Isoniazid-Derived Hydrazones Featuring Piperazine/Piperidine Rings: Design, Synthesis, and Investigation of Antitubercular Activity
Abstract
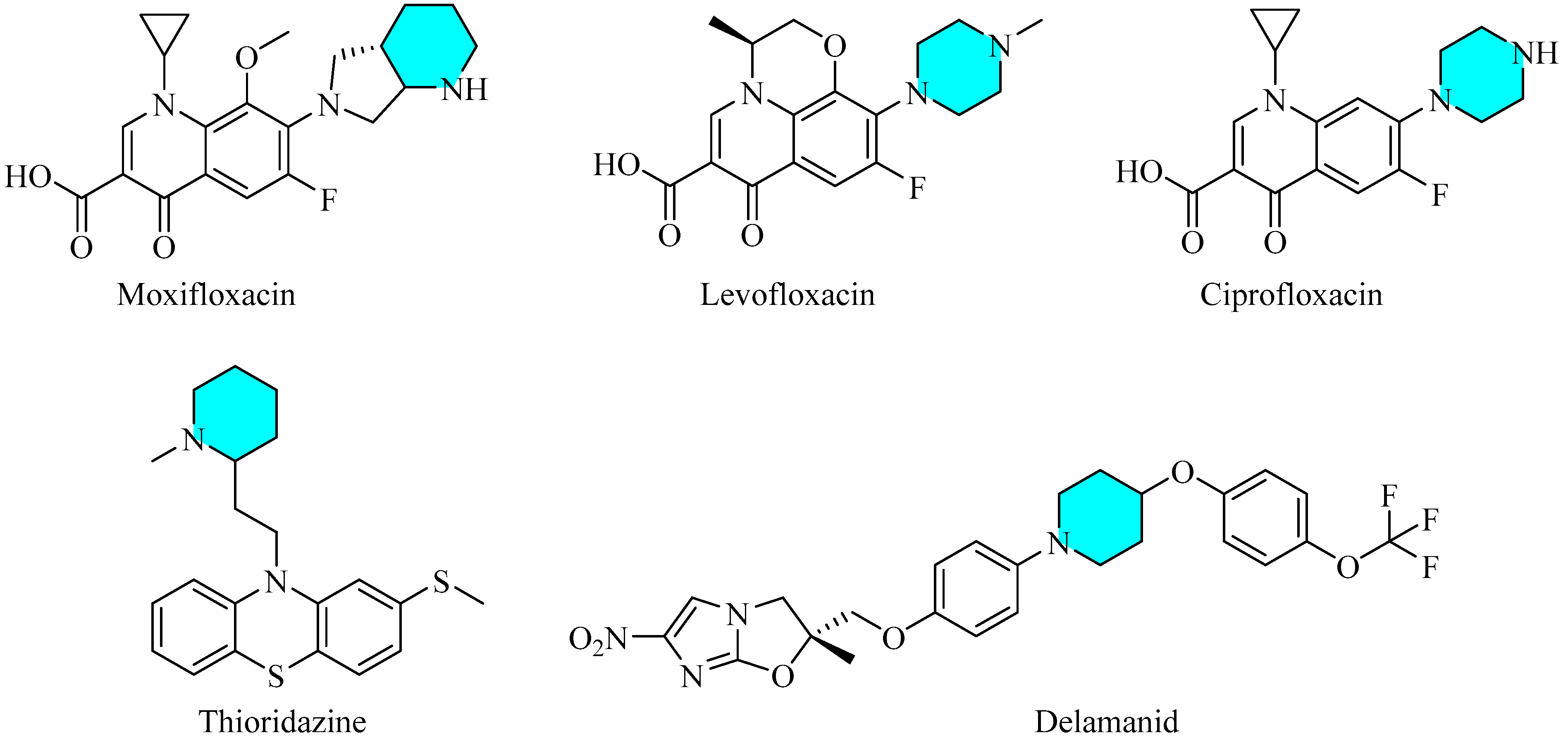
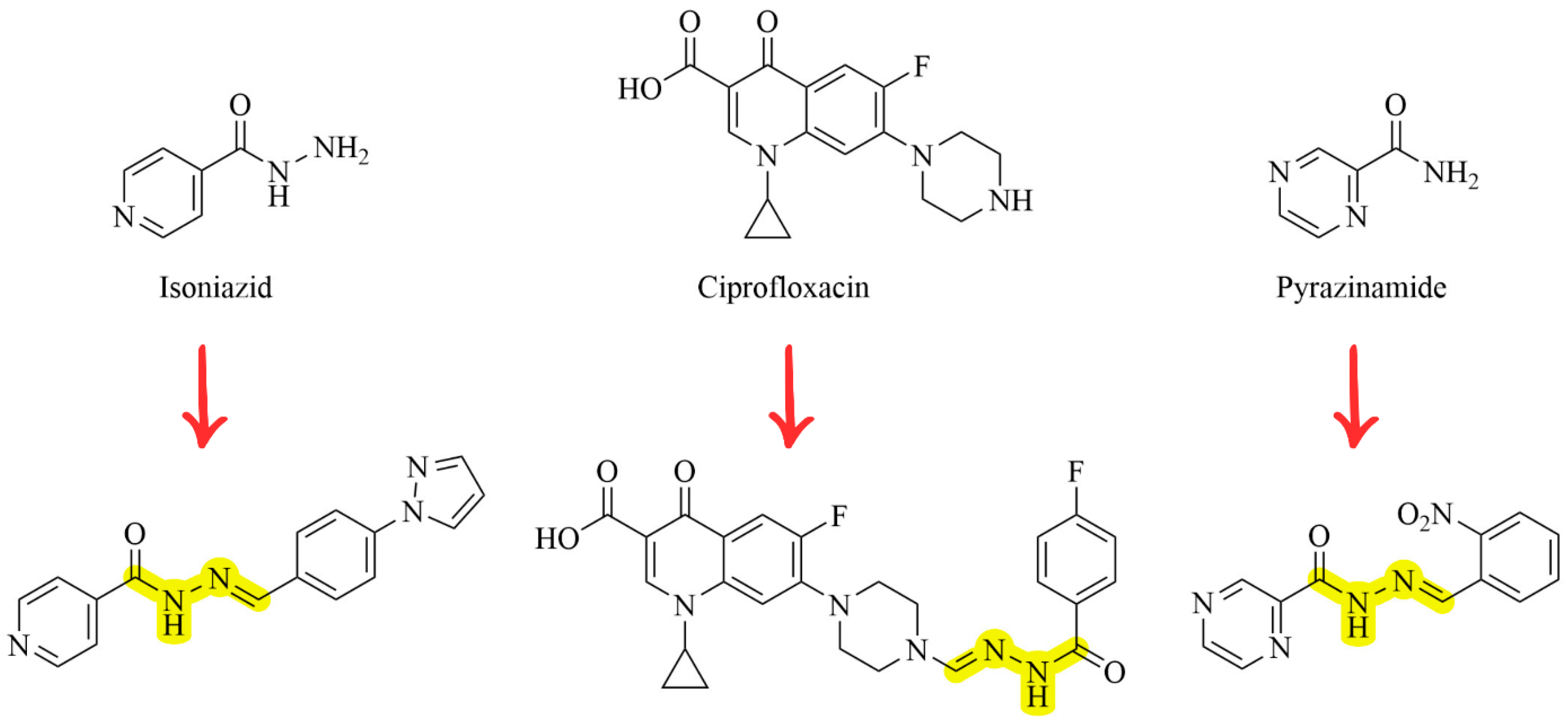
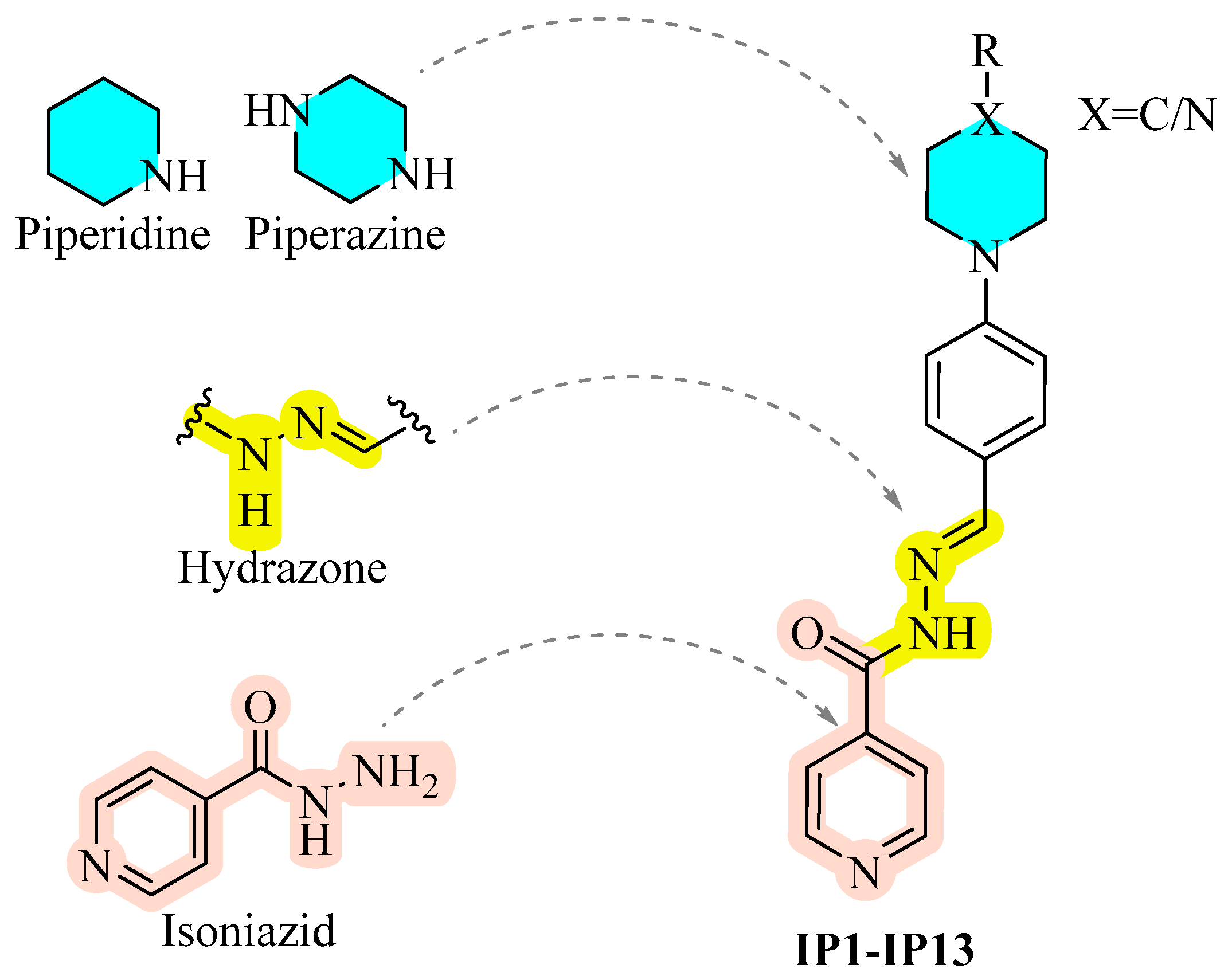
1. Introduction
2. Materials and Method
2.1. Chemistry
2.1.1. Experimental
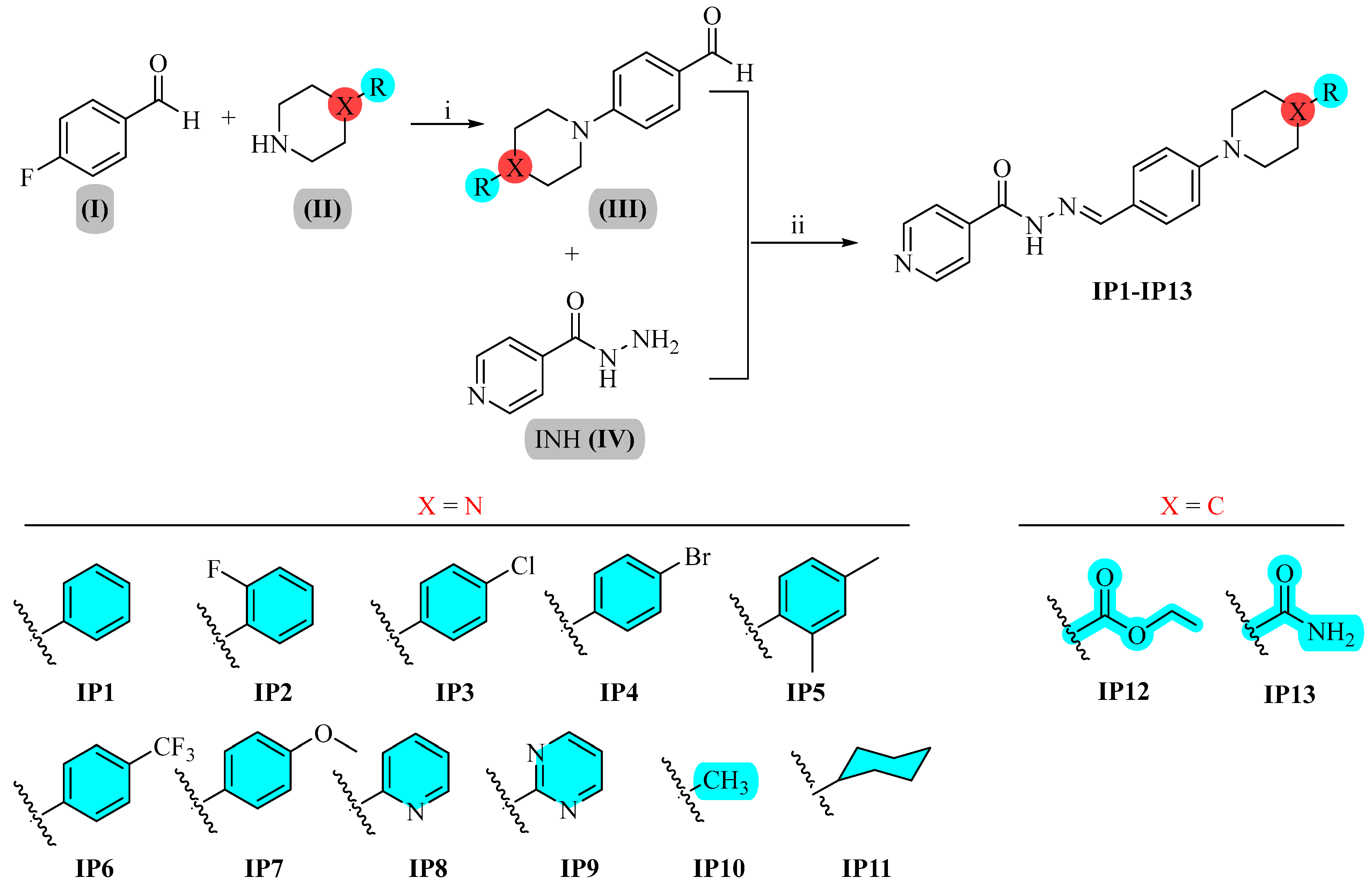
Preparation of Benzaldehydes Carrying Piperazine/Piperidine Derivatives (III)
General Procedure for the Synthesis of IP1–IP13
- N′-(4-(4-Phenylpiperazin-1-yl)benzylidene)isonicotinohydrazide (IP1)
- N′-(4-(4-(2-Fluorophenyl)piperazin-1-yl)benzylidene)isonicotinohydrazide (IP2)
- N′-(4-(4-(4-Chlorophenyl)piperazin-1-yl)benzylidene)isonicotinohydrazide (IP3)
- N′-(4-(4-(4-Bromophenyl)piperazin-1-yl)benzylidene)isonicotinohydrazide (IP4)
- N′-(4-(4-(2,4-Dimethylphenyl)piperazin-1-yl)benzylidene)isonicotinohydrazide (IP5)
- N′-(4-(4-(4-(Trifluoromethyl)phenyl)piperazin-1-yl)benzylidene)isonicotinohydrazide (IP6)
- N′-(4-(4-(4-Methoxyphenyl)piperazin-1-yl)benzylidene)isonicotinohydrazide (IP7)
- N′-(4-(4-(Pyridin-2-yl)piperazin-1-yl)benzylidene)isonicotinohydrazide (IP8)
- N′-(4-(4-(Pyrimidin-2-yl)piperazin-1-yl)benzylidene)isonicotinohydrazide (IP9)
- N′-(4-(4-Methylpiperazin-1-yl)benzylidene)isonicotinohydrazide (IP10)
- N′-(4-(4-Cyclohexylpiperazin-1-yl)benzylidene)isonicotinohydrazide (IP11)
- Ethyl 1-(4-((2-isonicotinoylhydrazono)methyl)phenyl)piperidine-4-carboxylate (IP12)
- 1-(4-((2-Isonicotinoylhydrazono)methyl)phenyl)piperidine-4-carboxamide (IP13)
2.2. Evaluation of Antitubercular Activity and Cytotoxicity
2.2.1. Microplate Alamar Blue Assay (MABA) Protocol for Antimycobacterial Testing
2.2.2. Assay for Host Cytotoxicity Determination
2.3. InhA Inhibition
2.4. Molecular Modeling Studies
2.4.1. Molecular Docking and Molecular Dynamics Simulations
2.4.2. Prediction of Drug-Likeness
3. Results and Discussion
3.1. Chemistry
3.2. Evaluation of Antimycobacterial Activity and Cytotoxicity
3.3. InhA Inhibition
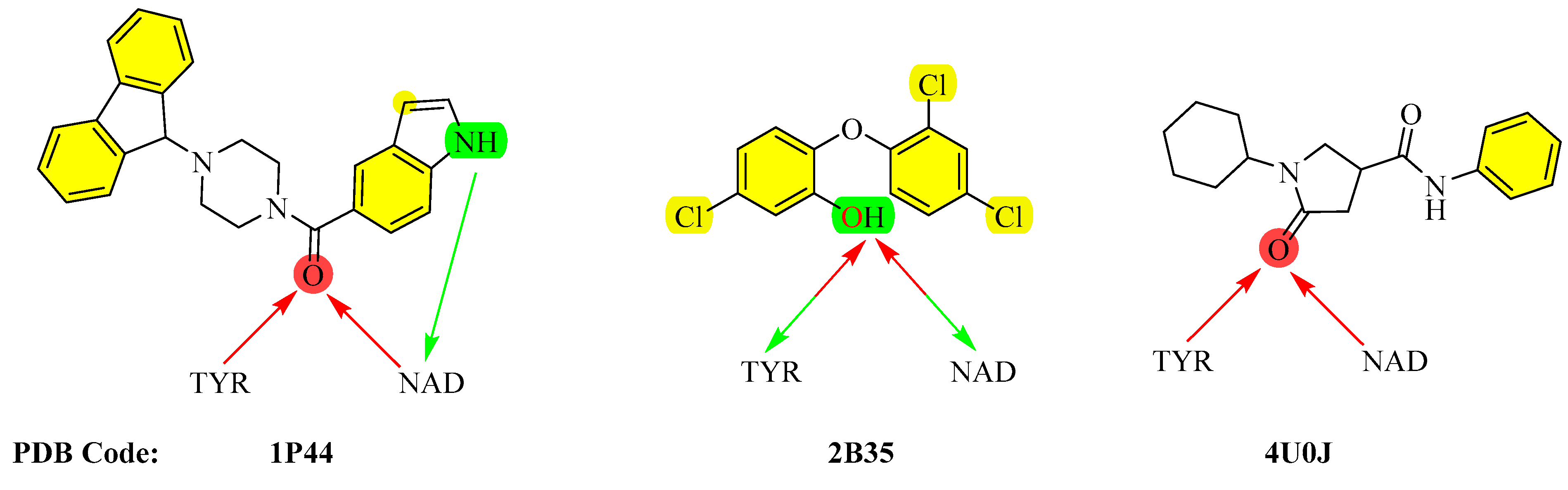
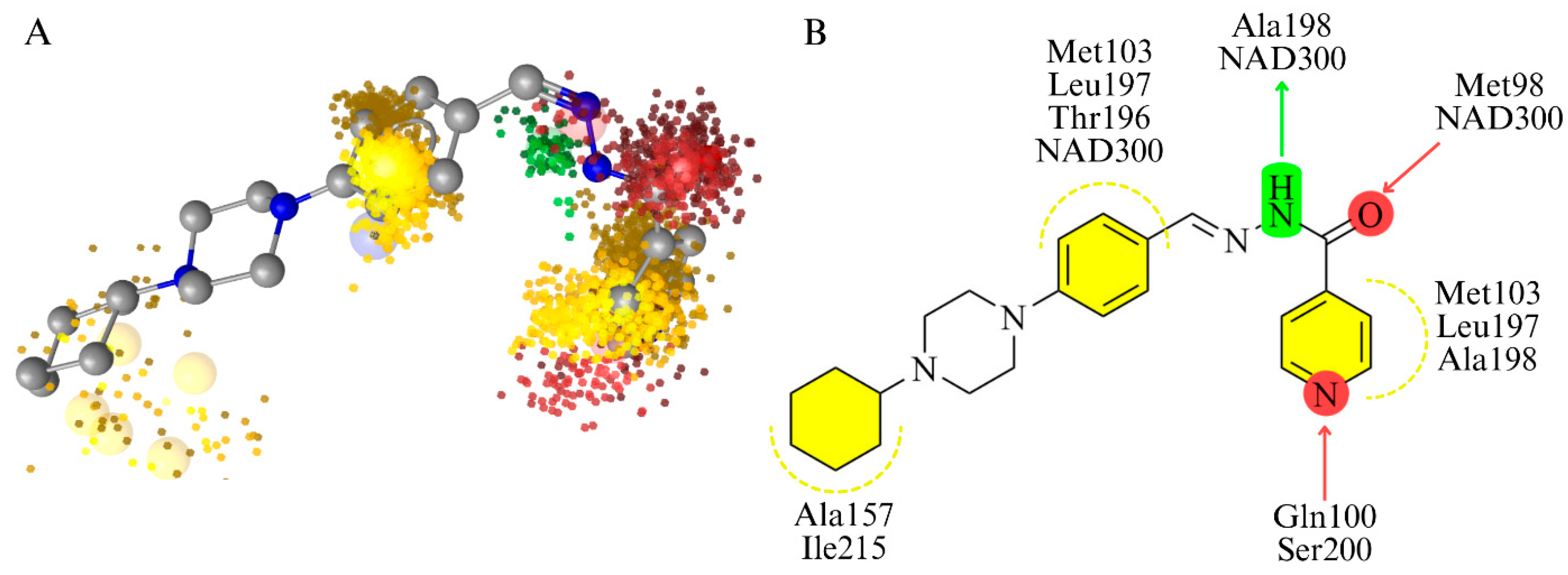
3.4. Molecular Modeling Studies
3.4.1. Molecular Docking and Molecular Dynamics Simulations
3.4.2. Prediction of Drug-Likeness
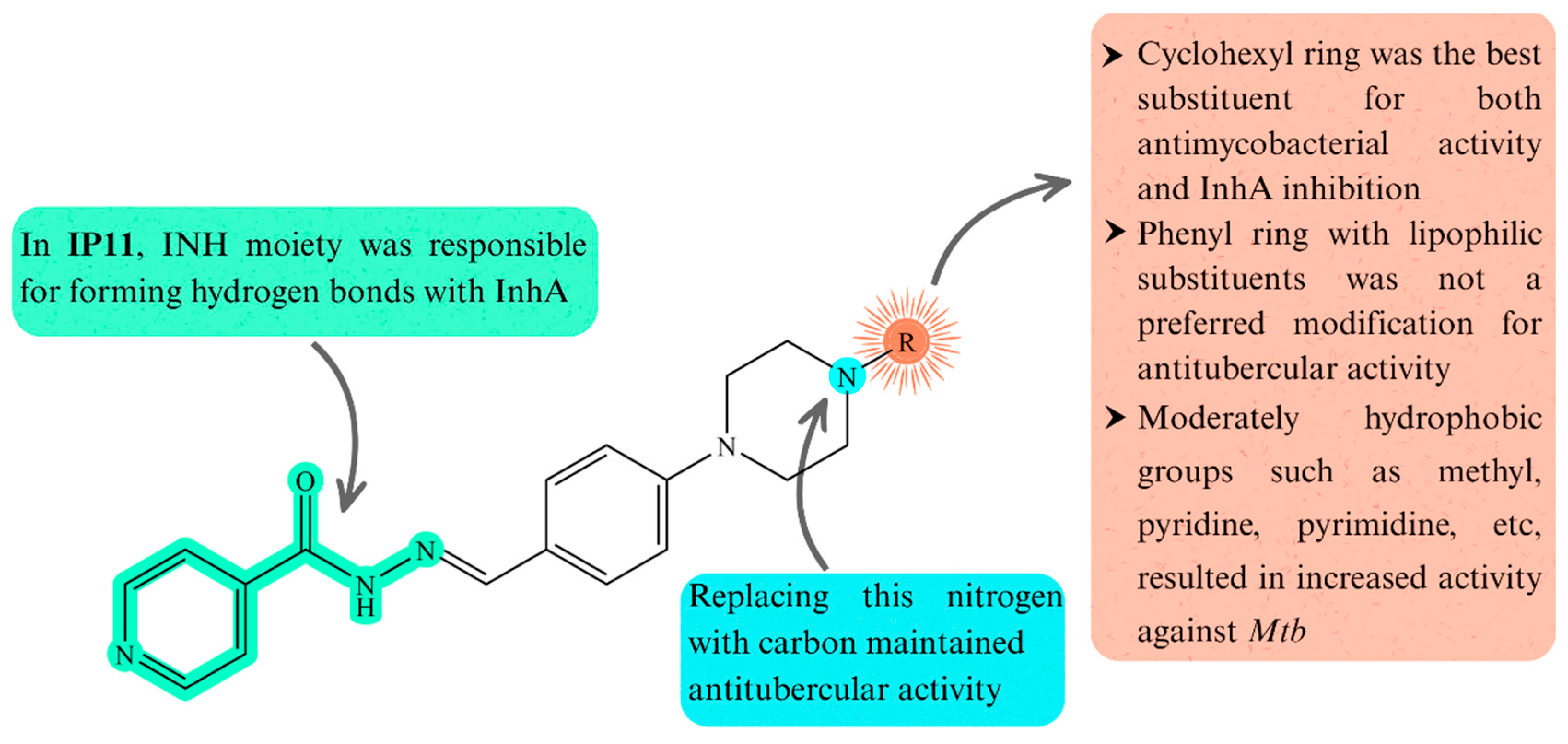
3.5. Structure-Activity Relationships
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Alsayed, S.S.R.; Gunosewoyo, H. Tuberculosis: Pathogenesis, Current Treatment Regimens and New Drug Targets. Int. J. Mol. Sci. 2023, 24, 5202. [Google Scholar] [CrossRef]
- Global Programme on Tuberculosis & Lung Health. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports (accessed on 22 July 2025).
- Sachan, R.S.K.; Mistry, V.; Dholaria, M.; Rana, A.; Devgon, I.; Ali, I.; Iqbal, J.; Eldin, S.M.; Mohammad Said Al-Tawaha, A.R.; Bawazeer, S.; et al. Overcoming Mycobacterium tuberculosis Drug Resistance: Novel Medications and Repositioning Strategies. ACS Omega 2023, 8, 32244–32257. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Mük, G.R.; Avram, S.; Vlad, I.M.; Limban, C.; Nuta, D.; Grumezescu, A.M.; Chifiriuc, M.C. Novel Strategies Based on Natural Products and Synthetic Derivatives to Overcome Resistance in Mycobacterium tuberculosis. Eur. J. Med. Chem. 2024, 269, 116268. [Google Scholar] [CrossRef]
- dos Santos Fernandes, G.F.; Salgado, H.R.N.; dos Santos, J.L. Isoniazid: A Review of Characteristics, Properties and Analytical Methods. Crit. Rev. Anal. Chem. 2017, 47, 298–308. [Google Scholar] [CrossRef]
- Reingewertz, T.H.; Meyer, T.; McIntosh, F.; Sullivan, J.; Meir, M.; Chang, Y.F.; Behr, M.A.; Barkana, D. Differential Sensitivity of Mycobacteria to Isoniazid Is Related to Differences in Katg-Mediated Enzymatic Activation of the Drug. Antimicrob. Agents Chemother. 2020, 64, e01899-19. [Google Scholar] [CrossRef]
- Wahan, S.K.; Bhargava, G.; Chawla, V.; Chawla, P.A. Unlocking InhA: Novel Approaches to Inhibit Mycobacterium tuberculosis. Bioorg. Chem. 2024, 146, 107250. [Google Scholar] [CrossRef]
- Bollela, V.R.; Namburete, E.I.; Feliciano, C.S.; Macheque, D.; Harrison, L.H.; Caminero, J.A. Detection of KatG and InhA Mutations to Guide Isoniazid and Ethionamide Use for Drug-Resistant Tuberculosis. Int. J. Tuberc. Lung Dis. 2016, 20, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Upton, A.M.; Mushtaq, A.; Victor, T.C.; Sampson, S.L.; Sandy, J.; Smith, D.-M.; van Helden, P.V.; Sim, E. Arylamine N-Acetyltransferase of Mycobacterium tuberculosis Is a Polymorphic Enzyme and a Site of Isoniazid Metabolism. Mol. Microbiol. 2001, 42, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Stagg, H.R.; Lipman, M.C.; McHugh, T.D.; Jenkins, H.E. Isoniazid-Resistant Tuberculosis: A Cause for Concern? Int. J. Tuberc. Lung Dis. 2017, 21, 129–139. [Google Scholar] [CrossRef]
- Tonge, P.J.; Kisker, C.; Slayden, R.A. Development of Modern InhA Inhibitors to Combat Drug Resistant Strains of Mycobacterium tuberculosis. Curr. Top. Med. Chem. 2007, 7, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Frolov, N.A.; Vereshchagin, A.N. Piperidine Derivatives: Recent Advances in Synthesis and Pharmacological Applications. Int. J. Mol. Sci. 2023, 24, 2937. [Google Scholar] [CrossRef]
- Shaquiquzzaman, M.; Verma, G.; Marella, A.; Akhter, M.; Akhtar, W.; Khan, M.F.; Tasneem, S.; Alam, M.M. Piperazine Scaffold: A Remarkable Tool in Generation of Diverse Pharmacological Agents. Eur. J. Med. Chem. 2015, 102, 487–529. [Google Scholar] [CrossRef]
- Pais, J.P.; Policarpo, M.; Pires, D.; Francisco, A.P.; Madureira, A.M.; Testa, B.; Anes, E.; Constantino, L. Fluoroquinolone Derivatives in the Treatment of Mycobacterium tuberculosis Infection. Pharmaceuticals 2022, 15, 1213. [Google Scholar] [CrossRef]
- Pieroni, M.; Machado, D.; Azzali, E.; Santos Costa, S.; Couto, I.; Costantino, G.; Viveiros, M. Rational Design and Synthesis of Thioridazine Analogues as Enhancers of the Antituberculosis Therapy. J. Med. Chem. 2015, 58, 5842–5853. [Google Scholar] [CrossRef]
- Khoshnood, S.; Taki, E.; Sadeghifard, N.; Kaviar, V.H.; Haddadi, M.H.; Farshadzadeh, Z.; Kouhsari, E.; Goudarzi, M.; Heidary, M. Mechanism of Action, Resistance, Synergism, and Clinical Implications of Delamanid Against Multidrug-Resistant Mycobacterium tuberculosis. Front. Microbiol. 2021, 12, 717045. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Sharma, D.; Sharma, A.; Saini, N.; Goyal, R.; Ola, M.; Chawla, R.; Thakur, V.K. Hydrazone Comprising Compounds as Promising Anti-Infective Agents: Chemistry and Structure-Property Relationship. Mater. Today Chem. 2020, 18, 100349. [Google Scholar] [CrossRef]
- Teneva, Y.; Simeonova, R.; Valcheva, V.; Angelova, V.T. Recent Advances in Anti-Tuberculosis Drug Discovery Based on Hydrazide–Hydrazone and Thiadiazole Derivatives Targeting InhA. Pharmaceuticals 2023, 16, 484. [Google Scholar] [CrossRef]
- Koçak Aslan, E.; Krishna, V.S.; Armaković, S.J.; Armaković, S.; Şahin, O.; Tønjum, T.; Gündüz, M.G. Linking Azoles to Isoniazid via Hydrazone Bridge: Synthesis, Crystal Structure Determination, Antitubercular Evaluation and Computational Studies. J. Mol. Liq. 2022, 354, 118873. [Google Scholar] [CrossRef]
- Vavříková, E.; Polanc, S.; Kočevar, M.; Horváti, K.; Bősze, S.; Stolaříková, J.; Vávrová, K.; Vinšová, J. New Fluorine-Containing Hydrazones Active against MDR-Tuberculosis. Eur. J. Med. Chem. 2011, 46, 4937–4945. [Google Scholar] [CrossRef]
- Vergara, F.M.F.; Lima, C.H.d.S.; Henriques, M.d.G.M.d.O.; Candéa, A.L.P.; Lourenço, M.C.S.; Ferreira, M.d.L.; Kaiser, C.R.; de Souza, M.V.N. Synthesis and Antimycobacterial Activity of N′-[(E)-(Monosubstituted-Benzylidene)]-2-Pyrazinecarbohydrazide Derivatives. Eur. J. Med. Chem. 2009, 44, 4954–4959. [Google Scholar] [CrossRef]
- Marquês, J.T.; Frazão De Faria, C.; Reis, M.; Machado, D.; Santos, S.; Santos, M.d.S.; Viveiros, M.; Martins, F.; De Almeida, R.F.M. In Vitro Evaluation of Isoniazid Derivatives as Potential Agents Against Drug-Resistant Tuberculosis. Front. Pharmacol. 2022, 13, 868545. [Google Scholar] [CrossRef]
- Sampiron, E.G.; Costacurta, G.F.; Baldin, V.P.; Almeida, A.L.; Ieque, A.L.; Santos, N.C.S.; Alves-Olher, V.G.; Vandresen, F.; Gimenes, A.C.R.; Siqueira, V.L.D.; et al. Hydrazone, Benzohydrazones and Isoniazid-Acylhydrazones as Potential Antituberculosis Agents. Future Microbiol. 2019, 14, 981–994. [Google Scholar] [CrossRef]
- Lone, M.S.; Mubarak, M.M.; Nabi, S.A.; Wani, F.R.; Amin, S.; Nabi, S.; Kantroo, H.A.; Samim, M.; Shafi, S.; Ahmad, S.; et al. Isonicotinoyl-Butanoic Acid Hydrazone Derivatives as Anti-Tubercular Agents: In-Silico Studies, Synthesis, Spectral Characterization and Biological Evaluation. Med. Chem. Res. 2023, 32, 808–826. [Google Scholar] [CrossRef]
- Koçak Aslan, E.; Han, M.İ.; Krishna, V.S.; Tamhaev, R.; Dengiz, C.; Doğan, Ş.D.; Lherbet, C.; Mourey, L.; Tønjum, T.; Gündüz, M.G. Isoniazid Linked to Sulfonate Esters via Hydrazone Functionality: Design, Synthesis, and Evaluation of Antitubercular Activity. Pharmaceuticals 2022, 15, 1301. [Google Scholar] [CrossRef]
- Mishra, C.B.; Kumari, S.; Manral, A.; Prakash, A.; Saini, V.; Lynn, A.M.; Tiwari, M. Design, Synthesis, in-Silico and Biological Evaluation of Novel Donepezil Derivatives as Multi-Target-Directed Ligands for the Treatment of Alzheimer’s Disease. Eur. J. Med. Chem. 2017, 125, 736–750. [Google Scholar] [CrossRef] [PubMed]
- Şimşek, T.; Özcan, E.; Çetinkaya, Y.; Aleksic, I.; Skaro Bogojevic, S.; Nikodinovic-Runic, J.; Gündüz, M.G.; Doğan, Ş.D. Hydrazone-Bridged 5-Nitrofuran and Piperidine/Piperazine Derivatives: Synthesis, DFT Studies, and Evaluation of Anticancer and Antimicrobial Activity. J. Mol. Struct. 2025, 1334, 141863. [Google Scholar] [CrossRef]
- Krishna, V.S.; Zheng, S.; Rekha, E.M.; Nallangi, R.; Sai Prasad, D.V.; George, S.E.; Guddat, L.W.; Sriram, D. Design and Development of ((4-Methoxyphenyl)Carbamoyl) (5-(5-Nitrothiophen-2-Yl)-1,3,4-Thiadiazol-2-Yl)Amide Analogues as Mycobacterium tuberculosis Ketol-Acid Reductoisomerase Inhibitors. Eur. J. Med. Chem. 2020, 193, 112178. [Google Scholar] [CrossRef]
- Sarkar, S.; Mayer Bridwell, A.E.; Good, J.A.D.; Wang, E.R.; McKee, S.R.; Valenta, J.; Harrison, G.A.; Flentie, K.N.; Henry, F.L.; Wixe, T.; et al. Design, Synthesis, and Evaluation of Novel Δ2-Thiazolino 2-Pyridone Derivatives That Potentiate Isoniazid Activity in an Isoniazid-Resistant Mycobacterium tuberculosis Mutant. J. Med. Chem. 2023, 66, 11056–11077. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.; Saffon, N.; Sammartino, J.C.; Degiacomi, G.; Pasca, M.R.; Lherbet, C. First Triclosan-Based Macrocyclic Inhibitors of InhA Enzyme. Bioorg. Chem. 2020, 95, 103498. [Google Scholar] [CrossRef]
- Kuo, M.R.; Morbidoni, H.R.; Alland, D.; Sneddon, S.F.; Gourlie, B.B.; Staveski, M.M.; Leonard, M.; Gregory, J.S.; Janjigian, A.D.; Yee, C.; et al. Targeting Tuberculosis and Malaria through Inhibition of Enoyl Reductase: COMPOUND ACTIVITY AND STRUCTURAL DATA. J. Biol. Chem. 2003, 278, 20851–20859. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Wolber, G.; Langer, T. LigandScout: 3-D Pharmacophores Derived from Protein-Bound Ligands and Their Use as Virtual Screening Filters. J. Chem. Inf. Model. 2005, 45, 160–169. [Google Scholar] [CrossRef]
- Schrödinger Release 2024-4: Maestro; Schrödinger, LLC: New York, NY, USA, 2024.
- Bowers, K.J.; Chow, D.E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. In Proceedings of the 2006 ACM/IEEE Conference on Supercomputing, Tampa, FL, USA, 11–17 November 2006; p. 84. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Schaller, D.; Šribar, D.; Noonan, T.; Deng, L.; Nguyen, T.N.; Pach, S.; Machalz, D.; Bermudez, M.; Wolber, G. Next Generation 3D Pharmacophore Modeling. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2020, 10, e1468. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Karagüzel, A.; Buran Uğur, S.; Çetinkaya, Y.; Doğan, Ş.D.; Stevanovic, M.; Nikodinovic-Runic, J.; Gündüz, M.G. Azole Rings Linked to COX Inhibitors via Hydrazone Bridge: Synthesis, Stereochemical Analysis, and Investigation of Antimicrobial Activity. J. Mol. Struct. 2024, 1306, 137787. [Google Scholar] [CrossRef]
- Cumming, B.M.; Baig, Z.; Addicott, K.W.; Chen, D.; Steyn, A.J.C. Host Bioenergetic Parameters Reveal Cytotoxicity of Antituberculosis Drugs Undetected Using Conventional Viability Assays. Antimicrob. Agents Chemother. 2021, 65, e00932-21. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.Y.; Lai, L.Y.; Hsieh, P.F.; Lin, T.L.; Lin, W.H.; Tasi, H.Y.; Lee, W.T.; Jou, R.; Wang, J.T. Two Novel KatG Mutations Conferring Isoniazid Resistance in Mycobacterium tuberculosis. Front. Microbiol. 2020, 11, 556918. [Google Scholar] [CrossRef] [PubMed]
- Encinas, L.; Li, S.Y.; Rullas-Trincado, J.; Tasneen, R.; Tyagi, S.; Soni, H.; Garcia-Perez, A.; Lee, J.; González Del Río, R.; De Mercado, J.; et al. Contribution of Direct InhA Inhibitors to Novel Drug Regimens in a Mouse Model of Tuberculosis. Antimicrob. Agents Chemother. 2024, 68, e0035724. [Google Scholar] [CrossRef]
- Chollet, A.; Maveyraud, L.; Lherbet, C.; Bernardes-Génisson, V. An Overview on Crystal Structures of InhA Protein: Apo-Form, in Complex with Its Natural Ligands and Inhibitors. Eur. J. Med. Chem. 2018, 146, 318–343. [Google Scholar] [CrossRef]



 | |||||||
|---|---|---|---|---|---|---|---|
| MIC (μM) | Toxicity IC50 (μM) * | ||||||
| Compound | X | R | H37Rv | inhA +(a) | katG +(b) | HEK 293 | A549 |
| IP1 | N | phenyl | 50 | - | - | - | - |
| IP2 | N | 2-fluorophenyl | >100 | - | - | - | - |
| IP3 | N | 4-chlorophenyl | 50 | - | - | - | - |
| IP4 | N | 4-bromophenyl | 100 | - | - | - | - |
| IP5 | N | 2,4-dimethylphenyl | 50 | - | - | - | - |
| IP6 | N | 4-trifluoromethylphenyl | 12.5 | - | - | - | - |
| IP7 | N | 4-methoxyphenyl | 0.78 | 25 | 100 | - | - |
| IP8 | N | pyridin-2-yl | 0.39 | 1.56 | 50 | >100 | >100 |
| IP9 | N | pyrimidin-2-yl | 0.78 | 3.12 | 25 | >100 | >100 |
| IP10 | N | methyl | 0.39 | 1.56 | 25 | >100 | >100 |
| IP11 | N | cyclohexyl | 0.39 | 0.78 | 12.5 | >100 | >100 |
| IP12 | C | ethylcarboxylate | 0.78 | 1.56 | 25 | >100 | >100 |
| IP13 | C | carboxamide | 0.78 | 1.56 | 50 | >100 | >100 |
| INH | 0.39 | 1.56 | 6.25 | - | - | ||
| Compound | MW a | LogP b | HBD c | HBA d | NRB e | TPSA f | Lipinski’s Violation |
|---|---|---|---|---|---|---|---|
| IP1 | 385.46 | 2.84 | 1 | 3 | 6 | 60.83 | 0 |
| IP2 | 403.45 | 3.13 | 1 | 4 | 6 | 60.83 | 0 |
| IP3 | 419.91 | 3.37 | 1 | 3 | 6 | 60.83 | 0 |
| IP4 | 464.36 | 3.46 | 1 | 3 | 6 | 60.83 | 0 |
| IP5 | 413.51 | 3.49 | 1 | 3 | 6 | 60.83 | 0 |
| IP6 | 453.46 | 3.86 | 1 | 6 | 7 | 60.83 | 0 |
| IP7 | 415.49 | 2.86 | 1 | 4 | 7 | 70.06 | 0 |
| IP8 | 386.45 | 2.26 | 1 | 4 | 6 | 73.72 | 0 |
| IP9 | 387.44 | 1.77 | 1 | 5 | 6 | 86.61 | 0 |
| IP10 | 323.39 | 1.66 | 1 | 4 | 5 | 60.83 | 0 |
| IP11 | 391.51 | 2.99 | 1 | 4 | 6 | 60.83 | 0 |
| IP12 | 380.44 | 2.54 | 1 | 5 | 8 | 83.89 | 0 |
| IP13 | 351.40 | 1.42 | 2 | 4 | 6 | 100.68 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özcan, E.; Vagolu, S.K.; Tamhaev, R.; Lherbet, C.; Mourey, L.; Tønjum, T.; Gündüz, M.G.; Doğan, Ş.D. Isoniazid-Derived Hydrazones Featuring Piperazine/Piperidine Rings: Design, Synthesis, and Investigation of Antitubercular Activity. Biomolecules 2025, 15, 1305. https://doi.org/10.3390/biom15091305
Özcan E, Vagolu SK, Tamhaev R, Lherbet C, Mourey L, Tønjum T, Gündüz MG, Doğan ŞD. Isoniazid-Derived Hydrazones Featuring Piperazine/Piperidine Rings: Design, Synthesis, and Investigation of Antitubercular Activity. Biomolecules. 2025; 15(9):1305. https://doi.org/10.3390/biom15091305
Chicago/Turabian StyleÖzcan, Esma, Siva Krishna Vagolu, Rasoul Tamhaev, Christian Lherbet, Lionel Mourey, Tone Tønjum, Miyase Gözde Gündüz, and Şengül Dilem Doğan. 2025. "Isoniazid-Derived Hydrazones Featuring Piperazine/Piperidine Rings: Design, Synthesis, and Investigation of Antitubercular Activity" Biomolecules 15, no. 9: 1305. https://doi.org/10.3390/biom15091305
APA StyleÖzcan, E., Vagolu, S. K., Tamhaev, R., Lherbet, C., Mourey, L., Tønjum, T., Gündüz, M. G., & Doğan, Ş. D. (2025). Isoniazid-Derived Hydrazones Featuring Piperazine/Piperidine Rings: Design, Synthesis, and Investigation of Antitubercular Activity. Biomolecules, 15(9), 1305. https://doi.org/10.3390/biom15091305







