Natural Compounds as Modulators of Ferroptosis: Mechanistic Insights and Therapeutic Prospects in Breast Cancer
Abstract
1. Introduction
2. The Molecular Regulatory Network of Ferroptosis
2.1. Regulation of Iron Metabolism
2.2. Lipid Peroxidation
2.3. Antioxidant Defense System
3. The Intrinsic Link Between Ferroptosis and Breast Cancer Therapy
3.1. Intrinsic Mechanisms Underlying Ferroptosis Sensitivity in Breast Cancer Cells
3.2. Differences in Ferroptosis Sensitivity Across Molecular Subtypes of Breast Cancer
3.3. Genetic Regulatory Network of Ferroptosis Sensitivity
3.4. Strategies for Using Ferroptosis Induction to Overcome Drug Resistance in Breast Cancer
4. Natural Products as Modulators of Ferroptosis in Breast Cancer
4.1. Polyphenols
| Compound | Strength of Evidence | Chemical Class | Model/ Disease | Effect | Reference |
|---|---|---|---|---|---|
| Quercetin | Strong | Flavonoid | MCF-7, MDA-MB-231 (breast cancer) | Induces ferroptosis by driving TFEB nuclear translocation, activating ferritinophagy, expanding the labile iron pool and intensifying lipid peroxidation. | [113] |
| Robustaflavone A | Strong | Flavonoid (biflavonoids) | Breast cancer cell lines | Elicit ferroptosis through mitochondrial dysfunction–associated escalation of lipid peroxidation. | [119] |
| Genistein | Strong | Isoflavone | MDA-MB-231 (TNBC) | Triggers ferroptosis characterized by increased lipid ROS and MDA with concomitant GPX4 suppression. | [120] |
| Daidzein | Strong | Isoflavone | MDA-MB-231 (TNBC) | Promotes ferroptosis via enhanced lipid peroxidation and GPX4 downregulation. | [120] |
| Resveratrol | Strong | Polyphenol (stilbene) | TNBC cells and xenografts | Induces ferroptosis through NEDD4L-mediated ubiquitination and degradation of GPX4, leading to lipid peroxide accumulation. | [116] |
| Oxyresveratrol | Strong | Polyphenol (stilbene) | Breast cancer cells | Suppresses the EGFR/PI3K/AKT pathway, downregulates GPX4, and thereby provokes ferroptosis. | [81] |
| Formononetin | Strong | Isoflavone | TNBC cells | Inhibits the mTORC1/SREBP1/SCD1 axis, reduces MUFA biosynthesis, and facilitates ferroptosis via augmented lipid peroxidation. | [117] |
| Rosmarinic acid | Strong | Phenolic acid | TNBC cells | Promotes mitochondrial fission and consequently drives ferroptosis through intensified lipid oxidative damage. | [118] |
| Curcumin | Strong | Curcuminoid | MCF-7, MDA-MB-231 (breast cancer) | Orchestrates ferroptosis by repressing the p53/SLC7A11 axis to deplete GSH and inactivate GPX4, while upregulating HO-1 to enlarge the labile iron pool and enhancing SLC1A5-dependent metabolic stress. | [121] |
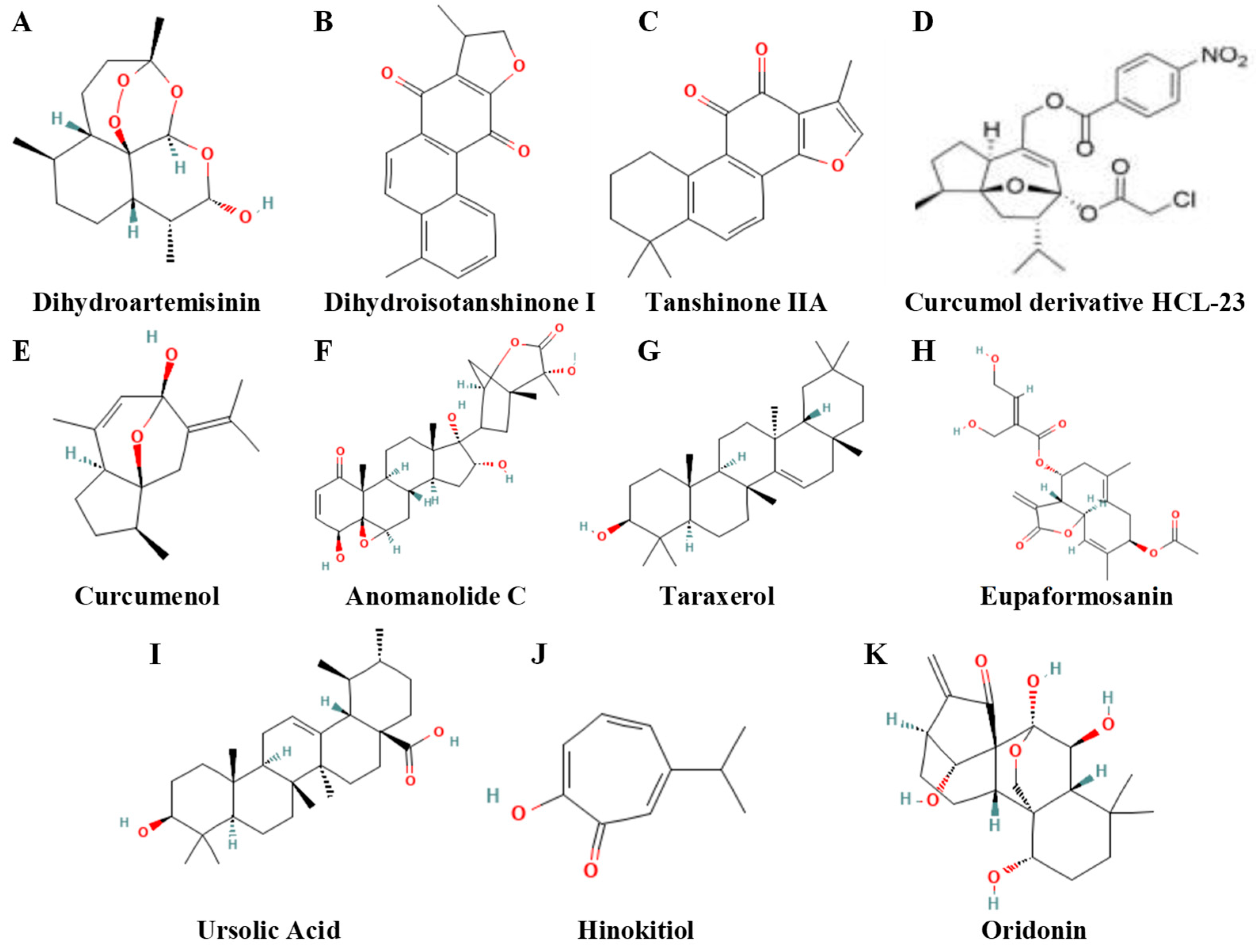
4.2. Terpenoids and Their Derivatives
| Compound | Strength of Evidence | Chemical subclass | Model/Disease | Effect (Concise Academic Wording) | Reference |
|---|---|---|---|---|---|
| Dihydroartemisinin (DHA) | Strong | Sesquiterpene lactone | Breast cancer (radiation model) | Targets ferroptosis signaling via hsa_circ_0001610 to increase radiosensitivity. | [144] |
| Dihydroisotanshinone I | Strong | Abietane diterpenoid (tanshinone family) | Breast cancer cells | Induces both ferroptosis and apoptosis. | [158] |
| Tanshinone IIA | Strong | Abietane diterpenoid | Breast cancer cells | Destabilizes SLC7A11 through KDM1A–PIAS4–mediated SUMOylation, promoting ferroptosis. | [146] |
| Curcumol derivative HCL-23 | Strong | Sesquiterpenoid | TNBC | Induces HO-1-dependent ferroptosis and apoptosis, inhibiting malignant phenotype. | [135] |
| Curcumenol | Strong | Sesquiterpenoid | TNBC | Promotes ferroptosis via the SLC7A11/NF-κB/TGF-β pathway and suppresses malignant progression. | [147] |
| Anomanolide C | Strong | Sesquiterpene lactone | TNBC | Triggers autophagy-dependent ferroptosis by promoting GPX4 ubiquitination, suppressing progression and metastasis. | [148] |
| Taraxerol | Strong | Triterpenoid | Breast cancer cells | Inhibits Nrf2 transcriptional activity, facilitating MIB2-mediated GPX4 ubiquitination and ferroptosis. | [149] |
| Eupaformosanin | Strong | Sesquiterpene lactone | TNBC | Induces apoptosis and ferroptosis through ubiquitination of mutant p53. | [150] |
| Ursolic acid | Strong | Triterpenoid | TNBC stem-like cells | Inhibits proliferation through NRF2-mediated ferroptosis. | [151] |
| Hinokitiol | Strong | Monoterpenoid (tropolone) complex | TNBC (in vitro and in vivo) | Acts as a ferroptosis inducer to inhibit tumor growth. | [152] |
| Oridonin | Strong | Diterpenoid | Breast cancer cells | Potentiates RSL3-induced ferroptosis via JNK/Nrf2/HO-1 oxidative-stress signaling. | [153] |
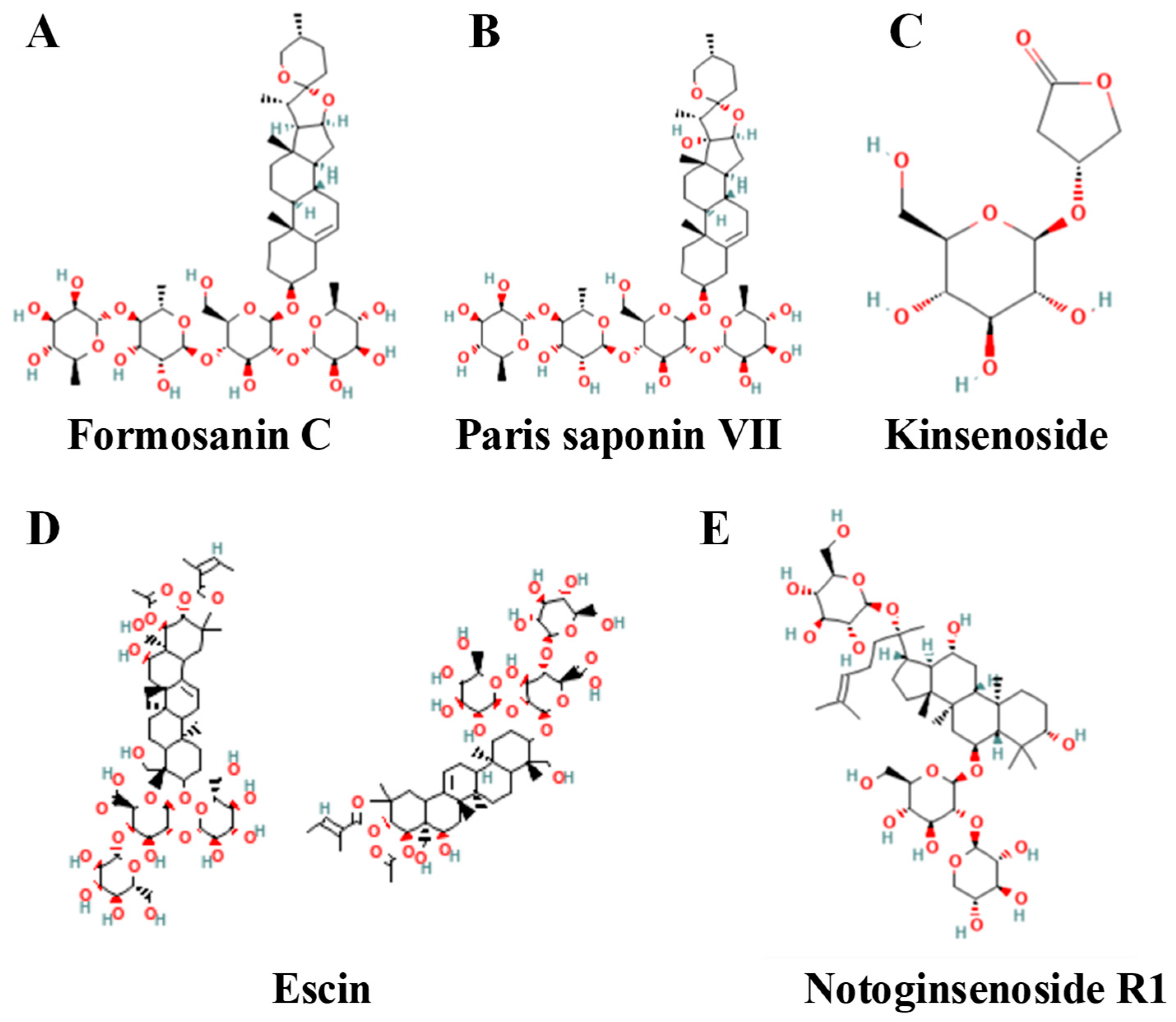
4.3. Saponins
| Compound | Strength of Evidence | Chemical Class | Model/ Disease | Effect | Reference |
|---|---|---|---|---|---|
| Formosanin C | Strong | Steroidal saponin | MDA-MB-231 (TNBC), MCF-7 (Luminal A) | Induces ferroptosis characterized by elevated ROS and MDA, depletion of glutathione, and increased intracellular ferrous iron. | [166] |
| Paris saponin VII | Strong | Steroidal saponin | MCF-7 (ER+) | Promotes ferroptosis by inhibiting the Nrf2/GPX4 axis and intensifying oxidative lipid damage. | [165] |
| Kinsenoside | Strong | Diterpenoid saponin | MDA-MB-231 (TNBC) | Triggers ferroptosis by inhibiting DGAT1-mediated lipid droplet biogenesis and enhancing lipid peroxidation. | [167] |
| Escin | Strong | Steroidal saponin | Breast cancer cell lines | Elicits ferroptosis through lipid peroxidation and synergizes with cisplatin to enhance antitumor efficacy. | [168] |
| Notoginsenoside R1 (NGR1) | Strong | Steroidal saponin | Breast cancer cell lines | Accelerates ferroptosis by repressing RUNX2 and modulating the AGE-RAGE pathway, thereby amplifying oxidative stress. | [164] |
4.4. Alkaloids
| Compound | Strength of Evidence | Chemical Class | Model/ Disease | Effect | Reference |
|---|---|---|---|---|---|
| Peiminine | Strong | Natural alkaloid | Breast cancer cells | Induces ferroptosis through modulation of the Nrf2 pathway, leading to oxidative and lipid peroxidative stress. | [172] |
| Indirubin | Strong | Indole alkaloid | 4T1 murine breast cancer (in vitro and in vivo) | Suppresses tumor growth by inducing ferroptosis marked by GPX4 downregulation and lipid peroxidation. | [173] |
4.5. Other Chemical Classes
| Compound | Strength of Evidence | Chemical Class | Model/ Disease | Effect | Reference |
|---|---|---|---|---|---|
| Tetrastigma hemsleyanum polysaccharide + doxorubicin | Strong | Polysaccharide (combination) | TNBC cells | Produces a synergistic antitumor effect by promoting ferroptosis and modulating antitumor immunity. | [92] |
| Plumbagin | Moderate | Quinone | (Glioma; mechanistic reference) | Acts as a GPX4-targeting ferroptosis inducer, offering mechanistic insight transferable to breast cancer contexts. | [176] |
| Juglone | Moderate | Quinone | (Endometrial cancer; mechanistic reference) | Induces ferroptosis via GPX4 inhibition, providing a mechanistic template relevant to breast cancer. | [186] |
| β-Lapachone | Moderate | Quinone | Colorectal cancer | Activates NCOA4-mediated ferritinophagy and JNK signaling to trigger ferroptosis; the mechanism is informative for breast cancer translation. | [177] |
| Bufalin | Strong | Sterol (cardiac glycoside) | Breast cancer cells | Induces ferroptosis by perturbing the DECR1–SLC7A11 axis and enhancing lipid peroxidation. | [35] |
| Erigoster B | Strong | Sterol | Breast cancer cells | Targets DECR1 to reprogram phosphatidylcholine/arachidonic acid metabolism and enforce ferroptosis. | [184] |
5. Conclusion, Current Limitations, and Future Perspectives
5.1. A Critical Review of Gaps and Limitations
5.2. Future Perspectives and Strategies to Surmount Obstacles
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sopik, V. International Variation in Breast Cancer Incidence and Mortality in Young Women. Breast Cancer Res. Treat. 2021, 186, 497–507. [Google Scholar] [CrossRef]
- Kinnel, B.; Singh, S.K.; Oprea-Ilies, G.; Singh, R. Targeted Therapy and Mechanisms of Drug Resistance in Breast Cancer. Cancers 2023, 15, 1320. [Google Scholar] [CrossRef]
- Riggio, A.I.; Varley, K.E.; Welm, A.L. The Lingering Mysteries of Metastatic Recurrence in Breast Cancer. Br. J. Cancer 2021, 124, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Serrano García, L.; Jávega, B.; Llombart Cussac, A.; Gión, M.; Pérez-García, J.M.; Cortés, J.; Fernández-Murga, M.L. Patterns of Immune Evasion in Triple-Negative Breast Cancer and New Potential Therapeutic Targets: A Review. Front. Immunol. 2024, 15, 1513421. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.J.; Daniels, B.; Kiely, B.E.; O’Connell, D.L.; Beith, J.; Pearson, S.; Chiew, K.-L.; Bulsara, M.K.; Houssami, N. Long Term Risk of Distant Metastasis in Women with Non-Metastatic Breast Cancer and Survival after Metastasis Detection: A Population-Based Linked Health Records Study. Med. J. Aust. 2022, 217, 402–409. [Google Scholar] [CrossRef]
- Pasha, N.; Turner, N.C. Understanding and Overcoming Tumor Heterogeneity in Metastatic Breast Cancer Treatment. Nat. Cancer 2021, 2, 680–692. [Google Scholar] [CrossRef]
- Chen, W.; Shen, L.; Jiang, J.; Zhang, L.; Zhang, Z.; Pan, J.; Ni, C.; Chen, Z. Antiangiogenic Therapy Reverses the Immunosuppressive Breast Cancer Microenvironment. Biomark. Res. 2021, 9, 59. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, Biology and Role in Disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, N.; Ding, C.; Zhang, H.; Liu, D.; Liu, S. Ferroptosis and Emt Resistance in Cancer: A Comprehensive Review of the Interplay. Front. Oncol. 2024, 14, 1344290. [Google Scholar] [CrossRef]
- Zou, W.; Wang, X.; Xia, X.; Zhang, T.; Nie, M.; Xiong, J.; Fang, X. Resveratrol Protected against the Development of Endometriosis by Promoting Ferroptosis through Mir-21-3p/P53/Slc7a11 Signaling Pathway. Biochem. Biophys. Res. Commun. 2024, 692, 149338. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Liu, Y.; Li, M.; Luo, Z. Emerging Roles of Ferroptosis in the Tumor Immune Landscape: From Danger Signals to Anti-Tumor Immunity. FEBS J. 2022, 289, 3655–3665. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Jahan, S.; Singh, R.; Saxena, J.; Ashraf, S.A.; Khan, A.; Choudhary, R.K.; Balakrishnan, S.; Badraoui, R.; Bardakci, F. Plants in Anticancer Drug Discovery: From Molecular Mechanism to Chemoprevention. BioMed Res. Int. 2022, 2022, 5425485. [Google Scholar] [CrossRef]
- Arslan, A.K.K.; Uzunhisarcıklı, E.; Yerer, M.B.; Bishayee, A. The Golden Spice Curcumin in Cancer: A Perspective on Finalized Clinical Trials During the Last 10 Years. J. Cancer Res. Ther. 2022, 18, 19–26. [Google Scholar] [CrossRef]
- Song, B.; Wang, W.; Tang, X.; Goh, R.M.W.-J.; Thuya, W.L.; Ho, P.C.L.; Chen, L.; Wang, L. Inhibitory Potential of Resveratrol in Cancer Metastasis: From Biology to Therapy. Cancers 2023, 15, 2758. [Google Scholar] [CrossRef] [PubMed]
- Ge, A.; He, Q.; Zhao, D.; Li, Y.; Chen, J.; Deng, Y.; Xiang, W.; Fan, H.; Wu, S.; Li, Y. Mechanism of Ferroptosis in Breast Cancer and Research Progress of Natural Compounds Regulating Ferroptosis. J. Cell. Mol. Med. 2024, 28, e18044. [Google Scholar] [PubMed]
- Diao, J.; Jia, Y.; Dai, E.; Liu, J.; Kang, R.; Tang, D.; Han, L.; Zhong, Y.; Meng, L. Ferroptotic Therapy in Cancer: Benefits, Side Effects, and Risks. Mol. Cancer 2024, 23, 89. [Google Scholar] [CrossRef]
- Ge, C.; Zhang, S.; Mu, H.; Zheng, S.; Tan, Z.; Huang, X.; Xu, C.; Zou, J.; Zhu, Y.; Feng, D. Emerging Mechanisms and Disease Implications of Ferroptosis: Potential Applications of Natural Products. Front. Cell Dev. Biol. 2022, 9, 774957. [Google Scholar] [CrossRef]
- von Samson-Himmelstjerna, F.A.; Kolbrink, B.; Riebeling, T.; Kunzendorf, U.; Krautwald, S. Progress and Setbacks in Translating a Decade of Ferroptosis Research into Clinical Practice. Cells 2022, 11, 2134. [Google Scholar] [CrossRef]
- Song, Y.-H.; Lei, H.-X.; Yu, D.; Zhu, H.; Hao, M.-Z.; Cui, R.-H.; Meng, X.-S.; Sheng, X.-H.; Zhang, L. Endogenous Chemicals Guard Health through Inhibiting Ferroptotic Cell Death. Biofactors 2024, 50, 266–293. [Google Scholar] [CrossRef]
- Zhu, M.; Peng, L.; Huo, S.; Peng, D.; Gou, J.; Shi, W.; Tao, J.; Jiang, T.; Jiang, Y.; Wang, Q. Stat3 Signaling Promotes Cardiac Injury by Upregulating Ncoa4-Mediated Ferritinophagy and Ferroptosis in High-Fat-Diet Fed Mice. Free. Radic. Biol. Med. 2023, 201, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, S.; Wan, X.; Wang, R.; Luo, H.; Chang, C.; Dai, P.; Gan, Y.; Guo, Y.; Hou, Y.; et al. Tryptophan 2,3-Dioxygenase-Positive Matrix Fibroblasts Fuel Breast Cancer Lung Metastasis Via Kynurenine-Mediated Ferroptosis Resistance of Metastatic Cells and T Cell Dysfunction. Cancer Commun. 2024, 44, 1261–1286. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Q.; Shan, X.; Gao, W.; Chen, Q. Atm Orchestrates Ferritinophagy and Ferroptosis by Phosphorylating Ncoa4. Autophagy 2023, 19, 2062–2077. [Google Scholar] [CrossRef]
- Cañeque, T.; Baron, L.; Müller, S.; Carmona, A.; Colombeau, L.; Versini, A.; Solier, S.; Gaillet, C.; Sindikubwabo, F.; Sampaio, J.L. Activation of Lysosomal Iron Triggers Ferroptosis in Cancer. Nature 2025, 642, 492–500. [Google Scholar] [CrossRef]
- Huang, L.; Feng, J.; Zhu, J.; Yang, J.; Xiong, W.; Lu, X.; Chen, S.; Yang, S.; Li, Y.; Xu, Y. A Strategy of Fenton Reaction Cycloacceleration for High-Performance Ferroptosis Therapy Initiated by Tumor Microenvironment Remodeling. Adv. Healthc. Mater. 2023, 12, 2203362. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Liu, C.; Li, L.; Yang, M.; Jiang, N.; Luo, S.; Sun, L. Acyl-Coa Synthase Acsl4: An Essential Target in Ferroptosis and Fatty Acid Metabolism. Chin. Med. J. 2023, 136, 2521–2537. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lai, Y.; Lu, F.; Wang, W. Targeting Acsls to Modulate Ferroptosis and Cancer Immunity. Trends Endocrinol. Metab. 2024, 36, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Liu, L.; Shi, X.; Liu, Y.; Wang, J.; Fang, X.; Chen, Z.; Ai, D.; Zhu, Y.; Zhang, X. Alox15/15-Hpete Aggravates Myocardial Ischemia-Reperfusion Injury by Promoting Cardiomyocyte Ferroptosis. Circulation 2023, 147, 1444–1460. [Google Scholar] [CrossRef]
- Mortensen, M.S.; Ruiz, J.; Watts, J.L. Polyunsaturated Fatty Acids Drive Lipid Peroxidation During Ferroptosis. Cells 2023, 12, 804. [Google Scholar] [CrossRef]
- Sen, U.; Coleman, C.; Sen, T. Stearoyl Coenzyme a Desaturase-1: Multitasker in Cancer, Metabolism, and Ferroptosis. Trends Cancer 2023, 9, 480–489. [Google Scholar] [CrossRef]
- Xie, X.; Tian, L.; Zhao, Y.; Liu, F.; Dai, S.; Gu, X.; Ye, Y.; Zhou, L.; Liu, X.; Sun, Y. Bach1-Induced Ferroptosis Drives Lymphatic Metastasis by Repressing the Biosynthesis of Monounsaturated Fatty Acids. Cell Death Dis. 2023, 14, 48. [Google Scholar] [CrossRef]
- Dar, N.J.; John, U.; Bano, N.; Khan, S.; Bhat, S.A. Oxytosis/Ferroptosis in Neurodegeneration: The Underlying Role of Master Regulator Glutathione Peroxidase 4 (Gpx4). Mol. Neurobiol. 2024, 61, 1507–1526. [Google Scholar] [CrossRef]
- Tan, M.; Yin, Y.; Ma, X.; Zhang, J.; Pan, W.; Tan, M.; Zhao, Y.; Yang, T.; Jiang, T.; Li, H. Glutathione System Enhancement for Cardiac Protection: Pharmacological Options against Oxidative Stress and Ferroptosis. Cell Death 2023, 14, 131. [Google Scholar] [CrossRef]
- Sun, L.-L.; He, H.-Y.; Li, W.; Jin, W.-L.; Wei, Y.-J. The Solute Carrier Transporters (Slcs) Family in Nutrient Metabolism and Ferroptosis. Biomark. Res. 2024, 12, 94. [Google Scholar] [CrossRef]
- Li, T.; Yi, J.; Wu, H.; Wang, K.; Zhou, B. Slc7a11 in Hepatocellular Carcinoma: Potential Mechanisms, Regulation, and Clinical Significance. Am. J. Cancer Res. 2024, 14, 2326. [Google Scholar] [CrossRef]
- Wu, S.; Wu, X.; Wang, Q.; Chen, Z.; Li, L.; Chen, H.; Qi, H. Bufalin Induces Ferroptosis by Modulating the 2,4-Dienoyl-Coa Reductase (Decr1)-Slc7a11 Axis in Breast Cancer. Phytomedicine 2024, 135, 156130. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Wei, X.; Zhao, J.; Zhang, D.; Luo, Y.; Yang, Y.; Xiang, Y.; Liu, X. Inhibition of Fsp1: A New Strategy for the Treatment of Tumors. Oncol. Rep. 2024, 52, 105. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liang, L.; Liu, S.; Yi, H.; Zhou, Y. Fsp1: A Key Regulator of Ferroptosis. Trends Mol. Med. 2023, 29, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Fu, L.; Liang, H.; Ai, X.; Liu, F.; Li, N.; Wu, L.; Li, S.; Yang, X.; Lin, Y. Inhibition of Mitochondrial Complex I Induces Mitochondrial Ferroptosis by Regulating Coqh2 Levels in Cancer. Cell Death 2025, 16, 254. [Google Scholar] [CrossRef]
- Liu, Z.; Kang, R.; Yang, N.; Pan, X.; Yang, J.; Yu, H.; Deng, W.; Jia, Z.; Zhang, J.; Shen, Q. Tetrahydrobiopterin Inhibitor-Based Antioxidant Metabolic Strategy for Enhanced Cancer Ferroptosis-Immunotherapy. J. Colloid 2024, 658, 100–113. [Google Scholar] [CrossRef]
- Yan, R.; Lin, B.; Jin, W.; Tang, L.; Hu, S.; Cai, R. Nrf2, a Superstar of Ferroptosis. Antioxidants 2023, 12, 1739. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, J.; Huang, S.; Chen, X.; Chang, A.C.Y.; Wang, C.; Zhang, J.; Zhang, H. Hydrogen Sulfide Protects Cardiomyocytes from Doxorubicin-Induced Ferroptosis through the Slc7a11/Gsh/Gpx4 Pathway by Keap1 S-Sulfhydration and Nrf2 Activation. Redox Biol. 2024, 70, 103066. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, T.-a.; Zhang, W.-Y.; Huang, S.-R.; Hu, Y.; Sun, J. Rhein Attenuates Cerebral Ischemia-Reperfusion Injury Via Inhibition of Ferroptosis through Nrf2/Slc7a11/Gpx4 Pathway. Exp. Neurol. 2023, 369, 114541. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, W.; Zhang, W. Ferroptosis and the Bidirectional Regulatory Factor P53. Cell Death Discov. 2023, 9, 197. [Google Scholar] [CrossRef]
- Dibra, D.; Xiong, S.; Moyer, S.M.; El-Naggar, A.K.; Qi, Y.; Su, X.; Kong, E.K.; Korkut, A.; Lozano, G. Mutant P53 Protects Triple-Negative Breast Adenocarcinomas from Ferroptosis In Vivo. Sci. Adv. 2024, 10, eadk1835. [Google Scholar] [CrossRef]
- Peng, C.; Chen, Y.; Jiang, M. Targeting Ferroptosis: A Promising Strategy to Overcome Drug Resistance in Breast Cancer. Front. Oncol. 2024, 14, 1499125. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wen, X.; Qin, J.; Zhang, X.; Wang, Y.; Wang, Z.; Zhou, T.; Di, Y.; He, W. Metabolism-Regulated Ferroptosis in Cancer Progression and Therapy. Cell Death Dis. 2024, 15, 196. [Google Scholar] [CrossRef]
- Yang, F.; Xiao, Y.; Ding, J.-H.; Jin, X.; Ma, D.; Li, D.-Q.; Shi, J.-X.; Huang, W.; Wang, Y.-P.; Jiang, Y.-Z. Ferroptosis Heterogeneity in Triple-Negative Breast Cancer Reveals an Innovative Immunotherapy Combination Strategy. Cell Metab. 2023, 35, 84–100.e8. [Google Scholar] [CrossRef]
- Qiu, Z.; Deng, C.; Zhou, F.; Chen, Y.; Chen, X.; Liu, X.; Ye, C.; Jin, N. Ferroptosis Heterogeneity within the Tumor Microenvironment Revealed a Genetic Blueprint of Breast Cancer. Environ. Toxicol. 2024, 39, 2741–2752. [Google Scholar] [CrossRef]
- Huang, L.; Wei, Y.; Ni, M.; Hu, H.; Xi, L.; Wang, C.; Zhu, Z.; Yang, B.; Zhao, H. Novel Withanolides from Tubocapsicum Anomalum Suppress Triple-Negative Breast Cancer by Triggering Apoptosis and P53-Asct2-Slc7a11-Mediated Ferroptosis. Molecules 2024, 29, 1838. [Google Scholar] [CrossRef]
- Yu, X.; Guo, Q.; Zhang, H.; Wang, X.; Han, Y.; Yang, Z. Hypoxia-Inducible Factor-1α Can Reverse the Adriamycin Resistance of Breast Cancer Adjuvant Chemotherapy by Upregulating Transferrin Receptor and Activating Ferroptosis. FASEB J. 2024, 38, e23876. [Google Scholar] [CrossRef]
- Huang, G.; Cai, Y.; Ren, M.; Zhang, X.; Fu, Y.; Cheng, R.; Wang, Y.; Miao, M.; Zhu, L.; Yan, T. Salidroside Sensitizes Triple-Negative Breast Cancer to Ferroptosis by Scd1-Mediated Lipogenesis and Ncoa4-Mediated Ferritinophagy. J. Adv. Res. 2024, 74, 589–607. [Google Scholar] [CrossRef]
- Pope, L.E.; Dixon, S.J. Regulation of Ferroptosis by Lipid Metabolism. Trends Cell Biol. 2023, 33, 1077–1087. [Google Scholar] [CrossRef]
- Luo, M.; Yan, J.; Hu, X.; Li, H.; Li, H.; Liu, Q.; Chen, Y.; Zou, Z. Targeting Lipid Metabolism for Ferroptotic Cancer Therapy. Apoptosis 2023, 28, 81–107. [Google Scholar] [CrossRef]
- Sha, R.; Xu, Y.; Yuan, C.; Sheng, X.; Wu, Z.; Peng, J.; Wang, Y.; Lin, Y.; Zhou, L.; Xu, S. Predictive and Prognostic Impact of Ferroptosis-Related Genes Acsl4 and Gpx4 on Breast Cancer Treated with Neoadjuvant Chemotherapy. EBioMedicine 2021, 71, 103560. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Tuo, Q.-Z.; Meng, J.; Wu, X.-L.; Li, C.-L.; Lei, P. Thrombin Induces Ferroptosis in Triple-Negative Breast Cancer through the Cpla2α/Acsl4 Signaling Pathway. Transl. Oncol. 2024, 39, 101817. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, Y.; Tian, X.; Miao, Y.; Ma, L.; Zhang, C.; Xu, X.; Wang, J.; Fang, W.; Zhang, X. Lpcat3 Is Transcriptionally Regulated by Yap/Zeb/Ep300 and Collaborates with Acsl4 and Yap to Determine Ferroptosis Sensitivity. Antioxid. Redox Signal. 2023, 39, 491–511. [Google Scholar] [CrossRef]
- Luo, J.-Y.; Deng, Y.-L.; Lu, S.-Y.; Chen, S.-Y.; He, R.-Q.; Qin, D.-Y.; Chi, B.-T.; Chen, G.; Yang, X.; Peng, W. Current Status and Future Directions of Ferroptosis Research in Breast Cancer: Bibliometric Analysis. Interact. J. Med. Res. 2025, 14, e66286. [Google Scholar] [CrossRef]
- Mokhtarpour, K.; Razi, S.; Rezaei, N. Ferroptosis as a Promising Targeted Therapy for Triple Negative Breast Cancer. Breast Cancer Res. Treat. 2024, 207, 497–513. [Google Scholar] [CrossRef]
- Khan, M.; Sunkara, V.; Yadav, M.; Bokhari, S.F.H.; Rehman, A.; Maheen, A.; Shehryar, A.; Chilla, S.P.; Nasir, M.; Niaz, H. Ferroptosis and Triple-Negative Breast Cancer: A Systematic Overview of Prognostic Insights and Therapeutic Potential. Cureus 2024, 16, e51719. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.-K.; Liu, J.; Ma, C.-Z.; Huang, S.; He, F.-H.; Long, Y.; Zheng, Z.-S.; Liang, J.-L.; Xu, N.; Wang, G. Iron-Dependent Cell Death: Exploring Ferroptosis as a Unique Target in Triple-Negative Breast Cancer Management. Cancer Manag. Res. 2025, 17, 625–637. [Google Scholar] [CrossRef]
- Hausman, R.; Brown, W.; McDonald, P.; Awrey, S.; Sun, G.; Montell, D.; Dedhar, S. Increased Ferroptosis Sensitivity and Epithelial to Mesenchymal Transition of Breast Cancer Cells Overcoming Chemotherapeutic Mediated Apoptotic Caspase Activation. Cancer Res. 2024, 84, 6002. [Google Scholar] [CrossRef]
- Ren, Y.; Mao, X.; Xu, H.; Dang, Q.; Weng, S.; Zhang, Y.; Chen, S.; Liu, S.; Ba, Y.; Zhou, Z. Ferroptosis and Emt: Key Targets for Combating Cancer Progression and Therapy Resistance. Cellular 2023, 80, 263. [Google Scholar] [CrossRef]
- Mu, W.; Zhou, Z.; Shao, L.; Wang, Q.; Feng, W.; Tang, Y.; He, Y.; Wang, Y. Advances in the Relationship between Ferroptosis and Epithelial–Mesenchymal Transition in Cancer. Front. Oncol. 2023, 13, 1257985. [Google Scholar] [CrossRef]
- Schwab, A.; Rao, Z.; Zhang, J.; Gollowitzer, A.; Siebenkäs, K.; Bindel, N.; D’Avanzo, E.; van Roey, R.; Hajjaj, Y.; Özel, E. Zeb1 Mediates Emt/Plasticity-Associated Ferroptosis Sensitivity in Cancer Cells by Regulating Lipogenic Enzyme Expression and Phospholipid Composition. Nat. Cell Biol. 2024, 26, 1470–1481. [Google Scholar] [CrossRef]
- Winkelkotte, A.M.; Schulze, A. A Fatty Acid Switch Drives Ferroptosis in Emt. Nat. Cell Biol. 2024, 26, 1375–1376. [Google Scholar] [CrossRef] [PubMed]
- Kandettu, A.; Ghosal, J.; Tharayil, J.S.; Kuthethur, R.; Mallya, S.; Narasimhamurthy, R.K.; Mumbrekar, K.D.; Subbannayya, Y.; Kumar, N.A.; Radhakrishnan, R. Inhibition of Mitochondrial Genome-Encoded Mitomir-3 Contributes to Zeb1 Mediated Gpx4 Downregulation and Pro-Ferroptotic Lipid Metabolism to Induce Ferroptosis in Breast Cancer Cells. Free. Radic. Biol. Radhakrishnan Med. 2025, 234, 151–168. [Google Scholar] [CrossRef]
- Cui, Y.; Li, Y.; Xu, Y.; Liu, X.; Kang, X.; Zhu, J.; Long, S.; Han, Y.; Xue, C.; Sun, Z. Slc7a11 Protects Luminal a Breast Cancer Cells against Ferroptosis Induced by Cdk4/6 Inhibitors. Redox Biol. 2024, 76, 103304. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Peng, C.; Liu, Y. Regulation of Ferroptosis by Pi3k/Akt Signaling Pathway: A Promising Therapeutic Axis in Cancer. Front. Cell Dev. Biol. 2024, 12, 1372330. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Z.; Wang, Y.; Chen, C.; Li, Y.; Dong, H.; Yao, T.; Jin, G.; Wang, Z. Tyms Knockdown Suppresses Cells Proliferation, Promotes Ferroptosis Via Inhibits Pi3k/Akt/Mtor Signaling Pathway Activation in Triple Negative Breast Cancer. Cell Biochem. Wang Biophys. 2024, 82, 2717–2726. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, A.; Needham, K.; Lane, D.J.; Ayton, S.; Redvers, R.P.; John, M.; Selistre-de-Araujo, H.S.; Denoyer, D.; Pouliot, N. Integrin Avβ3 Is a Master Regulator of Resistance to Tki-Induced Ferroptosis in Her2-Positive Breast Cancer. Cancers 2023, 15, 1216. [Google Scholar] [CrossRef]
- Park, S.Y.; Jeong, K.J.; Poire, A.; Zhang, D.; Tsang, Y.H.; Blucher, A.S.; Mills, G.B. Irreversible Her2 Inhibitors Overcome Resistance to the Rsl3 Ferroptosis Inducer in Non-Her2 Amplified Luminal Breast Cancer. Cell Death Dis. 2023, 14, 532. [Google Scholar] [CrossRef]
- Dixon, S.J.; Olzmann, J.A. The Cell Biology of Ferroptosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 424–442. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C. Regulation of Ferroptotic Cancer Cell Death by Gpx4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Cao, J.; Zhou, T.; Wu, T.; Lin, R.; Huang, J.; Shi, D.; Yu, J.; Ren, Y.; Qian, C.; He, L. Targeting Estrogen-Regulated System Xc− Promotes Ferroptosis and Endocrine Sensitivity of Er+ Breast Cancer. Cell Death 2025, 16, 30. [Google Scholar] [CrossRef]
- Cao, J.; Wu, T.; Zhou, T.; Jiang, Z.; Ren, Y.; Yu, J.; Wang, J.; Qian, C.; Wu, G.; He, L. Usp35 Promotes the Growth of Er Positive Breast Cancer by Inhibiting Ferroptosis Via Brd4-Slc7a11 Axis. Commun. Biol. 2025, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Zhang, Y.-L.; Huang, F.-Y.; Chen, H.-Y.; Chen, M.-H.; Wu, R.-H.; Dai, S.-Z.; He, G.-S.; Tan, G.-H.; Zheng, W.-P. Gankyrin Inhibits Ferroptosis through the P53/Slc7a11/Gpx4 Axis in Triple-Negative Breast Cancer Cells. Sci. Rep. 2023, 13, 21916. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Tan, X.-N.; Li, L.-P.; Gao, W.-H.; Tian, X.-F.; Zeng, P.-H. Brazilin Actuates Ferroptosis in Breast Cancer Cells Via P53/Slc7a11/Gpx4 Signaling Pathway. Chin. J. Integr. Med. 2024, 30, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhao, H.; Dai, H.; Li, J.; Pan, X.; Pan, W.; Xia, C.; Liu, F. Fxr Deficiency Induced Ferroptosis Via Modulation of the Cbp-Dependent P53 Acetylation to Suppress Breast Cancer Growth and Metastasis. Cell Death Liu Dis. 2024, 15, 826. [Google Scholar] [CrossRef]
- Rathnayake, D.S.; Dlamini, S.; Elkalawozgy, K.; Tillekeratne, L.V.; Taylor, W.R. Mutant P53 Reactivators Protect Breast Cancer Cells from Ferroptosis. Cell Biochem. Taylor Funct. 2024, 42, e4036. [Google Scholar] [CrossRef]
- Xiang, L.; Li, Q.; Guan, Z.; Wang, G.; Yu, X.; Zhang, X.; Zhang, G.; Hu, J.; Yang, X.; Li, M. Oxyresveratrol as a Novel Ferroptosis Inducer Exhibits Anticancer Activity against Breast Cancer Via the Egfr/Pi3k/Akt/Gpx4 Signalling Axis. Front. Pharmacol. 2025, 15, 1527286. [Google Scholar] [CrossRef]
- Wu, S.; Yang, G.; Wen, X.; Lin, Y.; Wang, S.; Wang, J.; Liu, Q.; Luo, D. Aldo-Keto Reductase 1b10 (Akr1b10) Suppresses Sensitivity of Ferroptosis in Tnbc by Activating the Akt/Gsk3β/Nrf2/Gpx4 Axis. Front. Biosci.-Landmark 2025, 30, 36615. [Google Scholar] [CrossRef]
- Wu, X.; Liu, C.; Li, Z.; Gai, C.; Ding, D.; Chen, W.; Hao, F.; Li, W. Regulation of Gsk3β/Nrf2 Signaling Pathway Modulated Erastin-Induced Ferroptosis in Breast Cancer. Mol. Cell. Biochem. 2020, 473, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.; Mao, C.; Horbath, A.D.; Yan, Y.; Cai, S.; Yao, J.; Jiang, Y.; Sun, M.; Liu, X.; Cheng, J. Brca1-Mediated Dual Regulation of Ferroptosis Exposes a Vulnerability to Gpx4 and Parp Co-Inhibition in Brca1-Deficient Cancers. Cancer Discov. 2024, 14, 1476–1495. [Google Scholar] [CrossRef]
- Qu, S.; Timmermans, A.M.; Heemskerk-Gerritsen, B.A.; Trapman-Jansen, A.M.; Broeren-Foekens, R.; Prager-van der Smissen, W.J.; El Hassnaoui, H.; van Tienhoven, T.; Bes-Stobbe, C.K.; Westenend, P.J. Expression and Localization of Ferritin-Heavy Chain Predicts Recurrence for Breast Cancer Patients with a Brca1/2 Mutation. Cancers 2023, 16, 28. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, R.; Huang, P.; Chen, M.; Chen, H.; Zeng, X.; Liu, J.; Zhang, J.; Huang, D.; Lao, L. Ferroptotic Neutrophils Induce Immunosuppression and Chemoresistance in Breast Cancer. Cancer Res. 2025, 85, 477–496. [Google Scholar] [CrossRef]
- Shang, Y.; Cao, T.; Ma, X.; Huang, L.; Wu, M.; Xu, J.; Wang, J.; Wang, H.; Wu, S.; Pandey, V. Estrogen-Induced Fxr1 Promotes Endocrine Resistance and Bone Metastasis in Breast Cancer Via Bcl2 and Gpx4. Front. Cell Dev. Biol. 2025, 13, 1563353. [Google Scholar] [CrossRef] [PubMed]
- Vinik, Y.; Maimon, A.; Dubey, V.; Raj, H.; Abramovitch, I.; Malitsky, S.; Itkin, M.; Ma’ayan, A.; Westermann, F.; Gottlieb, E. Programming a Ferroptosis-to-Apoptosis Transition Landscape Revealed Ferroptosis Biomarkers and Repressors for Cancer Therapy. Adv. Sci. 2024, 11, 2307263. [Google Scholar] [CrossRef]
- Frye, W.J.; Huff, L.M.; Dalmasy, J.M.G.; Salazar, P.; Carter, R.M.; Gensler, R.T.; Esposito, D.; Robey, R.W.; Ambudkar, S.V.; Gottesman, M.M. The Multidrug Resistance Transporter P-Glycoprotein Confers Resistance to Ferroptosis Inducers. Cancer Drug Resist. 2023, 6, 468. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, Y.; Rong, D.; Li, J.; Li, Z.; Qiu, H.; Chen, Q.; Yang, J.; Wang, C.; Huang, J. A Novel Taxane Sb-T-101141 Triggers a Noncanonical Ferroptosis to Overcome Paclitaxel Resistance of Breast Cancer Via Iron Homeostasis-Related Khsrp. Cell Death Dis. 2025, 16, 403. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chen, M.; Wang, D.; He, Y.; Ge, G.; Zeng, Z.; Shu, J.; Guo, W.; Wu, S.X.; Xiong, W. Doxorubicin and Iron-Doped Mesoporous Silica Nanoparticles for Chemodynamic Therapy and Chemotherapy of Breast Cancer. New J. Chem. 2024, 48, 17294–17309. [Google Scholar] [CrossRef]
- Shang, Y.; Zhao, M.; Chen, S.; Chen, Y.; Liu, X.; Zhou, F.; Li, Y.; Long, M.; Xu, K.; Ding, Z. Tetrastigma Hemsleyanum Polysaccharide Combined with Doxorubicin Promote Ferroptosis and Immune Function in Triple-Negative Breast Cancer. Int. J. Biol. Macromol. 2024, 275, 133424. [Google Scholar] [CrossRef]
- Shen, M.; Cao, S.; Long, X.; Xiao, L.; Yang, L.; Zhang, P.; Li, L.; Chen, F.; Lei, T.; Gao, H. Dnajc12 Causes Breast Cancer Chemotherapy Resistance by Repressing Doxorubicin-Induced Ferroptosis and Apoptosis Via Activation of Akt. Redox Biol. 2024, 70, 103035. [Google Scholar] [CrossRef]
- Zhu, Z.; Shen, H.; Xu, J.; Fang, Z.; Wo, G.; Ma, Y.; Yang, K.; Wang, Y.; Yu, Q.; Tang, J.-h. Gata3 Mediates Doxorubicin Resistance by Inhibiting Cyb5r2-Catalyzed Iron Reduction in Breast Cancer Cells. Drug Resist. Updates 2023, 69, 100974. [Google Scholar] [CrossRef]
- Hao, T.; Guo, H.; Wang, C.; Jing, S.; Zhang, W.; Zeng, Y.; Hou, J.; Song, Z.; Li, W. Mitochondria-Targeted Microneedles Reverse Doxorubicin Resistance Via Apoptosis-Ferroptosis Synergy. ACS Nano 2025, 19, 23315–23333. [Google Scholar] [CrossRef]
- Hua, Y.; Duan, N.; Sun, C.; Yang, F.; Tian, M.; Sun, Y.; Zhao, S.; Gong, J.; Liu, Q.; Huang, X. Targeting Slc7a11-Mediated Cysteine Metabolism for the Treatment of Trastuzumab-Resistant Her2-Positive Breast Cancer. eLife 2025, 14, RP103953. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Abreu, M.; Guan, J.; Khalid, U.; Ning, J.; Costa, M.; Chan, J.; Li, Q.; Fortin, J.; Wong, W.; Perampalam, P. Inhibition of Gpx4 Enhances Cdk4/6 Inhibitor and Endocrine Therapy Activity in Breast Cancer. Nat. Commun. 2024, 15, 9550. [Google Scholar] [CrossRef] [PubMed]
- Pottier, C.; Montero-Ruiz, L.; Jehay, R.; Wery, C.; Baiwir, D.; Mazzucchelli, G.; Bekisz, S.; Thissen, R.; Josse, C.; Rorive, A. Targeting Ferroptosis Resistance Resensitizes Metastatic Hr+ Her2− Breast Cancer Cells to Palbociclib-Hormone Therapy. Cancer Commun. 2025, 45, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, X.; Sun, W.; Xu, F.; Kou, H.; Hu, W.; Zhang, Y.; Jiang, Q.; Tang, J.; Xu, Y. Relb-Activated Gpx4 Inhibits Ferroptosis and Confers Tamoxifen Resistance in Breast Cancer. Redox Biol. 2023, 68, 102952. [Google Scholar] [CrossRef]
- Gu, T.; Wang, K.; Yuan, X.; Tang, H.; Wang, S.; Zhao, Z. The Dual Role of Gpx4 in Breast Cancer: Mechanisms of Therapeutic Resistance and Potential for Novel Targeted Therapies. Cancer Gene Ther. 2025, 32, 913–922. [Google Scholar] [CrossRef]
- Ye, F.; Wu, J.; Zhang, F. Mettl16 Epigenetically Enhances Gpx4 Expression Via M6a Modification to Promote Breast Cancer Progression by Inhibiting Ferroptosis. Biochem. Biophys. Res. Commun. 2023, 638, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gong, R.; Wan, X.; Jiang, S.; Guan, Y.; Li, Y.; Jiang, T.; Chen, Z.; Zhong, C.; He, L.; Xiang, Z. Gpx4-Autac Induces Ferroptosis in Breast Cancer by Promoting the Selective Autophagic Degradation of Gpx4 Mediated by Traf6-P62. Cell Death Xiang Differ. 2025, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Azizi, R.; Ahmed, H.H.; Kareem, R.A.; Waam, W.M.T.; Alwan, M.; Jawad, M.J.; Hamad, A.K.; Darzi, S. Slc7a11 Inhibitors Represent a Promising Therapeutic Target by Facilitating the Induction of Ferroptosis in Breast Cancer. Int. J. Mol. Cell. Med. 2025, 14, 496. [Google Scholar]
- Liu, W.; Jing, Y.; Chen, Y.; Sun, H.; Xu, W.; Liang, R.; Liu, W.; Zhang, Z.; Liu, H. Research Progress on the Cross-Regulation between Ferroptosis and Immunogenic Cell Death in Tumor Micro-Environment. Front. Oncol. 2025, 15, 1581951. [Google Scholar] [CrossRef]
- Yu, L.; Huang, K.; Liao, Y.; Wang, L.; Sethi, G.; Ma, Z. Targeting Novel Regulated Cell Death: Ferroptosis, Pyroptosis and Necroptosis in Anti-Pd-1/Pd-L1 Cancer Immunotherapy. Cell Prolif. 2024, 57, e13644. [Google Scholar] [CrossRef]
- Luo, B.; Zheng, H.; Liang, G.; Luo, Y.; Zhang, Q.; Li, X. Hmgb3 Contributes to Anti-Pd-1 Resistance by Inhibiting Ifn-Γ-Driven Ferroptosis in Tnbc. Mol. Carcinog. 2025, 64, 490–501. [Google Scholar] [CrossRef]
- Liu, W.; Luo, G. Cav1 Inhibits Xc-System through Ifngr1 to Promote Ferroptosis to Inhibit Stemness and Improves Anti-Pd-1 Efficacy in Breast Cancer. Transl. Oncol. 2024, 50, 102149. [Google Scholar] [CrossRef] [PubMed]
- Desterke, C.; Xiang, Y.; Elhage, R.; Duruel, C.; Chang, Y.; Hamaï, A. Ferroptosis Inducers Upregulate Pd-L1 in Recurrent Triple-Negative Breast Cancer. Cancers 2023, 16, 155. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, J. Induction of Ferroptosis by Natural Phenols: A Promising Strategy for Cancer Therapy. Phytother. Res. 2024, 38, 2041–2076. [Google Scholar] [CrossRef]
- Lesjak, M.; Simin, N.; Srai, S.K. Can Polyphenols Inhibit Ferroptosis? Antioxidants 2022, 11, 150. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; Pang, Y. Phytochemicals Targeting Ferroptosis: Therapeutic Opportunities and Prospects for Treating Breast Cancer. Pharmaceuticals 2022, 15, 1360. [Google Scholar] [CrossRef]
- Sichetti, M.; Giuseffi, M.; Giglio, E.; Marino, G.; Mecca, M. Effect of Natural Polyphenols on Breast Cancer Chemoprevention and Treatment. Mol. Nutr. Mecca Food Res. 2025, 69, e70055. [Google Scholar] [CrossRef]
- An, S.; Hu, M. Quercetin Promotes Tfeb Nuclear Translocation and Activates Lysosomal Degradation of Ferritin to Induce Ferroptosis in Breast Cancer Cells. Comput. Intell. Neurosci. 2022, 2022, 5299218. [Google Scholar] [CrossRef]
- Zhu, Q.; Han, Y.; He, Y.; Meng, P.; Fu, Y.; Yang, H.; He, G.; Long, M.; Shi, Y. Quercetin Inhibits Neuronal Ferroptosis and Promotes Immune Response by Targeting Lipid Metabolism-Related Gene Ptgs2 to Alleviate Breast Cancer-Related Depression. Phytomedicine 2024, 130, 155560. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Liu, Y.; Chen, Y. Iron Metabolism: An Emerging Therapeutic Target Underlying the Anti-Cancer Effect of Quercetin. Free. Radic. Res. 2021, 55, 296–303. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, Y.; Zhang, H.; Li, X.; Su, Y.; Cui, J.; Xu, R.; Mao, X.; Sang, M.; Lin, Z. Resveratrol Induces Ferroptosis in Triple-Negative Breast Cancer through Nedd4l-Mediated Gpx4 Ubiquitination and Degradation. Free. Radic. Biol. Med. 2025, 235, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Jiang, Y.; Wang, H.; Zhu, L.; Huang, S.; Liu, S.; Zhang, W.; Li, T. Formononetin Triggers Ferroptosis in Triple-Negative Breast Cancer Cells by Regulating the Mtorc1/Srebp1/Scd1 Pathway. Front. Pharmacol. 2024, 15, 1441105. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Chan, L.; Pang, Y.; Shang, Y.; Wang, W.; Zhao, L. Rosmarinic Acid Promotes Mitochondrial Fission and Induces Ferroptosis in Triple-Negative Breast Cancer Cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 10461–10475. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, X.; Li, J.; Yao, X.-C.; Liu, W.-L.; Kang, F.-H.; Zou, Z.-X.; Xu, K.-P.; Xu, P.-S.; Tan, G.-S. Identification of a New Natural Biflavonoids against Breast Cancer Cells Induced Ferroptosis Via the Mitochondrial Pathway. Bioorganic Chem. 2021, 109, 104744. [Google Scholar] [CrossRef]
- Arzuk, E.; Armağan, G. Genistein and Daidzein Induce Ferroptosis in Mda-Mb-231 Cells. J. Pharm. Pharmacol. 2024, 76, 1599–1608. [Google Scholar] [CrossRef]
- Cao, X.; Li, Y.; Wang, Y.; Yu, T.; Zhu, C.; Zhang, X.; Guan, J. Curcumin Suppresses Tumorigenesis by Ferroptosis in Breast Cancer. PLoS ONE 2022, 17, e0261370. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Gao, Y.; Cao, B. Targeting Intrinsic and Extrinsic Pathways of Ferroptosis: A Novel Anticancer Strategy of Curcumin. Pharmacogn. Mag. 2024, 20, 1061–1070. [Google Scholar] [CrossRef]
- Cao, X.; Li, Y.; Wang, Y.; Zhang, X.; Yu, T.; Zhu, C.; Guan, J. Curcumin Suppresses Tumorigenesis Via Promoting Slc1a5-Mediated Ferroptosis in Breast Cancer. 2020. Available online: https://europepmc.org/article/ppr/ppr232690 (accessed on 9 August 2025).
- Li, R.; Zhang, J.; Zhou, Y.; Gao, Q.; Wang, R.; Fu, Y.; Zheng, L.; Yu, H. Transcriptome Investigation and In Vitro Verification of Curcumin-Induced Ho-1 as a Feature of Ferroptosis in Breast Cancer Cells. Oxidative Med. Cell. Longev. 2020, 2020, 3469840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, C.; Peng, C.; Peng, F. Potential Roles and Mechanisms of Curcumin and Its Derivatives in the Regulation of Ferroptosis. Int. J. Biol. Sci. 2024, 20, 4838. [Google Scholar] [CrossRef]
- Xiao, W.; Xu, C. Cystine/Cysteine Metabolism Regulates the Progression and Response to Treatment of Triple-Negative Breast Cancer. Oncol. Lett. 2024, 28, 521. [Google Scholar] [CrossRef]
- Guo, D.; Lin, Q.; Liu, N.; Jin, Q.; Liu, C.; Wang, Y.; Zhu, X.; Zong, L. Copper-Based Metal–Organic Framework Co-Loaded Doxorubicin and Curcumin for Anti-Cancer with Synergistic Apoptosis and Ferroptosis Therapy. Int. J. Pharm. 2024, 666, 124744. [Google Scholar] [CrossRef]
- Xu, C.; Chen, Y.; Yu, Q.; Song, J.; Jin, Y.; Gao, X. Compounds Targeting Ferroptosis in Breast Cancer: Progress and Their Therapeutic Potential. Front. Pharmacol. 2023, 14, 1243286. [Google Scholar] [CrossRef]
- Mishra, S.D.; Mendonca, P.; Kaur, S.; Soliman, K.F. Silibinin Anticancer Effects through the Modulation of the Tumor Immune Microenvironment in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2025, 26, 6265. [Google Scholar] [CrossRef]
- Rambaran, T.F.; Nordström, A. Medical and Pharmacokinetic Effects of Nanopolyphenols: A Systematic Review of Clinical Trials. Food Front. 2021, 2, 140–152. [Google Scholar] [CrossRef]
- Quesada-Vázquez, S.; Eseberri, I.; Les, F.; Pérez-Matute, P.; Herranz-López, M.; Atgié, C.; Lopez-Yus, M.; Aranaz, P.; Oteo, J.A.; Escoté, X. Polyphenols and Metabolism: From Present Knowledge to Future Challenges. J. Physiol. Biochem. 2024, 80, 603–625. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Rakshit, G.; Singh, R.P.; Garse, S.; Khan, J.; Chakraborty, S. Dietary Polyphenols: Review on Chemistry/Sources, Bioavailability/Metabolism, Antioxidant Effects, and Their Role in Disease Management. Antioxidants 2024, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, D.; Yu, J.; Zhang, Y.; Zhou, Y. Applications of Metal–Phenolic Networks in Nanomedicine: A Review. Biomater. Sci. 2022, 10, 5786–5808. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, M.; Gao, Y.; Cheng, X.; Liu, X.; Tang, S.; Peng, Y.; Wang, N.; Hu, D.; Peng, H. Biomimetic Erythrocytes Engineered Drug Delivery for Cancer Therapy. Chem. Eng. J. 2022, 433, 133498. [Google Scholar] [CrossRef]
- Li, F.; Qi, Q.; Qiao, Y.; Huang, Y.; Lu, Y.; Gu, K.; Liu, H.; Gao, C.; Liu, S.; Wu, H. Curcumenol Inhibits Malignant Progression and Promotes Ferroptosis Via the Slc7a11/Nf-Κb/Tgf-Β Pathway in Triple-Negative Breast Cancer. Int. J. Mol. Med. 2025, 56, 111. [Google Scholar] [CrossRef] [PubMed]
- Van Kessel, A.T.; Cosa, G. Lipid-Derived Electrophiles Inhibit the Function of Membrane Channels During Ferroptosis. Proc. Natl. Acad. Sci. USA 2024, 121, e2317616121. [Google Scholar] [CrossRef]
- Koeberle, S.C.; Kipp, A.P.; Stuppner, H.; Koeberle, A. Ferroptosis-Modulating Small Molecules for Targeting Drug-Resistant Cancer: Challenges and Opportunities in Manipulating Redox Signaling. Med. Res. Rev. 2023, 43, 614–682. [Google Scholar] [CrossRef]
- Guo, J.; Huang, M.; Hou, S.; Yuan, J.; Chang, X.; Gao, S.; Zhang, Z.; Wu, Z.; Li, J. Therapeutic Potential of Terpenoids in Cancer Treatment: Targeting Mitochondrial Pathways. Cancer Rep. 2024, 7, e70006. [Google Scholar] [CrossRef] [PubMed]
- O’neill, P.M.; Barton, V.E.; Ward, S.A. The Molecular Mechanism of Action of Artemisinin—The Debate Continues. Molecules 2010, 15, 1705–1721. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. Ros-Induced Lipid Peroxidation Modulates Cell Death Outcome: Mechanisms Behind Apoptosis, Autophagy, and Ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef]
- Li, Z.; Wu, X.; Wang, W.; Gai, C.; Zhang, W.; Li, W.; Ding, D. Fe (Ii) and Tannic Acid-Cloaked Mof as Carrier of Artemisinin for Supply of Ferrous Ions to Enhance Treatment of Triple-Negative Breast Cancer. Nanoscale Res. Lett. 2021, 16, 37. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, Z.-N.; Jiang, X.-Y.; Tian, X.; Deng, M.-H.; Cheng, M.-S.; Yang, H.-L.; Liu, Y. dentification of Novel Artemisinin Hybrids Induce Apoptosis and Ferroptosis in Mcf-7 Cells. Int. J. Mol. Sci. 2022, 23, 15768. [Google Scholar] [CrossRef]
- Ito, N.; Nabil, A.; Uto, K.; Ebara, M. Poly (Artema), a Novel Artesunate-Based Polymer Induces Ferroptosis in Breast Cancer Cells. Sci. Technol. Adv. Mater. 2025, 26, 2482514. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, S.; Zeng, F.; Pan, D.; Cai, L.; Zhou, Y.; Wang, H.; Qin, G.; Zhang, C.; Chen, W. Dihydroartemisinin Enhances the Radiosensitivity of Breast Cancer by Targeting Ferroptosis Signaling Pathway through Hsa_Circ_0001610. Eur. J. Pharmacol. 2024, 983, 176943. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Li, A.; Huang, W.; Chen, S.; Han, F.; Wang, L. Dihydroartemisinin Induces Pyroptosis by Promoting the Aim2/Caspase-3/Dfna5 Axis in Breast Cancer Cells. Chem.-Biol. Interact. 2021, 340, 109434. [Google Scholar] [CrossRef]
- Luo, N.; Zhang, K.; Li, X.; Hu, Y.; Guo, L. Tanshinone Iia Destabilizes Slc7a11 by Regulating Pias4-Mediated Sumoylation of Slc7a11 through Kdm1a, and Promotes Ferroptosis in Breast Cancer. J. Adv. Res. 2025, 69, 313–327. [Google Scholar] [CrossRef]
- Zhao, P.; Song, H.; Gao, F.; Chen, L.; Qiu, J.; Jin, J.; Pan, C.; Tang, Y.; Chen, M.; Pan, Y. A Novel Derivative of Curcumol, Hcl-23, Inhibits the Malignant Phenotype of Triple-Negative Breast Cancer and Induces Apoptosis and Ho-1-Dependent Ferroptosis. Molecules 2023, 28, 3389. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Xu, W.; Liu, Y.; Zhang, J.-H.; Yang, Y.-Y.; Wang, Z.-w.; Sun, D.-J.; Li, H.; Liu, B.; Chen, L.-X. Anomanolide C Suppresses Tumor Progression and Metastasis by Ubiquitinating Gpx4-Driven Autophagy-Dependent Ferroptosis in Triple Negative Breast Cancer. Int. J. Biol. Sci. 2023, 19, 2531. [Google Scholar] [CrossRef]
- Du, P.; Han, A.; Liu, J.; Li, W.; Feng, X.; Chen, L. Taraxerol Induces Ferroptosis in Breast Cancer by Targeting Nrf2 Transcriptional Activity to Promote Mib2-Mediated Gpx4 Ubiquitination. Phytomedicine 2025, 145, 157024. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhu, Z.; Hu, H.; Guan, J.; Yang, B.; Zhao, H. Eupaformosanin Induces Apoptosis and Ferroptosis through Ubiquitination of Mutant P53 in Triple-Negative Breast Cancer. Eur. J. Pharmacol. 2022, 924, 174970. [Google Scholar] [CrossRef]
- Yang, X.; Liang, B.; Zhang, L.; Zhang, M.; Ma, M.; Qing, L.; Yang, H.; Huang, G.; Zhao, J. Ursolic Acid Inhibits the Proliferation of Triple-Negative Breast Cancer Stem-Like Cells through Nrf2-Mediated Ferroptosi. Oncol. Rep. 2024, 52, 94. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, M.; Zhang, J.; Sun, Z.; Zhang, W.; Dong, W.; Cheng, C.; Yao, Y.; Li, K. Hinokitiol-Iron Complex Is a Ferroptosis Inducer to Inhibit Triple-Negative Breast Tumor Growth. Cell Biosci. 2023, 13, 87. [Google Scholar] [CrossRef]
- Ye, S.; Hu, X.; Sun, S.; Su, B.; Cai, J.; Jiang, J. Oridonin Promotes Rsl3-Induced Ferroptosis in Breast Cancer Cells by Regulating the Oxidative Stress Signaling Pathway Jnk/Nrf2/Ho-1. Eur. J. Pharmacol. 2024, 974, 176620. [Google Scholar] [CrossRef]
- Bakar-Ates, F.; Ozkan, E. Cucurbitacin B and Erastin Co-Treatment Synergistically Induced Ferroptosis in Breast Cancer Cells Via Altered Iron-Regulating Proteins and Lipid Peroxidation. Toxicol. Vitr. 2024, 94, 105732. [Google Scholar] [CrossRef] [PubMed]
- Siraj, M.A.; Islam, M.A.; Al Fahad, M.A.; Kheya, H.R.; Xiao, J.; Simal-Gandara, J. Cancer Chemopreventive Role of Dietary Terpenoids by Modulating Keap1-Nrf2-Are Signaling System—A Comprehensive Update. Appl. Sci. 2021, 11, 10806. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N.; Zhou, Y.; Wang, K.; Sun, Y.; Yan, H.; Han, W.; Wang, X.; Wei, B.; Ke, Y. Oridonin Induces Ferroptosis by Inhibiting Gamma-Glutamyl Cycle in Te1 Cells. Phytother. Res. 2021, 35, 494–503. [Google Scholar] [CrossRef]
- Nie, A.; Shen, C.; Zhou, Z.; Wang, J.; Sun, B.; Zhu, C. Ferroptosis: Potential Opportunities for Natural Products in Cancer Therapy. Phytother. Res. 2024, 38, 1173–1190. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Shen, Y.-C.; Wu, C.-Y.; Tsai, Y.-Y.; Yang, Y.-H.; Lin, Y.-Y.; Kuan, F.-C.; Lu, C.-N.; Chang, G.-H.; Tsai, M.-S. Danshen Improves Survival of Patients with Breast Cancer and Dihydroisotanshinone I Induces Ferroptosis and Apoptosis of Breast Cancer Cells. Front. Pharmacol. 2019, 10, 1226. [Google Scholar] [CrossRef]
- Ouyang, M.; Wu, J.; Hu, X.; Liu, C.; Zhou, D. Decoding the Power of Saponins in Ferroptosis Regulation and Disease Intervention: A Review. J. Pharm. 2025, 77, 593–608. [Google Scholar] [CrossRef]
- Elekofehinti, O.O.; Iwaloye, O.; Olawale, F.; Ariyo, E.O. Saponins in Cancer Treatment: Current Progress and Future Prospects. Pathophysiology 2021, 28, 250–272. [Google Scholar] [CrossRef]
- Wei, G.; Sun, J.; Hou, Z.; Luan, W.; Wang, S.; Cui, S.; Cheng, M.; Liu, Y. Novel Antitumor Compound Optimized from Natural Saponin Albiziabioside a Induced Caspase-Dependent Apoptosis and Ferroptosis as a P53 Activator through the Mitochondrial Pathway. Eur. J. Med. Chem. 2018, 157, 759–772. [Google Scholar] [CrossRef]
- Wei, G.; Sun, J.; Luan, W.; Hou, Z.; Wang, S.; Cui, S.; Cheng, M.; Liu, Y. Natural Product Albiziabioside a Conjugated with Pyruvate Dehydrogenase Kinase Inhibitor Dichloroacetate to Induce Apoptosis-Ferroptosis-M2-Tams Polarization for Combined Cancer Therapy. J. Med. Chem. 2019, 62, 8760–8772. [Google Scholar] [CrossRef]
- Zhai, F.-G.; Liang, Q.-C.; Wu, Y.-Y.; Liu, J.-Q.; Liu, J.-W. Red Ginseng Polysaccharide Exhibits Anticancer Activity through Gpx4 Downregulation-Induced Ferroptosis. Pharm. Biol. 2022, 60, 909–914. [Google Scholar] [CrossRef]
- Li, W.; Guo, Y.; Xu, Z.; Li, F.; Dong, Y.; Xu, F. Notoginsenoside R1 (Ngr1) Regulates the Age-Rage Signaling Pathway by Inhibiting Runx2 Expression to Accelerate Ferroptosis in Breast Cancer Cells. Aging 2024, 16, 10446. [Google Scholar] [CrossRef]
- Yan, C.; Xuan, F. Paris Saponin Vii Promotes Ferroptosis to Inhibit Breast Cancer Via Nrf2/Gpx4 Axis. Biochem. Biophys. Res. Commun. 2024, 697, 149524. [Google Scholar] [CrossRef]
- Chen, H.-C.; Tang, H.-H.; Hsu, W.-H.; Wu, S.-Y.; Cheng, W.-H.; Wang, B.-Y.; Su, C.-L. Vulnerability of Triple-Negative Breast Cancer to Saponin Formosanin C-Induced Ferroptosis. Antioxidants 2022, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, D.; Zhu, Y.; Zhang, M.; Zhao, H. Kinsenoside Suppresses Dgat1-Mediated Lipid Droplet Formation to Trigger Ferroptosis in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2025, 26, 2322. [Google Scholar] [CrossRef]
- Li, C.; He, Z.; Yao, F.; Liao, S.; Sun, K.; Sun, S.; Li, Z.; Wang, Z. Role of Escin in Breast Cancer Therapy: Potential Mechanism for Inducing Ferroptosis and Synergistic Antitumor Activity with Cisplatin. Apoptosis 2023, 28, 1154–1167. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Wang, F.-Y.; Yang, L.-M.; Wang, S.-S.; Lu, H.; Wang, X.-S.; Lu, Y.; Ni, W.-X.; Liang, H.; Huang, K.-B. Cycloplatinated (Ii) Complex Based on Isoquinoline Alkaloid Elicits Ferritinophagy-Dependent Ferroptosis in Triple-Negative Breast Cancer Cells. J. Med. Chem. 2024, 67, 6738–6748. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, S.-S.; Li, M.-Y.; Liu, R.; Zhu, M.-F.; Yang, L.-M.; Wang, F.-Y.; Huang, K.-B.; Liang, H. Cyclometalated Iridium (Iii) Complex Based on Isoquinoline Alkaloid Synergistically Elicits the Icd Response and Ido Inhibition Via Autophagy-Dependent Ferroptosis. Acta Pharm. Sin. B 2025, 15, 424–437. [Google Scholar] [CrossRef]
- Yi, N.; Wang, L.; Jiang, Z.; Xu, G.; Li, L.; Zhang, Y.; Tan, Y. Peiminine Triggers Ferroptosis to Inhibit Breast Cancer Growth through Triggering Nrf2 Signaling. Tissue Cell 2024, 87, 102323. [Google Scholar] [CrossRef]
- Kuang, X.P.; Huang, J.W.; Jiang, Y.N.; Guo, Y.Z.; Yan, C.Y.; Li, W.X. Indirubin Suppresses 4t1 Murine Breast Cancer In Vitro and In Vivo by Induction of Ferroptosis. TMR Mod. Herb. Med. 2022, 5, 1. [Google Scholar] [CrossRef]
- Du, X.; Zhang, J.; Liu, L.; Xu, B.; Han, H.; Dai, W.; Pei, X.; Fu, X.; Hou, S. A Novel Anticancer Property of Lycium Barbarum Polysaccharide in Triggering Ferroptosis of Breast Cancer Cells. J. Zhejiang Univ.-Sci. B Hou 2022, 23, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Wang, J.; Zhao, Z.; Liu, B.; Liu, M.; Liu, M.; Shi, C.; Feng, X.; Fu, Y.; Shi, D. Phenolic Compounds Induce Ferroptosis-Like Death by Promoting Hydroxyl Radical Generation in the Fenton Reaction. Commun. Biol. 2024, 7, 199. [Google Scholar] [CrossRef]
- Zhan, S.; Lu, L.; Pan, S.-s.; Wei, X.-q.; Miao, R.-r.; Liu, X.-h.; Xue, M.; Lin, X.-k.; Xu, H.-l. Targeting Nqo1/Gpx4-Mediated Ferroptosis by Plumbagin Suppresses In Vitro and In Vivo Glioma Growth. Br. J. Cancer 2022, 127, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Miao, H.; Quan, M.; Wang, S.; Zhang, Y.; Zhou, H.; Zhang, X.; Lin, Z.; Piao, J. Β-Lapachone Induces Ferroptosis of Colorectal Cancer Cells Via Ncoa4-Mediated Ferritinophagy by Activating Jnk Pathway. Chem.-Biol. Interact. 2024, 389, 110866. [Google Scholar] [CrossRef]
- de Carvalho, E.P.; de Souza Pessoa, A.; Iano, F.G.; Ribeiro, L.; Leme, B.; Borges, L.F.; Sanches, M.L.R.; Ximenes, V.F.; de Oliveira, R.C. Antitumor Effect of Bromo-Naphthoquinone Associated with Tannic Acid in Triple Negative Breast Cancer Cells. Int. J. Biochem. Cell Biol. 2024, 177, 106697. [Google Scholar] [CrossRef]
- Wang, T.-X.; Duan, K.-L.; Huang, Z.-X.; Xue, Z.-A.; Liang, J.-Y.; Dang, Y.; Zhang, A.; Xiong, Y.; Ding, C.; Guan, K.-L. Tanshinone Functions as a Coenzyme That Confers Gain of Function of Nqo1 to Suppress Ferroptosis. Life Sci. Alliance 2023, 6, e202201667. [Google Scholar] [CrossRef]
- Yu, J.; Zhong, B.; Zhao, L.; Hou, Y.; Ai, N.; Lu, J.-J.; Ge, W.; Chen, X. Fighting Drug-Resistant Lung Cancer by Induction of Nad (P) H: Quinone Oxidoreductase 1 (Nqo1)-Mediated Ferroptosis. Drug Resist. Updates 2023, 70, 100977. [Google Scholar] [CrossRef]
- Prassas, I.; Diamandis, E.P. Novel Therapeutic Applications of Cardiac Glycosides. Nat. Rev. Drug Discov. 2008, 7, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Orta, M.L.; Maldonado-Navas, D.; García-Domínguez, I.; López-Lázaro, M. Evaluating the Cancer Therapeutic Potential of Cardiac Glycosides. BioMed Res. Int. 2014, 2014, 794930. [Google Scholar] [CrossRef] [PubMed]
- Nassar, Z.D.; Mah, C.Y.; Dehairs, J.; Burvenich, I.J.; Irani, S.; Centenera, M.M.; Helm, M.; Shrestha, R.K.; Moldovan, M.; Don, A.S. Human Decr1 Is an Androgen-Repressed Survival Factor That Regulates Pufa Oxidation to Protect Prostate Tumor Cells from Ferroptosis. Elife 2020, 9, e54166. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yin, S.; Yi, Z.; Li, Y.; Wu, S.; Xu, P.; Tang, M.; Sun, Y.; Qi, H.; Zhang, F. Erigoster B Targeting Decr1 Induces Ferroptosis of Breast Cancer Cells Via Promoting Phosphatidylcholine/Arachidonic Acid Metabolism. NPJ Precis. Oncol. 2025, 9, 162. [Google Scholar] [CrossRef]
- Mo, X.; Lan, J.; Wang, Y.; Zhong, H.; He, H.; Yang, Z.; Zhang, S.; Pan, W. Design of Photoactivatable Methylene Blue-Bufalin Conjugate for Gpx4-Targeted Degradation to Induce Ferroptosis-like Death in Breast Cancer Therapy. Bioorganic Chem. 2025, 163, 108629. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Ni, Z.-J.; Elam, E.; Zhang, F.; Thakur, K.; Wang, S.; Zhang, J.-G.; Wei, Z.-J. Juglone, a Novel Activator of Ferroptosis, Induces Cell Death in Endometrial Carcinoma Ishikawa Cells. Food Funct. 2021, 12, 4947–4959. [Google Scholar] [CrossRef]
- Kabir, A.; Muth, A. Polypharmacology: The Science of Multi-Targeting Molecules. Pharmacol. Res. 2022, 176, 106055. [Google Scholar] [CrossRef]
- Firouzjaei, A.A.; Aghaee-Bakhtiari, S.H.; Tafti, A.; Sharifi, K.; Abadi, M.H.J.N.; Rezaei, S.; Mohammadi-Yeganeh, S. Impact of Curcumin on Ferroptosis-Related Genes in Colorectal Cancer: Insights from In-Silico and In-Vitro Studies. Cell Biochem. Funct. 2023, 41, 1488–1502. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, Y.; Zhang, J.; Meng, A.; Liu, C. Salidroside Induces Ferroptosis in Breast Cancer Cells and Enhances the Anticancer Effect of Oxaliplatin. J. Funct. Foods 2025, 130, 106932. [Google Scholar] [CrossRef]
- Cai, B.; Qi, M.; Zhang, X.; Zhang, D. Integrating Network Pharmacology with In Vitro Experiments to Validate the Efficacy of Celastrol against Hepatocellular Carcinoma through Ferroptosis. Drug Des. Zhang Dev. Ther. 2024, 18, 3121–3141. [Google Scholar] [CrossRef]
- Wang, L.; Huang, H.; Li, X.; Ouyang, L.; Wei, X.; Xie, J.; Liu, D.; Tan, P.; Hu, Z. A Review on the Research Progress of Traditional Chinese Medicine with Anti-Cancer Effect Targeting Ferroptosis. Chin. Med. 2023, 18, 132. [Google Scholar] [CrossRef]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving Curcumin Bioavailability: Current Strategies and Future Perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, R.; Zhang, Q.; Luo, M.; Lu, F.; He, Z.; Jiang, Q.; Zhang, T. Dual Strategy for Improving the Oral Bioavailability of Resveratrol: Enhancing Water Solubility and Inhibiting Glucuronidation. J. Agric. Food Chem. 2021, 69, 9249–9258. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Li, Y.; Li, S.; Liu, J.; Yang, X.; Xia, G.; Wang, G. Natural Products and Derivatives for Breast Cancer Treatment: From Drug Discovery to Molecular Mechanism. Phytomedicine 2024, 129, 155600. [Google Scholar] [CrossRef]
- Aljabali, A.A.; Obeid, M.A.; Bashatwah, R.M.; Qnais, E.; Gammoh, O.; Alqudah, A.; Mishra, V.; Mishra, Y.; Khan, M.A.; Parvez, S. Phytochemicals in Cancer Therapy: A Structured Review of Mechanisms, Challenges, and Progress in Personalized Treatment. Chem. Parvez Biodivers. 2025, 22, e202402479. [Google Scholar] [CrossRef]
- Carvalho, S.M.; Mansur, A.A.; da Silveira, I.B.; Pires, T.F.; Victória, H.F.; Krambrock, K.; Leite, M.F.; Mansur, H.S. Nanozymes with Peroxidase-Like Activity for Ferroptosis-Driven Biocatalytic Nanotherapeutics of Glioblastoma Cancer: 2d and 3d Spheroids Models. Pharmaceutics 2023, 15, 1702. [Google Scholar] [CrossRef]
- Ye, L.; Zhong, F.; Sun, S.; Ou, X.; Yuan, J.; Zhu, J.; Zeng, Z. Tamoxifen Induces Ferroptosis in Mcf-7 Organoid. J. Cancer Res. Ther. 2023, 19, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.; Zhuang, L.; Gan, B. The Roles of Ferroptosis in Cancer: Tumor Suppression, Tumor Microenvironment, and Therapeutic Interventions. Cancer Cell 2024, 42, 513–534. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Qiu, J.; Hu, Y.; Wang, Y.; Yu, C.; Wu, Y. Efficacy of Ferscore in Predicting Sensitivity to Ferroptosis Inducers in Breast Cancer. NPJ Breast Cancer 2024, 10, 74. [Google Scholar] [CrossRef]
- Imam, M.; Ji, J.; Zhang, Z.; Yan, S. Targeting the Initiator to Activate Both Ferroptosis and Cuproptosis for Breast Cancer Treatment: Progress and Possibility for Clinical Application. Front. Pharmacol. 2025, 15, 1493188. [Google Scholar] [CrossRef]
- Cao, P.H.A.; Dominic, A.; Lujan, F.E.; Senthilkumar, S.; Bhattacharya, P.K.; Frigo, D.E.; Subramani, E. Unlocking Ferroptosis in Prostate Cancer—The Road to Novel Therapies and Imaging Markers. Nat. Rev. Urol. Subramani 2024, 21, 615–637. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, G.; Chen, X. Mechanism of Ferroptosis Resistance in Cancer Cells. Cancer Drug Resist. 2024, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-H.; Ding, W.; Zhang, N.; Zhou, Z.-Y.; Ling, Z.; Li, W.-J.; Chen, S.; Tang, Q.-Z. Activation of Ampkα2 Attenuated Doxorubicin-Induced Cardiotoxicity Via Inhibiting Lipid Peroxidation Associated Ferroptosis. Free. Radic. Biol. Medicine. 2023, 205, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, A.C.; Besanson, J.; Williams, Q.; Hoang, N.; Edwards, K.; Bishop, G.R.; Chen, Y.; Zeng, H.; Chen, J.-X. Ferrostatin-1 Specifically Targets Mitochondrial Iron-Sulfur Clusters and Aconitase to Improve Cardiac Function in Sirtuin 3 Cardiomyocyte Knockout Mice. J. Mol. Cell. Cardiol. 2024, 192, 36–47. [Google Scholar] [CrossRef]
- Bayır, H.; Dixon, S.J.; Tyurina, Y.Y.; Kellum, J.A.; Kagan, V.E. Ferroptotic Mechanisms and Therapeutic Targeting of Iron Metabolism and Lipid Peroxidation in the Kidney. Nat. Rev. Nephrol. 2023, 19, 315–336. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Chen, Y.; Mo, D.; Jin, R.; Huang, Y.; Zhang, L.; Zhang, C.; Gao, H.; Yan, Q. Inhibition of Acsl4 Ameliorates Tubular Ferroptotic Cell Death and Protects against Fibrotic Kidney Disease. Commun. Biol. 2023, 6, 907. [Google Scholar] [CrossRef]
- Sripetchwandee, J.; Kongkaew, A.; Kumfu, S.; Chunchai, T.; Chattipakorn, N.; Chattipakorn, S.C. Ferrostatin-1 and Z-Vad-Fmk Potentially Attenuated Iron-Mediated Neurotoxicity and Rescued Cognitive Function in Iron-Overloaded Rats. Life Sci. Chattipakorn 2023, 313, 121269. [Google Scholar] [CrossRef]
- Chen, T.; Leng, J.; Tan, J.; Zhao, Y.; Xie, S.; Zhao, S.; Yan, X.; Zhu, L.; Luo, J.; Kong, L. Discovery of Novel Potent Covalent Glutathione Peroxidase 4 Inhibitors as Highly Selective Ferroptosis Inducers for the Treatment of Triple-Negative Breast Cancer. J. Med. Chem. Kong 2023, 66, 10036–10059. [Google Scholar] [CrossRef]
- Ren, M.; Liang, S.; Lin, S.; Huang, R.; Chen, Y.; Zhang, Y.; Xu, Y. Design, Synthesis and Biological Evaluation of Artesunate-Se Derivatives as Anticancer Agents by Inducing Gpx4-Mediated Ferroptosis. Bioorganic Chem. 2024, 152, 107733. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Z.; Li, X.; Zhang, X.; Fan, X.; Wang, Y. Diversity-Oriented Synthesis toward the Discovery of Ferrocenophane-Appended Gpx4 Inhibitors as Potent Ferroptosis Inducers with Drug Likeness. J. Med. Chem. 2025, 68, 15828–15848. [Google Scholar] [CrossRef]
- Zeng, N.; Ye, G.; Zheng, M.; Liu, G.; Zhang, S.; Ma, S.; Xia, Z.; Zhou, Y.; Wang, S.; Xia, Q. Discovery and Validation of Indole Nitroolefins as Novel Covalent Gpx4 Inhibitors for Inducing Ferroptosis in Urological Cancers. Chin. J. Cancer Res. 2025, 37, 404. [Google Scholar] [CrossRef]
- Yasir, M.; Patra, J.; Maurya, R.K.; Tripathi, A.S.; Pathan, H.K. Exploring Natural Products for Allosteric Inhibition of Glutathione Peroxidase 4 in Drug-Resistant Cancers Via Molecular Docking and Dynamics. Anti-Cancer Drugs 2025. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Liu, X.; Zhang, W.; Zhang, K.; Pan, L.; Zhu, M.; Qin, H.; Zou, C.; Wang, W.; Zhang, C. Biomimetic Macrophage Membrane-Camouflaged Nanoparticles Induce Ferroptosis by Promoting Mitochondrial Damage in Glioblastoma. Acs Nano 2023, 17, 23746–23760. [Google Scholar] [CrossRef]
- Mu, Y.; Fan, Y.; He, L.; Hu, N.; Xue, H.; Guan, X.; Zheng, Z. Enhanced Cancer Immunotherapy through Synergistic Ferroptosis and Immune Checkpoint Blockade Using Cell Membrane-Coated Nanoparticles. Cancer Nanotechnol. 2023, 14, 83. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Zhang, K.; Xu, C.; Wang, J.; Li, Z.; Zhou, Y.; Liu, S.; Zhao, X.; Li, Z. Genetically Engineered Membrane-Coated Nanoparticles for Enhanced Prostate-Specific Membrane Antigen Targeting and Ferroptosis Treatment of Castration-Resistant Prostate Cancer. Adv. Sci. 2024, 11, 2401095. [Google Scholar] [CrossRef]
- Song, W.-F.; Zeng, J.-Y.; Ji, P.; Han, Z.-Y.; Sun, Y.-X.; Zhang, X.-Z. Self-Assembled Copper-Based Nanoparticles for Glutathione Activated and Enzymatic Cascade-Enhanced Ferroptosis and Immunotherapy in Cancer Treatment. Small 2023, 19, 2301148. [Google Scholar] [CrossRef]
- Zhang, G.; Duan, J.; Yu, F.; Qi, Y.; Zhao, S.; Zhao, Y.; Han, X.; Liu, H.; Sang, Y.; Yu, D. Gsh-Responsive Mn2+ Burst Nanoboxes as Mitophagy Intervention Agents Augment Ferroptosis and Chemoimmunotherapy in Triple-Negative Breast Cancer. Adv. Funct. Mater. 2025, 2500491. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, J.; Ren, B.; Cai, H.; Li, Z.; Fan, Q.; Xiong, W.; Feng, J.; Yan, C.; Wen, G. A Hollow Mesoporous Iron Oxide Nanoparticle to Strengthen Fenton Reaction and Weaken Antioxidant Defense Systems for High Efficacy Tumor Ferroptosis Therapy. Chem. Eng. J. 2024, 497, 154470. [Google Scholar] [CrossRef]
- Wang, J.; Fang, Z.; Zhao, C.; Sun, Z.; Gao, S.; Zhang, B.; Qiu, D.; Yang, M.; Sheng, F.; Gao, S. Intelligent Size-Switchable Iron Carbide-Based Nanocapsules with Cascade Delivery Capacity for Hyperthermia-Enhanced Deep Tumor Ferroptosis. Adv. Mater. 2024, 36, 2307006. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Tan, H.; Ye, Y.; Xu, W.; Gao, J.; Liu, L.; Zhang, L.; Jiang, J.; Tian, H.; Peng, F. Nir-Actuated Ferroptosis Nanomotor for Enhanced Tumor Penetration and Therapy. Adv. Mater. 2024, 36, 2412227. [Google Scholar] [CrossRef]
- Gong, G.; Ganesan, K.; Liu, Y.; Huang, Y.; Luo, Y.; Wang, X.; Zhang, Z.; Zheng, Y. Danggui Buxue Tang Improves Therapeutic Efficacy of Doxorubicin in Triple Negative Breast Cancer Via Ferroptosis. J. Ethnopharmacol. 2024, 323, 117655. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, C.; Jiang, C.; Liu, N.; Yang, Z.; Xing, H. Rsl3 Induces Ferroptosis by Activating the Nf-Κb Signalling Pathway to Enhance the Chemosensitivity of Triple-Negative Breast Cancer Cells to Paclitaxel. Sci. Rep. 2025, 15, 1654. [Google Scholar] [CrossRef]
- Zhong, T.; Li, Y.; Jin, M.; Liu, J.; Wu, Z.; Zhu, F.; Zhao, L.; Fan, Y.; Xu, L.; Ji, J. Downregulation of 4-Hne and Foxo4 Collaboratively Promotes Nsclc Cell Migration and Tumor Growth. Cell Death Dis. 2024, 15, 546. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, N.; Ma, X.; Li, M.; Feng, H. The Dual Role of Ferroptosis in Anthracycline-Based Chemotherapy Includes Reducing Resistance and Increasing Toxicity. Cell Death Discov. 2023, 9, 184. [Google Scholar] [CrossRef] [PubMed]





| Molecular Subtype | Baseline Ferroptosis Sensitivity | Key Mechanisms | Reference |
|---|---|---|---|
| TNBC | High | ZEB1 reshapes lipid composition (↓MUFA, ↑PUFA); mitomiR-3–ZEB1–GPX4 inhibition; salidroside amplifies lipid peroxidation via SCD1 inhibition and NCOA4-driven ferritinophagy | [51,64,66] |
| Luminal A/B (ER+/PR+) | Low/tolerant | ERα upregulates System Xc− (SLC7A11, SLC3A2) to suppress ferroptosis; CDK4/6 inhibitor response depends on SLC7A11 downregulation; USP35–BRD4–SLC7A11 axis maintains antioxidant defense | [67,75,76] |
| HER2+ | Medium–low | HER2–PI3K–AKT–mTOR enhances antioxidant capacity; integrin αvβ3 mediates dual resistance to TKIs and ferroptosis; irreversible HER2 inhibitors (e.g., neratinib) restore sensitivity to RSL3 | [70,71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Yu, H.; Zhou, H.; Cui, G.; Shao, M. Natural Compounds as Modulators of Ferroptosis: Mechanistic Insights and Therapeutic Prospects in Breast Cancer. Biomolecules 2025, 15, 1308. https://doi.org/10.3390/biom15091308
He H, Yu H, Zhou H, Cui G, Shao M. Natural Compounds as Modulators of Ferroptosis: Mechanistic Insights and Therapeutic Prospects in Breast Cancer. Biomolecules. 2025; 15(9):1308. https://doi.org/10.3390/biom15091308
Chicago/Turabian StyleHe, Haotong, Haoyang Yu, Hefeng Zhou, Guozhen Cui, and Min Shao. 2025. "Natural Compounds as Modulators of Ferroptosis: Mechanistic Insights and Therapeutic Prospects in Breast Cancer" Biomolecules 15, no. 9: 1308. https://doi.org/10.3390/biom15091308
APA StyleHe, H., Yu, H., Zhou, H., Cui, G., & Shao, M. (2025). Natural Compounds as Modulators of Ferroptosis: Mechanistic Insights and Therapeutic Prospects in Breast Cancer. Biomolecules, 15(9), 1308. https://doi.org/10.3390/biom15091308








