Single-Vesicle Molecular Profiling by dSTORM Imaging in a Liquid Biopsy Assay Predicts Early Relapse in Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Enrollment, Blood Sampling, and Plasma Isolation
2.2. Mismatch Repair Status Evaluation
2.3. Isolation of Extracellular Vesicles
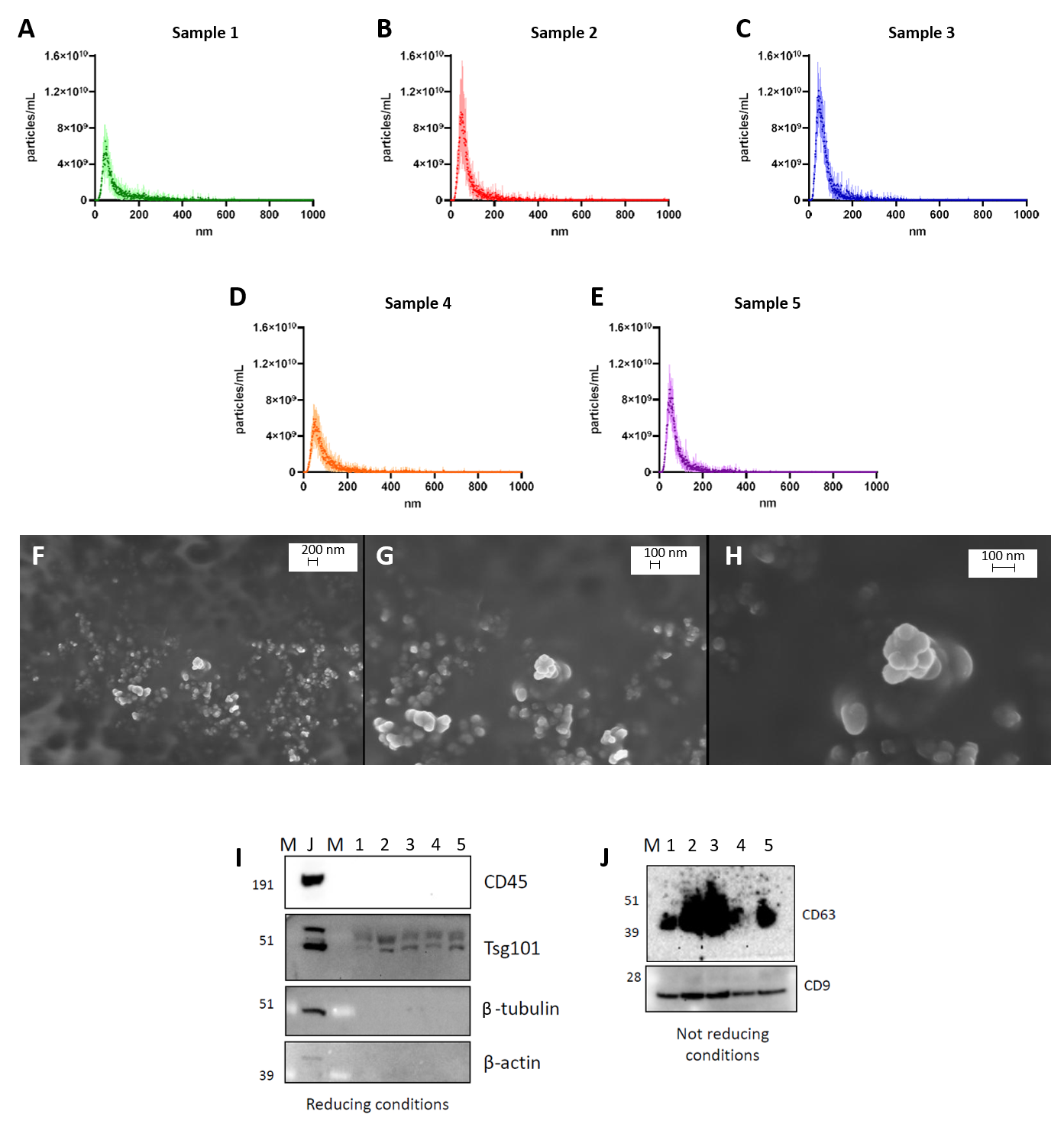
2.4. Characterization of EVs
2.4.1. Nanoparticle Tracking Analysis
2.4.2. Scanning Electron Microscopy (SEM)
2.4.3. Western Blot Analysis
2.4.4. Super-Resolution Microscopy
2.5. Statistical Analysis
3. Results
3.1. Characterization of sEVs
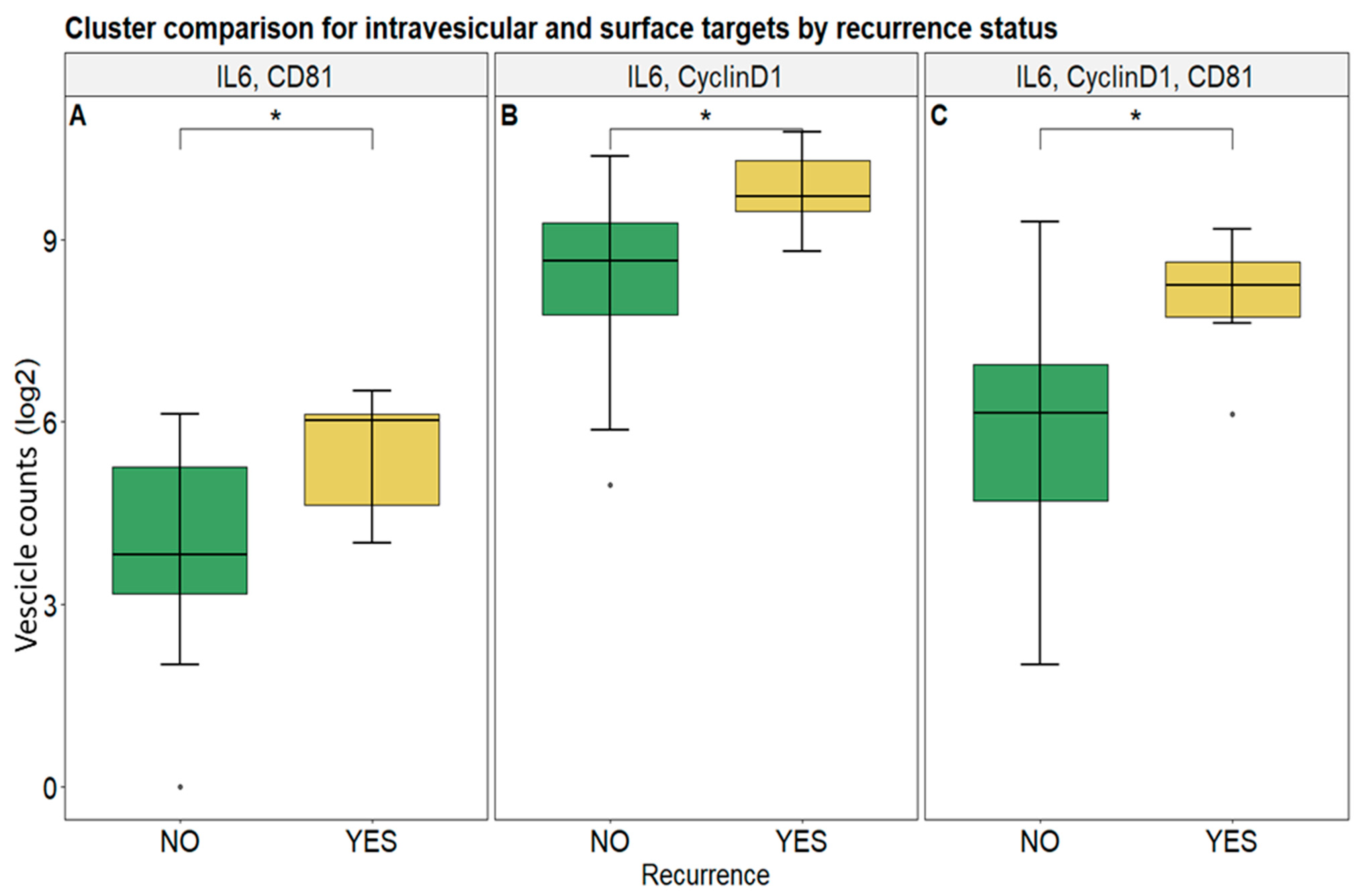
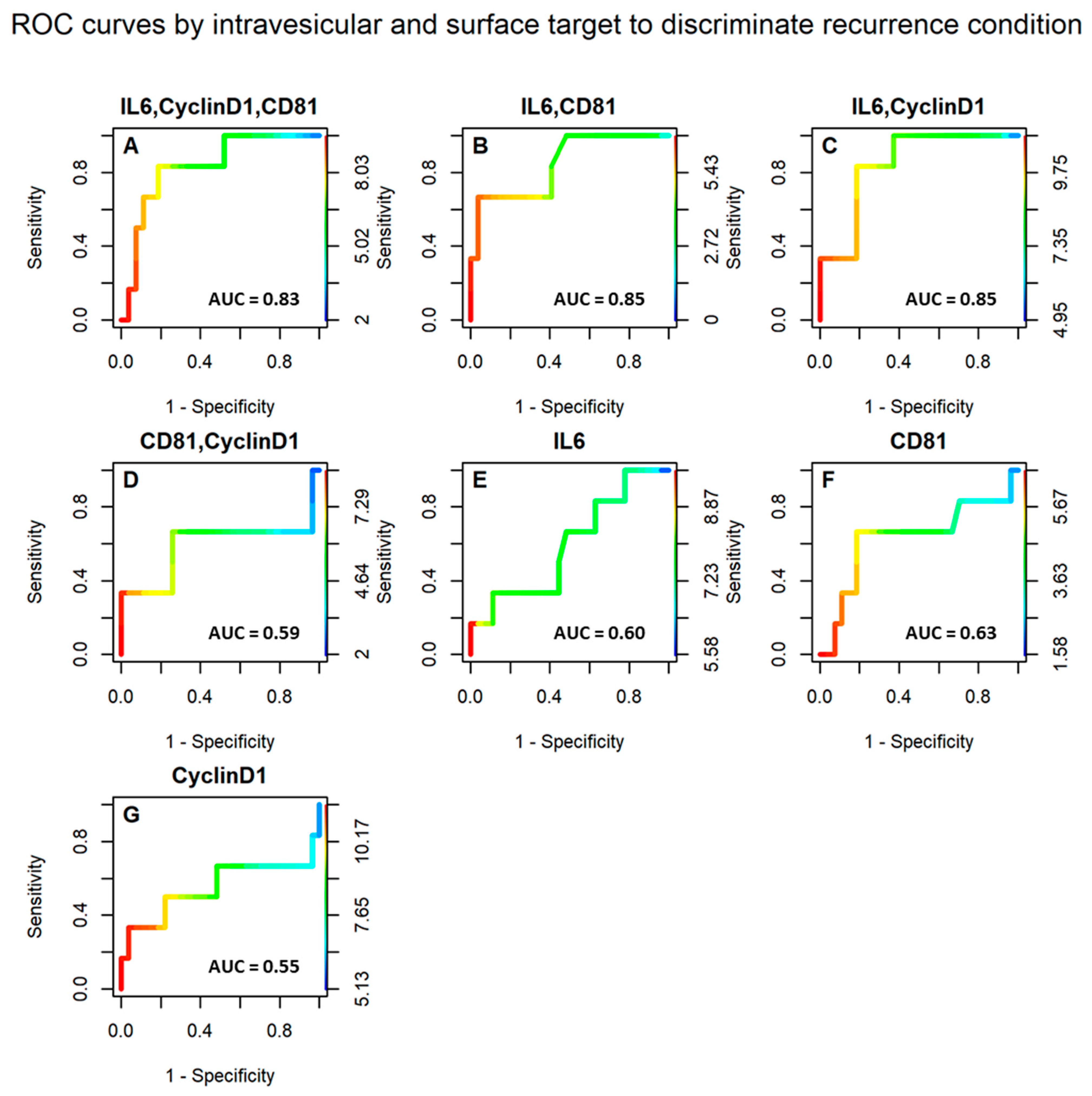
3.2. Selected Vesicular Markers Correlate with Poor Prognosis in Colorectal Cancer Patients
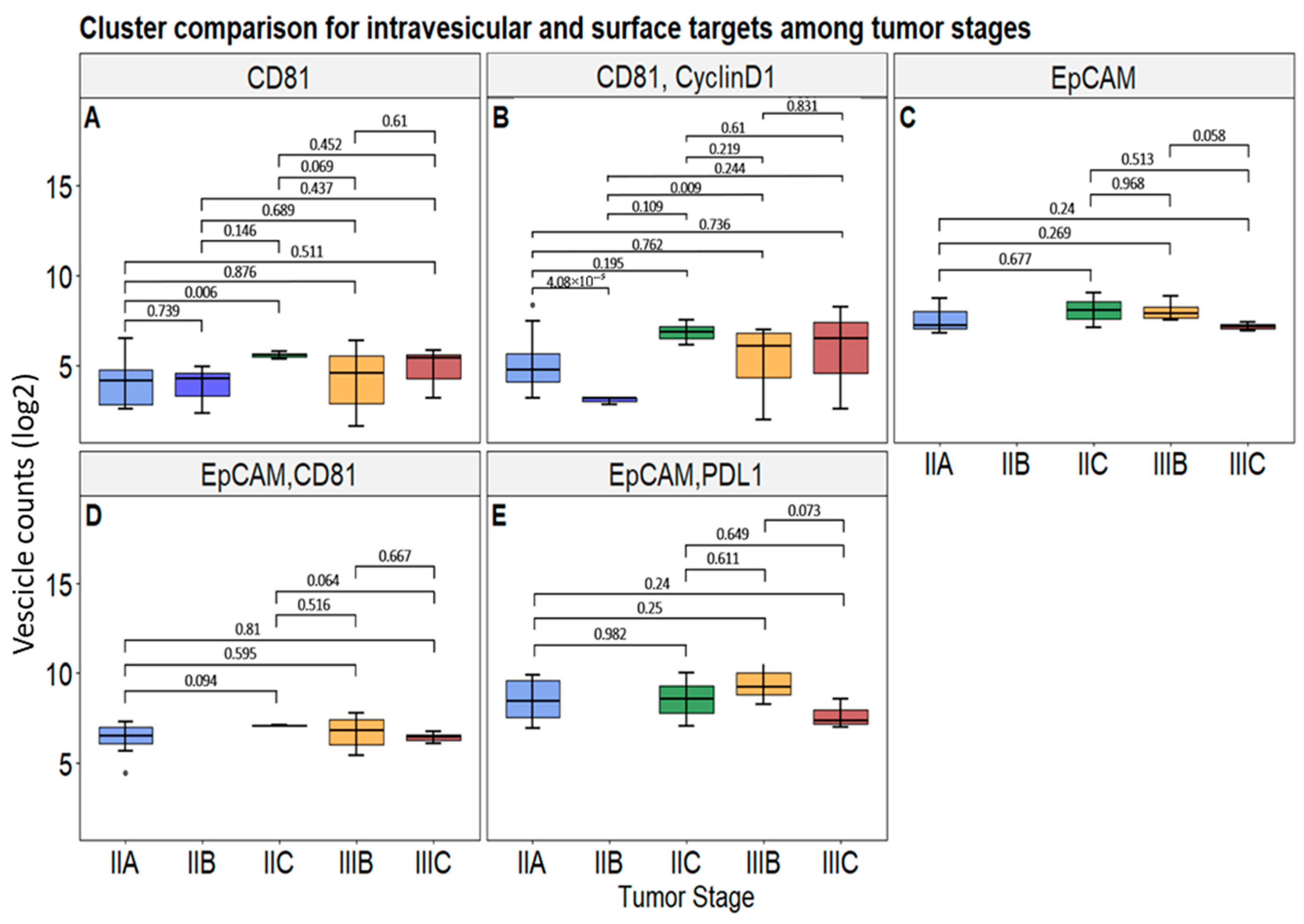
3.3. Correlation of Selected Vesicular Markers with CRC Patients’ Tumor Clinical Features
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Alexa Fluor dye |
| AUC | Area under the curve |
| CF | Cyanine-based far red fluorescent dye |
| CRC | Colorectal cancer |
| ctDNA | Circulating tumor DNA |
| CTCs | Circulating tumor cells |
| dMMR | Deficient mismatch repair |
| dSTORM | Direct stochastic optical reconstruction microscopy |
| EpCAM | Epithelial cell adhesion molecule |
| ESCRT | Endosomal sorting complexes required for transport |
| EVs | Extracellular vesicles |
| HDI | Human development index |
| ICI | Immune checkpoint inhibitor |
| IHC | Immunohistochemistry |
| IL-6 | Interleukin-6 |
| KRAS | Kirsten rat sarcoma virus |
| MMR | Mismatch repair |
| MSI | Microsatellite instability |
| NRAS | Neuroblastoma RAS oncogene |
| NTA | Nanoparticle tracking analysis |
| PD-L1 | Programmed cell death 1 ligand 1 |
| ROC | Receiver operating characteristic |
| RT | Room temperature |
| SEM | Scanning electron microscopy |
| UC | Ultracentrifugation |
| WB | Western blot |
References
- World Health Organization Website. World Health Statistics 2023: Monitoring Health for the SDGs, Sustainable Development Goals. Available online: https://www.who.int/publications/i/item/9789240074323 (accessed on 20 November 2023).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. ESMO Guidelines Committee. Electronic address: Clinicalguidelines@esmo.org. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.R.; Leshchiner, I.; Elagina, L.; Goyal, L.; Levovitz, C.; Siravegna, G.; Livitz, D.; Rhrissorrakrai, K.; Martin, E.E.; Van Seventer, E.E.; et al. Liquid versus tissue biopsy for detecting acquired resistance and tumor heterogeneity in gastrointestinal cancers. Nat. Med. 2019, 25, 1415–1421, Erratum in Nat. Med. 2019, 25, 1949. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Russo, M.; Siravegna, G.; Blaszkowsky, L.S.; Corti, G.; Crisafulli, G.; Ahronian, L.G.; Mussolin, B.; Kwak, E.L.; Buscarino, M.; Lazzari, L.; et al. Tumor Heterogeneity and Lesion-Specific Response to Targeted Therapy in Colorectal Cancer. Cancer Discov. 2016, 6, 147–153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siena, S.; Sartore-Bianchi, A.; Garcia-Carbonero, R.; Karthaus, M.; Smith, D.; Tabernero, J.; Van Cutsem, E.; Guan, X.; Boedigheimer, M.; Ang, A.; et al. Dynamic molecular analysis and clinical correlates of tumor evolution within a phase II trial of panitumumab-based therapy in metastatic colorectal cancer. Ann. Oncol. 2018, 29, 119–126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nors, J.; Iversen, L.H.; Erichsen, R.; Gotschalck, K.A.; Andersen, C.L. Incidence of Recurrence and Time to Recurrence in Stage I to III Colorectal Cancer: A Nationwide Danish Cohort Study. JAMA Oncol. 2023, 10, e235098. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zafar, S.N.; Hu, C.Y.; Snyder, R.A.; Cuddy, A.; You, Y.N.; Lowenstein, L.M.; Volk, R.J.; Chang, G.J. Predicting Risk of Recurrence After Colorectal Cancer Surgery in the United States: An Analysis of a Special Commission on Cancer National Study. Ann. Surg. Oncol. 2020, 27, 2740–2749. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Nozawa, H.; Hata, K.; Kiyomatsu, T.; Tanaka, T.; Nishikawa, T.; Sugihara, K.; Watanabe, T. Nomogram Predicting Survival After Recurrence in Patients with Stage I to III Colon Cancer: A Nationwide Multicenter Study. Dis. Colon. Rectum. 2018, 61, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Okamura, R.; Hida, K.; Nishizaki, D.; Sugihara, K.; Sakai, Y. Proposal of a stage-specific surveillance strategy for colorectal cancer patients: A retrospective analysis of Japanese large cohort. Eur. J. Surg. Oncol. 2018, 44, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Pugh, S.A.; Shinkins, B.; Fuller, A.; Mellor, J.; Mant, D.; Primrose, J.N. Site and Stage of Colorectal Cancer Influence the Likelihood and Distribution of Disease Recurrence and Postrecurrence Survival: Data from the FACS Randomized Controlled Trial. Ann. Surg. 2016, 263, 1143–1147. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; He, Y.; Wang, Y.; Li, X.; Young, J.; Ioannidis, J.P.A.; Dunlop, M.G.; Theodoratou, E. Risk factors and risk prediction models for colorectal cancer metastasis and recurrence: An umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid biopsy: A step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer 2022, 21, 79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tucci, M.; Mannavola, F.; Passarelli, A.; Stucci, L.S.; Cives, M.; Silvestris, F. Exosomes in melanoma: A role in tumor progression, metastasis and impaired immune system activity. Oncotarget 2018, 9, 20826–20837. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lugli, A.; Iezzi, G.; Hostettler, I.; Muraro, M.G.; Mele, V.; Tornillo, L.; Carafa, V.; Spagnoli, G.; Terracciano, L.; Zlobec, I. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br. J. Cancer. 2010, 103, 382–390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, S.; Zong, S.; Shi, Q.; Li, H.; Liu, S.; Yang, W.; Li, W.; Hou, F. Is Ep-CAM Expression a Diagnostic and Prognostic Biomarker for Colorectal Cancer? A Systematic Meta-Analysis. EBioMedicine 2017, 20, 61–69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.H.; Bae, J.M.; Song, Y.S.; Cho, N.Y.; Lee, H.S.; Kang, G.H. Clinicopathologic, molecular, and prognostic implications of the loss of EPCAM expression in colorectal carcinoma. Oncotarget 2016, 7, 13372–13387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, K.X.; Istl, A.C.; Quan, D.; Skaro, A.; Tang, E.; Zheng, X. PD-1 and PD-L1 inhibitors in cold colorectal cancer: Challenges and strategies. Cancer Immunol. Immunother. 2023, 72, 3875–3893. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dank, M.; Mühl, D.; Herold, M.; Hornyák, L.; Szasz, A.M.; Herold, Z. Does Elevated Pre-Treatment Plasma PD-L1 Level Indicate an Increased Tumor Burden and Worse Prognosis in Metastatic Colorectal Cancer? J. Clin. Med. 2022, 11, 4815. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, Y.; Zhang, X.; Zhu, M.; He, W.; Hua, H.; Ye, F.; Zhou, X.; Chen, N.; Li, Y.; Zhong, W.; et al. Soluble PD-L1: A potential dynamic predictive biomarker for immunotherapy in patients with proficient mismatch repair colorectal cancer. J. Transl. Med. 2023, 21, 25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, X.; Du, Z.; Huang, M.; Wang, D.; Fong, W.P.; Liang, J.; Fan, L.; Wang, Y.; Yang, H.; Chen, Z.; et al. Circulating PD-L1 is associated with T cell infiltration and predicts prognosis in patients with CRLM following hepatic resection. Cancer Immunol. Immunother. 2022, 71, 661–674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raimondi, L.; Raimondi, F.M.; Di Benedetto, L.; Cimino, G.; Spinelli, G.P. PD-L1 Expression on Circulating Tumor Cells May Be Predictive of Response to Regorafenib in Patients Diagnosed with Chemorefractory Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 6907. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jun, S.Y.; Kim, J.; Yoon, N.; Maeng, L.S.; Byun, J.H. Prognostic Potential of Cyclin D1 Expression in Colorectal Cancer. J. Clin. Med. 2023, 12, 572. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogino, S.; Nosho, K.; Irahara, N.; Kure, S.; Shima, K.; Baba, Y.; Toyoda, S.; Chen, L.; Giovannucci, E.L.; Meyerhardt, J.A.; et al. A cohort study of cyclin D1 expression and prognosis in 602 colon cancer cases. Clin. Cancer Res. 2009, 15, 4431–4438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Wei, J.; Xu, C.; Zhao, Z.; You, T. Prognostic significance of cyclin D1 expression in colorectal cancer: A meta-analysis of observational studies. PLoS ONE 2014, 9, e94508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taha, A.; Taha-Mehlitz, S.; Petzold, S.; Achinovich, S.L.; Zinovkin, D.; Enodien, B.; Pranjol, M.Z.I.; Nadyrov, E.A. Prognostic Value of Immunohistochemical Markers for Locally Advanced Rectal Cancer. Molecules 2022, 27, 596. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, J.; Huang, L.; Zhao, H.; Yan, Y.; Lu, J. The Role of Interleukins in Colorectal Cancer. Int. J. Biol. Sci. 2020, 16, 2323–2339. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, J.; Wang, X.; Yu, J.; Zhan, Z.; Guo, Z. IL-6, TNF-α and IL-12p70 levels in patients with colorectal cancer and their predictive value in anti-vascular therapy. Front. Oncol. 2022, 12, 997665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Circulating Interleukin-6 Level, Dietary Antioxidant Capacity, and Risk of Colorectal Cancer. Antioxidants 2019, 8, 595. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vainer, N.; Dehlendorff, C.; Johansen, J.S. Systematic literature review of IL-6 as a biomarker or treatment target in patients with gastric, bile duct, pancreatic and colorectal cancer. Oncotarget 2018, 9, 29820–29841. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lehtomäki, K.; Mustonen, H.; Kellokumpu-Lehtinen, P.L.; Joensuu, H.; Hermunen, K.; Soveri, L.M.; Boisen, M.K.; Dehlendorff, C.; Johansen, J.S.; Haglund, C.; et al. Lead Time and Prognostic Role of Serum CEA, CA19-9, IL-6, CRP, and YKL-40 after Adjuvant Chemotherapy in Colorectal Cancer. Cancers 2021, 13, 3892. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tseng-Rogenski, S.S.; Hamaya, Y.; Choi, D.Y.; Carethers, J.M. Interleukin 6 alters localization of hMSH3, leading to DNA mismatch repair defects in colorectal cancer cells. Gastroenterology 2015, 148, 579–589. [Google Scholar] [CrossRef]
- Mazzocca, A.; Liotta, F.; Carloni, V. Tetraspanin CD81-regulated cell motility plays a critical role in intrahepatic metastasis of hepatocellular carcinoma. Gastroenterology 2008, 135, 244–256.e1. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.H.; Ryu, B.K.; Lee, M.G.; Chi, S.G. CD81 is a candidate tumor suppressor gene in human gastric cancer. Cell Oncol. 2013, 36, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Luga, V.; Zhang, L.; Viloria-Petit, A.M.; Ogunjimi, A.A.; Inanlou, M.R.; Chiu, E.; Buchanan, M.; Hosein, A.N.; Basik, M.; Wrana, J.L. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 2012, 151, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Vences-Catalán, F.; Rajapaksa, R.; Kuo, C.C.; Miller, C.L.; Lee, A.; Ramani, V.C.; Jeffrey, S.S.; Levy, R.; Levy, S. Targeting the tetraspanin CD81 reduces cancer invasion and metastasis. Proc. Natl. Acad. Sci. USA 2021, 118, e2018961118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uretmen Kagiali, Z.C.; Sanal, E.; Karayel, Ö.; Polat, A.N.; Saatci, Ö.; Ersan, P.G.; Trappe, K.; Renard, B.Y.; Önder, T.T.; Tuncbag, N.; et al. Systems-level Analysis Reveals Multiple Modulators of Epithelial-mesenchymal Transition and Identifies DNAJB4 and CD81 as Novel Metastasis Inducers in Breast Cancer. Mol. Cell Proteomics 2019, 18, 1756–1771. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mizoshiri, N.; Shirai, T.; Terauchi, R.; Tsuchida, S.; Mori, Y.; Hayashi, D.; Kishida, T.; Arai, Y.; Mazda, O.; Nakanishi, T.; et al. The tetraspanin CD81 mediates the growth and metastases of human osteosarcoma. Cell Oncol. (Dordr). 2019, 42, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.K.; Byun, H.J.; Lee, J.; Jin, Y.J.; Wang, S.J.; Jeoung, D.I.; Kim, Y.M.; Lee, H. The tetraspanin CD81 protein increases melanoma cell motility by up-regulating metalloproteinase MT1-MMP expression through the pro-oncogenic Akt-dependent Sp1 activation signaling pathways. J. Biol. Chem. 2014, 289, 15691–15704. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vences-Catalán, F.; Rajapaksa, R.; Srivastava, M.K.; Marabelle, A.; Kuo, C.C.; Levy, R.; Levy, S. Tetraspanin CD81 promotes tumor growth and metastasis by modulating the functions of T regulatory and myeloid-derived suppressor cells. Cancer Res. 2015, 75, 4517–4526. [Google Scholar] [CrossRef] [PubMed]
- Martorana, E.; Raciti, G.; Giuffrida, R.; Bruno, E.; Ficarra, V.; Ludovico, G.M.; Suardi, N.R.; Iraci, N.; Leggio, L.; Bussolati, B.; et al. A Novel Liquid Biopsy Method Based on Specific Combinations of Vesicular Markers Allows Us to Discriminate Prostate Cancer from Hyperplasia. Cells 2024, 13, 1286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leggio, L.; L’Episcopo, F.; Magrì, A.; Ulloa-Navas, M.J.; Paternò, G.; Vivarelli, S.; Bastos, C.A.P.; Tirolo, C.; Testa, N.; Caniglia, S.; et al. Small Extracellular Vesicles Secreted by Nigrostriatal Astrocytes Rescue Cell Death and Preserve Mitochondrial Function in Parkinson’s Disease. Adv. Healthc. Mater. 2022, 11, e2201203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- R Studio Team. Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2021. [Google Scholar]
- Kassambara, A. ggpubr: ‘ggplot2′ Based Publication Ready Plots. 2023. Available online: https://rpkgs.datanovia.com/ggpubr/ (accessed on 20 May 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Sing, T.; Sander, O.; Beerenwinkel, N.; Lengauer, T. ROCR: Visualizing classifier performance in R. Bioinformatics 2005, 21, 3940–3941. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.J.; Bates, M.; Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 2006, 3, 793–795. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, B.; Wang, W.; Bates, M.; Zhuang, X. Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science 2008, 319, 810–813. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, C.; Zong, S.; Wang, Z.; Lu, J.; Zhu, D.; Zhang, Y.; Cui, Y. Imaging and Intracellular Tracking of Cancer-Derived Exosomes Using Single-Molecule Localization-Based Super-Resolution Microscope. ACS Appl. Mater. Interfaces 2016, 8, 25825–25833. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404, Erratum in: J. Extracell. Vesicles 2024, 13, e12451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McNamara, R.P.; Zhou, Y.; Eason, A.B.; Landis, J.T.; Chambers, M.G.; Willcox, S.; Peterson, T.A.; Schouest, B.; Maness, N.J.; MacLean, A.G.; et al. Imaging of surface microdomains on individual extracellular vesicles in 3-D. J. Extracell. Vesicles 2022, 11, e12191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maire, C.L.; Fuh, M.M.; Kaulich, K.; Fita, K.D.; Stevic, I.; Heiland, D.H.; Welsh, J.A.; Jones, J.C.; Görgens, A.; Ricklefs, T.; et al. Genome-wide methylation profiling of glioblastoma cell-derived extracellular vesicle DNA allows tumor classification. Neuro Oncol. 2021, 23, 1087–1099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verta, R.; Grange, C.; Skovronova, R.; Tanzi, A.; Peruzzi, L.; Deregibus, M.C.; Camussi, G.; Bussolati, B. Generation of Spike-Extracellular Vesicles (S-EVs) as a Tool to Mimic SARS-CoV-2 Interaction with Host Cells. Cells 2022, 11, 146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taieb, J.; Svrcek, M.; Cohen, R.; Basile, D.; Tougeron, D.; Phelip, J.M. Deficient mismatch repair/microsatellite unstable colorectal cancer: Diagnosis, prognosis and treatment. Eur. J. Cancer 2022, 175, 136–157. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, G.; Southward, K.; Handley, K.; Magill, L.; Beaumont, C.; Stahlschmidt, J.; Richman, S.; Chambers, P.; Seymour, M.; Kerr, D.; et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J. Clin. Oncol. 2011, 29, 1261–1270, Erratum in J. Clin. Oncol. 2011, 29, 2949. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A.; Foster, N.R.; Thibodeau, S.N.; Marsoni, S.; Monges, G.; Labianca, R.; Kim, G.P.; Yothers, G.; Allegra, C.; Moore, M.J.; et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J. Natl. Cancer Inst. 2011, 103, 863–875, Erratum in J. Natl. Cancer Inst. 2011, 103, 1639. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency, N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Llosa, N.J.; Cruise, M.; Tam, A.; Wicks, E.C.; Hechenbleikner, E.M.; Taube, J.M.; Blosser, R.L.; Fan, H.; Wang, H.; Luber, B.S.; et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015, 5, 43–51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. KEYNOTE-177 Investigators Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer, N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, Z.; Liu, C.; Shi, D.; Li, D.; Zheng, M.; Han-Zhang, H.; Lizaso, A.; Xiang, J.; Lv, J.; et al. Detection of Microsatellite Instability from Circulating Tumor DNA by Targeted Deep Sequencing. J. Mol. Diagn. 2020, 22, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.; Lefterova, M.I.; Artyomenko, A.; Kasi, P.M.; Nakamura, Y.; Mody, K.; Catenacci, D.V.T.; Fakih, M.; Barbacioru, C.; Zhao, J.; et al. Validation of Microsatellite Instability Detection Using a Comprehensive Plasma-Based Genotyping Panel. Clin. Cancer Res. 2019, 25, 7035–7045. [Google Scholar] [CrossRef] [PubMed]
- Cabel, L.; Riva, F.; Servois, V.; Livartowski, A.; Daniel, C.; Rampanou, A.; Lantz, O.; Romano, E.; Milder, M.; Buecher, B.; et al. Circulating tumor DNA changes for early monitoring of anti-PD1 immunotherapy: A proof-of-concept study. Ann. Oncol. 2017, 28, 1996–2001. [Google Scholar] [CrossRef] [PubMed]
- Georgiadis, A.; Durham, J.N.; Keefer, L.A.; Bartlett, B.R.; Zielonka, M.; Murphy, D.; White, J.R.; Lu, S.; Verner, E.L.; Ruan, F.; et al. Noninvasive Detection of Microsatellite Instability and High Tumor Mutation Burden in Cancer Patients Treated with PD-1 Blockade. Clin. Cancer Res. 2019, 25, 7024–7034. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kasi, P.M.; Budde, G.; Krainock, M.; Aushev, V.N.; Koyen Malashevich, A.; Malhotra, M.; Olshan, P.; Billings, P.R.; Aleshin, A. Circulating tumor DNA (ctDNA) serial analysis during progression on PD-1 blockade and later CTLA-4 rescue in patients with mismatch repair deficient metastatic colorectal cancer. J. Immunother. Cancer 2022, 10, e003312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



| Antibody | Dilution | Brand | Catalog Number |
|---|---|---|---|
| Mouse monoclonal anti-CD45 | 1:500 | R&D Systems | MAB14303 |
| Rabbit monoclonal anti-Tsg101 | 1:1000 | Abcam | ab125011 |
| Mouse monoclonal anti-β-Actin | 1:4000 | Sigma Aldrich | A1978 |
| Mouse monoclonal anti-β-Tubulin | 1:1000 | Cell Signaling technology | 86298 |
| Mouse monoclonal anti-CD63 | 1:1000 | Invitrogen | 10628D |
| Mouse monoclonal anti-CD9 | 1:1000 | Invitrogen | 10626D |
| Goat anti-mouse IgG (H + L) secondary antibody, Alexa Fluor Plus 555 | 1:5000 | Invitrogen | A32727 |
| Goat anti-mouse IgG (H + L) secondary antibody, Alexa Fluor Plus 488 | 1:5000 | Invitrogen | A32766 |
| Goat anti-rabbit IgG (H + L) secondary antibody, Alexa Fluor Plus 800 | 1:5000 | Invitrogen | A32808 |
| HRP-conjugated anti-rabbit secondary antibody | 1:10,000 | Invitrogen | 31460 |
| HRP-conjugated anti-mouse secondary antibody | 1:10,000 | Dako | P0447 |
| AGE (y.o.) | 67.4 (37–82) |
| GENDER | |
| Males | 18 (55%) |
| Females | 15 (45%) |
| STAGING | |
| IIA | 17 (52%) |
| IIB | 3 (9%) |
| IIC | 2 (6%) |
| IIIB | 8 (24%) |
| IIIC | 3 (9%) |
| TUMOR SIZE (T STATUS) | |
| T3 | 26 (79%) |
| T4 | 6 (18%) |
| Not Available | 1 (3%) |
| LYMPH NODE STATUS | |
| N0 | 21 (64%) |
| N1 | 9 (27%) |
| N2 | 2 (6%) |
| Not Available | 1 (3%) |
| MMR STATUS | |
| Proficient | 26 (79%) |
| Deficient | 5 (15%) |
| Not Available | 2 (6%) |
| RECURRENCE | |
| No | 27 (85%) |
| Yes | 6 (15%) |
| NO | YES | Sensitivity | Specifcity | Accuracy | |
|---|---|---|---|---|---|
| IL6,CyclinD1,CD81 ≥ 7.62 | 5 | 4 | 0.81 | 0.67 | 0.79 |
| IL6,CyelinD1,CD81 < 7.62 | 22 | 2 | |||
| IL6,CD81 ≥ 5.21 | 8 | 4 | 0.70 | 0.67 | 0.70 |
| IL6,CD81 ≤ 5.21 | 19 | 2 | |||
| IL6,CyclinD1 ≥ 9.56 | 5 | 4 | 0.81 | 0.67 | 0.79 |
| IL6,CyclinD1 < 9.56 | 22 | 2 | |||
| CD81,CyclinD1 ≥ 6.13 | 8 | 4 | 0.70 | 0.67 | 0.70 |
| CD81,CyclinD1 < 6.13 | 19 | 2 | |||
| IL6 ≥ 7.55 | 12 | 3 | 0.56 | 0.50 | 0.55 |
| IL6 < 7.55 | 15 | 3 | |||
| CD81 ≥ 4.91 | 8 | 4 | 0.70 | 0.67 | 0.70 |
| CD81 < 4.91 | 19 | 2 | |||
| CyclinD1 ≥ 9.06 | 13 | 3 | 0.52 | 0.50 | 0.52 |
| CyclinD1 < 9.06 | 14 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raciti, G.; Cavallaro, G.; Giuffrida, R.; Grange, C.; Leggio, L.; Catania, M.; Iraci, N.; Bruno, E.; Giaimi, L.A.; Lombardo, S.P.; et al. Single-Vesicle Molecular Profiling by dSTORM Imaging in a Liquid Biopsy Assay Predicts Early Relapse in Colorectal Cancer. Biomolecules 2025, 15, 1307. https://doi.org/10.3390/biom15091307
Raciti G, Cavallaro G, Giuffrida R, Grange C, Leggio L, Catania M, Iraci N, Bruno E, Giaimi LA, Lombardo SP, et al. Single-Vesicle Molecular Profiling by dSTORM Imaging in a Liquid Biopsy Assay Predicts Early Relapse in Colorectal Cancer. Biomolecules. 2025; 15(9):1307. https://doi.org/10.3390/biom15091307
Chicago/Turabian StyleRaciti, Gabriele, Giulia Cavallaro, Raffaella Giuffrida, Cristina Grange, Loredana Leggio, Marco Catania, Nunzio Iraci, Elena Bruno, Luca Antonio Giaimi, Sofia Paola Lombardo, and et al. 2025. "Single-Vesicle Molecular Profiling by dSTORM Imaging in a Liquid Biopsy Assay Predicts Early Relapse in Colorectal Cancer" Biomolecules 15, no. 9: 1307. https://doi.org/10.3390/biom15091307
APA StyleRaciti, G., Cavallaro, G., Giuffrida, R., Grange, C., Leggio, L., Catania, M., Iraci, N., Bruno, E., Giaimi, L. A., Lombardo, S. P., Chisari, G., Mare, M., Deiana, E., Memeo, L., Bussolati, B., & Forte, S. (2025). Single-Vesicle Molecular Profiling by dSTORM Imaging in a Liquid Biopsy Assay Predicts Early Relapse in Colorectal Cancer. Biomolecules, 15(9), 1307. https://doi.org/10.3390/biom15091307










