1. Introduction
Nucleic acid (NA) amplification methods are widely used in biotechnology, molecular biology, genetics, forensics and medicine for molecular diagnostic assays. Currently, two main strategies are used to amplify defined sequences of nucleic acids: thermocycling DNA amplification by polymerase chain reaction (PCR) and isothermal amplification.
The “gold standard” of sequence-specific DNA amplification is polymerase chain reaction (PCR), with it being the oldest and most widely used [
1,
2,
3]. This reaction relies upon instrument-based thermal cycling on the denaturation of a template DNA, followed by the annealing of primers at specific sites on the denatured template and their extension by a thermostable DNA polymerase (such as Taq DNA polymerase [
4,
5,
6]), allowing for an exponential increase in the amount of DNA [
2]. The main advantages of this method include its high sensitivity, the specificity of the reaction and the prevalence of the required equipment.
Isothermal amplification methods emerged in the early 1990s and gradually gained popularity thanks to the simple equipment required and the speed of the process in isothermal mode. Though these methods may require an initial high-temperature incubation to denature the template DNA for the initiation of the process, DNA amplification takes place at one defined temperature, while the separation of the template DNA strands during amplification is carried out by enzymatic strand displacement activity. A variety of isothermal amplification methods have been developed, including the following: nucleic acid sequence-based amplification (NASBA) [
7], strand displacement amplification (SDA) [
8,
9], rolling circle amplification (RCA) [
10], cross priming amplification (CPA) [
11], nickase-dependent amplification (NDA) [
12] and loop-mediated amplification (LAMP) [
13]. These methods, like many other isothermal amplification methods, require the use of a DNA polymerase with strong strand displacement activity [
14,
15], such as Phi29 [
16] or Bst DNA polymerase [
15,
17]. Generally, isothermal amplification provides higher reaction yields and faster assay rates than PCR amplification, but PCR provides a higher assay sensitivity [
18,
19]. Although the sensitivity of PCR and isothermal amplification methods may vary depending on the object and conditions of the analysis, the experience gained during the COVID-19 pandemic has clearly shown that at low viral loads, the sensitivity of various isothermal methods is inferior to that of PCR [
18,
20].
Upon the creation of DNA polymerases that possess both high thermal stability and high strand displacement activity, such as SD DNA polymerase [
14], it became possible to combine the processes of thermocyclic and isothermal amplification in one reaction. This has led to the emergence of a number of new hybrid methods that combine the advantages of isothermal amplification and PCR. Among the hybrid NA amplification methods, those of particular interest include polymerase chain displacement reaction (PCDR) [
14,
21], coupled PCR-LAMP [
18] and pulse-RCA [
22,
23]. PCDR is a hybrid technique combining PCR with strand displacement amplification; coupled PCR-LAMP combines a PCR-like thermocycling mode at the initial stage of amplification with an isothermal LAMP mode during the following stage; and pulse-RCA is a combination of rolling circle amplification with PCR. The hybrid methods advantageously combine both the high sensitivity of PCR and the fast assay rate of isothermal amplification [
18].
PCDR was the first method of DNA amplification, which combined PCR and isothermal amplification. The technique requires heat denaturation of dsDNA, as in conventional PCR, as well as the strand displacement activity of DNA polymerase, as in SDA. In PCDR, at least four primers are employed, and amplification is initiated simultaneously from the outer primers and the inner primers. By using a DNA polymerase with the strand displacement activity, the inner DNA strands are displaced during DNA synthesis from the outer primers and can be used as additional template strands for DNA amplification. As a result, PCDR increases DNA amplification ratio more than two-times per cycle as compared with the conventional two-primer PCR. The schematic, mechanism and kinetics of PCDR amplification have been described previously [
14,
21].
Coupled PCR-LAMP is the second method of hybrid amplification. LAMP is a fast and specific isothermal method for DNA amplification. However, LAMP provides robust detection of DNA only if a few hundred (or more) copies of a target DNA are present in the reaction assay. Coupled PCR-LAMP combines LAMP and PCR, where several PCR cycles were added before the isothermal amplification. In the case of a low amount of DNA, the addition of PCR cycles provides amplification of the starting material to the DNA copy level, which is sufficient to ensure the start of LAMP. For example, 6 PCR cycles can amplify 5 copies of a target DNA up to 320 copies, which is sufficient for the fast and specific initiation of LAMP. The mechanism and practical application of PCR-LAMP have been described in [
18].
Pulse-RCA was developed as a part of a single-molecule mutation sequencing (SMM-seq) protocol for the accurate cost-effective assessment of somatic mutations in DNA extracted from normal cells and tissues [
22]. Rolling circle–based linear amplification (RCA) was used to produce single-stranded DNA molecules. The amplification was carried out using an artificial thermostable polymerase having a strong strand displacement activity (SD DNA polymerase). This allowed multiple cycles of denaturation-annealing-extension to ensure efficient and less biased amplification in a reaction termed pulse-RCA [
22].
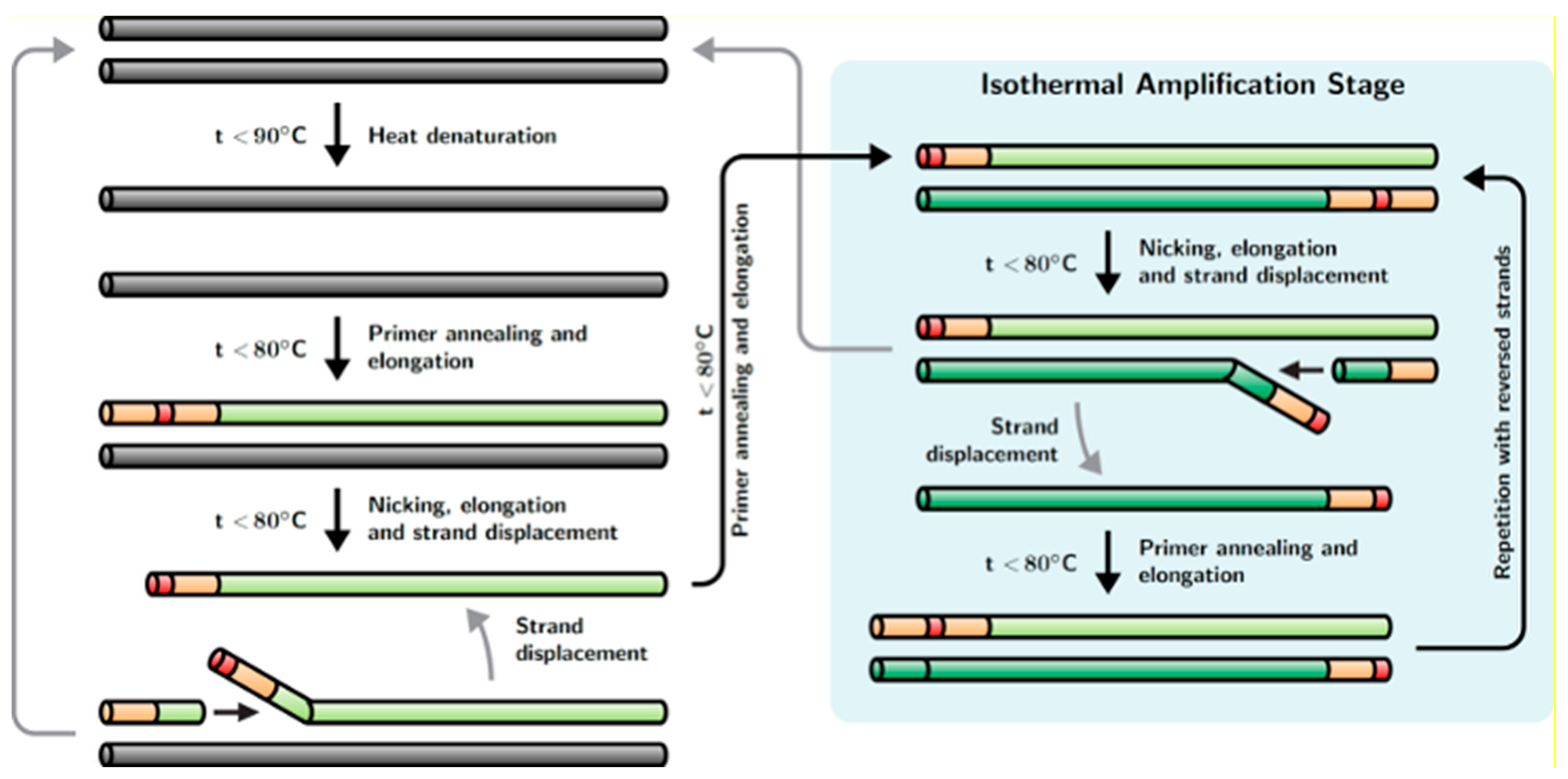
Recently, a new method of hybrid DNA amplification was developed by Dong et al., named strand displacement chain reaction (SDCR) [
24]; the reaction scheme of this method is shown in
Figure 1. This method appears as a hybrid of nickase-dependent isothermal amplification and PCR. SDCR requires deoxyribooligonucleotide primers that flank the DNA fragment to be amplified, similar to primers used for PCR, and contain one (or more) ribonucleotides. Said ribonucleotides should be located within the oligonucleotide sequences of the primers, dividing the primers into 3′ and 5′ terminal parts. In addition to the primers, the reaction mixture should contain a thermostable DNA polymerase with a strong strand displacement activity (SD DNA polymerase) and a thermostable ribonuclease H type II (RNase H2), which acts as a nickase in the reaction. SDCR amplification of the target DNA sequence is performed under a thermal cycling mode, for which the reaction mixture is successively heated to the denaturation temperature of dsDNA and then cooled to the temperature at which primer annealing and the enzymatic synthesis of new DNA chains occurs. During the annealing and elongation of primers on the template DNA, thermostable RNase H2 introduces a single-strand break (nick) in the nucleotide sequence of the annealed primers immediately upstream of the ribonucleotide(s) contained in the primers. The resulting nicks serve as the initiation sites for the synthesis of a new DNA chain, and the chain initially synthesized as a result of primer elongation is displaced by the chain-displacing activity of DNA polymerase. The result is a double-stranded DNA amplification product and displaced single-stranded DNA. The displaced DNA strand is primed by a complementary oligonucleotide primer containing a ribonucleotide(s), which again leads to primer elongation, the introduction of a break in the nucleotide sequence of the primer, and the displacement of the new DNA strand with the simultaneous formation of a double-stranded amplification product. Thus, at the primer annealing and elongation step, the process of DNA synthesis is continuous and performed in an isothermal mode. This ensures a more than twofold increase in the copy number of the amplified DNA template in one thermal cycle. It should be noted that the double-stranded DNA amplification product is not involved in isothermal amplification. In order to involve double-stranded DNA in the amplification process, a thermal denaturation step is required, resulting in double-stranded DNA (dsDNA) being converted into single-stranded DNA (ssDNA), which serves as a template for the synthesis of new DNA strands. The SDCR amplification protocol, combining the primer annealing and elongation phases, closely resembles a protocol for two-step PCR, but in contrast to PCR, SDCR increases DNA amplification ratio by a complementary isothermal amplification process during the elongation step. The SDCR mechanism is also described in [
24].
The potential advantages of SDCR include a remarkably higher amplification efficiency than that of PCR, the ability to use standard PCR analysis equipment (the method does not require a special equipment), and the ability to perform the reaction in “real-time” mode using a hydrolysis probe [
24]. In comparison with intercalating dyes, the use of hydrolysis probes capable of complementary interactions with the amplified sequences enables a significant increase in the specificity of the analysis and can eliminate the risk of false-positive results. To perform SDCR with a hydrolysis probe, the latter should contain a ribonucleotide in its sequence to ensure its hydrolysis by RNase H2 after hybridization with the target sequence.
Conceivably, the use of SDCR could greatly improve amplification assays, but there have not yet been any examples of practical applications of the technique, except for amplifications from plasmid templates [
24]. In the present work, we evaluate the performance of quantitative SDCR (qSDCR) and compare it with quantitative PCR (qPCR) in the real-time amplification assays of target sequences from human cDNA and bacterial and viral gDNA.
2. Materials and Methods
2.1. Enzymes and Reagents
The following materials were obtained from their respective sources: SD HotStart DNA polymerase (10 U/μL), SmartHot Taq DNA polymerase (5 U/μL), and 10× SD polymerase reaction buffer from Bioron GmbH (Römerberg, Germany); dNTPs from Bioline Limited (London, UK); oligonucleotide primers and probes were synthesized by Syntol JSC (Moscow, Russia); the cDNA library of human embryonic kidney 293 (HEK 293) cells and viral gDNA of lambda bacteriophage (Lambdavirus lambda) from Evrogen JSC (Moscow, Russia); and human gDNA and bacterial gDNA of Mycobacterium bovis (stain Russia BCG-1) from Syntol JSC (Moscow, Russia). Concentrations of the used DNA samples (laboratory controls) were verified using the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen by ThermoFisher Scientific, Inc., Waltham, MA, USA).
Thermostable RNase H2 (Pa RNase H2) was isolated from a recombinant
E. coli strain that carries the RNase H2 gene from
Pyrococcus abyssi. The cloning, expression, and purification of recombinant Pa RNase H2 are fully described in [
25]. The enzyme is also commercially available from Integrated DNA Technologies, Inc. (Coralville, IA, USA).
The real-time amplification reactions were performed using a CFX96 Touch Real-Time Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
2.2. cDNA of RNA Isolated from FFPE Blocks
Samples of total RNA were isolated from two separate formalin-fixed, paraffin-embedded (FFPE) blocks containing fixed human tissues using the RNeasy FFPE Kit for RNA Extraction (Qiagen GmbH, Hilden, Germany). Total RNA concentration was measured with a Qubit RNA High-Sensitivity kit and a Qubit-4 fluorimeter (ThermoFisher Scientific, Inc., Carlsbad, CA, USA). RNA integrity was analyzed using capillary electrophoresis Agilent 4200 TapeStation (Agilent Technologies, Inc., Santa Clara, CA, USA). The RNA integrity number (RIN) for each sample was 1.1.
cDNA was obtained from 500 ng of each RNA sample by reverse transcription using the HiScript III 1st Strand cDNA Synthesis Kit (Vazyme, Nanjing, China) according to the protocol with DNase I treatment in a volume of 20 μL. A specific primer to beta-2-microglobulin (B2M) RNA (5′-CAATCCAAATGCGGCATCTTC) was used for priming the 1st strand of cDNA. The synthesized cDNA was used in the following assays without additional purification.
2.3. Real-Time qSDCR Amplification
qSDCR assays were performed in three technical replicates, in real-time mode with a hydrolysis oligonucleotide probe and modified primers for SDCR, all of which contained ribonucleotide in the middle of their sequences. The 3′ terminal parts of the SDCR primers and the corresponding conventional PCR primers were completely identical and complementary to the amplified sequences, but the SDCR primers additionally contained artificially generated 5′-end sequences upstream of the ribonucleotide (the actual sequences are provided in
Section 2.5,
Section 2.6 and
Section 2.7). The reactions were carried out with an enzyme mixture of SD HotStart DNA polymerase and Pa RNase H2. The SD polymerase has no nuclease activity, so the reactions with this DNA polymerase contained Pa RNase H2 for the probe hydrolysis and for the nicking of SDCR primers. The reaction mixtures (25 μL) contained 0.25 mM dNTP (each); two modified primers comprising a ribonucleotide (0.2 μM, each); a hydrolysis oligonucleotide probe containing a ribonucleotide (0.3 μM); 3 mM MgCl
2; a specified amount of a template DNA, or a no-template control (NTC); SD HotStart DNA polymerase (20 U); Pa RNase H2 (2.5 ng); and 1X SD-reaction buffer. Preliminarily, we optimized the concentration of the SD polymerase and found that concentrations over 0.8 U/μL did not improve the performance of SDCR (
Figure S1).
The following conditions were used for SDCR performance: (a) initial denaturation at 92 °C for 90 s; and (b) amplification via 40 thermocycles at 92 °C for 30 s, and 66 °C for 60 s.
2.4. Real-Time qPCR Amplification
qPCR assays were performed in three technical replicates using a hydrolysis oligonucleotide probe, conventional PCR primers, and Taq polymerase (qPCR-2) or an enzyme mixture of SD polymerase and Pa RNase H2 (qPCR-1). The SD polymerase has no nuclease activity, so RNase H2 was needed for the probe hydrolysis. The reaction mixtures (25 μL) contained 0.25 mM dNTP (each); two non-modified primers (0.2 μM, each); a hydrolysis oligonucleotide probe containing a ribonucleotide (0.3 μM); 3 mM MgCl
2; a specified amount of a template DNA, or a no-template control (NTC); SmartHot Taq DNA polymerase (5 U), or an enzyme mixture of SD HotStart DNA polymerase (20 U) with Pa RNase H2 (2.5 ng); and 1X SD-reaction buffer. Preliminarily, we optimized concentrations of the enzymes used in the reactions. We found that Taq polymerase concentrations over 0.2 U/μL or SD polymerase concentrations over 0.4 U/μL did not improve the performance of PCR [
14].
The following conditions were used for PCR performance: (a) initial denaturation at 92 °C for 90 s; and (b) amplification via 40 thermocycles at 92 °C for 30 s, and 66 °C for 60 s.
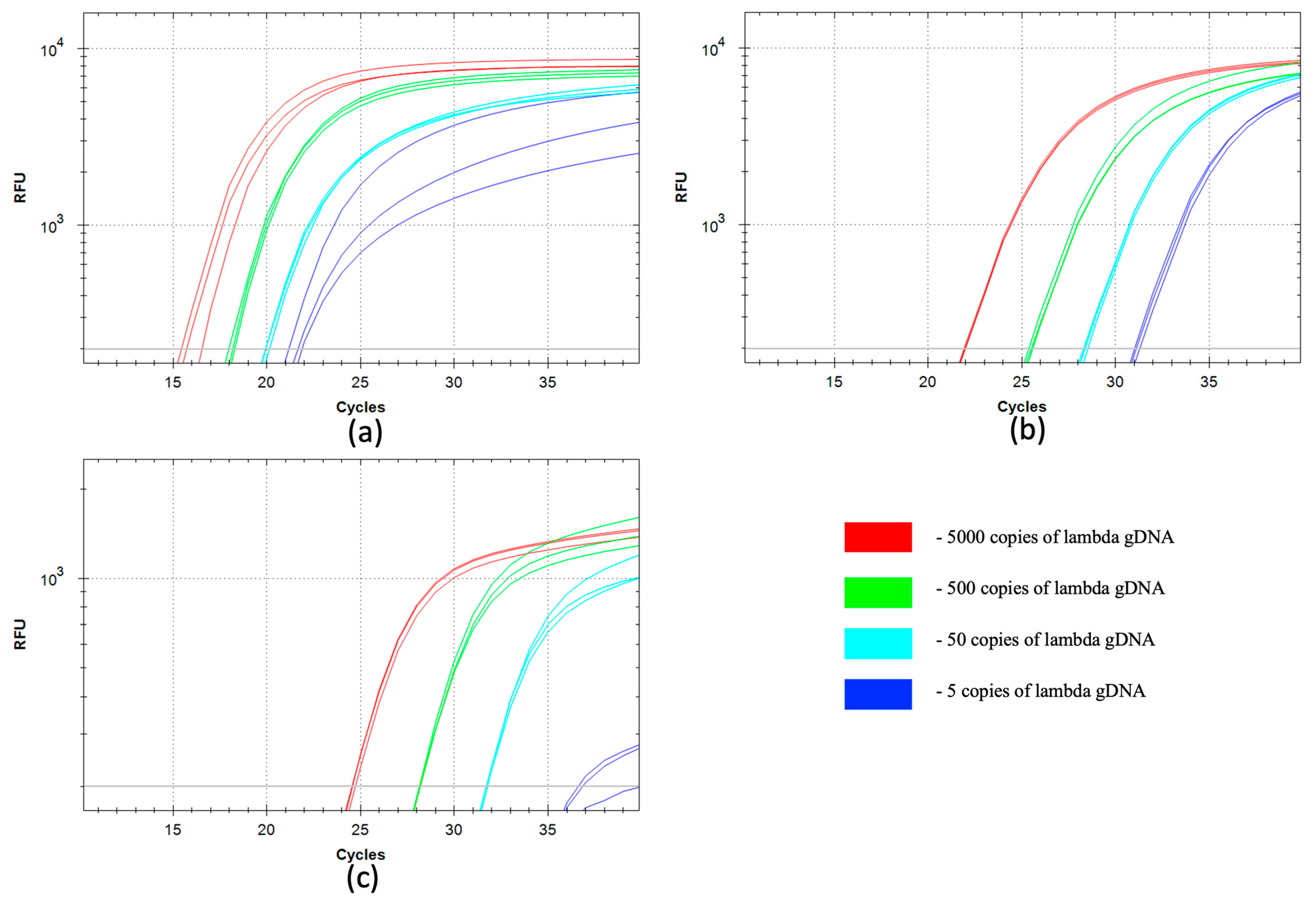
2.5. qSDCR and qPCR Assays of Lambda Bacteriophage gDNA
The real-time SDCR assay was performed as described in
Section 2.3 with the following probe and primers:
PL-probe (5′(ROX)-CCGGCG(rG)CTGCAGGT(BHQ2)GCAGAGTG-(P)), and primers
PL-f1 (5′-GTGCCGTGAAGACGACAGAGTT(rG)GTGGTCTGCCCTGGCTGAGTGA) and
PL-r1 (5′-GCAGTGGTATCTCGCAGGAGTT(rG)GCAGACCAGCCACCACGGCA).
The PCR detection of lambda gDNA (GenBank Accession NC_001416) was performed as described in
Section 2.4 with the following probe and primers:
PL-probe (above); primers
PL-f2 (5′-GGTGGTCTGCCCTGGCTGAGTGA) and
PL-r2 (5′-GGCAGACCAGCCACCACGGCA).
The size of the target amplified sequence was 76 bp. The reactions were performed with a series of 10-fold dilutions of the input template, from 250 fg to 0.25 fg of lambda gDNA per reaction. All reactions (including a no-template control, NTC) contained 50 ng of human gDNA.
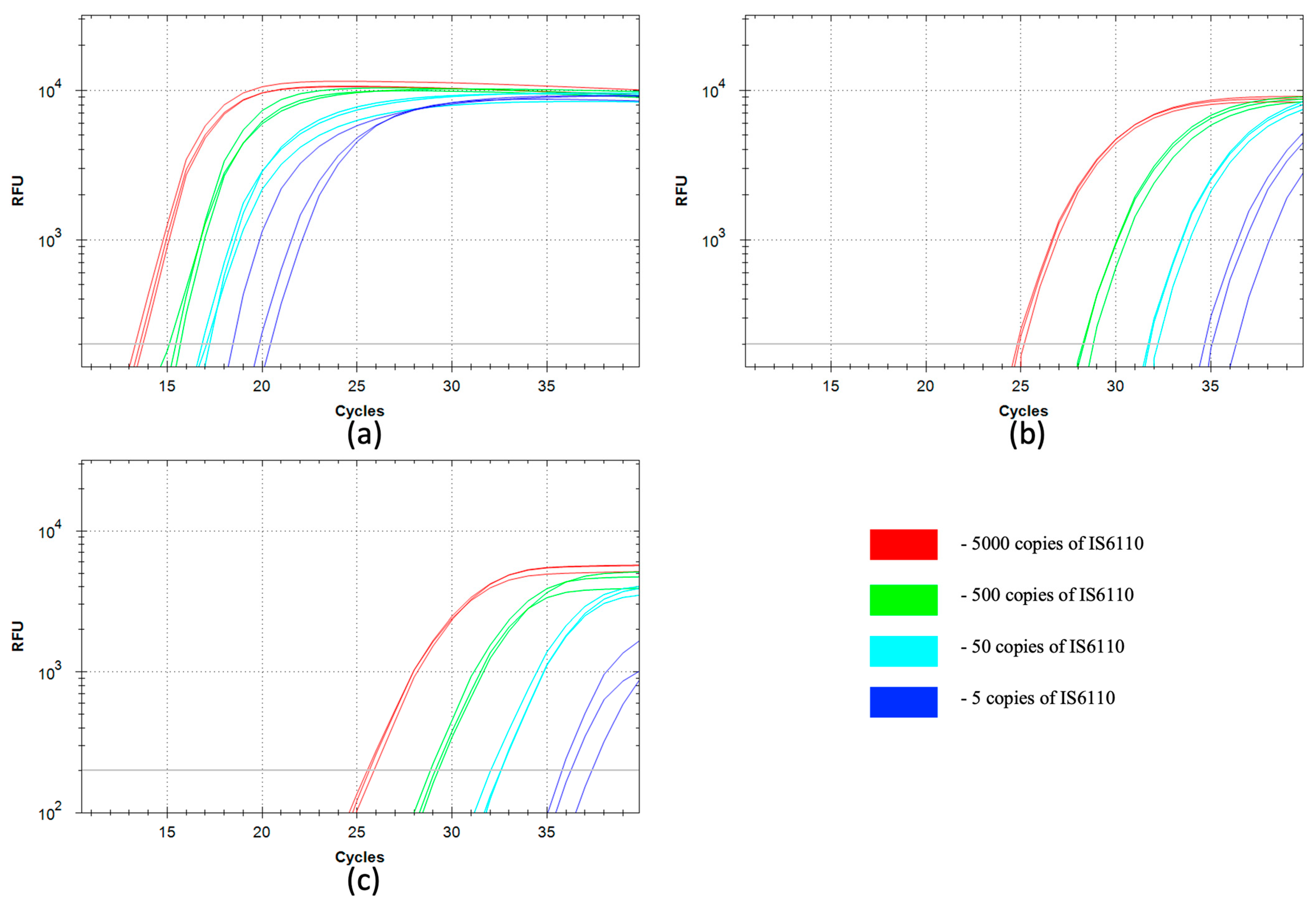
2.6. qSDCR and qPCR Assays of Mycobacterium bovis gDNA
The real-time assays were performed as described in
Section 2.3 and
Section 2.4. For the detection of
M. bovis gDNA (GenBank Accession NZ_CP013741) by SDCR amplification, the following probe and primers were used:
MT-probe (5′-(ROX)-GCGGATCTCT(rG)CGACCAT(BHQ2)CCGCACCGCCCGC-(P)), and primers
MT-f1 (5′-GTGCCGTGACGACGACAGCGTT(rG)CTGCCCACTCCGAATCGTGCT) and
MT-r1 (5′-GCAGTGGTCTC TCGCAGGCGTT(rG)TACCCGCCGGAGCTGCGT).
For the PCR assays. we used MT-probe (above) and primers MT-f2 (5′-GCTGCCCACTCCGAATCGTGCT) and MT-r2 (5′-GTACCCGCCGGAGCTGCGT).
The size of the target amplified sequence was 78 bp. The reactions were carried out with a series of 10-fold dilutions of the input template, from 11 pg to 11 fg of M. bovis gDNA per reaction. Also, all reactions (including a no-template control, NTC) contained 50 ng of human gDNA.
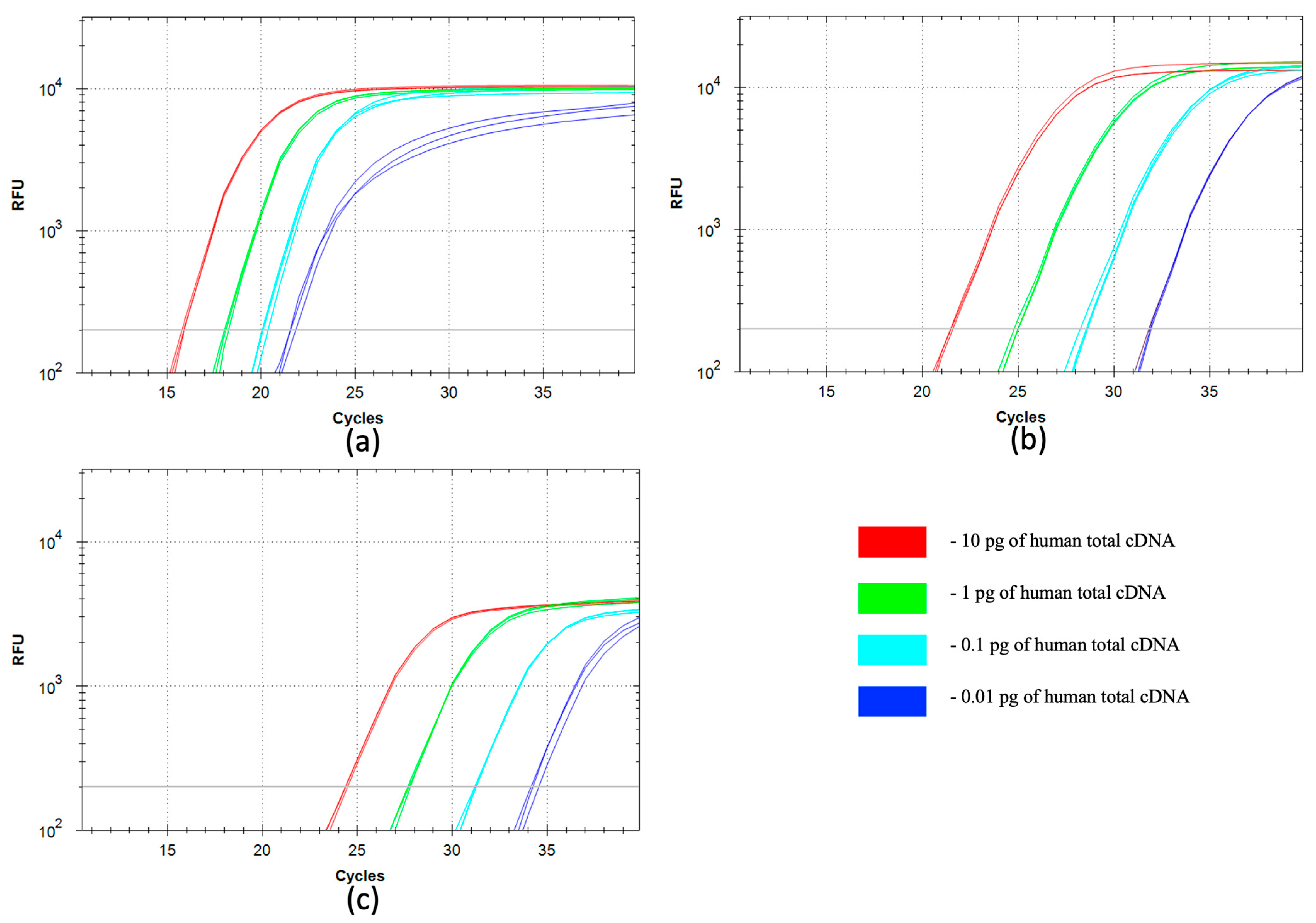
2.7. qSDCR and qPCR Assays of Human B2M cDNA
The real-time SDCR and PCR assays of human beta-2-microglobulin (B2M) cDNA (GenBank Accession BC_064910) were performed as described in
Section 2.3 and
Section 2.4. The human cDNA library or B2M cDNA synthesized from RNA isolated from FFPE blocks (
Section 2.2) was used as a template. The reactions using the cDNA library were carried out with a series of 10-fold dilutions of the input template, from 10 pg to 0.01 pg of the library per reaction. The reactions with the B2M cDNA of RNA isolated from FFPE blocks were performed with 1 μL of the non-purified cDNA.
The following probe and primers were used for the SDCR assays: B2M-probe (5′-(ROX)-CACAGCCCAA(rG)ATAGTT(BHQ2)AAGTGGGATCGAGAC-(P)), and primers B2M-f1 (5′-GTGCCGTGAAGACGACAGAGTT(rG)CCTGCCGTGTGAACCATGTGACT) and B2M-r1 (5′-GCAGTGGTATCTCGCAGGAGTT(rG)CCAAATGCGGCATCTTCAAACCTCCA).
The B2M-probe (above) and the following primers were used for the PCR assays: B2M-f2 (5′-GCCTGCCGTGTGAACCATGTGACT) and B2M-r2 (5′-CCAAATGCGGCATCTTCAAACCTCCA).
The size of the B2M amplified sequence was 101 bp.
2.8. Data Analysis
All data processing was performed using R software version 4.5.1 [
26]. Core R language and several extension packages were used, including RDML for raw amplification data manipulation [
27], chipPCR for Cq calculation [
28], and ggplot2 for the generation of plots [
29].
The efficiency of each amplification variant was estimated by standard curve (dilution series of template DNA). The log of each concentration in the dilution series (x-axis) was plotted against the Cq value for that concentration (y-axis). Then, efficiency was determined by the following equation, using the slope of the standard curve: Efficiency = 10(−1/slope) − 1. Amplification per one cycle (amplification factor) was determined as 10(−1/slope).
4. Discussion
The SDCR is a newly developed hybrid amplification technique which combines the advantages of isothermal amplification (fast assay rate) and PCR (high sensitivity) for NA detection, as other hybrid methods. We have compared qSDCR and qPCR techniques in the detection assays of human B2M cDNA, the bacterial gDNA of
M. bovis and the viral gDNA of lambda bacteriophage. Selecting these objects for the tests allowed us to try out SDCR assays for different applications. B2M is a commonly used reference gene for gene expression studies and particularly in cancer research [
31].
Mycobacterium bovis is a classical bacterial pathogen which belongs to the Mycobacterium tuberculosis complex (MBTC) and can primarily cause tuberculosis in cattle and other animals [
30]. Lambda bacteriophage (
Lambdavirus lambda) is a model double-stranded DNA virus containing a 48.5 kb DNA genome.
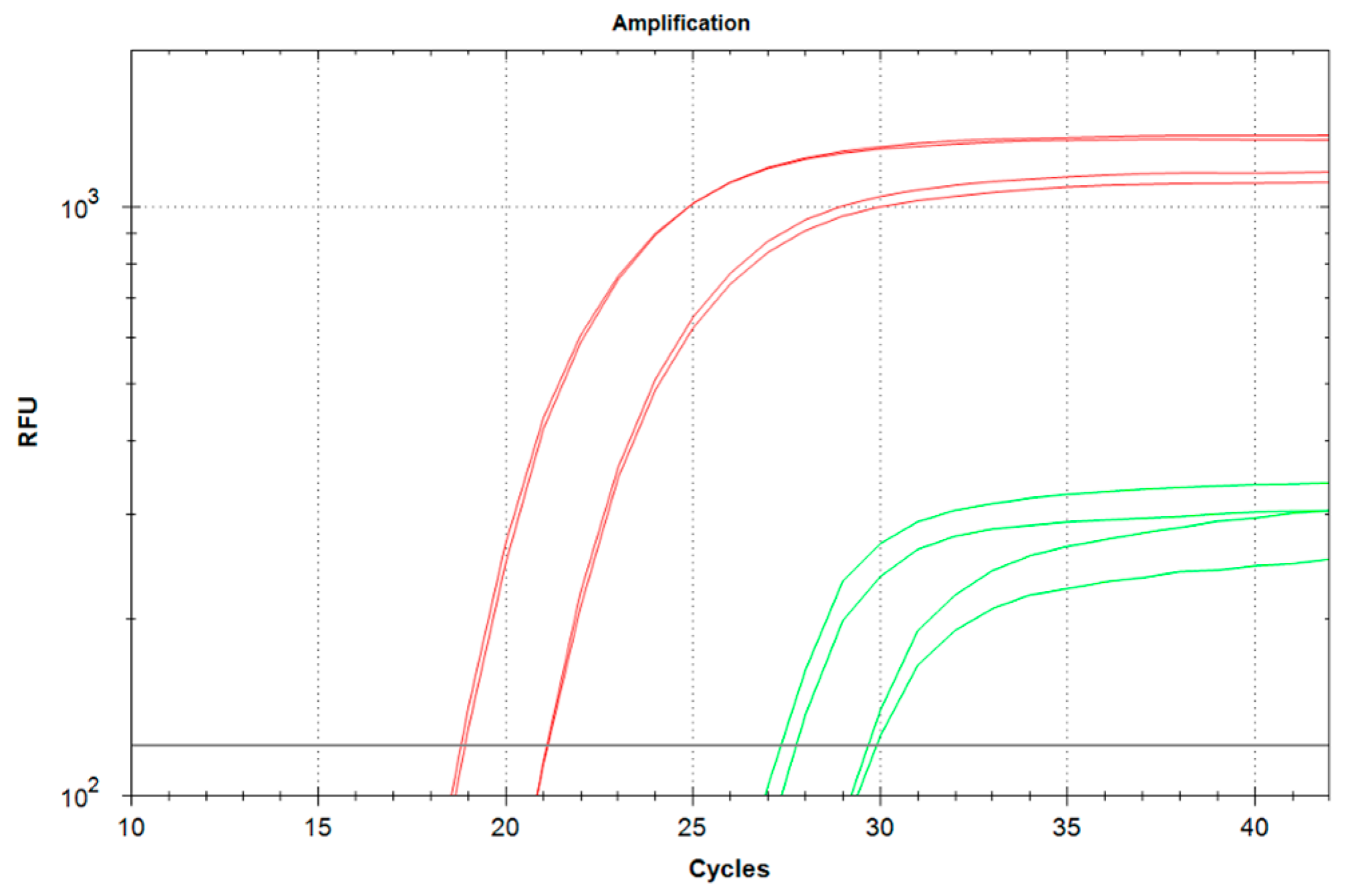
The detection of viral and bacterial species, and particularly pathogen species, is one of the most important tasks of molecular diagnostics. We have successfully used SDCR for the assays of lambda bacteriophage and
Mycobacterium bovis gDNA (
Section 3.2 and
Section 3.3), which provided a very high sensitivity and allowed us to detect single copies of viral and bacterial genomes in the reactions. It should be noted that the reactions were performed in the presence of human gDNA because clinical samples are typically contaminated by gDNA, and the reaction mixtures can contain gDNA up to several tens of nanograms. Further, qSDCR surpassed qPCR with remarkable results in terms of amplification efficiency. The amplification factor, which shows a value of amplification per one thermocycle, exceeded 3 for the SDCR tests, versus approximately 2 for the PCR assays. As a result, the SDCR technique, in comparison with PCR, allowed for a reduction in the number of thermocycles needed for the detection of the species by a third, and shortened assay time. (It should be specified that for SDCR the mentioned qPCR efficiency above 200% is a mathematical outcome of applying the qPCR slope formula to the hybrid amplification method that produces more than a two-fold increase in template per cycle.) The use of qSDCR allowed us to detect as few as five copies of the target sequences in the reactions in fewer than 22 cycles (see
Table 1 and
Table 2), which took about 35 min. The demonstrated characteristics of the qSDCR assays, including high sensitivity and short analysis time, are very close to those previously found for other hybrid amplification techniques, such as qPCDR and q(PCR-LAMP) [
18].
Another area of NA amplification is gene expression studies, which are important for biological, medical and cancer research. We have compared qSDCR and qPCR techniques in the expression analysis of a standard reference gene: human B2M. The target B2M cDNA was successfully detected by qSDCR in the library of total human cDNA and in the samples of the genetic material obtained from FFPE blocks. The materials from FFPE blocks are widely used in cancer research, but a high level of NA degradation drastically complicates the assays. Thus, these detection assays need high sensitivity and amplification efficiency. The qSDCR noticeably outperforms qPCR in the B2M tests (see
Section 3.3 and
Section 3.4), which makes it suitable for the expression assays with complicated templates. The SDCR amplification factor was 3.32 (versus approximately 2 for PCR), and Cq values were reduced by a third in comparison with qPCR (
Table 3). Thus, qSDCR appears to be a very promising application technique in gene expression studies.
Compared with other hybrid amplification methods, such as PCR-LAMP and PCDR, SDCR provides clear technological advantages in reaction architecture, primer design complexity, and enzyme utilization. LAMP and PCDR require four to six primers, which increases the risk of primer dimer formation and necessitates extensive optimization to ensure amplification efficiency. In contrast, SDCR employs only two conventional primers containing a single internal ribonucleotide, preserving the simplicity of classical PCR design while enabling strand displacement-based amplification. This streamlined primer architecture facilitates assay development and supports broad applicability across diverse nucleic acid targets.
SDCR also benefits from a simplified thermal profile. While PCR-LAMP involves a transition from an initial thermal cycling phase to an isothermal amplification stage, and PCDR requires continuous thermocycling with fine-tuned annealing conditions, SDCR uses a consistent two-step protocol with a single annealing and elongation temperature throughout the reaction. This uniform thermal regime eliminates the need for phase-specific transitions and reduces programming complexity, thereby enhancing reproducibility and assay robustness on standard real-time PCR platforms.
The usage of RNase H2 hydrolysis probes for qSDCR “real-time” amplifications provided a high assay specificity. Although the detections of the bacterial and viral gDNA were carried out in the presence of excess of human gDNA, the assays did not generate any non-specific or false-positive results. The high specificity of the qSDCRs is determined by two factors: specific probe hybridization with a target sequence and the specific hydrolysis of the probe by RNase H2. Thermostable Pa RNase H2 would cleave the probe only in the case of a perfect matched probe–DNA duplex formation [
25]. This characteristic of Pa RNase H2 suggests the possible use of qSDCR for single nucleotide polymorphism (SNP) detection.
Although SDCR employs a combination of two enzymes, SD DNA polymerase and thermostable RNase H2, the enzymatic system remains relatively simple. Both enzymatic functions, including strand displacement synthesis and site-specific nicking or probe hydrolysis, operate under the same thermal conditions and do not require stepwise enzyme addition. This simplifies the reaction workflow and reduces the potential for technical error. Moreover, the DNA polymerase used in SDCR does not require nuclease activity, as all cleavage and activation steps are mediated by RNase H2, enhancing both the specificity and modularity of the method. These features position SDCR as a technically efficient and operationally practical alternative among hybrid nucleic acid amplification strategies.
Along with the above-described advantages of SDCR over traditionally used PCR NA detection, the SDCR technique may have some limitations in use. First of all, it seems problematic to carry out a coupled reaction of reverse transcription and SDCR amplification in the “one-tube” format due to the presence of RNase H activity. We also found that SDCR exhibits the highest efficiency in the amplification of short (<200 bp) DNA fragments, so the size of the amplicons in our tests was about 100 base pairs (see
Section 2.5,
Section 2.6 and
Section 2.7). It should be mentioned that SDCR primers require an internal ribonucleotide and greater length, making them more complex and costly in terms of design and synthesis than conventional PCR primers. However, despite possible limitations, SDCR can be a very helpful tool in NA detection, especially in diagnostics, where speed, specificity and high sensitivity are required.
Additionally, several practical aspects must be considered for broader laboratory implementation of the method. Beyond its technical merits, SDCR requires specific reagents such as SD DNA polymerase, thermostable RNase H2, and ribonucleotide-containing primers, which increase assay costs compared to the single-enzyme format of conventional qPCR. For instance, SD polymerase is approximately 2.5 times more expensive than Taq polymerase. However, the markedly reduced number of cycles and shorter run time can improve throughput and partially offset these expenses, especially in high-throughput or time-critical applications.
Importantly, SDCR is fully compatible with standard real-time PCR instruments, allowing seamless integration into existing workflows without additional equipment. To support broader implementation and to confirm the economic and operational feasibility, further studies should include comprehensive cost–benefit analyses, evaluation of large-scale reagent availability, and validations in routine diagnostic settings. Such investigations will provide essential insights into the scalability and sustainability of SDCR for widespread clinical and research applications.