Pharmacological and Therapeutic Potential of Chrysopogon zizanioides (Vetiver): A Comprehensive Review of Its Medicinal Applications and Future Prospects
Abstract
1. Introduction
2. Anti-Inflammatory Effects
3. Analgesic Effects
4. Antioxidant Properties
5. Antimicrobial Effects
6. Wound Healing Potential
7. Anticancer Activity
8. Conclusions
9. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rao, R.R.; Suseela, M.R. Vetiveria zizanioides (Linn.) Nash—A multipurpose eco–friendly grass of India. ICV–2 Held Cha–Am Phetchaburi Thail. 2000, 18–22. [Google Scholar]
- Anjum, D.S.; Varshney, D.S. Usheera (Vetiveria zizanioides Linn.): Ayurvedic Insights and Modern Pharmacological Evidence. TIJER—Int. Res. J. 2025, 12, a280–a292. Available online: https://tijer.org/tijer/viewpaperforall.php?paper=TIJER2507029 (accessed on 12 June 2025).
- Pareek, A.; Kumar, A. Ethnobotanical and pharmaceutical uses of Vetiveria zizanioides (linn) nash: A medicinal plant of Rajasthan. Int. J. Life Sci. Pharma Res. 2013, 3, L-12–L-18. Available online: https://www.semanticscholar.org/paper/ETHNOBOTANICAL-AND-PHARMACEUTICAL-USES-OF-VETIVERIA-Pareek-Kumar/801c3de40786552ef952fca56eede9e3eb92732d (accessed on 12 June 2025).
- Barcellos-Silva, I.G.C.; Dos Santos, F.K.F.; Kharkwal, H.; Chander, S.; Kharkwal, A.C.; Awasthi, R.; Dhiman, N.; Sharma, B.; Kulkarni, G.T.; Larssen, H.; et al. Vetiver, Vetiveria zizanioides (L.) Nash: Biotechnology, Biorefineries, and the Production of Volatile Phytochemicals. Plants 2025, 14, 1435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Belhassen, E.; Filippi, J.-J.; Brévard, H.; Joulain, D.; Baldovini, N. Volatile constituents of vetiver: A review. Flavour. Fragr. J. 2014, 30, 26–82. [Google Scholar] [CrossRef]
- Ramírez-Rueda, R.; Marinho, J.; Salvador, M. Bioguided identification of antimicrobial compounds from Chrysopogon zizaniodes (L.) roberty root essential oil. Future Microbiol. 2019, 14, 1179–1189. [Google Scholar] [CrossRef]
- Devprakash, D.; Prashant Singh, P.S.; Srinivasan, K.K.; Subburaju, T.; Singh, S.K. Antifungal activity of alcoholic and aqueous extracts of Vetiveria zizanioides. J. Pharm. Res. Opin. 2011, 1, 85–88. [Google Scholar]
- Arafat, M.A.M.; Khalil, M.N.A.; Mohamed, O.G.; Abd El-Ghafar, O.A.M.; Tripathi, A.; Mahrous, E.A.; Abd El-kader, E.M.; El-Hawary, S. Vetiver aerial parts and roots ameliorate rheumatoid arthritis in complete Freund’s adjuvant rat model, a phytochemical profiling and mechanistic study. J. Ethnopharmacol. 2023, 317, 116764. [Google Scholar] [CrossRef]
- Grover, M.; Behl, T.; Bungau, S.; Aleya, L. Potential therapeutic effect of Chrysopogon zizanioides (Vetiver) as an anti-inflammatory agent. Env. Sci. Pollut. Res. Int. 2021, 28, 15597–15606. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nunes, C.D.R.; Barreto Arantes, M.; Menezes de Faria Pereira, S.; Leandro da Cruz, L.; de Souza Passos, M.; Pereira de Moraes, L.; Vieira, I.J.C.; Barros de Oliveira, D. Plants as Sources of Anti-Inflammatory Agents. Molecules 2020, 25, 3726. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beg, S.; Swain, S.; Hasan, H.; Barkat, M.A.; Hussain, M.S. Systematic review of herbals as potential anti-inflammatory agents: Recent advances, current clinical status and future perspectives. Pharmacogn. Rev. 2011, 5, 120–137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gonfa, Y.H.; Tessema, F.B.; Bachheti, A.; Rai, N.; Tadesse, M.G.; Singab, A.N.; Chaubey, K.K.; Bachheti, R.K. Anti-inflammatory activity of phytochemicals from medicinal plants and their nanoparticles: A review. Curr. Res. Biotechnol. 2023, 6, 100152. [Google Scholar] [CrossRef]
- Lunz, K.; Stappen, I. Back to the Roots-An Overview of the Chemical Composition and Bioactivity of Selected Root-Essential Oils. Molecules 2021, 26, 3155. [Google Scholar] [CrossRef]
- Chou, S.T.; Lai, C.P.; Lin, C.C.; Shih, Y. Study of the chemical composition, antioxidant activity and anti-inflammatory activity of essential oil from Vetiveria zizanioides. Food Chem. 2012, 134, 262–268. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Narkhede, M.B.; Wagh, A.E.; Rathi, A.M. Anti-inflammatory activity of Vetiveria zizanioides (linn.) Root. J. Pharm. Res. 2012, 5, 2016–2017. [Google Scholar]
- Mai, H.P.; Lê, K.K.L. Evaluation of the anti-inflammatory activity of Vetiver root extract in Dak Lak, Viet Nam. HCMUE J. Sci. 2024, 21, 407. [Google Scholar] [CrossRef]
- Frow, E.K.; Reckless, J.; Grainger, D.J. Tools for anti-inflammatory drug design: In vitro models of leukocyte migration. Med. Res. Rev. 2004, 24, 276–298. [Google Scholar] [CrossRef]
- Singh, D.; Rai, V.; Agrawal, D.K. Regulation of Collagen I and Collagen III in Tissue Injury and Regeneration. Cardiol. Cardiovasc. Med. 2023, 7, 5–16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lima, J.T.; Rocha, R.F.; Moreira, J.C.F.; Araújo, A.A.S. Phytochemical screening, antinociceptive and anti-inflammatory activities of Chrysopogon zizanioides essential oil. Rev. Bras. Farmacogn. 2012, 22, 443–450. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boyce, B.F.; Xiu, Y.; Li, J.; Xing, L.; Yao, Z. NF-κB-Mediated Regulation of Osteoclastogenesis. Endocrinol. Metab. 2015, 30, 35–44. [Google Scholar] [CrossRef]
- Ageeva, T.; Rizvanov, A.; Mukhamedshina, Y. NF-κB and JAK/STAT Signaling Pathways as Crucial Regulators of Neuroinflammation and Astrocyte Modulation in Spinal Cord Injury. Cells 2024, 13, 581. [Google Scholar] [CrossRef]
- Nakano, R.; Kitanaka, T.; Namba, S.; Kitanaka, N.; Suwabe, Y.; Konno, T.; Yamazaki, J.; Nakayama, T.; Sugiya, H. Non-Transcriptional and Translational Function of Canonical NF-κ B Signaling in Activating ERK1/2 in IL-1 β-Induced COX-2 Expression in Synovial Fibroblasts. Front. Immunol. 2020, 11, 579266. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, J.R.; Chebib, M.; Johnston, G.A. Flavonoid modulation of GABA(A) receptors. Br. J. Pharmacol. 2011, 163, 234–245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wasowski, C.; Marder, M. Flavonoids as GABAA receptor ligands: The whole story? J. Exp. Pharmacol. 2012, 4, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, J.C.; Shin, N.S. Efficacy of opioids for chronic pain: A review of the evidence. Clin. J. Pain 2008, 24, 469–478. [Google Scholar] [CrossRef]
- Milani, D.A.Q.; Davis, D.D. Pain management medications. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560692/ (accessed on 12 June 2025).
- O’Brien, T.; Christrup, L.L.; Drewes, A.M.; Fallon, M.T.; Kress, H.G.; McQuay, H.J.; Mikus, G.; Morlion, B.J.; Perez-Cajaraville, J.; Pogatzki-Zahn, E.; et al. European Pain Federation position paper on appropriate opioid use in chronic pain management. Eur. J. Pain 2017, 21, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. Sci. 2013, 16, 821–847. [Google Scholar] [CrossRef] [PubMed]
- Borde, B.; Dubey, S.; Agrawal, S.; Bhalerao, A.; Kadam, K.; Borde, G. Vetiver zizanioides and their Pharmacological Activity. Introduction 2023, 28, 240–247. Available online: https://ijppr.humanjournals.com/wp-content/uploads/2023/12/18.Bhagyashri-Borde-Suchita-Dubey-Shivam-Agrawal-Ankush-Bhalerao-Kamlesh-Kadam-Dr.-Geeta-Borde.pdf (accessed on 12 June 2025).
- McCurdy, C.R.; Scully, S.S. Analgesic substances derived from natural products (natureceuticals). Life Sci. 2005, 78, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Akash, S.; Akter, M.A.; Talukder, C.; Mim, S.A.; Rahman, A.; Obaid, A.A.; Islam, M.; Himu, J.I.; Aziz, T.; Rashid, M.A.; et al. Antioxidant, Antidiabetic, Analgesic, and Antibacterial Properties of Chrysopogon zizanioides Leaf Extract: An In Vivo, In Vitro, and In Silico Evaluation. preprint (Version 1). 2024. Available online: https://www.researchsquare.com/article/rs-5219178/v1 (accessed on 12 June 2025).
- Paredes, S.; Cantillo, S.; Candido, K.D.; Knezevic, N.N. An Association of Serotonin with Pain Disorders and Its Modulation by Estrogens. Int. J. Mol. Sci. 2019, 20, 5729. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, S.; Zhang, B.; Wang, D.; Hu, S.; Wang, W.; Liu, C.; Wu, Z.; Yang, C. Role of GABAergic system in the comorbidity of pain and depression. Brain Res. Bull. 2023, 200, 110691. [Google Scholar] [CrossRef]
- Wang, Z.J.; Heinbockel, T. Essential Oils and Their Constituents Targeting the GABAergic System and Sodium Channels as Treatment of Neurological Diseases. Molecules 2018, 23, 1061. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Böhm, C.; Wiessler, A.L.; Janzen, D.; Nausester, J.; Slavik, B.; Loos, H.M.; Villmann, C.; Buettner, A. Modulatory effect of various essential oils on different GABAA receptor subtypes present in the central nervous system. Phytomedicine Plus 2025, 5, 100852. [Google Scholar] [CrossRef]
- Fung, T.K.H.; Lau, B.W.M.; Ngai, S.P.C.; Tsang, H.W.H. Therapeutic Effect and Mechanisms of Essential Oils in Mood Disorders: Interaction between the Nervous and Respiratory Systems. Int. J. Mol. Sci. 2021, 22, 4844. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hao, C.W.; Lai, W.S.; Ho, C.T.; Sheen, L.Y. Antidepressant-like effect of lemon essential oil is through a modulation in the levels of norepinephrine, dopamine, and serotonin in mice: Use of the tail suspension test. J. Funct. Foods 2013, 5, 370–379. [Google Scholar] [CrossRef]
- Guimarães, A.G.; Quintans, J.S.; Quintans, L.J., Jr. Monoterpenes with analgesic activity—A systematic review. Phytother. Res. 2013, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- de Cássia Da Silveira e Sá, R.; Andrade, L.N.; De Sousa, D.P. Sesquiterpenes from essential oils and anti-inflammatory activity. Nat. Prod. Commun. 2015, 10, 1934578X1501001033. [Google Scholar] [CrossRef]
- Kiokias, S.; Oreopoulou, V. A Review of the Health Protective Effects of Phenolic Acids against a Range of Severe Pathologic Conditions (Including Coronavirus-Based Infections). Molecules 2021, 26, 5405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, H.J.; Chen, F.; Wang, X.; Chung, H.Y.; Jin, Z. Evaluation of antioxidant activity of vetiver (Vetiveria zizanioides L.) oil and identification of its antioxidant constituents. J. Agric. Food Chem. 2005, 53, 7691–7695. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, X.; Kim, H. Antioxidant, Anticarcinogenic and Termiticidal Activities of Vetiver Oil. 2003. Available online: https://www.semanticscholar.org/paper/Antioxidant-%2C-Anticarcinogenic-and-Termiticidal-of-Chen-Wang/6cdfdbaf5bd8a1924ed96c5a97e822871bcca064 (accessed on 12 June 2025).
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.P.; Sreenivasan, K.K.; Subburaju, T. Comparative antioxidant studies of ethanol extract and fresh aqueous extract of Vetiveria zizanioides. Int. J. Pharm. Pharm. Sci. 2011, 3, 325–331. [Google Scholar]
- Luqman, S.; Kumar, R.; Kaushik, S.; Srivastava, S.; Darokar, M.P.; Khanuja, S.P. Antioxidant potential of the root of Vetiveria zizanioides (L.) Nash. Indian J. Biochem. Biophys. 2009, 46, 122–125. [Google Scholar]
- El Ayadi, A.; Salsbury, J.R.; Enkhbaatar, P.; Herndon, D.N.; Ansari, N.H. Metal chelation attenuates oxidative stress, inflammation, and vertical burn progression in a porcine brass comb burn model. Redox Biol. 2021, 45, 102034. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wassmann, S.; Wassmann, K.; Nickenig, G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension 2004, 44, 381–386. [Google Scholar] [CrossRef]
- Grover, M.; Behl, T.; Virmani, T. Phytochemical Screening, Antioxidant Assay and Cytotoxic Profile for Different Extracts of Chrysopogon zizanioides Roots. Chem. Biodivers. 2021, 18, e2100012. [Google Scholar] [CrossRef]
- Tarai, D.K.; Nayak, S.; Karan, S. In vitro free radical scavenging activity of Vetiveria zizanioides. J. Pharm. Res. 2010, 3, 681–683. [Google Scholar]
- Jeddi, M.; Fikri-Benbrahim, K.; El Hachlafi, N.; Benkhaira, N.; Aboussemdai, A.; Ouaritini, Z.B. Chemical Composition of Thymus vulgaris, Origanum compactumand Vetiveria zizanoides Essential oils and their Antibacterial and Antioxidant Activities. Trop. J. Nat. Prod. Res. 2023, 7, 2244–2250. [Google Scholar] [CrossRef]
- Samaan, M.; Ebid, M.; Thabet, M. GC-MS analysis, antioxidant capacity and antimicrobic action of Vetiveria zizanioides. Essential oil cultivated in north Egypt. J. Plant Prod. 2022, 13, 699–704. [Google Scholar] [CrossRef]
- Soidrou, S.H.; Farah, A.; Satrani, B.; Ghanmi, M.; Lachkar, M.; Mohamed, A.S. Chemical composition, antioxidant and antimicrobial activity of Vetiveria zizanioides roots essential oil harvested in Ndzuwani, Comoros. World J. Pharm. Pharm. Sci. 2020, 9, 82–95. [Google Scholar]
- Hewawasam, R.P.; Jayatilaka, K.A.P.W. Antioxidant effect of crude water extract of Vetiveria zizanioides (Gramineae) in mice with acetaminophen induced hepatotoxicity. Int. J. Pharmacogn. 2015, 2, 11–20. [Google Scholar] [CrossRef]
- Prajna, J.; Richa, J.; Dipjyoti, C. HPLC quantification of phenolic acids from Vetiveria zizanioides (L.) Nash and its antioxidant and antimicrobial activity. J. Pharm. 2013, 2013, 270472. [Google Scholar] [CrossRef]
- Hassanpour, S.H.; Doroudi, A. Review of the antioxidant potential of flavonoids as a subgroup of polyphenols and partial substitute for synthetic antioxidants. Avicenna J. Phytomed. 2023, 13, 354–376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- David, A.; Wang, F.; Sun, X.; Li, H.; Lin, J.; Li, P.; Deng, G. Chemical composition, antioxidant, and antimicrobial activities of Vetiveria zizanioides (L.) nash essential oil extracted by carbon dioxide expanded ethanol. Molecules 2019, 24, 1897. [Google Scholar] [CrossRef]
- Subhadradevi, V.; Asokkumar, K.; Umamaheswari, M.; Sivashanmugam, A.T.; Sankaranand, R. In vitro antioxidant activity of Vetiveria zizanioides root extract. Tanzania J. Health Res. 2010, 12, 138–143. [Google Scholar] [CrossRef]
- Ali, S.; Arthanari, A.; Shanmugam, R. Antioxidant activity of silver nanoparticles synthesized using Vetiveria zizanioides-in vitro study. J. Res. Med. Dent. Sci. 2021, 9, 199–203. Available online: https://www.jrmds.in/articles/antioxidant-activity-of-silver-nanoparticles-synthesized-using-vetiveria-zizanioidesin-vitro-study.pdf (accessed on 12 June 2025).
- Muthukrishnan, S.; Manogaran, P. Phytochemical analysis and free radical scavenging potential activity of Vetiveria zizanioides Linn. J. Pharmacogn. Phytochem. 2018, 7, 1955–1960. Available online: https://www.phytojournal.com/archives/2018/vol7issue2/PartAB/7-2-24-402.pdf (accessed on 12 June 2025).
- Kandsi, F.; Abdnim, R.; Benkhaira, N.; Zahra Lafdil, F.; Bnouham, M.; Yamani, B.; Naceiri Mrabti, H.; Wondmie, G.F.; Bin Jardan, Y.A.; Ibenmoussa, S.; et al. Integrated assessment of phytochemicals, Antilipase, hemoglobin antiglycation, antihyperglycemic, antifungal and antibacterial properties of Vetiveria zizanioides (L.) Nash. Int. J. Food Prop. 2024, 27, 1150–1166. [Google Scholar] [CrossRef]
- Devi, S.V.; Kumar, K.A.; Maheswari, M.U.; Shanmugam, A.T.S.; Anand, R.S. In vitro antibacterial activity of ethanolic extract of Vetiveria zizaniodes root. Int. J. Pharm. Sci. Res. 2010, 1, 120–124. [Google Scholar] [CrossRef]
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef]
- Hulankova, R. Methods for Determination of Antimicrobial Activity of Essential Oils In Vitro-A Review. Plants 2024, 13, 2784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oliveira, T.A.S.; Vieira, T.M.; Esperandim, V.R.; Martins, C.H.G.; Magalhães, L.G.; Miranda, M.L.D.; Crotti, A.E.M. Antibacterial, Antiparasitic, and Cytotoxic Activities of Chemical Characterized Essential Oil of Chrysopogon zizanioides Roots. Pharmaceuticals 2022, 15, 967. [Google Scholar] [CrossRef]
- Dos Santos, D.S.; Oberger, J.V.; Niero, R.; Wagner, T.; Delle Monache, F.; Cruz, A.B.; Martin-Quintal, Z.; Cechinel Filho, V. Seasonal phytochemical study and antimicrobial potential of Vetiveria zizanioides roots. Acta Pharm. 2014, 64, 495–501. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Shinjyo, Y.; Midorikawa, N.; Matsumoto, T.; Sugaya, Y.; Ozawa, Y.; Oana, A.; Horie, C.; Yoshikawa, H.; Takahashi, Y.; Hasegawa, T.; et al. Analysis of cell death in Bacillus subtilis caused by sesquiterpenes from Chrysopogon zizanioides (L.) Roberty. J. Gen. Appl. Microbiol. 2022, 68, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.; Parab, M.; Jain, T.; Gharat, Y.; Koli, J. Essential Oil of Chrysopogon Zizanioides Increases Membrane Permeability, Disturbs Cell Membrane Integrity, and Suppresses the Growth of Methicillin-Resistant Staphylococcus Aureus (MRSA). J. Pharm. Drug Res. 2023, 6, 638–649. [Google Scholar]
- Nantachit, K.; Bunchoo, M.; Khantava, B.; Khamvan, C. Antimicrobial activity of alkaloid from roots of Vetiveria zizanoides (L.) Nash ex Small. Thai Pharm. Health Sci. J. 2010, 5, 99–102. [Google Scholar]
- Devanathadesikan Seshadri, V.; Vijayaraghavan, P.; Kim, Y.O.; Kim, H.J.; Ahmed Al-Ghamdi, A.; Elshikh, M.S.; Al-Dosary, M.A.; Alsubaie, Q.D. In vitro antioxidant and cytotoxic activities of polyherbal extracts from Vetiveria zizanioides, Trichosanthes cucumerina, and Mollugo cerviana on HeLa and MCF-7 cell lines. Saudi J. Biol. Sci. 2020, 27, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Luqman, S.; Srivastava, S.; Darokar, M.P.; Khanuja, S.P. Detection of Antibacterial Activity in Spent Roots of Two Genotypes of Aromatic Grass Vetiveria zizanioides. Pharm. Biol. 2005, 43, 732–736. [Google Scholar] [CrossRef][Green Version]
- Burger, P.; Landreau, A.; Watson, M.; Janci, L.; Cassisa, V.; Kempf, M.; Azoulay, S.; Fernandez, X. Vetiver Essential Oil in Cosmetics: What Is New? Medicines 2017, 4, 41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Premjanu, N.; MR, R.K.; Srividya, S.S.; Malathy, B.R.; Revathy, R. Antibiofilm activity of ethanolic root extract of Vetiveria zizanioides against dental pathogens. Curr. Trends Biotechnol. Pharm. 2024, 18, 88–105. [Google Scholar] [CrossRef]
- Ghosh, T.; Biswas, M.K.; Maity, D.; Dutta, P. Study of Antibacterial Activity of Chrysopogon zizanioids (vetiver) and its Anti-venom Potential Aspect. Res. J. Agric. Sci. 2020, 11, 889–894. [Google Scholar]
- De Zoysa, M.H.N.; Rathnayake, H.; Hewawasam, R.P.; Wijayaratne, W.M.D.G.B. Determination of In Vitro Antimicrobial Activity of Five Sri Lankan Medicinal Plants against Selected Human Pathogenic Bacteria. Int. J. Microbiol. 2019, 2019, 7431439. [Google Scholar] [CrossRef]
- Rathnayake, H.; De Zoysa, M.H.N.; Hewawasam, R.P.; Wijayaratne, W.M.D.G.B. Comparison of In Vitro Antibacterial Activity of Epaltes divaricata and Vetiveria zizanioides against Methicillin-Resistant Staphylococcus aureus. Scientifica 2020, 2020, 8239053. [Google Scholar] [CrossRef]
- Orchard, A.; Viljoen, A.; van Vuuren, S. Wound Pathogens: Investigating Antimicrobial Activity of Commercial Essential Oil Combinations against Reference Strains. Chem. Biodivers. 2018, 15, e1800405. [Google Scholar] [CrossRef]
- Seil, J.T.; Webster, T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomed. 2012, 7, 2767–2781. [Google Scholar] [CrossRef]
- Gupta, N.; Rai, D.B.; Jangid, A.K.; Kulhari, H. Use of nanotechnology in antimicrobial therapy. In Methods in Microbiology; Academic Press: Cambridge, MA, USA, 2019; Volume 46, pp. 143–172. [Google Scholar] [CrossRef]
- Ram, G.D.; Kumar, S.P.; Srinivasan, T.K.; Aravind, T.; Ramya, S.; Lingaraja, D.; Bhuvaneshwari, G. Green synthesis of silver nanoparticles using Chrysopogon zizanioides root extract and their antibacterial activities. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- He, X.; Gao, X.; Xie, W. Research Progress in Skin Aging, Metabolism, and Related Products. Int. J. Mol. Sci. 2023, 24, 15930. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Polito, M.P.; Romaldini, A.; Rinaldo, S.; Enzo, E. Coordinating energy metabolism and signaling pathways in epithelial self-renewal and differentiation. Biol. Direct. 2024, 19, 63. [Google Scholar] [CrossRef]
- Pfisterer, K.; Shaw, L.E.; Symmank, D.; Weninger, W. The Extracellular Matrix in Skin Inflammation and Infection. Front. Cell Dev. Biol. 2021, 9, 682414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Tollenaere, M.; Chapuis, E.; Lapierre, L.; Bracq, M.; Hubert, J.; Lambert, C.; Sandré, J.; Auriol, D.; Scandolera, A.; Reynaud, R. Overall renewal of skin lipids with Vetiver extract for a complete anti-ageing strategy. Int. J. Cosmet. Sci. 2021, 43, 165–180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, X.; Parker, T.L. Biological activity of vetiver (Vetiveria zizanioides) essential oil in human dermal fibroblasts. Cogent Med. 2017, 4, 1298176. [Google Scholar] [CrossRef]
- Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U.; et al. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int. J. Mol. Sci. 2023, 24, 4607. [Google Scholar] [CrossRef] [PubMed]
- Arafat, M.; Khalil, M.; Mahrous, E.A.; El Hawary, S. Volatiles composition of fresh aroma and hydrodistilled volatile oil of Chrysopogon zizanioides roots growing in Egypt along with the cytotoxic activities of the hydrodistilled oil. Egypt. J. Chem. 2024, 67, 299–305. [Google Scholar] [CrossRef]
- Hanifa, M.; Wulandari, R.; Zulfin, U.M.; Nugroho, E.P.; Haryanti, S.; Meiyanto, E. Different Cytotoxic Effects of Vetiver Oil on Three Types of Cancer Cells, Mainly Targeting CNR2 on TNBC. Asian Pac. J. Cancer Prev. 2022, 23, 241–251. [Google Scholar] [CrossRef]
- Intan, C.J.; Mualifah, M.; Dwiranti, A.; Saifudin, S. Comparative study on sources of vetiver extracts (Vetiveria zizanioides L.) against HeLa cells growth using mitotic index (MI) analysis. AIP Conf. Proc. 2024, 3163, 070004. [Google Scholar] [CrossRef]
- Saputra, L.O.; Fawzy, S.Y.; Wardani, R.K.; Lukitaningsih, E. The Exploration of Vetiver (Vetiveria zizanioides) as Co-Chemotherapy of Lung Cancer Selectively Targets AKR1C1: Bioinformatics Approach. Indones. J. Cancer Chemoprevention 2022, 13, 114–127. [Google Scholar] [CrossRef]
- Sunitha, V.S.; Midhun, S.J.; Sunil, M.A.; Radhakrishnan, E.K.; Mathew, J. Valencene-rich fraction from Vetiveria zizanioides exerts immunostimulatory effects in vitro and in mice. Asian Pac. J. Trop. Biomed. 2021, 11, 335–343. [Google Scholar] [CrossRef]
- Divya, P.V.; Sukesh, K. Cytotoxicity of Amaranthus viridis and Vetiveria zizanioides against human embryonic kidney cells. J. Phytopharm. 2025, 14, 10–13. [Google Scholar] [CrossRef]
- Peng, H.Y.; Lai, C.C.; Lin, C.C.; Chou, S.T. Effect of Vetiveria zizanioides essential oil on melanogenesis in melanoma cells: Downregulation of tyrosinase expression and suppression of oxidative stress. Sci. World J. 2014, 2014, 213013. [Google Scholar] [CrossRef]
- Sinha, S.; Jothiramajayam, M.; Ghosh, M.; Jana, A.; Chatterji, U.; Mukherjee, A. Vetiver oil (Java) attenuates cisplatin-induced oxidative stress, nephrotoxicity and myelosuppression in Swiss albino mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2015, 81, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Fadhillah, S.S.; Aini, A.Q.; Mualifah, S.; Bowolaksono, A.; Dwiranti, A. Effects of Vetiveria zizanioides on Cytotoxicity of Human Epithelial Cervical Cancer Cells. J. Hunan Univ. Nat. Sci. 2022, 49, 329–334. [Google Scholar]
- Grover, M.; Behl, T.; Virmani, T.; Sanduja, M.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Meraya, A.M.; Bungau, S.G. Exploration of Cytotoxic Potential of Longifolene/Junipene Isolated from Chrysopogon zizanioides. Molecules 2022, 27, 5764. [Google Scholar] [CrossRef]

| Study Design | Experimental Model | Extract Type and Bioactive Compounds Involved | Dosage | Main Observed Effects | References |
|---|---|---|---|---|---|
| In vitro | RAW 264.7 macrophages (LPS-induced) | Essential oil; major constituents: cedr-8-en-13-ol (12.4%), α-amorphene (7.8%), β-vatirenene (5.94%), α-gurjunene (5.91%) | 5–12.5 μg/mL | NO ↓ (to 26.5% at 12.5 μg/mL), iNOS ↓ (~67%), COX-2 ↓ (~75%), TNF-α ↓ (≥23%), IL-1β ↓ (up to 81%), IFN-β ↓; HO-1 ↑ (10–25%); superoxide anion ↓ 12–20%; lipid peroxidation ↓; apoptosis ↓ | [16] |
| In vivo | CFA-induced rheumatoid arthritis in Wistar albino rats | C. zizanioides aerial (CA, phenolic-rich: flavonoids, lignans, flavolignans) and root (CR, triterpene- and sesquiterpene-rich) ethanolic extracts | 200 mg/kg | ACPA ↓, IL-6 ↓, TNF-α ↓, IL-10 ↑ (CA > CR); JAK2/STAT3 ↓, SOCs3 ↑; ERK1/ERK2 ↓; TRAF6/c-FOS/NFATC1 ↓ (CA stronger), NF-κB ↓; RANKL ↓; synovial inflammation, pannus formation, cartilage destruction ↓ | [8] |
| In vivo | Carrageenan-induced paw edema and cotton pellet-induced granuloma in Wistar rats | Root methanol extract | 300–600 mg/kg | Acute: paw edema ↓ 66.17% at 600 mg/kg (6 h); chronic: granuloma formation ↓ 53.69% at 600 mg/kg; dose-dependent inhibition of early (histamine, serotonin, kinins) and late (prostaglandin) mediators; fibroblast proliferation and collagen synthesis ↓ | [18] |
| In vitro | Albumin denaturation assay | Root ethanol extract; bioactives: vetiverol, vetivones | 0–240 µg/mL | Protein denaturation ↓ dose-dependently; IC50 = 157.63 µg/mL (~2.8 × less potent than diclofenac) | [19] |
| Extract Type | Bioactive Compound Involved | Model/Test Used | Key Findings | Comparative Efficacy | References |
|---|---|---|---|---|---|
| Root essential oil | khusimol, E-isovalencenol, α-/β-vetivone |
|
| Aspirin inhibited writhing ~75%; vetiver EO achieved ~65% inhibition. Demonstrated peripheral-only analgesic action. | [22] |
| Ethanolic leave extract | GC/MS profiled 63 compounds including esters, sesquiterpenes, alcohols, hydrocarbons. Major compounds: 9,19-Cyclolanostan-3-ol acetate (8.8%), 13-Docosenamide (8.35%), γ-Sitosterol (5.2%) |
| ~66.08% analgesic activity | Compared to 91.11% for diclofenac sodium. | [35] |
| Study Design | Extract Type and Bioactive Compounds Identified | Assay/Model | Key Findings | Mechanism | References |
|---|---|---|---|---|---|
| In vitro | Ethanolic leaf extract of C. zizanoides; 63 phytoconstituents identified including 9,19-Cyclolanostan-3-ol acetate (3β) and Phytol | DPPH radical scavenging assay | Moderate antioxidant activity (IC50 = 257.23 µg/mL) compared to ascorbic acid. | Antioxidant via radical scavenging and SOD, catalase, GPx | [35] |
| In vitro | Aqueous and ethanolic whole plant extracts; glycosides, carbohydrates, phenols, flavonoids, saponins, gums, mucilage | FRAP, Nitric oxide scavenging, Hydrogen peroxide scavenging, DPPH-RSA | Ethanolic extract showed higher antioxidant activity than aqueous; correlated with phenolic and flavonoid content. | Antioxidant activity via free radical scavenging and electron donation | [49] |
| In vitro | Crude oil; β-vetivenene, β-vetivone, α-vetivone, khusimol, bicyclovetivenol | DPPH radical scavenging, Fe2+ metal chelating | Strong DPPH scavenging (~93% at 10 µL/mL); weak metal chelation. | Free radical scavenging via terpenoid constituents | [45] |
| In vitro | Essential oil; cedr-8-en-13-ol, α-amorphene, β-vatirenene, α-gurjunene | LPS-stimulated RAW 264.7 macrophages; superoxide anion, MDA, SOD assays | ↓ Superoxide anion (12–20%), ↓ MDA, ↓ NO, ↓ apoptosis. | Anti-inflammatory: ↓ HO-1, iNOS, COX-2, TNF-α, IL-1β, IFN-β; Antioxidant: ↓ oxidative stress and lipid peroxidation | [16] |
| In vitro | C. zizanioides oil; complex mixture of terpenoids | DPPH radical scavenging assay | Strong antioxidant activity (93% scavenging at 10 µL/mL), ~α-tocopherol, >BHT. | Free radical scavenging by components of C. zizanioides oil | [46] |
| In vitro | Hexane root extracts of two genotypes; phenolics and flavonoids | FRAP, DPPH, TAC, RP, TPC; oxidative stress in erythrocytes (H2O2, t-BHP) | KS1 genotype (spent root KSD) showed the highest antioxidant activity (FRAP, DPPH, TPC); protected GSH and ↓ MDA under H2O2 stress. | Free radical scavenging via phenolics; antioxidant protection of erythrocytes | [50] |
| In vitro | Root essential oil extracted via CXE, HD, IVD, and SFE; major components valerenol, valerenal, β-cadinene, β-vetivenene | DPPH radical scavenging | CXE oil showed moderate antioxidant activity (IC50 3.71 mg/mL). | Free radical scavenging via terpenoid constituents | [61] |
| In vitro | Root ethanolic extract; contains alkaloids, flavonoids, tannins, phenols, saponins, triterpenoids | Reducing power assay, superoxide anion scavenging, deoxyribose degradation, total antioxidant capacity, total phenolics and flavonoids | Dose-dependent strong antioxidant activity; superoxide IC50 130.36 µg/mL; high phenolic and flavonoid content. | Free radical scavenging; hydroxyl radical neutralization; lipid peroxidation inhibition | [62] |
| In vitro | Silver nanoparticles synthesized from aqueous extract of Vetiveria zizanioides | DPPH free radical scavenging assay | Dose-dependent antioxidant activity; max inhibition 72.4% at 50 µL; comparable to standard. | Free radical scavenging by nanoparticle-mediated electron donation | [63] |
| In vitro | Aqueous, methanolic, and ethanol root extracts; bioactive compounds include phenolics, flavonoids, alkaloids, saponins, tannins. | DPPH, FRAP, ABTS radical scavenging assays | Dose-dependent antioxidant activity; high phenolics correlated with strong scavenging; ABTS and FRAP confirm potent activity. | Free radical scavenging via electron donation and H-atom transfer by phenolics and flavonoids | [64] |
| In vitro | Water-soluble, glycoside, and cell wall-bound phenolic acids; major: p-coumaric, p-dihydroxybenzoic, ferulic acids | ABTS assay (TEAC) | Alkaline water-soluble (cell wall-bound) fraction: highest phenolics and antioxidant activity; strong correlation (r = 0.988) with TEAC. | Antioxidant via free radical scavenging by phenolic acids; higher lignin-bound phenolics ↑ stress mitigation | [59] |
| In vitro | Essential oil; major compounds: khusimol, isovalencenol, 2-isopropyl-5-methyl-9-methylene-bicyclo [4.4.0]decene, α-vetivol, beta-maalene, vetiselinenol, γ-selinenes, zizanol, khusiol, β-vatirenes | Phosphomolybdenum assay | Essential oil showed 75.5% total antioxidant capacity at 0.1 mg/mL. | Antioxidant activity likely mediated by phenolic and other bioactive constituents | [56] |
| In vitro | Essential oil; major compounds: Khusimol (25.60%), Bicyclo-vetivenol (11.47%), α-Vetivone (7.76%) | DPPH radical scavenging assay | Dose-dependent antioxidant activity; highest activity 60.43% at 0.5 mg/mL, lowest 52.74% at 0.03125 mg/mL; activity lower than BHT. | Antioxidant activity mainly from terpenic alcohols and phenolics; both major and minor constituents contribute | [57] |
| In vivo | Crude water extract (roots) | Biochemical assays for serum ALT, AST, ALP; liver antioxidant enzymes: GSH, GR, GST, GPx; lipid peroxidation (MDA) | Pre/post Vetiveria extract ↓ ALT, AST, ALP; ↑ GSH, GR, GST; ↓ lipid peroxidation; pre-treatment > post-treatment. | Protection via maintaining hepatic antioxidants, ↑ GR and GST, ↓ oxidative stress and lipid peroxidation; partially mimics NAC | [58] |
| In vitro | Essential oil; main components: longiverbenone (27.31%), longipinocarvone (26.88%), Cedr-8-en-13-ol (26.26%) | DPPH free radical scavenging assay, FRAP assay | Dose-dependent DPPH and FRAP activity; FRAP IC50 = 184.8 ± 1.02 μg/mL; activity linked to phenolics. | Antioxidant activity from high EO phenolics; electron donation and free radical neutralization | [55] |
| In vitro | Ethanol and ethyl acetate extracts; rich in flavonoids and phenolic compounds | DPPH free radical scavenging assay | Both extracts: dose-dependent scavenging; 140 µg/mL inhibition: ethanol 40.7%, ethyl acetate 59.3%; IC50: ethanol 157.38, ethyl acetate 112.79 µg/mL. | Antioxidant activity via H-donation by phenolics and flavonoids, quenching free radicals | [54] |
| In vitro | Ethanolic root extract; major compounds include Khusenic acid, Ascorbic acid, Junipen, gamma-Himachalene, alpha-Guaiene | DPPH radical scavenging assay | Strong antioxidant activity (IC50 10.73 μg/mL; ascorbic acid 4.61 μg/mL) | Antioxidant-mediated free radical scavenging contributes to cytotoxicity in cancer cells | [53] |
| Study Design/Assay | Extract Type and Bioactive Compounds Involved | Target Microorganism | Key Findings | Mechanism of Action | References |
|---|---|---|---|---|---|
| MIC assay | Ethanol, hexane, methanol extracts; Essential oil; Phenolic acids; Flavonoids; Terpenoids | S. aureus, B. subtilis, MRSA, E. coli, P. aeruginosa, Candida spp. |
|
| [36,37,38,44,45,46,48,49,51] |
| Mechanistic Observations (descriptive studies) | Essential oils, methanol/ethanol extracts; Terpenoids; Flavonoids; Phenolic acids | Broad spectrum (Gram-positive, Gram-negative, fungi) |
|
| [65,73,74,75,79] |
| Disk diffusion/ Well diffusion | Essential oils; Root, leaf, and methanol extracts; Terpenoids; Flavonoids; Phenolic acids | S. aureus, MRSA strains, E. coli, K. pneumoniae, P. aeruginosa, Candida spp. |
|
| [73,74,75,76,77,81,82] |
| Nanoparticle-mediated assay | Silver nanoparticles synthesized using root aqueous extract; Phytochemicals | S. aureus, P. aeruginosa | Potent antibacterial effect; Effective at low concentration (25 μg/mL) |
| [85] |
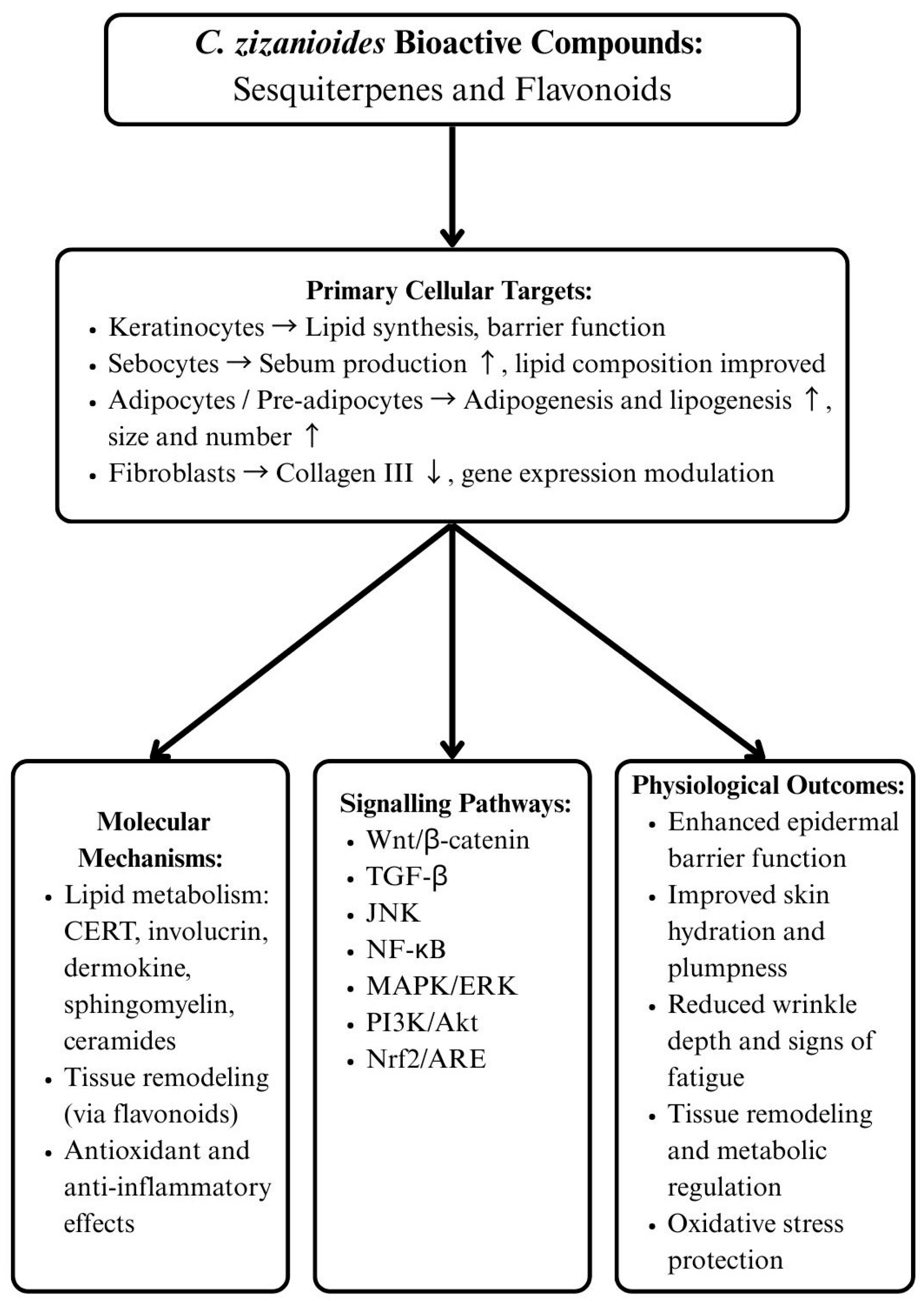
| Extract Type | Target Cells and Pathways | Mechanisms of Action | Observed Effects | Clinical Implications | References |
|---|---|---|---|---|---|
| Root Extract | Keratinocytes, sebocytes, adipocytes, skin explants |
|
| Hydration, anti-aging, barrier strengthening | [89] |
| Essential Oil | Pre-inflamed fibroblasts |
|
| Anti-fibrotic potential, metabolic skin support, wound repair | [90] |
| Flavonoids | Multiple signaling pathways (Wnt/β-catenin, Hippo, TGF-β, Hedgehog, JNK, NF-κB, MAPK/ERK, PI3K/Akt, Nrf2/ARE) |
|
| Basis for linking C. zizanioides activity to broader skin repair mechanisms | [91] |
| Extract Type | Bioactive Compound Involved | Cell Line Model | Effect | References |
|---|---|---|---|---|
| Essential oil | Cedr-8-en-13-ol, α-/β-pinene, α-/γ-terpinene | B16 melanoma cells | Non-cytotoxic; ↓ melanin, tyrosinase activity, ↑ antioxidant enzymes, ↓ oxidative stress | [98] |
| Ethanolic root extract | Ellagic acid, Ascorbic acid, Linoleic acid, α-/β-Sitosterol | SCC-29B (oral), DU-145 (prostate), Vero | Cytotoxic to SCC-29B, DU-145; induces DNA damage, apoptosis; minimal Vero toxicity | [53] |
| C. zizanioides acetate oil | Not specified | In vivo: Swiss albino mice treated with cisplatin | Protects from nephrotoxicity, DNA/chromosomal damage; restores GSH/enzymes; ↑ antioxidant defenses | [99] |
| Essential oil | Sesquiterpene lactones | HeLa (human cervical cancer) | Cytotoxic (IC50 0.05%); induces apoptosis, ROS, mitochondrial depolarization | [100] |
| Methanol extract (polyherbal including C. zizanioides) | Carbohydrates, alkaloids, steroids, saponins, flavonoids, tannins | HeLa, MCF-7 | Cytotoxic to HeLa; low toxicity to MCF-7; likely antioxidant mediated | [75] |
| Ethanolic root extract | Longifolene | DU-145 (prostate cancer), SCC-29B (oral cancer), Vero (healthy kidney) | Cytotoxic to prostate/oral cancer; minimal Vero toxicity | [101] |
| Aqueous root extract | Valencene | L929 fibroblasts (cytotoxicity) | Cytotoxic; ↑ TNF-α, IL-6; immunomodulatory | [96] |
| Essential Oil | β-caryophyllene, α-humulene, caryophyllene oxide | In vitro: WiDr (colon), 4T1 (TNBC), T47D (luminal breast) cancer cells; MTT assay | Cytotoxic; ↑ ROS, apoptosis; docking confirms binding | [93] |
| C. zizanioides crude oil and commercial essential oil | Not specified | In vitro: HeLa cervical cancer cells; Mitotic index (MI) assay | Stronger anticancer (MI 1.70%) vs. commercial EO (3.26%) and control (5.57%); ↓ MI → ↑ antimitotic activity; active components not identified. | [94] |
| C. zizanioides oil | Khusimol, aristol-1(10)en-9-ol, cyclocopacamphenol, bicyclo[5.2.0]nonane-2-methylene-4,8,8-trimethyl-4-vinyl | In vitro: Human lung (A549) and hepatocellular (HepG2) cancer cell lines; MTT assay | Moderate cytotoxicity; inhibits proliferation | [92] |
| C. zizanioides oil | Beta vetispirene | In silico: Molecular docking; bioinformatics | Selectively inhibits AKR1C1/2; ↑ ROS, apoptosis (lung cancer) | [95] |
| Methanolic root extract | Not specified | In vitro: MTT assay on HEK 293 cells | Non-cytotoxic; no viability reduction | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunasekar, C.J.; Majdalawieh, A.F.; Abu-Yousef, I.A.; Al Refaai, S.A. Pharmacological and Therapeutic Potential of Chrysopogon zizanioides (Vetiver): A Comprehensive Review of Its Medicinal Applications and Future Prospects. Biomolecules 2025, 15, 1312. https://doi.org/10.3390/biom15091312
Gunasekar CJ, Majdalawieh AF, Abu-Yousef IA, Al Refaai SA. Pharmacological and Therapeutic Potential of Chrysopogon zizanioides (Vetiver): A Comprehensive Review of Its Medicinal Applications and Future Prospects. Biomolecules. 2025; 15(9):1312. https://doi.org/10.3390/biom15091312
Chicago/Turabian StyleGunasekar, Conjeevaram J., Amin F. Majdalawieh, Imad A. Abu-Yousef, and Sham A. Al Refaai. 2025. "Pharmacological and Therapeutic Potential of Chrysopogon zizanioides (Vetiver): A Comprehensive Review of Its Medicinal Applications and Future Prospects" Biomolecules 15, no. 9: 1312. https://doi.org/10.3390/biom15091312
APA StyleGunasekar, C. J., Majdalawieh, A. F., Abu-Yousef, I. A., & Al Refaai, S. A. (2025). Pharmacological and Therapeutic Potential of Chrysopogon zizanioides (Vetiver): A Comprehensive Review of Its Medicinal Applications and Future Prospects. Biomolecules, 15(9), 1312. https://doi.org/10.3390/biom15091312











