Translating Alzheimer’s Disease Mechanisms into Therapeutic Opportunities
Abstract
1. Introduction
2. Pathogenesis of AD
2.1. Genetic Factors
2.2. Amyloid-Cascade Hypothesis
2.3. Tau Pathology
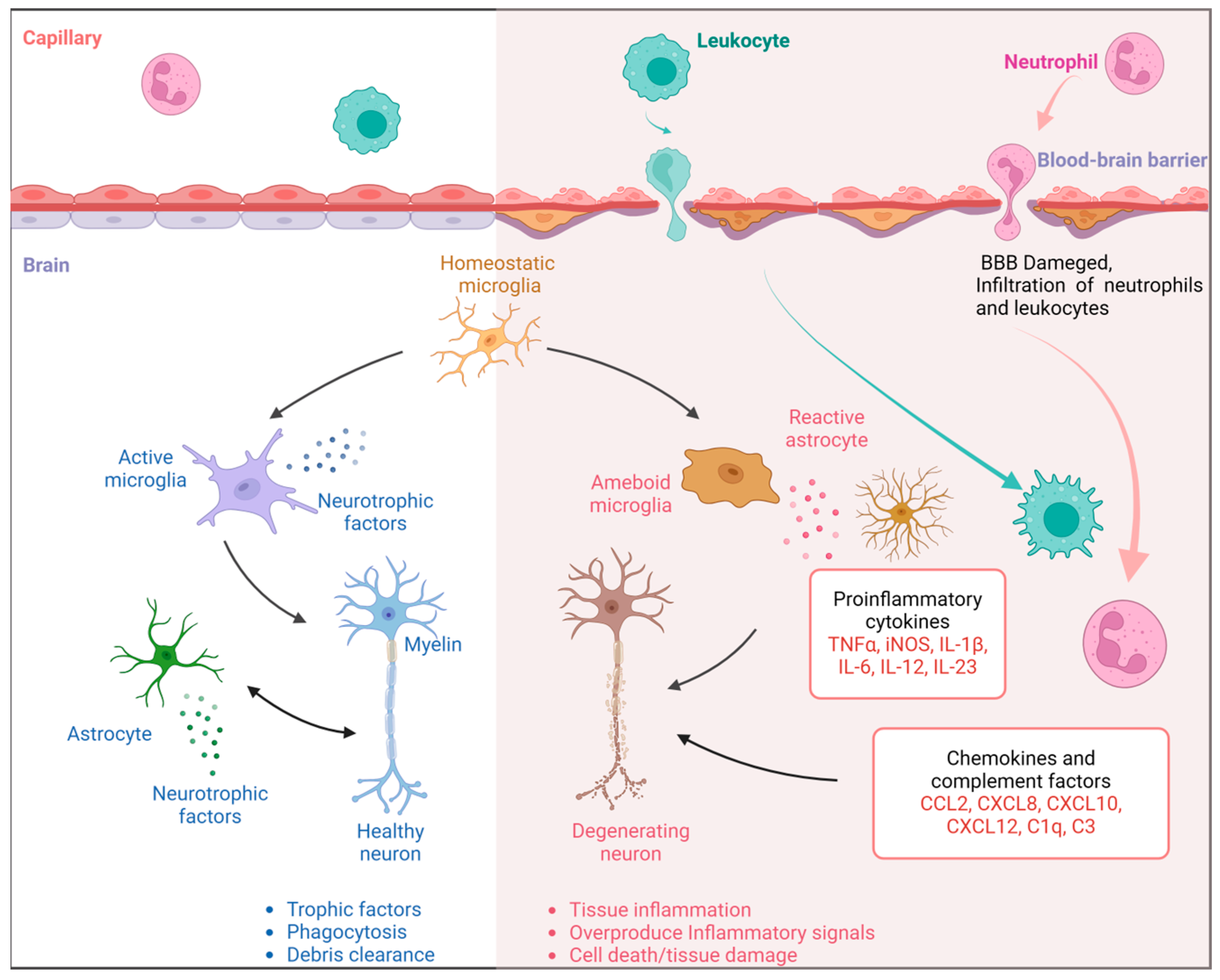
2.4. Neuroinflammation and Immunity
2.5. Vascular Pathology
2.6. Oxidative Stress
3. Biomarkers of AD
3.1. CSF Biomarkers
3.2. Imaging Biomarkers
4. Progress of Treatments
4.1. Symptomatic Treatment
4.2. Target Aβ and Tau Directly
4.3. Immunological Therapy
4.4. Target out of Aβ and Tau
4.5. Non-Pharmaceutical Treatment
| References | Description | Drug’s Name | Category | |
|---|---|---|---|---|
| [88,89,90] | Enhance acetylcholine availability through the inhibition of its degradation in the synaptic cleft | Donepezil | Acetyl-cholinesterase inhibitors (AChEIs) | Symptomatic treatment |
| [88,89,90] | Galantamine | |||
| [88,89,90] | Rivastigmine | |||
| [90,91] | Reduce L-glutamate excitatory neurotoxicity | Memantine | N-methyl-D-aspartate receptor antagonist | |
| [92] | Restrain Aβ production | BACE1 | β-secretase inhibitors | Target Aβ and Tau |
| [93] | LY450139 | γ-secretase inhibitors | ||
| [93] | GSM-2 | γ-secretase modulator | ||
| [94] | Anti-Aβ aggregation, mitigate mitochondrial dysfunction and enhance spatial learning and memory | ABAD | Aβ peptide-binding alcohol dehydrogenase | |
| [95,96] | Active cannabinoid system to mitigate the buildup of intracellular Aβ and avert proteotoxicity and inflammation | LRP1 | Lipoprotein receptor-related protein | |
| [97,98] | Induce antibodies | AN1792 | Aβ vaccine | Immunological therapy |
| [99] | Induce antibodies without T-cell to avoid the adverse events | CAD106 | ||
| [101,102] | Bapineuzumab | anti-Aβ antibodies | ||
| [103] | Solanezumab | |||
| [105,106] | Crenezumab | |||
| [100] | Inhibit or modulate the aggregation and the spread of tau | anti-tau antibodies | ||
| [107] | Enhance memory function affected by Aβ and reduce Aβ levels and plaque accumulation. | SuHeXiang Wan | Taditional Chinese Medicine | Target out of Aβ and Tau |
| [108] | Modulate cognitive behavior and neuropsychiatric symptoms | Ginkgo biloba extract EGb 761® | ||
| [109,110,111,112] | Against cytotoxicity and oxidative damag | Salidroside | ||
| [113,114] | Anti-inflammation and anti-oxidation | Curcumin | ||
| [118] | Reduce mitochondrial dysfunction | Resveratrol | ||
| [120] | Enhance spatial learning and memory impairments caused by LPS-induced neuroinflammation | S-propargyl cysteine | ||
| [135,136] | Alterate gut microbiota composition, stimulate differentiation and proliferation of Th1 cells, improve cognition | GV-971 | Intestinal microbiological regulator | |
| [121] | Reverse the impaired glucose utilization | Deep Brain Stimulation | Non-pharmaceutical treatment | |
| [122,123] | Temporarily compromise the tight junctions of the BBB, increasing local permeability | Scanning ultrasound | ||
| [124,125,126] | Aerobic and resistance training |
4.6. Limitations and Challenges
5. Concluding Remarks
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vermunt, L.; Sikkes, S.A.M.; van den Hout, A.; Handels, R.; Bos, I.; van der Flier, W.M.; Kern, S.; Ousset, P.J.; Maruff, P.; Skoog, I.; et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimers Dement 2019, 15, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; et al. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013, 12, 207–216. [Google Scholar] [CrossRef]
- Bateman, R.J.; Aisen, P.S.; De Strooper, B.; Fox, N.C.; Lemere, C.A.; Ringman, J.M.; Salloway, S.; Sperling, R.A.; Windisch, M.; Xiong, C. Autosomal-dominant Alzheimer’s disease: A review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res. Ther. 2011, 3, 1. [Google Scholar] [CrossRef]
- Morris, R.G.; Salmon, D.P. The centennial of Alzheimer’s disease and the publication of “Uber eine eigenartige Erkankung der Hirnrinde” by Alois Alzheimer. Cortex 2007, 43, 821–825. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, G.; Yang, J.; Pang, L.; Li, X. Pathological mechanisms and treatment progression of Alzheimer’s disease. Eur. J. Med. Res. 2025, 30, 625. [Google Scholar] [CrossRef]
- Hong, X.; Huang, L.; Lei, F.; Li, T.; Luo, Y.; Zeng, M.; Wang, Z. The Role and Pathogenesis of Tau Protein in Alzheimer’s Disease. Biomolecules 2025, 15, 824. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Hou, Y.; Wang, Y.; Liu, L.; Yi, X.; Xia, N. Photothermal and Photodynamic Strategies for Diagnosis and Therapy of Alzheimer’s Disease by Modulating Amyloid-beta Aggregation. Biosensors 2025, 15, 480. [Google Scholar] [CrossRef]
- Jansen, I.E.; Savage, J.E.; Watanabe, K.; Bryois, J.; Williams, D.M.; Steinberg, S.; Sealock, J.; Karlsson, I.K.; Hagg, S.; Athanasiu, L.; et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 2019, 51, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Kim, J.; Stewart, F.R.; Jiang, H.; DeMattos, R.B.; Patterson, B.W.; Fagan, A.M.; Morris, J.C.; Mawuenyega, K.G.; Cruchaga, C.; et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci. Transl. Med. 2011, 3, 89ra57. [Google Scholar] [CrossRef]
- Bell, R.D.; Winkler, E.A.; Singh, I.; Sagare, A.P.; Deane, R.; Wu, Z.; Holtzman, D.M.; Betsholtz, C.; Armulik, A.; Sallstrom, J.; et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012, 485, 512–516. [Google Scholar] [CrossRef]
- Holstege, H.; van der Lee, S.J.; Hulsman, M.; Wong, T.H.; van Rooij, J.G.; Weiss, M.; Louwersheimer, E.; Wolters, F.J.; Amin, N.; Uitterlinden, A.G.; et al. Characterization of pathogenic SORL1 genetic variants for association with Alzheimer’s disease: A clinical interpretation strategy. Eur. J. Hum. Genet. 2017, 25, 973–981. [Google Scholar] [CrossRef]
- Jonsson, T.; Stefansson, H.; Steinberg, S.; Jonsdottir, I.; Jonsson, P.V.; Snaedal, J.; Bjornsson, S.; Huttenlocher, J.; Levey, A.I.; Lah, J.J.; et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 107–116. [Google Scholar] [CrossRef]
- Cuyvers, E.; De Roeck, A.; Van den Bossche, T.; Van Cauwenberghe, C.; Bettens, K.; Vermeulen, S.; Mattheijssens, M.; Peeters, K.; Engelborghs, S.; Vandenbulcke, M.; et al. Mutations in ABCA7 in a Belgian cohort of Alzheimer’s disease patients: A targeted resequencing study. Lancet Neurol. 2015, 14, 814–822. [Google Scholar] [CrossRef]
- Veteleanu, A.; Stevenson-Hoare, J.; Keat, S.; Daskoulidou, N.; Zetterberg, H.; Heslegrave, A.; Escott-Price, V.; Williams, J.; Sims, R.; Zelek, W.M.; et al. Alzheimer’s disease-associated complement gene variants influence plasma complement protein levels. J. Neuroinflamm. 2023, 20, 169. [Google Scholar] [CrossRef]
- Escott-Price, V.; Sims, R.; Bannister, C.; Harold, D.; Vronskaya, M.; Majounie, E.; Badarinarayan, N.; Perades, G.; Consortia, I.; Morgan, K.; et al. Common polygenic variation enhances risk prediction for Alzheimer’s disease. Brain 2015, 138, 3673–3684. [Google Scholar] [CrossRef]
- Reiman, E.M.; Arboleda-Velasquez, J.F.; Quiroz, Y.T.; Huentelman, M.J.; Beach, T.G.; Caselli, R.J.; Chen, Y.; Su, Y.; Myers, A.J.; Hardy, J.; et al. Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5000-person neuropathological study. Nat. Commun. 2020, 11, 667. [Google Scholar] [CrossRef]
- Verghese, P.B.; Castellano, J.M.; Holtzman, D.M. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011, 10, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Strickland, M.R.; Soranno, A.; Holtzman, D.M. Apolipoprotein E: Structural Insights and Links to Alzheimer Disease Pathogenesis. Neuron 2021, 109, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, T.; Atwal, J.K.; Steinberg, S.; Snaedal, J.; Jonsson, P.V.; Bjornsson, S.; Stefansson, H.; Sulem, P.; Gudbjartsson, D.; Maloney, J.; et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 2012, 488, 96–99. [Google Scholar] [CrossRef]
- Sims, R.; van der Lee, S.J.; Naj, A.C.; Bellenguez, C.; Badarinarayan, N.; Jakobsdottir, J.; Kunkle, B.W.; Boland, A.; Raybould, R.; Bis, J.C.; et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat. Genet. 2017, 49, 1373–1384. [Google Scholar] [CrossRef]
- Samuelsson, J.; Najar, J.; Wallengren, O.; Kern, S.; Wetterberg, H.; Mellqvist Fassberg, M.; Zetterberg, H.; Blennow, K.; Lissner, L.; Rothenberg, E.; et al. Interactions between dietary patterns and genetic factors in relation to incident dementia among 70-year-olds. Eur. J. Nutr. 2022, 61, 871–884. [Google Scholar] [CrossRef]
- Pedrero-Chamizo, R.; Zhuang, K.; Juarez, A.; Janabi, M.; Jagust, W.J.; Landau, S.M. Alzheimer’s disease prevention: Apolipoprotein e4 moderates the effect of physical activity on brain beta-amyloid deposition in healthy older adults. J. Sci. Med. Sport 2024, 27, 402–407. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef]
- Wolfe, M.S. Targeting gamma-Secretase for Familial Alzheimer’s Disease. Med. Chem. Res. 2021, 30, 1321–1327. [Google Scholar] [CrossRef]
- Manescu, M.D.; Catalin, B.; Baldea, I.; Mateescu, V.O.; Rosu, G.C.; Boboc, I.K.S.; Istrate-Ofiteru, A.M.; Liliac, I.M.; Streba, C.T.; Kumar-Singh, S.; et al. Aquaporin 4 modulation drives amyloid burden and cognitive abilities in an APPPS1 mouse model of Alzheimer’s disease. Alzheimers Dement 2025, 21, e70164. [Google Scholar] [CrossRef]
- Andersson, E.; Lindblom, N.; Janelidze, S.; Salvado, G.; Gkanatsiou, E.; Soderberg, L.; Moller, C.; Lannfelt, L.; Ge, J.; Hanrieder, J.; et al. Soluble cerebral Abeta protofibrils link Abeta plaque pathology to changes in CSF Aβ42/Aβ40 ratios, neurofilament light and tau in Alzheimer’s disease model mice. Nat. Aging 2025, 5, 366–375. [Google Scholar] [CrossRef]
- Almeida, Z.L.; Vaz, D.C.; Brito, R.M.M. Morphological and Molecular Profiling of Amyloid-beta Species in Alzheimer’s Pathogenesis. Mol. Neurobiol. 2025, 62, 4391–4419. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Rafael Guimaraes, T.; Todd, N.; Ferguson, C.; Weiss, K.M.; Stauffer, F.R.; McDermott, B.; Hurtle, B.T.; Saito, T.; Saido, T.C.; et al. G protein-biased GPR3 signaling ameliorates amyloid pathology in a preclinical Alzheimer’s disease mouse model. Proc. Natl. Acad. Sci. USA 2022, 119, e2204828119. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, D.M.; Carrillo, M.C.; Hendrix, J.A.; Bain, L.J.; Catafau, A.M.; Gault, L.M.; Goedert, M.; Mandelkow, E.; Mandelkow, E.M.; Miller, D.S.; et al. Tau: From research to clinical development. Alzheimers Dement 2016, 12, 1033–1039. [Google Scholar] [CrossRef]
- Ranasinghe, K.G.; Kudo, K.; Syed, F.; Yballa, C.; Kramer, J.H.; Miller, B.L.; Rankin, K.P.; Garcia, P.A.; Kirsch, H.E.; Vossel, K.; et al. Distinct manifestations of excitatory-inhibitory imbalance associated with amyloid-beta and tau in patients with Alzheimer’s disease. Nat. Commun. 2025, 16, 7957. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; van der Kant, R.; Hansson, O. Tau biomarkers in Alzheimer’s disease: Towards implementation in clinical practice and trials. Lancet Neurol. 2022, 21, 726–734. [Google Scholar] [CrossRef]
- Prusiner, S.B. Cell biology. A unifying role for prions in neurodegenerative diseases. Science 2012, 336, 1511–1513. [Google Scholar] [CrossRef]
- Gibbons, G.S.; Lee, V.M.Y.; Trojanowski, J.Q. Mechanisms of Cell-to-Cell Transmission of Pathological Tau: A Review. JAMA Neurol 2019, 76, 101–108. [Google Scholar] [CrossRef]
- Estus, S.; Shaw, B.C.; Devanney, N.; Katsumata, Y.; Press, E.E.; Fardo, D.W. Evaluation of CD33 as a genetic risk factor for Alzheimer’s disease. Acta Neuropathol. 2019, 138, 187–199. [Google Scholar] [CrossRef]
- Zhang, L.; Xiang, X.; Li, Y.; Bu, G.; Chen, X.F. TREM2 and sTREM2 in Alzheimer’s disease: From mechanisms to therapies. Mol. Neurodegener. 2025, 20, 43. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Fruhwurth, S.; Zetterberg, H.; Paludan, S.R. Microglia and amyloid plaque formation in Alzheimer’s disease—Evidence, possible mechanisms, and future challenges. J. Neuroimmunol. 2024, 390, 578342. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, R.; Shahrokhi Nejad, S.; Falah Tafti, M.; Karimi, Z.; Sadr, S.R.; Ramadhan Hussein, D.; Talebian, N.; Esmaeilpour, K. Microglial activation as a hallmark of neuroinflammation in Alzheimer’s disease. Metab. Brain Dis. 2025, 40, 207. [Google Scholar] [CrossRef]
- Osborn, L.M.; Kamphuis, W.; Wadman, W.J.; Hol, E.M. Astrogliosis: An integral player in the pathogenesis of Alzheimer’s disease. Prog. Neurobiol. 2016, 144, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Govindpani, K.; McNamara, L.G.; Smith, N.R.; Vinnakota, C.; Waldvogel, H.J.; Faull, R.L.; Kwakowsky, A. Vascular Dysfunction in Alzheimer’s Disease: A Prelude to the Pathological Process or a Consequence of It? J. Clin. Med. 2019, 8, 651. [Google Scholar] [CrossRef]
- O’Brien, J.T.; Erkinjuntti, T.; Reisberg, B.; Roman, G.; Sawada, T.; Pantoni, L.; Bowler, J.V.; Ballard, C.; DeCarli, C.; Gorelick, P.B.; et al. Vascular cognitive impairment. Lancet Neurol. 2003, 2, 89–98. [Google Scholar] [CrossRef]
- Montagne, A.; Nation, D.A.; Sagare, A.P.; Barisano, G.; Sweeney, M.D.; Chakhoyan, A.; Pachicano, M.; Joe, E.; Nelson, A.R.; D’Orazio, L.M.; et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature 2020, 581, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Raghavan, N.S.; Bhattarai, P.; Siddiqui, T.; Sariya, S.; Reyes-Dumeyer, D.; Flowers, X.E.; Cardoso, S.A.L.; De Jager, P.L.; Bennett, D.A.; et al. FMNL2 regulates gliovascular interactions and is associated with vascular risk factors and cerebrovascular pathology in Alzheimer’s disease. Acta Neuropathol. 2022, 144, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Wang, Z.B.; Liu, X.P.; Mao, Z.Q.; Alzheimer’s Disease Neuroimaging Initiative. Elevated serum sodium is linked to increased amyloid-dependent tau pathology, neurodegeneration, and cognitive impairment in Alzheimer’s disease. J. Neurochem. 2025, 169, e16257. [Google Scholar] [CrossRef]
- Erickson, M.A.; Johnson, R.S.; Damodarasamy, M.; MacCoss, M.J.; Keene, C.D.; Banks, W.A.; Reed, M.J. Data-independent acquisition proteomic analysis of the brain microvasculature in Alzheimer’s disease identifies major pathways of dysfunction and upregulation of cytoprotective responses. Fluids Barriers CNS 2024, 21, 84. [Google Scholar] [CrossRef]
- Qiu, J.; Peng, S.; Qu, R.; Wu, L.; Xing, L.; Zhang, L.; Sun, J. New evidence of vascular defects in neurodegenerative diseases revealed by single cell RNA sequencing. Clin. Sci. 2024, 138, 1377–1394. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, A.; Perry, G.; Aliev, G.; Hirai, K.; Takeda, A.; Balraj, E.K.; Jones, P.K.; Ghanbari, H.; Wataya, T.; Shimohama, S.; et al. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001, 60, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Ahmed, U.; Thornalley, P.J.; Hager, K.; Fleischer, G.; Münch, G. Protein glycation, oxidation and nitration adduct residues and free adducts of cerebrospinal fluid in Alzheimer’s disease and link to cognitive impairment. J. Neurochem. 2005, 92, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.N.; Schmitt, F.A.; Scheff, S.W.; Ding, Q.; Chen, Q.; Butterfield, D.A.; Markesbery, W.R. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology 2005, 64, 1152–1156. [Google Scholar] [CrossRef]
- Roberts, L.J., 2nd; Montine, T.J.; Markesbery, W.R.; Tapper, A.R.; Hardy, P.; Chemtob, S.; Dettbarn, W.D.; Morrow, J.D. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J. Biol. Chem. 1998, 273, 13605–13612. [Google Scholar] [CrossRef]
- Leuzy, A.; Cullen, N.C.; Mattsson-Carlgren, N.; Hansson, O. Current advances in plasma and cerebrospinal fluid biomarkers in Alzheimer’s disease. Curr. Opin. Neurol. 2021, 34, 266–274. [Google Scholar] [CrossRef]
- Pereira, J.B.; Westman, E.; Hansson, O.; Alzheimer’s Disease Neuroimaging Initiative. Association between cerebrospinal fluid and plasma neurodegeneration biomarkers with brain atrophy in Alzheimer’s disease. Neurobiol. Aging 2017, 58, 14–29. [Google Scholar] [CrossRef]
- Toledo, J.B.; Zetterberg, H.; van Harten, A.C.; Glodzik, L.; Martinez-Lage, P.; Bocchio-Chiavetto, L.; Rami, L.; Hansson, O.; Sperling, R.; Engelborghs, S.; et al. Alzheimer’s disease cerebrospinal fluid biomarker in cognitively normal subjects. Brain 2015, 138, 2701–2715. [Google Scholar] [CrossRef]
- Kandimalla, R.J.; Prabhakar, S.; Wani, W.Y.; Kaushal, A.; Gupta, N.; Sharma, D.R.; Grover, V.K.; Bhardwaj, N.; Jain, K.; Gill, K.D. CSF p-Tau levels in the prediction of Alzheimer’s disease. Biol. Open 2013, 2, 1119–1124. [Google Scholar] [CrossRef]
- Barthelemy, N.R.; Saef, B.; Li, Y.; Gordon, B.A.; He, Y.; Horie, K.; Stomrud, E.; Salvado, G.; Janelidze, S.; Sato, C.; et al. CSF tau phosphorylation occupancies at T217 and T205 represent improved biomarkers of amyloid and tau pathology in Alzheimer’s disease. Nat. Aging 2023, 3, 391–401. [Google Scholar] [CrossRef]
- Khalafi, M.; Dartora, W.J.; McIntire, L.B.J.; Butler, T.A.; Wartchow, K.M.; Hojjati, S.H.; Razlighi, Q.R.; Shirbandi, K.; Zhou, L.; Chen, K.; et al. Diagnostic accuracy of phosphorylated tau217 in detecting Alzheimer’s disease pathology among cognitively impaired and unimpaired: A systematic review and meta-analysis. Alzheimers Dement 2025, 21, e14458. [Google Scholar] [CrossRef]
- Halbgebauer, S.; Steinacker, P.; Riedel, D.; Oeckl, P.; Anderl-Straub, S.; Lombardi, J.; von Arnim, C.A.F.; Nagl, M.; Giese, A.; Ludolph, A.C.; et al. Visinin-like protein 1 levels in blood and CSF as emerging markers for Alzheimer’s and other neurodegenerative diseases. Alzheimers Res. Ther. 2022, 14, 175. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhang, M.; Chen, S.J.; Miao, W.; Wang, Z.X.; Zhou, Y.J.; Yu, S.Q.; Sun, Z.W.; Zhou, X.; Yu, X.F.; et al. Neuroinflammation-mediated YKL-40 correlates with tau pathology and predicts longitudinal cognitive impairment and brain atrophy in Alzheimer’s disease, with hypertensive dependency. Front. Aging Neurosci. 2025, 17, 1630022. [Google Scholar] [CrossRef]
- Wolner, S.H.; Gleerup, H.S.; Musaeus, C.S.; Hogh, P.; Ashton, N.J.; Brinkmalm, A.; Nilsson, J.; Grotschel, L.; Zetterberg, H.; Blennow, K.; et al. Synaptosomal-Associated Protein 25 kDA (SNAP-25) Levels in Cerebrospinal Fluid: Implications for Alzheimer’s Disease Diagnosis and Monitoring. Synapse 2025, 79, e70010. [Google Scholar] [CrossRef] [PubMed]
- Duits, F.H.; Brinkmalm, G.; Teunissen, C.E.; Brinkmalm, A.; Scheltens, P.; Van der Flier, W.M.; Zetterberg, H.; Blennow, K. Synaptic proteins in CSF as potential novel biomarkers for prognosis in prodromal Alzheimer’s disease. Alzheimers Res. Ther. 2018, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Galasko, D.; Xiao, M.; Xu, D.; Smirnov, D.; Salmon, D.P.; Dewit, N.; Vanbrabant, J.; Jacobs, D.; Vanderstichele, H.; Vanmechelen, E.; et al. Synaptic biomarkers in CSF aid in diagnosis, correlate with cognition and predict progression in MCI and Alzheimer’s disease. Alzheimers Dement 2019, 5, 871–882. [Google Scholar] [CrossRef]
- Hoglund, K.; Schussler, N.; Kvartsberg, H.; Smailovic, U.; Brinkmalm, G.; Liman, V.; Becker, B.; Zetterberg, H.; Cedazo-Minguez, A.; Janelidze, S.; et al. Cerebrospinal fluid neurogranin in an inducible mouse model of neurodegeneration: A translatable marker of synaptic degeneration. Neurobiol. Dis. 2020, 134, 104645. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Hertze, J.; Zetterberg, H.; Landqvist Waldo, M.; Santillo, A.; Blennow, K.; Hansson, O. Cerebrospinal fluid neurogranin and YKL-40 as biomarkers of Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2016, 3, 12–20. [Google Scholar] [CrossRef]
- Kim, K.Y.; Shin, K.Y.; Chang, K.A. GFAP as a Potential Biomarker for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Cells 2023, 12, 1309. [Google Scholar] [CrossRef]
- Kandimalla, R.J.; Anand, R.; Veeramanikandan, R.; Wani, W.Y.; Prabhakar, S.; Grover, V.K.; Bharadwaj, N.; Jain, K.; Gill, K.D. CSF ubiquitin as a specific biomarker in Alzheimer’s disease. Curr. Alzheimer Res. 2014, 11, 340–348. [Google Scholar] [CrossRef]
- Sjodin, S.; Hansson, O.; Ohrfelt, A.; Brinkmalm, G.; Zetterberg, H.; Brinkmalm, A.; Blennow, K. Mass Spectrometric Analysis of Cerebrospinal Fluid Ubiquitin in Alzheimer’s Disease and Parkinsonian Disorders. Proteom. Clin. Appl. 2017, 11, 1700100. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wu, H.T.; Qin, X.Y.; Cao, C.; Liu, Y.; Cao, Z.Z.; Cheng, Y. Postmortem Brain, Cerebrospinal Fluid, and Blood Neurotrophic Factor Levels in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Mol. Neurosci. 2018, 65, 289–300. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Bocchetta, M.; Chetelat, G.; Rabinovici, G.D.; de Leon, M.J.; Kaye, J.; Reiman, E.M.; Scheltens, P.; Barkhof, F.; Black, S.E.; et al. Imaging markers for Alzheimer disease: Which vs how. Neurology 2013, 81, 487–500. [Google Scholar] [CrossRef]
- Drzezga, A.; Barthel, H. Imaging and Fluid Biomarkers of Alzheimer Disease: Complementation Rather Than Competition. J. Nucl. Med. 2025, 66, S32–S44. [Google Scholar] [CrossRef]
- Rabinovici, G.D.; Gatsonis, C.; Apgar, C.; Chaudhary, K.; Gareen, I.; Hanna, L.; Hendrix, J.; Hillner, B.E.; Olson, C.; Lesman-Segev, O.H.; et al. Association of Amyloid Positron Emission Tomography With Subsequent Change in Clinical Management Among Medicare Beneficiaries With Mild Cognitive Impairment or Dementia. JAMA 2019, 321, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.; Sabbagh, M. Amyloid Imaging: Poised for Integration into Medical Practice. Neurotherapeutics 2017, 14, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Guerra, U.P.; Nobili, F.M.; Padovani, A.; Perani, D.; Pupi, A.; Sorbi, S.; Trabucchi, M. Recommendations from the Italian Interdisciplinary Working Group (AIMN, AIP, SINDEM) for the utilization of amyloid imaging in clinical practice. Neurol. Sci. 2015, 36, 1075–1081. [Google Scholar] [CrossRef]
- Laforce, R.; Rosa-Neto, P.; Soucy, J.P.; Rabinovici, G.D.; Dubois, B.; Gauthier, S. Canadian Consensus Guidelines on Use of Amyloid Imaging in Canada: Update and Future Directions from the Specialized Task Force on Amyloid imaging in Canada. Can. J. Neurol. Sci. 2016, 43, 503–512. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chetelat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Chen, M.K.; Mecca, A.P.; Naganawa, M.; Finnema, S.J.; Toyonaga, T.; Lin, S.F.; Najafzadeh, S.; Ropchan, J.; Lu, Y.; McDonald, J.W.; et al. Assessing Synaptic Density in Alzheimer Disease With Synaptic Vesicle Glycoprotein 2A Positron Emission Tomographic Imaging. JAMA Neurol. 2018, 75, 1215–1224. [Google Scholar] [CrossRef]
- Leuzy, A.; Chiotis, K.; Lemoine, L.; Gillberg, P.G.; Almkvist, O.; Rodriguez-Vieitez, E.; Nordberg, A. Tau PET imaging in neurodegenerative tauopathies-still a challenge. Mol. Psychiatry 2019, 24, 1112–1134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lu, J.; Wang, L.; Jiao, F.; Wang, M.; Shi, K.; Zuo, C.; Jiang, J. Evaluating 18F-Florzolotau tau PET for Alzheimer’s disease diagnosis with 18F-Flortaucipir as reference. J. Neurol. 2025, 272, 597. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; Coomans, E.M.; Apostolova, L.G.; Baker, S.L.; Barthel, H.; Beach, T.G.; Benzinger, T.L.S.; Betthauser, T.; Bischof, G.N.; Bottlaender, M.; et al. Tau PET positivity in individuals with and without cognitive impairment varies with age, amyloid-beta status, APOE genotype and sex. Nat. Neurosci. 2025, 28, 1610–1621. [Google Scholar] [CrossRef] [PubMed]
- Liebsch, F.; Kulic, L.; Teunissen, C.; Shobo, A.; Ulku, I.; Engelschalt, V.; Hancock, M.A.; van der Flier, W.M.; Kunach, P.; Rosa-Neto, P.; et al. Aβ34 is a BACE1-derived degradation intermediate associated with amyloid clearance and Alzheimer’s disease progression. Nat. Commun. 2019, 10, 2240. [Google Scholar] [CrossRef]
- Mattsson, N.; Cullen, N.C.; Andreasson, U.; Zetterberg, H.; Blennow, K. Association Between Longitudinal Plasma Neurofilament Light and Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol. 2019, 76, 791–799. [Google Scholar] [CrossRef]
- Spitzer, P.; Mulzer, L.M.; Oberstein, T.J.; Munoz, L.E.; Lewczuk, P.; Kornhuber, J.; Herrmann, M.; Maler, J.M. Microvesicles from cerebrospinal fluid of patients with Alzheimer’s disease display reduced concentrations of tau and APP protein. Sci. Rep. 2019, 9, 7089. [Google Scholar] [CrossRef]
- Cai, Z.; Li, S.; Matuskey, D.; Nabulsi, N.; Huang, Y. PET imaging of synaptic density: A new tool for investigation of neuropsychiatric diseases. Neurosci. Lett. 2019, 691, 44–50. [Google Scholar] [CrossRef]
- Davies, P.; Maloney, A.J. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 1976, 2, 1403. [Google Scholar] [CrossRef]
- Li, D.D.; Zhang, Y.H.; Zhang, W.; Zhao, P. Meta-Analysis of Randomized Controlled Trials on the Efficacy and Safety of Donepezil, Galantamine, Rivastigmine, and Memantine for the Treatment of Alzheimer’s Disease. Front Neurosci. 2019, 13, 472. [Google Scholar] [CrossRef]
- Howard, R.; McShane, R.; Lindesay, J.; Ritchie, C.; Baldwin, A.; Barber, R.; Burns, A.; Dening, T.; Findlay, D.; Holmes, C.; et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N. Engl. J. Med. 2012, 366, 893–903. [Google Scholar] [CrossRef]
- McShane, R.; Westby, M.J.; Roberts, E.; Minakaran, N.; Schneider, L.; Farrimond, L.E.; Maayan, N.; Ware, J.; Debarros, J. Memantine for dementia. Cochrane Database Syst. Rev. 2019, 3, CD003154. [Google Scholar] [CrossRef] [PubMed]
- Mitani, Y.; Yarimizu, J.; Saita, K.; Uchino, H.; Akashiba, H.; Shitaka, Y.; Ni, K.; Matsuoka, N. Differential effects between gamma-secretase inhibitors and modulators on cognitive function in amyloid precursor protein-transgenic and nontransgenic mice. J. Neurosci. 2012, 32, 2037–2050. [Google Scholar] [CrossRef]
- May, P.C.; Dean, R.A.; Lowe, S.L.; Martenyi, F.; Sheehan, S.M.; Boggs, L.N.; Monk, S.A.; Mathes, B.M.; Mergott, D.J.; Watson, B.M.; et al. Robust central reduction of amyloid-beta in humans with an orally available, non-peptidic beta-secretase inhibitor. J. Neurosci. 2011, 31, 16507–16516. [Google Scholar] [CrossRef]
- Yao, J.; Du, H.; Yan, S.; Fang, F.; Wang, C.; Lue, L.F.; Guo, L.; Chen, D.; Stern, D.M.; Gunn Moore, F.J.; et al. Inhibition of amyloid-beta (Abeta) peptide-binding alcohol dehydrogenase-Abeta interaction reduces Abeta accumulation and improves mitochondrial function in a mouse model of Alzheimer’s disease. J. Neurosci. 2011, 31, 2313–2320. [Google Scholar] [CrossRef]
- Bachmeier, C.; Beaulieu-Abdelahad, D.; Mullan, M.; Paris, D. Role of the cannabinoid system in the transit of beta-amyloid across the blood-brain barrier. Mol. Cell. Neurosci. 2013, 56, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Currais, A.; Quehenberger, O.; Armando, A.M.; Daugherty, D.; Maher, P.; Schubert, D. Amyloid proteotoxicity initiates an inflammatory response blocked by cannabinoids. npj Aging Mech. Dis. 2016, 2, 16012. [Google Scholar] [CrossRef] [PubMed]
- Orgogozo, J.M.; Gilman, S.; Dartigues, J.F.; Laurent, B.; Puel, M.; Kirby, L.C.; Jouanny, P.; Dubois, B.; Eisner, L.; Flitman, S.; et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 2003, 61, 46–54. [Google Scholar] [CrossRef]
- Vellas, B.; Black, R.; Thal, L.J.; Fox, N.C.; Daniels, M.; McLennan, G.; Tompkins, C.; Leibman, C.; Pomfret, M.; Grundman, M.; et al. Long-term follow-up of patients immunized with AN1792: Reduced functional decline in antibody responders. Curr. Alzheimer Res. 2009, 6, 144–151. [Google Scholar] [CrossRef]
- Winblad, B.; Andreasen, N.; Minthon, L.; Floesser, A.; Imbert, G.; Dumortier, T.; Maguire, R.P.; Blennow, K.; Lundmark, J.; Staufenbiel, M.; et al. Safety, tolerability, and antibody response of active Abeta immunotherapy with CAD106 in patients with Alzheimer’s disease: Randomised, double-blind, placebo-controlled, first-in-human study. Lancet Neurol. 2012, 11, 597–604. [Google Scholar] [CrossRef]
- Chai, X.; Wu, S.; Murray, T.K.; Kinley, R.; Cella, C.V.; Sims, H.; Buckner, N.; Hanmer, J.; Davies, P.; O’Neill, M.J.; et al. Passive immunization with anti-Tau antibodies in two transgenic models: Reduction of Tau pathology and delay of disease progression. J. Biol. Chem. 2011, 286, 34457–34467. [Google Scholar] [CrossRef]
- Salloway, S.; Sperling, R.; Fox, N.C.; Blennow, K.; Klunk, W.; Raskind, M.; Sabbagh, M.; Honig, L.S.; Porsteinsson, A.P.; Ferris, S.; et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2014, 370, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, R.; Rinne, J.O.; Boada, M.; Katayama, S.; Scheltens, P.; Vellas, B.; Tuchman, M.; Gass, A.; Fiebach, J.B.; Hill, D.; et al. Bapineuzumab for mild to moderate Alzheimer’s disease in two global, randomized, phase 3 trials. Alzheimers Res. Ther. 2016, 8, 18. [Google Scholar] [CrossRef]
- Doody, R.S.; Thomas, R.G.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; Raman, R.; Sun, X.; Aisen, P.S.; et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2014, 370, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Hatami, A.; Albay, R., 3rd; Monjazeb, S.; Milton, S.; Glabe, C. Monoclonal antibodies against Abeta42 fibrils distinguish multiple aggregation state polymorphisms in vitro and in Alzheimer disease brain. J. Biol. Chem. 2014, 289, 32131–32143. [Google Scholar] [CrossRef]
- Adolfsson, O.; Pihlgren, M.; Toni, N.; Varisco, Y.; Buccarello, A.L.; Antoniello, K.; Lohmann, S.; Piorkowska, K.; Gafner, V.; Atwal, J.K.; et al. An effector-reduced anti-β-amyloid (Aβ) antibody with unique abeta binding properties promotes neuroprotection and glial engulfment of Abeta. J. Neurosci. 2012, 32, 9677–9689. [Google Scholar] [CrossRef]
- Garber, K. Genentech’s Alzheimer’s antibody trial to study disease prevention. Nat. Biotechnol. 2012, 30, 731–732. [Google Scholar] [CrossRef]
- Jeon, S.; Bose, S.; Hur, J.; Jun, K.; Kim, Y.K.; Cho, K.S.; Koo, B.S. A modified formulation of Chinese traditional medicine improves memory impairment and reduces Abeta level in the Tg-APPswe/PS1dE9 mouse model of Alzheimer’s disease. J. Ethnopharmacol. 2011, 137, 783–789. [Google Scholar] [CrossRef]
- Ihl, R.; Tribanek, M.; Bachinskaya, N.; Group, G.S. Efficacy and tolerability of a once daily formulation of Ginkgo biloba extract EGb 761(R) in Alzheimer’s disease and vascular dementia: Results from a randomised controlled trial. Pharmacopsychiatry 2012, 45, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.I.; Pae, H.O.; Choi, B.M.; Oh, G.S.; Jeong, S.; Lee, H.J.; Kim, H.Y.; Kang, K.J.; Yun, Y.G.; Kim, Y.C.; et al. Salidroside from Rhodiola sachalinensis protects neuronal PC12 cells against cytotoxicity induced by amyloid-beta. Immunopharmacol. Immunotoxicol. 2003, 25, 295–304. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.; Zhao, X.; Lin, X.; Tan, C.; Cao, G.; Wang, Z. Neuroprotective effects of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Neurochem. Int. 2010, 57, 547–555. [Google Scholar] [CrossRef]
- Qu, Z.Q.; Zhou, Y.; Zeng, Y.S.; Lin, Y.K.; Li, Y.; Zhong, Z.Q.; Chan, W.Y. Protective effects of a Rhodiola crenulata extract and salidroside on hippocampal neurogenesis against streptozotocin-induced neural injury in the rat. PLoS ONE 2012, 7, e29641. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Banach, M. Botanicals and phytochemicals active on cognitive decline: The clinical evidence. Pharmacol. Res. 2018, 130, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef]
- Lou, S.; Gong, D.; Yang, M.; Qiu, Q.; Luo, J.; Chen, T. Curcumin Improves Neurogenesis in Alzheimer’s Disease Mice via the Upregulation of Wnt/beta-Catenin and BDNF. Int. J. Mol. Sci. 2024, 25, 5123. [Google Scholar] [CrossRef]
- Panickar, K.S.; Qin, B.; Anderson, R.A. Ischemia-induced endothelial cell swelling and mitochondrial dysfunction are attenuated by cinnamtannin D1, green tea extract, and resveratrol in vitro. Nutr. Neurosci. 2015, 18, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Manczak, M.; Mao, P.; Calkins, M.J.; Cornea, A.; Reddy, A.P.; Murphy, M.P.; Szeto, H.H.; Park, B.; Reddy, P.H. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J. Alzheimers Dis. 2010, 20 (Suppl. S2), S609–S631. [Google Scholar] [CrossRef]
- Galasko, D.R.; Peskind, E.; Clark, C.M.; Quinn, J.F.; Ringman, J.M.; Jicha, G.A.; Cotman, C.; Cottrell, B.; Montine, T.J.; Thomas, R.G.; et al. Antioxidants for Alzheimer disease: A randomized clinical trial with cerebrospinal fluid biomarker measures. Arch. Neurol. 2012, 69, 836–841. [Google Scholar] [CrossRef]
- Puranik, N.; Kumari, M.; Tiwari, S.; Dhakal, T.; Song, M. Resveratrol as a Therapeutic Agent in Alzheimer’s Disease: Evidence from Clinical Studies. Nutrients 2025, 17, 2557. [Google Scholar] [CrossRef]
- Dysken, M.W.; Sano, M.; Asthana, S.; Vertrees, J.E.; Pallaki, M.; Llorente, M.; Love, S.; Schellenberg, G.D.; McCarten, J.R.; Malphurs, J.; et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: The TEAM-AD VA cooperative randomized trial. JAMA 2014, 311, 33–44. [Google Scholar] [CrossRef]
- Gong, Q.H.; Wang, Q.; Pan, L.L.; Liu, X.H.; Xin, H.; Zhu, Y.Z. S-propargyl-cysteine, a novel hydrogen sulfide-modulated agent, attenuates lipopolysaccharide-induced spatial learning and memory impairment: Involvement of TNF signaling and NF-kappaB pathway in rats. Brain. Behav. Immun. 2011, 25, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Laxton, A.W.; Tang-Wai, D.F.; McAndrews, M.P.; Zumsteg, D.; Wennberg, R.; Keren, R.; Wherrett, J.; Naglie, G.; Hamani, C.; Smith, G.S.; et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann. Neurol. 2010, 68, 521–534. [Google Scholar] [CrossRef]
- Leinenga, G.; Gotz, J. Scanning ultrasound removes amyloid-beta and restores memory in an Alzheimer’s disease mouse model. Sci. Transl. Med. 2015, 7, 278ra33. [Google Scholar] [CrossRef] [PubMed]
- Leinenga, G.; To, X.V.; Bodea, L.G.; Yousef, J.; Richter-Stretton, G.; Palliyaguru, T.; Chicoteau, A.; Dagley, L.; Nasrallah, F.; Gotz, J. Scanning ultrasound-mediated memory and functional improvements do not require amyloid-beta reduction. Mol. Psychiatry 2024, 29, 2408–2423. [Google Scholar] [CrossRef]
- De la Rosa, A.; Olaso-Gonzalez, G.; Arc-Chagnaud, C.; Millan, F.; Salvador-Pascual, A.; Garcia-Lucerga, C.; Blasco-Lafarga, C.; Garcia-Dominguez, E.; Carretero, A.; Correas, A.G.; et al. Physical exercise in the prevention and treatment of Alzheimer’s disease. J. Sport Health Sci. 2020, 9, 394–404. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef]
- Li, X.; Jin, Y.; Ding, X.; Zhu, T.; Wei, C.; Yao, L. Long-term exercise training inhibits inflammation by suppressing hippocampal NLRP3 in APP/PS1 mice. Sports Med. Health Sci. 2023, 5, 329–335. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Wang, Y.; Li, J.; Chang, J.; Jia, Q. Effect of Physical Exercise on Cognitive Function of Alzheimer’s Disease Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trial. Front. Psychiatry 2022, 13, 927128. [Google Scholar] [CrossRef] [PubMed]
- Tari, A.R.; Walker, T.L.; Huuha, A.M.; Sando, S.B.; Wisloff, U. Neuroprotective mechanisms of exercise and the importance of fitness for healthy brain ageing. Lancet 2025, 405, 1093–1118. [Google Scholar] [CrossRef]
- Barnes, L.L.; Dhana, K.; Liu, X.; Carey, V.J.; Ventrelle, J.; Johnson, K.; Hollings, C.S.; Bishop, L.; Laranjo, N.; Stubbs, B.J.; et al. Trial of the MIND Diet for Prevention of Cognitive Decline in Older Persons. N. Engl. J. Med. 2023, 389, 602–611. [Google Scholar] [CrossRef]
- Agarwal, P.; Barnes, L.L.; Dhana, K.; Liu, X.; Zhang, Y.; Beck, T.; Cornelis, M.C.; Tangney, C.; Rajan, K.B. Association of MIND diet with cognitive decline among Black and White older adults. Alzheimers Dement 2024, 20, 8461–8469. [Google Scholar] [CrossRef]
- Welty, F.K. Omega-3 fatty acids and cognitive function. Curr. Opin. Lipidol. 2023, 34, 12–21. [Google Scholar] [CrossRef]
- Patel, S.; Thornton, A.; Parmar, M.S. Resveratrol’s Multifaceted Potential in Alzheimer’s Disease: Insights from Preclinical and Clinical Evidence. Mol. Neurobiol. 2025. [Google Scholar] [CrossRef]
- Sowmiya, S.; Dhivya, L.S.; Harikrishnan, N.; Ankul Singh, S. Exploring the potential of probiotics in Alzheimer’s disease and gut dysbiosis. IBRO Neurosci. Rep. 2024, 17, 441–455. [Google Scholar]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.E.; Dodiya, H.B.; Michalkiewicz, J.; Lee, C.; Shaik, S.M.; Weigle, I.Q.; Zhang, C.; Osborn, J.; Nambiar, A.; Patel, P.; et al. Sodium oligomannate alters gut microbiota, reduces cerebral amyloidosis and reactive microglia in a sex-specific manner. Mol. Neurodegener. 2024, 19, 18. [Google Scholar] [CrossRef]
- Ornish, D.; Madison, C.; Kivipelto, M.; Kemp, C.; McCulloch, C.E.; Galasko, D.; Artz, J.; Rentz, D.; Lin, J.; Norman, K.; et al. Effects of intensive lifestyle changes on the progression of mild cognitive impairment or early dementia due to Alzheimer’s disease: A randomized, controlled clinical trial. Alzheimers Res. Ther. 2024, 16, 122. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, L.; Zhang, X.; Shi, J.; Zhu, Y.; Wang, H.; Zhu, X.; Zhu, Q.; Luo, J.-L. Translating Alzheimer’s Disease Mechanisms into Therapeutic Opportunities. Biomolecules 2025, 15, 1290. https://doi.org/10.3390/biom15091290
Li J, Wang L, Zhang X, Shi J, Zhu Y, Wang H, Zhu X, Zhu Q, Luo J-L. Translating Alzheimer’s Disease Mechanisms into Therapeutic Opportunities. Biomolecules. 2025; 15(9):1290. https://doi.org/10.3390/biom15091290
Chicago/Turabian StyleLi, Jiejia, Liyun Wang, Xiaodan Zhang, Jianhua Shi, Yizhun Zhu, Han Wang, Xiangyang Zhu, Qing Zhu, and Jia-Lie Luo. 2025. "Translating Alzheimer’s Disease Mechanisms into Therapeutic Opportunities" Biomolecules 15, no. 9: 1290. https://doi.org/10.3390/biom15091290
APA StyleLi, J., Wang, L., Zhang, X., Shi, J., Zhu, Y., Wang, H., Zhu, X., Zhu, Q., & Luo, J.-L. (2025). Translating Alzheimer’s Disease Mechanisms into Therapeutic Opportunities. Biomolecules, 15(9), 1290. https://doi.org/10.3390/biom15091290








