, 154, 311–324. [Google Scholar] [CrossRef]
- Fettig, L.M.; McGinn, O.; Finlay-Schultz, J.; LaBarbera, D.V.; Nordeen, S.K.; A Sartorius, C. Cross talk between progesterone receptors and retinoic acid receptors in regulation of cytokeratin 5-positive breast cancer cells. Oncogene 2017, 36, 6074–6084. [Google Scholar] [CrossRef] [Green Version]
- Maden, M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat. Rev. Neurosci. 2007, 8, 755–765. [Google Scholar] [CrossRef]
- Janesick, A.; Wu, S.C.; Blumberg, B. Retinoic acid signaling and neuronal differentiation. Cell. Mol. Life Sci. 2015, 72, 1559–1576. [Google Scholar] [CrossRef] [Green Version]
- Haushalter, C.; Asselin, L.; Fraulob, V.; Dollé, P.; Rhinn, M. Retinoic acid controls early neurogenesis in the developing mouse cerebral cortex. Dev. Boil. 2017, 430, 129–141. [Google Scholar] [CrossRef]
- E Purton, L.; Bernstein, I.D.; Collins, S.J. All-trans retinoic acid enhances the long-term repopulating activity of cultured hematopoietic stem cells. Blood 2000, 95, 470–477. [Google Scholar] [CrossRef]
- Collins, S.J. Retinoic acid receptors, hematopoiesis and leukemogenesis. Curr. Opin. Hematol. 2008, 15, 346–351. [Google Scholar] [CrossRef]
- Masetti, R.; Vendemini, F.; Zama, D.; Biagi, C.; Gasperini, P.; Pession, A. All-transretinoic acid in the treatment of pediatric acute promyelocytic leukemia. Expert Rev. Anticancer. Ther. 2012, 12, 1191–1204. [Google Scholar] [CrossRef]
- Kusmartsev, S.; Cheng, F.; Yu, B.; Nefedova, Y.; Sotomayor, E.; Lush, R.; Gabrilovich, D. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003, 63, 4441–4449. [Google Scholar]
- Bazewicz, C.G.; Dinavahi, S.S.; Schell, T.D.; Robertson, G.P. Aldehyde dehydrogenase in regulatory T-cell development, immunity and cancer. Immunology 2019, 156, 47–55. [Google Scholar] [CrossRef]
- Zhou, D.; Conrad, C.; Xia, F.; Park, J.-S.; Payer, B.; Yin, Y.; Lauwers, G.Y.; Thasler, W.; Lee, J.T.; Avruch, J.; et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 2009, 16, 425–438. [Google Scholar] [CrossRef]
- Xu, M.Z.; Yao, T.-J.; Lee, N.P.Y.; Ng, I.O.L.; Chan, Y.-T.; Zender, L.; Lowe, S.W.; Poon, R.T.P.; Luk, J.M. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer 2009, 115, 4576–4585. [Google Scholar] [CrossRef] [Green Version]
- Avruch, J.; Zhou, D.; Bardeesy, N. YAP oncogene overexpression supercharges colon cancer proliferation. Cell Cycle 2012, 11, 1090–1096. [Google Scholar] [CrossRef] [Green Version]
- Bleul, T.; Rühl, R.; Bulashevska, S.; Karakhanova, S.; Werner, J.; Bazhin, A.V. Reduced retinoids and retinoid receptors’ expression in pancreatic cancer: A link to patient survival. Mol. Carcinog. 2015, 54, 870–879. [Google Scholar] [CrossRef]
- Herreros-Villanueva, M.; Er, T.K.; Bujanda, L. Retinoic Acid Reduces Stem Cell–Like Features in Pancreatic Cancer Cells. Pancreas 2015, 44, 918–924. [Google Scholar] [CrossRef]
- Young, M.-J.; Wu, Y.-H.; Chiu, W.-T.; Weng, T.-Y.; Huang, Y.-F.; Chou, C.-Y. All-trans retinoic acid downregulates ALDH1-mediated stemness and inhibits tumour formation in ovarian cancer cells. Carcinog. 2015, 36, 498–507. [Google Scholar] [CrossRef] [Green Version]
- Endo, T.; Freinkman, E.; De Rooij, D.G.; Page, D.C. Periodic production of retinoic acid by meiotic and somatic cells coordinates four transitions in mouse spermatogenesis. Proc. Natl. Acad. Sci. USA 2017, 114, E10132–E10141. [Google Scholar] [CrossRef] [Green Version]
- Endo, T.; Romer, K.A.; Anderson, E.L.; Baltus, A.E.; De Rooij, D.G.; Page, D.C. Periodic retinoic acid–STRA8 signaling intersects with periodic germ-cell competencies to regulate spermatogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, E2347–E2356. [Google Scholar] [CrossRef]
- Velte, E.K.; Niedenberger, B.A.; Serra, N.D.; Singh, A.; Roa-DeLaCruz, L.; Hermann, B.P.; Geyer, C.B. Differential RA responsiveness directs formation of functionally distinct spermatogonial populations at the initiation of spermatogenesis in the mouse. Development 2019, 146, dev173088. [Google Scholar] [CrossRef]
- Peer, N.R.; Law, S.M.; Murdoch, B.; Goulding, E.H.; Eddy, E.M.; Kim, K. Germ Cell–Specific Retinoic Acid Receptor α Functions in Germ Cell Organization, Meiotic Integrity, and Spermatogonia. Endocrinol. 2018, 159, 3403–3420. [Google Scholar] [CrossRef]
- Stocum, D.L. Mechanisms of urodele limb regeneration. Regeneration 2017, 4, 159–200. [Google Scholar] [CrossRef]
- Canino, C.; Luo, Y.; Marcato, P.; Blandino, G.; Pass, H.I.; Cioce, M. A STAT3-NFkB/DDIT3/CEBPβ axis modulates ALDH1A3 expression in chemoresistant cell subpopulations. Oncotarget 2015, 6, 12637–12653. [Google Scholar] [CrossRef]
- Jia, D.; Yang, W.; Li, L.; Liu, H.; Tan, Y.; Ooi, S.; Chi, L.; Filion, L.G.; Figeys, D.; Wang, L. β-Catenin and NF-κB co-activation triggered by TLR3 stimulation facilitates stem cell-like phenotypes in breast cancer. Cell Death Differ 2015, 22, 298–310. [Google Scholar] [CrossRef]
- Jia, D.; Wang, L. The other face of TLR3: A driving force of breast cancer stem cells. Mol. Cell. Oncol. 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Bondhopadhyay, B.; Moirangthem, A.; Basu, A. Innate adjuvant receptor Toll-like receptor 3 can promote breast cancer through cell surface. Tumour Biol. 2015, 36, 1261–1271. [Google Scholar] [CrossRef]
- Venkatesh, A.; Nandigam, H.; Muccioli, M.; Singh, M.; Loftus, T.; Lewis, D.; Pate, M.; Benencia, F. Regulation of inflammatory factors by double-stranded RNA receptors in breast cancer cells. Immunobiology 2018, 223, 466–476. [Google Scholar] [CrossRef]
- Malta, T.M.; Sokolov, A.; Gentles, A.J.; Burzykowski, T.; Poisson, L.; Kamińska, B.; Huelsken, J.; Gevaert, O.; Colaprico, A.; Czerwińska, P.; et al. Machine Learning Identifies Stemness Features Associated with Oncogenic Dedifferentiation. Cell 2018, 173, 338–354.e15. [Google Scholar] [CrossRef] [Green Version]



| Cell Type | Action | Signaling Pathway | RA Dose-Time | References |
|---|---|---|---|---|
| Pluripotent stem cells | Stemness | Inhibition of Wnt. Activation of Akt-mTOR | 0.5 µM (24 h) | [22] |
| Breast cancer cells T47D403 | Stemness | Lack of expression of RARα tumor suppressor genes and activation of RARα-PI3K-AKT | 1 µM (72 h) | [23] |
| Breast cancer cells MDA-MB-231 | Stemness | Upregulation of 1286 genes, among them MUC4. Activation of the axis Src-YAP-IL6 | 0.1 µM (18 h) 5 µM (48 h) | [24] [25] |
| Breast cancer cells MDA-MB-468 | Differentiation | Upregulation of 1358 genes, among them HOXA1 Inhibition of the axis Src-YAP-IL6 | 0.1 µM (18 h) 5 µM (48 h) | [24] [25] |
| Breast cancer cells MCF-7 | Stemness | Activation of ALDH1A1-HIF1α-VEGF | 1 µM (48 h) | [26] |
| Mammary MCF12A cells and T47D breast cancer cells | Differentiation | RARβ/TET2-miR200c-Suppression of PKCζ | 1 µM (24 h) | [27] |
| Adult hippocampus | Stemness | Activation of HIF1α-VEGF | 1 µM (24 h) | [28] |
| Glioblastoma T1440, T1452 and T1464 | Stemness | Increased SOX2 expression | 1 µM (7d) | [29] |
| Glioblastoma T1338 | Differentiation | Decreased SOX2 expression | 1 µM (7d) | [29] |
| Dormant hematopoietic cells | Stemness | Attenuation of C-MYC expression | 5 µM (24–48 h) | [30] |
| Hematopoietic stem cells | Differentiation or stemness | Differentiation through RARα Stemness through RARγ NOTCH1 expression | 1 µM (14d) | [31] |
| Colorectal cancer cells | Differentiation | RARγ-inhibition of YAP-increased E-cadherin expression | 1 µM (30 min) | [32] |
| Hepatocelular carcinoma cells | Stemness | RARγ-PI3K-AKT-NFκB | 1 µM (48 h) | [33] |
| Pancreatic ductal adenocarcinoma | Differentiation | Decrease ALDH1, SOX2 and NANOG | 10 μM (48 h) | [34] |
| Spermatogonial stem cells | Differentiation | Upregulation of STRA8, AGPAT3, FAM57A, WDR91 | 0.1 μM (24 h) | [35] |
| Regeneration of keratinocytes | Stemness | TLR3-STAT3 and NFkB-ALDH1-RA-RAR | 0.1µM (48 h) | [36] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezquita, B.; Mezquita, C. Two Opposing Faces of Retinoic Acid: Induction of Stemness or Induction of Differentiation Depending on Cell-Type. Biomolecules 2019, 9, 567. https://doi.org/10.3390/biom9100567
Mezquita B, Mezquita C. Two Opposing Faces of Retinoic Acid: Induction of Stemness or Induction of Differentiation Depending on Cell-Type. Biomolecules. 2019; 9(10):567. https://doi.org/10.3390/biom9100567
Chicago/Turabian StyleMezquita, Belén, and Cristóbal Mezquita. 2019. "Two Opposing Faces of Retinoic Acid: Induction of Stemness or Induction of Differentiation Depending on Cell-Type" Biomolecules 9, no. 10: 567. https://doi.org/10.3390/biom9100567
APA StyleMezquita, B., & Mezquita, C. (2019). Two Opposing Faces of Retinoic Acid: Induction of Stemness or Induction of Differentiation Depending on Cell-Type. Biomolecules, 9(10), 567. https://doi.org/10.3390/biom9100567