Specialized Roles for Actin in Osteoclasts: Unanswered Questions and Therapeutic Opportunities
Abstract
1. Introduction
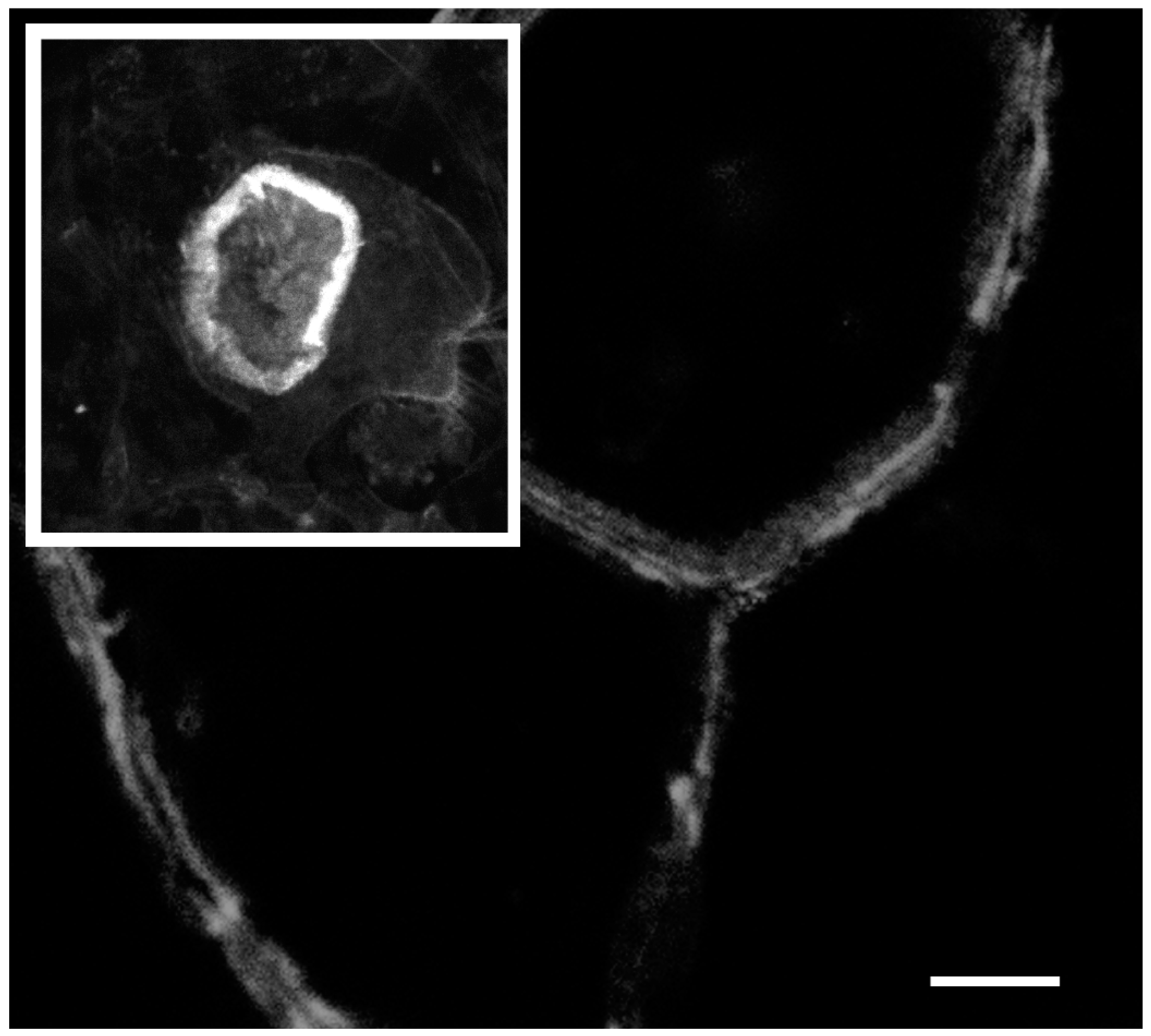
2. Actin Dynamics in the Actin Ring
3. Mechanisms Regulating Microfilament Dynamics in the Actin Ring
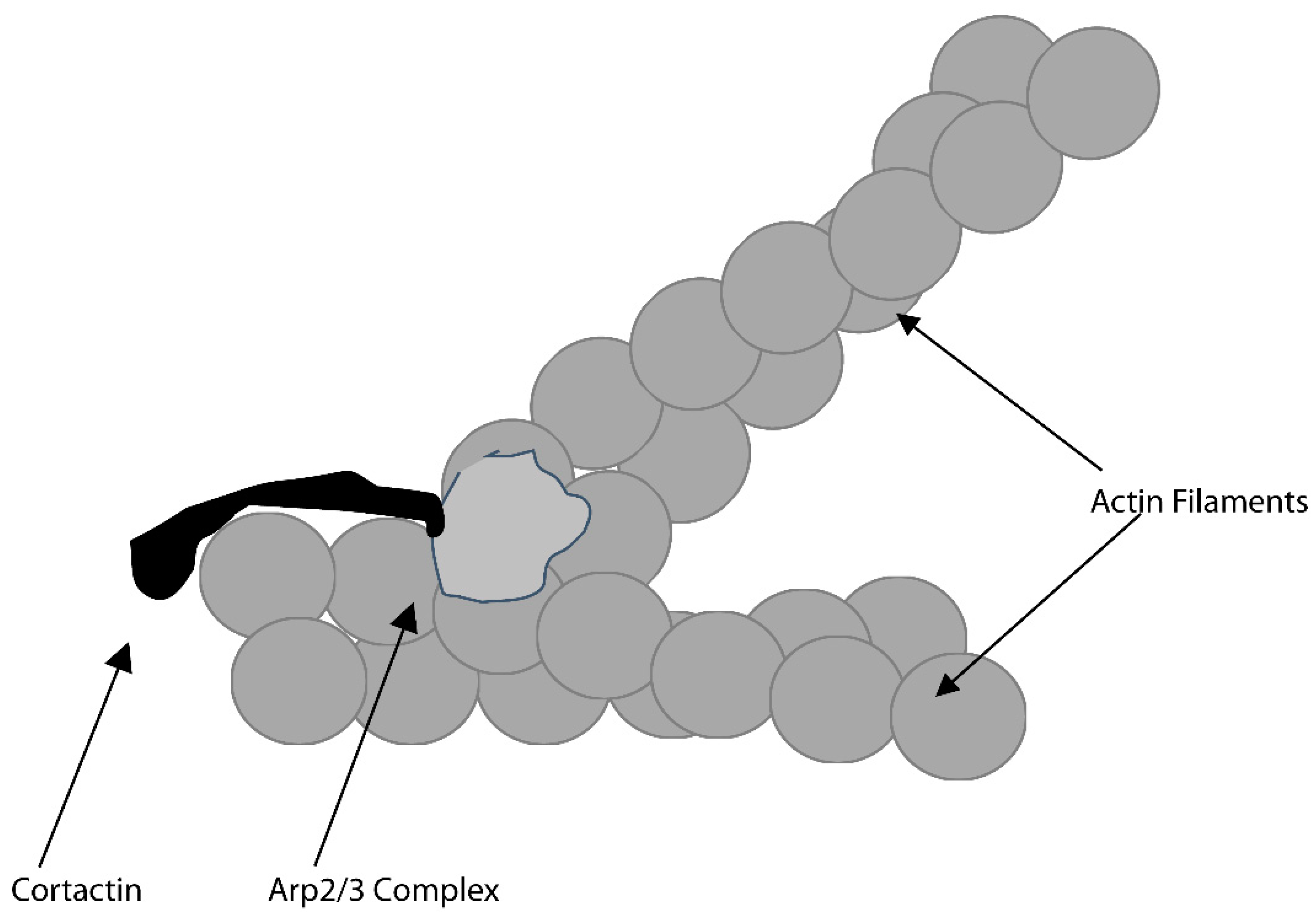
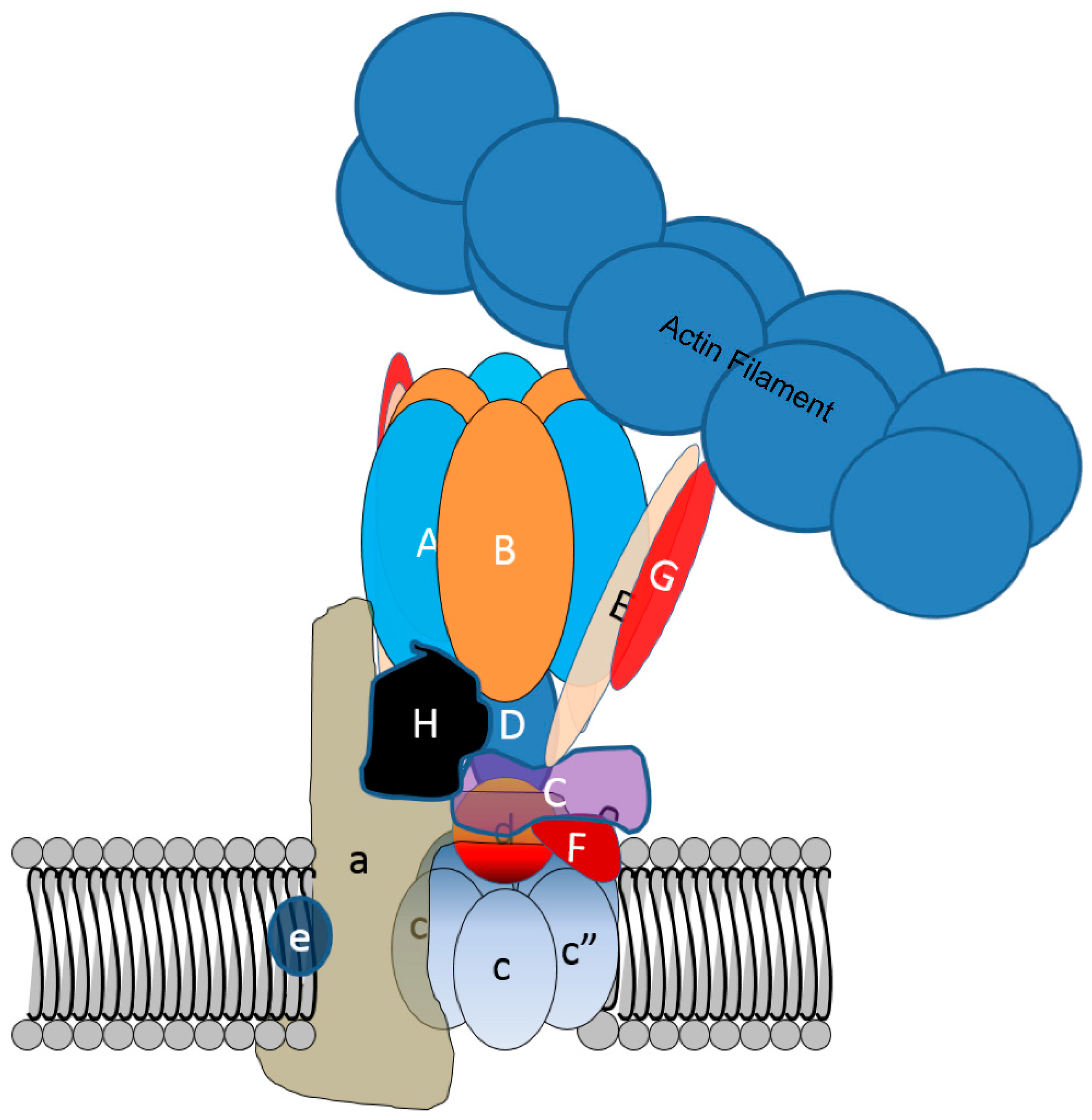
3.1. Actin-Related (Arp)-2/3 Complex and the Osteoclast Actin Ring and Resorption Compartment
3.2. Triggering of Actin Ring Formation-Integrins, CD44, Bone Mineral, Other Candidates
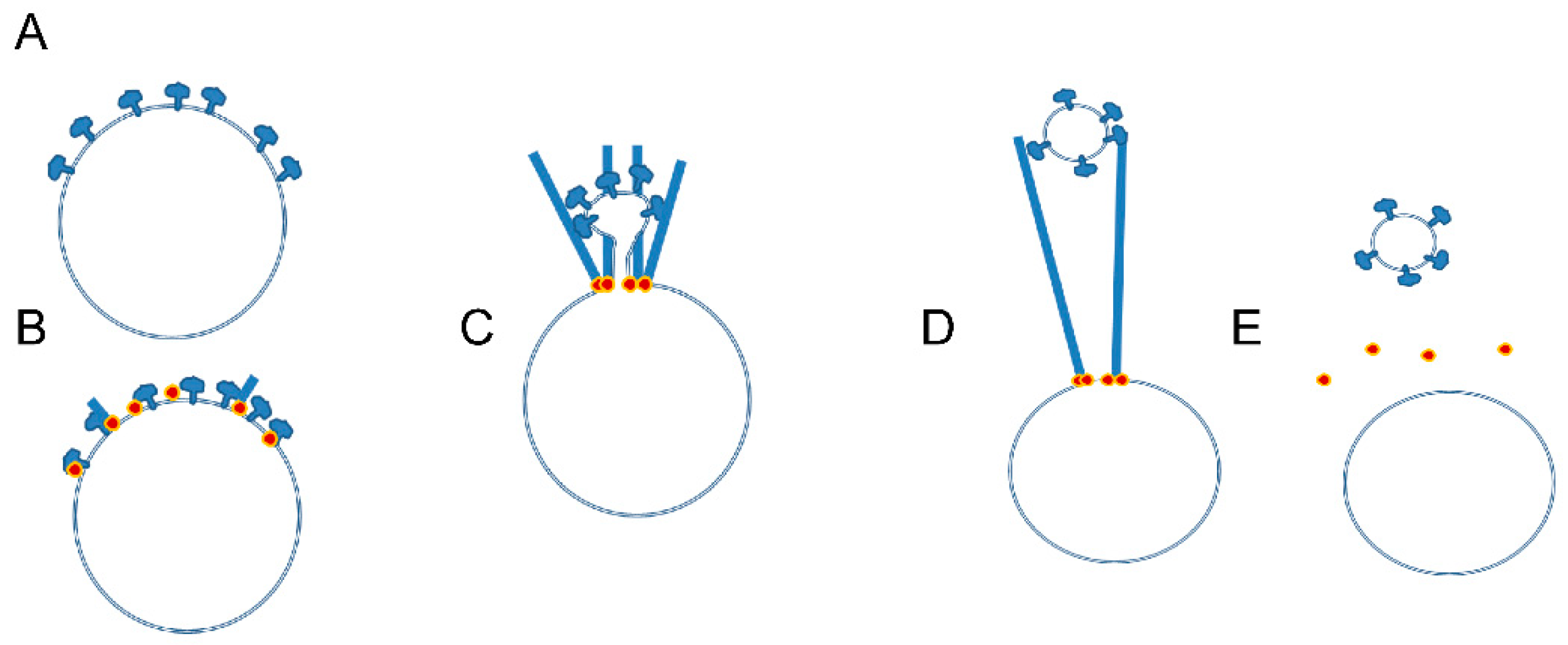
3.3. Unanswered Questions Regarding Mechanisms of Actin Ring Formation
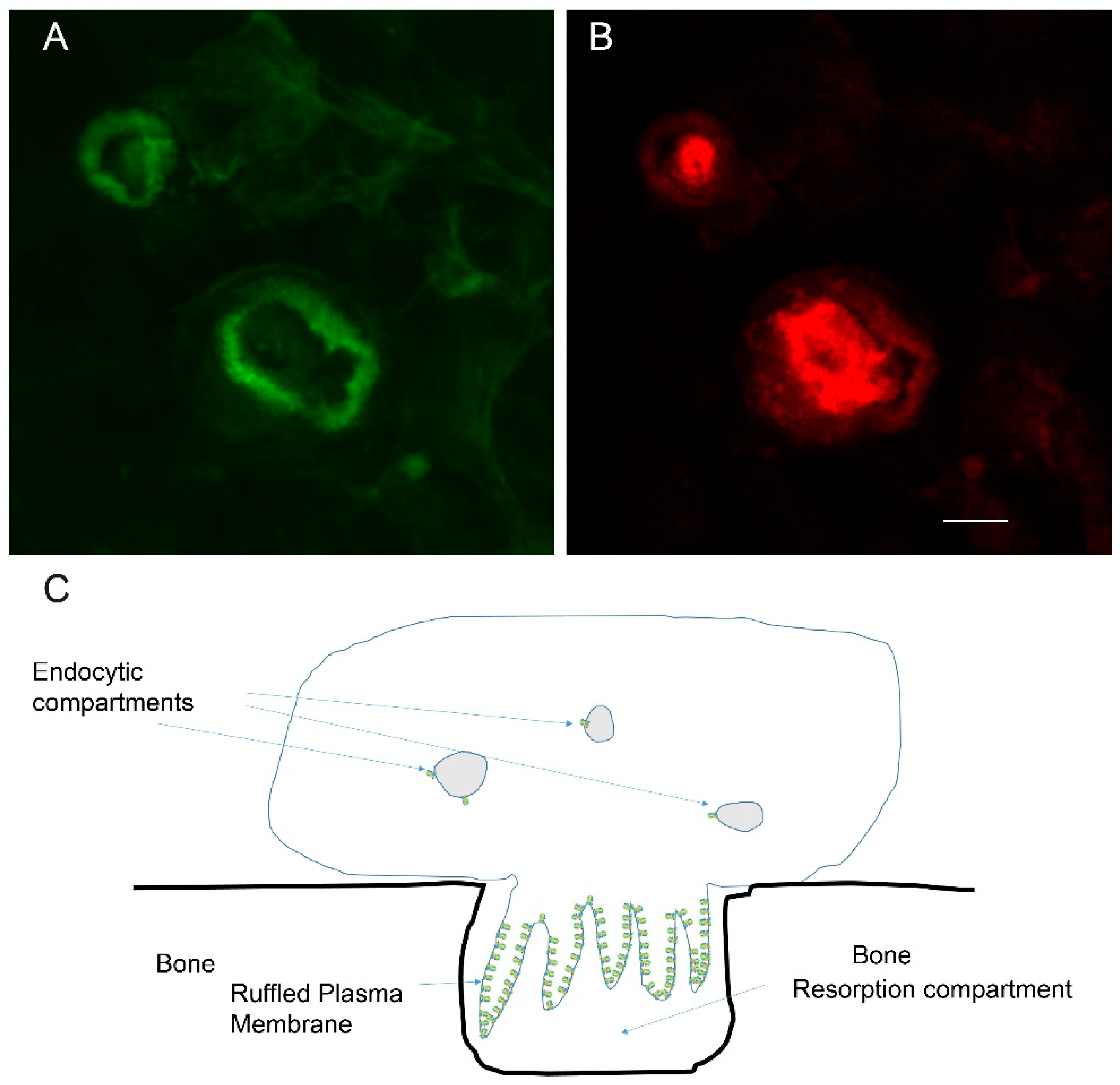
3.4. Actin and the Formation of the Ruffle Membrane
4. Summary and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Toro, E.J.; Ostrov, D.A.; Wronski, T.J.; Holliday, L.S. Rational Identification of Enoxacin as a Novel V-ATPase-Directed Osteoclast Inhibitor. Curr. Protein Pept. Sci. 2012, 13, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L. Osteoclasts: what do they do and how do they do it? Am. J. Pathol. 2007, 170, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.C.; Teitelbaum, S.L.; Ghiselli, R.; Gluck, S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science 1989, 245, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Vaananen, H.K.; Karhukorpi, E.K.; Sundquist, K.; Wallmark, B.; Roininen, I.; Hentunen, T.; Tuukkanen, J.; Lakkakorpi, P. Evidence for the presence of a proton pump of the vacuolar H(+)-ATPase type in the ruffled borders of osteoclasts. J. Cell Biol. 1990, 111, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Bromme, D.; Okamoto, K.; Wang, B.B.; Biroc, S. Human cathepsin O2, a matrix protein-degrading cysteine protease expressed in osteoclasts. Functional expression of human cathepsin O2 in Spodoptera frugiperda and characterization of the enzyme. J. Biol. Chem. 1996, 271, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Drake, F.H.; Dodds, R.A.; James, I.E.; Connor, J.R.; Debouck, C.; Richardson, S.; Lee-Rykaczewski, E.; Coleman, L.; Rieman, D.; Barthlow, R.; et al. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J. Biol. Chem. 1996, 271, 12511–12516. [Google Scholar] [CrossRef]
- King, G.J.; Holtrop, M.E. Actin-like filaments in bone cells of cultured mouse calvaria as demonstrated by binding to heavy meromyosin. J. Cell Biol. 1975, 66, 445–451. [Google Scholar] [CrossRef]
- Soriano, P.; Montgomery, C.; Geske, R.; Bradley, A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 1991, 64, 693–702. [Google Scholar] [CrossRef]
- Lowe, C.; Yoneda, T.; Boyce, B.F.; Chen, H.; Mundy, G.R.; Soriano, P. Osteopetrosis in Src-deficient mice is due to an autonomous defect of osteoclasts. Proc. Natl. Acad. Sci. USA 1993, 90, 4485–4489. [Google Scholar] [CrossRef]
- Destaing, O.; Sanjay, A.; Itzstein, C.; Horne, W.C.; Toomre, D.; De Camilli, P.; Baron, R. The tyrosine kinase activity of c-Src regulates actin dynamics and organization of podosomes in osteoclasts. Mol. Biol. Cell 2008, 19, 394–404. [Google Scholar] [CrossRef]
- Lakkakorpi, P.; Tuukkanen, J.; Hentunen, T.; Jarvelin, K.; Vaananen, K. Organization of osteoclast microfilaments during the attachment to bone surface in vitro. J. Bone Miner. Res. 1989, 4, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Lakkakorpi, P.T.; Vaananen, H.K. Cytoskeletal changes in osteoclasts during the resorption cycle. Microsc. Res. Tech. 1996, 33, 171–181. [Google Scholar] [CrossRef]
- Lakkakorpi, P.T.; Vaananen, H.K. Kinetics of the osteoclast cytoskeleton during the resorption cycle in vitro. J. Bone Miner. Res. 1991, 6, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Hiura, K.; Lim, S.S.; Little, S.P.; Lin, S.; Sato, M. Differentiation-Dependent Expression of Tensin and Cortactin in Chicken Osteoclasts. Cell Motil. Cytoskeleton 1995, 30, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Lakkakorpi, P.T.; Nakamura, I.; Nagy, R.M.; Parsons, J.T.; Rodan, G.A.; Duong, L.T. Stable association of PYK2 and p130(Cas) in osteoclasts and their co-localization in the sealing zone. J. Biol. Chem. 1999, 274, 4900–4907. [Google Scholar] [CrossRef]
- Zambonin-Zallone, A.; Teti, A.; Grano, M.; Rubinacci, A.; Abbadini, M.; Gaboli, M.; Marchisio, P.C. Immunocytochemical distribution of extracellular matrix receptors in human osteoclasts: a β3 integrin is colocalized with vinculin and talin in the podosomes of osteoclastoma giant cells. Exp. Cell Res. 1989, 182, 645–652. [Google Scholar] [CrossRef]
- Zambonin, Z.A.; Teti, A.; Gaboli, M.; Marchisio, P.C. β3 subunit of vitronectin receptor is present in osteoclast adhesion structures and not in other monocyte-macrophage derived cells. Connect. Tissue Res. 1989, 20, 143–149. [Google Scholar]
- Dolce, C.; Vakani, A.; Archer, L.; Morris-Wiman, J.A.; Holliday, L.S. Effects of echistatin and an RGD peptide on orthodontic tooth movement. J. Dent. Res. 2003, 82, 682–686. [Google Scholar] [CrossRef]
- Lark, M.W.; Stroup, G.B.; Dodds, R.A.; Kapadia, R.; Hoffman, S.J.; Hwang, S.M.; James, I.E.; Lechowska, B.; Liang, X.; Rieman, D.J.; et al. Antagonism of the osteoclast vitronectin receptor with an orally active nonpeptide inhibitor prevents cancellous bone loss in the ovariectomized rat. J. Bone Miner. Res. 2001, 16, 319–327. [Google Scholar] [CrossRef]
- Lark, M.W.; Stroup, G.B.; Hwang, S.M.; James, I.E.; Rieman, D.J.; Drake, F.H.; Bradbeer, J.N.; Mathur, A.; Erhard, K.F.; Newlander, K.A.; et al. Design and characterization of orally active Arg-Gly-Asp peptidomimetic vitronectin receptor antagonist SB 265123 for prevention of bone loss in osteoporosis. J. Pharmacol. Exp. Ther. 1999, 291, 612–617. [Google Scholar]
- Carron, C.P.; Meyer, D.M.; Engleman, V.W.; Rico, J.G.; Ruminski, P.G.; Ornberg, R.L.; Westlin, W.F.; Nickols, G.A. Peptidomimetic antagonists of alphavbeta3 inhibit bone resorption by inhibiting osteoclast bone resorptive activity, not osteoclast adhesion to bone. J. Endocrinol. 2000, 165, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Engleman, V.W.; Nickols, G.A.; Ross, F.P.; Horton, M.A.; Griggs, D.W.; Settle, S.L.; Ruminski, P.G.; Teitelbaum, S.L. A peptidomimetic antagonist of the alpha(v)beta3 integrin inhibits bone resorption in vitro and prevents osteoporosis in vivo. J. Clin. Invest. 1997, 99, 2284–2292. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.E.; Caulfield, M.P.; Sato, M.; Quartuccio, H.A.; Gould, R.J.; Garsky, V.M.; Rodan, G.A.; Rosenblatt, M. Inhibition of osteoclastic bone resorption in vivo by echistatin, an “arginyl-glycyl-aspartyl” (RGD)-containing protein. Endocrinology 1993, 132, 1411–1413. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.H.; Alberts, D.P.; Bhatnagar, P.K.; Bondinell, W.E.; Callahan, J.F.; Calvo, R.R.; Cousins, R.D.; Erhard, K.F.; Heerding, D.A.; Keenan, R.M.; et al. Discovery of orally active nonpeptide vitronectin receptor antagonists based on a 2-benzazepine Gly-Asp mimetic. J. Med. Chem. 2000, 43, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Nakchbandi, I.; Ruppert, R.; Kawelke, N.; Hess, M.W.; Pfaller, K.; Jurdic, P.; Fässler, R.; Moser, M. Kindlin-3-mediated signaling from multiple integrin classes is required for osteoclast-mediated bone resorption. J. Cell Biol. 2011, 192, 883–897. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.P.; Hodivala-Dilke, K.; Zheng, M.H.; Namba, N.; Lam, J.; Novack, D.; Feng, X.; Ross, F.P.; Hynes, R.O.; Teitelbaum, S.L. Mice lacking β3 integrins are osteosclerotic because of dysfunctional osteoclasts. J. Clin. Invest. 2000, 105, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Holliday, L.S.; Welgus, H.G.; Fliszar, C.J.; Veith, G.M.; Jeffrey, J.J.; Gluck, S.L. Initiation of osteoclast bone resorption by interstitial collagenase. J. Biol. Chem. 1997, 272, 22053–22058. [Google Scholar] [CrossRef]
- Gluck, S.L.; Lee, B.S.; Wang, S.P.; Underhill, D.; Nemoto, J.; Holliday, L.S. Plasma membrane V-ATPases in proton-transporting cells of the mammalian kidney and osteoclast. Acta Physiol. Scand. Suppl. 1998, 643, 203–212. [Google Scholar]
- Marchisio, P.C.; Cirillo, D.; Naldini, L.; Primavera, M.V.; Teti, A.; Zambonin-Zallone, A. Cell-substratum interaction of cultured avian osteoclasts is mediated by specific adhesion structures. J. Cell Biol. 1984, 99, 1696–1705. [Google Scholar] [CrossRef]
- Zambonin-Zallone, A.; Teti, A.; Carano, A.; Marchisio, P.C. The distribution of podosomes in osteoclasts cultured on bone laminae: Effect of retinol. J. Bone Miner. Res. 1988, 3, 517–523. [Google Scholar] [CrossRef]
- Destaing, O.; Saltel, F.; Geminard, J.C.; Jurdic, P.; Bard, F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol. Biol. Cell 2003, 14, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Saltel, F.; Destaing, O.; Bard, F.; Eichert, D.; Jurdic, P. Apatite-mediated actin dynamics in resorbing osteoclasts. Mol. Biol. Cell 2004, 15, 5231–5241. [Google Scholar] [CrossRef] [PubMed]
- Korn, E.D. Actin polymerization and its regulation by proteins from nonmuscle cells. Physiol. Rev. 1982, 62, 672–737. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Breton, S. Mitochondria-rich, proton-secreting epithelial cells. J. Exp. Biol. 1996, 199, 2345–2358. [Google Scholar]
- Zhang, Y.; Rohatgi, N.; Veis, D.J.; Schilling, J.; Teitelbaum, S.L.; Zou, W. PGC1beta Organizes the Osteoclast Cytoskeleton by Mitochondrial Biogenesis and Activation. J. Bone Miner. Res. 2018, 33, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Lehenkari, P.; Hentunen, T.A.; Laitala-Leinonen, T.; Tuukkanen, J.; Vaananen, H.K. Carbonic anhydrase II plays a major role in osteoclast differentiation and bone resorption by effecting the steady state intracellular pH and Ca2+. Exp. Cell Res. 1998, 242, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Holliday, L.S.; Zhang, L.; Dunn, W.A., Jr.; Gluck, S.L. Interaction between aldolase and vacuolar H+-ATPase: evidence for direct coupling of glycolysis to the ATP-hydrolyzing proton pump. J. Biol. Chem. 2001, 276, 30407–30413. [Google Scholar] [CrossRef]
- Lu, M.; Sautin, Y.Y.; Holliday, L.S.; Gluck, S.L. The glycolytic enzyme aldolase mediates assembly, expression, and activity of vacuolar H+-ATPase. J. Biol. Chem. 2004, 279, 8732–8739. [Google Scholar] [CrossRef]
- Lemma, S.; Sboarina, M.; Porporato, P.E.; Zini, N.; Sonveaux, P.; Di Pompo, G.; Baldini, N.; Avnet, S. Energy metabolism in osteoclast formation and activity. Int. J. Biochem. Cell Biol. 2016, 79, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Blanchoin, L.; Boujemaa-Paterski, R.; Sykes, C.; Plastino, J. Actin dynamics, architecture, and mechanics in cell motility. Physiol. Rev. 2014, 94, 235–263. [Google Scholar] [CrossRef]
- Bubb, M.R.; Knutson, J.R.; Porter, D.K.; Korn, E.D. Actobindin induces the accumulation of actin dimers that neither nucleate polymerization nor self-associate. J. Biol. Chem. 1994, 269, 25592–25597. [Google Scholar] [PubMed]
- Pollard, T.D. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 451–477. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Parsons, J.T. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J. Cell Biol. 1993, 120, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Weaver, A.M.; Karginov, A.V.; Kinley, A.W.; Weed, S.A.; Li, Y.; Parsons, J.T.; Cooper, J.A. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr. Biol. 2001, 11, 370–374. [Google Scholar] [CrossRef]
- Hurst, I.R.; Zuo, J.; Jiang, J.; Holliday, L.S. Actin-related protein 2/3 complex is required for actin ring formation. J. Bone Miner. Res. 2004, 19, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, P.; Besson, A. Cortactin function in invadopodia. Small GTPases. 2017, 31, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Luxenburg, C.; Parsons, J.T.; Addadi, L.; Geiger, B. Involvement of the Src-cortactin pathway in podosome formation and turnover during polarization of cultured osteoclasts. J. Cell Sci. 2006, 119, 4878–4888. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Myoui, A.; Ikeda, F.; Hata, K.; Yoshikawa, H.; Nishimura, R.; Yoneda, T. Critical role of cortactin in actin ring formation and osteoclastic bone resorption. J. Bone Miner. Metab 2006, 24, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, S.; Faccio, R.; Chandrasekar, I.; Ross, F.P.; Cooper, J.A. Cortactin has an essential and specific role in osteoclast actin assembly. Mol. Biol. Cell 2006, 17, 2882–2895. [Google Scholar] [CrossRef] [PubMed]
- Campellone, K.G.; Welch, M.D. A nucleator arms race: Cellular control of actin assembly. Nat. Rev. Mol. Cell Biol. 2010, 11, 237–251. [Google Scholar] [CrossRef]
- Calle, Y.; Jones, G.E.; Jagger, C.; Fuller, K.; Blundell, M.P.; Chow, J.; Chambers, T.; Thrasher, A.J. WASp deficiency in mice results in failure to form osteoclast sealing zones and defects in bone resorption. Blood 2004, 103, 3552–35561. [Google Scholar] [CrossRef] [PubMed]
- Carnell, M.; Zech, T.; Calaminus, S.D.; Ura, S.; Hagedorn, M.; Johnston, S.A.; May, R.C.; Soldati, T.; Machesky, L.M.; Insall, R.H. Actin polymerization driven by WASH causes V-ATPase retrieval and vesicle neutralization before exocytosis. J. Cell Biol. 2011, 193, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Toro, E.J.; Zuo, J.; Ostrov, D.A.; Catalfamo, D.; Bradaschia-Correa, V.; Arana-Chavez, V.; Caridad, A.R.; Neubert, J.K.; Wronski, T.J.; Wallet, S.M.; et al. Enoxacin directly inhibits osteoclastogenesis without inducing apoptosis. J. Biol. Chem. 2012, 287, 17894–17904. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, J.T.; Gomez, T.S.; Schoon, R.A.; Mangalam, A.K.; Billadeau, D.D. WASH knockout T cells demonstrate defective receptor trafficking, proliferation, and effector function. Mol. Cell Biol. 2013, 33, 958–973. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.; Jones, G.E.; Machesky, L.M.; Anton, I.M. WIP: WASP-interacting proteins at invadopodia and podosomes. Eur. J. Cell Biol. 2012, 91, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Banon-Rodriguez, I.; Monypenny, J.; Ragazzini, C.; Franco, A.; Calle, Y.; Jones, G.E.; Anton, I.M. The cortactin-binding domain of WIP is essential for podosome formation and extracellular matrix degradation by murine dendritic cells. Eur. J. Cell Biol. 2011, 90, 213–223. [Google Scholar] [CrossRef]
- Siar, C.H.; Rahman, Z.A.; Tsujigiwa, H.; Mohamed Om, A.K.; Nagatsuka, H.; Ng, K.H. Invadopodia proteins, cortactin, N-WASP and WIP differentially promote local invasiveness in ameloblastoma. J. Oral Pathol. Med. 2016, 45, 591–598. [Google Scholar] [CrossRef]
- Destaing, O.; Saltel, F.; Gilquin, B.; Chabadel, A.; Khochbin, S.; Ory, S.; Jurdic, P. A novel Rho-mDia2-HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J. Cell Sci. 2005, 118, 2901–2911. [Google Scholar] [CrossRef]
- Mersich, A.T.; Miller, M.R.; Chkourko, H.; Blystone, S.D. The formin FRL1 (FMNL1) is an essential component of macrophage podosomes. Cytoskeleton (Hoboken.) 2010, 67, 573–585. [Google Scholar] [CrossRef]
- Miller, M.R.; Blystone, S.D. Human Macrophages Utilize the Podosome Formin FMNL1 for Adhesion and Migration. Cellbio. (Irvine. Calif.) 2015, 4, 1–11. [Google Scholar] [CrossRef]
- Chellaiah, M.; Kizer, N.; Silva, M.; Alvarez, U.; Kwiatkowski, D.; Hruska, K.A. Gelsolin deficiency blocks podosome assembly and produces increased bone mass and strength. J. Cell Biol. 2000, 148, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Blangy, A.; Touaitahuata, H.; Cres, G.; Pawlak, G. Cofilin activation during podosome belt formation in osteoclasts. PLoS ONE 2012, 7, e45909. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Zhu, M.; Troiano, N.; Horowitz, M.; Bian, J.; Gundberg, C.; Kolodziejczak, K.; Insogna, K. LIM kinase 1 deficient mice have reduced bone mass. Bone 2013, 52, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Zalli, D.; Neff, L.; Nagano, K.; Shin, N.Y.; Witke, W.; Gori, F.; Baron, R. The Actin-Binding Protein Cofilin and Its Interaction With Cortactin Are Required for Podosome Patterning in Osteoclasts and Bone Resorption In Vivo and In Vitro. J. Bone Miner. Res. 2016, 31, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.A.; Schafer, D.A. Control of actin assembly and disassembly at filament ends. Curr. Opin. Cell Biol. 2000, 12, 97–103. [Google Scholar] [CrossRef]
- Chellaiah, M.; Fitzgerald, C.; Alvarez, U.; Hruska, K. c-Src is required for stimulation of gelsolin-associated phosphatidylinositol 3-kinase. J. Biol. Chem. 1998, 273, 11908–11916. [Google Scholar] [CrossRef] [PubMed]
- Holliday, L.S.; Welgus, H.G.; Hanna, J.; Lee, B.S.; Lu, M.; Jeffrey, J.J.; Gluck, S.L. Interstitial Collagenase Activity Stimulates the Formation of Actin Rings and Ruffled Membranes in Mouse Marrow Osteoclasts. Calcif. Tissue Int. 2003, 72, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.E.; Thula-Mata, T.; Toro, E.J.; Yeh, Y.W.; Holt, C.; Holliday, L.S.; Gower, L.B. Multifunctional role of osteopontin in directing intrafibrillar mineralization of collagen and activation of osteoclasts. Acta Biomater. 2014, 10, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, M.H.; Nesbitt, S.A.; Dorey, E.L.; Horton, M.A. Rat osteoclasts adhere to a wide range of RGD (Arg-Gly-Asp) peptide-containing proteins, including the bone sialoproteins and fibronectin, via a beta β3 integrin. J. Bone Miner. Res. 1992, 7, 335–343. [Google Scholar] [CrossRef]
- Chellaiah, M.A.; Kizer, N.; Biswas, R.; Alvarez, U.; Strauss-Schoenberger, J.; Rifas, L.; Rittling, S.R.; Denhardt, D.T.; Hruska, K.A. Osteopontin deficiency produces osteoclast dysfunction due to reduced CD44 surface expression. Mol. Biol. Cell 2003, 14, 173–189. [Google Scholar] [CrossRef]
- Georgess, D.; Machuca-Gayet, I.; Blangy, A.; Jurdic, P. Podosome organization drives osteoclast-mediated bone resorption. Cell Adh. Migr. 2014, 8, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Spessotto, P.; Rossi, F.M.; Degan, M.; Di Francia, R.; Perris, R.; Colombatti, A.; Gattei, V. Hyaluronan-CD44 interaction hampers migration of osteoclast-like cells by down-regulating MMP-9. J. Cell Biol. 2002, 158, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Marhaba, R.; Klingbeil, P.; Nuebel, T.; Nazarenko, I.; Buechler, M.W.; Zoeller, M. CD44 and EpCAM: cancer-initiating cell markers. Curr. Mol. Med. 2008, 8, 784–804. [Google Scholar] [CrossRef] [PubMed]
- Skruber, K.; Read, T.A.; Vitriol, E.A. Reconsidering an active role for G-actin in cytoskeletal regulation. J. Cell Sci. 2018, 131, jcs203760. [Google Scholar] [CrossRef] [PubMed]
- Shestakova, E.A.; Singer, R.H.; Condeelis, J. The physiological significance of beta -actin mRNA localization in determining cell polarity and directional motility. Proc. Natl. Acad. Sci. USA 2001, 98, 7045–7050. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.A.; Muench, S.P. The Vacuolar ATPase—A Nano-scale Motor That Drives Cell Biology. Subcell. Biochem. 2018, 87, 405–459. [Google Scholar]
- Scimeca, J.C.; Quincey, D.; Parrinello, H.; Romatet, D.; Grosgeorge, J.; Gaudray, P.; Philip, N.; Fischer, A.; Carle, G.F. Novel mutations in the TCIRG1 gene encoding the a3 subunit of the vacuolar proton pump in patients affected by infantile malignant osteopetrosis. Hum. Mutat. 2003, 21, 151–157. [Google Scholar] [CrossRef]
- Chen, S.H.; Bubb, M.R.; Yarmola, E.G.; Zuo, J.; Jiang, J.; Lee, B.S.; Lu, M.; Gluck, S.L.; Hurst, I.R.; Holliday, L.S. Vacuolar H+-ATPase binding to microfilaments: regulation in response to phosphatidylinositol 3-kinase activity and detailed characterization of the actin-binding site in subunit B. J. Biol. Chem. 2004, 279, 7988–7998. [Google Scholar] [CrossRef]
- Zuo, J.; Jiang, J.; Chen, S.H.; Vergara, S.; Gong, Y.; Xue, J.; Huang, H.; Kaku, M.; Holliday, L.S. Actin Binding Activity of Subunit B of Vacuolar H(+)-ATPase Is Involved in Its Targeting to Ruffled Membranes of Osteoclasts. J. Bone Miner. Res. 2006, 21, 714–721. [Google Scholar] [CrossRef]
- Holliday, L.S.; Lu, M.; Lee, B.S.; Nelson, R.D.; Solivan, S.; Zhang, L.; Gluck, S.L. The amino-terminal domain of the B subunit of vacuolar H+-ATPase contains a filamentous actin binding site. J. Biol. Chem. 2000, 275, 32331–32337. [Google Scholar] [CrossRef]
- Zuo, J.; Vergara, S.; Kohno, S.; Holliday, L.S. Biochemical and functional characterization of the actin-binding activity of the B subunit of yeast vacuolar H+-ATPase. J. Exp. Biol. 2008, 211, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Muench, S.P.; Huss, M.; Song, C.F.; Phillips, C.; Wieczorek, H.; Trinick, J.; Harrison, M.A. Cryo-electron microscopy of the vacuolar ATPase motor reveals its mechanical and regulatory complexity. J. Mol. Biol. 2009, 386, 989–999. [Google Scholar] [CrossRef]
- Ostrov, D.A.; Magis, A.T.; Wronski, T.J.; Chan, E.K.; Toro, E.J.; Donatelli, R.E.; Sajek, K.; Haroun, I.N.; Nagib, M.I.; Piedrahita, A.; et al. Identification of enoxacin as an inhibitor of osteoclast formation and bone resorption by structure-based virtual screening. J. Med. Chem. 2009, 52, 5144–5151. [Google Scholar] [CrossRef] [PubMed]
- Herczegh, P.; Buxton, T.B.; McPherson, J.C., III; Kovacs-Kulyassa, A.; Brewer, P.D.; Sztaricskai, F.; Stroebel, G.G.; Plowman, K.M.; Farcasiu, D.; Hartmann, J.F. Osteoadsorptive bisphosphonate derivatives of fluoroquinolone antibacterials. J. Med. Chem. 2002, 45, 2338–2341. [Google Scholar] [CrossRef] [PubMed]
- Toro, E.J.; Zuo, J.; Guiterrez, A.; La Rosa, R.L.; Gawron, A.J.; Bradaschia-Correa, V.; Arana-Chavez, V.; Dolce, C.; Rivera, M.F.; Kesavalu, L.; et al. Bis-enoxacin Inhibits Bone Resorption and Orthodontic Tooth Movement. J. Dent. Res. 2013, 92, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.F.; Chukkapalli, S.S.; Velsko, I.M.; Lee, J.Y.; Bhattacharyya, I.; Dolce, C.; Toro, E.J.; Holliday, L.S.; Kesavalu, L. Bis-enoxacin blocks rat alveolar bone resorption from experimental periodontitis. PLoS ONE 2014, 9, e92119. [Google Scholar] [CrossRef] [PubMed]
- Oktay, S.; Chukkapalli, S.S.; Rivera-Kweh, M.F.; Velsko, I.M.; Holliday, L.S.; Kesavalu, L. Periodontitis in rats induces systemic oxidative stress that is controlled by bone-targeted antiresorptives. J. Periodontol. 2015, 86, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qu, X.; Nie, T.; Zhai, Z.; Li, H.; Ouyang, Z.; Qin, A.; Zhang, S.; Zhang, S.; Fan, Q.; et al. The Beneficial Effects of Bisphosphonate-enoxacin on Cortical Bone Mass and Strength in Ovariectomized Rats. Front. Pharmacol. 2017, 8, 355. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Liu, X.; Mao, C.; Qin, A.; Lu, E. Bisenoxacin blocks alveolar bone resorption in rats with ovariectomyinduced osteoporosis. Mol. Med. Rep. 2018, 17, 3232–3238. [Google Scholar] [PubMed]
- Karsdal, M.A.; Martin, T.J.; Bollerslev, J.; Christiansen, C.; Henriksen, K. Are nonresorbing osteoclasts sources of bone anabolic activity? J. Bone Miner. Res. 2007, 22, 487–494. [Google Scholar] [CrossRef]
- Gluck, S.L.; Underhill, D.M.; Iyori, M.; Holliday, L.S.; Kostrominova, T.Y.; Lee, B.S. Physiology and biochemistry of the kidney vacuolar H+-ATPase. Annu. Rev. Physiol. 1996, 58, 427–445. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qu, X.; Wu, C.; Zhai, Z.; Tian, B.; Li, H.; Ouyang, Z.; Xu, X.; Wang, W.; Fan, Q.; et al. The effect of enoxacin on osteoclastogenesis and reduction of titanium particle-induced osteolysis via suppression of JNK signaling pathway. Biomaterials 2014, 35, 5721–5730. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, G.; Zuo, J.; Holliday, L.S. Specialized Roles for Actin in Osteoclasts: Unanswered Questions and Therapeutic Opportunities. Biomolecules 2019, 9, 17. https://doi.org/10.3390/biom9010017
Han G, Zuo J, Holliday LS. Specialized Roles for Actin in Osteoclasts: Unanswered Questions and Therapeutic Opportunities. Biomolecules. 2019; 9(1):17. https://doi.org/10.3390/biom9010017
Chicago/Turabian StyleHan, Guanghong, Jian Zuo, and Lexie Shannon Holliday. 2019. "Specialized Roles for Actin in Osteoclasts: Unanswered Questions and Therapeutic Opportunities" Biomolecules 9, no. 1: 17. https://doi.org/10.3390/biom9010017
APA StyleHan, G., Zuo, J., & Holliday, L. S. (2019). Specialized Roles for Actin in Osteoclasts: Unanswered Questions and Therapeutic Opportunities. Biomolecules, 9(1), 17. https://doi.org/10.3390/biom9010017




