Nature’s Synergy: Cellular and Molecular Evaluation of Snail Slime and Its Principal Component, Glycolic Acid, on Keratinocytes, with Preliminary Evidence from Endothelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. SS Extraction
2.2. Cell Culture
2.3. Cell Treatments
2.4. MTT Assay
2.5. Lactate Dehydrogenase (LDH) Cytotoxicity Assay
2.6. Flow Cytometry Analysis of ROS Release
2.7. Western Blot Analysis
2.8. RNA Extraction
2.9. Reverse Transcription and Real-Time Polymerase Chain Reaction (RT-PCR)
2.10. IL-6 Secretion ELISA Assay
2.11. Wound Healing Assay
2.12. Statistical Analysis
3. Results
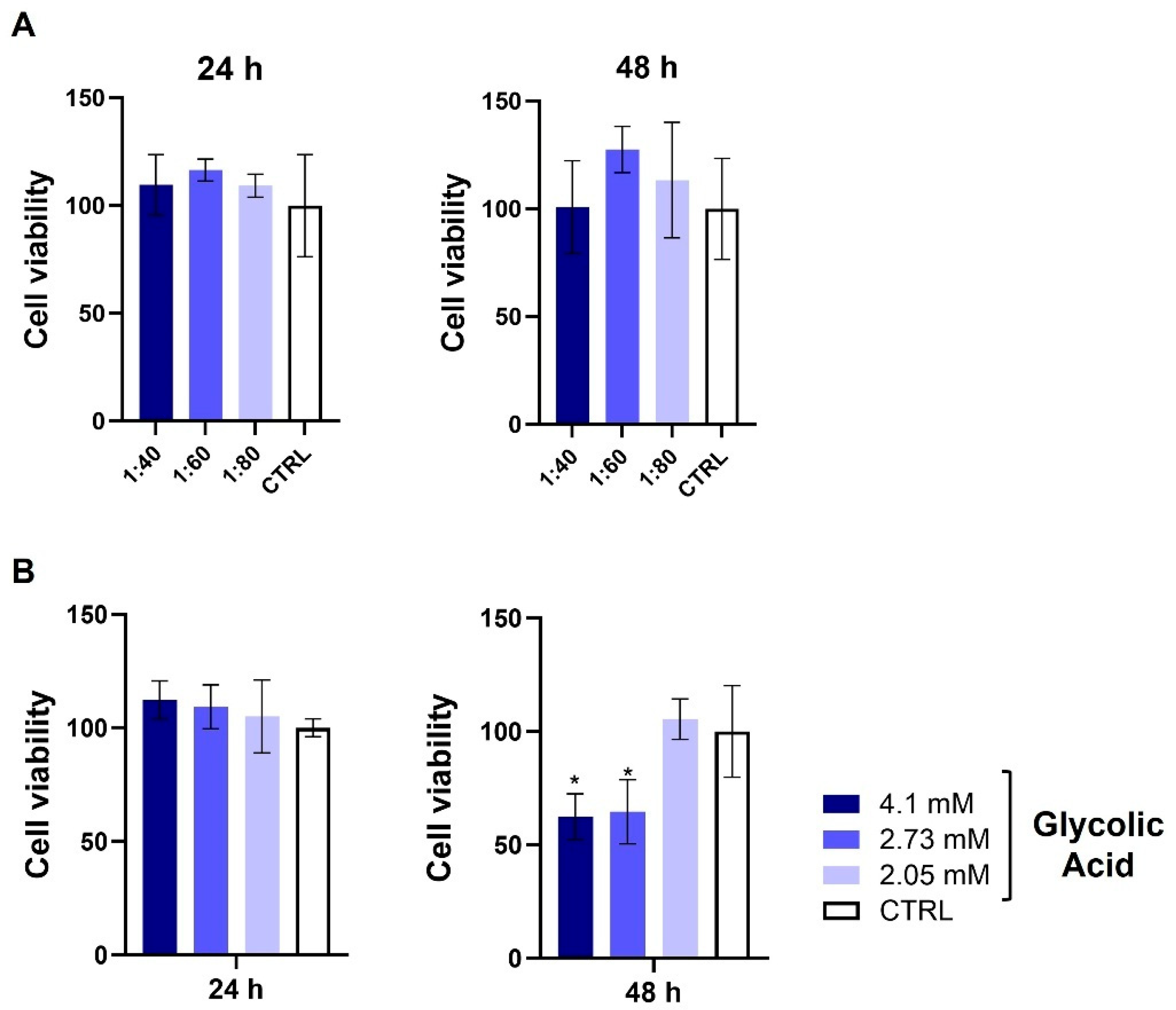
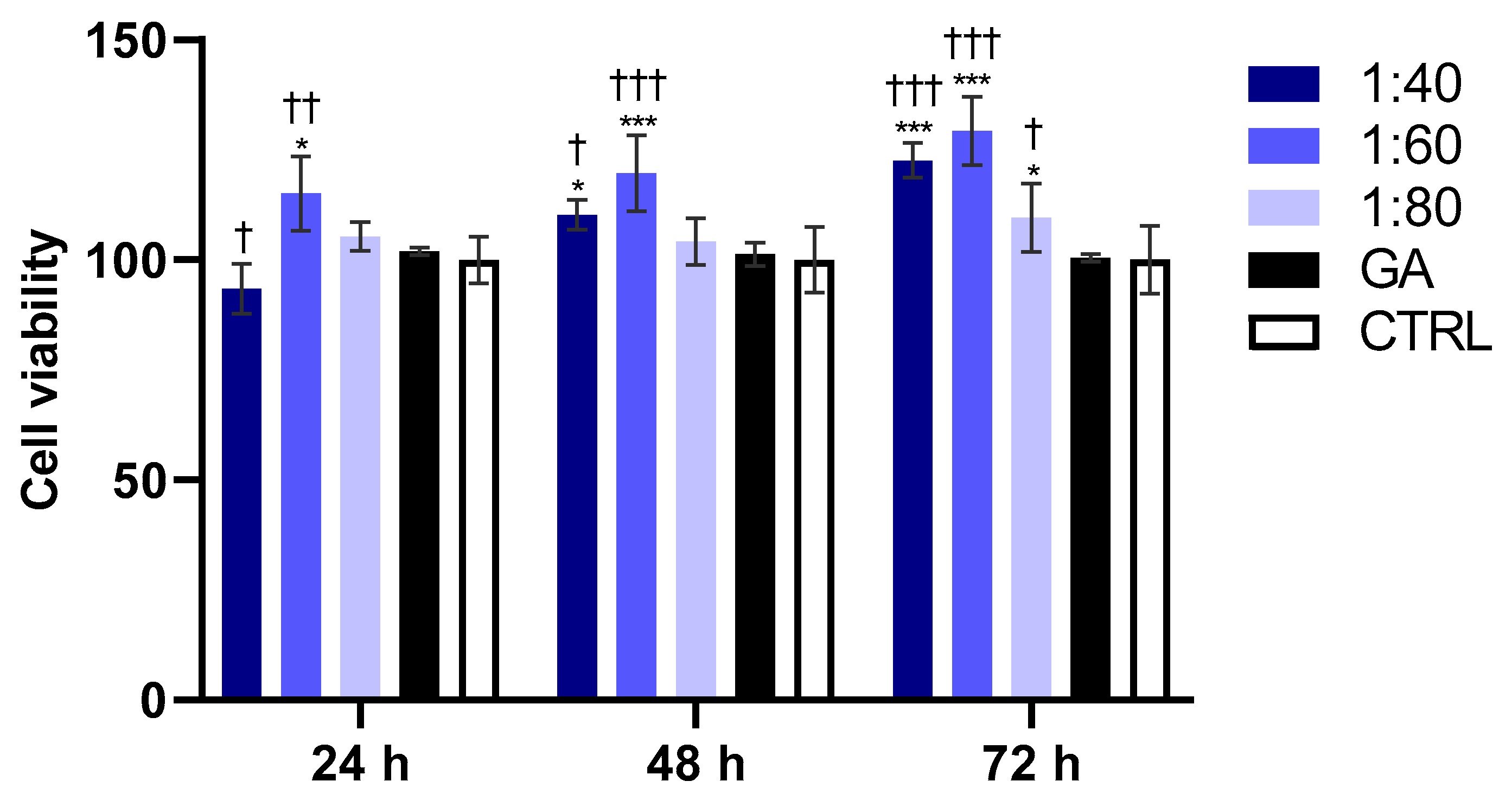
3.1. Assessment of HaCat Viability After SS and GA Treatment
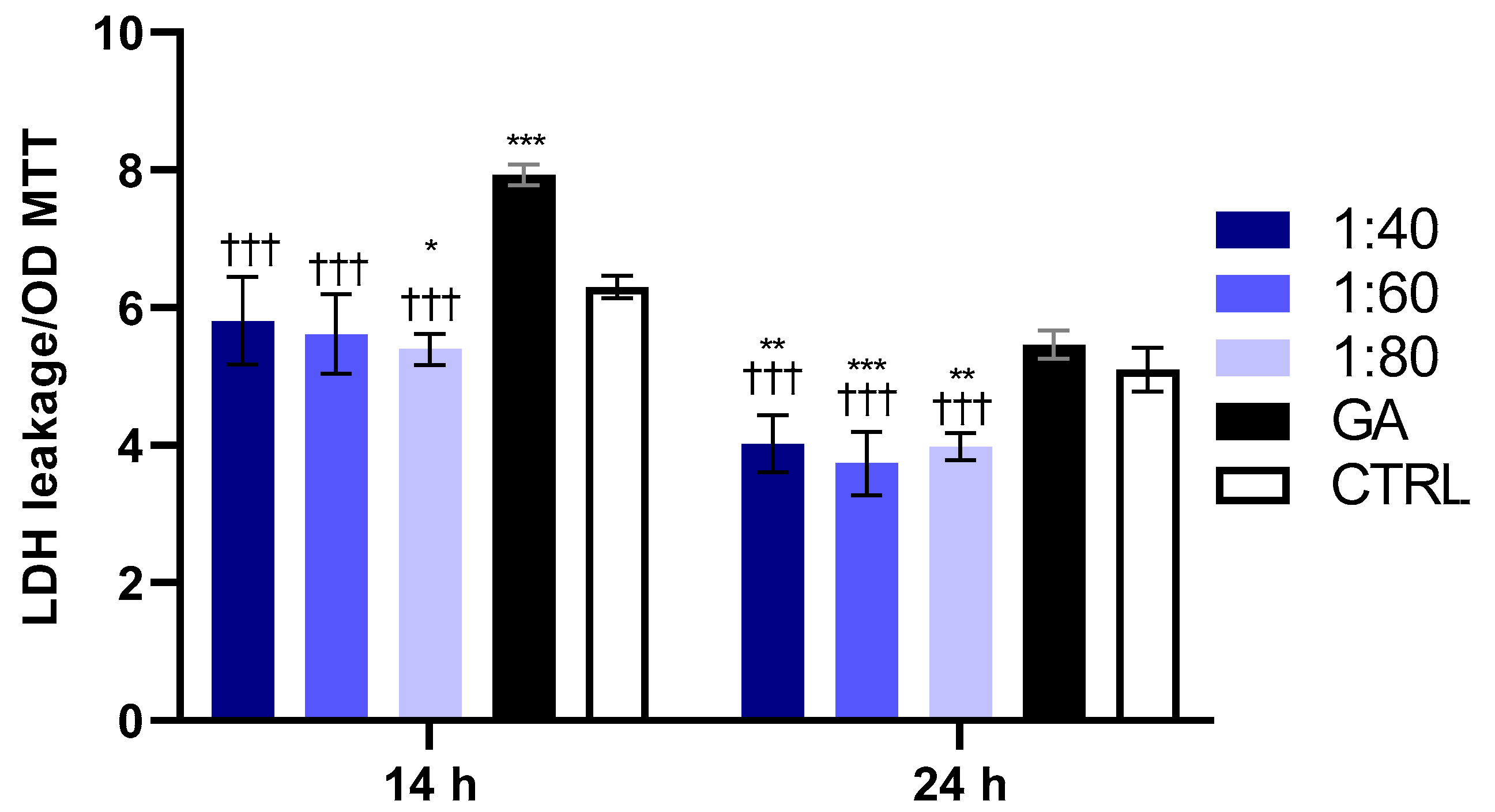
3.2. Evaluation of GA’s and SS’s Effects on HaCaT Cytotoxicity
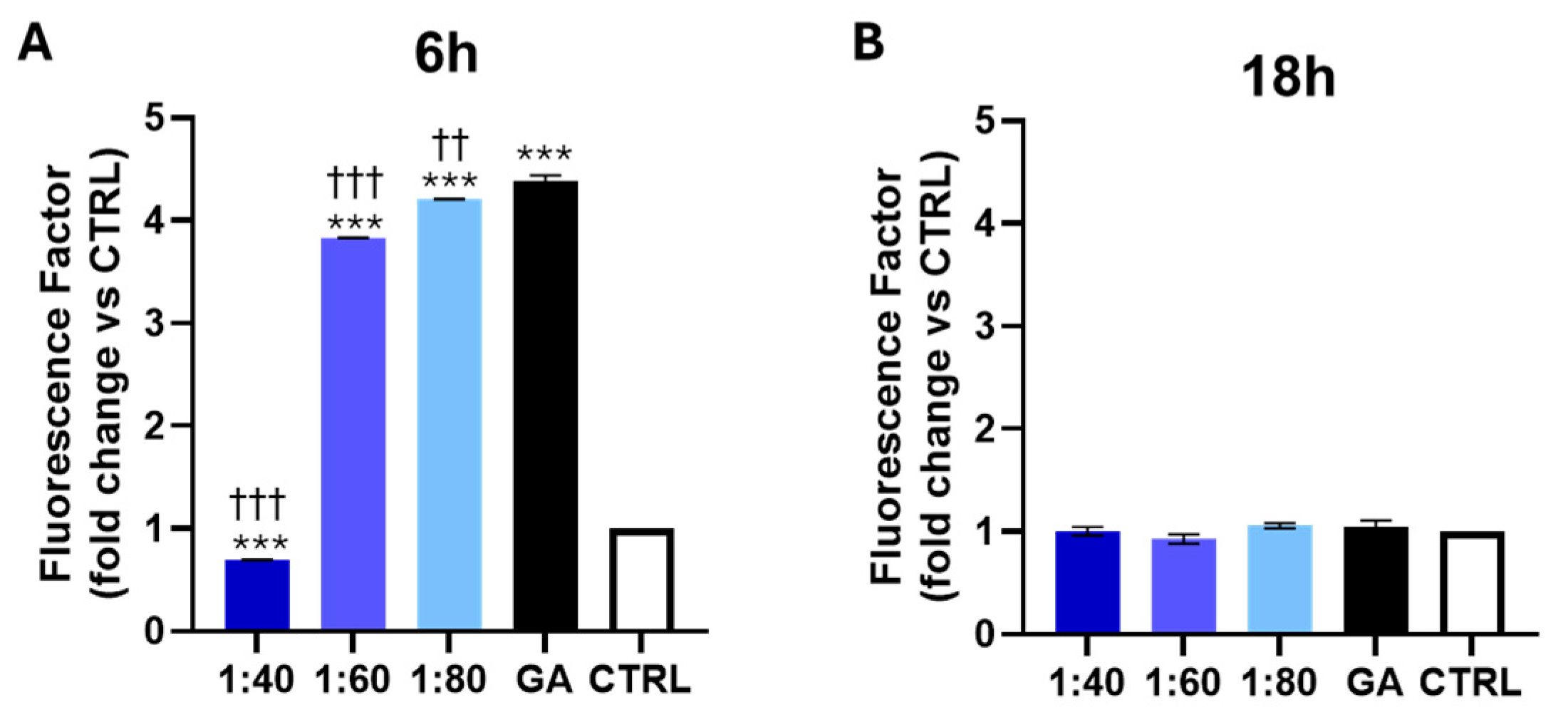
3.3. Measurement of ROS Level After SS and GA Treatment
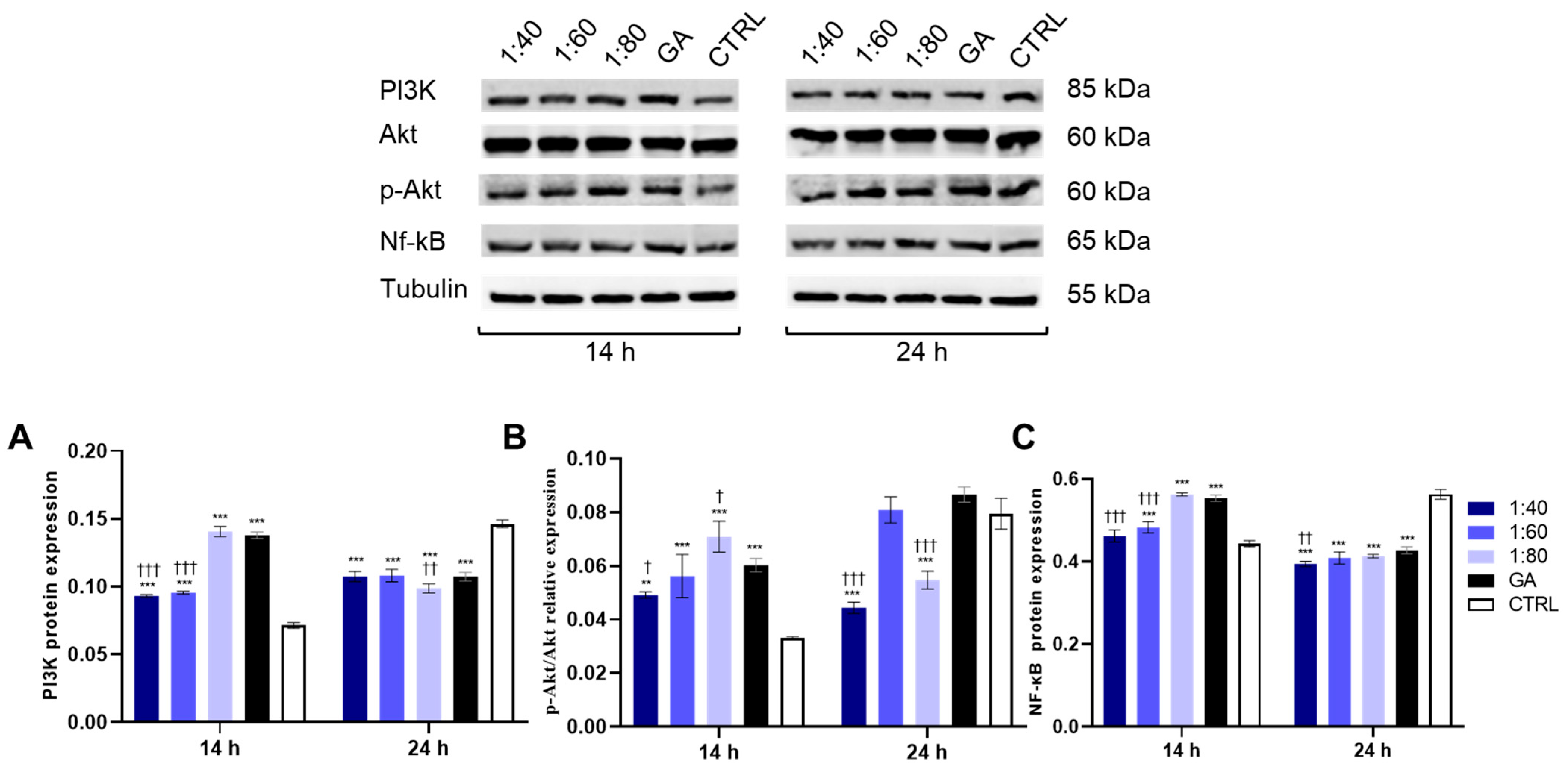
3.4. Effect of SS and GA Treatment on PI3K/Akt/NF-κB Protein Levels
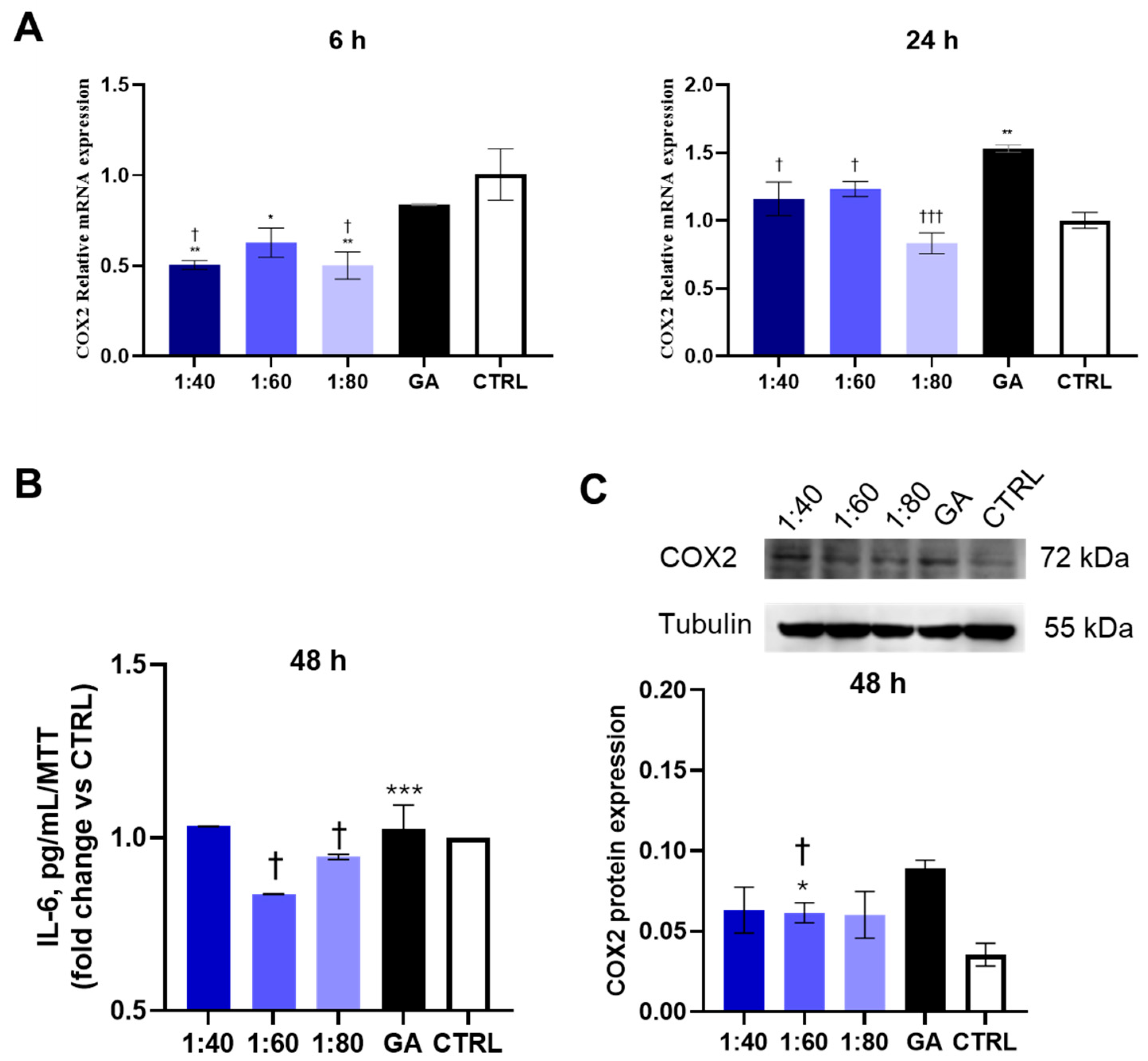
3.5. Assessment of Inflammatory Response
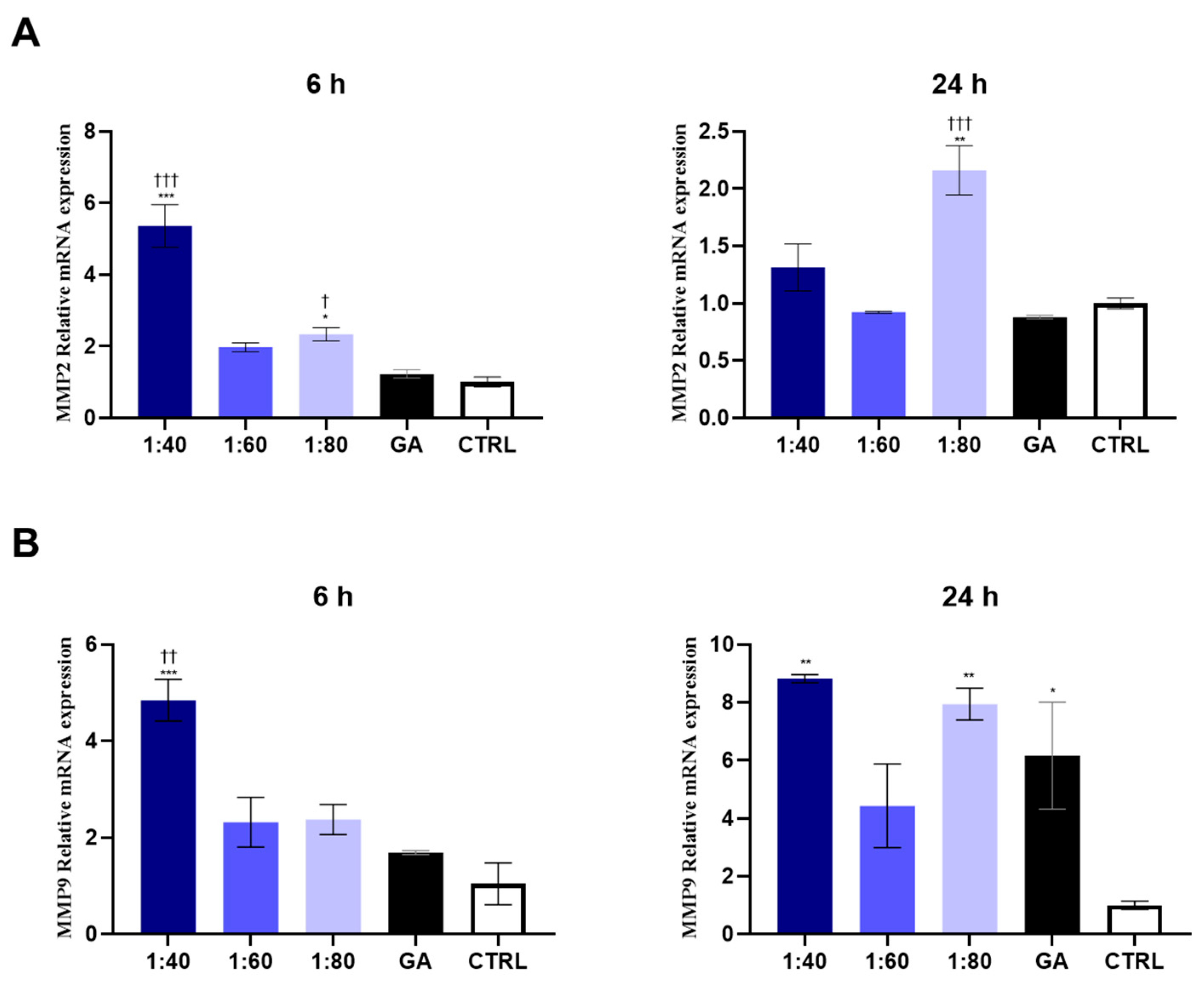
3.6. Analysis of MMP Gene Expression in Response to SS and GA Treatment
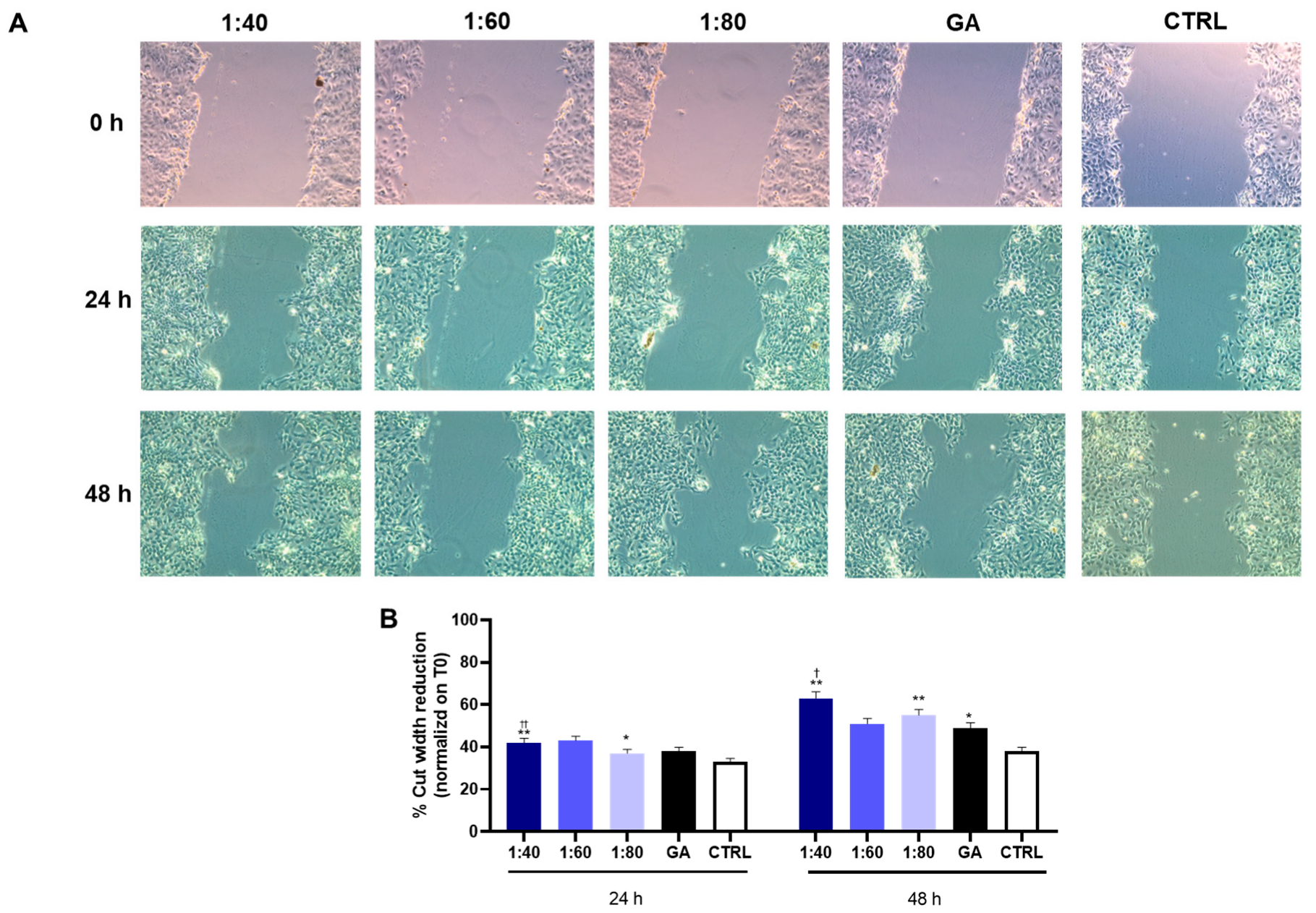
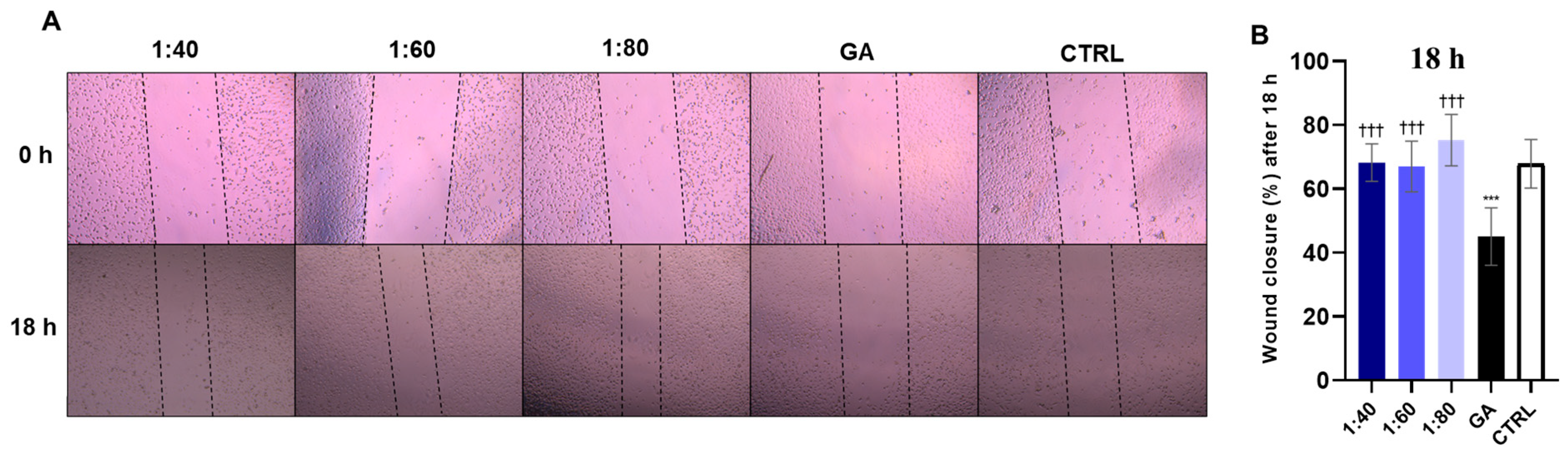
3.7. Wound Healing Assessment in HaCaT Cells After SS and GA Treatment
3.8. Evaluation of Endothelial Cell Viability
3.9. Wound Healing Assessment in Endothelial Cells After SS and GA Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cilia, G.; Fratini, F. Antimicrobial properties of terrestrial snail and slug mucus. J. Complement. Integr. Med. 2018, 15, 20170168. [Google Scholar] [CrossRef]
- Rashad, M.; Sampò, S.; Cataldi, A.; Zara, S. Biological activities of gastropods secretions: Snail and slug slime. Nat. Prod. Bioprospecting 2023, 13, 42. [Google Scholar] [CrossRef]
- Adikwu, M.; Nnamani, P. Some physiological and toxicological properties of snail mucin extracted from Archachatina marginata. Bio-Res. 2006, 3, 1–6. [Google Scholar] [CrossRef]
- Barajas-Ledesma, E.; Holland, C. Probing the compositional and rheological properties of gastropod locomotive mucus. Front. Soft Matter 2023, 3, 1201511. [Google Scholar] [CrossRef]
- Olaniyan, O.T.; Adetunji, C.O. Biological, Biochemical, and Biodiversity of Biomolecules from Marine-Based Beneficial Microorganisms: Industrial Perspective; Adetunji, C.O., Panpatte, D.G., Jhala, Y.K., Eds.; Springer: Singapore, 2021; Volume 27, pp. 57–81. [Google Scholar]
- Rashad, M.; Sampò, S.; Cataldi, A.; Zara, S. From Nature to Nurture: The Science and Applications of Snail Slime in Health and Beauty. J. Cosmet. Dermatol. 2025, 24, e70002. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Zhang, Z.; Li, G.; Sun, J.; Gu, T.; Ain, N.U.; Zhang, X.; Li, D. Extraction, structure, pharmacological activities and applications of polysaccharides and proteins isolated from snail mucus. Int. J. Biol. Macromol. 2023, 258, 128878. [Google Scholar] [CrossRef]
- Liegertová, M.; Malý, J. Gastropod Mucus: Interdisciplinary Perspectives on Biological Activities, Applications, and Strategic Priorities. ACS. Biomater. Sci. Eng. 2023, 9, 5567–5579. [Google Scholar] [CrossRef]
- Cabibbo, M.; Scialabba, C.; Drago, S.E.; Craparo, E.F.; Cavallaro, G. From nature to medicine: Snail slime-based functional excipients for oral dosage forms. Int. J. Pharm. 2025, 682, 125914. [Google Scholar] [CrossRef]
- Rattanawiwatpong, P.; Wanitphakdeedecha, R.; Bumrungpert, A.; Maiprasert, M. Anti-aging and brightening effects of a topical treatment containing vitamin C, vitamin E, and raspberry leaf cell culture extract: A split-face, randomized controlled trial. J. Cosmet. Dermatol. 2020, 19, 671–676. [Google Scholar] [CrossRef]
- Dinica, R.M.; Sandu, C.; Botezatu, A.V.D.; Busuioc, A.C.; Balanescu, F.; Mihaila, M.D.I.; Dumitru, C.N.; Furdui, B.; Iancu, A.V. Allantoin from Valuable Romanian Animal and Plant Sources with Promising Anti-Inflammatory Activity as a Nutricosmetic Ingredient. Sustainability 2021, 13, 10170. [Google Scholar] [CrossRef]
- Kim, Y.; Sim, W.-J.; Lee, J.-S.; Lim, T.-G. Snail mucin is a functional food ingredient for skin. J. Funct. Foods 2022, 92, 105053. [Google Scholar] [CrossRef]
- Kim, K.H.; Chung, C.B.; Kim, Y.H.; Kim, K.S.; Han, C.S.; Kim, C.H. Cosmeceutical properties of levan produced by Zy-momonas mobilis. J. Cosmet. Sci. 2005, 56, 395–406. [Google Scholar] [CrossRef]
- Narda, M.; Trullas, C.; Brown, A.; Piquero-Casals, J.; Granger, C.; Fabbrocini, G. Glycolic acid adjusted to pH 4 stimulates collagen production and epidermal renewal without affecting levels of proinflammatory TNF-alpha in human skin explants. J. Cosmet. Dermatol. 2020, 20, 513–521. [Google Scholar] [CrossRef]
- Van Scott, E.J.; Ditre, C.M.; Yu, R.J. Alpha-hydroxyacids in the treatment of signs of photoaging. Clin. Dermatol. 1996, 14, 217–226. [Google Scholar] [CrossRef]
- Tsoutsos, D.; Kakagia, D.; Tamparopoulos, K. The efficacy of Helix. aspersa Müller extract in the healing of partial thickness burns: A novel treatment for open burn management protocols. J. Dermatol. Treat. 2009, 20, 219–222. [Google Scholar] [CrossRef]
- Brieva, A.; Philips, N.; Tejedor, R.; Guerrero, A.; Pivel, J.P.; Alonso-Lebrero, J.L.; Gonzalez, S. Molecular basis for the re-generative properties of a secretion of the mollusk Cryptomphalus aspersa. Skin Pharmacol. Physiol. 2008, 21, 15–22. [Google Scholar] [CrossRef]
- Dhiman, V.; Pant, D. Human health and snails. J. Immunoass. Immunochem. 2020, 42, 211–235. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.; Cerullo, A.R.; Parziale, J.; Achrak, E.; Sultana, S.; Ferd, J.; Samad, S.; Deng, W.; Braunschweig, A.B.; Holford, M. Advancing Discovery of Snail Mucins Function and Application. Front. Bioeng. Biotechnol. 2021, 9, 734023. [Google Scholar] [CrossRef]
- Ricci, A.; Gallorini, M.; Feghali, N.; Sampò, S.; Cataldi, A.; Zara, S. Snail Slime Extracted by a Cruelty Free Method Preserves Viability and Controls Inflammation Occurrence: A Focus on Fibroblasts. Molecules 2023, 28, 1222. [Google Scholar] [CrossRef] [PubMed]
- Pitt, S.J.; Graham, M.A.; Dedi, C.G.; Taylor-Harris, P.M.; Gunn, A. Antimicrobial properties of mucus from the brown garden snail Helix aspersa. Br. J. Biomed. Sci. 2015, 72, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wang, J.; Chen, X.; Shi, Y.; Xie, J. Epidermal Stem Cells in Wound Healing and Regeneration. Stem Cells Int. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Germolec, D.R.; Shipkowski, K.A.; Frawley, R.P.; Evans, E. Markers of Inflammation. Methods Mol. Biol. 2018, 1803, 57–79. [Google Scholar] [CrossRef]
- De Colli, M.; Tortorella, P.; Marconi, G.D.; Agamennone, M.; Campestre, C.; Tauro, M.; Cataldi, A.; Zara, S. In vitro comparison of new bisphosphonic acids and zoledronate effects on human gingival fibroblasts viability, inflammation and matrix turnover. Clin. Oral Investig. 2015, 20, 2013–2021. [Google Scholar] [CrossRef]
- di Giacomo, V.; Balaha, M.; Pinti, M.; Di Marcantonio, M.C.; Cela, I.; Acharya, T.R.; Kaushik, N.K.; Choi, E.H.; Mincione, G.; Sala, G.; et al. Cold atmospheric plasma activated media selectively affects human head and neck cancer cell lines. Oral. Dis. 2024, 31, 401–416. [Google Scholar] [CrossRef]
- Rizzi, V.; Gubitosa, J.; Fini, P.; Nuzzo, S.; Agostiano, A.; Cosma, P. Snail slime-based gold nanoparticles: An interesting potential ingredient in cosmetics as an antioxidant, sunscreen, and tyrosinase inhibitor. J. Photochem. Photobiol. B Biol. 2021, 224, 112309. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Fini, P.; Fanelli, F.; Sibillano, T.; Corriero, N.; Cosma, P. Chitosan/snail slime films as multifunctional platforms for potential biomedical and cosmetic applications: Physical and chemical characterization. J. Mater. Chem. B 2022, 11, 2638–2649. [Google Scholar] [CrossRef]
- Agada, D.E.; Sar, T.T.; Ujoh, J.A.; Ameh, L.O. Antibacterial susceptibility of staphylococcus aureus, salmonella typhi, bacillus subtilis and escherichia coli to snail slime. Afr. Health Sci. 2023, 23, 177–182. [Google Scholar] [CrossRef]
- Aouji, M.; Rkhaila, A.; Bouhaddioui, B.; Zirari, M.; Harifi, H.; Taboz, Y.; Lrhorfi, L.A.; Bengueddour, R. Chemical composi-tion, mineral profile, anti-bacterial, and wound healing properties of snail slime of Helix aspersa Müller. Biomedicine 2023, 13, 10–19. [Google Scholar] [CrossRef]
- Ellijimi, C.; Ben Hammouda, M.; Othman, H.; Moslah, W.; Jebali, J.; Ben Mabrouk, H.; Morjen, M.; Haoues, M.; Luis, J.; Marrakchi, N.; et al. Helix aspersa maxima mucus exhibits antimelanogenic and antitumoral effects against melanoma cells. Biomed. Pharmacother. 2018, 101, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, M.F.; Albertini, B.; Dolci, L.S.; Bonvicini, F.; Bigi, A.; Gentilomi, G.A.; Passerini, N.; Panzavolta, S. Novel drug-loaded film forming patch based on gelatin and snail slime. Int. J. Pharm. 2021, 598, 120408. [Google Scholar] [CrossRef]
- Perpelek, M.; Tamburaci, S.; Aydemir, S.; Tihminlioglu, F.; Baykara, B.; Karakasli, A.; Havitcioglu, H. Bioactive snail mucus-slime extract loaded chitosan scaffolds for hard tissue regeneration: The effect of mucoadhesive and antibacterial extracts on physical characteristics and bioactivity of chitosan matrix. Biomed. Mater. 2021, 16, 065008. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Fini, P.; Laurenzana, A.; Fibbi, G.; Veiga-Villauriz, C.; Fanelli, F.; Fracassi, F.; Onzo, A.; Bianco, G.; et al. Biomolecules from snail mucus (Helix. aspersa) conjugated gold nanoparticles, exhibiting potential wound healing and anti-inflammatory activity. Soft Matter 2020, 16, 10876–10888. [Google Scholar] [CrossRef]
- Houshmand, E.B. Effect of glycolic acid, phytic acid, soothing complex containing Emulsion on Hyperpigmentation and skin luminosity: A clinical evaluation. J. Cosmet. Dermatol. 2021, 20, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, L.; Yang, J.; Lee, B.; Kim, S.; Kim, J.W. Current trends and requirements in sensors for hydroxy acid-based skincare treatments: A mini-review. J. Ind. Eng. Chem. 2024, 144, 723–734. [Google Scholar] [CrossRef]
- Fuchs, E.; Raghavan, S. Getting under the skin of epidermal morphogenesis. Nat. Rev. Genet. 2002, 3, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Knutsen-Larson, S.; Dawson, A.L.; Dunnick, C.A.; Dellavalle, R.P. Acne Vulgaris: Pathogenesis, Treatment, and Needs Assessment. Dermatol. Clin. 2012, 30, 99–106. [Google Scholar] [CrossRef]
- Conforti, C.; Zalaudek, I.; Vezzoni, R.; Retrosi, C.; Fai, A.; Fadda, S.; Di Michele, E.; Dianzani, C. Chemical peeling for acne and melasma: Current knowledge and innovations. Ital. J. Dermatol. Venereol. 2020, 155, 280–285. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflamma-tion-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Denda, S.; Denda, M.; Inoue, K.; Hibino, T. Glycolic acid induces keratinocyte proliferation in a skin equivalent model via TRPV1 activation. J. Dermatol. Sci. 2010, 57, 108–113. [Google Scholar] [CrossRef]
- Teng, Y.; Fan, Y.; Ma, J.; Lu, W.; Liu, N.; Chen, Y.; Pan, W.; Tao, X. The PI3K/Akt Pathway: Emerging Roles in Skin Homeostasis and a Group of Non-Malignant Skin Disorders. Cells 2021, 10, 1219. [Google Scholar] [CrossRef]
- Bai, D.; Ueno, L.; Vogt, P.K. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int. J. Cancer. 2009, 125, 2863–2870. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, C.S. Flavonoid myricetin inhibits TNF-α-stimulated production of inflammatory mediators by suppressing the Akt, mTOR and NF-κB pathways in human keratinocytes. Eur. J. Pharmacol. 2016, 784, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Romashkova, J.A.; Makarov, S.S. NF-κB is a target of AKT in anti-apoptotic PDGF signalling. Nature 1999, 401, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Kalinski, P. Regulation of Immune Responses by Prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef]
- Futagami, A.; Ishizaki, M.; Fukuda, Y.; Kawana, S.; Yamanaka, N. Wound Healing Involves Induction of Cyclooxygenase-2 Expression in Rat Skin. Mod. Pathol. 2002, 82, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, Y.; Simonenko, V.; Xu, J.J.; Liu, K.; Wang, D.; Shi, J.; Zhong, T.; Zhang, L.; Zeng, L.; et al. Simultaneous silencing of TGF-β1 and COX-2 reduces human skin hypertrophic scar through activation of fibroblast apoptosis. Oncotarget 2017, 8, 80651–80665. [Google Scholar] [CrossRef]
- Pfisterer, K.; Shaw, L.E.; Symmank, D.; Weninger, W. The Extracellular Matrix in Skin Inflammation and Infection. Front. Cell Dev. Biol. 2021, 9, 682414. [Google Scholar] [CrossRef]
- Russo, B.; Brembilla, N.C.; Chizzolini, C. Interplay Between Keratinocytes and Fibroblasts: A Systematic Review Providing a New Angle for Understanding Skin Fibrotic Disorders. Front. Immunol. 2020, 11, 648. [Google Scholar] [CrossRef]
- Newby, A. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc. Res. 2006, 69, 614–624. [Google Scholar] [CrossRef]
- Nabeshima, K.; Inoue, T.; Shimao, Y.; Sameshima, T. Matrix metalloproteinases in tumor invasion: Role for cell migration. Pathol. Int. 2002, 52, 255–264. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and wound healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Kamarazaman, I.S.; Kiong, L.S.; Hasan, M.K.N.; Basherudin, N.; Kasim, N.A.M.; Ali, A.A.; Ramli, S.; Maniam, S.; James, R.J.; Rojsitthisak, P.; et al. Baeckea frutescens L. Promotes wound healing by upregulating expression of TGF-β, IL-1 β, VEGF and MMP-2. Saudi Pharm. J. 2024, 32, 102110. [Google Scholar] [CrossRef]
- Krishnaswamy, V.R.; Mintz, D.; Sagi, I. Matrix metalloproteinases: The sculptors of chronic cutaneous wounds. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 2220–2227. [Google Scholar] [CrossRef]
- Han, Y.P.; Tuan, T.L.; Wu, H.; Hughes, M.; Garner, W.L. TNF-alpha stimulates activation of pro-MMP2 in human skin through NF-(kappa)B mediated induction of MT1-MMP. J. Cell Sci. 2001, 114, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; John, T.; Schulze-Tanzil, G.; Lehmann, I.; Mobasheri, A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Im-plications for the treatment of osteoarthritis. Biochem. Pharmacol. 2007, 73, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Ukaegbu, K.; Allen, E.; Svoboda, K.K.H. Reactive Oxygen Species and Antioxidants in Wound Healing: Mechanisms and Therapeutic Potential. Int. Wound J. 2025, 22, e70330. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, M.; Rashad, M.; Spano, M.; Mannina, L.; Garzoli, S.; Zengin, G.; Di Giacomo, S.; Bruni, P.; Di Profio, P.; Fontana, A.; et al. Characterization of Mucins, Glycosaminoglycans, and Bioactive Compounds in Helix aspersa’s slime by Spectroscopic and Biochemical Analysis. J. Mol. Struct. 2026, 1349, 143838. [Google Scholar] [CrossRef]
- Balaha, M.; Cataldi, A.; Ammazzalorso, A.; Cacciatore, I.; De Filippis, B.; Di Stefano, A.; Maccallini, C.; Rapino, M.; Ko-ro-na-Glowniak, I.; Przekora, A.; et al. CAPE derivatives: Multifaceted agents for chronic wound healing. Arch. Pharm. 2024, 357, e2400165. [Google Scholar] [CrossRef]
- Furtado, J.; Eichmann, A. Vascular development, remodeling and maturation. Curr. Top. Dev. Biol. 2024, 159, 344–370. [Google Scholar] [CrossRef] [PubMed]
- Bajbouj, K.; Ramakrishnan, R.K.; Hamid, Q.; Pirozzi, C.J. Role of Matrix Metalloproteinases in Angiogenesis and Its Implications in Asthma. J. Immunol. Res. 2021, 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Takeo, M.; Lee, W.; Ito, M. Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, S.; Genova, T.; Chinigò, G.; Roato, I.; Scarpellino, G.; Kopecka, J.; Altruda, F.; Tolosano, E.; Riganti, C.; Mussano, F.; et al. Endothelial Cells Promote Osteogenesis by Establishing a Functional and Metabolic Coupling with Human Mesenchymal Stem Cells. Front. Physiol. 2022, 12, 813547. [Google Scholar] [CrossRef]
- Rosanto, Y.B.; Hasan, C.Y.; Rahardjo, R.; Pangestiningsih, T.W. Effect of snail mucus on angiogenesis during wound healing. F1000Research 2021, 10, 181. [Google Scholar] [CrossRef]
| Gene | Sequence (5′ to 3′) | Source |
|---|---|---|
| GAPDH-Fr | GGGTGTGAACCATGAGAAGTA | Primer blast |
| GAPDH-Rv | ACTGTGGTCATGAGTCCTTC | Primer blast |
| COX2-Fr | CCCTTCTGCCTGACACCTTT | Primer blast |
| COX2-Rv | TTCTGTACTGCGGGTGGAAC | Primer blast |
| MMP-2-Fr | GCTACGATGGAGGCGCTAAT | Primer blast |
| MMP-2-Rv | GGGCAGCCATAGAAGGTGTT | Primer blast |
| MMP-9-Fr | CGACGTCTTCCAGTACCGAG | Primer blast |
| MMP-9-Rv | GTTGGTCCCAGTGGGGATTT | Primer blast |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashad, M.; Ricci, A.; Pilato, S.; Cataldi, A.; Balaha, M.; Zara, S. Nature’s Synergy: Cellular and Molecular Evaluation of Snail Slime and Its Principal Component, Glycolic Acid, on Keratinocytes, with Preliminary Evidence from Endothelial Cells. Biomolecules 2025, 15, 1302. https://doi.org/10.3390/biom15091302
Rashad M, Ricci A, Pilato S, Cataldi A, Balaha M, Zara S. Nature’s Synergy: Cellular and Molecular Evaluation of Snail Slime and Its Principal Component, Glycolic Acid, on Keratinocytes, with Preliminary Evidence from Endothelial Cells. Biomolecules. 2025; 15(9):1302. https://doi.org/10.3390/biom15091302
Chicago/Turabian StyleRashad, Muhammad, Alessia Ricci, Serena Pilato, Amelia Cataldi, Marwa Balaha, and Susi Zara. 2025. "Nature’s Synergy: Cellular and Molecular Evaluation of Snail Slime and Its Principal Component, Glycolic Acid, on Keratinocytes, with Preliminary Evidence from Endothelial Cells" Biomolecules 15, no. 9: 1302. https://doi.org/10.3390/biom15091302
APA StyleRashad, M., Ricci, A., Pilato, S., Cataldi, A., Balaha, M., & Zara, S. (2025). Nature’s Synergy: Cellular and Molecular Evaluation of Snail Slime and Its Principal Component, Glycolic Acid, on Keratinocytes, with Preliminary Evidence from Endothelial Cells. Biomolecules, 15(9), 1302. https://doi.org/10.3390/biom15091302








