Natural Product-Derived Drugs: Structural Insights into Their Biological Mechanisms
Abstract
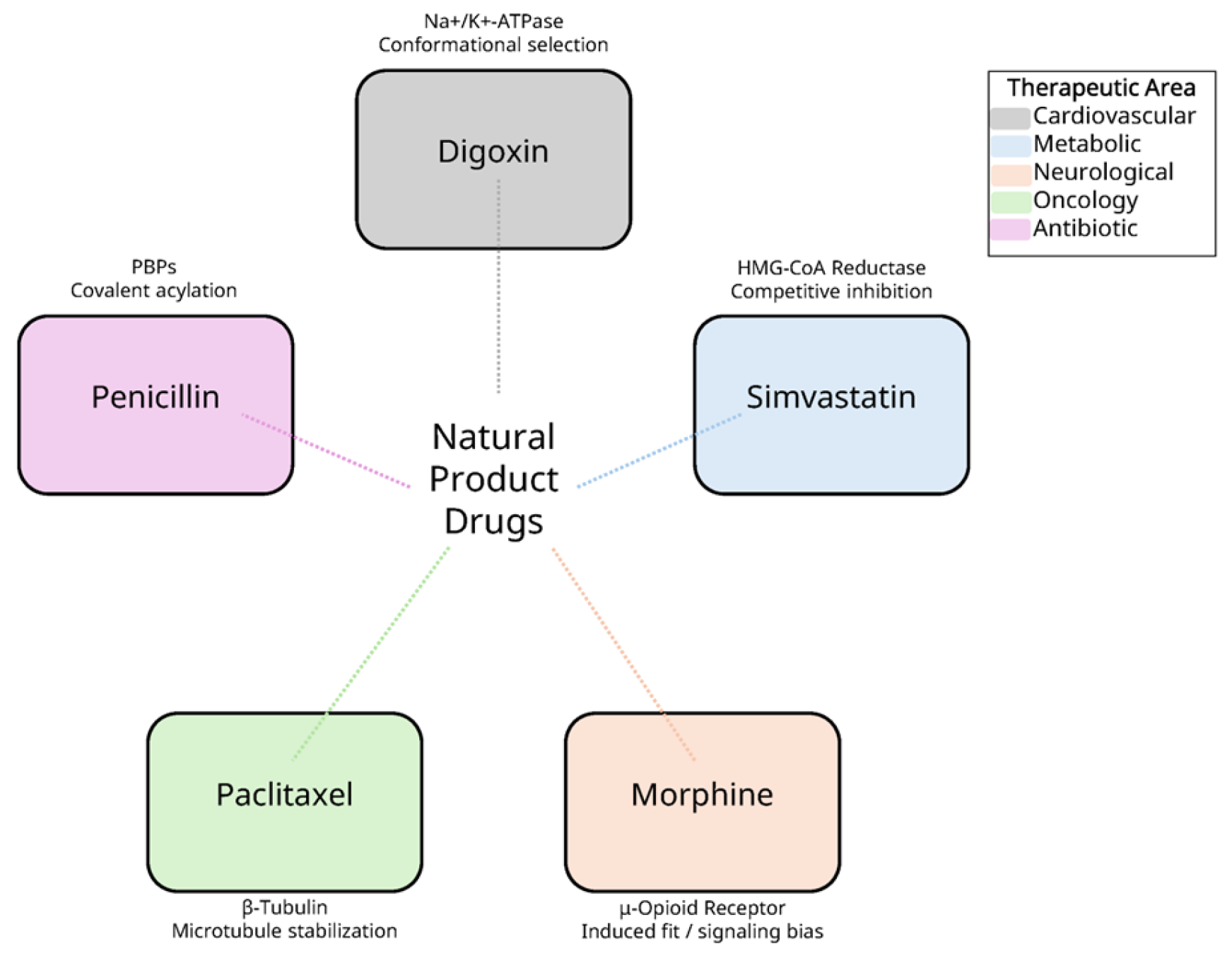
1. Introduction
2. Structural Analysis of Natural Product-Protein Complexes
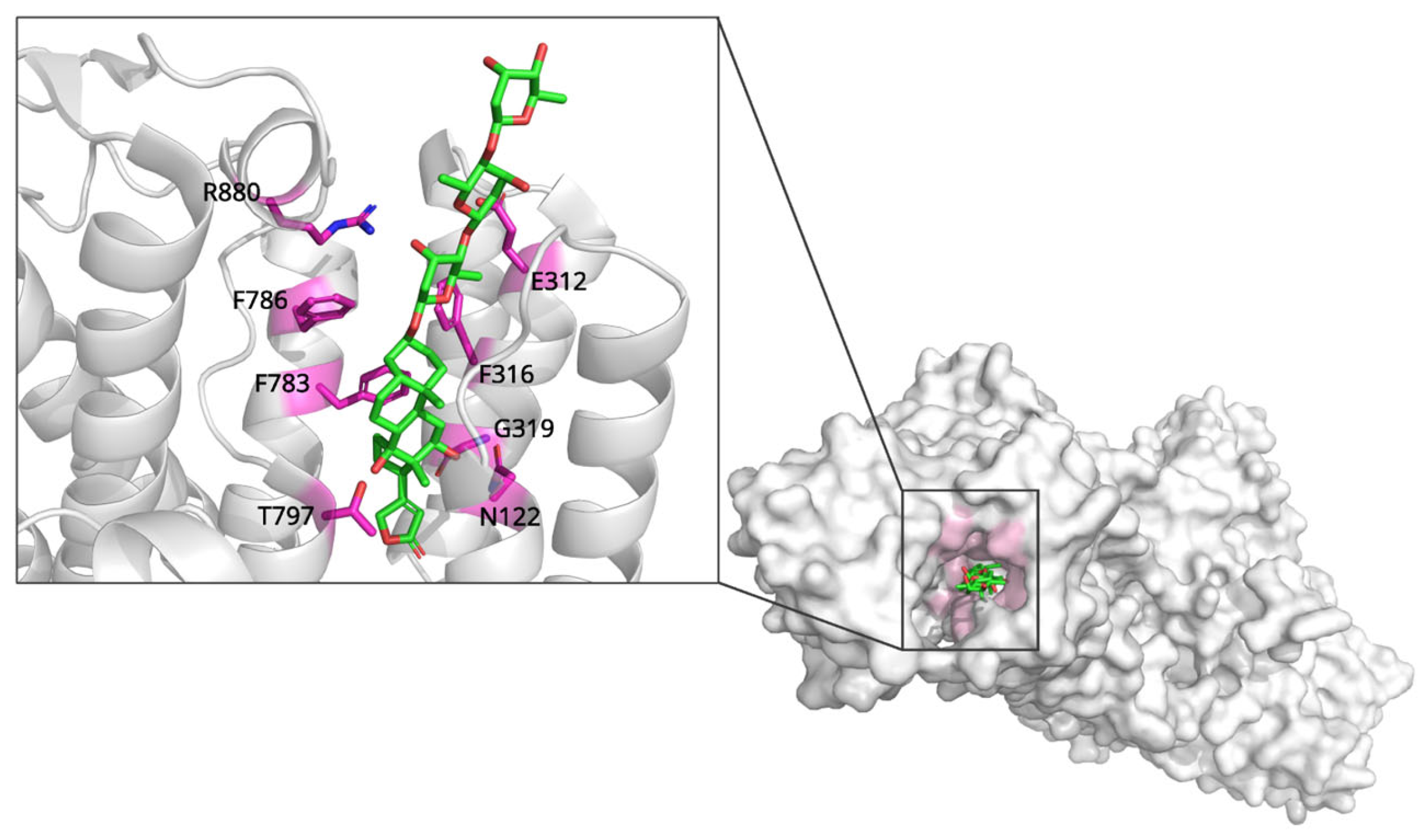
2.1. Digoxin and Na+/K+-ATPase: Mechanisms of Ion Transport Inhibition
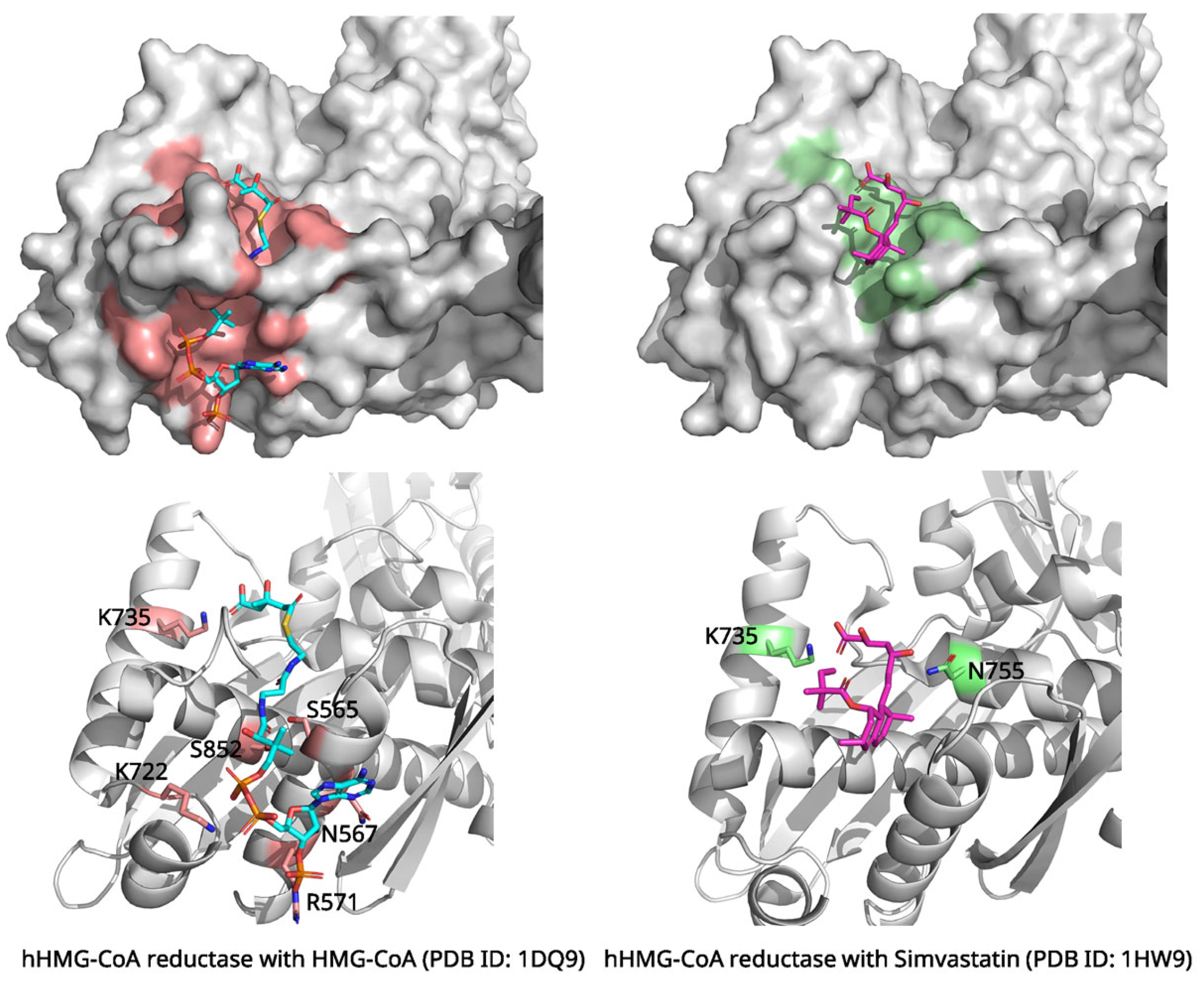
2.2. Simvastatin and HMG-CoA Reductase: Inhibition of Cholesterol Biosynthesis
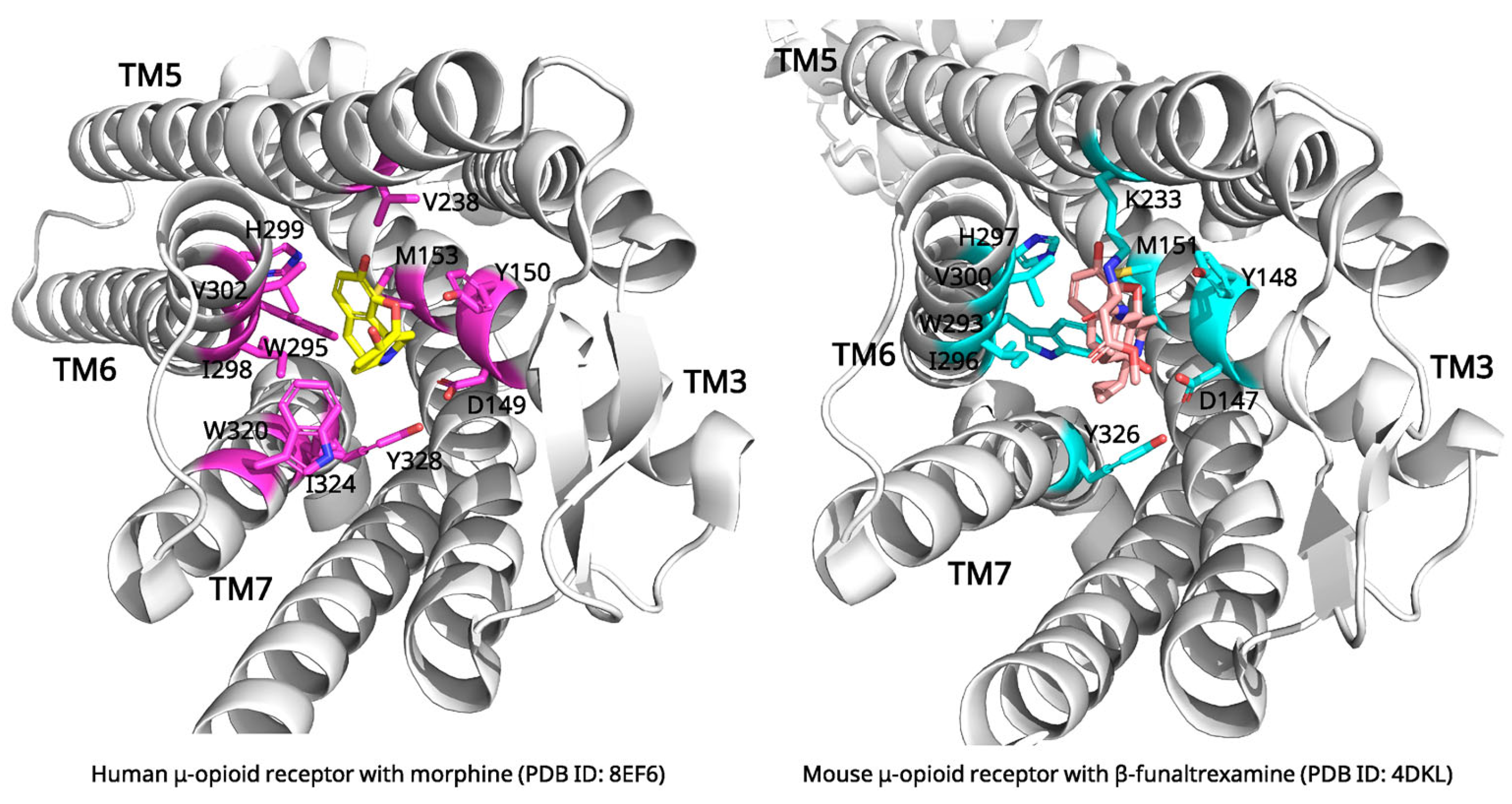
2.3. Morphine and μ-Opioid Receptor: Mechanisms of Pain Relief and Receptor Activation
2.4. Paclitaxel and β-Tubulin: Mechanisms of Microtubule Stabilization
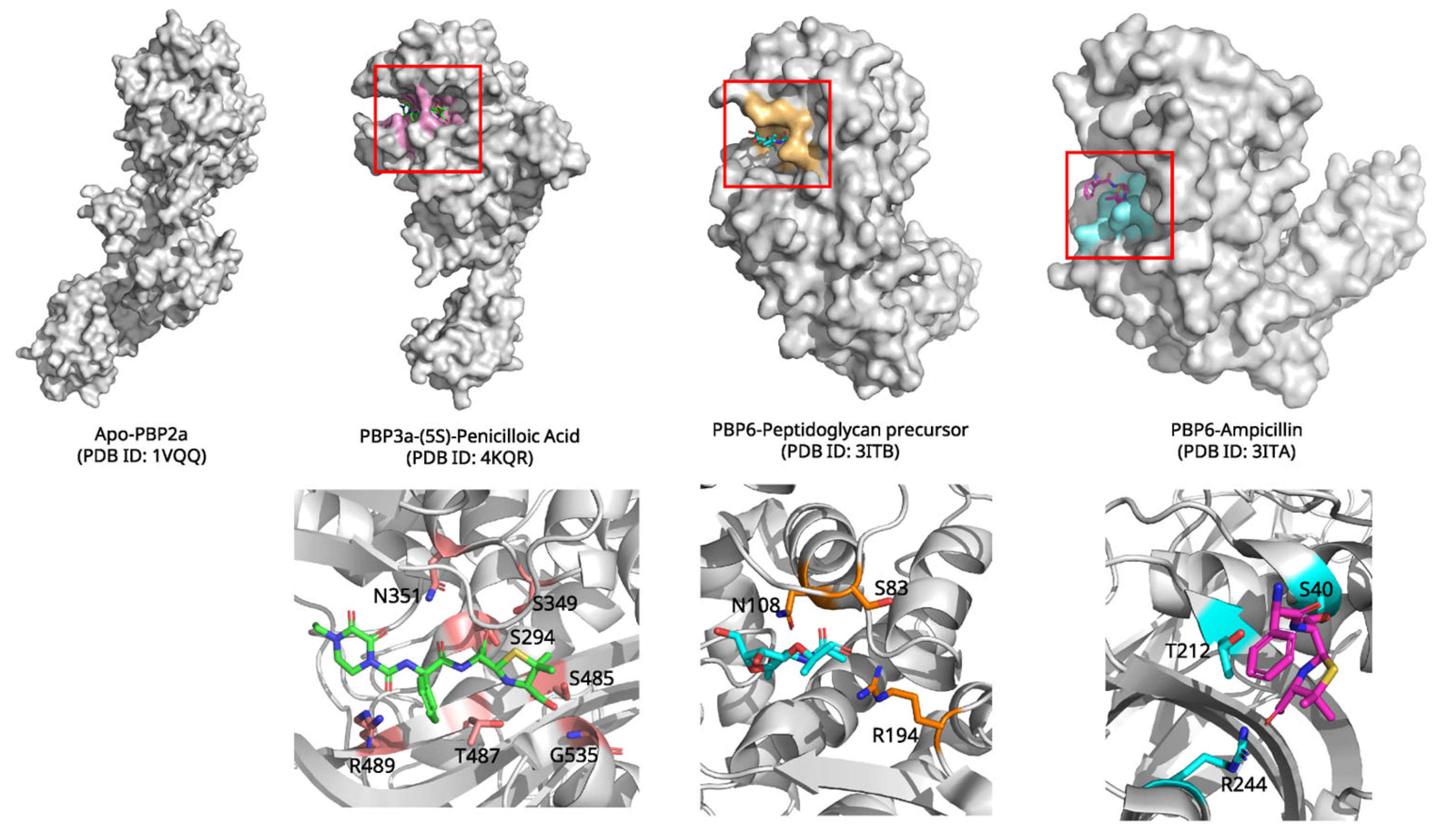
2.5. Penicillin and Penicillin-Binding Proteins: Inhibition of Bacterial Cell Wall Synthesis
3. Comparative Structural Analysis of Natural Products: Common Binding Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhan, F.; Zhu, J.; Xu, S.; Xu, J. The latest advances with natural products in drug discovery and opportunities for the future: A 2025 update. Expert. Opin. Drug Discov. 2025, 20, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Blundell, T.L.; Jhoti, H.; Abell, C. High-throughput crystallography for lead discovery in drug design. Nat. Rev. Drug Discov. 2002, 1, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C. The process of structure-based drug design. Chem. Biol. 2003, 10, 787–797. [Google Scholar] [CrossRef]
- Hauptman, P.J.; Kelly, R.A. Digitalis. Circulation 1999, 99, 1265–1270. [Google Scholar] [CrossRef]
- Lingrel, J.B. The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na,K-ATPase. Annu. Rev. Physiol. 2010, 72, 395–412. [Google Scholar] [CrossRef]
- Kanai, R.; Cornelius, F.; Ogawa, H.; Motoyama, K.; Vilsen, B.; Toyoshima, C. Binding of cardiotonic steroids to Na(+),K(+)-ATPase in the E2P state. Proc. Natl. Acad. Sci. USA 2021, 118, e2020438118. [Google Scholar] [CrossRef]
- Hilbers, F.; Kopec, W.; Isaksen, T.J.; Holm, T.H.; Lykke-Hartmann, K.; Nissen, P.; Khandelia, H.; Poulsen, H. Tuning of the Na,K-ATPase by the beta subunit. Sci. Rep. 2016, 6, 20442. [Google Scholar] [CrossRef]
- Ren, Y.; Wu, S.; Burdette, J.E.; Cheng, X.; Kinghorn, A.D. Structural Insights into the Interactions of Digoxin and Na(+)/K(+)-ATPase and Other Targets for the Inhibition of Cancer Cell Proliferation. Molecules 2021, 26, 3672. [Google Scholar] [CrossRef]
- Kanai, R.; Cornelius, F.; Vilsen, B.; Toyoshima, C. Cryoelectron microscopy of Na(+),K(+)-ATPase in the two E2P states with and without cardiotonic steroids. Proc. Natl. Acad. Sci. USA 2022, 119, e2123226119. [Google Scholar] [CrossRef] [PubMed]
- Prassas, I.; Diamandis, E.P. Novel therapeutic applications of cardiac glycosides. Nat. Rev. Drug Discov. 2008, 7, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.; Gregersen, J.L.; Yatime, L.; Nissen, P.; Fedosova, N.U. Structures and characterization of digoxin- and bufalin-bound Na+,K+-ATPase compared with the ouabain-bound complex. Proc. Natl. Acad. Sci. USA 2015, 112, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Akera, T.; Brody, T.M. The role of Na+,K+-ATPase in the inotropic action of digitalis. Pharmacol. Rev. 1977, 29, 187–220. [Google Scholar] [CrossRef]
- Altamirano, J.; Li, Y.; DeSantiago, J.; Piacentino, V., 3rd; Houser, S.R.; Bers, D.M. The inotropic effect of cardioactive glycosides in ventricular myocytes requires Na+-Ca2+ exchanger function. J. Physiol. 2006, 575, 845–854. [Google Scholar] [CrossRef]
- Blaustein, M.P. How does pressure overload cause cardiac hypertrophy and dysfunction? High-ouabain affinity cardiac Na(+) pumps are crucial. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H919–H930. [Google Scholar] [CrossRef]
- Tobert, J.A. Lovastatin and beyond: The history of the HMG-CoA reductase inhibitors. Nat. Rev. Drug Discov. 2003, 2, 517–526. [Google Scholar] [CrossRef]
- Schachter, M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: An update. Fundam. Clin. Pharmacol. 2005, 19, 117–125. [Google Scholar] [CrossRef]
- Serajuddin, A.T.; Ranadive, S.A.; Mahoney, E.M. Relative lipophilicities, solubilities, and structure-pharmacological considerations of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors pravastatin, lovastatin, mevastatin, and simvastatin. J. Pharm. Sci. 1991, 80, 830–834. [Google Scholar] [CrossRef]
- Istvan, E.S.; Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 2001, 292, 1160–1164. [Google Scholar] [CrossRef]
- Istvan, E.S.; Palnitkar, M.; Buchanan, S.K.; Deisenhofer, J. Crystal structure of the catalytic portion of human HMG-CoA reductase: Insights into regulation of activity and catalysis. EMBO J. 2000, 19, 819–830. [Google Scholar] [CrossRef]
- Gesto, D.S.; Pereira, C.M.S.; Cerqueira, N.; Sousa, S.F. An Atomic-Level Perspective of HMG-CoA-Reductase: The Target Enzyme to Treat Hypercholesterolemia. Molecules 2020, 25, 3891. [Google Scholar] [CrossRef]
- Ancuceanu, R.; Popovici, P.C.; Draganescu, D.; Busnatu, S.; Lascu, B.E.; Dinu, M. QSAR Regression Models for Predicting HMG-CoA Reductase Inhibition. Pharmaceuticals 2024, 17, 1448. [Google Scholar] [CrossRef] [PubMed]
- Alberts, A.W.; Chen, J.; Kuron, G.; Hunt, V.; Huff, J.; Hoffman, C.; Rothrock, J.; Lopez, M.; Joshua, H.; Harris, E.; et al. Mevinolin: A highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc. Natl. Acad. Sci. USA 1980, 77, 3957–3961. [Google Scholar] [CrossRef] [PubMed]
- Stancu, C.; Sima, A. Statins: Mechanism of action and effects. J. Cell Mol. Med. 2001, 5, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Sizar, O.; Khare, S.; Patel, P.; Talati, R. Statin Medications. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- D’Imprima, E.; Kuhlbrandt, W. Current limitations to high-resolution structure determination by single-particle cryoEM. Q. Rev. Biophys. 2021, 54, e4. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wang, Y.; He, B.; He, X.; Zhou, X.E.; Guo, S.; Rao, Q.; Yang, J.; Liu, J.; Zhou, Q.; et al. Molecular recognition of morphine and fentanyl by the human mu-opioid receptor. Cell 2022, 185, 4361–4375. [Google Scholar] [CrossRef]
- Huang, W.; Manglik, A.; Venkatakrishnan, A.J.; Laeremans, T.; Feinberg, E.N.; Sanborn, A.L.; Kato, H.E.; Livingston, K.E.; Thorsen, T.S.; Kling, R.C.; et al. Structural insights into micro-opioid receptor activation. Nature 2015, 524, 315–321. [Google Scholar] [CrossRef]
- Chen, J.; Gou, Q.; Chen, X.; Song, Y.; Zhang, F.; Pu, X. Exploring biased activation characteristics by molecular dynamics simulation and machine learning for the mu-opioid receptor. Phys. Chem. Chem. Phys. 2024, 26, 10698–10710. [Google Scholar] [CrossRef]
- Manglik, A.; Kruse, A.C.; Kobilka, T.S.; Thian, F.S.; Mathiesen, J.M.; Sunahara, R.K.; Pardo, L.; Weis, W.I.; Kobilka, B.K.; Granier, S. Crystal structure of the micro-opioid receptor bound to a morphinan antagonist. Nature 2012, 485, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Campomanes, P.; Kless, A.; Schapitz, I.; Wagener, M.; Koch, T.; Carloni, P. Structural Determinants for the Binding of Morphinan Agonists to the mu-Opioid Receptor. PLoS ONE 2015, 10, e0135998. [Google Scholar] [CrossRef]
- Koehl, A.; Hu, H.; Maeda, S.; Zhang, Y.; Qu, Q.; Paggi, J.M.; Latorraca, N.R.; Hilger, D.; Dawson, R.; Matile, H.; et al. Structure of the micro-opioid receptor-G(i) protein complex. Nature 2018, 558, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S. Peptidomimetics and Their Applications for Opioid Peptide Drug Discovery. Biomolecules 2022, 12, 1241. [Google Scholar] [CrossRef]
- Abrimian, A.; Kraft, T.; Pan, Y.X. Endogenous Opioid Peptides and Alternatively Spliced Mu Opioid Receptor Seven Transmembrane Carboxyl-Terminal Variants. Int. J. Mol. Sci. 2021, 22, 3779. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Prota, A.E.; Lucena-Agell, D.; Ma, Y.; Estevez-Gallego, J.; Li, S.; Bargsten, K.; Josa-Prado, F.; Altmann, K.H.; Gaillard, N.; Kamimura, S.; et al. Structural insight into the stabilization of microtubules by taxanes. eLife 2023, 12, e84791. [Google Scholar] [CrossRef]
- Sun, L.; Simmerling, C.; Ojima, I. Recent advances in the study of the bioactive conformation of taxol. ChemMedChem 2009, 4, 719–731. [Google Scholar] [CrossRef]
- Kellogg, E.H.; Hejab, N.M.A.; Howes, S.; Northcote, P.; Miller, J.H.; Diaz, J.F.; Downing, K.H.; Nogales, E. Insights into the Distinct Mechanisms of Action of Taxane and Non-Taxane Microtubule Stabilizers from Cryo-EM Structures. J. Mol. Biol. 2017, 429, 633–646. [Google Scholar] [CrossRef]
- Mitra, A.; Sept, D. Taxol allosterically alters the dynamics of the tubulin dimer and increases the flexibility of microtubules. Biophys. J. 2008, 95, 3252–3258. [Google Scholar] [CrossRef]
- Steinmetz, M.O.; Prota, A.E. Structure-based discovery and rational design of microtubule-targeting agents. Curr. Opin. Struct. Biol. 2024, 87, 102845. [Google Scholar] [CrossRef]
- Bozdaganyan, M.; Fedorov, V.; Kholina, E.; Kovalenko, I.; Gudimchuk, N.; Orekhov, P. Exploring tubulin-paclitaxel binding modes through extensive molecular dynamics simulations. Sci. Rep. 2025, 15, 8378. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, A.; Song, Y.; Liu, N.; Wang, J.; Li, Y.; Liang, X.; Li, G.; Chu, H.; Wang, H.W. Structural insights into the mechanism of GTP initiation of microtubule assembly. Nat. Commun. 2023, 14, 5980. [Google Scholar] [CrossRef]
- Prota, A.E.; Danel, F.; Bachmann, F.; Bargsten, K.; Buey, R.M.; Pohlmann, J.; Reinelt, S.; Lane, H.; Steinmetz, M.O. The novel microtubule-destabilizing drug BAL27862 binds to the colchicine site of tubulin with distinct effects on microtubule organization. J. Mol. Biol. 2014, 426, 1848–1860. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, R.B.; Gigant, B.; Curmi, P.A.; Jourdain, I.; Lachkar, S.; Sobel, A.; Knossow, M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004, 428, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Gigant, B.; Wang, C.; Ravelli, R.B.; Roussi, F.; Steinmetz, M.O.; Curmi, P.A.; Sobel, A.; Knossow, M. Structural basis for the regulation of tubulin by vinblastine. Nature 2005, 435, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.; Li, H.; Downing, K.H.; Nogales, E. Refined structure of alpha beta-tubulin at 3.5 A resolution. J. Mol. Biol. 2001, 313, 1045–1057. [Google Scholar] [CrossRef]
- Diaz, J.F.; Andreu, J.M. Assembly of purified GDP-tubulin into microtubules induced by taxol and taxotere: Reversibility, ligand stoichiometry, and competition. Biochemistry 1993, 32, 2747–2755. [Google Scholar] [CrossRef]
- Amos, L.A.; Lowe, J. How Taxol stabilises microtubule structure. Chem. Biol. 1999, 6, R65–R69. [Google Scholar] [CrossRef]
- Steinmetz, M.O.; Prota, A.E. Microtubule-Targeting Agents: Strategies To Hijack the Cytoskeleton. Trends Cell Biol. 2018, 28, 776–792. [Google Scholar] [CrossRef]
- Tipper, D.J.; Strominger, J.L. Mechanism of action of penicillins: A proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc. Natl. Acad. Sci. USA 1965, 54, 1133–1141. [Google Scholar] [CrossRef]
- van Berkel, S.S.; Nettleship, J.E.; Leung, I.K.; Brem, J.; Choi, H.; Stuart, D.I.; Claridge, T.D.; McDonough, M.A.; Owens, R.J.; Ren, J.; et al. Binding of (5S)-penicilloic acid to penicillin binding protein 3. ACS Chem. Biol. 2013, 8, 2112–2116. [Google Scholar] [CrossRef]
- Sainsbury, S.; Bird, L.; Rao, V.; Shepherd, S.M.; Stuart, D.I.; Hunter, W.N.; Owens, R.J.; Ren, J. Crystal structures of penicillin-binding protein 3 from Pseudomonas aeruginosa: Comparison of native and antibiotic-bound forms. J. Mol. Biol. 2011, 405, 173–184. [Google Scholar] [CrossRef]
- Ghuysen, J.M. Serine beta-lactamases and penicillin-binding proteins. Annu. Rev. Microbiol. 1991, 45, 37–67. [Google Scholar] [CrossRef]
- Lim, D.; Strynadka, N.C. Structural basis for the beta lactam resistance of PBP2a from methicillin-resistant Staphylococcus aureus. Nat. Struct. Biol. 2002, 9, 870–876. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Shi, Q.; Hesek, D.; Lee, M.; Mobashery, S.; Shoichet, B.K. Crystal structures of penicillin-binding protein 6 from Escherichia coli. J. Am. Chem. Soc. 2009, 131, 14345–14354. [Google Scholar] [CrossRef] [PubMed]
- Papp-Wallace, K.M.; Endimiani, A.; Taracila, M.A.; Bonomo, R.A. Carbapenems: Past, present, and future. Antimicrob. Agents Chemother. 2011, 55, 4943–4960. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Jacoby, G.A. Updated functional classification of beta-lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Flanders, P.L.; Contreras-Martel, C.; Brown, N.W.; Shirley, J.D.; Martins, A.; Nauta, K.N.; Dessen, A.; Carlson, E.E.; Ambrose, E.A. Combined Structural Analysis and Molecular Dynamics Reveal Penicillin-Binding Protein Inhibition Mode with beta-Lactones. ACS Chem. Biol. 2022, 17, 3110–3120. [Google Scholar] [CrossRef]
- Laursen, M.; Yatime, L.; Nissen, P.; Fedosova, N.U. Crystal structure of the high-affinity Na+K+-ATPase-ouabain complex with Mg2+ bound in the cation binding site. Proc. Natl. Acad. Sci. USA 2013, 110, 10958–10963. [Google Scholar] [CrossRef]
- Morth, J.P.; Pedersen, B.P.; Toustrup-Jensen, M.S.; Sorensen, T.L.; Petersen, J.; Andersen, J.P.; Vilsen, B.; Nissen, P. Crystal structure of the sodium-potassium pump. Nature 2007, 450, 1043–1049. [Google Scholar] [CrossRef]
- Rasmussen, S.G.; DeVree, B.T.; Zou, Y.; Kruse, A.C.; Chung, K.Y.; Kobilka, T.S.; Thian, F.S.; Chae, P.S.; Pardon, E.; Calinski, D.; et al. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature 2011, 477, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Alushin, G.M.; Lander, G.C.; Kellogg, E.H.; Zhang, R.; Baker, D.; Nogales, E. High-resolution microtubule structures reveal the structural transitions in alphabeta-tubulin upon GTP hydrolysis. Cell 2014, 157, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Hong, J. Role of natural product diversity in chemical biology. Curr. Opin. Chem. Biol. 2011, 15, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Van Drie, J.H.; Tong, L. Cryo-EM as a powerful tool for drug discovery. Bioorg Med. Chem. Lett. 2020, 30, 127524. [Google Scholar] [CrossRef]
- Murphy, C.; Deplazes, E.; Cranfield, C.G.; Garcia, A. The Role of Structure and Biophysical Properties in the Pleiotropic Effects of Statins. Int. J. Mol. Sci. 2020, 21, 8745. [Google Scholar] [CrossRef]
- Kise, R.; Inoue, A. GPCR signaling bias: An emerging framework for opioid drug development. J. Biochem. 2024, 175, 367–376. [Google Scholar] [CrossRef]
- Wilke, M.S.; Lovering, A.L.; Strynadka, N.C. Beta-lactam antibiotic resistance: A current structural perspective. Curr. Opin. Microbiol. 2005, 8, 525–533. [Google Scholar] [CrossRef]
- Renaud, J.P.; Chari, A.; Ciferri, C.; Liu, W.T.; Remigy, H.W.; Stark, H.; Wiesmann, C. Cryo-EM in drug discovery: Achievements, limitations and prospects. Nat. Rev. Drug Discov. 2018, 17, 471–492. [Google Scholar] [CrossRef]
- Ferreira, F.J.N.; Carneiro, A.S. AI-Driven Drug Discovery: A Comprehensive Review. ACS Omega 2025, 10, 23889–23903. [Google Scholar] [CrossRef]
- Seshadri, K.; Abad, A.N.D.; Nagasawa, K.K.; Yost, K.M.; Johnson, C.W.; Dror, M.J.; Tang, Y. Synthetic Biology in Natural Product Biosynthesis. Chem. Rev. 2025, 125, 3814–3931. [Google Scholar] [CrossRef]
- Torino, S.; Dhurandhar, M.; Stroobants, A.; Claessens, R.; Efremov, R.G. Time-resolved cryo-EM using a combination of droplet microfluidics with on-demand jetting. Nat. Methods 2023, 20, 1400–1408. [Google Scholar] [CrossRef]
- Hartmann, A.; Sreenivasa, K.; Schenkel, M.; Chamachi, N.; Schake, P.; Krainer, G.; Schlierf, M. An automated single-molecule FRET platform for high-content, multiwell plate screening of biomolecular conformations and dynamics. Nat. Commun. 2023, 14, 6511. [Google Scholar] [CrossRef]
- Kurzeder, C.; Nguyen-Strauli, B.D.; Krol, I.; Ring, A.; Castro-Giner, F.; Nuesch, M.; Asawa, S.; Zhang, Y.W.; Budinjas, S.; Gvozdenovic, A.; et al. Digoxin for reduction of circulating tumor cell cluster size in metastatic breast cancer: A proof-of-concept trial. Nat. Med. 2025, 31, 1120–1124. [Google Scholar] [CrossRef]
- Zeng, W.; Deng, H.; Luo, Y.; Zhong, S.; Huang, M.; Tomlinson, B. Advances in statin adverse reactions and the potential mechanisms: A systematic review. J. Adv. Res. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Lagunas-Rangel, F.A.; Liepinsh, E.; Fredriksson, R.; Alsehli, A.M.; Williams, M.J.; Dambrova, M.; Jonsson, J.; Schioth, H.B. Off-target effects of statins: Molecular mechanisms, side effects and the emerging role of kinases. Br. J. Pharmacol. 2024, 181, 3799–3818. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Kumar, A.; Zhang, X.; Martin, C.; Van Holsbeeck, K.; Raia, P.; Koehl, A.; Laeremans, T.; Steyaert, J.; Manglik, A.; et al. Structural basis of mu-opioid receptor targeting by a nanobody antagonist. Nat. Commun. 2024, 15, 8687. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.E.; Juechter, L.A.; Rocha, J.; Jones, L.D.; Outten, B.; Aishman, T.D.; Ivers, A.R.; Shields, G.C. Impact of Intracellular Proteins on mu-Opioid Receptor Structure and Ligand Binding. J. Phys. Chem. B 2025, 129, 71–87. [Google Scholar] [CrossRef]
- Fairbanks, C.A.; Peterson, C.D. The opioid receptor: Emergence through millennia of pharmaceutical sciences. Front Pain Res 2023, 4, 960389. [Google Scholar] [CrossRef]
- Chew, Y.M.; Cross, R.A. Taxol acts differently on different tubulin isotypes. Commun. Biol. 2023, 6, 946. [Google Scholar] [CrossRef]
- Hunashal, Y.; Kumar, G.S.; Choy, M.S.; D’Andrea, E.D.; Da Silva Santiago, A.; Schoenle, M.V.; Desbonnet, C.; Arthur, M.; Rice, L.B.; Page, R.; et al. Molecular basis of beta-lactam antibiotic resistance of ESKAPE bacterium E. faecium Penicillin Binding Protein PBP5. Nat. Commun. 2023, 14, 4268. [Google Scholar] [CrossRef]
- Sethuvel, D.P.M.; Bakthavatchalam, Y.D.; Karthik, M.; Irulappan, M.; Shrivastava, R.; Periasamy, H.; Veeraraghavan, B. beta-Lactam Resistance in ESKAPE Pathogens Mediated Through Modifications in Penicillin-Binding Proteins: An Overview. Infect. Dis. Ther. 2023, 12, 829–841. [Google Scholar] [CrossRef]
- Lavollay, M.; Buon, C.; Le Moigne, V.; Compain, F.; Guyonvarch, A.; Fonvielle, M. Exploration of the role of the penicillin binding protein 2c (Pbp2c) in inducible beta-lactam resistance in Corynebacteriaceae. Front. Microbiol. 2024, 15, 1327723. [Google Scholar] [CrossRef]
- Joshi, P.; Vendruscolo, M. Druggability of Intrinsically Disordered Proteins. Adv. Exp. Med. Biol. 2015, 870, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Kunakom, S.; Eustaquio, A.S. Natural Products and Synthetic Biology: Where We Are and Where We Need to Go. mSystems 2019, 4, 10–1128. [Google Scholar] [CrossRef] [PubMed]

| Drug | Protein Target | Binding Mechanism | PDB ID (Resolution, Method) | Structural Insights |
|---|---|---|---|---|
| Digoxin | Na+/K+-ATPase | Conformational selection; stabilizes E2P state, blocks catalytic cycle transitions | 7DDH (3.46 Å, X-ray) | Binds preformed cavity (M1, M2, M4-M6); hydrophobic contacts and H-bonds (Thr797, Gly319) |
| Simvastatin | HMG-CoA Reductase | Competitive inhibition; substrate mimicry | 1HW9 (2.30 Å, X-ray) | Hydroxy acid moiety mimics HMG; H-bonds with Lys735, Ser684, Asp690; hydrophobic decalin ring interactions (Leu562, Val683, etc.) |
| Morphine | μ-Opioid Receptor | Induced fit; receptor activation via TM6 displacement | 8EF6 (3.20 Å, Cryo-EM) | Ionic bond with Asp149; H-bonds with Tyr150, His299; stabilizes active conformation enabling G-protein/p-arrestin recruitment |
| Paclitaxel | β-Tubulin | Conformational selection; stabilizes microtubule assembly | 8BDF (1.90 Å, X-ray) | Binds taxane site (M-loop, H7); H-bonds with His229, Arg369; stabilizes lateral protofilament contacts |
| Penicillin | Penicillin-Binding Proteins | Covalent inhibition; acyl-enzyme intermediate formation | 4KQR (2.10 Å, X-ray) | Forms covalent bond with Ser294; blocks peptidoglycan cross-linking; P3–P4 loop rearrangements explain inhibition/selectivity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Kim, Y.; Boo, H.J.; Yoon, D.; Cha, J.S.; Yoo, J. Natural Product-Derived Drugs: Structural Insights into Their Biological Mechanisms. Biomolecules 2025, 15, 1303. https://doi.org/10.3390/biom15091303
Choi Y, Kim Y, Boo HJ, Yoon D, Cha JS, Yoo J. Natural Product-Derived Drugs: Structural Insights into Their Biological Mechanisms. Biomolecules. 2025; 15(9):1303. https://doi.org/10.3390/biom15091303
Chicago/Turabian StyleChoi, Yujeong, Younghyun Kim, Hye Joon Boo, Danbi Yoon, Jeong Seok Cha, and Jiho Yoo. 2025. "Natural Product-Derived Drugs: Structural Insights into Their Biological Mechanisms" Biomolecules 15, no. 9: 1303. https://doi.org/10.3390/biom15091303
APA StyleChoi, Y., Kim, Y., Boo, H. J., Yoon, D., Cha, J. S., & Yoo, J. (2025). Natural Product-Derived Drugs: Structural Insights into Their Biological Mechanisms. Biomolecules, 15(9), 1303. https://doi.org/10.3390/biom15091303






