Fumigant Antifungal Activity via Reactive Oxygen Species of Thymus vulgaris and Satureja hortensis Essential Oils and Constituents against Raffaelea quercus-mongolicae and Rhizoctonia solani
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strain and Culture Conditions
2.2. Plant Essential Oils and Chemicals
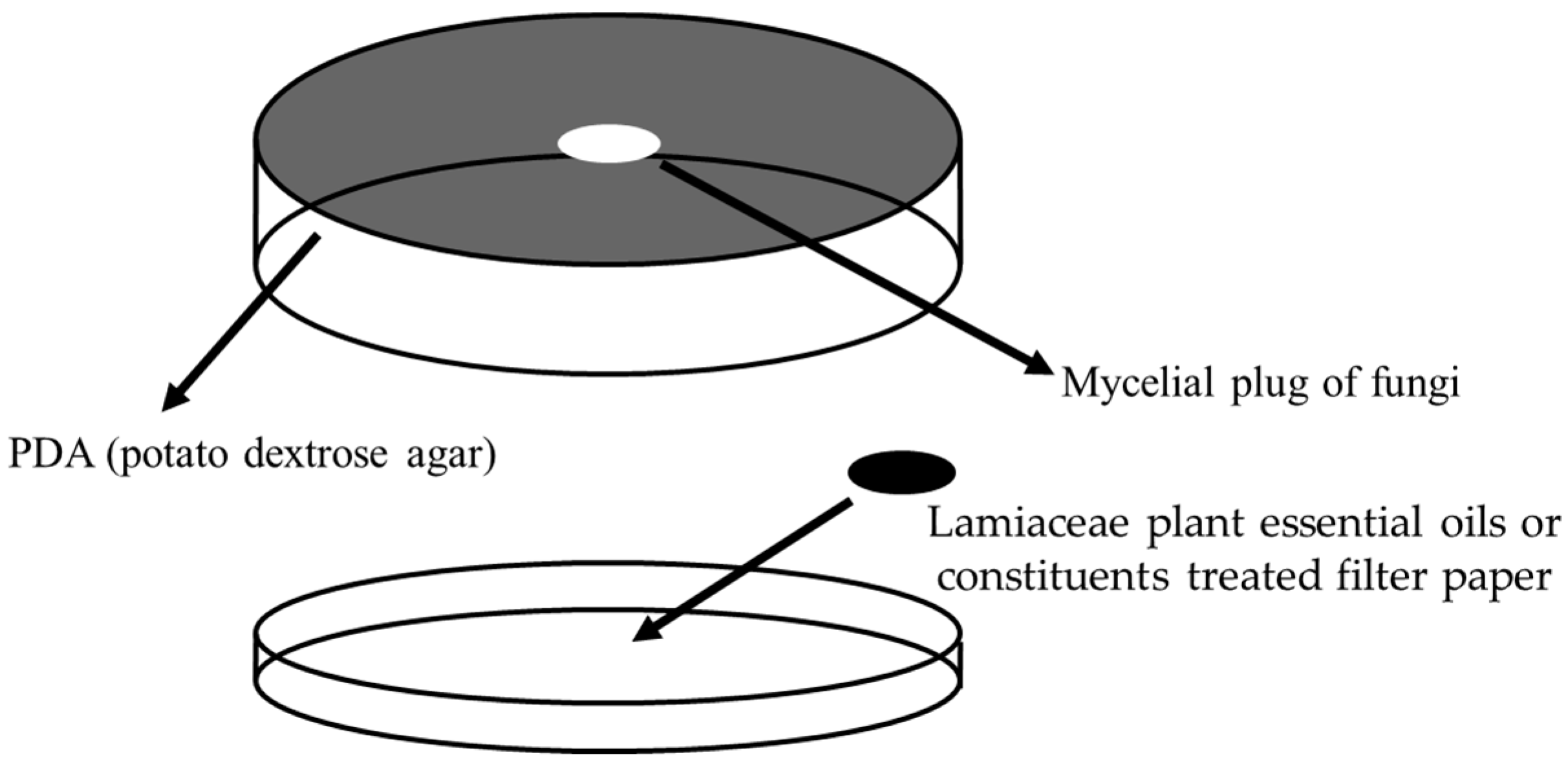
2.3. Fumigant Antifungal Activity Bioassays
2.4. Gas Chromatography
2.5. Gas Chromatography-Mass Spectrometry
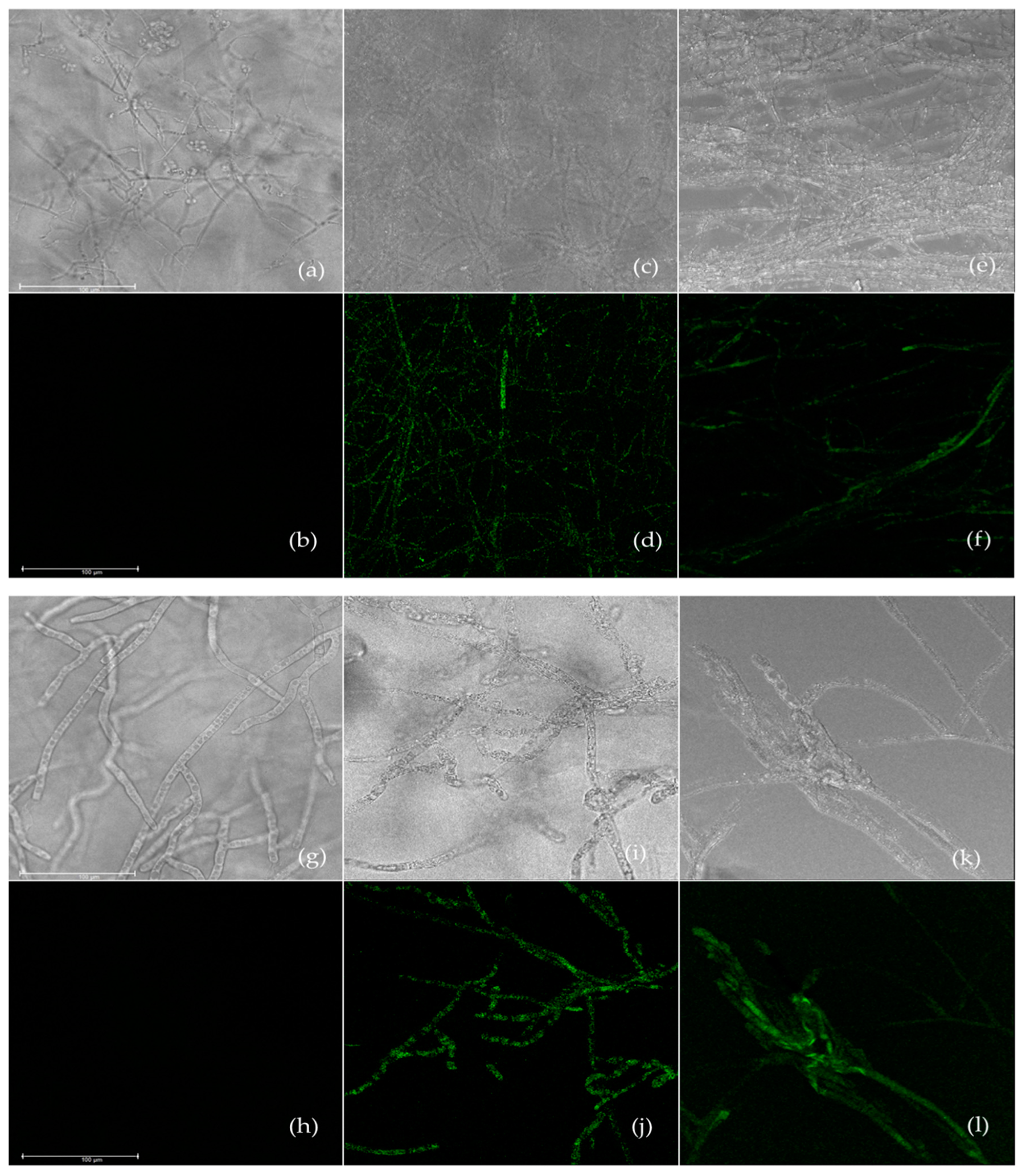
2.6. Assessment of Reactive Oxygen Species Generation
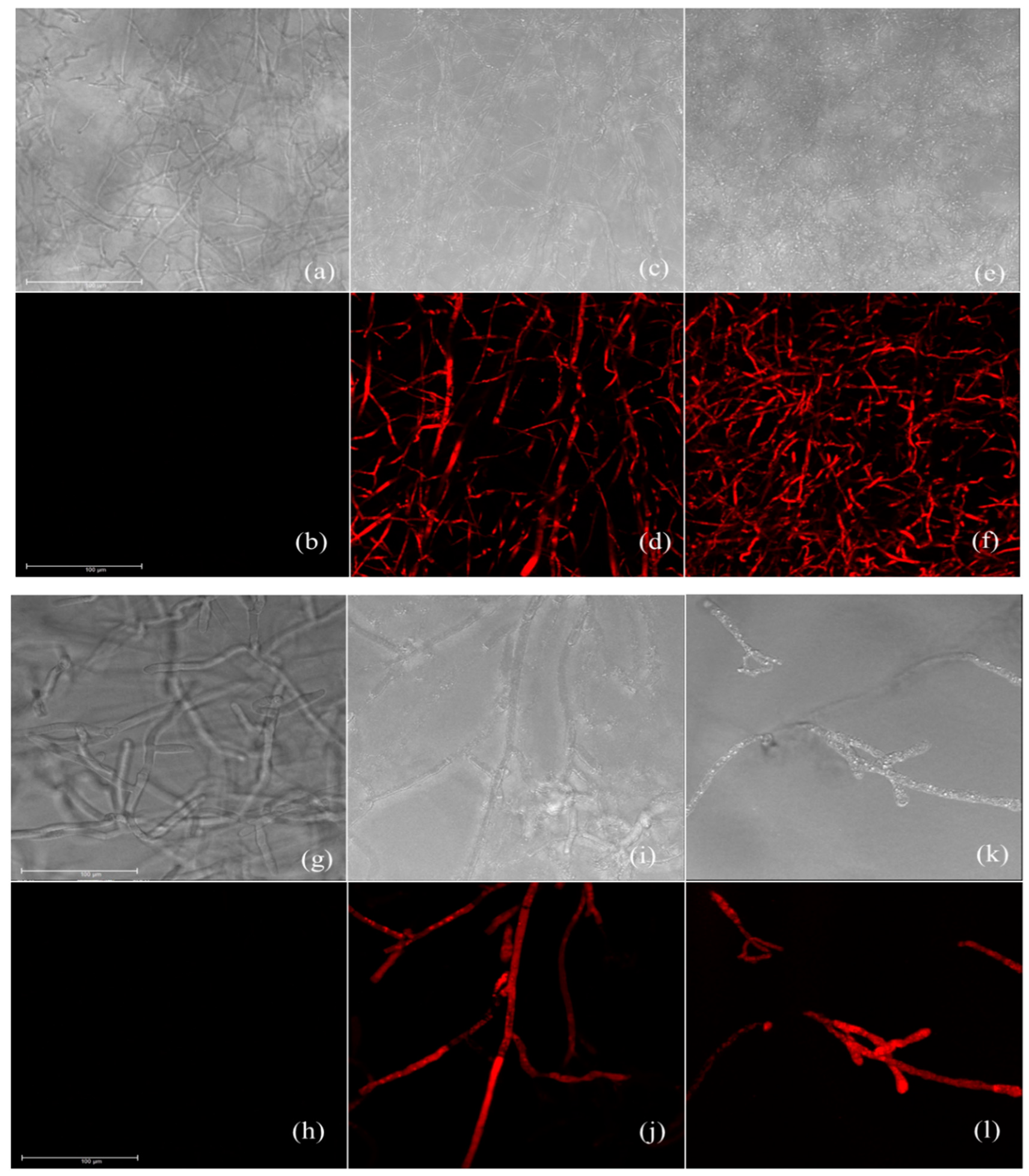
2.7. Cell Membrane Integrity Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Fumigant Antifungal Activities of Lamiaceae Plant Essential Oils
3.2. Chemical Composition of Summer Savory and Thyme White Essential Oils
3.3. Fumigant Antifungal Activities of Constituents from Summer Savory and Thyme White Essential Oils
3.4. Effect of Carvacrol and Thymol on Reactive Oxygen Species Generation
3.5. Effect of Carvacrol and Thymol on Cell Membrane Integrity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, J.; Lee, S.G.; Shin, S.C.; Kwon, Y.D.; Park, I.K. Male-produced aggregation pheromone blend in Platypus koryoensis. J. Agric. Food Chem. 2009, 57, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Park, I.K.; Nam, Y.; Seo, S.T.; Kim, S.W.; Jung, C.S.; Han, H.R. Development of a mass trapping device for the ambrosia beetle, Platypus koryoensis, an insect vector of oak wilt disease in Korea. J. Asia-Pacific Entomol. 2016, 19, 39–43. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, Y.J.; Seo, S.T.; Shin, H.D. Raffaelea quercus-mongolicae sp. Nov. associated with Platypus koryoensis on oak in Korea. Mycotaxon 2009, 110, 189–197. [Google Scholar] [CrossRef]
- Lee, B.Y.; Chung, Y.J. Insect pests of trees and shrubs in Korea; Seongandang: Seoul, Korea, 1997; p. 145. [Google Scholar]
- Lee, J.E. Antimicrobial Activity and Mode of Action of Four Plant Essential Oils, Their Constituents and trans-Cinnamaldehyde Derivatives against Three Tree Pathogens. Master’s Thesis, Seoul National University, Seoul, Korea, 2018. [Google Scholar]
- StĘpniewska-Jarosz, S.; Mańka, M.; Asiegbu, F. Studies on anastomosis groups of Rhizoctonia solani isolates causing disease in two forest nurseries in Poland. For. Pathol. 2006, 36, 97–109. [Google Scholar] [CrossRef]
- Kim, J.K.; Koh, S.H.; Koo, C.D.; Kim, K.W.; Kim, J.K.; Kim, J.J.; Park, G.S.; Park, Y.C.; Park, I.K.; Byun, B.K.; et al. Forest Protection; Haengmoonsa: Seoul, Korea, 2019. [Google Scholar]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; Melo, N.R.D.; Sanches-Silva, A. Use of essential oils in active food packing: Recent advances and future trends. Trends Food Sci. Tech. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Kim, E.; Park, I.K. Fumigant antifungal activity of Myrtaceae essential oils and constituents from Leptospermum petersonii against three Aspergillus species. Molecules 2012, 17, 10459–10469. [Google Scholar] [CrossRef]
- Van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Mendiburu, F.D. Agricolae: Statistical Procedures for Agricultural Research Version 1.2-8. Available online: http://tarwi.lamolina.edu.pe/~fmendiburu (accessed on 3 October 2019).
- Trivllini, A.; Lucchesini, M.; Maggini, R.; Mosadegh, H.; Villamarin, T.S.S.; Vernieri, P.; Mensuali-Sodi, A.; Pardossi, A. Lamiaceae phenols as multifaceted compounds: Bioactivity industrial prospects and role of “positive-stress”. Ind. Crops Prod. 2016, 83, 241–254. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.S.; Lee, S.G.; Shin, S.C.; Park, I.K. Fumigant antifungal activity of plant essential oils and components from West Indian bay (Pimenta racemose) and thyme (Thymus vulgaris) oils against two phytopathogenic fungi. Flavour Fragr. J. 2008, 23, 272–277. [Google Scholar] [CrossRef]
- Abdollahi, A.; Hassani, A.; Ghosta, Y.; Meshkatalsadat, M.H.; Shabani, R. Screening of antifungal properties of essential oils extracted from sweet basil, fennel, summer savory and thyme against postharvest phytopathogenic fungi. J. Food Saf. 2011, 31, 350–356. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef] [PubMed]
- Moosavi-Nasab, M.; Saharkhiz, M.J.; Ziaee, E.; Moayedi, F.; Koshani, R.; Azizu, R. Chemical compositions and antibacterial activities of five selected aromatic plants essential oils against food-borne pathogens and spoilage bacteria. J. Essent. Oil Res. 2016, 28, 241–251. [Google Scholar] [CrossRef]
- Zambonelli, A.; D’Aulrio, A.Z. Chemical composition and fungicidal activity of commercial essential oils of Thymus vulgaris L. J. Essent. Oil. Res. 2004, 16, 69–74. [Google Scholar] [CrossRef]
- Galabosi, B.; Peura, P. Agrobotanical features and oil content of wild and cultivated forms of caraway (Carum carvi L.). J. Essent. Oil. Res. 1996, 8, 389–397. [Google Scholar] [CrossRef]
- Salehi, B.; Mishr, A.P.; Shukla, I.; Sharifi-Rad Contreas, M. d. M.; Sgura-Carretro, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Contreras, M. d. M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive revies. Phyther. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Envrion. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Ultee, A.; Kets, E.P.W.; Smid, E.J. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 1999, 65, 4606–4610. [Google Scholar]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur. J. Clin. Microbiol. Infect Dis. 2011, 30, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.J.; Kohanski, M.A.; Collins, J.J. Role of reactive oxygen species in antibiotic action and resistance. Curr. Opin. Microbiol. 2009, 12, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef]
- Mello, E.O.; Ribeiro, S.F.F.; Carvalho, A.O.; Santos, I.S.; Da Cunha, M.; Santa-Catarina, C.; Gomes, V.M. Antifungal activity of PvD1 defensin involves plasma membrane permeabilization, inhibition of medium acidification, and induction of ROS in fungi cells. Curr. Biol. 2011, 62, 1209–1217. [Google Scholar] [CrossRef]
- Yan, L.; Li, M.; Cao, Y.; Gao, P.; Cao, Y.; Wang, Y.; Jiang, Y. The alternative oxidase of Candida albicans causes reduced fluconazole susceptibility. J. Antimicrob. Chemother. 2009, 4, 764–773. [Google Scholar] [CrossRef]
- Cheng, J.J.; Park, T.S.; Choi, L.G.; Fischl, A.S.; Ye, X.S. Induction of apoptosis by sphingoid long-chain bases in Aspergillus nidulans. Mol. Cell Biol. 2003, 23, 163–177. [Google Scholar] [CrossRef]
- Qi, G.; Zhu, F.; Du, P.; Yang, X.; Qiu, D.; Yu, Z.; Chen, J.; Zhao, X. Lipopeptide induces apoptosis in fungal cells by a mitochondria-dependent pathway. Peptides 2010, 31, 1978–1986. [Google Scholar] [CrossRef]
- Shen, Q.; Zhou, W.; Li, H.; Hu, L.; Mo, H. ROS involves the fungicidal actions of thymol against spores of Aspergillus flavus via the induction of nitric oxide. PLoS ONE 2016, 11, e0155647. [Google Scholar] [CrossRef]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Chen, Y.; Wang, Y. The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS ONE 2012, 7, e30147. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, T.; Samaranayake, Y.H.; Fang, H.H.P.; Yip, H.K.; Samaranayake, L.P. The use of new probes and stains for improved assessment of cell viability and extracellular polymeric substances in Candida albicans biofilms. Mycopathologia 2005, 159, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.M.; Shi, G.X.; Shao, J.; Wu, D.Q.; Yan, Y.Y.; Zhang, M.X.; Cui, Y.Y.; Wang, C.Z. In vitro antifungal activity of baicalin gains Candida albicans biofilms via apoptotic induction. Microb. Pathog. 2015, 87, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Tao, N.; Yang, W.; Jing, G. Cinnamaldehyde damaged the cell membrane of Alternaria alternata and induced the degradation of mycotoxins in vivo. Ind. Crop. Prod. 2018, 112, 427–433. [Google Scholar] [CrossRef]
| Common Name | Scientific Name | Source | Part |
|---|---|---|---|
| Basil | Ocimum basilicum L. | Jinarome | Leaves |
| Hyssop | Hyssopus officinalis L. | Osahdi | plants |
| Lavender | Lavandula angustifolia Mill. | Jinarome | Flowers |
| Marjoram | Origanum majorana L. | Jinarome | Fruits |
| Patchouli | Pogostemon cablin Benth. | Oshadi | Leaves |
| Pennyroyal | Mentha pulegium L. | Jinarome | Plants |
| Spanish Sage | Salvia lavandulaefolia Vahl. | Jinarome | Flowers |
| Spearmint | Mentha spicata L. | Jinarome | Flowering plants |
| Summer Savory | Satureja hortensis L. | Osahdi | Blossom/plants |
| Thyme White | Thymus vulgaris L. | Jinarome | Leaves |
| Essential Oils. | Inhibition Rate (%, mean ± SE) | |||||
|---|---|---|---|---|---|---|
| 10 1 | 5 | 2.5 | 1.25 | 0.625 | 0.3125 | |
| Basil | 58.42 ± 1.21b 2 | 0d | - 3 | - | - | - |
| Hyssop | 27.72 ± 2.38c | 0d | - | - | - | - |
| Lavender | 37.46 ± 8.53c | 0d | - | - | - | - |
| Marjoram | 37.30 ± 5.77c | 0d | - | - | - | - |
| Patchouli | 24.60 ± 4.27c | 0d | - | - | - | - |
| Pennyroyal | 72.38 ± 2.29b | 44.40 ± 0.35c | 34.62 ± 2.82b | 27.96 ± 2.70c | 0b | - |
| Spanish sage | 59.06 ± 1.77b | 0d | - | - | - | - |
| Spearmint | 91.76 ± 0.75a | 57.50 ± 1.55b | 37.06 ± 1.94b | 15.98 ± 2.29d | 0b | - |
| Summer savory | 100a | 100a | 100a | 91.50 ± 1.60a | 54.40 ± 0.78a | 34.86 ± 4.01 |
| Thyme white | 100a | 100a | 100a | 73.52 ± 0.64b | 50.18 ± 2.79a | 29.30 ± 2.01 |
| F9,40 = 60.81 | F9,40 = 7110 | F3,16 = 469 | F3,16 = 60.81 | F3,16 = 60.81 | F1,8 = 60.81 | |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p = 0.25 | |
| Essential Oils | Inhibition Rate (%, mean ± SE) | |||||
|---|---|---|---|---|---|---|
| 10 1 | 5 | 2.5 | 1.25 | 0.625 | 0.3125 | |
| Basil | 100a 2 | 54.40 ± 1.04e | 20.20 ± 1.24d | 0d | - | - |
| Hyssop | 63.96 ± 1.33d | 36.64 ± 1.54e | 11.96 ± 1.14e | 0d | - | - |
| Lavender | 86.38 ± 0.64b | 62.86 ± 1.13c | 19.28 ± 1.98d | 0d | - | - |
| Marjoram | 0e | - 3 | - | - | - | - |
| Patchouli | 0e | - | - | - | - | - |
| Pennyroyal | 100a | 87.96 ± 0.90b | 36.42 ± 2.29c | 21.96 ± 0.96c | 17.70 ± 0.70c | 0c |
| Spanish sage | 73.08 ± 0.95c | 57.50 ± 0.96d | 23.52 ± 0.88d | 0d | - | - |
| Spearmint | 100a | 91.30 ± 0.89b | 44.18 ± 1.12b | 25.28 ± 1.07c | 0d | - |
| Summer savory | 100a | 100a | 100a | 91.76 ± 2.07a | 82.18 ± 1.00a | 41.98 ± 0.81b |
| Thyme white | 100a | 100a | 100a | 85.72 ± 1.17b | 63.30 ± 1.25b | 54.38 ± 2.53a |
| F9,40 = 5264 | F7,32 = 631.3 | F7,32 = 730.9 | F7,32 = 1562 | F3,16 = 1926 | F2,12 = 346.4 | |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.001 | |
| No. | Compound | Retention Index | Composition Rate (%) | |
|---|---|---|---|---|
| DB-5 | Summer Savory | Thyme White | ||
| 1 | α-Pinene | 930 | 1.34 | 5.25 |
| 2 | Camphene | 946 | - 1 | 1.41 |
| 3 | β-Pinene | 973 | 0.43 | - |
| 4 | Myrcene | 989 | 1.81 | 3.35 |
| 5 | α-Terpinene | 1014 | 3.51 | - |
| 6 | p-Cymene | 1022 | 10.72 | 30.16 |
| 7 | Limonene | 1027 | 0.63 | 1.03 |
| 8 | γ-Terpinene | 1057 | 34.63 | 11.44 |
| 9 | Linalool | 1100 | - | 9.49 |
| 10 | Terpinen-4-ol | 1179 | 0.50 | - |
| 11 | Thymol | 1290 | 0.21 | 25.32 |
| 12 | Carvacrol | 1298 | 39.84 | 3.46 |
| 13 | β-Caryophyllene | 1415 | 2.40 | 1.75 |
| 14 | Caryophyllene oxide | 1577 | - | 1.15 |
| Sum | 96.04 | 93.81 | ||
| Compounds | Inhibition Rate (%, mean ± SE) | ||||
|---|---|---|---|---|---|
| 2.5 1 | 1.25 | 0.625 | 0.3125 | 0.15625 | |
| (+)-α-Pinene | 0e 2 | - 3 | - | - | - |
| (−)-α-Pinene | 0e | - | - | - | - |
| (+)-Camphene | 0e | - | - | - | - |
| (−)-Camphene | 0e | - | - | - | - |
| (+)-β-Pinene | 0e | - | - | - | - |
| (−)-β-Pinene | 0e | - | - | - | - |
| Myrcene | 0e | - | - | - | - |
| α-Terpinene | 0e | - | - | - | - |
| p-Cymene | 0e | - | - | - | - |
| (+)-Limonene | 0e | - | - | - | - |
| (−)-Limonene | 0e | - | - | - | - |
| γ-Terpinene | 0e | - | - | - | - |
| Linalool | 15.48 ± 2.25d | 0d | - | - | - |
| Terpinen-4-ol | 74.84 ± 0.89b | 59.28 ± 3.37b | 47.06 ± 1.47c | 21.74 ± 1.04c | 0b |
| Thymol | 100a | 100a | 100a | 53.30 ± 0.92a | 28.40 ± 2.45a |
| Carvacrol | 100a | 100a | 81.28 ± 2.20b | 30.68 ± 2.09b | 0b |
| β-Caryophyllene | 0e | - | - | - | - |
| Caryophyllene oxide | 46.18 ± 2.91c | 20.64 ± 2.56c | 10.38 ± 1.67d | 0d | - |
| F17,72 = 1601 | F4,20 = 578.2 | F3,16 = 534.8 | F3,16 = 310.1 | F2,12 = 134.7 | |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| Compounds | Inhibition Rate (%, mean ± SE) | ||||
|---|---|---|---|---|---|
| 2.5 1 | 1.25 | 0.625 | 0.3125 | 0.15625 | |
| (+)-α-Pinene | 47.52 ± 1.84c 2 | 33.52 ± 1.37b | 21.52 ± 1.35b | 0b | - |
| (−)-α-Pinene | 47.30 ± 1.88c | 18.38 ± 1.10c | 0d | - | - |
| (+)-Camphene | 0f | - 3 | - | - | - |
| (−)-Camphene | 0f | - | - | - | - |
| (+)-β-Pinene | 0f | - | - | - | - |
| (−)-β-Pinene | 0f | - | - | - | - |
| Myrcene | 0f | - | - | - | - |
| α-Terpinene | 0f | - | - | - | - |
| p-Cymene | 0f | - | - | - | - |
| (+)-Limonene | 22.64 ± 0.44d | 0d | - | - | - |
| (−)-Limonene | 0f | - | - | - | - |
| γ-Terpinene | 0f | - | - | - | - |
| Linalool | 42.62 ± 1.74c | 0d | - | - | - |
| Terpinen-4-ol | 71.30 ± 1.84b | 32.40 ± 1.29b | 15.28 ± 1.32c | 0b | - |
| Thymol | 100a | 100a | 100a | 78.64 ± 1.34a | 51.28 ± 6.98 |
| Carvacrol | 100a | 100a | 100a | 81.96 ± 3.23a | 55.50 ± 0.92 |
| β-Caryophyllene | 15.74 ± 1.77e | 0d | - | - | - |
| Caryophyllene oxide | 0f | - | - | - | - |
| F17,72 = 1338 | F7,32 = 2997 | F4,20 = 3331 | F3,16 = 703.9 | F1,8 = 0.359 | |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p = 0.566 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-E.; Lee, J.-E.; Huh, M.-J.; Lee, S.-C.; Seo, S.-M.; Kwon, J.H.; Park, I.-K. Fumigant Antifungal Activity via Reactive Oxygen Species of Thymus vulgaris and Satureja hortensis Essential Oils and Constituents against Raffaelea quercus-mongolicae and Rhizoctonia solani. Biomolecules 2019, 9, 561. https://doi.org/10.3390/biom9100561
Kim J-E, Lee J-E, Huh M-J, Lee S-C, Seo S-M, Kwon JH, Park I-K. Fumigant Antifungal Activity via Reactive Oxygen Species of Thymus vulgaris and Satureja hortensis Essential Oils and Constituents against Raffaelea quercus-mongolicae and Rhizoctonia solani. Biomolecules. 2019; 9(10):561. https://doi.org/10.3390/biom9100561
Chicago/Turabian StyleKim, Jeong-Eun, Ji-Eun Lee, Min-Jung Huh, Sung-Chan Lee, Seon-Mi Seo, Jun Hyeong Kwon, and Il-Kwon Park. 2019. "Fumigant Antifungal Activity via Reactive Oxygen Species of Thymus vulgaris and Satureja hortensis Essential Oils and Constituents against Raffaelea quercus-mongolicae and Rhizoctonia solani" Biomolecules 9, no. 10: 561. https://doi.org/10.3390/biom9100561
APA StyleKim, J.-E., Lee, J.-E., Huh, M.-J., Lee, S.-C., Seo, S.-M., Kwon, J. H., & Park, I.-K. (2019). Fumigant Antifungal Activity via Reactive Oxygen Species of Thymus vulgaris and Satureja hortensis Essential Oils and Constituents against Raffaelea quercus-mongolicae and Rhizoctonia solani. Biomolecules, 9(10), 561. https://doi.org/10.3390/biom9100561






