Giantin Is Required for Post-Alcohol Recovery of Golgi in Liver Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Cell Culture, Ethanol Administration, and Isolation of Rat Hepatocytes
2.3. Human Liver Tissues
2.4. Immunoprecipitation and Transfection
2.5. Confocal Immunofluorescence Microscopy
2.6. Plasma Membrane Protein Isolation and Glycan Assessment
2.7. Statistical Analysis
2.8. Miscellaneous
3. Results
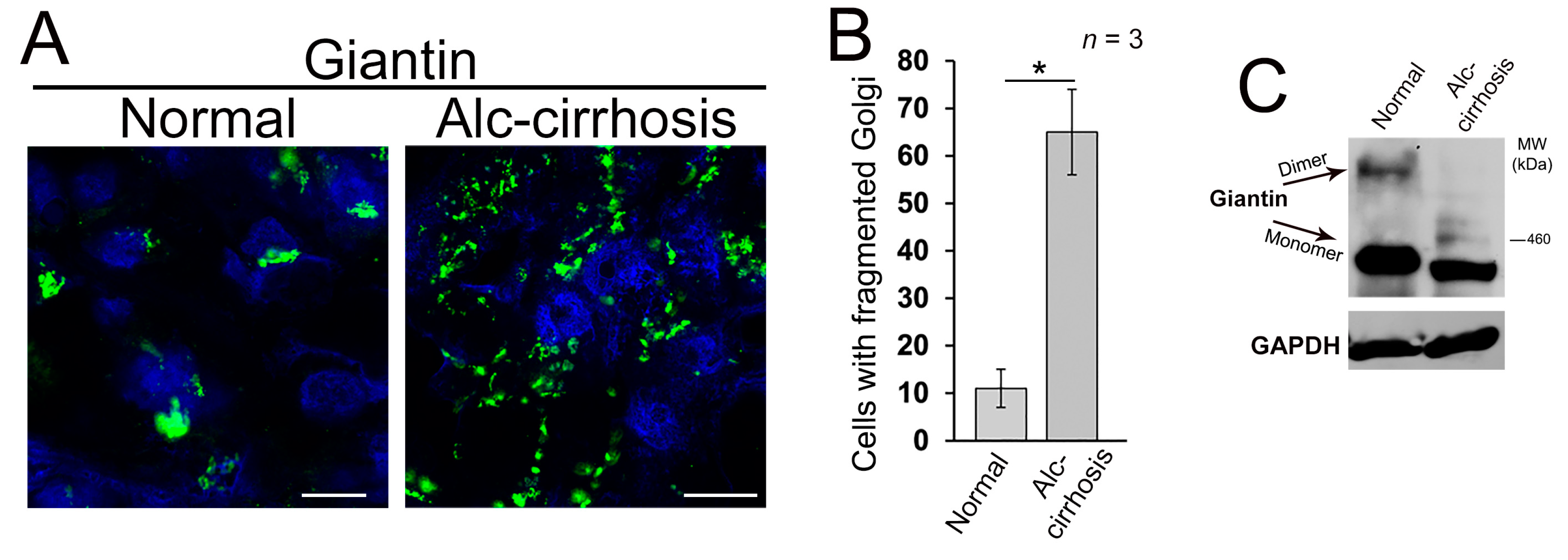
3.1. Structure of Golgi and Giantin in Patients with Alcoholic Liver Cirrhosis
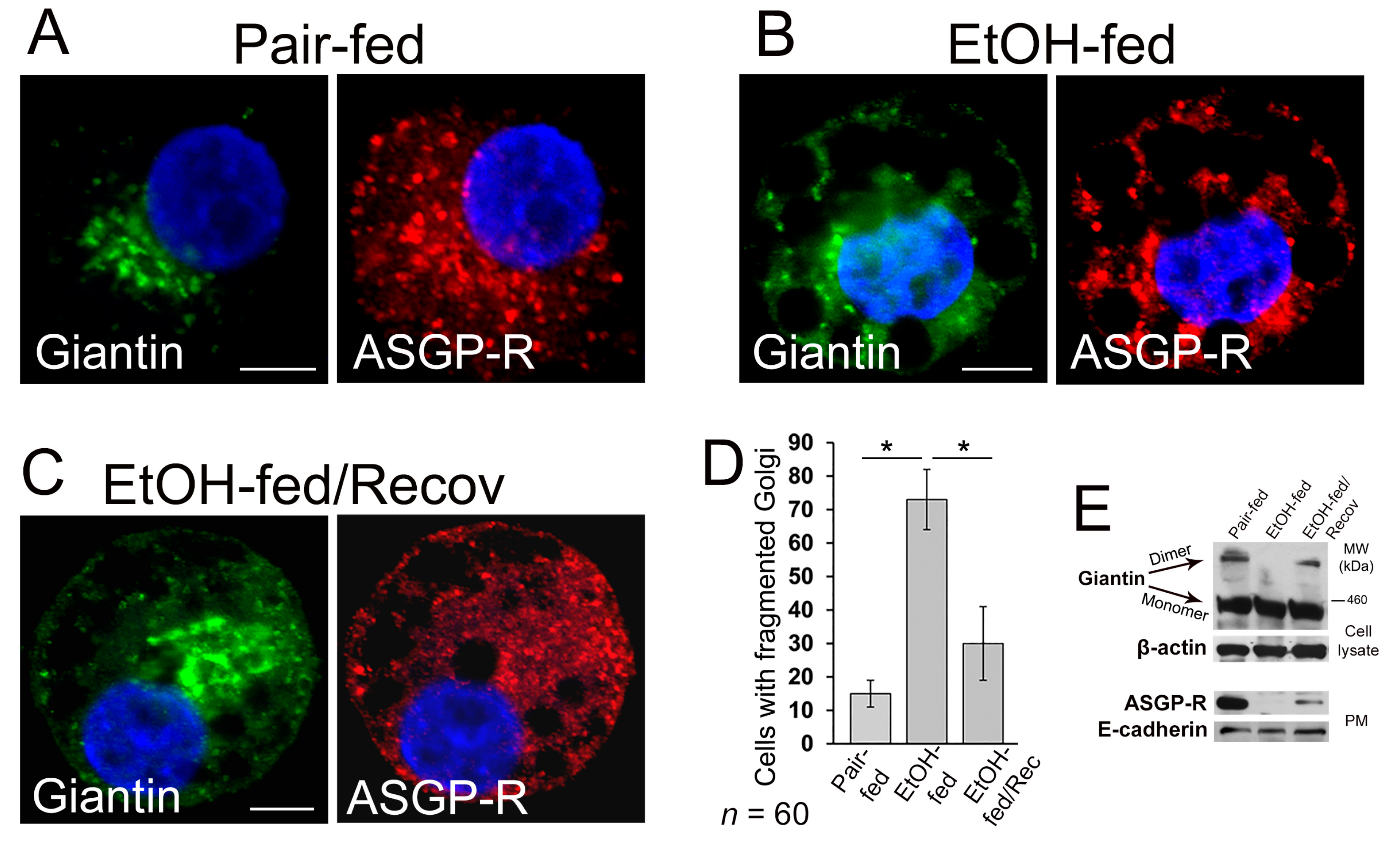
3.2. Post-Alcohol Golgi Recovery in the Rat Model
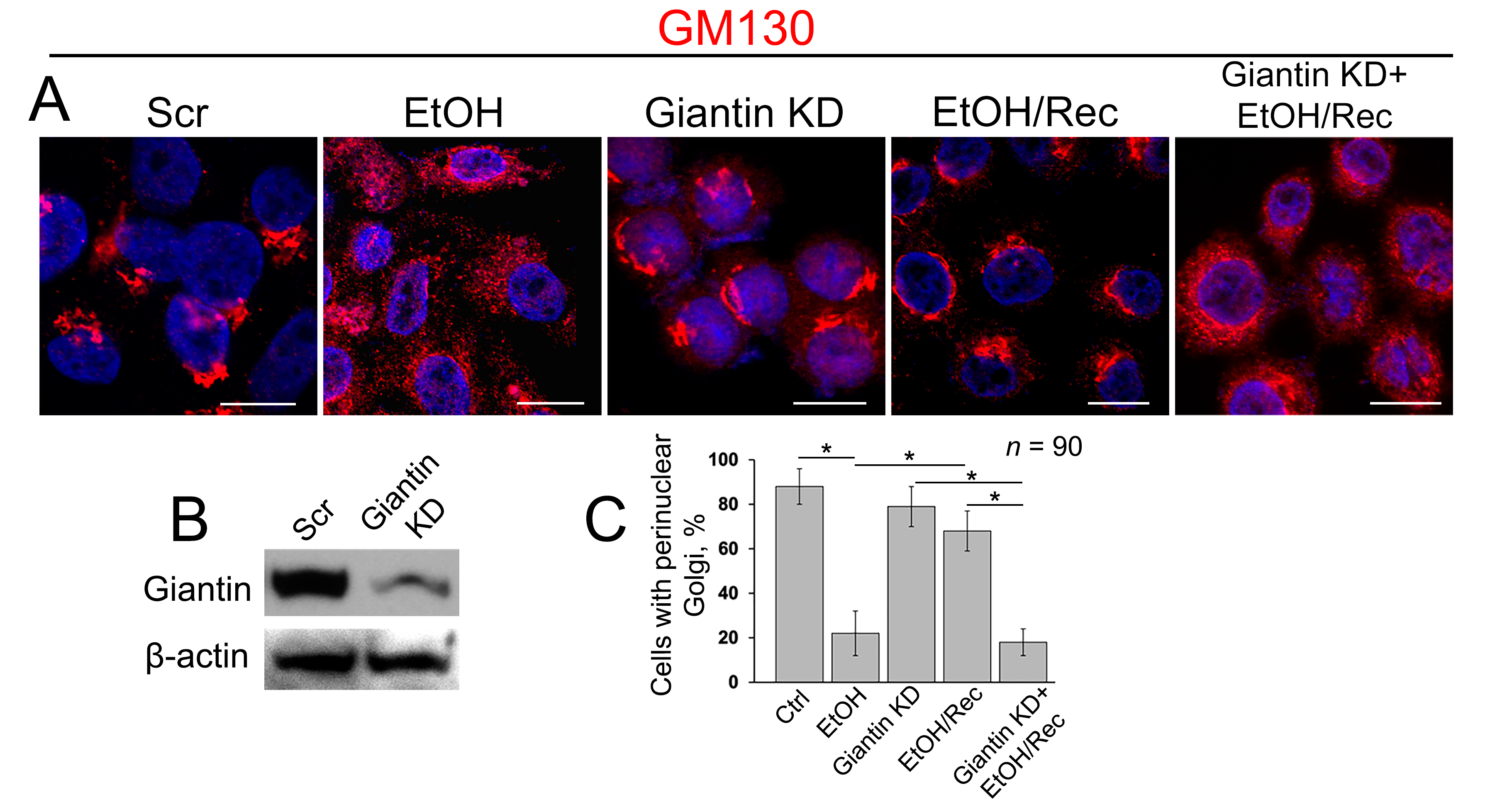
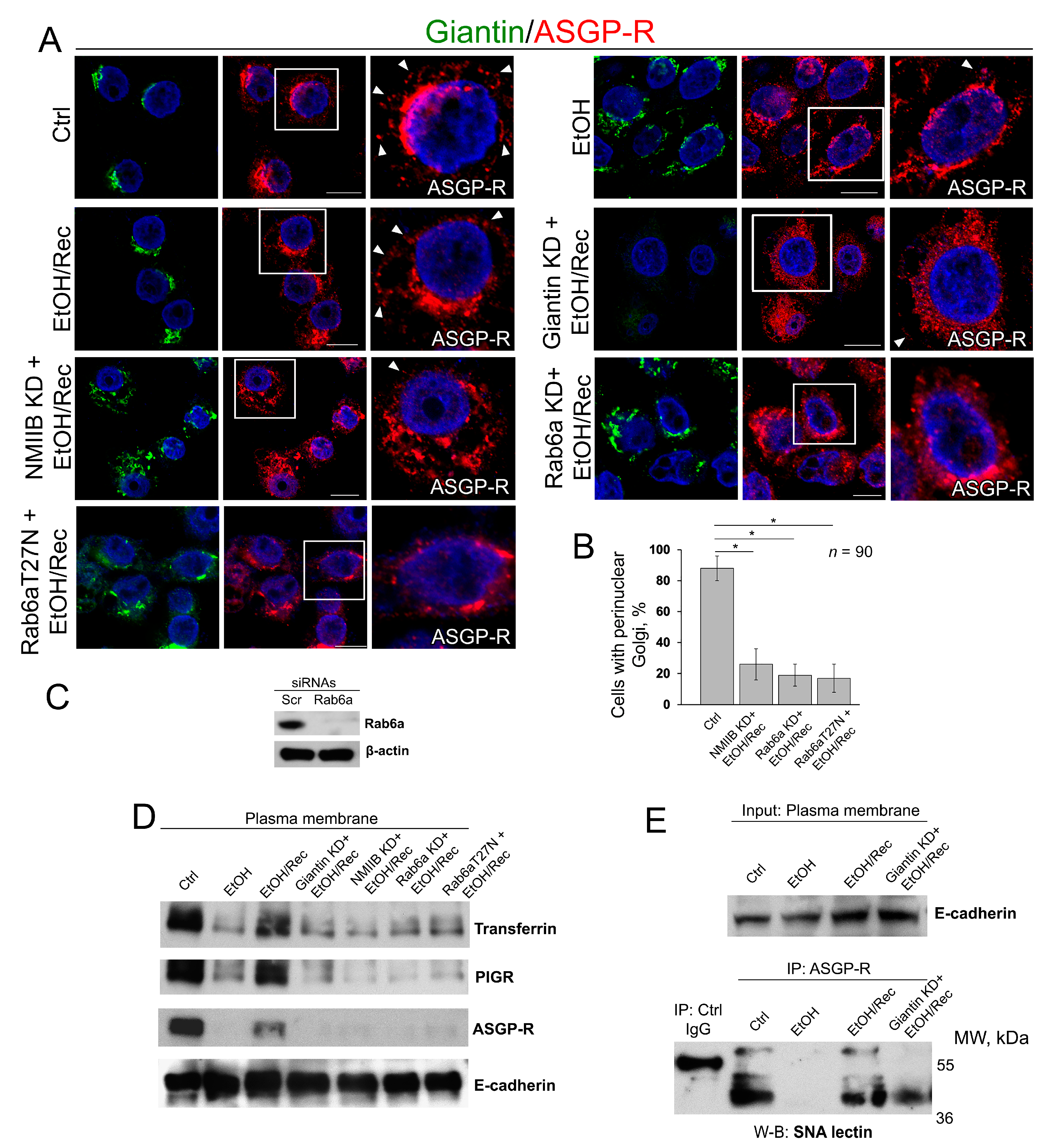
3.3. Post-Ethanol Recovery of Golgi in Giantin-Depletes Cells
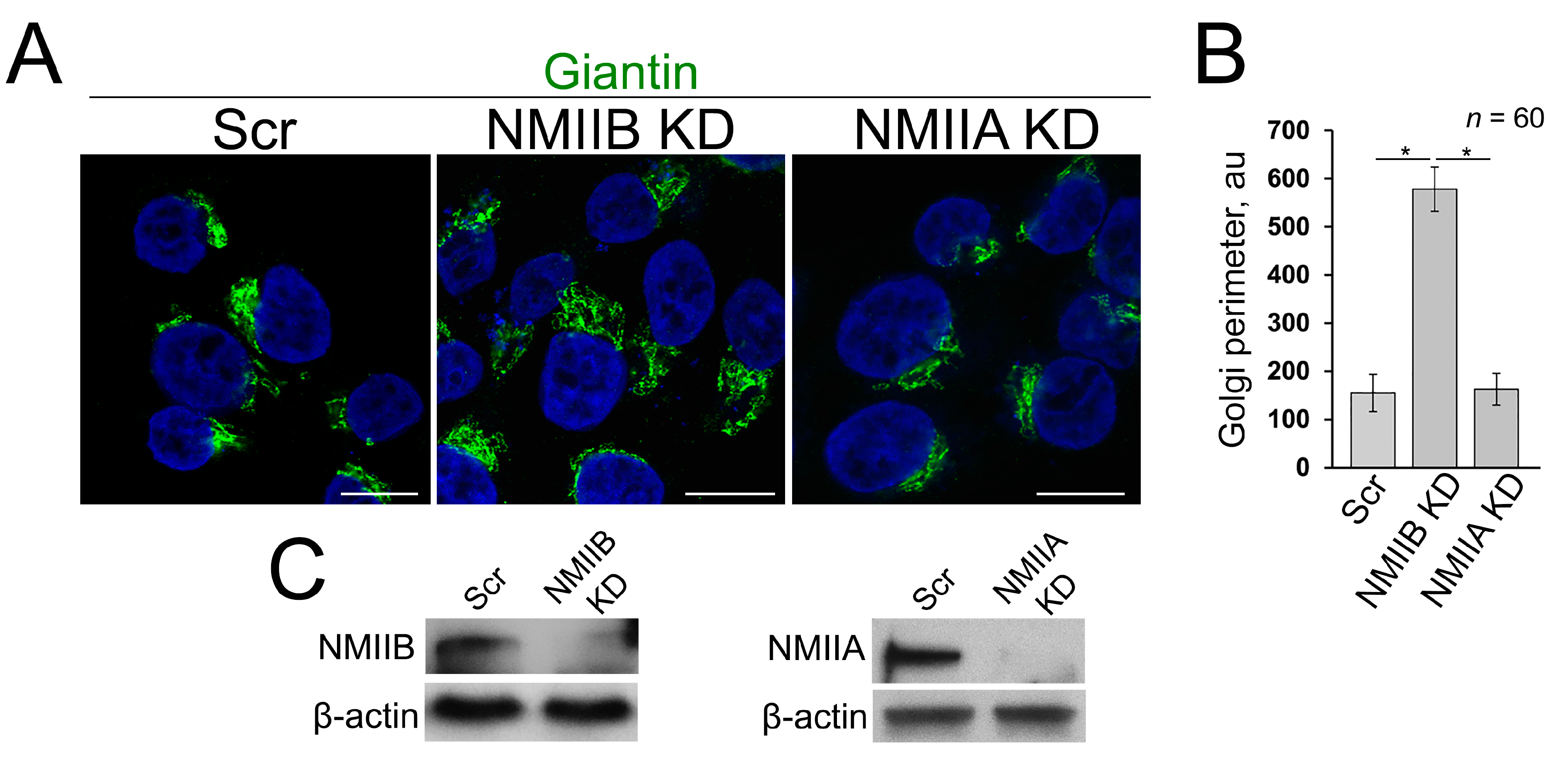
3.4. Post-Alcohol Recovery of Golgi and Trafficking of Hepatic Proteins Requires Non-Muscle Myosin IIB and Rab6a
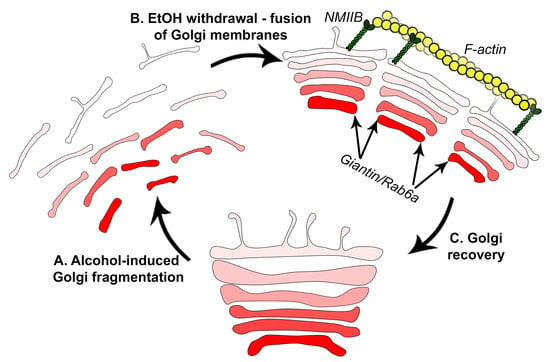
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nakamura, N.; Wei, J.H.; Seemann, J. Modular organization of the mammalian golgi apparatus. Curr. Opin. Cell Biol. 2012, 24, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Zaal, K.J.; Smith, C.L.; Polishchuk, R.S.; Altan, N.; Cole, N.B.; Ellenberg, J.; Hirschberg, K.; Presley, J.F.; Roberts, T.H.; Siggia, E.; et al. Golgi membranes are absorbed into and reemerge from the ER during mitosis. Cell 1999, 99, 589–601. [Google Scholar] [CrossRef]
- Petrosyan, A.; Cheng, P.W. Golgi fragmentation induced by heat shock or inhibition of heat shock proteins is mediated by non-muscle myosin IIA via its interaction with glycosyltransferases. Cell Stress Chaperones 2014, 19, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Pavelka, M.; Ellinger, A. Effect of colchicine on the Golgi complex of rat pancreatic acinar cells. J. Cell Biol. 1983, 97, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Rogalski, A.A.; Bergmann, J.E.; Singer, S.J. Effect of microtubule assembly status on the intracellular processing and surface expression of an integral protein of the plasma membrane. J. Cell Biol. 1984, 99, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Klausner, R.D.; Donaldson, J.G.; Lippincott-Schwartz, J. Brefeldin a: Insights into the control of membrane traffic and organelle structure. J. Cell Biol. 1992, 116, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.M.; Renau-Piqueras, J.; Marin, M.P.; Esteban-Pretel, G. Chronic alcohol exposure affects the cell components involved in membrane traffic in neuronal dendrites. Neurotox. Res. 2015, 27, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Renau-Piqueras, J.; Miragall, F.; Guerri, C.; Baguena-Cervellera, R. Prenatal exposure to alcohol alters the Golgi apparatus of newborn rat hepatocytes: A cytochemical study. J. Histochem. Cytochem. 1987, 35, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, H. Hepatotoxic Effects of Ethanol, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999. [Google Scholar]
- Ji, C. Dissecting the role of disturbed ER-Golgi trafficking in antivirals and alcohol abuse-induced pathogenesis of liver disorders. J. Drug Abuse 2017, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Mozo, L.; Simo, A.; Suarez, A.; Rodrigo, L.; Gutierrez, C. Autoantibodies to Golgi proteins in hepatocellular carcinoma: Case report and literature review. Eur. J. Gastroenterol. Hepatol. 2002, 14, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Sonnichsen, B.; Lowe, M.; Levine, T.; Jamsa, E.; Dirac-Svejstrup, B.; Warren, G. A role for giantin in docking copi vesicles to Golgi membranes. J. Cell Biol. 1998, 140, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Linstedt, A.D.; Hauri, H.P. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kda. Mol. Biol. Cell 1993, 4, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Linstedt, A.D.; Foguet, M.; Renz, M.; Seelig, H.P.; Glick, B.S.; Hauri, H.P. A c-terminally-anchored Golgi protein is inserted into the endoplasmic reticulum and then transported to the Golgi apparatus. Proc. Natl. Acad. Sci. USA 1995, 92, 5102–5105. [Google Scholar] [CrossRef] [PubMed]
- Petrosyan, A.; Holzapfel, M.S.; Muirhead, D.E.; Cheng, P.W. Restoration of compact Golgi morphology in advanced prostate cancer enhances susceptibility to galectin-1-induced apoptosis by modifying mucin o-glycan synthesis. Mol. Cancer Res. 2014, 12, 1704–1716. [Google Scholar] [CrossRef] [PubMed]
- Petrosyan, A.; Cheng, P.W.; Clemens, D.L.; Casey, C.A. Downregulation of the small gtpase sar1a: A key event underlying alcohol-induced Golgi fragmentation in hepatocytes. Sci. Rep. 2015, 5, 17127. [Google Scholar] [CrossRef] [PubMed]
- Koreishi, M.; Gniadek, T.J.; Yu, S.; Masuda, J.; Honjo, Y.; Satoh, A. The golgin tether giantin regulates the secretory pathway by controlling stack organization within Golgi apparatus. PLoS ONE 2013, 8, e59821. [Google Scholar] [CrossRef] [PubMed]
- Asante, D.; Maccarthy-Morrogh, L.; Townley, A.K.; Weiss, M.A.; Katayama, K.; Palmer, K.J.; Suzuki, H.; Westlake, C.J.; Stephens, D.J. A role for the Golgi matrix protein giantin in ciliogenesis through control of the localization of dynein-2. J. Cell Sci. 2013, 126, 5189–5197. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Takada, A.; Takase, S.; Sato, H. Accumulation of glycoprotein in the Golgi apparatus of hepatocytes in alcoholic liver injuries. Am. J. Gastroenterol. 1991, 86, 854–860. [Google Scholar] [PubMed]
- Guasch, R.; Renau-Piqueras, J.; Guerri, C. Chronic ethanol consumption induces accumulation of proteins in the liver Golgi apparatus and decreases galactosyltransferase activity. Alcohol. Clin. Exp. Res. 1992, 16, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Cottalasso, D.; Gazzo, P.; Dapino, D.; Domenicotti, C.; Pronzato, M.A.; Traverso, N.; Bellocchio, A.; Nanni, G.; Marinari, U.M. Effect of chronic ethanol consumption on glycosylation processes in rat liver microsomes and Golgi apparatus. Alcohol. Alcohol. 1996, 31, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Casey, C.A.; Bhat, G.; Holzapfel, M.S.; Petrosyan, A. Study of ethanol-induced Golgi disorganization reveals the potential mechanism of alcohol-impaired n-glycosylation. Alcohol. Clin. Exp. Res. 2016, 40, 2573–2590. [Google Scholar] [CrossRef] [PubMed]
- Ktistakis, N.T.; Brown, H.A.; Waters, M.G.; Sternweis, P.C.; Roth, M.G. Evidence that phospholipase d mediates adp ribosylation factor-dependent formation of Golgi coated vesicles. J. Cell Biol. 1996, 134, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Petrosyan, A.; Ali, M.F.; Cheng, P.W. Glycosyltransferase-specific Golgi-targeting mechanisms. J. Biol. Chem. 2012, 287, 37621–37627. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, N.L.; Bergen, D.J.M.; Skinner, R.E.H.; Kague, E.; Martin-Silverstone, E.; Robson Brown, K.A.; Hammond, C.L.; Stephens, D.J. Giantin-knockout models reveal a feedback loop between Golgi function and glycosyltransferase expression. J. Cell Sci. 2017, 130, 4132–4143. [Google Scholar] [CrossRef] [PubMed]
- Manca, S.; Frisbie, C.P.; LaGrange, C.A.; Casey, C.A.; Riethoven, J.M.; Petrosyan, A. The role of alcohol-induced Golgi fragmentation for androgen receptor signaling in prostate cancer. Mol. Cancer Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Solomons, H.D. Carbohydrate deficient transferrin and alcoholism. Germs 2012, 2, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Guirguis, J.; Chhatwal, J.; Dasarathy, J.; Rivas, J.; McMichael, D.; Nagy, L.E.; McCullough, A.J.; Dasarathy, S. Clinical impact of alcohol-related cirrhosis in the next decade: Estimates based on current epidemiological trends in the united states. Alcohol. Clin. Exp. Res. 2015, 39, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Pessione, F.; Ramond, M.J.; Peters, L.; Pham, B.N.; Batel, P.; Rueff, B.; Valla, D.C. Five-year survival predictive factors in patients with excessive alcohol intake and cirrhosis. Effect of alcoholic hepatitis, smoking and abstinence. Liver Int. 2003, 23, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Borowsky, S.A.; Strome, S.; Lott, E. Continued heavy drinking and survival in alcoholic cirrhotics. Gastroenterology 1981, 80, 1405–1409. [Google Scholar] [PubMed]
- Luca, A.; Garcia-Pagan, J.C.; Bosch, J.; Feu, F.; Caballeria, J.; Groszmann, R.J.; Rodes, J. Effects of ethanol consumption on hepatic hemodynamics in patients with alcoholic cirrhosis. Gastroenterology 1997, 112, 1284–1289. [Google Scholar] [CrossRef]
- Kamper-Jorgensen, M.; Gronbaek, M.; Tolstrup, J.; Becker, U. Alcohol and cirrhosis: Dose--response or threshold effect? J. Hepatol. 2004, 41, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Koch, O.R.; Roatta de Conti, L.L.; Bolanos, L.P.; Stoppani, A.O. Ultrastructural and biochemical aspects of liver mitochondria during recovery from ethanol-induced alterations. Experimental evidence of mitochondrial division. Am. J. Pathol. 1978, 90, 325–344. [Google Scholar] [PubMed]
- Kravos, M.; Malesic, I. Kinetics and isoforms of serum glutamate dehydrogenase in alcoholics. Alcohol. Alcohol. 2008, 43, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Casey, C.A.; Kragskow, S.L.; Sorrell, M.F.; Tuma, D.J. Ethanol-induced impairments in receptor-mediated endocytosis of asialoorosomucoid in isolated rat hepatocytes: Time course of impairments and recovery after ethanol withdrawal. Alcohol. Clin. Exp. Res. 1989, 13, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Misteli, T. The concept of self-organization in cellular architecture. J. Cell Biol. 2001, 155, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Lippincott-Schwartz, J.; Yuan, L.C.; Bonifacino, J.S.; Klausner, R.D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin a: Evidence for membrane cycling from Golgi to ER. Cell 1989, 56, 801–813. [Google Scholar] [CrossRef]
- Berger, E.G.; Grimm, K.; Bachi, T.; Bosshart, H.; Kleene, R.; Watzele, M. Double immunofluorescent staining of alpha 2,6 sialyltransferase and beta 1,4 galactosyltransferase in monensin-treated cells: Evidence for different Golgi compartments? J. Cell. Biochem. 1993, 52, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, D.A.; Siddhanta, A.; Shields, D. Fragmentation and re-assembly of the Golgi apparatus in vitro. A requirement for phosphatidic acid and phosphatidylinositol 4,5-bisphosphate synthesis. J. Biol. Chem. 2002, 277, 3030–3039. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, Y.; Alieva, N.O.; Miserey-Lenkei, S.; Lichtenstein, A.; Kam, Z.; Sabanay, H.; Bershadsky, A. Involvement of the rho-mdia1 pathway in the regulation of Golgi complex architecture and dynamics. Mol. Biol. Cell 2011, 22, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Clemens, D.L.; Forman, A.; Jerrells, T.R.; Sorrell, M.F.; Tuma, D.J. Relationship between acetaldehyde levels and cell survival in ethanol-metabolizing hepatoma cells. Hepatology 2002, 35, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S.; DeCarli, L.M. The feeding of alcohol in liquid diets: Two decades of applications and 1982 update. Alcohol. Clin. Exp. Res. 1982, 6, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Casey, C.A.; Kragskow, S.L.; Sorrell, M.F.; Tuma, D.J. Chronic ethanol administration impairs the binding and endocytosis of asialo-orosomucoid in isolated hepatocytes. J. Biol. Chem. 1987, 262, 2704–2710. [Google Scholar] [PubMed]
- Tworek, B.L.; Tuma, D.J.; Casey, C.A. Decreased binding of asialoglycoproteins to hepatocytes from ethanol-fed rats. Consequence of both impaired synthesis and inactivation of the asialoglycoprotein receptor. J. Biol. Chem. 1996, 271, 2531–2538. [Google Scholar] [CrossRef] [PubMed]
- Schaffert, C.S.; Sorrell, M.F.; Tuma, D.J. Expression and cytoskeletal association of integrin subunits is selectively increased in rat perivenous hepatocytes after chronic ethanol administration. Alcohol. Clin. Exp. Res. 2001, 25, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Thomes, P.G.; Trambly, C.S.; Thiele, G.M.; Duryee, M.J.; Fox, H.S.; Haorah, J.; Donohue, T.M., Jr. Proteasome activity and autophagosome content in liver are reciprocally regulated by ethanol treatment. Biochem. Biophys. Res. Commun. 2012, 417, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Dupre, D.J.; Robitaille, M.; Ethier, N.; Villeneuve, L.R.; Mamarbachi, A.M.; Hebert, T.E. Seven transmembrane receptor core signaling complexes are assembled prior to plasma membrane trafficking. J. Biol. Chem. 2006, 281, 34561–34573. [Google Scholar] [CrossRef] [PubMed]
- Petrosyan, A.; Cheng, P.W. A non-enzymatic function of Golgi glycosyltransferases: Mediation of Golgi fragmentation by interaction with non-muscle myosin IIA. Glycobiology 2013, 23, 690–708. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, M.F.; Casey, C.A.; Tuma, D.J. Recovery of ethanol-induced impairments in receptor-mediated endocytosis of asialoorosomucoid in isolated rat hepatocytes. Trans. Am. Clin. Climatol. Assoc. 1989, 100, 163–170. [Google Scholar] [PubMed]
- Casey, C. The Clinical Laboratory Analysis of the Serum Samples; UNMC: Omaha, NE, USA, 2018. [Google Scholar]
- Petrosyan, A.; Casey, C.A.; Cheng, P.W. The role of rab6a and phosphorylation of non-muscle myosin IIA tailpiece in alcohol-induced Golgi disorganization. Sci. Rep. 2016, 6, 31962. [Google Scholar] [CrossRef] [PubMed]
- Mochida, S.; Kobayashi, H.; Matsuda, Y.; Yuda, Y.; Muramoto, K.; Nonomura, Y. Myosin ii is involved in transmitter release at synapses formed between rat sympathetic neurons in culture. Neuron 1994, 13, 1131–1142. [Google Scholar] [CrossRef]
- Togo, T.; Steinhardt, R.A. Nonmuscle myosin IIA and IIB have distinct functions in the exocytosis-dependent process of cell membrane repair. Mol. Biol. Cell 2004, 15, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Kelley, C.A.; Sellers, J.R.; Gard, D.L.; Bui, D.; Adelstein, R.S.; Baines, I.C. Xenopus nonmuscle myosin heavy chain isoforms have different subcellular localizations and enzymatic activities. J. Cell Biol. 1996, 134, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Maupin, P.; Phillips, C.L.; Adelstein, R.S.; Pollard, T.D. Differential localization of myosin-ii isozymes in human cultured cells and blood cells. J. Cell Sci. 1994, 107, 3077–3090. [Google Scholar] [PubMed]
- Heimann, K.; Percival, J.M.; Weinberger, R.; Gunning, P.; Stow, J.L. Specific isoforms of actin-binding proteins on distinct populations of Golgi-derived vesicles. J. Biol. Chem. 1999, 274, 10743–10750. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Kovacs, M.; Hu, A.; Limouze, J.; Harvey, E.V.; Sellers, J.R. Kinetic mechanism of non-muscle myosin IIB: Functional adaptations for tension generation and maintenance. J. Biol. Chem. 2003, 278, 27439–27448. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, M.; Wang, F.; Hu, A.; Zhang, Y.; Sellers, J.R. Functional divergence of human cytoplasmic myosin ii: Kinetic characterization of the non-muscle IIA isoform. J. Biol. Chem. 2003, 278, 38132–38140. [Google Scholar] [CrossRef] [PubMed]
- Sandquist, J.C.; Bement, W.M. Hold on tightly, let go lightly: Myosin functions at adherens junctions. Nat. Cell Biol. 2010, 12, 633–635. [Google Scholar] [CrossRef] [PubMed]
- Norstrom, M.F.; Smithback, P.A.; Rock, R.S. Unconventional processive mechanics of non-muscle myosin IIB. J. Biol. Chem. 2010, 285, 26326–26334. [Google Scholar] [CrossRef] [PubMed]
- Petrosyan, A. Confocal Immunofluorescence Analysis; UNMC: Omaha, NE, USA, 2018. [Google Scholar]
- Cummings, R.D.; Etzler, M.E. Antibodies and lectins in glycan analysis. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor: Laurel Hollow, NY, USA, 2009. [Google Scholar]
- Schwartz, A.L. Trafficking of asialoglycoproteins and the asialoglycoprotein receptor. Targeted Diagn. Ther. 1991, 4, 3–39. [Google Scholar] [PubMed]
- Welti, M.; Hulsmeier, A.J. Ethanol-induced impairment in the biosynthesis of n-linked glycosylation. J. Cell. Biochem. 2014, 115, 754–762. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Johannes, L.; Mallard, F.; Girod, A.; Grill, S.; Reinsch, S.; Keller, P.; Tzschaschel, B.; Echard, A.; Goud, B.; et al. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J. Cell Biol. 1999, 147, 743–760. [Google Scholar] [CrossRef] [PubMed]
- Miserey-Lenkei, S.; Chalancon, G.; Bardin, S.; Formstecher, E.; Goud, B.; Echard, A. Rab and actomyosin-dependent fission of transport vesicles at the Golgi complex. Nat. Cell Biol. 2010, 12, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Petrosyan, A.; Manca, S.; Casey, C.; Thomes, P.; Riethoven, Je.; Clarke, J. Golgi Renaissance: the pivotal role of the largest Golgi protein giantin. Sci. Rep. 2018. submitted. [Google Scholar]
- Puthenveedu, M.A.; Bachert, C.; Puri, S.; Lanni, F.; Linstedt, A.D. Gm130 and grasp65-dependent lateral cisternal fusion allows uniform Golgi-enzyme distribution. Nat. Cell Biol. 2006, 8, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Marra, P.; Salvatore, L.; Mironov, A., Jr.; Di Campli, A.; Di Tullio, G.; Trucco, A.; Beznoussenko, G.; Mironov, A.; De Matteis, M.A. The biogenesis of the Golgi ribbon: The roles of membrane input from the ER and of gm130. Mol. Biol. Cell 2007, 18, 1595–1608. [Google Scholar] [CrossRef] [PubMed]
- Saheki, Y.; De Camilli, P. Endoplasmic reticulum-plasma membrane contact sites. Annu. Rev. Biochem. 2017, 86, 659–684. [Google Scholar] [CrossRef] [PubMed]
- Suski, J.M.; Lebiedzinska, M.; Wojtala, A.; Duszynski, J.; Giorgi, C.; Pinton, P.; Wieckowski, M.R. Isolation of plasma membrane-associated membranes from rat liver. Nat. Protoc. 2014, 9, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Ji, C. Advances and new concepts in alcohol-induced organelle stress, unfolded protein responses and organ damage. Biomolecules 2015, 5, 1099–1121. [Google Scholar] [CrossRef] [PubMed]
- Duran, J.M.; Valderrama, F.; Castel, S.; Magdalena, J.; Tomas, M.; Hosoya, H.; Renau-Piqueras, J.; Malhotra, V.; Egea, G. Myosin motors and not actin comets are mediators of the actin-based Golgi-to-endoplasmic reticulum protein transport. Mol. Biol. Cell 2003, 14, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Petrosyan, A.; Ali, M.F.; Verma, S.K.; Cheng, H.; Cheng, P.W. Non-muscle myosin IIA transports a Golgi glycosyltransferase to the endoplasmic reticulum by binding to its cytoplasmic tail. Int. J. Biochem. Cell Biol. 2012, 44, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Pavlos, N.J.; Xu, J.; Riedel, D.; Yeoh, J.S.; Teitelbaum, S.L.; Papadimitriou, J.M.; Jahn, R.; Ross, F.P.; Zheng, M.H. Rab3d regulates a novel vesicular trafficking pathway that is required for osteoclastic bone resorption. Mol. Cell Biol. 2005, 25, 5253–5269. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.M.; Cefaratti, C.; Berti-Mattera, L.; Romani, A. Delayed restoration of mg2+ content and transport in liver cells following ethanol withdrawal. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G621–G631. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Nahon, P.; Rausch, V.; Peccerella, T.; Silva, I.; Yagmur, E.; Straub, B.K.; Lackner, C.; Seitz, H.K.; Rufat, P.; et al. Caspase-cleaved keratin-18 fragments increase during alcohol withdrawal and predict liver-related death in patients with alcoholic liver disease. Hepatology 2017, 66, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Roth, N.C.; Saberi, B.; Macklin, J.; Kanel, G.; French, S.W.; Govindarajan, S.; Buzzanco, A.S.; Stolz, A.A.; Donovan, J.A.; Kaplowitz, N. Prediction of histologic alcoholic hepatitis based on clinical presentation limits the need for liver biopsy. Hepatol. Commun. 2017, 1, 1070–1084. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Adachi, M.; Fernandez-Checa, J.C.; Cederbaum, A.I.; Deaciuc, I.V.; Nanji, A.A. Role of apoptosis in alcoholic liver injury. Alcohol. Clin. Exp. Res. 2003, 27, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casey, C.A.; Thomes, P.; Manca, S.; Petrosyan, A. Giantin Is Required for Post-Alcohol Recovery of Golgi in Liver Cells. Biomolecules 2018, 8, 150. https://doi.org/10.3390/biom8040150
Casey CA, Thomes P, Manca S, Petrosyan A. Giantin Is Required for Post-Alcohol Recovery of Golgi in Liver Cells. Biomolecules. 2018; 8(4):150. https://doi.org/10.3390/biom8040150
Chicago/Turabian StyleCasey, Carol A., Paul Thomes, Sonia Manca, and Armen Petrosyan. 2018. "Giantin Is Required for Post-Alcohol Recovery of Golgi in Liver Cells" Biomolecules 8, no. 4: 150. https://doi.org/10.3390/biom8040150
APA StyleCasey, C. A., Thomes, P., Manca, S., & Petrosyan, A. (2018). Giantin Is Required for Post-Alcohol Recovery of Golgi in Liver Cells. Biomolecules, 8(4), 150. https://doi.org/10.3390/biom8040150