The Potential Role of the Proteases Cathepsin D and Cathepsin L in the Progression and Metastasis of Epithelial Ovarian Cancer
Abstract
:1. Introduction
2. Cathepsin D
2.1. Physiological Roles of CathD as Both AN Intracellular and Extracellular Protein
2.2. Expression of CathD in Ovarian Cancer
| Cancer Type | Metastasis | Invasion | Angiogenesis | References |
|---|---|---|---|---|
| Breast | ↑ | ↑ | ↑ | [45,46,47,48] |
| Ovarian | ND | ND | ↑ | [18] |
| Prostate | ↑ | ↑ | ↓ | [49,50,51] |
| Endometrial | ND | ↑ | ND | [58] |
| Melanocytic | ↑ | ↑ | ND | [53] |
| Glioma | ↑ | ↑ | ND | [54] |
| Lung | ND | ↑ | ND | [57] |
2.3. Role of CathD in Tumor Progression
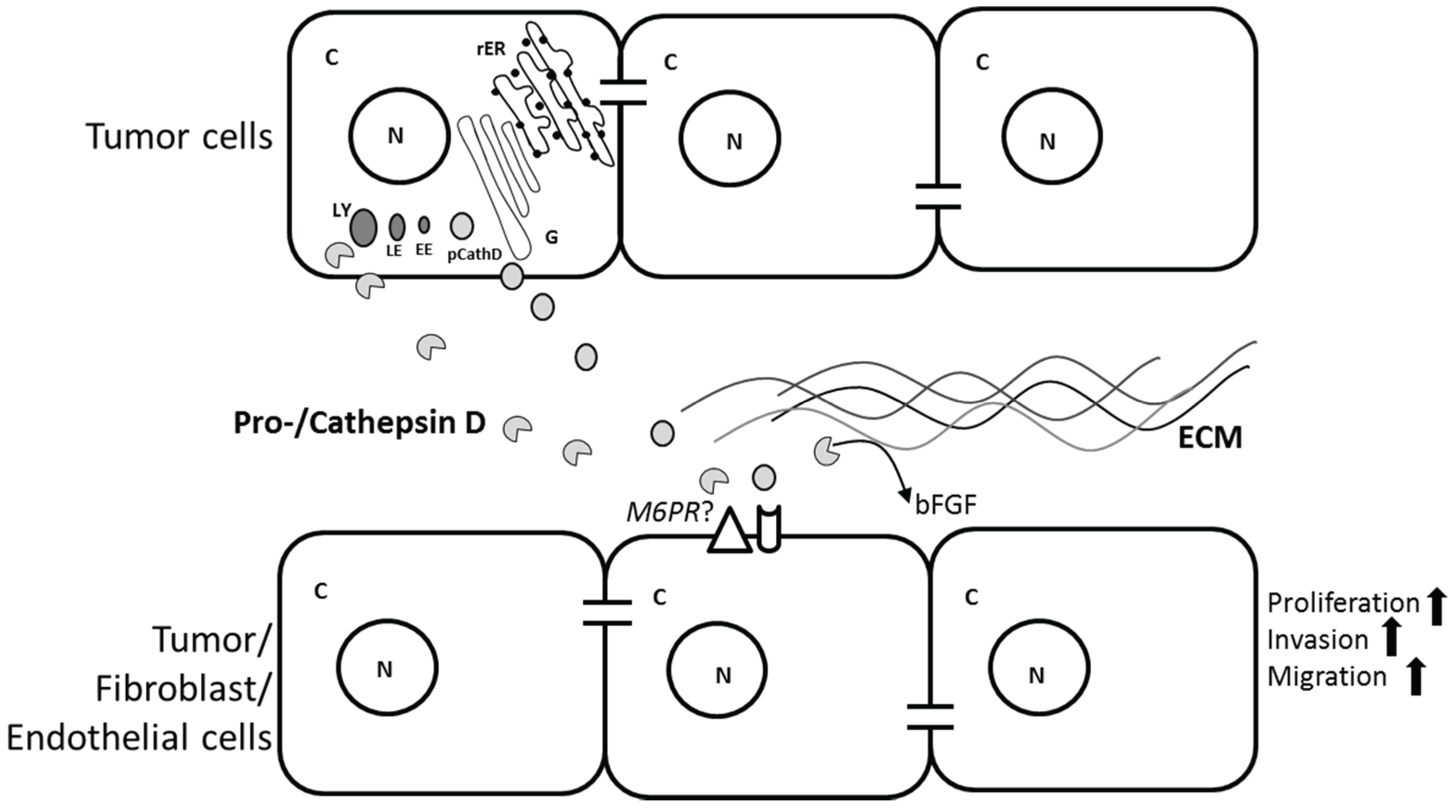
3. Cathepsin L
3.1. Physiological Roles of CathL
3.2. Cathepsin L Secretion
3.3. Expression of CathL in Ovarian Cancer
3.4. Role of CathL in Tumor Progression
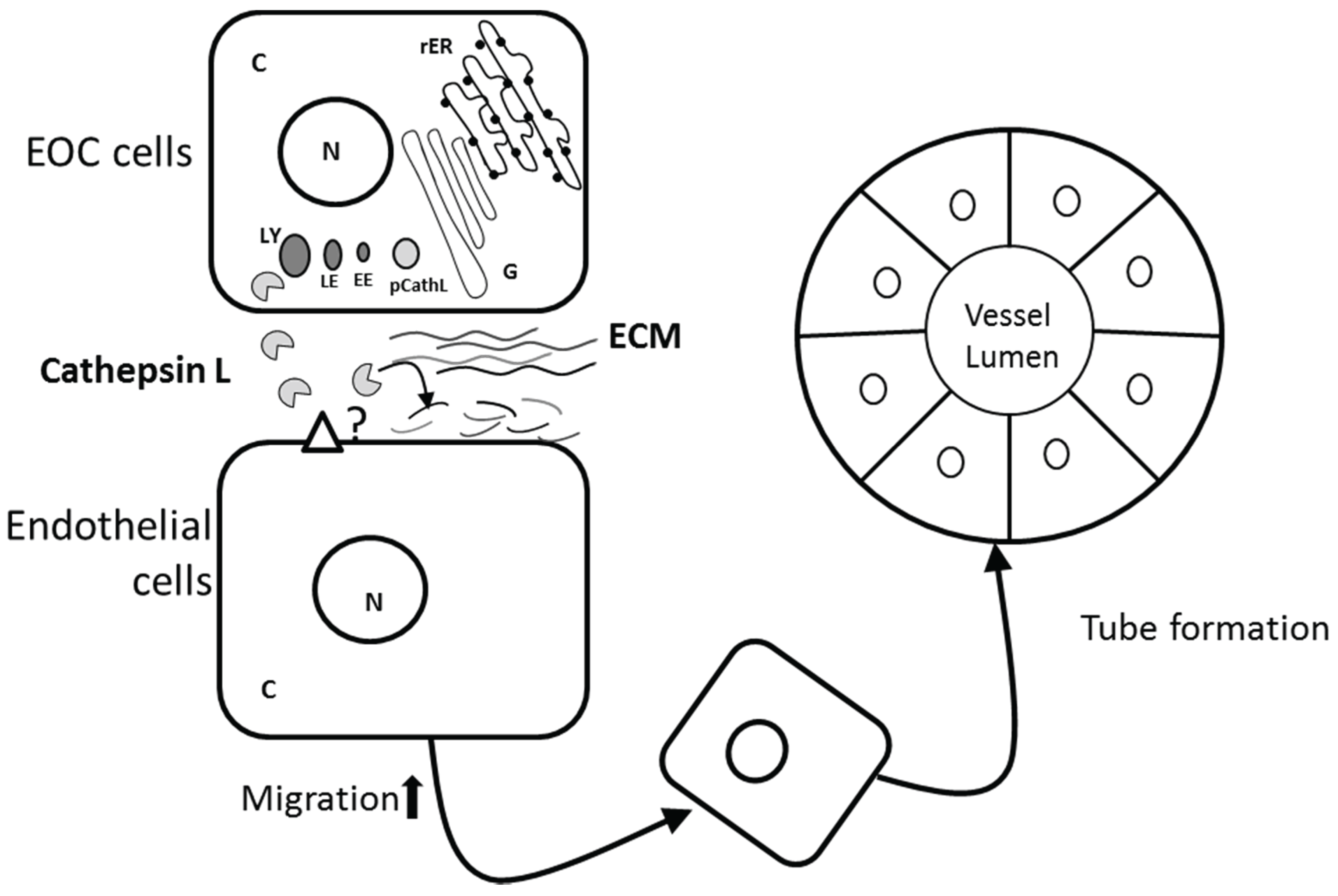
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Buy, J.N.; Moss, A.A.; Ghossain, M.A.; Sciot, C.; Malbec, L.; Vadrot, D.; Paniel, B.J.; Decroix, Y. Peritoneal implants from ovarian tumors: Ct findings. Radiology 1988, 169, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Johnatty, S.E.; Beesley, J.; Paul, J.; Fereday, S.; Spurdle, A.B.; Webb, P.M.; Byth, K.; Marsh, S.; McLeod, H.; Group, A.S.; et al. Abcb1 (Mdr 1) polymorphisms and progression-free survival among women with ovarian cancer following paclitaxel/carboplatin chemotherapy. Clin. Cancer Res. 2008, 14, 5594–5601. [Google Scholar] [CrossRef] [PubMed]
- Deraco, M.; Baratti, D.; Laterza, B.; Balestra, M.R.; Mingrone, E.; Macri, A.; Virzi, S.; Puccio, F.; Ravenda, P.S.; Kusamura, S. Advanced cytoreduction as surgical standard of care and hyperthermic intraperitoneal chemotherapy as promising treatment in epithelial ovarian cancer. Eur. J. Surg. Oncol. 2011, 37, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Thompson, E.W.; Quinn, M.A. Epithelial-mesenchymal interconversions in normal ovarian surface epithelium and ovarian carcinomas: An exception to the norm. J. Cell. Physiol. 2007, 213, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.A.; Kraut, N.; Beug, H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 2005, 17, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, U.; Christofori, G. Cell adhesion and signalling by cadherins and ig-cams in cancer. Nat. Rev. Cancer 2004, 4, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Patel, I.S.; Madan, P.; Getsios, S.; Bertrand, M.A.; MacCalman, C.D. Cadherin switching in ovarian cancer progression. Int. J. Cancer 2003, 106, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.G.; Zeineldin, R.; Stack, M.S. Phenotypic plasticity of neoplastic ovarian epithelium: Unique cadherin profiles in tumor progression. Clin. Exp. Metastasis 2008, 25, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Strobel, T.; Cannistra, S.A. Beta1-integrins partly mediate binding of ovarian cancer cells to peritoneal mesothelium in vitro. Gynecol. Oncol. 1999, 73, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Slack-Davis, J.K.; Atkins, K.A.; Harrer, C.; Hershey, E.D.; Conaway, M. Vascular cell adhesion molecule-1 is a regulator of ovarian cancer peritoneal metastasis. Cancer Res. 2009, 69, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Van der Bilt, A.R.; van der Zee, A.G.; de Vries, E.G.; de Jong, S.; Timmer-Bosscha, H.; ten Hoor, K.A.; den Dunnen, W.F.; Hollema, H.; Reyners, A.K. Multiple vegf family members are simultaneously expressed in ovarian cancer: A proposed model for bevacizumab resistance. Curr. Pharm. Des. 2012, 18, 3784–3792. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Liu, Y.; Sun, Y.; He, X. Expression of Ets-1, Ang-2 and maspin in ovarian cancer and their role in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2011, 30, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Miyamoto, S.; Suzuki, S.O.; Oki, E.; Yagi, H.; Sonoda, K.; Yamazaki, A.; Mizushima, H.; Maehara, Y.; Mekada, E.; et al. Clinical significance of heparin-binding epidermal growth factor-like growth factor and a disintegrin and metalloprotease 17 expression in human ovarian cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 4783–4792. [Google Scholar] [CrossRef] [PubMed]
- Toutirais, O.; Chartier, P.; Dubois, D.; Bouet, F.; Leveque, J.; Catros-Quemener, V.; Genetet, N. Constitutive expression of Tgf-beta1, interleukin-6 and interleukin-8 by tumor cells as a major component of immune escape in human ovarian carcinoma. Eur. Cytokine Netw. 2003, 14, 246–255. [Google Scholar] [PubMed]
- Winiarski, B.K.; Wolanska, K.I.; Rai, S.; Ahmed, T.; Acheson, N.; Gutowski, N.J.; Whatmore, J.L. Epithelial ovarian cancer-induced angiogenic phenotype of human omental microvascular endothelial cells may occur independently of vegf signaling. Transl. Oncol. 2013, 6, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Benes, P.; Vetvicka, V.; Fusek, M. Cathepsin D—Many functions of one aspartic protease. Crit. Rev. Oncol. Hematol. 2008, 68, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, M.M.; Grosch, H.W.; Locatelli-Hoops, S.; Werth, N.; Smolenova, E.; Nettersheim, M.; Sandhoff, K.; Hasilik, A. Purified recombinant human prosaposin forms oligomers that bind procathepsin D and affect its autoactivation. Biochem. J. 2004, 383, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Laurent-Matha, V.; Derocq, D.; Prebois, C.; Katunuma, N.; Liaudet-Coopman, E. Processing of human cathepsin D is independent of its catalytic function and auto-activation: Involvement of cathepsins L and B. J. Biochem. 2006, 139, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, H.; Sakaguchi, M.; Mihara, K.; Murakami, K.; Omura, T. Intracellular targeting of lysosomal cathepsin D in cos cells. J. Biochem. 1995, 118, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, Y.; Tsukuba, T.; Okamoto, K.; Kadowaki, T.; Yamamoto, K. The role of the cathepsin E propeptide in correct folding, maturation and sorting to the endosome. J. Biochem. 2005, 138, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J.; Fusek, M. Role of procathepsin D activation peptide in prostate cancer growth. Prostate 2000, 44, 1–7. [Google Scholar] [CrossRef]
- Gieselmann, V.; Hasilik, A.; von Figura, K. Processing of human cathepsin D in lysosomes in vitro. J. Biol. Chem. 1985, 260, 3215–3220. [Google Scholar] [PubMed]
- Yoshinari, M.; Taurog, A. Lysosomal digestion of thyroglobulin: Role of cathepsin D and thiol proteases. Endocrinology 1985, 117, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Kenessey, A.; Nacharaju, P.; Ko, L.W.; Yen, S.H. Degradation of tau by lysosomal enzyme cathepsin D: Implication for alzheimer neurofibrillary degeneration. J. Neurochem. 1997, 69, 2026–2038. [Google Scholar] [CrossRef] [PubMed]
- Roberg, K.; Johansson, U.; Ollinger, K. Lysosomal release of cathepsin D precedes relocation of cytochrome C and loss of mitochondrial transmembrane potential during apoptosis induced by oxidative stress. Free Radic. Biol. Med. 1999, 27, 1228–1237. [Google Scholar] [CrossRef]
- Kageyama, T.; Tatematsu, M.; Ichinose, M.; Yahagi, N.; Miki, K.; Moriyama, A.; Yonezawa, S. Development-dependent expression of cathepsins D and e in various rat tissues, with special reference to the high expression of cathepsin E in fetal liver. Zool. Sci. 1998, 15, 517–523. [Google Scholar] [PubMed]
- Tyynela, J.; Sohar, I.; Sleat, D.E.; Gin, R.M.; Donnelly, R.J.; Baumann, M.; Haltia, M.; Lobel, P. A mutation in the ovine cathepsin D gene causes a congenital lysosomal storage disease with profound neurodegeneration. EMBO J. 2000, 19, 2786–2792. [Google Scholar] [CrossRef] [PubMed]
- Tyynela, J.; Sohar, I.; Sleat, D.E.; Gin, R.M.; Donnelly, R.J.; Baumann, M.; Haltia, M.; Lobel, P. Congenital ovine neuronal ceroid lipofuscinosis—A cathepsin D deficiency with increased levels of the inactive enzyme. Eur. J. Paediatr. Neurol. 2001, 5, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Steinfeld, R.; Reinhardt, K.; Schreiber, K.; Hillebrand, M.; Kraetzner, R.; Bruck, W.; Saftig, P.; Gartner, J. Cathepsin d deficiency is associated with a human neurodegenerative disorder. Am. J. Hum. Genet. 2006, 78, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Fritchie, K.; Siintola, E.; Armao, D.; Lehesjoki, A.E.; Marino, T.; Powell, C.; Tennison, M.; Booker, J.M.; Koch, S.; Partanen, S.; et al. Novel mutation and the first prenatal screening of cathepsin D deficiency (Cln10). Acta Neuropathol. 2009, 117, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Siintola, E.; Partanen, S.; Stromme, P.; Haapanen, A.; Haltia, M.; Maehlen, J.; Lehesjoki, A.E.; Tyynela, J. Cathepsin d deficiency underlies congenital human neuronal ceroid-lipofuscinosis. Brain 2006, 129, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Awano, T.; Katz, M.L.; O’Brien, D.P.; Taylor, J.F.; Evans, J.; Khan, S.; Sohar, I.; Lobel, P.; Johnson, G.S. A mutation in the cathepsin D gene (CTSD) in american bulldogs with neuronal ceroid lipofuscinosis. Mol. Genet. Metab. 2006, 87, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Cullen, V.; Lindfors, M.; Ng, J.; Paetau, A.; Swinton, E.; Kolodziej, P.; Boston, H.; Saftig, P.; Woulfe, J.; Feany, M.B.; et al. Cathepsin D expression level affects alpha-synuclein processing, aggregation, and toxicity in vivo. Mol. Brain 2009. [Google Scholar] [CrossRef] [PubMed]
- Hilfiker-Kleiner, D.; Kaminski, K.; Podewski, E.; Bonda, T.; Schaefer, A.; Sliwa, K.; Forster, O.; Quint, A.; Landmesser, U.; Doerries, C.; et al. A cathepsin D-cleaved 16 kda form of prolactin mediates postpartum cardiomyopathy. Cell 2007, 128, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.M.; Li, X.; Wen, G.; Tauqeer, Z.; Brown, W.T.; Malik, M. Cathepsin D and apoptosis related proteins are elevated in the brain of autistic subjects. Neuroscience 2010, 165, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Vignon, F.; Capony, F.; Rochefort, H. Estradiol down-regulates the mannose-6-phosphate/insulin-like growth factor-ii receptor gene and induces cathepsin-D in breast cancer cells: A receptor saturation mechanism to increase the secretion of lysosomal proenzymes. Mol. Endocrinol. 1991, 5, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vagner, J.; Baudys, M.; Tang, J.; Foundling, S.I.; Fusek, M. Human breast milk contains procathepsin D—Detection by specific antibodies. Biochem. Mol. Biol. Int. 1993, 30, 921–928. [Google Scholar] [PubMed]
- Larsen, L.B.; Petersen, T.E. Identification of five molecular forms of cathepsin D in bovine milk. Adv. Exp. Med. Biol. 1995, 362, 279–283. [Google Scholar] [PubMed]
- Benes, P.; Koelsch, G.; Dvorak, B.; Fusek, M.; Vetvicka, V. Detection of procathepsin D in rat milk. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 133, 113–118. [Google Scholar] [CrossRef]
- Zuhlsdorf, M.; Imort, M.; Hasilik, A.; von Figura, K. Molecular forms of beta-hexosaminidase and cathepsin D in serum and urine of healthy subjects and patients with elevated activity of lysosomal enzymes. Biochem. J. 1983, 213, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Baechle, D.; Flad, T.; Cansier, A.; Steffen, H.; Schittek, B.; Tolson, J.; Herrmann, T.; Dihazi, H.; Beck, A.; Mueller, G.A.; et al. Cathepsin d is present in human eccrine sweat and involved in the postsecretory processing of the antimicrobial peptide dcd-1l. J. Biol. Chem. 2006, 281, 5406–5415. [Google Scholar] [CrossRef] [PubMed]
- Rochefort, H. Cathepsin d in breast cancer: A tissue marker associated with metastasis. Eur. J. Cancer 1992, 28, 1780–1783. [Google Scholar] [CrossRef]
- Ferrandina, G.; Scambia, G.; Bardelli, F.; Benedetti Panici, P.; Mancuso, S.; Messori, A. Relationship between cathepsin-D content and disease-free survival in node-negative breast cancer patients: A meta-analysis. Br. J. Cancer 1997, 76, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Foekens, J.A.; Look, M.P.; Bolt-de Vries, J.; Meijer-van Gelder, M.E.; van Putten, W.L.; Klijn, J.G. Cathepsin-D in primary breast cancer: Prognostic evaluation involving 2810 patients. Br. J. Cancer 1999, 79, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Briozzo, P.; Badet, J.; Capony, F.; Pieri, I.; Montcourrier, P.; Barritault, D.; Rochefort, H. Mcf7 mammary cancer cells respond to bfgf and internalize it following its release from extracellular matrix: A permissive role of cathepsin D. Exp. Cell Res. 1991, 194, 252–259. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Liu, W.; Zhu, J.; Zhao, X.; Wright, E.; Cao, L.; Ding, I.; Rodgers, G.P. Olfactomedin 4 suppresses prostate cancer cell growth and metastasis via negative interaction with cathepsin D and SDF-1. Carcinogenesis 2011, 32, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Konno, S.; Cherry, J.P.; Mordente, J.A.; Chapman, J.R.; Choudhury, M.S.; Mallouh, C.; Tazaki, H. Role of cathepsin D in prostatic cancer cell growth and its regulation by brefeldin A. World J. Urol. 2001, 19, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, W.; Yamamoto, K.; Ishikawa, S.; Takemoto, S.; Ono, M.; Fukushi, J.; Naito, S.; Nozaki, C.; Iwanaga, S.; Kuwano, M. Angiostatin generation by cathepsin D secreted by human prostate carcinoma cells. J. Biol. Chem. 2000, 275, 38912–38920. [Google Scholar] [CrossRef] [PubMed]
- Losch, A.; Kohlberger, P.; Gitsch, G.; Kaider, A.; Breitenecker, G.; Kainz, C. Lysosomal protease cathepsin D is a prognostic marker in endometrial cancer. Br. J. Cancer 1996, 73, 1525–1528. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wada, M.; Usagawa, Y.; Yasukochi, Y.; Yokoyama, A.; Wada, N.; Sakamoto, M.; Maekawa, T.; Miyazaki, R.; Yonenaga, E.; et al. Overexpression of cathepsin D in malignant melanoma. Fukuoka Igaku Zasshi 2013, 104, 370–375. [Google Scholar] [PubMed]
- Fukuda, M.E.; Iwadate, Y.; Machida, T.; Hiwasa, T.; Nimura, Y.; Nagai, Y.; Takiguchi, M.; Tanzawa, H.; Yamaura, A.; Seki, N. Cathepsin d is a potential serum marker for poor prognosis in glioma patients. Cancer Res. 2005, 65, 5190–5194. [Google Scholar] [CrossRef] [PubMed]
- Rochefort, H.; Garcia, M.; Glondu, M.; Laurent, V.; Liaudet, E.; Rey, J.M.; Roger, P. Cathepsin D in breast cancer: Mechanisms and clinical applications, a 1999 overview. Clin. Chim. Acta 2000, 291, 157–170. [Google Scholar] [CrossRef]
- Pruitt, F.L.; He, Y.; Franco, O.E.; Jiang, M.; Cates, J.M.; Hayward, S.W. Cathepsin d acts as an essential mediator to promote malignancy of benign prostatic epithelium. Prostate 2013, 73, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J.; Benes, P. Role of enzymatically inactive procathepsin D in lung cancer. Anticancer Res. 2004, 24, 2739–2743. [Google Scholar] [PubMed]
- Nazeer, T.; Malfetano, J.H.; Rosano, T.G.; Ross, J.S. Correlation of tumor cytosol Cathepsin D with differentiation and invasiveness of endometrial adenocarcinoma. Am. J. Clin. Pathol. 1992, 97, 764–769. [Google Scholar] [PubMed]
- Baekelandt, M.; Holm, R.; Trope, C.G.; Nesland, J.M.; Kristensen, G.B. The significance of metastasis-related factors cathepsin-D and nm23 in advanced ovarian cancer. Ann. Oncol. 1999, 10, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Ferrandina, G.; Scambia, G.; Fagotti, A.; D’Agostino, G.; Benedetti Panici, P.; Carbone, A.; Mancuso, S. Immunoradiometric and immunohistochemical analysis of cathepsin D in ovarian cancer: Lack of association with clinical outcome. Br. J. Cancer 1998, 78, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Henzen-Logmans, S.C.; Fieret, E.J.; Berns, E.M.; van der Burg, M.E.; Klijn, J.G.; Foekens, J.A. Ki-67 staining in benign, borderline, malignant primary and metastatic ovarian tumors: Correlation with steroid receptors, epidermal-growth-factor receptor and cathepsin D. Int. J. Cancer 1994, 57, 468–472. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Losch, A.; Schindl, M.; Kohlberger, P.; Lahodny, J.; Breitenecker, G.; Horvat, R.; Birner, P. Cathepsin D in ovarian cancer: Prognostic value and correlation with p53 expression and microvessel density. Gynecol. Oncol. 2004, 92, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Wu, W.; Zhou, C.; Zhou, J. The potential prognostic value of Cathepsin D protein in serous ovarian cancer. Arch. Gynecol. Obstet. 2012, 286, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Winiarski, B.K.; Cope, N.; Alexander, M.; Pilling, L.C.; Warren, S.; Acheson, N.; Gutowski, N.J.; Whatmore, J.L. Clinical relevance of increased endothelial and mesothelial expression of proangiogenic proteases and vegfa in the omentum of patients with metastatic ovarian high-grade serous carcinoma. Transl. Oncol. 2014, 7, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Brouillet, J.P.; Dufour, F.; Lemamy, G.; Garcia, M.; Schlup, N.; Grenier, J.; Mani, J.C.; Rochefort, H. Increased cathepsin D level in the serum of patients with metastatic breast carcinoma detected with a specific pro-cathepsin D immunoassay. Cancer 1997, 79, 2132–2136. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vektvickova, J.; Fusek, M. Effect of human procathepsin D on proliferation of human cell lines. Cancer Lett. 1994, 79, 131–135. [Google Scholar] [CrossRef]
- Ollinger, K. Inhibition of cathepsin D prevents free-radical-induced apoptosis in rat cardiomyocytes. Arch. Biochem. Biophys. 2000, 373, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Kagedal, K.; Johansson, U.; Ollinger, K. The lysosomal protease cathepsin D mediates apoptosis induced by oxidative stress. FASEB J. 2001, 15, 1592–1594. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Neumeyer, J.; Jakob, M.; Hallas, C.; Tchikov, V.; Winoto-Morbach, S.; Wickel, M.; Schneider-Brachert, W.; Trauzold, A.; Hethke, A.; et al. Cathepsin D links TNF-induced acid sphingomyelinase to Bid-mediated caspase-9 and -3 activation. Cell Death Differ. 2004, 11, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Blomgran, R.; Zheng, L.; Stendahl, O. Cathepsin-cleaved bid promotes apoptosis in human neutrophils via oxidative stress-induced lysosomal membrane permeabilization. J. Leukoc. Biol. 2007, 81, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.C.; Steen, H.; Ollinger, K.; Roberg, K. Cathepsin d mediates cytochrome C release and caspase activation in human fibroblast apoptosis induced by staurosporine. Cell Death Differ. 2003, 10, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte-Luis, V.; Montero, J.A.; Torre-Perez, N.; Garcia-Porrero, J.A.; Hurle, J.M. Cathepsin D gene expression outlines the areas of physiological cell death during embryonic development. Dev. Dyn. 2007, 236, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte-Luis, V.; Montero, J.A.; Kawakami, Y.; Izpisua-Belmonte, J.C.; Hurle, J.M. Lysosomal cathepsins in embryonic programmed cell death. Dev. Biol. 2007, 301, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Beaujouin, M.; Baghdiguian, S.; Glondu-Lassis, M.; Berchem, G.; Liaudet-Coopman, E. Overexpression of both catalytically active and -inactive cathepsin D by cancer cells enhances apoptosis-dependent chemo-sensitivity. Oncogene 2006, 25, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- Tardy, C.; Tyynela, J.; Hasilik, A.; Levade, T.; Andrieu-Abadie, N. Stress-induced apoptosis is impaired in cells with a lysosomal targeting defect but is not affected in cells synthesizing a catalytically inactive cathepsin D. Cell Death Differ. 2003, 10, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Ohri, S.S.; Vashishta, A.; Proctor, M.; Fusek, M.; Vetvicka, V. The propeptide of cathepsin D increases proliferation, invasion and metastasis of breast cancer cells. Int. J. Oncol. 2008, 32, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Briozzo, P.; Morisset, M.; Capony, F.; Rougeot, C.; Rochefort, H. In vitro degradation of extracellular matrix with Mr 52,000 cathepsin D secreted by breast cancer cells. Cancer Res. 1988, 48, 3688–3692. [Google Scholar] [PubMed]
- Westley, B.R.; May, F.E. Cathepsin D and breast cancer. Eur. J. Cancer 1996, 32, 15–24. [Google Scholar] [CrossRef]
- Crowe, D.L.; Shuler, C.F. Regulation of tumor cell invasion by extracellular matrix. Histol. Histopathol. 1999, 14, 665–671. [Google Scholar] [PubMed]
- Laurent-Matha, V.; Maruani-Herrmann, S.; Prebois, C.; Beaujouin, M.; Glondu, M.; Noel, A.; Alvarez-Gonzalez, M.L.; Blacher, S.; Coopman, P.; Baghdiguian, S.; et al. Catalytically inactive human cathepsin D triggers fibroblast invasive growth. J. Cell Biol. 2005, 168, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Derocq, D.; Pujol, P.; Rochefort, H. Overexpression of transfected cathepsin D in transformed cells increases their malignant phenotype and metastatic potency. Oncogene 1990, 5, 1809–1814. [Google Scholar] [PubMed]
- Liaudet, E.; Garcia, M.; Rochefort, H. Cathepsin D maturation and its stimulatory effect on metastasis are prevented by addition of kdel retention signal. Oncogene 1994, 9, 1145–1154. [Google Scholar] [PubMed]
- Liaudet, E.; Derocq, D.; Rochefort, H.; Garcia, M. Transfected cathepsin D stimulates high density cancer cell growth by inactivating secreted growth inhibitors. Cell Growth Differ. 1995, 6, 1045–1052. [Google Scholar] [PubMed]
- Glondu, M.; Coopman, P.; Laurent-Matha, V.; Garcia, M.; Rochefort, H.; Liaudet-Coopman, E. A mutated cathepsin-D devoid of its catalytic activity stimulates the growth of cancer cells. Oncogene 2001, 20, 6920–6929. [Google Scholar] [CrossRef] [PubMed]
- Berchem, G.; Glondu, M.; Gleizes, M.; Brouillet, J.P.; Vignon, F.; Garcia, M.; Liaudet-Coopman, E. Cathepsin-D affects multiple tumor progression steps in vivo: Proliferation, angiogenesis and apoptosis. Oncogene 2002, 21, 5951–5955. [Google Scholar] [CrossRef] [PubMed]
- Fusek, M.; Vetvicka, V. Mitogenic function of human procathepsin D: The role of the propeptide. Biochem. J. 1994, 303, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J.; Fusek, M. Effect of procathepsin D and its activation peptide on prostate cancer cells. Cancer Lett. 1998, 129, 55–59. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J.; Fusek, M. Anti-human procathepsin D activation peptide antibodies inhibit breast cancer development. Breast Cancer Res. Treat. 1999, 57, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J.; Hilgert, I.; Voburka, Z.; Fusek, M. Analysis of the interaction of procathepsin D activation peptide with breast cancer cells. Int. J. Cancer 1997, 73, 403–409. [Google Scholar] [CrossRef]
- Rochefort, H.; Capony, F.; Garcia, M.; Cavailles, V.; Freiss, G.; Chambon, M.; Morisset, M.; Vignon, F. Estrogen-induced lysosomal proteases secreted by breast cancer cells: A role in carcinogenesis? J. Cell. Biochem. 1987, 35, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Vignon, F.; Capony, F.; Chambon, M.; Freiss, G.; Garcia, M.; Rochefort, H. Autocrine growth stimulation of the Mcf 7 breast cancer cells by the estrogen-regulated 52 k protein. Endocrinology 1986, 118, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Clark-Lewis, I.; Buri, C.; Langen, H.; Lis, M.; Mazzucchelli, L. Cathepsin D specifically cleaves the chemokines macrophage inflammatory protein-1 alpha, macrophage inflammatory protein-1 beta, and SLC that are expressed in human breast cancer. Am. J. Pathol. 2003, 162, 1183–1190. [Google Scholar] [CrossRef]
- Hu, L.; Roth, J.M.; Brooks, P.; Luty, J.; Karpatkin, S. Thrombin up-regulates cathepsin D which enhances angiogenesis, growth, and metastasis. Cancer Res. 2008, 68, 4666–4673. [Google Scholar] [CrossRef] [PubMed]
- Kirschke, H.; Langner, J.; Wiederanders, B.; Ansorge, S.; Bohley, P. Cathepsin l. A new proteinase from rat-liver lysosomes. Eur. J. Biochem. 1977, 74, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Kominami, E.; Ueno, T.; Muno, D.; Katunuma, N. The selective role of cathepsins B and D in the lysosomal degradation of endogenous and exogenous proteins. FEBS Lett. 1991, 287, 189–192. [Google Scholar] [CrossRef]
- Kominami, E.; Tsukahara, T.; Hara, K.; Katunuma, N. Biosyntheses and processing of lysosomal cysteine proteinases in rat macrophages. FEBS Lett. 1988, 231, 225–228. [Google Scholar] [CrossRef]
- Nishimura, Y.; Furuno, K.; Kato, K. Biosynthesis and processing of lysosomal cathepsin L in primary cultures of rat hepatocytes. Arch. Biochem. Biophys. 1988, 263, 107–116. [Google Scholar] [CrossRef]
- Towatari, T.; Katunuma, N. Amino acid sequence of rat liver cathepsin L. FEBS Lett. 1988, 236, 57–61. [Google Scholar] [CrossRef]
- Ishidoh, K.; Towatari, T.; Imajoh, S.; Kawasaki, H.; Kominami, E.; Katunuma, N.; Suzuki, K. Molecular cloning and sequencing of cDNA for rat cathepsin L. FEBS Lett. 1987, 223, 69–73. [Google Scholar] [CrossRef]
- Dong, J.M.; Sahagian, G.G. Basis for low affinity binding of a lysosomal cysteine protease to the cation-independent mannose 6-phosphate receptor. J. Biol. Chem. 1990, 265, 4210–4217. [Google Scholar] [PubMed]
- Ishidoh, K.; Muno, D.; Sato, N.; Kominami, E. Molecular cloning of cDNA for rat cathepsin C. Cathepsin C, a cysteine proteinase with an extremely long propeptide. J. Biol. Chem. 1991, 266, 16312–16317. [Google Scholar] [PubMed]
- Ritonja, A.; Popovic, T.; Kotnik, M.; Machleidt, W.; Turk, V. Amino acid sequences of the human kidney cathepsins H and L. FEBS Lett. 1988, 228, 341–345. [Google Scholar] [CrossRef]
- Mason, R.W.; Gal, S.; Gottesman, M.M. The identification of the major excreted protein (MEP) from a transformed mouse fibroblast cell line as a catalytically active precursor form of cathepsin L. Biochem. J. 1987, 248, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.W.; Massey, S.D. Surface activation of pro-cathepsin L. Biochem. Biophys. Res. Commun. 1992, 189, 1659–1666. [Google Scholar] [CrossRef]
- Nakagawa, T.; Roth, W.; Wong, P.; Nelson, A.; Farr, A.; Deussing, J.; Villadangos, J.A.; Ploegh, H.; Peters, C.; Rudensky, A.Y. Cathepsin l: Critical role in II degradation and CD4 T cell selection in the thymus. Science 1998, 280, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.S.; deRoos, P.; Honey, K.; Beers, C.; Rudensky, A.Y. A role for cathepsin L and cathepsin S in peptide generation for Mhc class II presentation. J. Immunol. 2002, 168, 2618–2625. [Google Scholar] [CrossRef] [PubMed]
- Roth, W.; Deussing, J.; Botchkarev, V.A.; Pauly-Evers, M.; Saftig, P.; Hafner, A.; Schmidt, P.; Schmahl, W.; Scherer, J.; Anton-Lamprecht, I.; et al. Cathepsin l deficiency as molecular defect of furless: Hyperproliferation of keratinocytes and pertubation of hair follicle cycling. FASEB J. 2000, 14, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Potts, W.; Bowyer, J.; Jones, H.; Tucker, D.; Freemont, A.J.; Millest, A.; Martin, C.; Vernon, W.; Neerunjun, D.; Slynn, G.; et al. Cathepsin l-deficient mice exhibit abnormal skin and bone development and show increased resistance to osteoporosis following ovariectomy. Int. J. Exp. Pathol. 2004, 85, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Von Figura, K.; Hasilik, A. Lysosomal enzymes and their receptors. Annu. Rev. Biochem. 1986, 55, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Dahms, N.M.; Lobel, P.; Kornfeld, S. Mannose 6-phosphate receptors and lysosomal enzyme targeting. J. Biol. Chem. 1989, 264, 12115–12118. [Google Scholar] [PubMed]
- Dong, J.M.; Prence, E.M.; Sahagian, G.G. Mechanism for selective secretion of a lysosomal protease by transformed mouse fibroblasts. J. Biol. Chem. 1989, 264, 7377–7383. [Google Scholar] [PubMed]
- Prence, E.M.; Dong, J.M.; Sahagian, G.G. Modulation of the transport of a lysosomal enzyme by PDGF. J. Cell Biol. 1990, 110, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Achkar, C.; Gong, Q.M.; Frankfater, A.; Bajkowski, A.S. Differences in targeting and secretion of cathepsins B and L by BALB/3T3 fibroblasts and Moloney murine sarcoma virus-transformed BALB/3T3 fibroblasts. J. Biol. Chem. 1990, 265, 13650–13654. [Google Scholar] [PubMed]
- Cuvier, C.; Jang, A.; Hill, R.P. Exposure to hypoxia, glucose starvation and acidosis: Effect on invasive capacity of murine tumor cells and correlation with cathepsin (L + B) secretion. Clin. Exp. Metastasis 1997, 15, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Greenland, J.; Baluk, P.; Adams, A.; Bose, O.; McDonald, D.M.; Caughey, G.H. Cathepsin L protects mice from mycoplasmal infection and is essential for airway lymphangiogenesis. Am. J. Respir. Cell Mol. Biol. 2013, 49, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Maciewicz, R.A.; Wotton, S.F. Degradation of cartilage matrix components by the cysteine proteinases, cathepsins B and L. Biomed. Biochim. Acta 1991, 50, 561–564. [Google Scholar] [PubMed]
- Nguyen, Q.; Mort, J.S.; Roughley, P.J. Cartilage proteoglycan aggregate is degraded more extensively by cathepsin L than by cathepsin B. Biochem. J. 1990, 266, 569–573. [Google Scholar] [PubMed]
- Maciewicz, R.A.; Wotton, S.F.; Etherington, D.J.; Duance, V.C. Susceptibility of the cartilage collagens types II, IX and XI to degradation by the cysteine proteinases, cathepsins B and L. FEBS Lett. 1990, 269, 189–193. [Google Scholar] [CrossRef]
- Nosaka, A.Y.; Kanaori, K.; Teno, N.; Togame, H.; Inaoka, T.; Takai, M.; Kokubo, T. Conformational studies on the specific cleavage site of type i collagen (α-1) fragment (157–192) by cathepsins K and L by proton Nmr spectroscopy. Bioorg. Med. Chem. 1999, 7, 375–379. [Google Scholar] [CrossRef]
- Mason, R.W.; Johnson, D.A.; Barrett, A.J.; Chapman, H.A. Elastinolytic activity of human cathepsin L. Biochem. J. 1986, 233, 925–927. [Google Scholar] [CrossRef] [PubMed]
- Ishidoh, K.; Kominami, E. Procathepsin L degrades extracellular matrix proteins in the presence of glycosaminoglycans in vitro. Biochem. Biophys. Res. Commun. 1995, 217, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Kohno, K.; Kawamata, T.; Morimitsu, K.; Kuwano, M.; Miyakawa, I. Increased cathepsin L levels in serum in some patients with ovarian cancer: Comparison with CA125 and CA72-4. Gynecol. Oncol. 1995, 56, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, S.; Wang, Q.; Yang, Z.; Pan, Z.; Li, L. Overexpression of cysteine cathepsin L is a marker of invasion and metastasis in ovarian cancer. Oncol. Rep. 2014, 31, 1334–1342. [Google Scholar] [PubMed]
- Zhang, L.; Wei, L.; Shen, G.; He, B.; Gong, W.; Min, N.; Zhang, L.; Duan, Y.; Xie, J.; Luo, H.; et al. Cathepsin l is involved in proliferation and invasion of ovarian cancer cells. Mol. Med. Rep. 2015, 11, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.F.; Colella, R.; Denhardt, D.T.; Wilson, S.M. Increased expression of cathepsins L and B and decreased activity of their inhibitors in metastatic, ras-transformed NIH 3T3 cells. Mol. Carcinog. 1992, 5, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Frade, R.; Rodrigues-Lima, F.; Huang, S.; Xie, K.; Guillaume, N.; Bar-Eli, M. Procathepsin-L, a proteinase that cleaves human C3 (the third component of complement), confers high tumorigenic and metastatic properties to human melanoma cells. Cancer Res. 1998, 58, 2733–2736. [Google Scholar] [CrossRef]
- Ishidoh, K.; Kominami, E. Gene regulation and extracellular functions of procathepsin L. Biol. Chem. 1998, 379, 131–135. [Google Scholar] [PubMed]
- Yang, Z.; Cox, J.L. Cathepsin L increases invasion and migration of B16 melanoma. Cancer Cell Int. 2007. [Google Scholar] [CrossRef] [PubMed]
- Gocheva, V.; Zeng, W.; Ke, D.; Klimstra, D.; Reinheckel, T.; Peters, C.; Hanahan, D.; Joyce, J.A. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes Dev. 2006, 20, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Im, E.K.; Jin, T.W.; Lee, S.M.; Kim, S.H.; Choi, E.Y.; Shin, M.J.; Lee, K.H.; Jang, Y. Cathepsin l derived from skeletal muscle cells transfected with bfgf promotes endothelial cell migration. Exp. Mol. Med. 2011, 43, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Felbor, U.; Dreier, L.; Bryant, R.A.; Ploegh, H.L.; Olsen, B.R.; Mothes, W. Secreted cathepsin L generates endostatin from collagen XVIII. EMBO J. 2000, 19, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Urbich, C.; Heeschen, C.; Aicher, A.; Sasaki, K.; Bruhl, T.; Farhadi, M.R.; Vajkoczy, P.; Hofmann, W.K.; Peters, C.; Pennacchio, L.A.; et al. Cathepsin L is required for endothelial progenitor cell-induced neovascularization. Nat. Med. 2005, 11, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Fiebiger, E.; Maehr, R.; Villadangos, J.; Weber, E.; Erickson, A.; Bikoff, E.; Ploegh, H.L.; Lennon-Dumenil, A.M. Invariant chain controls the activity of extracellular cathepsin L. J. Exp. Med. 2002, 196, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Shimada, N.; Ohno-Matsui, K.; Iseki, S.; Koike, M.; Uchiyama, Y.; Wang, J.; Yoshida, T.; Sato, T.; Peters, C.; Mochizuki, M.; et al. Cathepsin L in bone marrow-derived cells is required for retinal and choroidal neovascularization. Am. J. Pathol. 2010, 176, 2571–2580. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pranjol, M.Z.I.; Gutowski, N.; Hannemann, M.; Whatmore, J. The Potential Role of the Proteases Cathepsin D and Cathepsin L in the Progression and Metastasis of Epithelial Ovarian Cancer. Biomolecules 2015, 5, 3260-3279. https://doi.org/10.3390/biom5043260
Pranjol MZI, Gutowski N, Hannemann M, Whatmore J. The Potential Role of the Proteases Cathepsin D and Cathepsin L in the Progression and Metastasis of Epithelial Ovarian Cancer. Biomolecules. 2015; 5(4):3260-3279. https://doi.org/10.3390/biom5043260
Chicago/Turabian StylePranjol, Md Zahidul Islam, Nicholas Gutowski, Michael Hannemann, and Jacqueline Whatmore. 2015. "The Potential Role of the Proteases Cathepsin D and Cathepsin L in the Progression and Metastasis of Epithelial Ovarian Cancer" Biomolecules 5, no. 4: 3260-3279. https://doi.org/10.3390/biom5043260
APA StylePranjol, M. Z. I., Gutowski, N., Hannemann, M., & Whatmore, J. (2015). The Potential Role of the Proteases Cathepsin D and Cathepsin L in the Progression and Metastasis of Epithelial Ovarian Cancer. Biomolecules, 5(4), 3260-3279. https://doi.org/10.3390/biom5043260





