Navigating Brain Organoid Maturation: From Benchmarking Frameworks to Multimodal Bioengineering Strategies
Abstract
1. Introduction
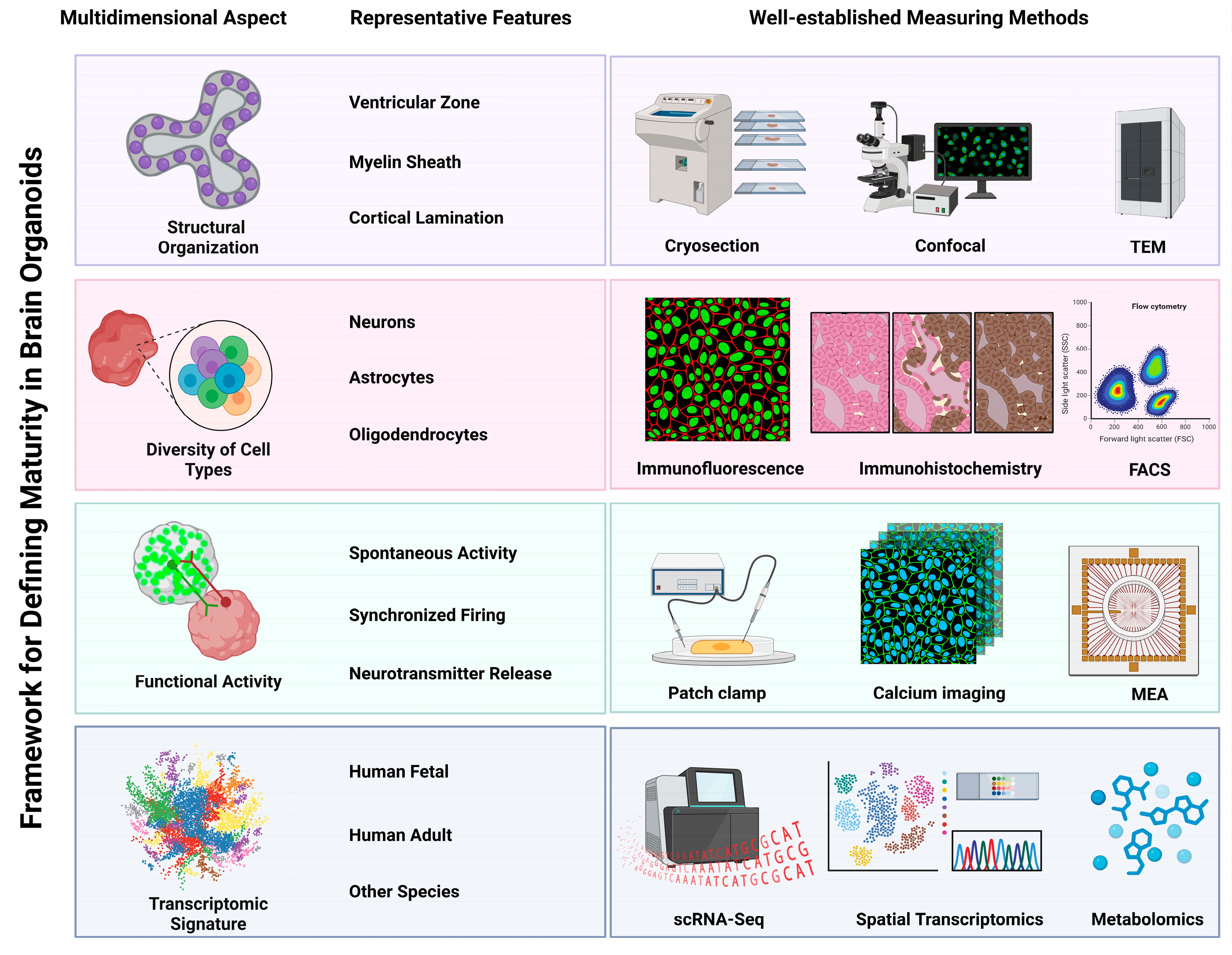
2. Multidimensional Framework for Assessing Brain Organoid Maturity
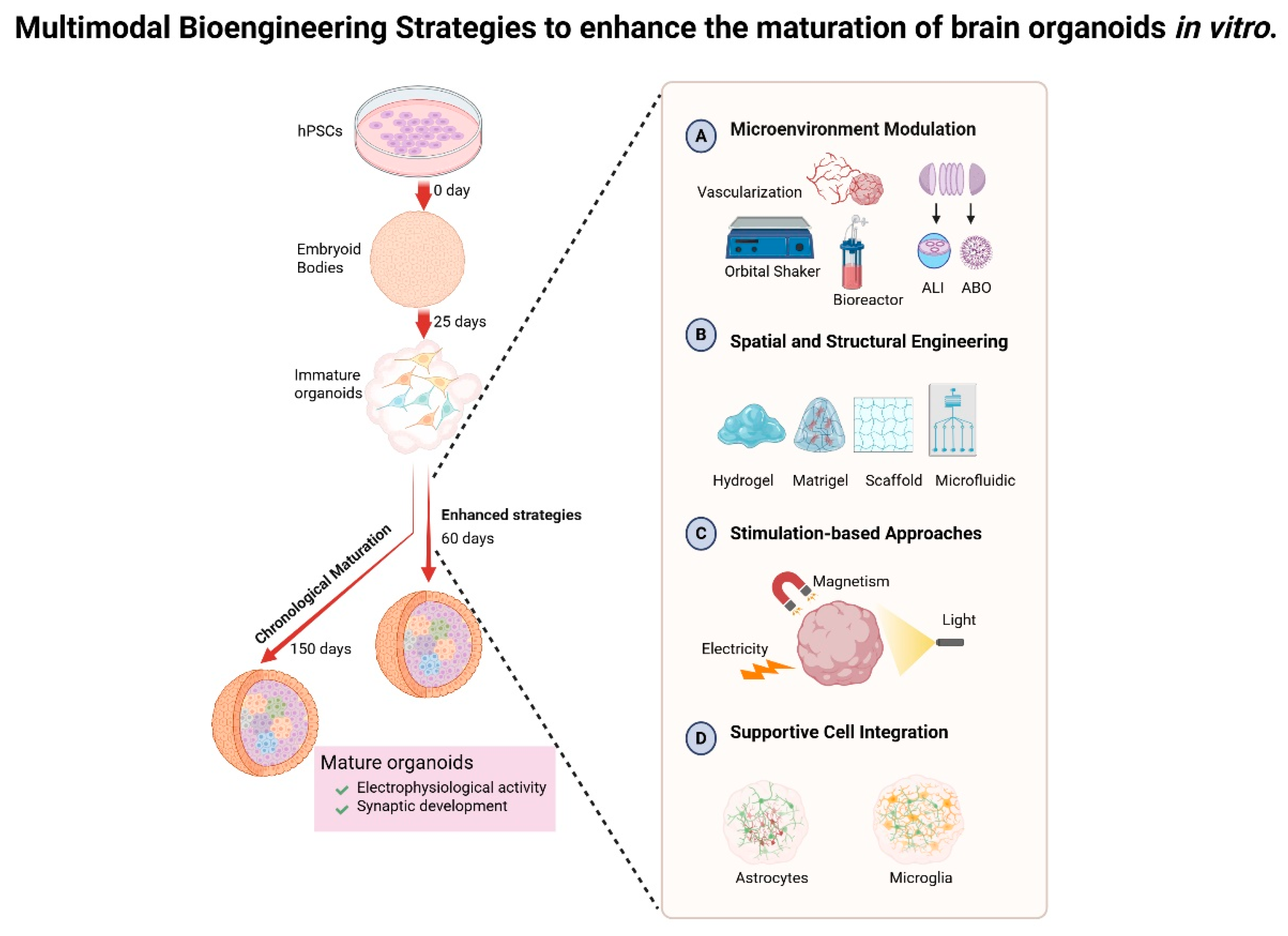
3. Temporal Dynamics and Engineering Paradigms for Brain Organoid Maturation
3.1. Chronological Maturation in Brain Organoids Rationale
3.2. Strategies to Enhance the Maturation of Brain Organoids In Vitro
3.2.1. Microenvironment Modulation
- Vascularization of organoids. The establishment of functional vascular networks significantly enhances the supply of oxygen and nutrients while facilitating the elimination of waste, thereby mitigating hypoxia-induced necrosis and enabling the prolonged culture of brain organoids. For instance, Shi et al. elucidated that vascularized organoids, achieved through the co-culturing of human pluripotent stem cells with endothelial cells (ECs), exhibited remarkable survival for up to 200 days, accompanied by an increase in neuronal diversity and synaptic density [34]. In another approach, a distinct group directly induced endothelial differentiation by overexpressing the transcription factor human EST Variant 2 (ETV2) within pluripotent stem cells, culminating in the generation of vascularized brain organoids that thrived for an extended duration of 120 days, thereby enhancing the maturation of the organoids and even imparting features reminiscent of the BBB [26]. In in vitro culture, ECs enhance brain organoid maturation through multifaceted mechanisms. These extend beyond perfusion support and BBB mimicry [36,60] to include neurogenesis modulation by EC-secreted angiocrine factors [61,62] and metabolic–neuronal activity synchronization via EC-derived signaling molecules such as nitric oxide (NO) and brain-derived neurotrophic factor (BDNF) [56,57]. While the aforementioned strategies focus on in vitro vascularization, an alternative approach leverages transplantation into rodent hosts to achieve functional vascular integration. The transplantation of these brain organoids into rodent brains capitalizes on host vascularization, resulting in an impressive survival rate of approximately 233 days, alongside increased cell survival, maturation, and synaptic connectivity within the in vivo vascularized organoid [63]. Though primarily an in vivo methodology, this strategy provides valuable mechanistic insights applicable to refining in vitro vascularization techniques.
- Bioreactor. Rotational systems serve to enhance nutrient diffusion and replicate fluid shear stress, thereby promoting the self-organization of three-dimensional structures. Lancaster et al. were pioneers in this innovative approach, employing a spinning bioreactor to extend the complexity of organoids and prolong their culture duration to an impressive 300 days [2]. Other bioreactors, such as the orbital shaker, offer a low-shear environment, allowing organoids to be cultured with a ‘single house’ manner across various media or conditions [37]. However, a notable limitation of both the spinning bioreactor and the orbital shaker lies in their substantial consumption of media enriched with growth factors and essential compounds, as well as the requirement for extensive incubator space. In response to these challenges, the miniaturized spinning bioreactor, dubbed ‘SpinΩ’, was developed to reduce culture volume, thereby increasing throughput and reproducibility of the organoids while maintaining their viability for over 200 days [30].
- Microfluidic system. Microfluidic devices adeptly simulate the dynamics of blood flow, facilitating long-term culture with enhanced oxygen exchange and nutritional supply. Evidence has demonstrated that microfluidic devices, when integrated with brain extracellular matrix, significantly improve oxygen delivery to the core regions of organoids at day in vitro 120, thereby enhancing cell survival and reducing apoptosis, ultimately promoting both structural and functional maturation of the organoids [33]. Additionally, the innovative combination of brain organoids and microfluidic systems, referred to as ‘brain organoids-on-chips’, serves to refine culture conditions by minimizing shear stress, augmenting oxygen supply, and optimizing material exchanges, thus fostering the development of cortical layers, increasing organoid size, and enhancing electrophysical functions, all of which are essential for more accurately modeling brain development [64].
- Slicing organoid technique. In the realm of brain organoids cultivated within the confines of suspension culture, a notable expansion is observed, reaching diameters of 3 to 4 mm. Yet, within the depths of these burgeoning structures, the cells grapple with hypoxia, culminating in the formation of a necrotic core that imposes limitations on both growth and maturation. The innovative sliced organoid system, characterized by its disk-shaped configuration, unveils the interior to the external culture milieu, thereby circumventing the constraints of diffusion. This advancement not only mitigates cell death but also fosters the development of a more complete architecture and diverse cell subtypes over an extended culture period, approximately 150 days [65].
- Air–liquid interface culture. A growing body of evidence underscores its efficacy in alleviating hypoxia and enhancing cellular viability within the core of organoids [66,67]. A particular study explored a novel approach that marries the traditional slice culture technique with the air–liquid interface system, yielding remarkable improvements in survival rates and the maturation of morphology, marked by extensive axon outgrowths. Notably, this method enables the brain organoid culture to endure for as long as one year [67].
- Adhesion-Based Culture Platform. In the context of the adhesion-based culture platform, this methodology addresses hypoxia by diminishing organoid thickness and promoting astrocyte migration, as evidenced in the long-term adhesion brain organoid (LT-ABO) system. In essence, Xianwei Chen and colleagues executed a meticulous slicing of brain organoids once the characteristic layered structure had been established, typically after 70 to 100 days. These organoid slices were then cultured upon Matrigel-coated plates, facilitating adhesion and extending the culture duration to an impressive one year [31].
3.2.2. Spatial and Structural Engineering Strategies
- Matrigel and the alternatives. The extracellular matrix (ECM) emerges as a pivotal architect in steering the maturation of brain organoids, furnishing biochemical, biomechanical, and structural cues that closely mirror the in vivo neural microenvironment [68,69]. The mechanical properties of the ECM directly influence neuronal differentiation and cortical layering. Matrigel, a basement membrane extract derived from mouse sarcoma, serves as an exogenous supplement of ECM. However, the implications of Matrigel on the architecture and cellular specification of brain organoids produced through various protocols remain a subject of contention [5,70,71]. The inherent complexity of Matrigel, coupled with its batch-to-batch variability stemming from compositional fluctuations, underscores its limitations in organoid culture. Recent strides in ECM engineering have concentrated on refining both natural and synthetic matrices as viable alternatives to Matrigel, aimed at augmenting neuronal differentiation, structural intricacy, and functional maturation.
- Decellularized brain tissue-derived ECM. Decellularized brain tissue-derived ECM presents a novel avenue, retaining region-specific biochemical signals—such as laminin, neurocan, and heparan sulfate—that are critical for neurogenesis, axon guidance, and synaptic formation. For instance, the application of human brain ECM (BEM) within microfluidic systems has been shown to significantly enhance cortical layering and electrophysiological activity in brain organoids when juxtaposed with Matrigel-based cultures at day 30 [33].
- Synthetic hydrogels. Synthetic hydrogels have also emerged as a promising frontier. Andrew et al. demonstrated that cortical organoids differentiated within neural cadherin peptide-functionalized gelatin methacryloyl hydrogels (GelMA-Cad) exhibited a greater frequency of spontaneous excitatory post-synaptic currents and action potentials in comparison to those cultivated in Matrigel, indicating that GelMA-Cad fosters the maturation of cortical organoids [72]. Other laboratories have formulated and employed hyaluronic acid-based matrices (HA-based matrices) to replicate the elastic modulus characteristic of brain tissue, thereby promoting the differentiation of neural progenitors into functional neurons. Notably, they discovered that HA-based matrices facilitate neuronal network formation by day 7 and 14, particularly in the presence of neighboring astrocytes [73].
3.2.3. Stimulation-Based Strategies
3.2.4. Integration of Supportive Cell Types
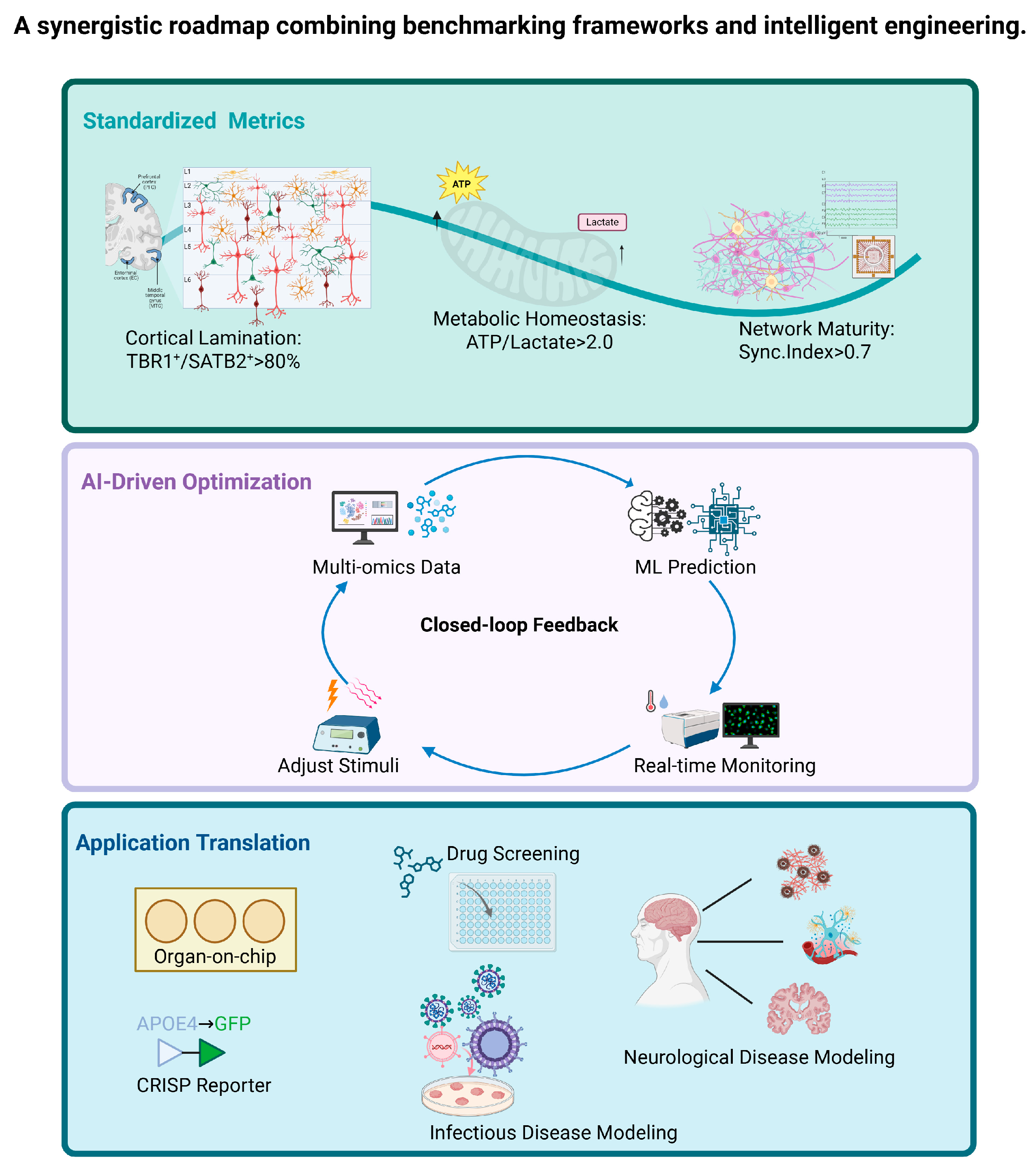
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three dimensions |
| TEM | Transmission electron microscopy |
| IF | Immunofluorescence |
| IHC | Immunohistochemistry |
| FACS | Fluorescence activating cell sorter |
| MEA | Multielectrode array |
| ScRNA-seq | Single-cell RNA sequencing |
| EM | Electron microscopy |
| MBP | Myelin basic protein |
| ECs | Endothelial cells |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| NO | Nitric oxide |
| ECM | Extracellular matrix |
| GelMA-Cad | Gelatin methacryloyl hydrogels |
| ES | Electrical stimulation |
| ACM | Astrocyte-conditioned medium |
| iPSCs | Patient-derived induced pluripotent stem cells |
| AI | Artificial intelligence |
| ML | Machine learning |
| ATP | Adenosine triphosphate |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| vhCOs | Vascularized brain organoids |
| hPSC | Human pluripotent stem cell |
References
- He, Z.; Dony, L.; Fleck, J.S.; Szalata, A.; Li, K.X.; Sliskovic, I.; Lin, H.C.; Santel, M.; Atamian, A.; Quadrato, G.; et al. An integrated transcriptomic cell atlas of human neural organoids. Nature 2024, 635, 690–698. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Jo, J.; Xiao, Y.; Sun, A.X.; Cukuroglu, E.; Tran, H.D.; Goke, J.; Tan, Z.Y.; Saw, T.Y.; Tan, C.P.; Lokman, H.; et al. Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell Stem Cell 2016, 19, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 2013, 80, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Pasca, A.M.; Sloan, S.A.; Clarke, L.E.; Tian, Y.; Makinson, C.D.; Huber, N.; Kim, C.H.; Park, J.Y.; O’Rourke, N.A.; Nguyen, K.D.; et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 2015, 12, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Mariani, J.; Coppola, G.; Zhang, P.; Abyzov, A.; Provini, L.; Tomasini, L.; Amenduni, M.; Szekely, A.; Palejev, D.; Wilson, M.; et al. FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell 2015, 162, 375–390. [Google Scholar] [CrossRef]
- Luo, C.; Lancaster, M.A.; Castanon, R.; Nery, J.R.; Knoblich, J.A.; Ecker, J.R. Cerebral Organoids Recapitulate Epigenomic Signatures of the Human Fetal Brain. Cell Rep. 2016, 17, 3369–3384. [Google Scholar] [CrossRef] [PubMed]
- Machairaki, V. Human Pluripotent Stem Cells as In Vitro Models of Neurodegenerative Diseases. Adv. Exp. Med. Biol. 2020, 1195, 93–94. [Google Scholar] [CrossRef]
- Chen, X.; Shi, Y. Generating Homogeneous Brain Organoids from Human iPSCs. Methods Mol. Biol. 2024, 2794, 157–167. [Google Scholar] [CrossRef]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef]
- Cederquist, G.Y.; Asciolla, J.J.; Tchieu, J.; Walsh, R.M.; Cornacchia, D.; Resh, M.D.; Studer, L. Specification of positional identity in forebrain organoids. Nat. Biotechnol. 2019, 37, 436–444. [Google Scholar] [CrossRef]
- Nasr, B.; Chatterton, R.; Yong, J.H.M.; Jamshidi, P.; D’Abaco, G.M.; Bjorksten, A.R.; Kavehei, O.; Chana, G.; Dottori, M.; Skafidas, E. Self-Organized Nanostructure Modified Microelectrode for Sensitive Electrochemical Glutamate Detection in Stem Cells-Derived Brain Organoids. Biosensors 2018, 8, 14. [Google Scholar] [CrossRef]
- de Jong, J.O.; Llapashtica, C.; Genestine, M.; Strauss, K.; Provenzano, F.; Sun, Y.; Zhu, H.; Cortese, G.P.; Brundu, F.; Brigatti, K.W.; et al. Cortical overgrowth in a preclinical forebrain organoid model of CNTNAP2-associated autism spectrum disorder. Nat. Commun. 2021, 12, 4087. [Google Scholar] [CrossRef]
- Khan, T.A.; Revah, O.; Gordon, A.; Yoon, S.J.; Krawisz, A.K.; Goold, C.; Sun, Y.; Kim, C.H.; Tian, Y.; Li, M.Y.; et al. Neuronal defects in a human cellular model of 22q11.2 deletion syndrome. Nat. Med. 2020, 26, 1888–1898. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, Y.; Yamazaki, Y.; Ren, Y.; Davis, M.D.; Liu, C.C.; Lu, W.; Wang, X.; Chen, K.; Cherukuri, Y.; et al. APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer’s disease patient iPSC-derived cerebral organoids. Nat. Commun. 2020, 11, 5540. [Google Scholar] [CrossRef]
- Arzua, T.; Yan, Y.; Jiang, C.; Logan, S.; Allison, R.L.; Wells, C.; Kumar, S.N.; Schafer, R.; Bai, X. Modeling alcohol-induced neurotoxicity using human induced pluripotent stem cell-derived three-dimensional cerebral organoids. Transl. Psychiatry 2020, 10, 347. [Google Scholar] [CrossRef]
- Watanabe, M.; Buth, J.E.; Vishlaghi, N.; de la Torre-Ubieta, L.; Taxidis, J.; Khakh, B.S.; Coppola, G.; Pearson, C.A.; Yamauchi, K.; Gong, D.; et al. Self-Organized Cerebral Organoids with Human-Specific Features Predict Effective Drugs to Combat Zika Virus Infection. Cell Rep. 2017, 21, 517–532. [Google Scholar] [CrossRef]
- Wang, S.N.; Wang, Z.; Xu, T.Y.; Cheng, M.H.; Li, W.L.; Miao, C.Y. Cerebral Organoids Repair Ischemic Stroke Brain Injury. Transl. Stroke Res. 2020, 11, 983–1000. [Google Scholar] [CrossRef]
- Bershteyn, M.; Nowakowski, T.J.; Pollen, A.A.; Di Lullo, E.; Nene, A.; Wynshaw-Boris, A.; Kriegstein, A.R. Human iPSC-Derived Cerebral Organoids Model Cellular Features of Lissencephaly and Reveal Prolonged Mitosis of Outer Radial Glia. Cell Stem Cell 2017, 20, 435–449 e434. [Google Scholar] [CrossRef]
- Trujillo, C.A.; Gao, R.; Negraes, P.D.; Gu, J.; Buchanan, J.; Preissl, S.; Wang, A.; Wu, W.; Haddad, G.G.; Chaim, I.A.; et al. Complex Oscillatory Waves Emerging from Cortical Organoids Model Early Human Brain Network Development. Cell Stem Cell 2019, 25, 558–569.e557. [Google Scholar] [CrossRef]
- Madhavan, M.; Nevin, Z.S.; Shick, H.E.; Garrison, E.; Clarkson-Paredes, C.; Karl, M.; Clayton, B.L.L.; Factor, D.C.; Allan, K.C.; Barbar, L.; et al. Induction of myelinating oligodendrocytes in human cortical spheroids. Nat. Methods 2018, 15, 700–706. [Google Scholar] [CrossRef]
- Gordon, A.; Yoon, S.J.; Tran, S.S.; Makinson, C.D.; Park, J.Y.; Andersen, J.; Valencia, A.M.; Horvath, S.; Xiao, X.; Huguenard, J.R.; et al. Long-term maturation of human cortical organoids matches key early postnatal transitions. Nat. Neurosci. 2021, 24, 331–342. [Google Scholar] [CrossRef]
- Sloan, S.A.; Darmanis, S.; Huber, N.; Khan, T.A.; Birey, F.; Caneda, C.; Reimer, R.; Quake, S.R.; Barres, B.A.; Pasca, S.P. Human Astrocyte Maturation Captured in 3D Cerebral Cortical Spheroids Derived from Pluripotent Stem Cells. Neuron 2017, 95, 779–790 e776. [Google Scholar] [CrossRef]
- Porciuncula, L.O.; Goto-Silva, L.; Ledur, P.F.; Rehen, S.K. The Age of Brain Organoids: Tailoring Cell Identity and Functionality for Normal Brain Development and Disease Modeling. Front. Neurosci. 2021, 15, 674563. [Google Scholar] [CrossRef]
- Lu, T.M.; Houghton, S.; Magdeldin, T.; Duran, J.G.B.; Minotti, A.P.; Snead, A.; Sproul, A.; Nguyen, D.T.; Xiang, J.; Fine, H.A.; et al. Pluripotent stem cell-derived epithelium misidentified as brain microvascular endothelium requires ETS factors to acquire vascular fate. Proc. Natl. Acad. Sci. USA 2021, 118, e2016950118. [Google Scholar] [CrossRef]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.S.; et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef]
- Stoberl, N.; Maguire, E.; Salis, E.; Shaw, B.; Hall-Roberts, H. Human iPSC-derived glia models for the study of neuroinflammation. J. Neuroinflammation 2023, 20, 231. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhauser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Bhaduri, A.; Andrews, M.G.; Mancia Leon, W.; Jung, D.; Shin, D.; Allen, D.; Jung, D.; Schmunk, G.; Haeussler, M.; Salma, J.; et al. Cell stress in cortical organoids impairs molecular subtype specification. Nature 2020, 578, 142–148. [Google Scholar] [CrossRef]
- Qian, X.; Jacob, F.; Song, M.M.; Nguyen, H.N.; Song, H.; Ming, G.L. Generation of human brain region-specific organoids using a miniaturized spinning bioreactor. Nat. Protoc. 2018, 13, 565–580. [Google Scholar] [CrossRef]
- Chen, X.; Sun, G.; Feng, L.; Tian, E.; Shi, Y. Human iPSC-derived microglial cells protect neurons from neurodegeneration in long-term cultured adhesion brain organoids. Commun. Biol. 2025, 8, 30. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Novak, S.W.; Yu, J.; Gallina, I.S.; Xu, L.L.; Lim, C.K.; Fernandes, S.; Shokhirev, M.N.; Williams, A.E.; et al. Morphological diversification and functional maturation of human astrocytes in glia-enriched cortical organoid transplanted in mouse brain. Nat. Biotechnol. 2025, 43, 52–62. [Google Scholar] [CrossRef]
- Cho, A.N.; Jin, Y.; An, Y.; Kim, J.; Choi, Y.S.; Lee, J.S.; Kim, J.; Choi, W.Y.; Koo, D.J.; Yu, W.; et al. Microfluidic device with brain extracellular matrix promotes structural and functional maturation of human brain organoids. Nat. Commun. 2021, 12, 4730. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, L.; Wang, M.; Liu, J.; Zhong, S.; Li, R.; Li, P.; Guo, L.; Fang, A.; Chen, R.; et al. Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol. 2020, 18, e3000705. [Google Scholar] [CrossRef]
- Chen, X.; Sun, G.; Tian, E.; Zhang, M.; Davtyan, H.; Beach, T.G.; Reiman, E.M.; Blurton-Jones, M.; Holtzman, D.M.; Shi, Y. Modeling Sporadic Alzheimer’s Disease in Human Brain Organoids under Serum Exposure. Adv. Sci. 2021, 8, e2101462. [Google Scholar] [CrossRef]
- Dao, L.; You, Z.; Lu, L.; Xu, T.; Sarkar, A.K.; Zhu, H.; Liu, M.; Calandrelli, R.; Yoshida, G.; Lin, P.; et al. Modeling blood-brain barrier formation and cerebral cavernous malformations in human PSC-derived organoids. Cell Stem Cell 2024, 31, 818–833 e811. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef]
- Miura, Y.; Li, M.Y.; Birey, F.; Ikeda, K.; Revah, O.; Thete, M.V.; Park, J.Y.; Puno, A.; Lee, S.H.; Porteus, M.H.; et al. Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells. Nat. Biotechnol. 2020, 38, 1421–1430. [Google Scholar] [CrossRef]
- Chen, X.; Saiyin, H.; Liu, Y.; Wang, Y.; Li, X.; Ji, R.; Ma, L. Human striatal organoids derived from pluripotent stem cells recapitulate striatal development and compartments. PLoS Biol. 2022, 20, e3001868. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, D.; Bi, C.; Mi, T.; Zhu, W.; Xia, L.; Teng, Z.; Hu, B.; Wu, Y. A Chemical Recipe for Generation of Clinical-Grade Striatal Neurons from hESCs. Stem Cell Rep. 2018, 11, 635–650. [Google Scholar] [CrossRef]
- Birey, F.; Pasca, S.P. Imaging neuronal migration and network activity in human forebrain assembloids. STAR Protoc. 2022, 3, 101478. [Google Scholar] [CrossRef]
- Gu, L.; Cai, H.; Chen, L.; Gu, M.; Tchieu, J.; Guo, F. Functional Neural Networks in Human Brain Organoids. BME Front. 2024, 5, 0065. [Google Scholar] [CrossRef]
- He, L.; Sun, Z.; Li, J.; Zhu, R.; Niu, B.; Tam, K.L.; Xiao, Q.; Li, J.; Wang, W.; Tsui, C.Y.; et al. Electrical stimulation at nanoscale topography boosts neural stem cell neurogenesis through the enhancement of autophagy signaling. Biomaterials 2021, 268, 120585. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Hu, N.; Chang, Z.H.; Shi, J.X.; Fan, X.; Chen, M.M.; Bao, S.Q.; Chen, C.; Zuo, J.C.; Zhang, X.W.; et al. Brain organoid maturation and implantation integration based on electrical signals input. J. Adv. Res. 2024, 73, 375–395. [Google Scholar] [CrossRef]
- Fitzgerald, M.Q.; Chu, T.; Puppo, F.; Blanch, R.; Chillon, M.; Subramaniam, S.; Muotri, A.R. Generation of ‘semi-guided’ cortical organoids with complex neural oscillations. Nat. Protoc. 2024, 19, 2712–2738. [Google Scholar] [CrossRef]
- Glass, M.R.; Waxman, E.A.; Yamashita, S.; Lafferty, M.; Beltran, A.A.; Farah, T.; Patel, N.K.; Singla, R.; Matoba, N.; Ahmed, S.; et al. Cross-site reproducibility of human cortical organoids reveals consistent cell type composition and architecture. Stem Cell Reports 2024, 19, 1351–1367. [Google Scholar] [CrossRef] [PubMed]
- Fiorenzano, A.; Sozzi, E.; Birtele, M.; Kajtez, J.; Giacomoni, J.; Nilsson, F.; Bruzelius, A.; Sharma, Y.; Zhang, Y.; Mattsson, B.; et al. Single-cell transcriptomics captures features of human midbrain development and dopamine neuron diversity in brain organoids. Nat. Commun. 2021, 12, 7302. [Google Scholar] [CrossRef] [PubMed]
- Velasco, S.; Kedaigle, A.J.; Simmons, S.K.; Nash, A.; Rocha, M.; Quadrato, G.; Paulsen, B.; Nguyen, L.; Adiconis, X.; Regev, A.; et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 2019, 570, 523–527. [Google Scholar] [CrossRef]
- Sato, Y.; Asahi, T.; Kataoka, K. Integrative single-cell RNA-seq analysis of vascularized cerebral organoids. BMC Biol. 2023, 21, 245. [Google Scholar] [CrossRef]
- Zheng, H.; Feng, Y.; Tang, J.; Yu, F.; Wang, Z.; Xu, J.; Hai, C.; Jiang, M.; Cheng, Y.; Shao, Z.; et al. Astrocyte-secreted cues promote neural maturation and augment activity in human forebrain organoids. Nat. Commun. 2025, 16, 2845. [Google Scholar] [CrossRef]
- Trevino, A.E.; Sinnott-Armstrong, N.; Andersen, J.; Yoon, S.J.; Huber, N.; Pritchard, J.K.; Chang, H.Y.; Greenleaf, W.J.; Pasca, S.P. Chromatin accessibility dynamics in a model of human forebrain development. Science 2020, 367, eaay1645. [Google Scholar] [CrossRef]
- Ramani, A.; Pasquini, G.; Gerkau, N.J.; Jadhav, V.; Vinchure, O.S.; Altinisik, N.; Windoffer, H.; Muller, S.; Rothenaigner, I.; Lin, S.; et al. Reliability of high-quantity human brain organoids for modeling microcephaly, glioma invasion and drug screening. Nat. Commun. 2024, 15, 10703. [Google Scholar] [CrossRef]
- Park, S.; Min, C.H.; Choi, E.; Choi, J.S.; Park, K.; Han, S.; Choi, W.; Jang, H.J.; Cho, K.O.; Kim, M. Long-term tracking of neural and oligodendroglial development in large-scale human cerebral organoids by noninvasive volumetric imaging. Sci. Rep. 2025, 15, 2536. [Google Scholar] [CrossRef]
- Sun, G.; Chiuppesi, F.; Chen, X.; Wang, C.; Tian, E.; Nguyen, J.; Kha, M.; Trinh, D.; Zhang, H.; Marchetto, M.C.; et al. Modeling Human Cytomegalovirus-Induced Microcephaly in Human iPSC-Derived Brain Organoids. Cell Rep. Med. 2020, 1, 100002. [Google Scholar] [CrossRef]
- Quadrato, G.; Nguyen, T.; Macosko, E.Z.; Sherwood, J.L.; Min Yang, S.; Berger, D.R.; Maria, N.; Scholvin, J.; Goldman, M.; Kinney, J.P.; et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 2017, 545, 48–53. [Google Scholar] [CrossRef]
- Rizzuti, M.; Melzi, V.; Brambilla, L.; Quetti, L.; Sali, L.; Ottoboni, L.; Meneri, M.; Ratti, A.; Verde, F.; Ticozzi, N.; et al. Shaping the Neurovascular Unit Exploiting Human Brain Organoids. Mol. Neurobiol. 2024, 61, 6642–6657. [Google Scholar] [CrossRef]
- Sun, X.Y.; Ju, X.C.; Li, Y.; Zeng, P.M.; Wu, J.; Zhou, Y.Y.; Shen, L.B.; Dong, J.; Chen, Y.J.; Luo, Z.G. Generation of vascularized brain organoids to study neurovascular interactions. Elife 2022, 11, e76707. [Google Scholar] [CrossRef] [PubMed]
- Marton, R.M.; Miura, Y.; Sloan, S.A.; Li, Q.; Revah, O.; Levy, R.J.; Huguenard, J.R.; Pasca, S.P. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat. Neurosci. 2019, 22, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Hergenreder, E.; Minotti, A.P.; Zorina, Y.; Oberst, P.; Zhao, Z.; Munguba, H.; Calder, E.L.; Baggiolini, A.; Walsh, R.M.; Liston, C.; et al. Combined small-molecule treatment accelerates maturation of human pluripotent stem cell-derived neurons. Nat. Biotechnol. 2024, 42, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, S.; Lawler, S.E.; Qu, Y.; Fadzen, C.M.; Wolfe, J.M.; Regan, M.S.; Pentelute, B.L.; Agar, N.Y.R.; Cho, C.F. Blood-brain-barrier organoids for investigating the permeability of CNS therapeutics. Nat. Protoc. 2018, 13, 2827–2843. [Google Scholar] [CrossRef]
- Nikolova, M.T.; He, Z.; Seimiya, M.; Jonsson, G.; Cao, W.; Okuda, R.; Wimmer, R.A.; Okamoto, R.; Penninger, J.M.; Camp, J.G.; et al. Fate and state transitions during human blood vessel organoid development. Cell 2025, 188, P3329–P3348.E31. [Google Scholar] [CrossRef]
- Song, L.; Yuan, X.; Jones, Z.; Griffin, K.; Zhou, Y.; Ma, T.; Li, Y. Assembly of Human Stem Cell-Derived Cortical Spheroids and Vascular Spheroids to Model 3-D Brain-like Tissues. Sci. Rep. 2019, 9, 5977. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.A.; Goncalves, J.T.; Bloyd, C.W.; Li, H.; Fernandes, S.; Quang, D.; Johnston, S.; Parylak, S.L.; Jin, X.; Gage, F.H. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 2018, 36, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Guo, Y.; Zhu, Y.; Qin, J. Engineering stem cell-derived 3D brain organoids in a perfusable organ-on-a-chip system. RSC Adv. 2018, 8, 1677–1685. [Google Scholar] [CrossRef]
- Qian, X.; Su, Y.; Adam, C.D.; Deutschmann, A.U.; Pather, S.R.; Goldberg, E.M.; Su, K.; Li, S.; Lu, L.; Jacob, F.; et al. Sliced Human Cortical Organoids for Modeling Distinct Cortical Layer Formation. Cell Stem Cell 2020, 26, 766–781 e769. [Google Scholar] [CrossRef]
- Ao, Z.; Cai, H.; Havert, D.J.; Wu, Z.; Gong, Z.; Beggs, J.M.; Mackie, K.; Guo, F. One-Stop Microfluidic Assembly of Human Brain Organoids To Model Prenatal Cannabis Exposure. Anal. Chem. 2020, 92, 4630–4638. [Google Scholar] [CrossRef]
- Giandomenico, S.L.; Mierau, S.B.; Gibbons, G.M.; Wenger, L.M.D.; Masullo, L.; Sit, T.; Sutcliffe, M.; Boulanger, J.; Tripodi, M.; Derivery, E.; et al. Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output. Nat. Neurosci. 2019, 22, 669–679. [Google Scholar] [CrossRef]
- Reginensi, D.; Ortiz, D.A.; Denis, B.; Castillo, S.; Burillo, A.; Khoury, N.; Xu, J.; Dam, M.L.; Escobar, A.A.H.; Dave, K.R.; et al. Region-specific brain decellularized extracellular matrix promotes cell recovery in an in vitro model of stroke. Sci. Rep. 2025, 15, 11921. [Google Scholar] [CrossRef] [PubMed]
- Dityatev, A.; Seidenbecher, C.I.; Schachner, M. Compartmentalization from the outside: The extracellular matrix and functional microdomains in the brain. Trends Neurosci. 2010, 33, 503–512. [Google Scholar] [CrossRef]
- Chiaradia, I.; Imaz-Rosshandler, I.; Nilges, B.S.; Boulanger, J.; Pellegrini, L.; Das, R.; Kashikar, N.D.; Lancaster, M.A. Tissue morphology influences the temporal program of human brain organoid development. Cell Stem Cell 2023, 30, 1351–1367.e1310. [Google Scholar] [CrossRef]
- Martins-Costa, C.; Pham, V.A.; Sidhaye, J.; Novatchkova, M.; Wiegers, A.; Peer, A.; Moseneder, P.; Corsini, N.S.; Knoblich, J.A. Morphogenesis and development of human telencephalic organoids in the absence and presence of exogenous extracellular matrix. EMBO J. 2023, 42, e113213. [Google Scholar] [CrossRef]
- Kjar, A.; Haschert, M.R.; Zepeda, J.C.; Simmons, A.J.; Yates, A.; Chavarria, D.; Fernandez, M.; Robertson, G.; Abdulrahman, A.M.; Kim, H.; et al. Biofunctionalized gelatin hydrogels support development and maturation of iPSC-derived cortical organoids. Cell Rep. 2024, 43, 114874. [Google Scholar] [CrossRef]
- Janzen, D.; Bakirci, E.; Faber, J.; Andrade Mier, M.; Hauptstein, J.; Pal, A.; Forster, L.; Hazur, J.; Boccaccini, A.R.; Detsch, R.; et al. Reinforced Hyaluronic Acid-Based Matrices Promote 3D Neuronal Network Formation. Adv. Healthc. Mater. 2022, 11, e2201826. [Google Scholar] [CrossRef]
- Chen, C.; Rengarajan, V.; Kjar, A.; Huang, Y. A matrigel-free method to generate matured human cerebral organoids using 3D-Printed microwell arrays. Bioact. Mater. 2021, 6, 1130–1139. [Google Scholar] [CrossRef]
- Medvedeva, V.P.; Pierani, A. How Do Electric Fields Coordinate Neuronal Migration and Maturation in the Developing Cortex? Front. Cell Dev. Biol. 2020, 8, 580657. [Google Scholar] [CrossRef]
- Park, Y.; Franz, C.K.; Ryu, H.; Luan, H.; Cotton, K.Y.; Kim, J.U.; Chung, T.S.; Zhao, S.; Vazquez-Guardado, A.; Yang, D.S.; et al. Three-dimensional, multifunctional neural interfaces for cortical spheroids and engineered assembloids. Sci. Adv. 2021, 7, eabf9153. [Google Scholar] [CrossRef]
- Li, T.L.; Liu, Y.; Forro, C.; Yang, X.; Beker, L.; Bao, Z.; Cui, B.; Pasca, S.P. Stretchable mesh microelectronics for the biointegration and stimulation of human neural organoids. Biomaterials 2022, 290, 121825. [Google Scholar] [CrossRef]
- Kim, E.; Jeong, E.; Hong, Y.M.; Jeong, I.; Kim, J.; Kwon, Y.W.; Park, Y.G.; Lee, J.; Choi, S.; Kim, J.Y.; et al. Magnetically reshapable 3D multi-electrode arrays of liquid metals for electrophysiological analysis of brain organoids. Nat. Commun. 2025, 16, 2011. [Google Scholar] [CrossRef]
- Osaki, T.; Duenki, T.; Chow, S.Y.A.; Ikegami, Y.; Beaubois, R.; Levi, T.; Nakagawa-Tamagawa, N.; Hirano, Y.; Ikeuchi, Y. Complex activity and short-term plasticity of human cerebral organoids reciprocally connected with axons. Nat. Commun. 2024, 15, 2945. [Google Scholar] [CrossRef] [PubMed]
- Schafer, S.T.; Mansour, A.A.; Schlachetzki, J.C.M.; Pena, M.; Ghassemzadeh, S.; Mitchell, L.; Mar, A.; Quang, D.; Stumpf, S.; Ortiz, I.S.; et al. An in vivo neuroimmune organoid model to study human microglia phenotypes. Cell 2023, 186, 2111–2126.e2120. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Zhang, T.; Fan, K.; Cai, W.; Liu, H. Astrocyte-Neuron Signaling in Synaptogenesis. Front. Cell Dev. Biol. 2021, 9, 680301. [Google Scholar] [CrossRef]
- VanderWall, K.B.; Vij, R.; Ohlemacher, S.K.; Sridhar, A.; Fligor, C.M.; Feder, E.M.; Edler, M.C.; Baucum, A.J., 2nd; Cummins, T.R.; Meyer, J.S. Astrocytes Regulate the Development and Maturation of Retinal Ganglion Cells Derived from Human Pluripotent Stem Cells. Stem Cell Rep. 2019, 12, 201–212. [Google Scholar] [CrossRef]
- Krencik, R.; Seo, K.; van Asperen, J.V.; Basu, N.; Cvetkovic, C.; Barlas, S.; Chen, R.; Ludwig, C.; Wang, C.; Ward, M.E.; et al. Systematic Three-Dimensional Coculture Rapidly Recapitulates Interactions between Human Neurons and Astrocytes. Stem Cell Rep. 2017, 9, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Ormel, P.R.; Vieira de Sa, R.; van Bodegraven, E.J.; Karst, H.; Harschnitz, O.; Sneeboer, M.A.M.; Johansen, L.E.; van Dijk, R.E.; Scheefhals, N.; Berdenis van Berlekom, A.; et al. Microglia innately develop within cerebral organoids. Nat. Commun. 2018, 9, 4167. [Google Scholar] [CrossRef] [PubMed]
- Kanton, S.; Boyle, M.J.; He, Z.; Santel, M.; Weigert, A.; Sanchis-Calleja, F.; Guijarro, P.; Sidow, L.; Fleck, J.S.; Han, D.; et al. Organoid single-cell genomic atlas uncovers human-specific features of brain development. Nature 2019, 574, 418–422. [Google Scholar] [CrossRef]
- Park, D.S.; Kozaki, T.; Tiwari, S.K.; Moreira, M.; Khalilnezhad, A.; Torta, F.; Olivie, N.; Thiam, C.H.; Liani, O.; Silvin, A.; et al. iPS-cell-derived microglia promote brain organoid maturation via cholesterol transfer. Nature 2023, 623, 397–405. [Google Scholar] [CrossRef]
- Sabate-Soler, S.; Nickels, S.L.; Saraiva, C.; Berger, E.; Dubonyte, U.; Barmpa, K.; Lan, Y.J.; Kouno, T.; Jarazo, J.; Robertson, G.; et al. Microglia integration into human midbrain organoids leads to increased neuronal maturation and functionality. Glia 2022, 70, 1267–1288. [Google Scholar] [CrossRef] [PubMed]
- Voitiuk, K.; Seiler, S.T.; Pessoa de Melo, M.; Geng, J.; van der Molen, T.; Hernandez, S.; Schweiger, H.E.; Sevetson, J.L.; Parks, D.F.; Robbins, A.; et al. A feedback-driven brain organoid platform enables automated maintenance and high-resolution neural activity monitoring. Internet Things 2025, 33, 101671. [Google Scholar] [CrossRef]
- Li, C.; Fleck, J.S.; Martins-Costa, C.; Burkard, T.R.; Themann, J.; Stuempflen, M.; Peer, A.M.; Vertesy, A.; Littleboy, J.B.; Esk, C.; et al. Single-cell brain organoid screening identifies developmental defects in autism. Nature 2023, 621, 373–380. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, R.; Kang, B.; Qian, S.; He, X.; Zhang, X. Single-cell long-read sequencing in human cerebral organoids uncovers cell-type-specific and autism-associated exons. Cell Rep. 2023, 42, 113335. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Chen, X.; Wettschurack, K.; Que, Z.; Deming, B.A.; Olivero-Acosta, M.I.; Cui, N.; Eaton, M.; Zhao, Y.; et al. Microglial over-pruning of synapses during development in autism-associated SCN2A-deficient mice and human cerebral organoids. Mol. Psychiatry 2024, 29, 2424–2437. [Google Scholar] [CrossRef]
- Liu, H.; Mei, F.; Ye, R.; Han, X.; Wang, S.; Ding, Y.; Zhi, Y.; Pang, K.; Guo, W.; Lu, B. APOE3ch alleviates Abeta and tau pathology and neurodegeneration in the human APP(NL-G-F) cerebral organoid model of Alzheimer’s disease. Cell Res. 2024, 34, 451–454. [Google Scholar] [CrossRef]
- Vanova, T.; Sedmik, J.; Raska, J.; Amruz Cerna, K.; Taus, P.; Pospisilova, V.; Nezvedova, M.; Fedorova, V.; Kadakova, S.; Klimova, H.; et al. Cerebral organoids derived from patients with Alzheimer’s disease with PSEN1/2 mutations have defective tissue patterning and altered development. Cell Rep. 2023, 42, 113310. [Google Scholar] [CrossRef]
- Samarasinghe, R.A.; Miranda, O.A.; Buth, J.E.; Mitchell, S.; Ferando, I.; Watanabe, M.; Allison, T.F.; Kurdian, A.; Fotion, N.N.; Gandal, M.J.; et al. Identification of neural oscillations and epileptiform changes in human brain organoids. Nat. Neurosci. 2021, 24, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Zivko, C.; Sagar, R.; Xydia, A.; Lopez-Montes, A.; Mintzer, J.; Rosenberg, P.B.; Shade, D.M.; Porsteinsson, A.P.; Lyketsos, C.G.; Mahairaki, V. iPSC-derived hindbrain organoids to evaluate escitalopram oxalate treatment responses targeting neuropsychiatric symptoms in Alzheimer’s disease. Mol. Psychiatry 2024, 29, 3644–3652. [Google Scholar] [CrossRef]
- Hao, H.; Xue, Y.; Wu, Y.; Wang, C.; Chen, Y.; Wang, X.; Zhang, P.; Ji, J. A paradigm for high-throughput screening of cell-selective surfaces coupling orthogonal gradients and machine learning-based cell recognition. Bioact. Mater. 2023, 28, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Greener, J.G.; Kandathil, S.M.; Moffat, L.; Jones, D.T. A guide to machine learning for biologists. Nat. Rev. Mol. Cell Biol. 2022, 23, 40–55. [Google Scholar] [CrossRef]
- Bai, L.; Wu, Y.; Li, G.; Zhang, W.; Zhang, H.; Su, J. AI-enabled organoids: Construction, analysis, and application. Bioact. Mater. 2024, 31, 525–548. [Google Scholar] [CrossRef] [PubMed]
- Fei, K.; Zhang, J.; Yuan, J.; Xiao, P. Present Application and Perspectives of Organoid Imaging Technology. Bioengineering 2022, 9, 121. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Zhu, Y.; Tang, J.; Liu, Y.; Lu, M.; Zhang, R.; Sun, A.X. Navigating Brain Organoid Maturation: From Benchmarking Frameworks to Multimodal Bioengineering Strategies. Biomolecules 2025, 15, 1118. https://doi.org/10.3390/biom15081118
Huang J, Zhu Y, Tang J, Liu Y, Lu M, Zhang R, Sun AX. Navigating Brain Organoid Maturation: From Benchmarking Frameworks to Multimodal Bioengineering Strategies. Biomolecules. 2025; 15(8):1118. https://doi.org/10.3390/biom15081118
Chicago/Turabian StyleHuang, Jingxiu, Yingli Zhu, Jiong Tang, Yang Liu, Ming Lu, Rongxin Zhang, and Alfred Xuyang Sun. 2025. "Navigating Brain Organoid Maturation: From Benchmarking Frameworks to Multimodal Bioengineering Strategies" Biomolecules 15, no. 8: 1118. https://doi.org/10.3390/biom15081118
APA StyleHuang, J., Zhu, Y., Tang, J., Liu, Y., Lu, M., Zhang, R., & Sun, A. X. (2025). Navigating Brain Organoid Maturation: From Benchmarking Frameworks to Multimodal Bioengineering Strategies. Biomolecules, 15(8), 1118. https://doi.org/10.3390/biom15081118




