Determinants of Glycosaminoglycan (GAG) Structure
Abstract
:1. Proteoglycans
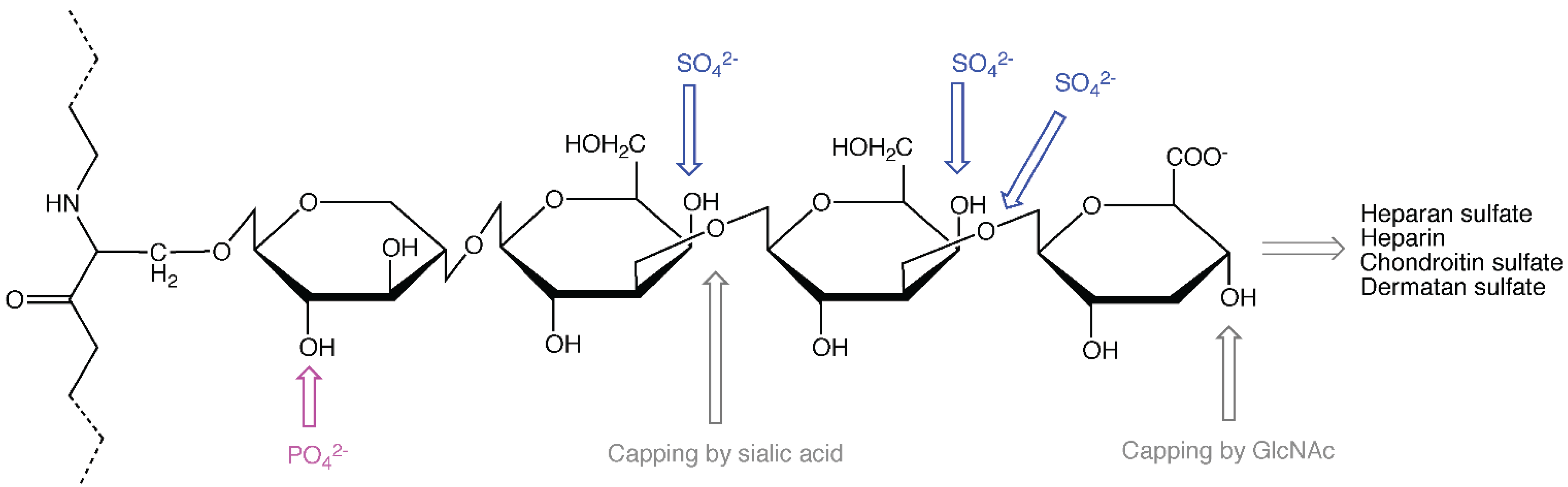
2. The Role of the Protein Core
3. Modification of the Linker Region
4. Keratan Sulfate Proteoglycans and Hyaluronic Acid Glycosaminoglycans
5. Sorting of Proteoglycans in the Secretory Pathway
6. The Environment of the Golgi Apparatus
7. Nucleotide Sugars, PAPS and Their Transporters
8. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Prydz, K.; Dalen, K.T. Synthesis and sorting of proteoglycans. J. Cell Sci. 2000, 113, 193–205. [Google Scholar]
- Lawrence, R.; Kuberan, B.; Lech, M.; Beeler, D.L.; Rosenberg, R.D. Mapping critical biological motifs and biosynthetic pathways of heparan sulfate. Glycobiology 2004, 14, 467–479. [Google Scholar]
- Esko, J.D.; Selleck, S.B. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 2002, 71, 435–471. [Google Scholar]
- Lindahl, U.; Li, J.P. Interactions between heparan sulfate and proteins—Design and functional implications. Int. Rev. Cell Mol. Biol. 2009, 276, 105–159. [Google Scholar]
- Mitzumoto, S.; Yamada, S.; Sugahara, K. Molecular interactions between chondroitin-dermatan sulfate and growth factors/receptors/matrix proteins. Curr. Opin. Struct. Biol. 2015, 34, 35–42. [Google Scholar]
- Iozzo, R.V.; Sanderson, R.D. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J. Cell. Mol. Med. 2011, 15, 1013–1031. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, A.; Lendorf, M.E.; Couchman, J.R.; Multhaupt, H.A. Breast and ovarian cancers: A survey and possible roles for the cell surface heparan sulfate proteoglycans. J. Histochem. Cytochem. 2012, 60, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef] [PubMed]
- Kolset, S.O.; Pejler, G. Serglycin: A structural and functional chameleon with wide impact on immune cells. J. Immunol. 2011, 187, 4927–4933. [Google Scholar] [CrossRef] [PubMed]
- Funderburgh, J.L. Keratan sulfate biosynthesis. IUBMB Life 2002, 54, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Götting, C.; Kuhn, J.; Kleesiek, K. Human xylosyltransferases in health and disease. Cell. Mol. Life Sci. 2007, 64, 1498–1517. [Google Scholar] [CrossRef] [PubMed]
- Malmström, A.; Bartolini, B.; Thelin, M.A.; Pacheco, B.; Maccarana, M. Iduronic acid in chondroitin/dermatan sulfate: Biosynthesis and biological function. J. Histochem. Cytochem. 2012, 60, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Le Jan, S.; Hayashi, M.; Kasza, Z.; Eriksson, I.; Bishop, J.R.; Weibrecht, I.; Heldin, J.; Holmborn, K.; Jakobsson, L.; Söderberg, O.; et al. Functional overlap between chondroitin and heparan sulfate proteoglycans during VEGF-induced sprouting angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.T.; Kitagawa, H.; Tanaka, J.; Tamura, J.; Sugahara, K. In vitro heparan sulfate polymerization: Crucial roles of core protein moieties of primer substrates in addition to the EXT1-EXT2 interaction. J. Biol. Chem. 2003, 278, 41618–41623. [Google Scholar] [CrossRef] [PubMed]
- Fransson, L.A.; Belting, M.; Cheng, F.; Jönsson, M.; Mani, K.; Sandgren, S. Novel aspects of glypican biology. Cell. Mol. Life Sci. 2004, 61, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.L.; Lander, A.D. Mechanisms underlying preferential assembly of heparan sulfate on glypican-1. J. Biol. Chem. 2001, 276, 7507–7517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Esko, J.D. Amino acid determinants that drive heparan sulfate assembly in a proteoglycan. J. Biol. Chem. 1994, 269, 19295–19299. [Google Scholar] [PubMed]
- Esko, J.D.; Zhang, L. Influence of core protein sequence on glycosaminoglycan assembly. Curr. Opin. Struct. Biol. 1996, 6, 663–670. [Google Scholar] [CrossRef]
- Zhang, L.; David, G.; Esko, J.D. Repetitive Ser-gly sequences enhance heparan sulfate assembly in proteoglycans. J. Biol. Chem. 1995, 270, 27127–27135. [Google Scholar] [CrossRef] [PubMed]
- Dolan, M.; Horchar, T.; Rigatti, B.; Hassell, J.R. Identification of sites in domain I of perlecan that regulate heparan sulfate synthesis. J. Biol. Chem. 1997, 272, 4316–4322. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, T.; Weilke, C.; Kleesiek, K. Recognition of acceptor proteins by UDP-d-xylose proteoglycan core protein beta-d-xylosyltransferase. J. Biol. Chem. 1997, 272, 11171–11175. [Google Scholar] [PubMed]
- Deepa, S.S.; Yamada, S.; Zako, M.; Goldberger, O.; Sugahara, K. Chondroitin sulfate chains on syndecan-1 and syndecan-4 from normal murine mammary gland epitelial cells are structurally and functionally distinct and cooperate with heparan sulfate chains to bind growth factors. A novel function to control binding of midkine, pleiotrophin, and basic fibroblast growth factor. J. Biol. Chem. 2004, 279, 37368–37376. [Google Scholar] [PubMed]
- Seidler, D.G.; Breuer, E.; Grande-Allen, K.J.; Hascall, V.C.; Kresse, H. Core protein dependence of epimerization of glucuronosyl residues in galactosaminoglycans. J. Biol. Chem. 2002, 277, 42409–42416. [Google Scholar] [CrossRef] [PubMed]
- Herzog, C.; Lippmann, I.; Grobe, K.; Zamfir, A.D.; Echtermeyer, F.; Seidler, D.G. The amino acid tryptophan prevents the biosynthesis of dermatan sulfate. Mol. Biosyst. 2011, 7, 2872–2881. [Google Scholar] [CrossRef] [PubMed]
- Fransson, L.A. Structure of dermatan sulfate III. The hybrid structure of dermatan sulfate from umbilical cord. J. Biol. Chem. 1968, 243, 1504–1510. [Google Scholar] [PubMed]
- Habuchi, H.; Yamagata, T.; Iwata, H.; Suzuki, S. The occurrence of a wide variety of dermatan sulfate-chondroitin sulfate copolymers in fibrous cartilage. J. Biol. Chem. 1973, 248, 6019–6028. [Google Scholar] [PubMed]
- Akatsu, C.; Mizumoto, S.; Kaneiwa, T.; Maccarana, M.; Malmström, A.; Yamada, S.; Sugahara, K. Dermatan sulfate epimerase 2 is the predominant isozyme in the formation of the chondroitin/dermatan sulfate hybrid structure in postnatal developing mouse brain. Glycobiology 2011, 21, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Esko, J.D.; Stewart, T.E.; Taylor, W.H. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA 1985, 82, 3197–3201. [Google Scholar] [CrossRef] [PubMed]
- Esko, J.D.; Weinke, J.L.; Taylor, W.H.; Ekborg, G.; Rodén, L.; Anantharamaiah, G.; Gawish, A. Inhibition of chondroitin and heparan sulfate biosynthesis in Chinese hamster ovary cell mutants defective in galactosyltransferase I. J. Biol. Chem. 1987, 262, 12189–12195. [Google Scholar] [PubMed]
- Bai, X.; Wei, G.; Esko, J.D. Chinese hamster ovary cell mutants defective in glycosaminoglycan assembly and glucuronosyltransferase I. J. Biol. Chem. 1989, 274, 13017–13024. [Google Scholar] [CrossRef]
- Kearns, A.E.; Campbell, S.C.; Westley, J.; Schwartz, N.B. Initiation of chondroitin sulfate biosynthesis: A kinetic analysis of UDP-d-xylose:core protein β-d-xylosyltransferase. Biochemistry 1991, 30, 7477–7483. [Google Scholar] [CrossRef] [PubMed]
- Kearns, A.E.; Vertel, B.M.; Schwartz, N.B. Topography of glycosylation and UDP-xylose production. J. Biol. Chem. 1993, 268, 11097–11104. [Google Scholar] [PubMed]
- Vertel, B.M.; Walters, L.M.; Flay, N.; Kearns, A.E.; Schwartz, N.B. Xylosylation is an endoplasmic reticulum to Golgi event. J. Biol. Chem. 1993, 268, 11105–11112. [Google Scholar] [PubMed]
- Jönsson, M.; Eklund, E.; Fransson, L.A.; Oldberg, A. Initiation of the decoringlycosaminoglycan chain in the endoplasmic reticulum-Golgi intermediate compartment. J. Biol. Chem. 2003, 278, 21415–21420. [Google Scholar] [CrossRef] [PubMed]
- Nuwayhid, N.; Glaser, J.H.; Johnson, J.C.; Conrad, H.E.; Hauser, S.C.; Hirschberg, C.B. Xylosylation and glucuronosylation reactions in rat liver Golgi apparatus and endoplasmic reticulum. J. Biol. Chem. 1986, 261, 12936–12941. [Google Scholar] [PubMed]
- Lohmander, L.S.; Shinomura, T.; Hascall, V.C.; Kimura, J.H. Xylosyl transfer to the core protein precursor of the rat chondrosarcoma proteoglycan. J. Biol. Chem. 1989, 264, 18775–18780. [Google Scholar] [PubMed]
- Oegma, T.R., Jr.; Kraft, E.L.; Jourdian, G.W.; van Valen, T.R. Phosphorylation of chondroitin sulfate in proteoglycans from the swarm rat chondrosarcoma. J. Biol. Chem. 1984, 259, 1720–1726. [Google Scholar]
- Sugahara, K.; Mizuno, N.; Okumura, Y.; Kawasaki, T. The phosphorylated and/or sulfated structure of the carbohydrate-protein linkage region isolated from chondroitin sulfate in the hybrid proteoglycans of Engelbreth-Holm-Swarm mouse tumor. Eur. J. Biochem. 1992, 204, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.; Ohi, Y.; Harada, T.; de Waard, P.; Vliegenthart, J.F. Structural studies on sulfated oligosaccharides derived from the carbohydrate-protein linkage region of chondroitin 6-sulfate proteoglycans of shark cartilage. I. Six compounds containing 0 or 1 sulfate and/or phosphate residues. J. Biol. Chem. 1992, 267, 6027–6035. [Google Scholar] [PubMed]
- Sugahara, K.; Yamada, S.; Yoshida, K.; de Waard, P.; Vliegenthart, J.F. A novel sulfated structure in the carbohydrate-protein linkage region isolated from porcine intestinal heparin. J. Biol. Chem. 1992, 267, 1528–1533. [Google Scholar] [PubMed]
- Fransson, L.A.; Silverberg, L.; Carlstedt, I. Structure of the heparan sulfate-protein linkage region. Demonstration of the sequence galactosyl-xylose-2-phosphate. J. Biol. Chem. 1985, 260, 14722–14726. [Google Scholar] [PubMed]
- Sugahara, K.; Ohkita, Y.; Shibata, Y.; Yoshida, K.; Ikegami, A. Structural studies on the hexasaccharide alditols isolated from the carbohydrate-protein linkage region of dermatan sulfate proteoglycans of bovine aorta. Demonstration of iduronic acid-containing components. J. Biol. Chem. 1995, 270, 7204–7212. [Google Scholar] [PubMed]
- Sugahara, K.; Tsuda, H.; Yoshida, K.; Yamada, S.; de Beer, T.; Vliegenthart, J.F. Structure determination of the octa- and decasaccharide sequences isolated from the carbohydrate-protein linkage region of porcine intestinal heparin. J. Biol. Chem. 1995, 270, 22914–22923. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Heinegård, G.; Fransson, L.; Bayliss, M.; Bielicki, J.; Hopwood, J.; Yoshida, K. Variations in the chondroitin sulfate-protein linkage region of aggrecans from bovine nasal and human articular cartilages. J. Biol. Chem. 1996, 271, 28572–28580. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Xiao, J.; Rahdar, M.; Choudhury, B.P.; Cui, J.; Taylor, G.S.; Esko, J.D.; Dixon, J.E. Xylose phosphorylation functions as a molecular switch to regulate proteoglycan synthesis. Proc. Natl. Acad. Sci. USA 2014, 111, 15723–15728. [Google Scholar] [CrossRef] [PubMed]
- Koike, T.; Izumikawa, T.; Sato, B.; Kitagawa, H. Identification of phosphatase that dephosphorylates xylose in the glycosaminoglycan-protein linkage region of proteoglycans. J. Biol. Chem. 2014, 289, 6695–6708. [Google Scholar] [CrossRef] [PubMed]
- Moses, J.; Oldberg, Å.; Fransson, L.A. Initiation of galactosaminoglycan biosynthesis. Separate galactosylation and dephosphorylation pathways for phosphoxylosylated decorin protein and exogenous xyloside. Eur. J. Biochem. 1999, 260, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Tone, Y.; Pedersen, L.C.; Yamamoto, T.; Izumikawa, T.; Kitagawa, H.; Nishihara, J.; Tamura, J.; Negishi, M.; Sugahara, K. 2-O-phosphorylation of xylose and 6-O-sulfation of galactose in the protein linkage region of glycosaminoglycans influence the glucuronyltransferase-I activity involved in linkage region synthesis. J. Biol. Chem. 2008, 283, 16801–16808. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.; Yamashina, L.; de Waard, P.; van Halbeek, H.; Vliegenthart, J.F. Structural studies on sulfated glycopeptides from the carbohydrate-protein linkage region of chondroitin 4-sulfate proteoglycans of swarm rat chondrosarcoma. Demonstration of the structure Gal (4-O-sulfate) beta 1-3 Gal beta 1-4XYL beta 1-O-Ser. J. Biol. Chem. 1988, 263, 10168–10174. [Google Scholar] [PubMed]
- Sugahara, K.; Masuda, M.; Harada, T.; Yamashina, L.; de Waard, P.; Vliegenthart, J.F. Structural studies on sulfated oligosaccharides derived from the carbohydrate-protein linkage region of chondroitin sulfate proteoglycans of whale cartilage. Eur. J. Biochem. 1991, 202, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, H.; Tone, Y.; Tamura, J.; Neumann, K.W.; Ogawa, T.; Oka, S.; Kawasaki, T.; Sugahara, K. Molecular cloning and expression of glucuronyltransferase I involved in the biosynthesis of the glycosaminoglycan-protein linkage region of proteoglycans. J. Biol. Chem. 1998, 273, 6615–6618. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, H.; Kano, Y.; Shimakawa, H.; Ogawa, T.; Okabe, H.; Sugahara, K. Identification and characterization of a novel UDPGalNAc:GlcA beta-R alpha 1,4-N-acetylgalactosaminyltransferase from a human sarcoma cell line. Glycobiology 1999, 9, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, H.; Tsutsumi, K.; Ikegami-Kuzuhara, A.; Nadanaka, S.; Goto, F.; Ogawa, T.; Sugahara, K. Sulfation of the galactose residues in the glycosaminoglycan-protein linkage region by recombinant human chondroitin 6-O-sulfotransferase-1. J. Biol. Chem. 2008, 283, 27438–27443. [Google Scholar] [CrossRef] [PubMed]
- Spiro, R.C.; Freeze, H.H.; Sampath, D.; Garcia, J.A. Uncoupling of chondroitin sulfate glycosaminoglycan synthesis by brefeldin A. J. Cell Biol. 1991, 115, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, G.; Katsman, M.; Silbert, J.E. Effects of brefeldin A on the localization of chondroitin sulfate-synthesizing enzymes. Activities in subfractions of the Golgi from chick embryo epiphyseal cartilage. J. Biol. Chem. 1992, 267, 8802–8806. [Google Scholar] [PubMed]
- Fransson, L.A.; Karlsson, P.; Schmidtchen, A. Effects of cycloheximide, brefeldin A, sumarin, heparin and primaquine on proteoglycan and glycosaminoglycan biosynthesis in human embryonic skin fibroblasts. Biochim. Biophys. Acta 1992, 1137, 287–297. [Google Scholar] [CrossRef]
- Uhlin-Hansen, L.; Yanagishita, M. Defferential effect of brefeldin A on the biosynthesis of heparan sulfate and chondroitin/dermatan sulfate proteoglycans in rat ovarian granulosa cells in culture. J. Biol. Chem. 1993, 268, 17370–17376. [Google Scholar] [PubMed]
- Calabro, A.; Hascall, V. Differential effects of brefeldin A on chondroitin sulfate and hyaluronan synthesis in rat chondrosarcoma cells. J. Biol. Chem. 1994, 269, 22764–22770. [Google Scholar] [PubMed]
- Nadanaka, S.; Zhou, S.; Kagiyama, S.; Shoji, N.; Sugahara, K.; Asano, M.; Kitagawa, H. EXTL2, a member of the EXT family of tumor suppressors, controls glycosaminoglycan biosynthesis in a xylose kinase-dependent manner. J. Biol. Chem. 2013, 288, 9321–9333. [Google Scholar] [CrossRef] [PubMed]
- Gulberti, S.; Lattard, V.; Fondeur, M.; Jacquinet, J.C.; Mulliert, G.; Netter, P.; Magdalou, J.; Ouzzine, M.; Fournel-Gigleux, S. Phosphorylation and sulfation of oligosaccharide substrates critically influence the activity of human beta1,4-galactosyltransferase 7 (GalT-I) and beta1,3-glucuronosyltransferase I (GlcAT-I) involved in the biosynthesis of the glycosaminoglycan-protein linkage region of proteoglycans. J. Biol. Chem. 2005, 280, 1417–1425. [Google Scholar] [PubMed]
- Nakagawa, N.; Izumikawa, T.; Kitagawa, H.; Oka, S. Sulfation of glucuronic acid in the linkage tetrasaccharide by HNK-1 sulfotransferase is an inhibitory signal for the expression of a chondroitin sulfate chain on thrombomodulin. Biochem. Biophys. Res. Commun. 2011, 415, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, T.; Mizumoto, S.; Nishimura, Y.; Tamura, J.; Yamada, S.; Sugahara, K. Involvement of human natural killer-1 (HNK-1) sulfotransferase in the biosynthesis of the GlcUA(3-O-sulfate)-Gal-Gal-Xyl tetrasaccharide found in α-thrombomodulin from human urine. J. Biol. Chem. 2011, 286, 33003–33011. [Google Scholar] [CrossRef] [PubMed]
- Fransson, L.A.; Havsmark, B.; Sakurai, K.; Suzuki, S. Sequence analysis of p-hydroxyphenyl-O-β-d-xyloside initiated and radio-iodinated dermatan sulfate from skin fibroblasts. Glycoconj. J. 1990, 9, 45–55. [Google Scholar] [CrossRef]
- Funderburgh, J.L. Keratan sulfate: Structure, biosynthesis, and function. Glycobiology 2000, 10, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Funderburgh, J.L.; Funderburgh, M.L.; Mann, M.M.; Conrad, G.W. Unique glycosylation of three keratan sulfate proteoglycan isoforms. J. Biol. Chem. 1991, 266, 14226–14231. [Google Scholar] [PubMed]
- Dunlevy, J.R.; Neame, P.J.; Vergnes, J.P.; Hassell, J.R. Identification of the N-linked oligosaccharide sites in chick corneal lumican and keratocan that receive keratan sulfate. J. Biol. Chem. 1998, 273, 9615–9621. [Google Scholar] [CrossRef] [PubMed]
- Barry, F.P.; Rosenberg, L.C.; Gaw, J.U.; Gaw, J.U.; Koob, T.J.; Neame, P.J. N- and O-linked keratan sulfate on the hyaluronan binding region of aggrecan from mature and immature bovine cartilage. J. Biol. Chem. 1995, 270, 20516–20524. [Google Scholar] [CrossRef] [PubMed]
- Barry, F.P.; Neame, P.J.; Sasse, J.; Pearson, D. Length variation in the keratan sulfate domain of mammalian aggrecan. Matrix Biol. 1994, 14, 323–328. [Google Scholar] [CrossRef]
- Krusius, T.; Finne, J.; Margolis, R.K.; Margolis, R.U. Identification of an O-glycosidic mannose-linked sialylated tetrasaccharide and keratan sulfate oligosaccharides in the chondroitin sulfate proteoglycan of brain. J. Biol. Chem. 1986, 261, 8237–8242. [Google Scholar] [PubMed]
- Brändli, A.W.; Hansson, G.C.; Rodriguez-Boulan, E.; Simons, K. A polarized epithelial cell mutant deficient in translocation of galactose into the Golgi complex. J. Biol. Chem. 1988, 263, 16283–16290. [Google Scholar] [PubMed]
- Toma, L.; Pinhal, M.A.; Dietrich, C.P.; Nader, H.B.; Hirschberg, C.B. Transport of UDP-galactose into the Golgi lumen regulates the biosynthesis of proteoglycans. J. Biol. Chem. 1996, 271, 3879–3901. [Google Scholar]
- Gardell, S.; Baker, J.; Caterson, B.; Heinegård, D.; Rodén, L. Link protein and a hyaluronic acid-binding region as components of aorta proteoglycan. Biochem. Biophys. Res. Commun. 1980, 95, 1823–1831. [Google Scholar] [CrossRef]
- Hardingham, T.E.; Ewins, R.J.F.; Muir, H. Cartilage proteoglycans. Structure and heterogeneity of the protein core and the effects of specific protein modifications on the binding to hyaluronate. Biochem. J. 1976, 157, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Von Figura, K.; Kiowsky, W.; Buddecke, E. Differently labelled glucosamine-precursor pooles for the biosynthesis of hyaluronate and heparan sulfate. Eur. J. Biochem. 1973, 40, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Rilla, K.; Oikari, S.; Jokela, T.A.; Hyttinen, J.M.T.; Kärnä, R.; Tammi, R.H.; Tammi, M.I. Hyaluronan synthase 1 (HAS1) requires higher cellular UDP-GlcNAc concentration than HAS2 and HAS3. J. Biol. Chem. 2013, 288, 5973–5983. [Google Scholar] [CrossRef] [PubMed]
- Itano, N.; Sawai, T.; Yoshida, M.; Lenas, P.; Yamada, Y.; Imagawa, M.; Shinomura, T.; Hamaguchi, M.; Yoshida, Y.; Ohnuki, Y.; et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 1999, 274, 25085–25092. [Google Scholar] [CrossRef] [PubMed]
- Kolset, S.O.; Prydz, K.; Pejler, G. Intracellular proteoglycans. Biochem. J. 2004, 379, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Haltiwanger, R.S.; Blomberg, G.; Hart, G.W. Glycosylation of nuclear and cytoplasmic proteins. Purification and characterization of a uridine diphospho-N-acetylglucosamine:polypeptide β-N-acetylglucosaminyltransferase. J. Biol. Chem. 1992, 267, 9005–9013. [Google Scholar] [PubMed]
- Vigetti, D.; Deleonibus, S.; Moretto, P.; Karousou, E.; Viola, M.; Bartolini, B.; Hascall, V.C.; Tammi, M.; de Luca, G.; Passi, A. Role of UDP-N-acetylglucosamine (GlcNAc) and O-GlcNAcylation of hyaluronan synthase 2 in the control of chondroitin and hyaluronan synthesis. J. Biol. Chem. 2012, 287, 35544–35555. [Google Scholar] [CrossRef] [PubMed]
- Mian, N. Analysis of cell-growth-phase-related variations in hyaluronate synthase activity of isolated plasma-membrane fractions of cultured human skin fibroblasts. Biochem. J. 1986, 237, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Bart, G.; Vico, N.O.; Hassinen, A.; Pujol, F.M.; Deen, A.J.; Ruusala, A.; Tammi, R.H.; Squire, A.; Heldin, P.; Kellokumpu, S.; et al. Fluorescence resonance energy transfer (FRET) and proximity ligation assays reveal functionally relevant homo- and heteromeric complexes among hyaluronan synthases HAS1, HAS2, and HAS3. J. Biol. Chem. 2015, 290, 11479–11490. [Google Scholar] [CrossRef] [PubMed]
- Mertens, G.; van der Scheuern, B.; van den Berghe, H.; David, G. Heparan sulfate expression in polarized epithelial cells: The apical sorting of glypican (GPI-anchored proteoglycan) is inversely related to its heparan sulfate content. J. Cell Biol. 1996, 132, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Paladino, S.; Lebreton, S.; Tivodar, S.; Formiggini, F.; Ossato, G.; Gratton, E.; Tramier, M.; Coppey-Moisan, M.; Zurzolo, C. Golgi sorting regulates organization and activity of GPI proteins at apical membranes. Nat. Chem. Biol. 2014, 10, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Svennevig, K.; Prydz, K.; Kolset, S.O. Proteoglycans in polarized epithelial Madin-Darby canine kidney cells. Biochem. J. 1995, 311, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Kolset, S.O.; Vuong, T.T.; Prydz, K. Apical secretion of chondroitin sulphate in polarized Madin-Darby canine kidney (MDCK) cells. J. Cell Sci. 1999, 112, 1797–1801. [Google Scholar] [PubMed]
- Tveit, H.; Dick, G.; Skibeli, V.; Prydz, K. A proteoglycan undergoes different modifications en route to the apical and basolateral surfaces of Madin-Darby canine kidney cells. J. Biol. Chem. 2005, 280, 29596–29603. [Google Scholar] [CrossRef] [PubMed]
- Vuong, T.T.; Prydz, K.; Tveit, H. Differences in the apical and basolateral pathways for glycosaminoglycan biosynthesis in Madin-Darby canine kidney cells. Glycobiology 2006, 16, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Prydz, K.; Dick, G.; Tveit, H. How many ways through the Golgi maze? Traffic 2008, 9, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Tveit, H.; Akslen, L.K.; Fagereng, G.L.; Tranulis, M.A.; Prydz, K. A secretory Golgi bypass route to the apical surface domain of epithelial MDCK cells. Traffic 2009, 10, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.; Grøndahl, F.; Prydz, K. Overexpression of the 3'-phosphoadenosine 5'-phosphosulfate (PAPS) transporter 1 increases sulfation of chondroitin sulfate in the apical pathway of MDCK II cells. Glycobiology 2008, 18, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Hafte, T.T.; Fagereng, G.L.; Prydz, K.; Grøndahl, F.; Tveit, H. Protein core-dependent glycosaminoglycan modification and glycosaminoglycan-dependent polarized sorting in epithelial Madin-Darby canine kidney cells. Glycobiology 2011, 21, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.C.; Thinakaran, G.; Slunt, H.H.; Sisodia, S.S. Metabolism of the amyloid precursor-like protein 2 in MDCK cells. Polarized trafficking occurs independent of the chondroitin sulfate glycosaminoglycan chain. J. Biol. Chem. 1995, 270, 12641–12645. [Google Scholar] [PubMed]
- Kobialka, S.; Beuret, N.; Ben-Tekaya, H.; Spiess, M. Glycosaminoglycan chains affect exocytic and endocytic protein traffic. Traffic 2009, 10, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Bexiga, M.G.; Simpson, J.C. Human diseases associated with form and function of the Golgi complex. Int. J. Mol. Sci. 2013, 14, 18670–18681. [Google Scholar] [CrossRef] [PubMed]
- Hennet, T.; Cabalzar, J. Congenital disorders of glycosylation: A concise chart of glycocalyx dysfunction. Trends Biochem. Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Palokangas, H.; Ying, M.; Väänänen, K.; Saraste, J. Retrograde transport from the pre-Golgi intermediate compartment and the Golgi complex is affected by the vacuolar H+-ATPase inhibitor bafilomycin A1. Mol. Biol. Cell 1998, 9, 3561–3578. [Google Scholar] [CrossRef] [PubMed]
- Seksek, O.; Biwersi, J.; Verkman, A.S. Direct measurement of trans-Golgi pH in living cells and regulation by second messengers. J. Biol. Chem. 1995, 270, 4967–4970. [Google Scholar] [CrossRef] [PubMed]
- Rivinoja, A.; Pojul, F.M.; Hassinen, A.; Kellokumpu, S. Golgi pH, its regulation and roles in human disease. Ann. Med. 2012, 44, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chang, A. pH-dependent cargo sorting from the Golgi. J. Biol. Chem. 2011, 286, 10058–10065. [Google Scholar] [CrossRef] [PubMed]
- Saroussi, S.; Nelson, N. Vacuolar H+-ATPase-an enzyme for all seasons. Pflugers. Arch. 2009, 457, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Holappa, K.; Muñoz, M.T.; Egea, G.; Kellokumpu, S. The AE2 anion exchanger is necessary for the structural integrity of the Golgi apparatus in mammalian cells. FEBS Lett. 2004, 564, 97–103. [Google Scholar] [CrossRef]
- Maeda, Y.; Ide, T.; Koike, M.; Uchiyama, Y.; Kinoshita, T. GPHR is a novel anion channel critical for acidification and functions of the Golgi apparatus. Nat. Cell Biol. 2008, 10, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Rosa, P.; Mantovani, S.; Rosboch, R.; Huttner, W. Monensin and brefeldin A differentially affect the phosphorylation and sulfation of secretory proteins. J. Biol. Chem. 1992, 267, 12227–12232. [Google Scholar] [PubMed]
- Axelsson, M.A.; Karlsson, N.G.; Steel, D.M.; Ouwendijk, J.; Nilsson, T.; Hansson, G.C. Neutralization of pH in the Golgi apparatus causes redistribution of glycosyltransferases and changes in the O-glycosylation of mucins. Glycobiology 2001, 11, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Rivinoja, A.; Hassinen, A.; Kokkonen, N.; Kauppila, A.; Kellokumpu, S. Elevated Golgi pH impairs terminal N-glycosylation by inducing mislocalization of Golgi glycosyltransferases. J. Cell. Physiol. 2009, 220, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Hassinen, A.; Pujol, F.M.; Kokkonen, N.; Pieters, C.; Kihlström, M.; Korhonen, K.; Kellokumpu, S. Functional organization of Golgi N- and O-glycosylation pathways involves pH-dependent complex formation that is impaired in cancer cells. J. Biol. Chem. 2011, 286, 38329–38340. [Google Scholar] [CrossRef] [PubMed]
- Kornak, U.; Reynders, E.; Dimopoulou, A.; van Reeuwijk, J.; Fischer, B.; Rajab, A.; Budde, B.; Nürnberg, P.; Foulquier, F.; ARCL Debré-type Study Group; et al. Impaired glycosylation and cutis laxa caused by mutations in the vesicular H+-ATPase subunit ATP6V0A2. Nat. Genet. 2008, 40, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Guillard, M.; Dimopoulou, A.; Fischer, B.; Morava, E.; Lefeber, D.J.; Kornak, U.; Wevers, R.A. Vacuolar H+-ATPase meets glycosylation in patients with cutis laxa. Biochim. Biophys. Acta 2009, 1792, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Rivinoja, A.; Kokkonen, N.; Kellokumpu, I.; Kellokumpu, S. Elevated Golgi pH in breast and colorectal cancer cells correlates with the expression of oncofetal carbohydrate T-antigen. J. Cell Physiol. 2006, 208, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Kizuka, Y. Glycans and cancer: Role of N-glycans in cancer biomarker, progression and metastasis, and therapeutics. Adv. Cancer Res. 2015, 126, 11–51. [Google Scholar] [PubMed]
- Caplan, M.J.; Stow, J.L.; Newman, A.P.; Madri, J.; Anderson, H.C.; Farquhar, M.G.; Palade, G.E.; Jamieson, J.D. Dependence on pH of polarized sorting of secreted proteins. Nature 1987, 329, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Grøndahl, F.; Tveit, H.; Prydz, K. Neutralization of endomembrane compartments in epithelial MDCK cells affects proteoglycan synthesis in the apical secretory pathway. Biochem. J. 2009, 418, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Pezzati, R.; Bossi, M.; Podini, P.; Meldolesi, J.; Grohovaz, F. High-resolution calcium mapping of the endoplasmic reticulum-Golgi-exocytic membrane system. Electron energy loss imaging analysis of quick frozen-freeze dried PC12 cells. Mol. Biol. Cell 1997, 8, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Aulestia, F.J.; Alonso, M.T.; García-Sancho, J. Differential calcium handling by the cis and trans regions of the Golgi apparatus. Biochem. J. 2015, 466, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Sannerud, R.; Flatmark, T.; Saraste, J. Colocalization of Ca2+-ATPase and GRP94 with p58 and the effects of thapsigargin on protein recycling suggest the participation of the pre-Golgi intermediate compartment in intracellular Ca2+ storage. Eur. J. Cell Biol. 2002, 81, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, A.; Peter, F.; Van, P.F.; Söling, H.D.; Hauri, H.P. A luminal calcium-binding protein with a KDEL endoplasmic reticulum retention motif in the ER-Golgi intermediate compartment. Eur. J. Cell Biol. 1993, 60, 366–370. [Google Scholar] [PubMed]
- Yamasaki, A.; Tani, K.; Yamamoto, A.; Kitamura, N.; Komada, M. The Ca2+-binding protein ALG-2 is recruited to endoplasmic reticulum exit sites by Sec31A and stabilizes the localization of Sec31A. Mol. Biol. Cell 2006, 17, 4876–4887. [Google Scholar] [CrossRef] [PubMed]
- Kienzle, C.; von Blume, J. Secretory cargo sorting at the trans-Golgi network. Trends Cell Biol. 2014, 24, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylova, M.; Reddy, P.P.; Munsch, T.; Landgraf, P.; Suman, S.K.; Smalla, K.H.; Gundelfinger, E.D.; Sharma, Y.; Kreutz, M.R. Calneurons provide a calcium threshold for trans-Golgi network to plasma membrane trafficking. Proc. Natl. Acad. Sci. USA 2009, 106, 9093–9098. [Google Scholar] [CrossRef] [PubMed]
- Faller, C.E.; Guvench, O. Sulfation and Cation Effects on the Conformational Properties of the Glycan Backbone of Chondroitin Sulfate Disaccharides. J. Phys. Chem. B. 2015. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Sakaguchi, K.; Yanagishita, M.; Aurbach, G.D.; Hascall, V.C. Extracellular calcium regulates distribution and transport of heparan sulfate proteoglycans in a rat parathyroid cell line. J. Biol. Chem. 1990, 265, 13661–13668. [Google Scholar] [PubMed]
- Yanagishita, M.; Brandi, M.L.; Sakaguchi, K. Extracellular calcium regulates distribution and transport of heparan sulfate proteoglycans in a rat parathyroid cell line. J. Biol. Chem. 1989, 264, 15714–15720. [Google Scholar] [PubMed]
- Muresan, Z.; MacGregor, R.R. The release of parathyroid hormone and the exocytosis of a proteoglycan are modulated by extracellular Ca2+ in a similar manner. Mol. Biol. Cell 1994, 5, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.C.; Iannotti, J.P.; Misra, S.; Richards, C.F. Effects of thapsigargin, an intracellular calcium-mobilizing agent, on synthesis and secretion of cartilage collagen and proteoglycan. J. Orthop. Res. 1994, 12, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Bouakka, M.; Legendre, P.; Jouis, V.; Langris, M.; Béliard, R.; Loyau, G.; Bocquet, J. Calcium ionophore and phorbol myristate acetate synergistically inhibited proteoglycan biosynthesis in articular chondrocytes by prostaglandin independent mechanism. Biochem. Biophys. Res. Commun. 1988, 153, 690–698. [Google Scholar] [CrossRef]
- Curwin, A.J.; von Blume, J.; Malhotra, V. Cofilin-mediated sorting and export of specific cargo from the Golgi apparatus in yeast. Mol. Biol. Cell 2012, 23, 2327–2338. [Google Scholar] [CrossRef] [PubMed]
- Kienzle, C.; Basnet, N.; Crevenna, A.H.; Beck, G.; Habermann, B.; Mizuno, N.; von Blume, J. Cofilin recruits F-actin to SPCA1 and promotes Ca2+-mediated secretory cargo sorting. J. Cell Biol. 2014, 206, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Gunn, P.A.; Gliddon, B.L.; Londrigan, S.L.; Lew, A.M.; van Driel, I.R.; Gleeson, P.A. The Golgi apparatus in the endomembrane-rich gastric parietal cells exist as functional stable mini-stacks dispersed throughout the cytoplasm. Biol. Cell 2011, 103, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Yamamoto-Hino, M.; Abe, M.; Kuwahara, R.; Haraguchi, S.; Kusaka, I.; Awano, W.; Kinoshita-Toyoda, A.; Goto, S. Distinct functional units of the Golgi complex in Drosophila cells. Proc. Natl. Acad. Sci. USA 2005, 102, 13467–13472. [Google Scholar] [CrossRef] [PubMed]
- Fuda, H.; Shimizu, C.; Lee, Y.C.; Akita, H.; Strot, C.A. Characterization and expression of human bifunctional 3′-phosphoadenosine 5′-phosphosulphate synthase isoforms. Biochem. J. 2002, 365, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, C.B.; Robbins, P.W.; Abeijon, C. Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annu. Rev. Biochem. 1998, 67, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Kawakita, M. Molecular physiology and pathology of the nucleotide sugar transporter family (SLC35). Pflugers. Arch. 2004, 447, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.Y.; Horvitz, H.R. The SQV-1 UDP-glucuronic acid decarboxylase and the SQV-7 nucleotide-sugar transporter may act in the Golgi apparatus to affect Caenorhabditis elegansvulval morphogenesis and embryonic development. Proc. Natl. Acad. Sci. USA 2002, 99, 14218–14223. [Google Scholar] [CrossRef] [PubMed]
- Dejima, K.; Seko, A.; Yamashita, K.; Gengyo-Ando, K.; Mitani, S.; Izumikawa, H.; Sugahara, K.; Mizuguchi, S.; Kazuya, N. Essential roles of 3′-phosphoadenosine 5′-phoshosulfate synthase in embryonic and larval development of the nematode Caenorhabditis elegans. J. Biol. Chem. 2006, 281, 11431–11440. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Stevens, L.M.; Stein, D. Synthesis of the sulfate donor PAPS in either the Drosophila germline or somatic follicle cells can support embryonic dorsal-ventral axis formation. Development 2007, 134, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Rijcken, W.R.P.; Overdijk, B.; van den Eijnden, D.H.; Ferwerda, W. The effect of increasing nucleotide sugar concentrations on the incorporation of sugars into glycoconjugates in rat hepatocytes. Biochem. J. 1995, 305, 865–870. [Google Scholar] [CrossRef]
- Cecchelli, R.; Cacan, R.; Porchet-Hennere, E.; Verbert, A. Dilatation of Golgi vesicles by monensin leads to enhanced accumulation of sugar nucleotides. Biosci. Rep. 1986, 6, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Caffaro, C.E.; Luhn, K.; Bakker, H.; Vestweber, D.; Samuelson, J.; Berninsone, P.; Hirschberg, C.B. A single Caenorhabditis elegans Golgi apparatus-type transporter of UDP-Glucose, UDP-Galactose, UDP-N-Acetylglucosamine, and UDP-N-Acetylgalactosamine. Biochemistry 2008, 47, 4337–4344. [Google Scholar] [CrossRef] [PubMed]
- Maszczak-Seneczko, D.; Olczak, T.; Jakimowicz, P.; Olczak, M. Overexpression of UDP-GlcNAc transporter partially corrects galactosylation defect caused by UDP-Gal transporter mutation. FEBS Lett. 2011, 585, 3090–3094. [Google Scholar] [CrossRef] [PubMed]
- Reiter, W.D.; Vanzin, G.F. Molecular genetics of nucleotide sugar interconversion pathways in plants. Plant Mol. Biol. 2001, 47, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Moriarity, J.L.; Hurt, K.J.; Resnick, A.C.; Storm, P.B.; Laroy, W.; Schnaar, R.L.; Snyder, S.H. UDP-glucuronate decarboxylase, a key enzyme in proteoglycan synthesis: Cloning, characterization, and localization. J. Biol. Chem. 2002, 277, 16968–16975. [Google Scholar] [CrossRef] [PubMed]
- Patterson, G.H.; Hirschberg, K.; Polishchuk, R.S.; Gerlich, D.; Phair, R.D.; Lippincott-Schwartz, J. Transport through the Golgi apparatus by rapid partitioning within a two-phase membrane system. Cell 2008, 133, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Miura, Y.; Etchison, J.R.; Freeze, H.H. Intact Golgi synthesize complex branched O-linked chains on glycoside primers: Evidence for the functional continuity of seven glycosyltransferases and three sugar nucleotide transporters. Glycoconj. J. 2001, 18, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Sprong, H.; Degroote, S.; Nilsson, T.; Kawakita, M.; Ishida, N.; van der Sluijs, P.; van Meer, G. Association of the Golgi UDP-galactose transporter with UDP-galactose:ceramide galactosyltransferase allows UDP-galactose import in the endoplasmic reticulum. Mol. Biol. Cell 2003, 14, 3482–3493. [Google Scholar] [CrossRef] [PubMed]
- Maszczak-Seneczko, D.; Sosicka, P.; Kaczmarek, B.; Majkowski, M.; Luzarowski, M.; Olczak, T.; Olczak, M. UDP-galactose (SLC35A2) and UDP-N-acetylglucosamine (SLC35A3) transporters form glycosylation-related complexes with mannoside acetylglucosaminyltransferases (Mgats). J. Biol. Chem. 2015, 290, 15475–15486. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, H. Large-scale measurement of absolute protein glycosylation stoichiometry. Anal. Chem. 2015, 87, 6479–6487. [Google Scholar] [CrossRef] [PubMed]
- Embery, G.; Hall, R.; Waddington, R.; Septier, D.; Goldberg, M. Proteoglycans in dentinogenesis. Crit. Rev. Oral Biol. Med. 2001, 12, 331–349. [Google Scholar] [CrossRef] [PubMed]
- Prydz, K.; Tveit, H.; Vedeler, A.; Saraste, J. Arrivals and departures at the plasma membrane: direct and indirect transport routes. Cell Tissue Res. 2013, 352, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Victor, X.V.; Nguyen, T.K.N.; Ethirajan, M.; Tran, V.M.; Nguyen, K.V.; Kuneran, B. Investigating the elusive mechanism of glycosaminoglycan biosynthesis. J. Biol. Chem. 2009, 284, 25842–25853. [Google Scholar] [CrossRef] [PubMed]
- Multhaupt, H.A.B.; Couchman, J.R. Heparan sulfate biosynthesis: Methods for investigation of the heparanosome. J. Histochem. Cytochem. 2012, 60, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Hassinen, A.; Kellokumpu, S. Organizational interplay of Golgi-N-glycosyltransferases involves organelle microenvironment-dependent transitions between enzyme homo- and heterodimers. J. Biol. Chem. 2014, 289, 26937–26948. [Google Scholar] [CrossRef] [PubMed]
- McCormick, C.; Duncan, G.; Goutsos, K.T.; Tufaro, F. The putative tumor suppressors EXT1 and EXT2 form a stable complex that accumulates in the Golgi apparatus and catalyzes the synthesis of heparan sulfate. Proc. Natl. Acad. Sci. USA 2000, 97, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Presto, J.; Thuveson, M.; Carlsson, P.; Busse, M.; Wilén, M.; Eriksson, I.; Kusche-Gullberg, M.; Kjellén, L. Heparan sulfate biosynthesis enzymes EXT1 and EXT2 affect NDST1 expression and heparan sulfate sulfation. Proc. Natl. Acad. Sci. USA 2008, 105, 4751–4756. [Google Scholar] [CrossRef] [PubMed]
- Dejima, K.; Takemura, M.; Nakato, E.; Peterson, J.; Hayashi, Y.; Kinoshita-Toyoda, A.; Toyoda, H.; Nakato, H. Analysis of Drosophila glucuronyl C5-epimerase. Implications for developmental roles of heparan sulfate sulfation compensation and 2-O-sulfated glucuronic acid. J. Biol. Chem. 2013, 288, 34384–34393. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, U. A personal voyage through the proteoglycan field. Matrix Biol. 2014, 35, 3–7. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prydz , K. Determinants of Glycosaminoglycan (GAG) Structure. Biomolecules 2015, 5, 2003-2022. https://doi.org/10.3390/biom5032003
Prydz K. Determinants of Glycosaminoglycan (GAG) Structure. Biomolecules. 2015; 5(3):2003-2022. https://doi.org/10.3390/biom5032003
Chicago/Turabian StylePrydz , Kristian. 2015. "Determinants of Glycosaminoglycan (GAG) Structure" Biomolecules 5, no. 3: 2003-2022. https://doi.org/10.3390/biom5032003
APA StylePrydz , K. (2015). Determinants of Glycosaminoglycan (GAG) Structure. Biomolecules, 5(3), 2003-2022. https://doi.org/10.3390/biom5032003




