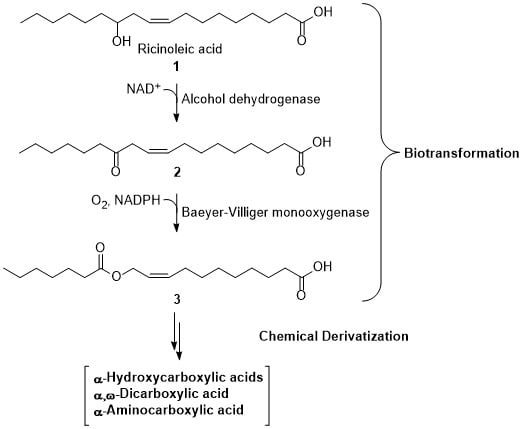
Bioenzymatic and Chemical Derivatization of Renewable Fatty Acids
Abstract
1. Introduction
2. Results and Discussion
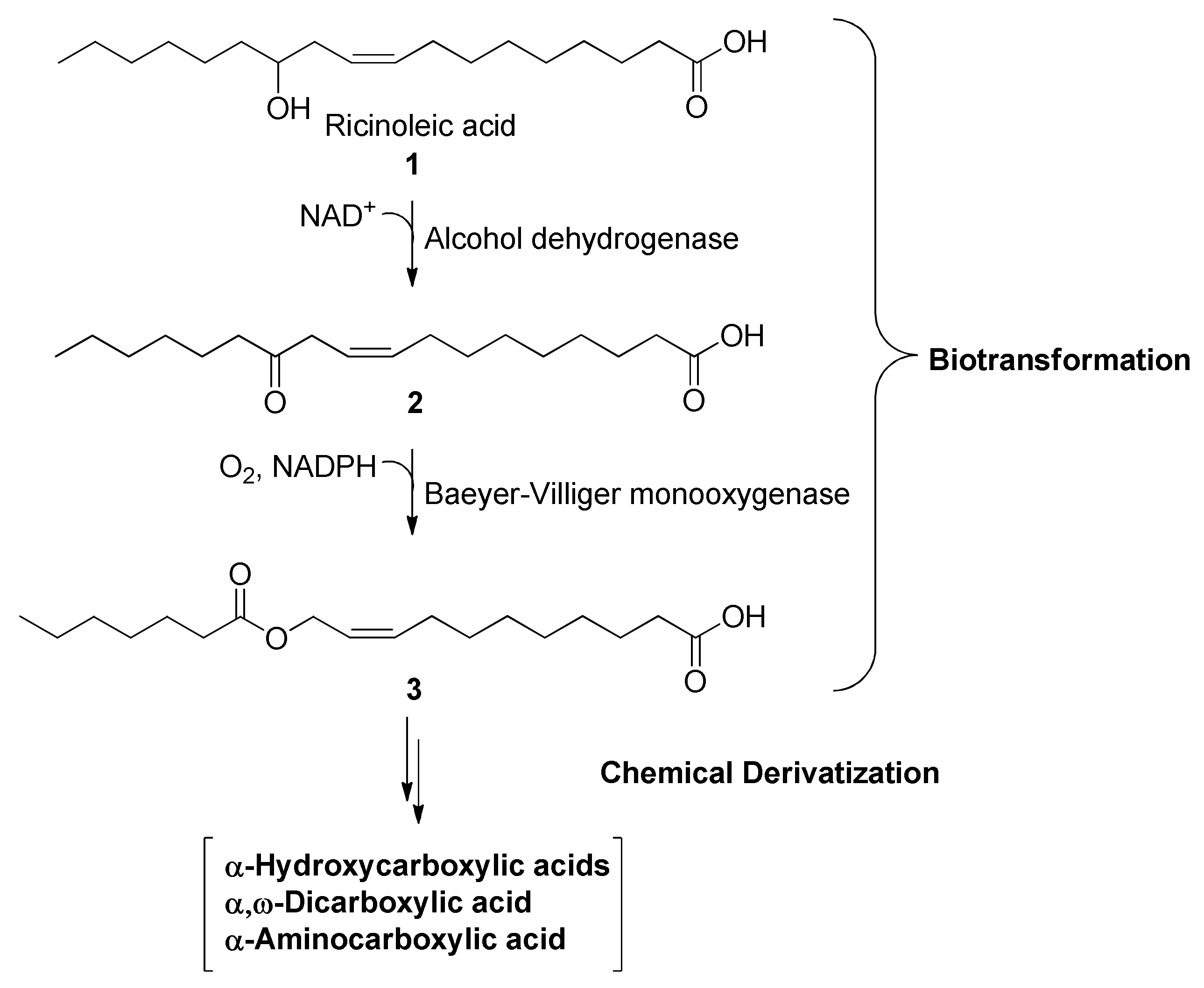
2.1. Chemical Derivatization of the Bioenzymatically Driven Ester Intermediate
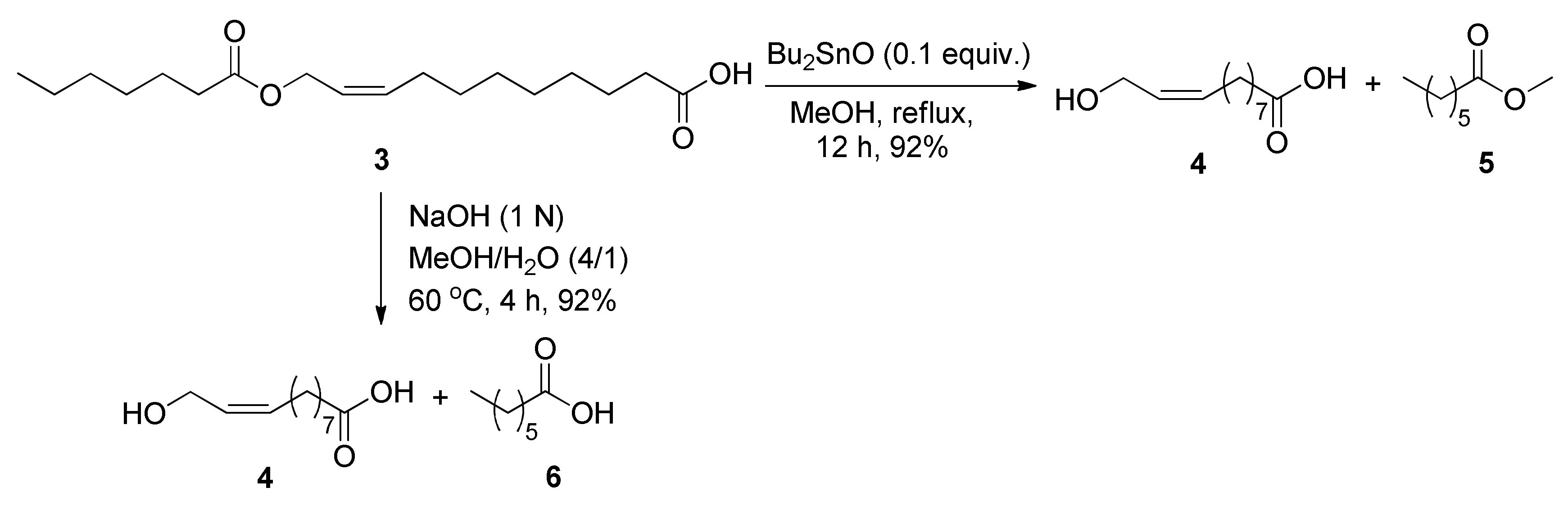
2.2. Oxidation of 11-Hydroxyundecanoic Acid to 1,11-Undecanedioic Acid
2.2.1. Optimization for Previously Developed Oxidation Conditions
2.2.2. Newly Developed Oxidation Methods
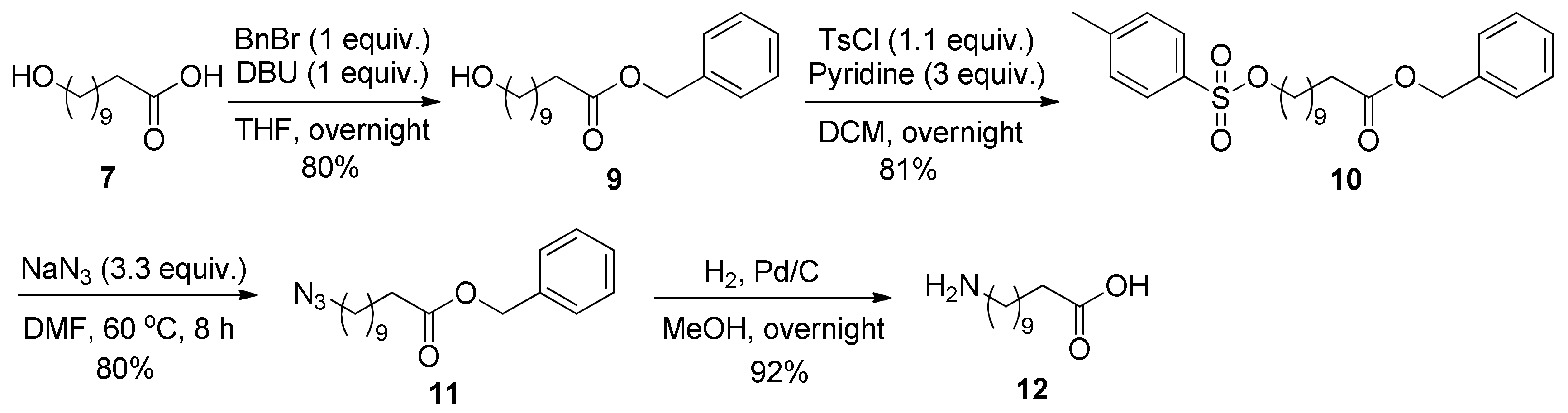
2.3. Chemical Conversion of 11-Hydroxyundecanoic Acid to 11-Aminoundecanoic Acid
3. Materials and Methods
3.1. General Experimental Methods
3.2. Chemical Derivatization of the Bioenzymatically-Driven Ester Intermediate (3)
3.2.1. Methanolysis of (Z)-11-(Heptanoyloxy)undec-9-enoic acid (3)
3.2.2. Hydrolysis of (Z)-11-(Heptanoyloxy)undec-9-enoic acid (3)
3.3. Oxidation of 11-Hydroxyundecanoic Acid (7) to 1,11-Undecanedioic Acid (8)
3.3.1. Pd/C-Catalyzed Oxidation Using NaBH4 in Basic Aqueous Alcohol [27]
3.3.2. 2-Iodobenzoic Acid/Oxone-Mediated Oxidation [28]
3.3.3. Nickel(II) Salt-Catalyzed Oxidation Using Aqueous Sodium Hypochlorite [29]
3.4. Chemical Conversion of 11-Hydroxyundecanoic Acid (7) to 11-Aminoundecanoic Acid (12)
3.4.1. Synthesis of Benzyl 11-Hydroxyundecanoate (9) [30]
3.4.2. Synthesis of Benzyl 11-(Tosyloxy)undecanoate (10)
3.4.3. Synthesis of Benzyl 11-Azidoundecanoate (11) [31]
3.4.4. Synthesis of 11-Aminoundecanoic Acid (12) [7]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhowmick, A.K.; Stephens, H.L. Handbook of Elastomers, 2nd ed.; CRC Press: New York, NY, USA, 2001; pp. 417–432. [Google Scholar]
- Drobny, J.G. Handbook of Thermoplastic Elastomers; William Andrew Publishing Norwich: New York, NY, USA, 2007; pp. 306–310. [Google Scholar]
- Ayorinde, F.O.; Nana, E.Y.; Nicely, P.D.; Woods, A.S.; Price, E.O.; Nwaonicha, C.P. Syntheses of 12-aminododecanoic and 11-aminoundecanoic acids from vernolic acid. J. Am. Oil Chem. Soc. 1997, 74, 531–538. [Google Scholar] [CrossRef]
- Kockritz, A.; Martin, A. Synthesis of azelaic acid from vegetable oil-based feed stocks. Eur. J. Lipid Sci. Technol. 2011, 113, 83–91. [Google Scholar] [CrossRef]
- Kroha, K. Industrial biotechnology provides opportunities for commercial production of new long-chain dibasic acids. Inform 2004, 15, 568–571. [Google Scholar]
- Schorken, U.; Kempers, P. Lipid biotechnology: industrially relevant production processes. Eur. J. Lipid Sci. Technol. 2009, 111, 627–645. [Google Scholar] [CrossRef]
- Koh, M.H.; Kim, H.; Shin, N.; Kim, H.S.; Yoo, D.; Kim, Y.G. Divergent process for c 10, c 11 and c 12 ω-amino acid and α,ω-dicarboxylic acid monomers of polyamides from castor oil as a renewable resource. Bull. Korean Chem. Soc. 2012, 33, 1873–1878. [Google Scholar] [CrossRef][Green Version]
- Jin, J.; Yang, X.; Liu, J.; Xiong, Y.; Poulikakos, P.; Karoulia, Z.; Wu, X.; Ahmed, T. Compositions and Methods for Treating CDK4/6-Mediated Cancer. WO Patent 2018106870 A1, 14 June 2018. [Google Scholar]
- Koval, V.S.; Arutyunyan, A.F.; Salyanov, V.L.; Klimova, R.R.; Kushch, A.A.; Rybalkina, E.Y.; Susova, O.Y.; Zhuze, A.L. DNA sequence-specific ligands. XVII. Synthesis, spectral properties, virological and biochemical studies of fluorescent dimeric bisbenzimidazoles DBA(n). Bioorg. Med. Chem. 2018, 26, 2302–2309. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Liu, K. Novel Compounds and Uses Thereof. WO Patent 2018013559 A1, 18 January 2018. [Google Scholar]
- Ni, J.; Guo, M.; Cao, Y.; Lei, L.; Liu, K.; Wang, B.; Lu, F.; Zhai, R.; Gao, X.; Yan, C.; et al. Discovery, synthesis of novel fusidic acid derivatives possessed amino-terminal groups at the 3-hydroxyl position with anticancer activity. Eur. J. Med. Chem. 2019, 162, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Tomar, R.; Bhattacharya, D.; Babu, S.A. Assembling of medium/long chain-based β-arylated unnatural amino acid derivatives via the Pd(II)-catalyzed sp3β-C-H arylation and a short route for rolipram-type derivatives. Tetrahedron 2019, 75, 2447–2465. [Google Scholar] [CrossRef]
- Bi, Y.; Liu, X.X.; Zhang, H.Y.; Yang, X.; Liu, Z.Y.; Lu, J.; Lewis, P.J.; Wang, C.Z.; Xu, J.Y.; Meng, Q.G.; et al. Synthesis and antibacterial evaluation of novel 3-substituted ocotillol-type derivatives as leads. Molecules 2017, 22, 590. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Lee, J.H.; Bornscheuer, U.T.; Park, J.B. Microbial synthesis of medium-chain α,ω-dicarboxylic acids and ω-aminocarboxylic acids from renewable long-chain fatty acids. Adv. Synth. Catal. 2014, 356, 1782–1788. [Google Scholar] [CrossRef]
- Das, G.; Trivedi, R.K.; Vasishtha, A.K. Heptaldehyde and undecylenic acid from castor oil. J. Am. Oil Chem. Soc. 1989, 66, 938–941. [Google Scholar] [CrossRef]
- Gao, X.; Du, C.; Pan, X.; Wang, P. Method for Preparing 11-Aminoundecanoic Acid and 12-Aminododecanoic Acid. CN Patent 109593045 A, 9 April 2019. [Google Scholar]
- Song, J.W.; Jeon, E.Y.; Song, D.H.; Jang, H.Y.; Bornscheuer, U.T.; Oh, D.K.; Park, J.B. Multistep enzymatic synthesis of long-chain α,ω-dicarboxylic and ω–hydroxy carboxylic acids from renewable fatty acids and plant oils. Angew. Chem. Int. Ed. 2013, 52, 2534–2537. [Google Scholar] [CrossRef] [PubMed]
- Woolley, K.L.; Nadikudi, M.; Koupaei, M.N.; Corban, M.; McCartney, P.; Bissember, A.C.; Lewis, T.W.; Gueven, N.; Smith, J.A. Amide linked redox-active naphthoquinones for the treatment of mitochondrial dysfunction. MedChemComm 2019, 10, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Mostyn, S.N.; Carland, J.E.; Shimmon, S.; Ryan, R.M.; Rawling, T.; Vandenberg, R.J. Synthesis and characterization of novel acyl-glycine inhibitors of GlyT2. ACS Chem. Neurosci. 2017, 8, 1949–1959. [Google Scholar] [CrossRef]
- Harling, J.D.; Neipp, C.E.; Pendrak, I.; Smith, I.E.D.; Terrell, L.R.; Youngman, M. Compounds for the modulation of RIP2 kinase activity. WO Patent 2017046036 A1, 23 March 2017. [Google Scholar]
- Jang, H.Y.; Singha, K.; Kim, H.M.; Kwon, Y.U.; Park, J.B. Chemo-enzymatic synthesis of 11-hydroxyundecanoic acid and 1,11-undecanedioic acid from ricinoleic acid. Green Chem. 2016, 18, 1089–1095. [Google Scholar] [CrossRef]
- Koppireddi, S.; Seo, J.H.; Jeon, E.J.; Chowdhury, P.S.; Jang, H.Y.; Park, J.B.; Kwon, Y.U. Combined biocatalytic and chemical transformations of oleic Acid to ω-hydroxynonanoic acid and α,ω- nonanedioic acid. Adv. Synth. Catal. 2016, 358, 3084–3092. [Google Scholar] [CrossRef]
- Narayana, C.; Periasamy, M. Acetoxyborohydride: a simple selective hydroborating agent. Tetrahedron. Lett. 1985, 26, 1757–1760. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kurita, O.; Kono, Y.; Hyakutake, H.; Sakuri, A. Structure of a new antifungal C11-hydroxyfatty acid isolated from leaves of wild rice (Oryza officinalis). Biosci. Biotech. Biochem. 1995, 59, 2049–2051. [Google Scholar] [CrossRef]
- Schrewe, M.; Julsing, M.K.; Lange, K.; Czarnotta, E.; Schmid, A.; Bühler, B. Reaction and catalyst engineering to exploit kinetically controlled whole-cell multistep biocatalysis for terminal FAME oxyfunctionalization. Biotechnol. Bioeng. 2014, 111, 1820–1830. [Google Scholar] [CrossRef] [PubMed]
- Ladkau, N.; Assmann, M.; Schrewe, M.; Julsing, M.K.; Schmid, A.; Bghler, B. Efficient production of the nylon-12 monomer ω-amino dodecanoic acid methyl ester from renewable dodecanoic acid methyl ester with engineered Escherichia coli. Metab. Eng. 2016, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Ahn, H.; De Castro, K.A.; Rhee, H. Pd/C and NaBH4 in basic aqueous alcohol: An efficient system for an environmentally benign oxidation of alcohols. Synthesis 2010, 3, 477–485. [Google Scholar] [CrossRef]
- Thottumkara, A.P.; Bowsher, M.S.; Vinod, T.K. In situ generation of o-iodoxybenzoic acid (IBX) and the catalytic use of it in oxidation reactions in the presence of oxone as a co-oxidant. Org. Lett. 2005, 7, 2933–2936. [Google Scholar] [CrossRef] [PubMed]
- Joseph, M.G.; James, W.O.; Stephen, A.M. An efficient and practical system for the catalytic oxidation of alcohols, aldehydes, and α,ω-unsaturated carboxylic acids. J. Org. Chem. 2006, 71, 9291–9296. [Google Scholar]
- Kubo, S.; Naiki, H. Fluorine-containing carboxylic acid. JP Patent 2017061420 A, 30 March 2017. [Google Scholar]
- Soumen, D.; Anupam, M.; Navin, S.; Madhava, B.M.; Haladhar, D.S.; Satbir, S.S.; Ashutosh, D. Synthesis and biodistribution studies of 99mTc labeled fatty acid derivatives prepared via click approach for potential use in cardiac imaging. J. Label. Comp. Radiophar. 2018, 61, 1048–1057. [Google Scholar]
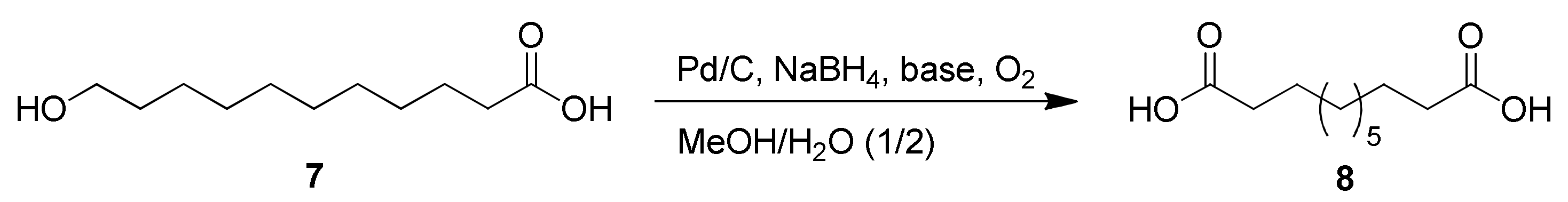
| Entry | Reagent | Tempa/Time | Yield (%) |
|---|---|---|---|
| 1 | Pd/C (10%, 5 mol%), NaBH4 (20 mol%), KOH (3 equiv.), O2 | rt/2 d | n.d.b |
| 2 | Pd/C (10%, 5 mol%), NaBH4 (20 mol%), K2CO3 (4 equiv.), air | rt/2 d | n.d.b |
| 3 | Pd/C (10%, 5 mol%), NaBH4 (10 mol%), KOH (6 equiv.), air | rt/3 d | 71% |
| 4 | Pd/C (10%, 5 mol%), NaBH4 (10 mol%), KOH (6 equiv.), air | rt/4 d | 85% |
| 5 | Pd/C (10 %, 5 mol%), NaBH4 (10 mol%), KOH (6 equiv.), air | rt/4 d | 50%c |
| 6 | Pt/C (10%, 5 mol%.), NaBH4 (10 mol%), KOH (6 equiv.), air | rt/2 d | 58% |
| 7 | Pt/C (10%, 5 mol%.), NaBH4 (10 mol%), KOH (6 equiv.), air | rt/4 d | 85% |
| 8 | Raney®-Ni (5 mol%.), NaBH4 (10 mol%), KOH (6 equiv.), air | rt/4 d | n.d.b |
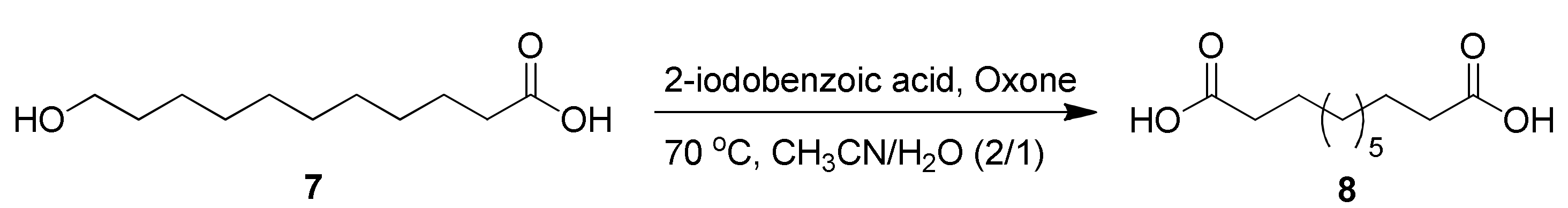
| Entry | Reagent | Time (h) | Yield (%) |
|---|---|---|---|
| 1 | 2-IBAcid (0.3 equiv.), Oxone (1.3 equiv.) | 6 | 76% |
| 2 | 2-IBAcid (0.2 equiv.), Oxone (3.2 equiv.) | 9 | 32% |
| 3 | 2-IBAcid (0.4 equiv.), Oxone (1.6 equiv.) | 9 | 85% |
| 4 | 2-IBAcid (0.4 equiv.), Oxone (1.6 equiv.) | 24 | 97% |
| 5 | 2-IBAcid (0.4 equiv.), Oxone (1.6 equiv.) | 9 | no reactiona |

| Entry | Solventb | Reagent | Temp/Time | Yield (%) |
|---|---|---|---|---|
| 1 | H2O/DCM | NiCl2 (5 mol%), NaOCl (9 mL) | 0 °C/2 h → rt/2 h | 82 |
| 2 | H2O/DCM | NiCl2 (5 mol%), NaOCl (18 mL) | 0 °C/2 h → rt/2 h | 94 |
| 3 | H2O/ACN | NiCl2 (5 mol%), NaOCl (18 mL) | 0 °C/2 h → rt/2 h | 94 |
| 4 | H2O/THF | NiCl2 (5 mol%), NaOCl (18 mL) | 0 °C/2 h → rt/2 h | 90 |
| 5 | H2O/1,4-dioxane | NiCl2 (5 mol%), NaOCl (18 mL) | 0 °C/2 h → rt/2 h | 94 |
| 6 | H2O | NiCl2 (5 mol%), NaOCl (18 mL) | 0 °C/2 h → rt/2 h | 85 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akula, R.K.; Kwon, Y.-U. Bioenzymatic and Chemical Derivatization of Renewable Fatty Acids. Biomolecules 2019, 9, 566. https://doi.org/10.3390/biom9100566
Akula RK, Kwon Y-U. Bioenzymatic and Chemical Derivatization of Renewable Fatty Acids. Biomolecules. 2019; 9(10):566. https://doi.org/10.3390/biom9100566
Chicago/Turabian StyleAkula, Ravi Kumar, and Yong-Uk Kwon. 2019. "Bioenzymatic and Chemical Derivatization of Renewable Fatty Acids" Biomolecules 9, no. 10: 566. https://doi.org/10.3390/biom9100566
APA StyleAkula, R. K., & Kwon, Y.-U. (2019). Bioenzymatic and Chemical Derivatization of Renewable Fatty Acids. Biomolecules, 9(10), 566. https://doi.org/10.3390/biom9100566