A Review on Low-Dimensional Nanoarchitectonics for Neurochemical Sensing and Modulation in Responsive Neurological Outcomes
Abstract
1. Introduction
2. Neurochemicals and Their Modulation Challenges
3. Classification and Functionalities of Low-Dimensional Nanohybrids
4. LDNHs in Neurochemical Sensing
4.1. Electrochemical Sensing
4.2. Optical Sensing (Fluorescence, Raman, SERS, etc.)
4.3. Quantitative Benchmarks for Sensor Stability and Fouling Resistance
5. Multiplexed and Wireless Sensing Systems for Neurochemical Monitoring
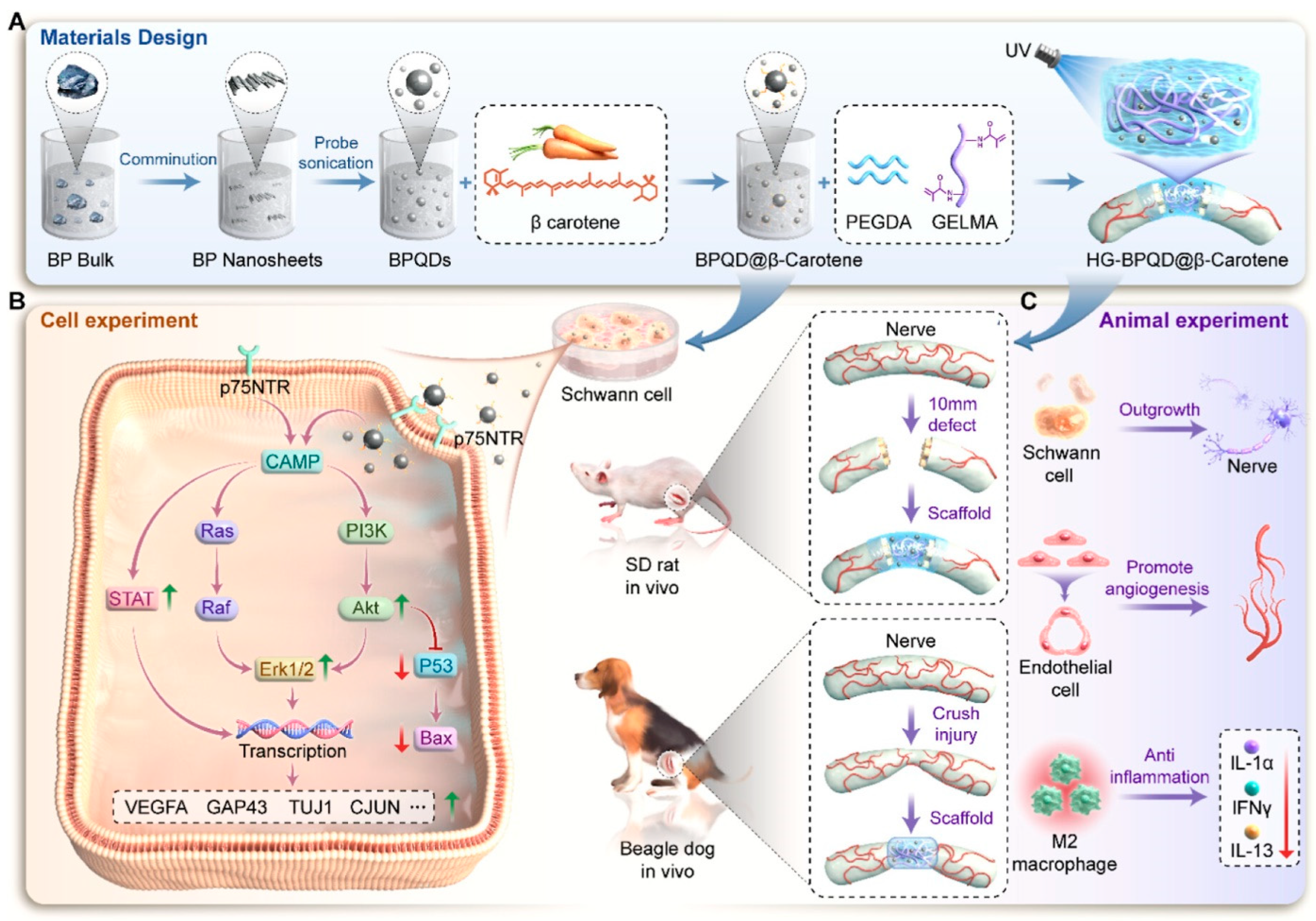
6. LDNHs in Neuromodulation
6.1. Electrical Neuromodulation
6.2. Optogenetics and Photo-Modulation
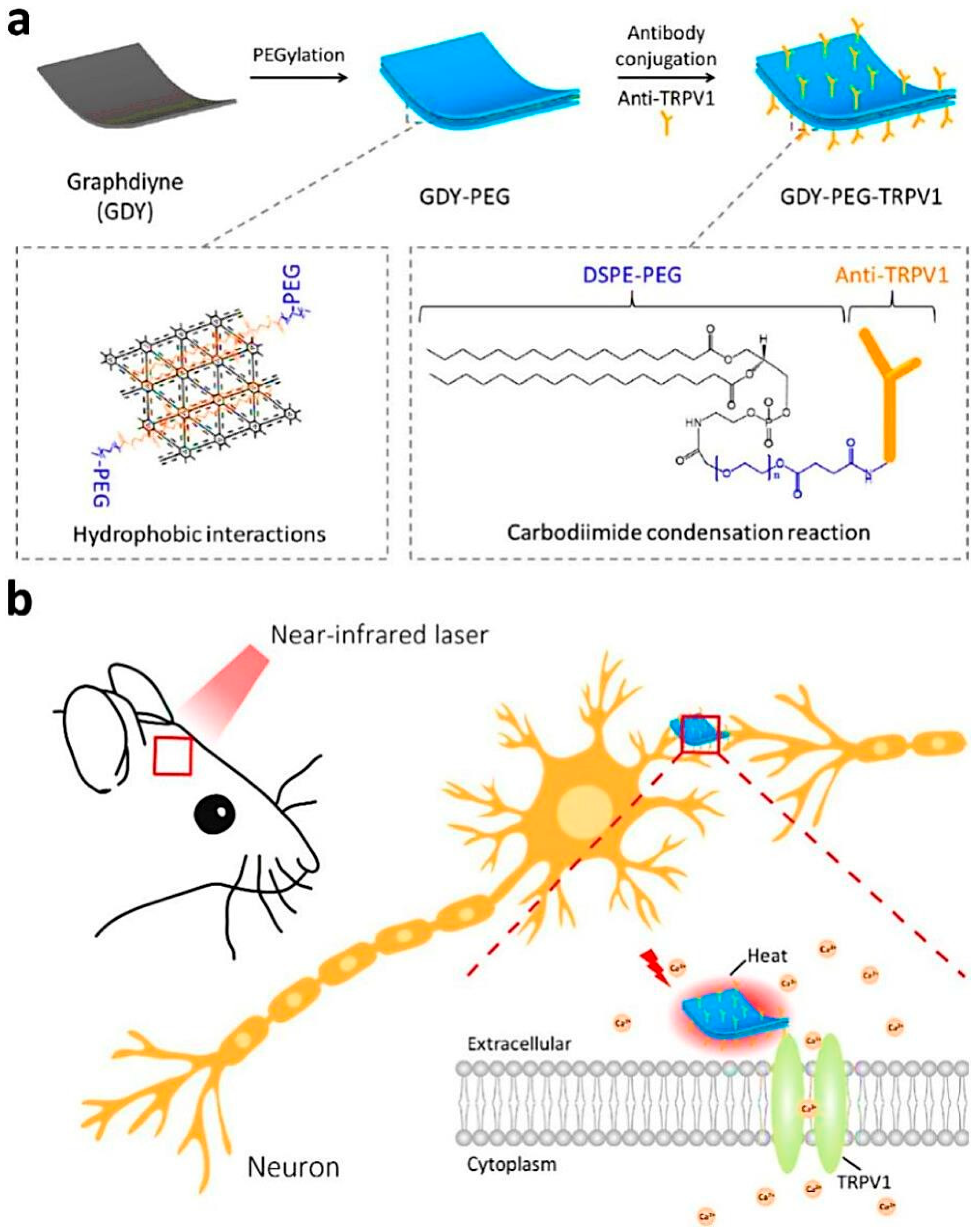
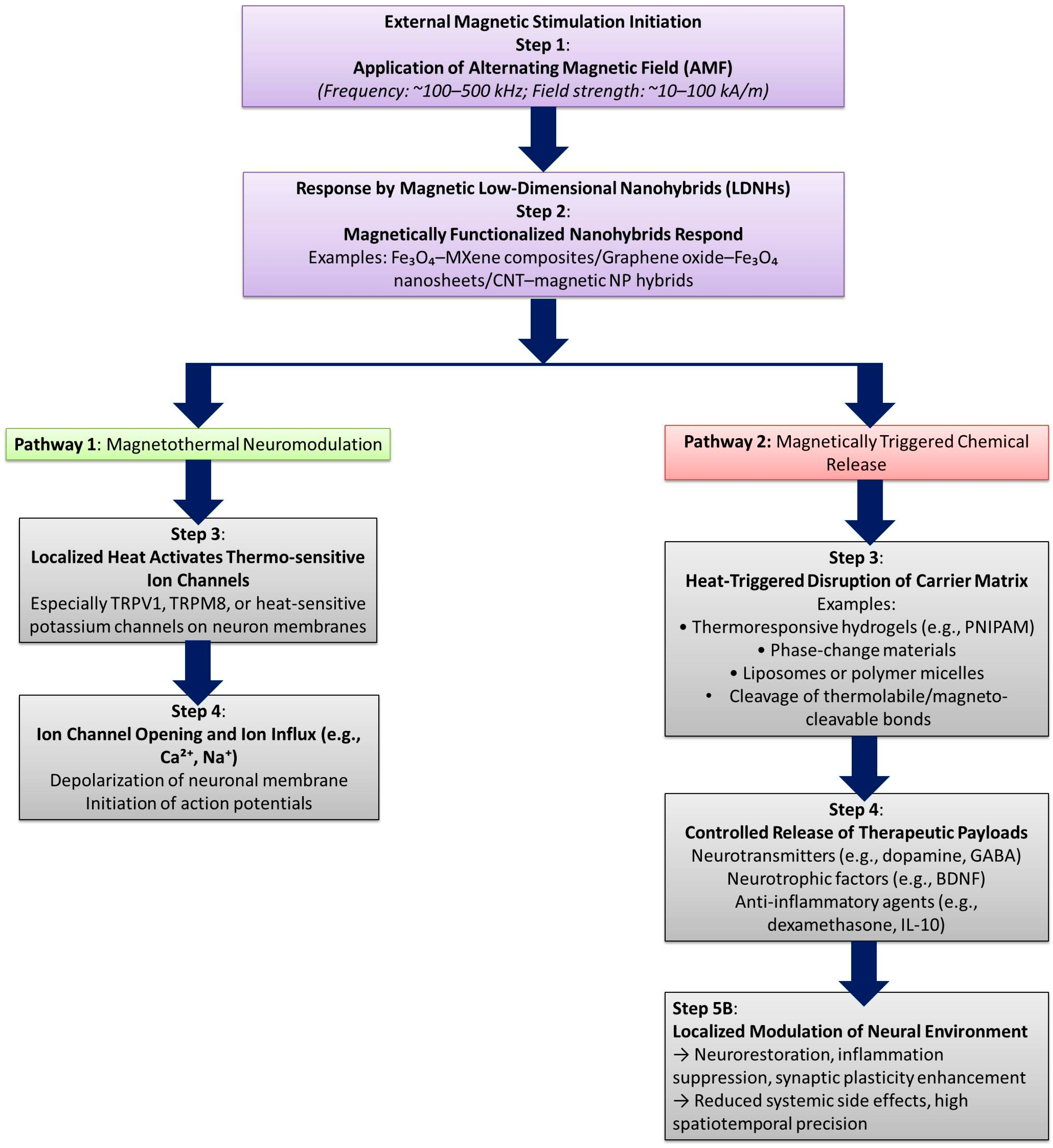
7. Magnetothermal and Chemical Modulation
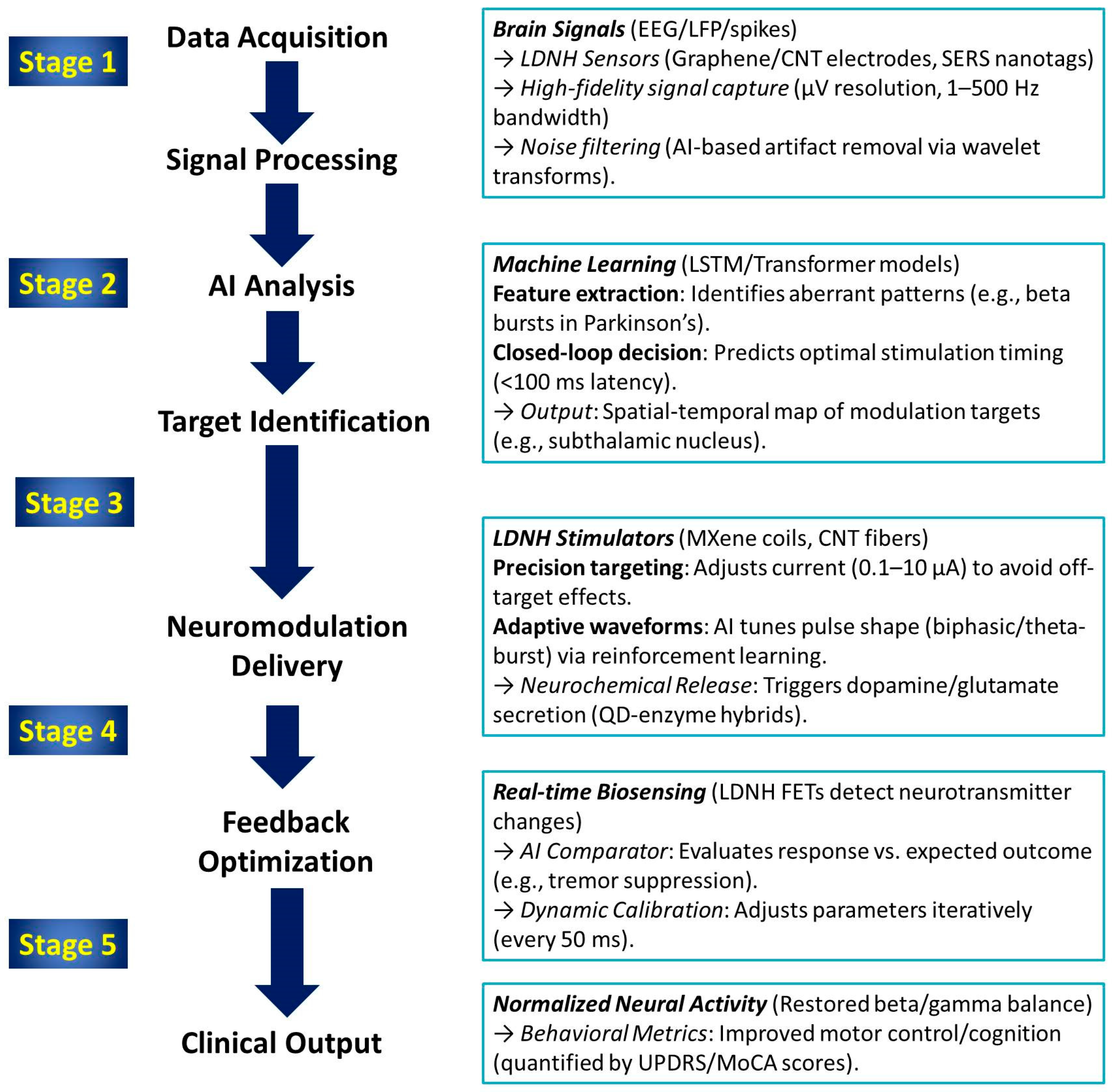
8. Biointerface Engineering, Brain Integration and Artificial Intelligence (AI)
9. Strategies for Reducing Immune Response, Glial Scar Formation
10. Outlook and Future Perspectives
11. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Neurological Disorders: Public Health Challenges; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Amarnath, C.A.; Nanda, S.S.; Papaefthymiou, G.C.; Yi, D.K.; Paik, U. Nanohybridization of low-dimensional nanomaterials: Synthesis, classification, and application. Crit. Rev. Solid State Mater. Sci. 2013, 38, 1–56. [Google Scholar] [CrossRef]
- Singh, S.; Melnik, R. Coupled multiphysics modelling of sensors for chemical, biomedical, and environmental applications with focus on smart materials and low-dimensional nanostructures. Chemosensors 2022, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Ghallab, Y.K.; Elassal, O.S. Biochemical and Neuropharmacology of Psychiatric Disorders. In Nutrition and Psychiatric Disorders: An Evidence-Based Approach to Understanding the Diet-Brain Connection; Springer: Berlin/Heidelberg, Germany, 2024; pp. 25–47. [Google Scholar]
- Ascenzi, B.M.; Myers, M.W.; Buccilli, B. Neurochemistry of the central nervous system. In From Anatomy to Function of the Central Nervous System; Elsevier: Amsterdam, The Netherlands, 2025; pp. 59–107. [Google Scholar]
- Qu, S.; Yu, Z.; Zhou, Y.; Wang, S.; Jia, M.; Chen, T.; Zhang, X. Gut microbiota modulates neurotransmitter and gut-brain signaling. Microbiol. Res. 2024, 287, 127858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pei, X.; Jing, L.; Zhang, Q.; Zhao, H. Lead induced cerebellar toxicology of developmental Japanese quail (Coturnix japonica) via oxidative stress-based Nrf2/Keap1 pathway inhibition and glutathione-mediated apoptosis signaling activation. Environ. Pollut. 2024, 352, 124114. [Google Scholar] [CrossRef]
- Barros, B.J.; Cunha, J.P. Neurophotonics: A comprehensive review, current challenges and future trends. Front. Neurosci. 2024, 18, 1382341. [Google Scholar] [CrossRef]
- Zhi, W.; Li, Y.; Wang, L.; Hu, X. Advancing Neuroscience and Therapy: Insights into Genetic and Non-Genetic Neuromodulation Approaches. Cells 2025, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Tan, A.; Wong, J.; Spagnoli, J.C.; Lam, J.; Blevins, B.D.; Thorne, L.; Ashkan, K.; Xie, J.; Liu, H. Nanotechnology for neuroscience: Promising approaches for diagnostics, therapeutics and brain activity mapping. Adv. Funct. Mater. 2017, 27, 1700489. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Banerjee, S.; Das, N.C. Graphene based emergent nanolights: A short review on the synthesis, properties and application. Res. Chem. Intermed. 2019, 45, 3823–3853. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Margel, S.; Gedanken, A. Tailor made magnetic nanolights: Fabrication to cancer theranostics applications. Nanoscale Adv. 2021, 3, 6762–6796. [Google Scholar] [CrossRef]
- García de Arquer, F.P.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor quantum dots: Technological progress and future challenges. Science 2021, 373, eaaz8541. [Google Scholar] [CrossRef]
- Gidwani, B.; Sahu, V.; Shukla, S.S.; Pandey, R.; Joshi, V.; Jain, V.K.; Vyas, A. Quantum dots: Prospectives, toxicity, advances and applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102308. [Google Scholar] [CrossRef]
- Sun, P.; Xing, Z.; Li, Z.; Zhou, W. Recent advances in quantum dots photocatalysts. Chem. Eng. J. 2023, 458, 141399. [Google Scholar] [CrossRef]
- Das, P.; Maruthapandi, M.; Saravanan, A.; Natan, M.; Jacobi, G.; Banin, E.; Gedanken, A. Carbon dots for heavy-metal sensing, pH-sensitive cargo delivery, and antibacterial applications. ACS Appl. Nano Mater. 2020, 3, 11777–11790. [Google Scholar] [CrossRef]
- Das, P.; Sherazee, M.; Marvi, P.K.; Ahmed, S.R.; Gedanken, A.; Srinivasan, S.; Rajabzadeh, A.R. Waste-derived sustainable fluorescent nanocarbon-coated breathable functional fabric for antioxidant and antimicrobial applications. ACS Appl. Mater. Interfaces 2023, 15, 29425–29439. [Google Scholar] [CrossRef]
- Saravanan, A.; Maruthapandi, M.; Das, P.; Luong, J.H.; Gedanken, A. Green synthesis of multifunctional carbon dots with antibacterial activities. Nanomaterials 2021, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Maruthapandi, M.; Das, P.; Ganguly, S.; Margel, S.; Luong, J.H.; Gedanken, A. Applications of N-doped carbon dots as antimicrobial agents, antibiotic carriers, and selective fluorescent probes for nitro explosives. ACS Appl. Bio Mater. 2020, 3, 8023–8031. [Google Scholar] [CrossRef] [PubMed]
- Maruthapandi, M.; Saravanan, A.; Das, P.; Luong, J.H.; Gedanken, A. Microbial inhibition and biosensing with multifunctional carbon dots: Progress and perspectives. Biotechnol. Adv. 2021, 53, 107843. [Google Scholar] [CrossRef] [PubMed]
- Salah, L.S.; Ouslimani, N.; Bousba, D.; Huynen, I.; Danlée, Y.; Aksas, H. Carbon nanotubes (CNTs) from synthesis to functionalized (CNTs) using conventional and new chemical approaches. J. Nanomater. 2021, 2021, 4972770. [Google Scholar] [CrossRef]
- Siahkouhi, M.; Razaqpur, G.; Hoult, N.; Baghban, M.H.; Jing, G. Utilization of carbon nanotubes (CNTs) in concrete for structural health monitoring (SHM) purposes: A review. Constr. Build. Mater. 2021, 309, 125137. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, N.C. Rheological properties of polymer–carbon composites. In Carbon-Containing Polymer Composites; Springer: Singapore, 2018; pp. 271–294. [Google Scholar]
- Ganguly, S.; Das, N.C. Chlorosulphonated polyethylene and its composites for electronic applications. In Flexible and Stretchable Electronic Composites; Springer: Cham, Switzerland, 2015; pp. 229–259. [Google Scholar]
- Derakhshi, M.; Daemi, S.; Shahini, P.; Habibzadeh, A.; Mostafavi, E.; Ashkarran, A.A. Two-dimensional nanomaterials beyond graphene for biomedical applications. J. Funct. Biomater. 2022, 13, 27. [Google Scholar] [CrossRef]
- Li, J.; Zeng, H.; Zeng, Z.; Zeng, Y.; Xie, T. Promising graphene-based nanomaterials and their biomedical applications and potential risks: A comprehensive review. ACS Biomater. Sci. Eng. 2021, 7, 5363–5396. [Google Scholar] [CrossRef]
- Ganguly, S. Preparation/processing of polymer-graphene composites by different techniques. In Polymer Nanocomposites Containing Graphene; Elsevier: Amsterdam, The Netherlands, 2022; pp. 45–74. [Google Scholar]
- Ganguly, S.; Ghosh, S.; Das, P.; Das, T.K.; Ghosh, S.K.; Das, N.C. Poly (N-vinylpyrrolidone)-stabilized colloidal graphene-reinforced poly (ethylene-co-methyl acrylate) to mitigate electromagnetic radiation pollution. Polym. Bull. 2020, 77, 2923–2943. [Google Scholar] [CrossRef]
- Laraba, S.R.; Luo, W.; Rezzoug, A.; Zahra, Q.u.a.; Zhang, S.; Wu, B.; Chen, W.; Xiao, L.; Yang, Y.; Wei, J. Graphene-based composites for biomedical applications. Green Chem. Lett. Rev. 2022, 15, 724–748. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Perelshtein, I.; Margel, S.; Gedanken, A. Acoustic green synthesis of graphene-gallium nanoparticles and PEDOT: PSS hybrid coating for textile to mitigate electromagnetic radiation pollution. ACS Appl. Nano Mater. 2022, 5, 1644–1655. [Google Scholar] [CrossRef]
- Lee, I.-C.; Li, Y.-C.E.; Thomas, J.L.; Lee, M.-H.; Lin, H.-Y. Recent advances using MXenes in biomedical applications. Mater. Horiz. 2024, 11, 876–902. [Google Scholar] [CrossRef]
- Das, P.; Marvi, P.K.; Ganguly, S.; Tang, X.; Wang, B.; Srinivasan, S.; Rajabzadeh, A.R.; Rosenkranz, A. MXene-based elastomer mimetic stretchable sensors: Design, properties, and applications. Nano-Micro Lett. 2024, 16, 135. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Zheng, M.; Ma, Z.; Chen, Y.; Deng, L.; Guang, F.; Khademolqorani, S.; Banitaba, S.N.; Osman, A.I. Synergistic integration of MXene nanostructures into electrospun fibers for advanced biomedical engineering applications. Nanoscale Horiz. 2024, 9, 1703–1724. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Ganguly, S.; Rosenkranz, A.; Wang, B.; Yu, J.; Srinivasan, S.; Rajabzadeh, A.R. MXene/0D nanocomposite architectures: Design, properties and emerging applications. Mater. Today Nano 2023, 24, 100428. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Saha, A.; Noked, M.; Margel, S.; Gedanken, A. Carbon-dots-initiated photopolymerization: An in situ synthetic approach for MXene/poly (norepinephrine)/copper hybrid and its application for mitigating water pollution. ACS Appl. Mater. Interfaces 2021, 13, 31038–31050. [Google Scholar] [CrossRef]
- Nowsherwan, G.A.; Khan, M.; Iqbal, M.A.; Nowsherwan, N.; Ahmad, M.; Haider, S.; Ali, T.; Morsy, K.; Hussain, S.S. Reviewing black phosphorus for biomedical and optoelectronic applications. Inorg. Chem. Commun. 2024, 160, 111912. [Google Scholar] [CrossRef]
- Qi, W.; Zhang, R.; Wang, Z.; Du, H.; Zhao, Y.; Shi, B.; Wang, Y.; Wang, X.; Wang, P. Advances in the application of black phosphorus-based composite biomedical materials in the field of tissue engineering. Pharmaceuticals 2024, 17, 242. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Jena, B.K. Review and Perspectives on Multifunctional Applications of Hexagonal Boron Nitride Nanosheets and Quantum Dots in Energy Conversions. Energy Fuels 2025, 39, 4119–4150. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Marvi, P.K.; Sherazee, M.; Tang, X.; Srinivasan, S.; Rajabzadeh, A.R. Carbon Dots Infused 3D Printed Cephalopod Mimetic Bactericidal and Antioxidant Hydrogel for Uniaxial Mechano-Fluorescent Tactile Sensor. Adv. Mater. 2024, 36, 2409819. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Ganguly, S.; Bose, M.; Ray, D.; Ghosh, S.; Mondal, S.; Aswal, V.K.; Das, A.K.; Banerjee, S.; Das, N.C. Surface quaternized nanosensor as a one-arrow-two-hawks approach for fluorescence turn “on–off–on” bifunctional sensing and antibacterial activity. New J. Chem. 2019, 43, 6205–6219. [Google Scholar] [CrossRef]
- Nisa, F.u.; Tahir, M.; Khalid, S.; Amin, N.; Yin, H.; Long, Y.; Tang, H.; Iiaz, K.; Khan, A.U.; Naseem, M. Revolutionizing Micro-Scale Energy Storage by 0D Carbon Nanostructures: Synthesis, Integration, Performance Optimization Mechanisms and Sustainable Applications. Adv. Funct. Mater. 2025, 35, 2418053. [Google Scholar] [CrossRef]
- Yin, X.; Pang, H.; Liu, H.; Zhao, J.; Zhang, B.; Liu, D.; Shi, Y. Achieving ultralow friction under high pressure through operando formation of PbS QDs/graphene heterojunction with 0D/1D nanostructure. Carbon 2024, 218, 118748. [Google Scholar] [CrossRef]
- Bai, M.; Shao, X.; Wang, C.; Wang, J.; Wang, X.; Guan, P.; Hu, X. Application of carbon-based nanomaterials in Alzheimer’s disease. Mater. Horiz. 2025, 12, 673–693. [Google Scholar] [CrossRef]
- Al-Muhanna, M.K.; Alghamdi, A.A.; Alrfaei, B.; Afzal, M.; Al-Subaiee, R.; Haddadi, R. Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging. Nanotechnol. Rev. 2024, 13, 20240128. [Google Scholar] [CrossRef]
- Khan, R.; Rahman, N.U.; Hayat, M.F.; Ghernaout, D.; Salih, A.A.; Ashraf, G.A.; Samad, A.; Mahmood, M.A.; Rahman, N.; Sohail, M. Unveiling cutting-edge developments: Architectures and nanostructured materials for application in optoelectronic artificial synapses. Nanoscale 2024, 16, 14589–14620. [Google Scholar] [CrossRef]
- Kour, A.; Panda, H.S.; Singh, I.R.; Kumar, A.; Panda, J.J. Peptide-metal nanohybrids (PMN): Promising entities for combating neurological maladies. Adv. Colloid Interface Sci. 2023, 318, 102954. [Google Scholar] [CrossRef]
- Aminabad, E.D.; Hasanzadeh, M.; Saadati, A.; Feizi, M.A.H.; Safaralizadeh, R.; Mobed, A. An innovative biodevice towards monitoring of miR-153 using specific DNA immobilized on the surface of poly (chitosan) decorated AgNPrs/GQDs-CysA conductive nano-ink: Early-stage diagnosis of Parkinson’s disease using biosensor technology. Mater. Sci. Eng. B 2022, 286, 116017. [Google Scholar] [CrossRef]
- Verma, R.; Yadav, S.K.; Singh, D.; Singh, J. Fabrication of a mesoporous CoFe2O4/rGO nanohybrid and laccase interface biosensor for rapid detection of adrenaline for neurodegenerative disease diagnosis. Mater. Adv. 2025, 6, 1988–2001. [Google Scholar] [CrossRef]
- Wu, C.; Chen, S.; Zhou, T.; Wu, K.; Qiao, Z.; Zhang, Y.; Xin, N.; Liu, X.; Wei, D.; Sun, J. Antioxidative and conductive nanoparticles-embedded cell niche for neural differentiation and spinal cord injury repair. ACS Appl. Mater. Interfaces 2021, 13, 52346–52361. [Google Scholar] [CrossRef]
- Amaro Junior, E. Artificial intelligence and Big Data in neurology. Arq. Neuro-Psiquiatr. 2022, 80, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Huang, T.; Wang, X.; Niu, J.; Zhao, W.; Xu, H.; Lu, L. Application of big data and artificial intelligence approaches in diagnosis and treatment of neuropsychiatric diseases. In Big Data in Psychiatry & Neurology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 305–323. [Google Scholar]
- Sardana, V. From big data to better outcomes: The promising role of artificial intelligence in neuroscience. J. Mod. Med. 2023, 1, 1–2. [Google Scholar] [CrossRef]
- Gupta, N.S.; Kumar, P. Perspective of artificial intelligence in healthcare data management: A journey towards precision medicine. Comput. Biol. Med. 2023, 162, 107051. [Google Scholar] [CrossRef]
- Alkon, D.L.; Sakakibara, M.; Naito, S.; Heldman, E.; Lederhendler, I. The role of neurochemical modulation in learning. Neurosci. Res. 1986, 3, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Pan, C.; Mao, L.; Yu, P. Neuromodulation with chemicals: Opportunities and challenges. Fundam. Res. 2025, 5, 55–62. [Google Scholar] [CrossRef]
- Uddin, M.S.; Mamun, A.; Kabir, M.T.; Nasrullah, M.; Wahid, F.; Begum, M.M. Neurochemistry of neurochemicals: Messengers of brain functions. J. Intellect. Disabil. Diagn. Treat 2018, 5, 137–151. [Google Scholar] [CrossRef]
- Cools, R.; Arnsten, A.F. Neuromodulation of prefrontal cortex cognitive function in primates: The powerful roles of monoamines and acetylcholine. Neuropsychopharmacology 2022, 47, 309–328. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Park, N.H.; Lee, Y.C.; Kim, K.-H.; Hong, J. Advances and challenges in neurochemical profiling of biological samples using mass spectrometry coupled with separation methods. TrAC Trends Anal. Chem. 2018, 106, 159–168. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Drug delivery systems, CNS protection, and the blood brain barrier. BioMed Res. Int. 2014, 2014, 869269. [Google Scholar] [CrossRef]
- Reinhold, A.; Rittner, H. Barrier function in the peripheral and central nervous system—A review. Pflügers Arch.-Eur. J. Physiol. 2017, 469, 123–134. [Google Scholar] [CrossRef]
- Pachter, J.S.; de Vries, H.E.; Fabry, Z. The blood-brain barrier and its role in immune privilege in the central nervous system. J. Neuropathol. Exp. Neurol. 2003, 62, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.Y.; Shafiei, G.; Markello, R.D.; Smart, K.; Cox, S.M.; Nørgaard, M.; Beliveau, V.; Wu, Y.; Gallezot, J.-D.; Aumont, É. Mapping neurotransmitter systems to the structural and functional organization of the human neocortex. Nat. Neurosci. 2022, 25, 1569–1581. [Google Scholar] [CrossRef]
- Hörtnagl, H.; Berger, M.; Sperk, G.; Pifl, C. Regional heterogeneity in the distribution of neurotransmitter markers in the rat hippocampus. Neuroscience 1991, 45, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Lawn, T.; Howard, M.A.; Turkheimer, F.; Misic, B.; Deco, G.; Martins, D.; Dipasquale, O. From neurotransmitters to networks: Transcending organisational hierarchies with molecular-informed functional imaging. Neurosci. Biobehav. Rev. 2023, 150, 105193. [Google Scholar] [CrossRef] [PubMed]
- Zilles, K.; Palomero-Gallagher, N. Multiple transmitter receptors in regions and layers of the human cerebral cortex. Front. Neuroanat. 2017, 11, 78. [Google Scholar] [CrossRef]
- Lam, K.S.; Aman, M.G.; Arnold, L.E. Neurochemical correlates of autistic disorder: A review of the literature. Res. Dev. Disabil. 2006, 27, 254–289. [Google Scholar] [CrossRef]
- McIntyre, C.C.; Anderson, R.W. Deep brain stimulation mechanisms: The control of network activity via neurochemistry modulation. J. Neurochem. 2016, 139, 338–345. [Google Scholar] [CrossRef]
- Albert, G.; Cook, C.; Prato, F.; Thomas, A. Deep brain stimulation, vagal nerve stimulation and transcranial stimulation: An overview of stimulation parameters and neurotransmitter release. Neurosci. Biobehav. Rev. 2009, 33, 1042–1060. [Google Scholar] [CrossRef]
- Grill, W.M.; Mclntyre, C.C. Extracellular excitation of central neurons: Implications for the mechanisms of deep brain stimulation. Thalamus Relat. Syst. 2001, 1, 269–277. [Google Scholar] [CrossRef]
- Boutet, A.; Jain, M.; Gwun, D.; Rusjan, P.; Neudorfer, C.; Elias, G.J.; Germann, J.; Bilbily, A.; Kucharczyk, W.; Fasano, A. Modulation of CNS functions by deep brain stimulation: Insights provided by molecular imaging. PET SPECT Neurol. 2021, 1177–1244. [Google Scholar] [CrossRef]
- Deniau, J.M.; Degos, B.; Bosch, C.; Maurice, N. Deep brain stimulation mechanisms: Beyond the concept of local functional inhibition. Eur. J. Neurosci. 2010, 32, 1080–1091. [Google Scholar] [CrossRef]
- Wilson, D.; Moehlis, J. Clustered desynchronization from high-frequency deep brain stimulation. PLoS Comput. Biol. 2015, 11, e1004673. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, M.; Xiao, L.; Gui, L.; Zheng, W.; Bai, L.; Su, B.; Li, B.; Xu, Y.; Pan, W. Potential therapeutic mechanism of deep brain stimulation of the nucleus accumbens in obsessive-compulsive disorder. Front. Cell. Neurosci. 2023, 16, 1057887. [Google Scholar] [CrossRef]
- Sweeney-Reed, C.M.; Lee, H.; Rampp, S.; Zaehle, T.; Buentjen, L.; Voges, J.; Holtkamp, M.; Hinrichs, H.; Heinze, H.-J.; Schmitt, F.C. Thalamic interictal epileptiform discharges in deep brain stimulated epilepsy patients. J. Neurol. 2016, 263, 2120–2126. [Google Scholar] [CrossRef]
- Lozano, A.M.; Lipsman, N.; Bergman, H.; Brown, P.; Chabardes, S.; Chang, J.W.; Matthews, K.; McIntyre, C.C.; Schlaepfer, T.E.; Schulder, M. Deep brain stimulation: Current challenges and future directions. Nat. Rev. Neurol. 2019, 15, 148–160. [Google Scholar] [CrossRef]
- Westfall, T.C. Local regulation of adrenergic neurotransmission. Physiol. Rev. 1977, 57, 659–728. [Google Scholar] [CrossRef] [PubMed]
- Madhurantakam, S.; Karnam, J.B.; Brabazon, D.; Takai, M.; Ahad, I.U.; Balaguru Rayappan, J.B.; Krishnan, U.M. “Nano”: An emerging avenue in electrochemical detection of neurotransmitters. ACS Chem. Neurosci. 2020, 11, 4024–4047. [Google Scholar] [CrossRef] [PubMed]
- Adam, T.; Gopinath, S.C. Zero to Multi-dimensional Nanohybrid Materials: Synthesis and Characterization. In Hybrid-Nanomaterials: Fabrication, Characterization and Applications; Springer: Berlin/Heidelberg, Germany, 2024; pp. 73–87. [Google Scholar]
- Jiang, A.; Xing, S.; Lin, H.; Chen, Q.; Li, M. Role of pyramidal low-dimensional semiconductors in advancing the field of optoelectronics. Photonics 2024, 11, 370. [Google Scholar] [CrossRef]
- Yan, Y.; Xin, Y.; Zhao, Q. Advanced Low-Dimensional Carbon Nanomaterials for Oxygen Electrocatalysis. Nanomaterials 2025, 15, 254. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Lopez, J.A.; Villalpando, I.; Salazar, M.I.; Torres-Torres, C. Hierarchical Nanobiosensors at the End of the SARS-CoV-2 Pandemic. Biosensors 2024, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, Q.; Wang, Y. Piezoelectric applications of low-dimensional composites and porous materials. Materials 2024, 17, 844. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zhao, C.; Li, M.; Xu, H. Recent Progress in Surface Acoustic Wave Sensors Based on Low-Dimensional Materials and Their Applications. Chemosensors 2024, 12, 255. [Google Scholar] [CrossRef]
- Subrati, M.; Lyra, K.M.; Spyrou, K.; Magdalini Toliou, I.; Petrou, G.; Manganiaris, P.; Papavasiliou, A.; Sakellis, E.; Athanasekou, C.P.; Glisenti, A. Valorization of Industrial Waste Graphite Fines into Graphene Oxide-Based Nanohybrids. Chempluschem 2025, 90, e202400692. [Google Scholar] [CrossRef]
- Lin, M.-H.; Hong, S.-H.; Ding, J.-F.; Liu, C.-L. Organic Porous Materials and Their Nanohybrids for Next-Generation Thermoelectric Application. ACS Appl. Mater. Interfaces 2024, 16, 67116–67133. [Google Scholar] [CrossRef]
- Keshtkari, L.; Rabczuk, T. Pyrazinoquinoxaline graphdiyne: A novel high-capacity anode material for metal-ion batteries. Phys. E Low-Dimens. Syst. Nanostructures 2024, 161, 115955. [Google Scholar] [CrossRef]
- Wang, X.; Lu, T.; Cai, Z.; Han, D.; Ye, X.; Liu, Z. A photoelectrochemical sensor for real-time monitoring of neurochemical signals in the brain of awake animals. Anal. Chem. 2024, 96, 6079–6088. [Google Scholar] [CrossRef]
- Zhu, J.; Shao, D.; Wen, W.; Tian, Z.; Zhang, X.; Wang, S. Self-powered electrochemical sensor based on photoelectrode: An up-to-date review. Coord. Chem. Rev. 2024, 518, 216095. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, H.; Liu, X.; Huang, Y.; Xu, X.; Alharbi, N.S.; Hayat, T.; Li, J. Hybrid 0D–2D Nanoheterostructures: In Situ Growth of Amorphous Silver Silicates Dots on g-C3N4 Nanosheets for Full-Spectrum Photocatalysis. ACS Appl. Mater. Interfaces 2016, 8, 35138–35149. [Google Scholar] [CrossRef] [PubMed]
- Gunjakar, J.L.; Kim, I.Y.; Lee, J.M.; Jo, Y.K.; Hwang, S.-J. Exploration of Nanostructured Functional Materials Based on Hybridization of Inorganic 2D Nanosheets. J. Phys. Chem. C 2014, 118, 3847–3863. [Google Scholar] [CrossRef]
- Hong, F.; Chen, R.; Lu, P.; Li, L.; Xiao, R.; Chen, Y.; Yang, H. A universal, portable, and ultra-sensitive pipet immunoassay platform for deoxynivalenol detection based on dopamine self-polymerization-mediated bioconjugation and signal amplification. J. Hazard. Mater. 2022, 436, 129257. [Google Scholar] [CrossRef]
- Choi, H.K.; Choi, J.-H.; Yoon, J. An updated review on electrochemical nanobiosensors for neurotransmitter detection. Biosensors 2023, 13, 892. [Google Scholar] [CrossRef]
- Panicker, L.R.; Shamsheera, F.; Narayan, R.; Kotagiri, Y.G. Wearable Electrochemical Microneedle Sensors Based on the Graphene-Silver-Chitosan Nanocomposite for Real-Time Continuous Monitoring of the Depression Biomarker Serotonin. ACS Appl. Nano Mater. 2023, 6, 20601–20611. [Google Scholar] [CrossRef]
- Chen, J.; Xia, F.; Ding, X.; Zhang, D. Highly Sensitive and Biocompatible Microsensor for Selective Dynamic Monitoring of Dopamine in Rat Brain. ACS Sens. 2024, 9, 6207–6217. [Google Scholar] [CrossRef]
- Li, Y.; Chang, C.-C.; Wang, C.; Wu, W.-T.; Wang, C.-M.; Tu, H.-L. Microfluidic Biosensor Decorated with an Indium Phosphate Nanointerface for Attomolar Dopamine Detection. ACS Sens. 2023, 8, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xia, F.; Ding, X.; Zhang, D. Universal Covalent Grafting Strategy of an Aptamer on a Carbon Fiber Microelectrode for Selective Determination of Dopamine In Vivo. Anal. Chem. 2024, 96, 10322–10331. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Gu, C.; Xiao, Q.; Chen, J.; Yin, Z.-Z.; Liu, H.; Fan, K.; Li, L. A highly selective and sensitive biosensor for dopamine based on a surface molecularly imprinted layer to coordinate nano-interface functionalized acupuncture needle. Chem. Eng. J. 2022, 436, 135203. [Google Scholar] [CrossRef]
- Thakur, N.; Gupta, D.; Mandal, D.; Nagaiah, T.C. Ultrasensitive electrochemical biosensors for dopamine and cholesterol: Recent advances, challenges and strategies. Chem. Commun. 2021, 57, 13084–13113. [Google Scholar] [CrossRef]
- Ye, C.; Shi, D.; Zhu, Y.; Shi, P.; Zhao, N.; Sun, Z.; Zhang, Z.; Zhang, D.; Lv, Y.; Wu, W.; et al. Graphene electrochemical biosensors combining effervescent solid-phase extraction (ESPE) for rapid, ultrasensitive, and simultaneous determination of DA, AA, and UA. Biosens. Bioelectron. 2025, 268, 116899. [Google Scholar] [CrossRef]
- Zhou, D.-W.; Yin, M.; Shen, Y.; Wang, X.-X.; Wang, C.-Y.; Chen, K.-Z.; Fang, Q.; Qiao, S.-L. LDHzyme-assisted high-performance on-site tracking of levodopa pharmacokinetics for Parkinson’s disease management. Biosens. Bioelectron. 2025, 268, 116926. [Google Scholar] [CrossRef]
- Dong, H.; Chen, W.; Xu, K.; Zheng, L.; Wei, B.; Liu, R.; Yang, J.; Wang, T.; Zhou, Y.; Zhang, Y.; et al. High Selectivity Fluorescence and Electrochemical Dual-Mode Detection of Glutathione in the Serum of Parkinson’s Disease Model Mice and Humans. Anal. Chem. 2025, 97, 1318–1328. [Google Scholar] [CrossRef]
- Chavan, S.G.; Rathod, P.R.; Koyappayil, A.; Hwang, S.; Lee, M.-H. Recent advances of electrochemical and optical point-of-care biosensors for detecting neurotransmitter serotonin biomarkers. Biosens. Bioelectron. 2024, 267, 116743. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, C.; Chen, S.; Li, J. Miniaturized Electrochemical Sensing Platforms for Quantitative Monitoring of Glutamate Dynamics in the Central Nervous System. Angew. Chem. Int. Ed. 2024, 63, e202406867. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Hwang, Y.; An, S.; Park, J.; Kwon, Y.-B.; Cho, B.; Kwon, D.; Kim, Y.; Kang, S. Electromagnetized MXenes Enhance the Efficient Direct Reprogramming of Dopamine Neurons for Parkinson’s Disease Therapy. ACS Nano 2025, 19, 16744–16759. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Mao, G.; Zhu, G.; Yi, Y. Self-reduction of gold@ platinum bimetallic nanoparticles on Ti 3 C 2 T x MXene nanoribbons coupled with hydrogel and smartphone technology for colorimetric detection of silver ions. Anal. Methods 2025, 17, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Shen, T.; Duan, B.; Liu, Z.; Wang, C. In vivo electrochemical biosensing Technologies for Neurochemicals: Recent advances in electrochemical sensors and devices. ACS Sens. 2025, 10, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Zhang, Y.; Wang, L.; Geng, Q.; Liu, D.; Duan, L.; Wang, Y.; Cui, J. Ratiometric dual signal-enhancing-based electrochemical biosensor for ultrasensitive kanamycin detection. ACS Appl. Mater. Interfaces 2020, 12, 52713–52720. [Google Scholar] [CrossRef]
- Sugunan, A.; Rethnakumaran, A.V.; Menamparambath, M.M. A review on Ti3C2Tx based nanocomposites for the electrochemical sensing of clinically relevant biomarkers. Sens. Diagn. 2024, 3, 1769–1788. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Nataraj, N.; Meyyappan, M.; Pal, U. Graphene-Based Field-Effect Transistors in Biosensing and Neural Interfacing Applications: Recent Advances and Prospects. Anal. Chem. 2023, 95, 2590–2622. [Google Scholar] [CrossRef]
- Gupta, P.; Goyal, R.N.; Shim, Y.-B. Simultaneous analysis of dopamine and 5-hydroxyindoleacetic acid at nanogold modified screen printed carbon electrodes. Sens. Actuators B Chem. 2015, 213, 72–81. [Google Scholar] [CrossRef]
- Min, J.; Tu, J.; Xu, C.; Lukas, H.; Shin, S.; Yang, Y.; Solomon, S.A.; Mukasa, D.; Gao, W. Skin-interfaced wearable sweat sensors for precision medicine. Chem. Rev. 2023, 123, 5049–5138. [Google Scholar] [CrossRef]
- Manoharan Nair Sudha Kumari, S.; Thankappan Suryabai, X. Sensing the Future─Frontiers in Biosensors: Exploring Classifications, Principles, and Recent Advances. ACS Omega 2024, 9, 48918–48987. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Gao, N.; Chang, G.; Wu, Y. Biosensors for Early Detection of Parkinson’s Disease: Principles, Applications, and Future Prospects. Biosensors 2025, 15, 280. [Google Scholar] [CrossRef] [PubMed]
- Nisa, M.U.; Yaqub, A.; Khan, M.H.; Yaseen, F.; Jilani, S.; Ajab, H.; Shah, N.S.; Al-Anazi, A. Nanotechnology-driven electrochemical neurotransmitter sensing as a fundamental approach towards improving diagnostics and therapeutics: A review. Sens. Actuators Rep. 2025, 9, 100292. [Google Scholar] [CrossRef]
- Amouzadeh Tabrizi, M. A facile method for the fabrication of the microneedle electrode and its application in the enzymatic determination of glutamate. Biosensors 2023, 13, 828. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.J.; Hollmann, C.A.; Zhang, Z.; Suffern, D.; Bradforth, S.E.; Dimitrijevic, N.M.; Minarik, W.G.; Nadeau, J.L. Photophysics of dopamine-modified quantum dots and effects on biological systems. Nat. Mater. 2006, 5, 409–417. [Google Scholar] [CrossRef]
- Kaur, H.; Siwal, S.S.; Saini, R.V.; Singh, N.; Thakur, V.K. Significance of an Electrochemical Sensor and Nanocomposites: Toward the Electrocatalytic Detection of Neurotransmitters and Their Importance within the Physiological System. ACS Nanosci. Au 2023, 3, 1–27. [Google Scholar] [CrossRef]
- Sobhanan, J.; Rival, J.V.; Anas, A.; Shibu, E.S.; Takano, Y.; Biju, V. Luminescent quantum dots: Synthesis, optical properties, bioimaging and toxicity. Adv. Drug Deliv. Rev. 2023, 197, 114830. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, S.; Liu, Z.; Wang, H.; Huang, D. CdSe-ZnS Quantum Dots for Selective and Sensitive Detection and Quantification of Hypochlorite. Anal. Chem. 2010, 82, 9775–9781. [Google Scholar] [CrossRef]
- Liu, X.; Dang, A.; Li, T.; Lee, T.-C.; Sun, Y.; Liu, Y.; Ye, F.; Ma, S.; Yang, Y.; Deng, W. Triple-enhanced Raman scattering sensors from flexible MXene/Au nanocubes platform via attenuating the coffee ring effect. Biosens. Bioelectron. 2023, 237, 115531. [Google Scholar] [CrossRef] [PubMed]
- Pienpinijtham, P.; Kitahama, Y.; Ozaki, Y. Progress of tip-enhanced Raman scattering for the last two decades and its challenges in very recent years. Nanoscale 2022, 14, 5265–5288. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Huang, Y.; Shen, Z.; Chen, X. Synthesis and bioapplications of Ag2S quantum dots with near-infrared fluorescence. Adv. Mater. 2021, 33, 2007768. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, C.; Song, S.; Ding, Y.; Meng, N.; Liu, X.; Zhang, Y.; Gong, L.; Wu, W. Ultrasonic enhancement of microdroplet-based interfacial reaction for improving the synthesis of Ag2S QDs. Ultrason. Sonochemistry 2023, 95, 106411. [Google Scholar] [CrossRef]
- Jiang, P.; Chen, Z. Ligand effect on the synthesis of emission-tunable near-infrared Ag 2 S quantum dots. New J. Chem. 2017, 41, 5707–5712. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Zhao, Y.-W.; Zhang, Z.-Y.; Xiong, H.-M.; Yu, S.-N. One-pot synthesis of water-dispersible Ag2S quantum dots with bright fluorescent emission in the second near-infrared window. Nanotechnology 2013, 24, 055706. [Google Scholar] [CrossRef]
- Xing, L.; Xu, S.; Cui, J.; Wang, L. Solvent tailored strategy for synthesis of ultrasmall Ag2S quantum dots with near-infrared-II luminescence. J. Nanosci. Nanotechnol. 2019, 19, 4549–4555. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.T.; Roy, I.; Ding, H.; Bergey, E.J.; Prasad, P.N. Biocompatible near-infrared quantum dots as ultrasensitive probes for long-term in vivo imaging applications. Small 2009, 5, 1997–2004. [Google Scholar] [CrossRef]
- Bentolila, L.A.; Ebenstein, Y.; Weiss, S. Quantum dots for in vivo small-animal imaging. J. Nucl. Med. 2009, 50, 493–496. [Google Scholar] [CrossRef]
- Zhang, J.; Han, S.; Zhao, Z.; Zhou, C.; Chen, H.; Hou, J.; Wu, J. Ultrasmall Black Phosphorus Quantum Dots with Robust Antioxidative Properties for Acute Kidney and Liver Injury Therapy. Small 2025, 21, 2407543. [Google Scholar] [CrossRef]
- Das, P.; Saha, S.; Kumar Guha, P.; Kumar Bhunia, A. Quantum dot-protein interface: Interaction of the CdS quantum dot with human hemoglobin for the study of the energy transfer process and binding mechanism along with detection of the unfolding of hemoglobin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 324, 124937. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Ganguly, S.; Agarwal, T.; Maity, P.; Ghosh, S.; Choudhary, S.; Gangopadhyay, S.; Maiti, T.K.; Dhara, S.; Banerjee, S.; et al. Heteroatom doped blue luminescent carbon dots as a nano-probe for targeted cell labeling and anticancer drug delivery vehicle. Mater. Chem. Phys. 2019, 237, 121860. [Google Scholar] [CrossRef]
- Hu, M.; Liang, C.; Wang, D. Implantable bioelectrodes: Challenges, strategies, and future directions. Biomater. Sci. 2024, 12, 270–287. [Google Scholar] [CrossRef]
- Zhao, Y.; Mejia, E.; Xiao, C.; Song, J.; Zhu, W.; Lezec, H.; Agrawal, A.; Zhou, W. Nanolaminate Nano-Optoelectrodes Enable Dual-Channel Plasmon-Enhanced Raman Spectroscopy for Electrochemistry. Small Methods 2025, 9, 2402107. [Google Scholar] [CrossRef]
- Roberts, C.; Lin, J.; Kohlman, E.; Jain, N.; Amekor, M.; Alcaraz, A.J.; Hogan, N.; Hecker, M.; Brinkmann, M. Acute and subchronic toxicity of 6PPD-quinone to early life stage lake trout (Salvelinus namaycush). Environ. Sci. Technol. 2025, 59, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Sharma, N.; Dalal, A.; Panghal, P.; Sharma, A.K.; Kumar, S. Cutting-edge regeneration technologies for saturated adsorbents: A systematic review on pathways to circular wastewater treatment system. Environ. Monit. Assess. 2025, 197, 215. [Google Scholar] [CrossRef] [PubMed]
- Yasri, N.G.; Roberts, E.P. Electrochemical regeneration of adsorbents: An Electrochemist’s perspective. Curr. Opin. Electrochem. 2024, 46, 101504. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Zhang, K.; Beitollahi, H.; Van Le, Q.; Jang, H.W.; Shokouhimehr, M. Carbon and graphene quantum dots: A review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC Adv. 2020, 10, 15406–15429. [Google Scholar] [CrossRef]
- Han, M.; Karatum, O.; Nizamoglu, S. Optoelectronic neural interfaces based on quantum dots. ACS Appl. Mater. Interfaces 2022, 14, 20468–20490. [Google Scholar] [CrossRef]
- Delehanty, J.B.; Susumu, K.; Manthe, R.L.; Algar, W.R.; Medintz, I.L. Active cellular sensing with quantum dots: Transitioning from research tool to reality; a review. Anal. Chim. Acta 2012, 750, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Lai, W.; Man, T.; Chang, B.; Li, L.; Chandrasekaran, A.R.; Pei, H. Rationally Engineered Nucleic Acid Architectures for Biosensing Applications. Chem. Rev. 2019, 119, 11631–11717. [Google Scholar] [CrossRef]
- Proppe, A.H.; Berkinsky, D.B.; Zhu, H.; Šverko, T.; Kaplan, A.E.; Horowitz, J.R.; Kim, T.; Chung, H.; Jun, S.; Bawendi, M.G. Highly stable and pure single-photon emission with 250 ps optical coherence times in InP colloidal quantum dots. Nat. Nanotechnol. 2023, 18, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Sadhu, S.; Haldar, K.K.; Patra, A. Size dependent resonance energy transfer between semiconductor quantum dots and dye using FRET and kinetic model. J. Phys. Chem. C 2010, 114, 3891–3897. [Google Scholar] [CrossRef]
- Hou, Z. The New Era of Neural Modulation Led by Smart Nanomaterials. Int. J. Nanomed. 2024, 19, 12287–12295. [Google Scholar] [CrossRef]
- Wu, L.; Wang, J.; Gao, N.; Ren, J.; Zhao, A.; Qu, X. Electrically pulsatile responsive drug delivery platform for treatment of Alzheimer’s disease. Nano Res. 2015, 8, 2400–2414. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Zeng, M.; Zhang, Y.; Wei, D.; Sun, J.; Fan, H. Functional material-mediated wireless physical stimulation for neuro-modulation and regeneration. J. Mater. Chem. B 2023, 11, 9056–9083. [Google Scholar] [CrossRef]
- Sun, F.; Shen, H.; Yang, Q.; Yuan, Z.; Chen, Y.; Guo, W.; Wang, Y.; Yang, L.; Bai, Z.; Liu, Q.; et al. Dual Behavior Regulation: Tether-Free Deep-Brain Stimulation by Photothermal and Upconversion Hybrid Nanoparticles. Adv. Mater. 2023, 35, 2210018. [Google Scholar] [CrossRef]
- Huang, P.-Y.; Jiang, B.-Y.; Chen, H.-J.; Xu, J.-Y.; Wang, K.; Zhu, C.-Y.; Hu, X.-Y.; Li, D.; Zhen, L.; Zhou, F.-C.; et al. Neuro-inspired optical sensor array for high-accuracy static image recognition and dynamic trace extraction. Nat. Commun. 2023, 14, 6736. [Google Scholar] [CrossRef]
- Lu, Q.; Sun, Y.; Liang, Z.; Zhang, Y.; Wang, Z.; Mei, Q. Nano-optogenetics for Disease Therapies. ACS Nano 2024, 18, 14123–14144. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, M.S.; Sharma, R.; Veetil, S.K.; Srivastava, S.K.; Kateriya, S. Modular Diversity of the BLUF Proteins and Their Potential for the Development of Diverse Optogenetic Tools. Appl. Sci. 2019, 9, 3924. [Google Scholar] [CrossRef]
- Yoo, S.; Park, J.-H.; Nam, Y. Single-Cell Photothermal Neuromodulation for Functional Mapping of Neural Networks. ACS Nano 2019, 13, 544–551. [Google Scholar] [CrossRef]
- Li, H.; Zha, S.; Li, H.; Liu, H.; Wong, K.-L.; All, A.H. Polymeric Dendrimers as Nanocarrier Vectors for Neurotheranostics. Small 2022, 18, 2203629. [Google Scholar] [CrossRef]
- Shao, L.; Wei, H.; Liu, J.; Ma, W.; Yu, P.; Wang, M.; Mao, L. Graphdiyne as a Highly Efficient and Neuron-Targeted Photothermal Transducer for in Vivo Neuromodulation. ACS Nano 2024, 18, 15607–15616. [Google Scholar] [CrossRef]
- Shen, J.; Sun, Y.; Liu, X.; Chai, Y.; Wang, C.; Xu, J. Nerve Regeneration Potential of Antioxidant-Modified Black Phosphorus Quantum Dots in Peripheral Nerve Injury. ACS Nano 2024, 18, 23518–23536. [Google Scholar] [CrossRef]
- Lu, X.; Li, G.; Jiao, W.; Li, K.; Zhang, T.; Liu, X.; Fan, H. Magnetic nanomaterials-mediated neuromodulation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1890. [Google Scholar] [CrossRef] [PubMed]
- Tommasini, G.; Sol-Fernández, S.D.; Flavián-Lázaro, A.C.; Lewinska, A.; Wnuk, M.; Tortiglione, C.; Moros, M. Remote Magneto–Thermal Modulation of Reactive Oxygen Species Balance Enhances Tissue Regeneration In Vivo. Adv. Funct. Mater. 2024, 34, 2405282. [Google Scholar] [CrossRef]
- Fu, H.; Chen, L.; Fang, W.; Hu, P.; Shi, J. Mild magnetothermal enhanced nanocatalytic immunotherapy for solid tumors by immune cell activation and intratumoral infiltration. Nano Today 2023, 52, 101987. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. 3D printed magnetic polymer composite hydrogels for hyperthermia and magnetic field driven structural manipulation. Prog. Polym. Sci. 2022, 131, 101574. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. Magnetic Polymeric Conduits in Biomedical Applications. Micromachines 2025, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Margel, S. Magnetic Ionogel and Its Applications. Gels 2025, 11, 219. [Google Scholar] [CrossRef]
- Darawsheh, M.; Barrios, L.A.; Roubeau, O.; Teat, S.J.; Aromí, G. Guest-, Light-and Thermally-Modulated Spin Crossover in [FeII2] Supramolecular Helicates. Chem.-A Eur. J. 2016, 22, 8635–8645. [Google Scholar] [CrossRef]
- Greeson, E.M.; Madsen, C.S.; Makela, A.V.; Contag, C.H. Magnetothermal control of temperature-sensitive repressors in superparamagnetic iron nanoparticle-coated bacillus subtilis. ACS Nano 2022, 16, 16699–16712. [Google Scholar] [CrossRef]
- Wang, M.; Sun, R.; Chen, H.; Yoshitomi, T.; Mamiya, H.; Takeguchi, M.; Kawazoe, N.; Yang, Y.; Chen, G. Differential intracellular influence of cancer cells and normal cells on magnetothermal properties and magnetic hyperthermal effects of magnetic nanoparticles. Mater. Horiz. 2025, 12, 4363–4378. [Google Scholar] [CrossRef]
- Shen, Z.; Miyajima, H.; Fujita, S. Alternating Magnetic Field (AMF)-Controlled Magnetothermal Actuation of Hybrid Hydrogel Fiber Mats for Soft Robotics. ACS Appl. Polym. Mater. 2025, 7, 6814–6824. [Google Scholar] [CrossRef]
- Lv, M.; Wang, L.; Ding, W.; Tan, J.; Zhang, N.; Chen, L.; Zhai, Y.; Liu, X.; Xie, H.; Wang, J. Synthesis of magneto-thermal biresponsive temperature-sensitive copolymer/inorganic hybrid green nanoemulsifier with tunable LCST behavior and simulation and calculation of LCST transition behavior of its copolymer. J. Mol. Liq. 2024, 393, 123686. [Google Scholar] [CrossRef]
- Shokrani, H.; Shokrani, A.; Seidi, F.; Kucińska-Lipka, J.; Makurat-Kasprolewicz, B.; Saeb, M.R.; Ramakrishna, S. Artificial intelligence for biomedical engineering of polysaccharides: A short overview. Curr. Opin. Biomed. Eng. 2023, 27, 100463. [Google Scholar] [CrossRef]
- Khan, W.U.; Shen, Z.; Mugo, S.M.; Wang, H.; Zhang, Q. Implantable hydrogels as pioneering materials for next-generation brain–computer interfaces. Chem. Soc. Rev. 2025, 54, 2832–2880. [Google Scholar] [CrossRef]
- Yang, G.; Zheng, Y.; Tian, B. Perspective on Nano-Enabled Photostimulation Biointerfaces. ACS Nano 2025, 19, 21189–21205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, H.; Hu, X.; Muhammad, W.; Liu, C.; Liu, W. Inflammation-modulating polymeric nanoparticles: Design strategies, mechanisms, and therapeutic applications. EBioMedicine 2025, 118, 105837. [Google Scholar] [CrossRef] [PubMed]
- Ai, M.; Wang, J.; Chen, J.; Wang, W.; Xu, X.; Gan, Y.; Li, X.; Gou, X.; Cao, J.; Lv, Z. Plasma brain-derived neurotrophic factor (BDNF) concentration and the BDNF Val66Met polymorphism in suicide: A prospective study in patients with depressive disorder. Pharmacogenomics Pers. Med. 2019, 12, 97–106. [Google Scholar] [CrossRef]
- Xie, Y. A multiscale brain emulation-based artificial intelligence framework for dynamic environments. Sci. Rep. 2025, 15, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A. Stretchable Electronics: Advances in Elastic Conductive Fibers for Multifunctional Applications. Org. Electron. 2024, 135, 107145. [Google Scholar] [CrossRef]
- Gong, S.; Lu, Y.; Yin, J.; Levin, A.; Cheng, W. Materials-driven soft wearable bioelectronics for connected healthcare. Chem. Rev. 2024, 124, 455–553. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.S.; Kim, K. Recent Trends of functional composites and structures for electromechanical sensors: A review. Adv. Intell. Syst. 2024, 6, 2300730. [Google Scholar] [CrossRef]
- Poudel, P.; Park, S. Recent advances in the treatment of Alzheimer’s disease using nanoparticle-based drug delivery systems. Pharmaceutics 2022, 14, 835. [Google Scholar] [CrossRef]
- Lenders, V.; Koutsoumpou, X.; Phan, P.; Soenen, S.J.; Allegaert, K.; de Vleeschouwer, S.; Toelen, J.; Zhao, Z.; Manshian, B.B. Modulation of engineered nanomaterial interactions with organ barriers for enhanced drug transport. Chem. Soc. Rev. 2023, 52, 4672–4724. [Google Scholar] [CrossRef]
- Hettiarachchi, S.D.; Zhou, Y.; Seven, E.; Lakshmana, M.K.; Kaushik, A.K.; Chand, H.S.; Leblanc, R.M. Nanoparticle-mediated approaches for Alzheimer’s disease pathogenesis, diagnosis, and therapeutics. J. Control. Release 2019, 314, 125–140. [Google Scholar] [CrossRef]
- Roghani, A.K.; Garcia, R.I.; Roghani, A.; Reddy, A.; Khemka, S.; Reddy, R.P.; Pattoor, V.; Jacob, M.; Reddy, P.H.; Sehar, U. Treating Alzheimer’s disease using nanoparticle-mediated drug delivery strategies/systems. Ageing Res. Rev. 2024, 97, 102291. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, T.T.D.; Nguyen, T.K.O.; Vo, T.K.; Vo, V.G. Advances in developing therapeutic strategies for Alzheimer’s disease. Biomed. Pharmacother. 2021, 139, 111623. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, C.; Napolitano, F.; Montesarchio, D.; Sampaolo, S.; Melone, M.A.B. Nanoparticle-guided brain drug delivery: Expanding the therapeutic approach to neurodegenerative diseases. Pharmaceutics 2021, 13, 1897. [Google Scholar] [CrossRef]
- Modi, G.; Pillay, V.; Choonara, Y.E.; Ndesendo, V.M.; du Toit, L.C.; Naidoo, D. Nanotechnological applications for the treatment of neurodegenerative disorders. Prog. Neurobiol. 2009, 88, 272–285. [Google Scholar] [CrossRef]
- Kakinen, A.; Jiang, Y.; Davis, T.P.; Teesalu, T.; Saarma, M. Brain targeting nanomedicines: Pitfalls and promise. Int. J. Nanomed. 2024, 19, 4857–4875. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, C.; Guo, Q.; Wan, X.; Shao, X.; Liu, Q.; Zhang, Q. Dual-functional nanoparticles for precise drug delivery to Alzheimer’s disease lesions: Targeting mechanisms, pharmacodynamics and safety. Int. J. Pharm. 2017, 525, 237–248. [Google Scholar] [CrossRef]
- Gidwani, M.; Singh, A.V. Nanoparticle enabled drug delivery across the blood brain barrier: In vivo and in vitro models, opportunities and challenges. Curr. Pharm. Biotechnol. 2013, 14, 1201–1212. [Google Scholar] [CrossRef]
- Walther, C.C. Where: Humans, technology, and humane technology. In Human Leadership for Humane Technology: The New AI: Agency Ignited; Springer: Berlin/Heidelberg, Germany, 2024; pp. 103–194. [Google Scholar] [CrossRef]
- Rickli, J.-M.; Ienca, M. The security and military implications of neurotechnology and artificial intelligence. In Clinical Neurotechnology Meets Artificial Intelligence: Philosophical, Ethical, Legal and Social Implications; Springer International Publishing: Cham, Switzerland, 2021; pp. 197–214. [Google Scholar]
- Lucas, G.; Syse, H.; Helkala, K.; Barrett, E. Ethical Challenges in AI-Enhanced Military Operations; Frontiers Media SA: Lausanne, Switzerland, 2023. [Google Scholar]
- Raposo, C.; Schwartz, M. Glial scar and immune cell involvement in tissue remodeling and repair following acute CNS injuries. Glia 2014, 62, 1895–1904. [Google Scholar] [CrossRef]
- Otte, E.; Vlachos, A.; Asplund, M. Engineering strategies towards overcoming bleeding and glial scar formation around neural probes. Cell Tissue Res. 2022, 387, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 3879. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Yue, C.; Li, Z.; Ma, L.; Wang, T.; Zhang, H.; Wang, J.; Yang, S. MXene/Silk Fibroin Strengthened PVA–Based Eutectogel with Excellent Self–Healing Ability and Environmental Adaptability: Design, Synthesis, and Sensing Application. Chem.–Asian J. 2024, 19. [Google Scholar] [CrossRef]
- Nicaise, A.M.; D’Angelo, A.; Ionescu, R.-B.; Krzak, G.; Willis, C.M.; Pluchino, S. The role of neural stem cells in regulating glial scar formation and repair. Cell Tissue Res. 2022, 387, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Park, C.B. Magnetoelectric dissociation of Alzheimer’s β-amyloid aggregates. Sci. Adv. 2022, 8, eabn1675. [Google Scholar] [CrossRef]
- Habib, S.; Singh, M. Angiopep-2-modified nanoparticles for brain-directed delivery of therapeutics: A review. Polymers 2022, 14, 712. [Google Scholar] [CrossRef]
- Janetanakit, W.; Wang, L.; Santacruz-Gomez, K.; Landon, P.B.; Sud, P.L.; Patel, N.; Jang, G.; Jain, M.; Yepremyan, A.; Kazmi, S.A.; et al. Gold-Embedded Hollow Silica Nanogolf Balls for Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 27533–27543. [Google Scholar] [CrossRef]
- Li, C.; Feng, K.; Xie, N.; Zhao, W.; Ye, L.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Mesoporous Silica-Coated Gold Nanorods with Designable Anchor Peptides for Chemo-Photothermal Cancer Therapy. ACS Appl. Nano Mater. 2020, 3, 5070–5078. [Google Scholar] [CrossRef]
- Huang, P.; Lin, J.; Wang, S.; Zhou, Z.; Li, Z.; Wang, Z.; Zhang, C.; Yue, X.; Niu, G.; Yang, M.; et al. Photosensitizer-conjugated silica-coated gold nanoclusters for fluorescence imaging-guided photodynamic therapy. Biomaterials 2013, 34, 4643–4654. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Orozco, C.; Urban, C.; Knight, M.W.; Urban, A.S.; Neumann, O.; Bishnoi, S.W.; Mukherjee, S.; Goodman, A.M.; Charron, H.; Mitchell, T.; et al. Au Nanomatryoshkas as Efficient Near-Infrared Photothermal Transducers for Cancer Treatment: Benchmarking against Nanoshells. ACS Nano 2014, 8, 6372–6381. [Google Scholar] [CrossRef] [PubMed]
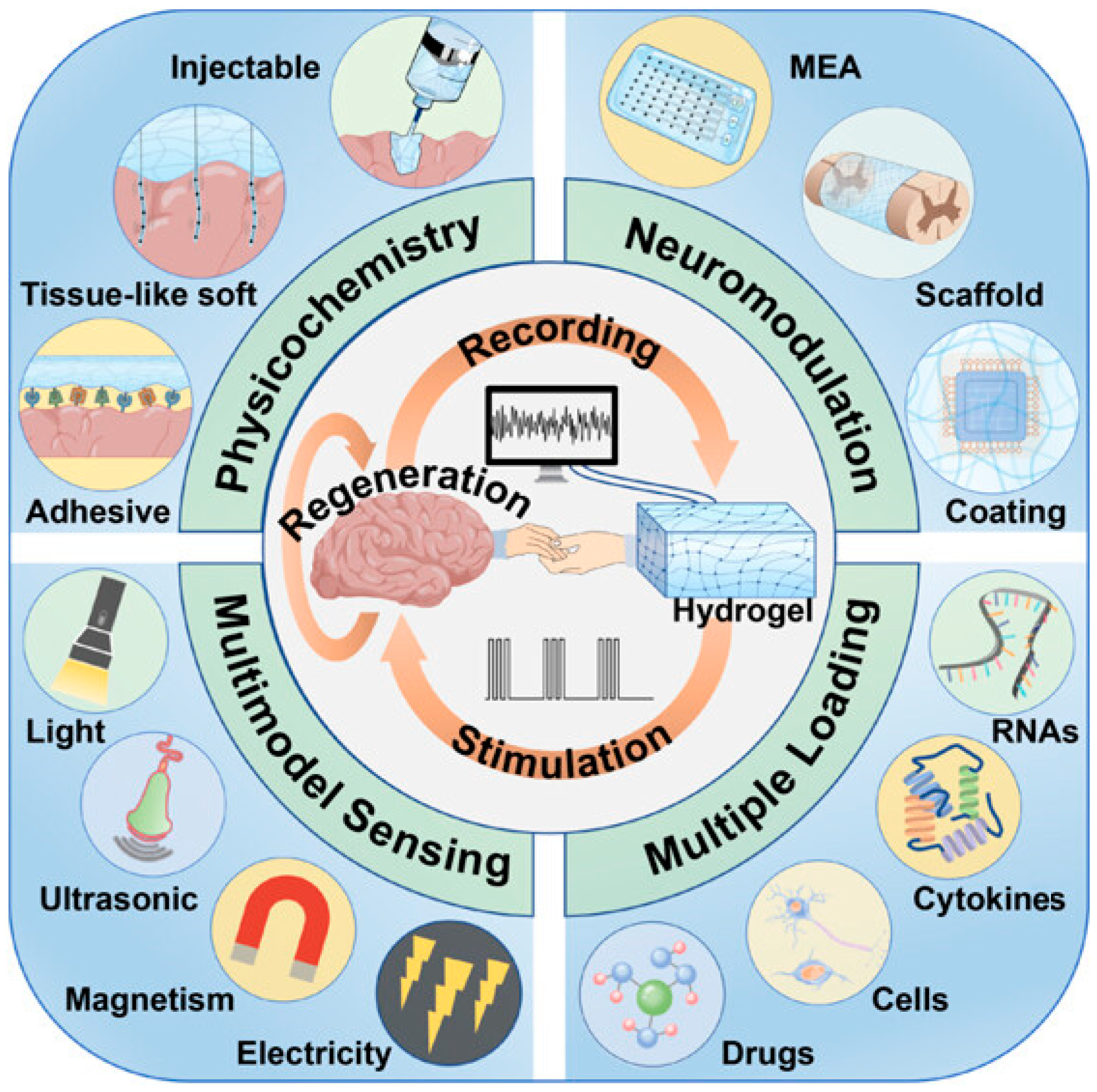
- Zhao, Y.; Sun, R.; Wang, Z.; Ma, S.; Wang, R.; Li, F.; Geng, H. Engineered Hydrogels as Functional Components of Controllable Neuromodulation for Translational Therapeutics. ACS Appl. Bio Mater. 2025, 8, 7587–7615. [Google Scholar] [CrossRef]

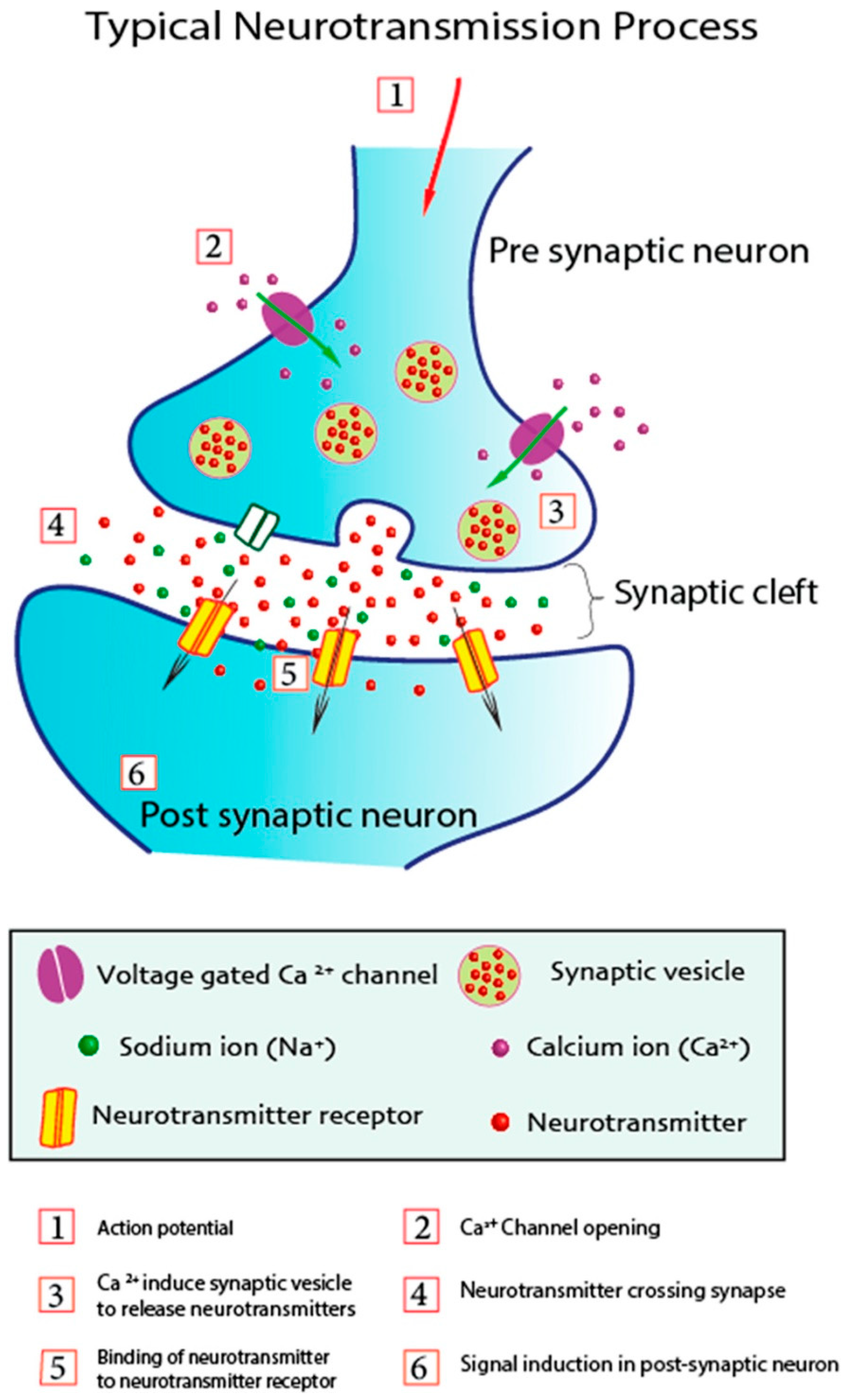
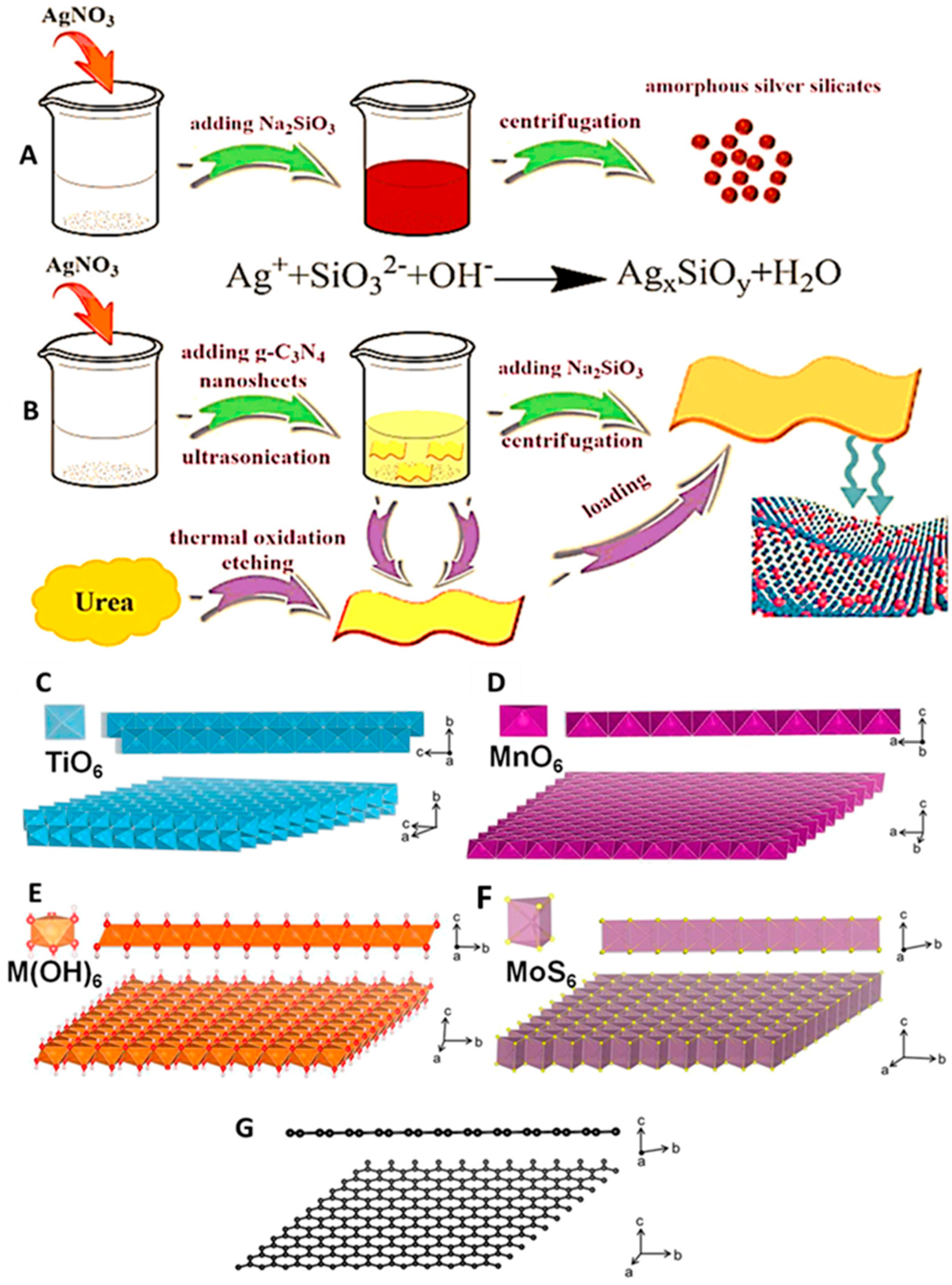
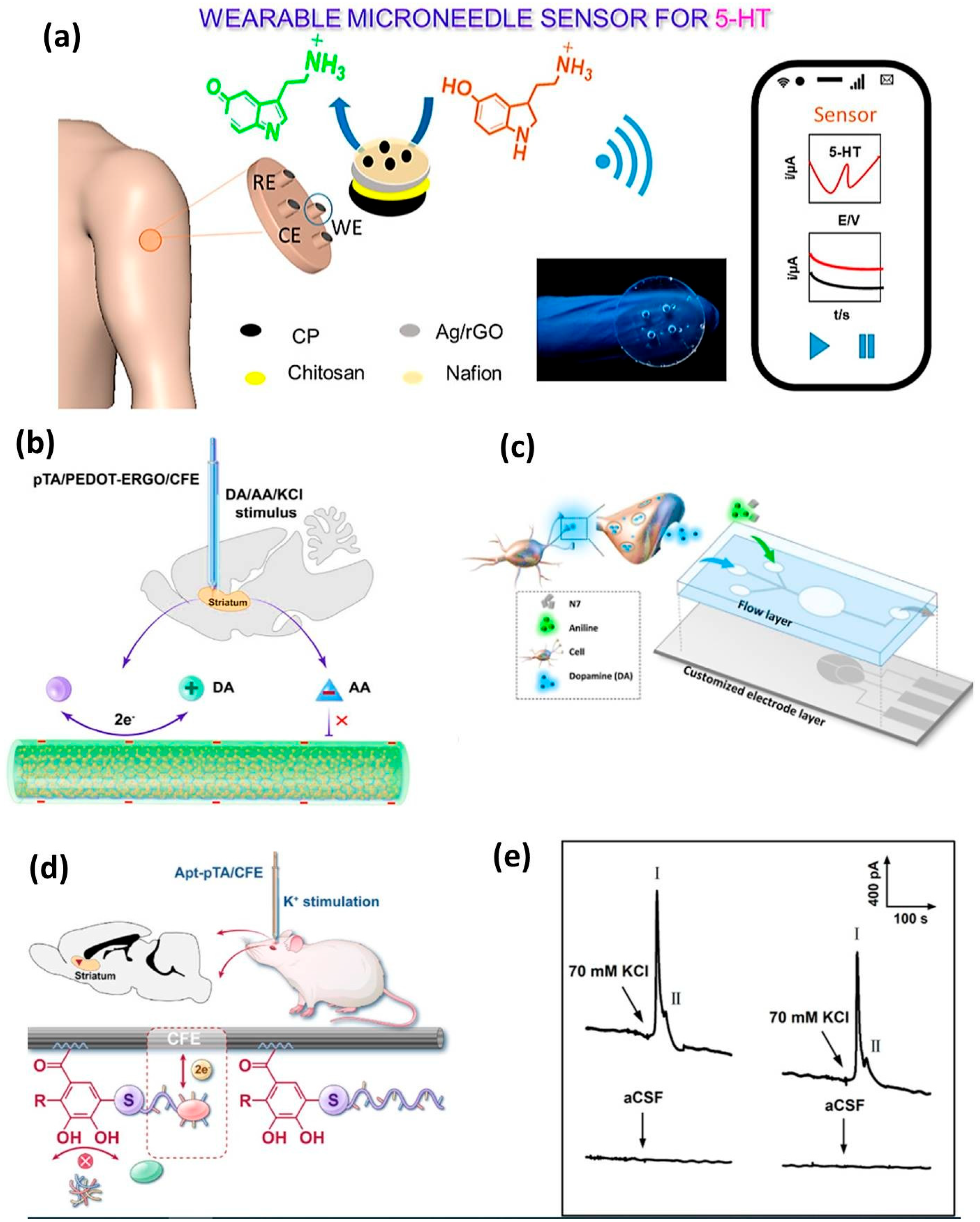
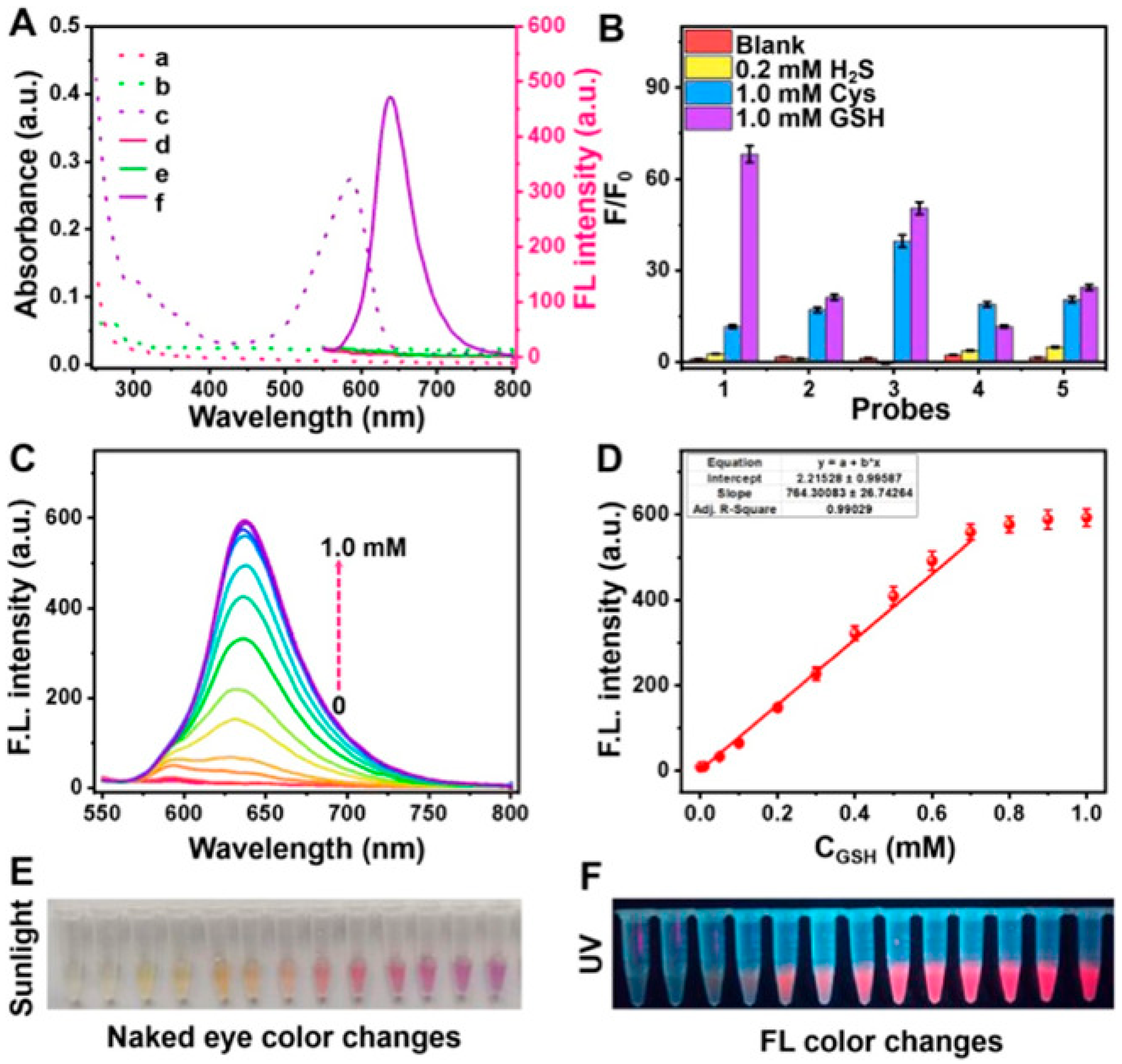
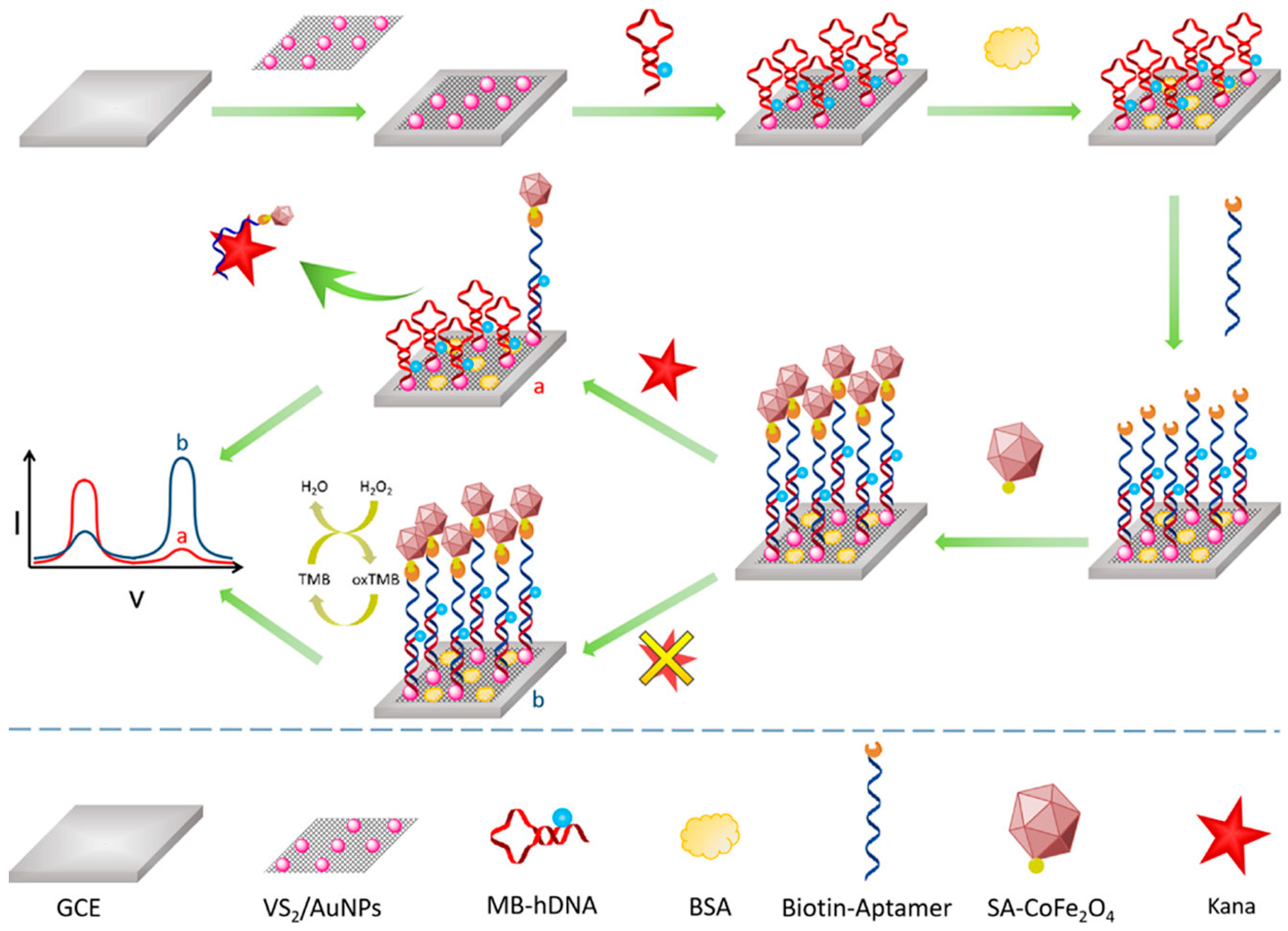
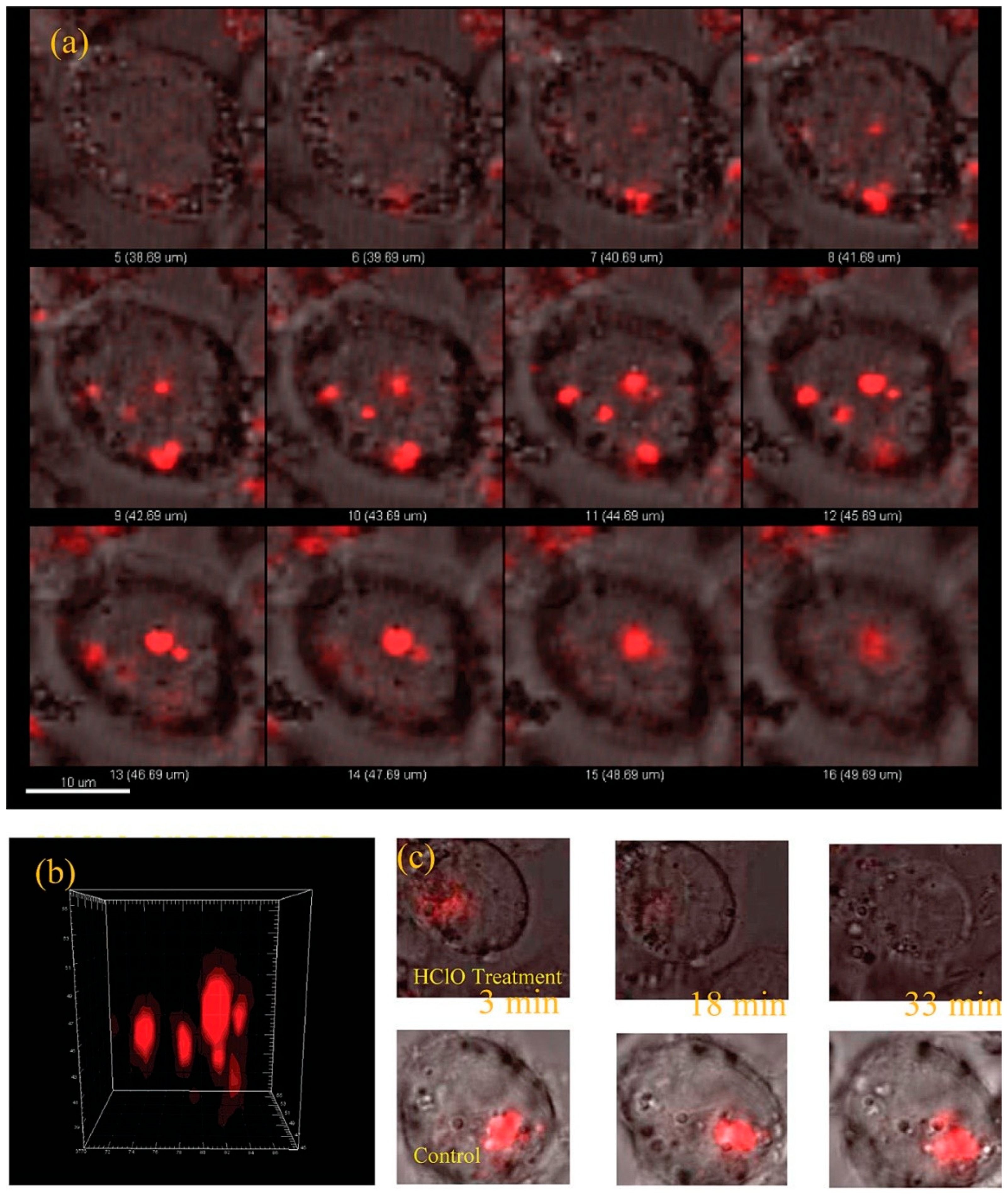
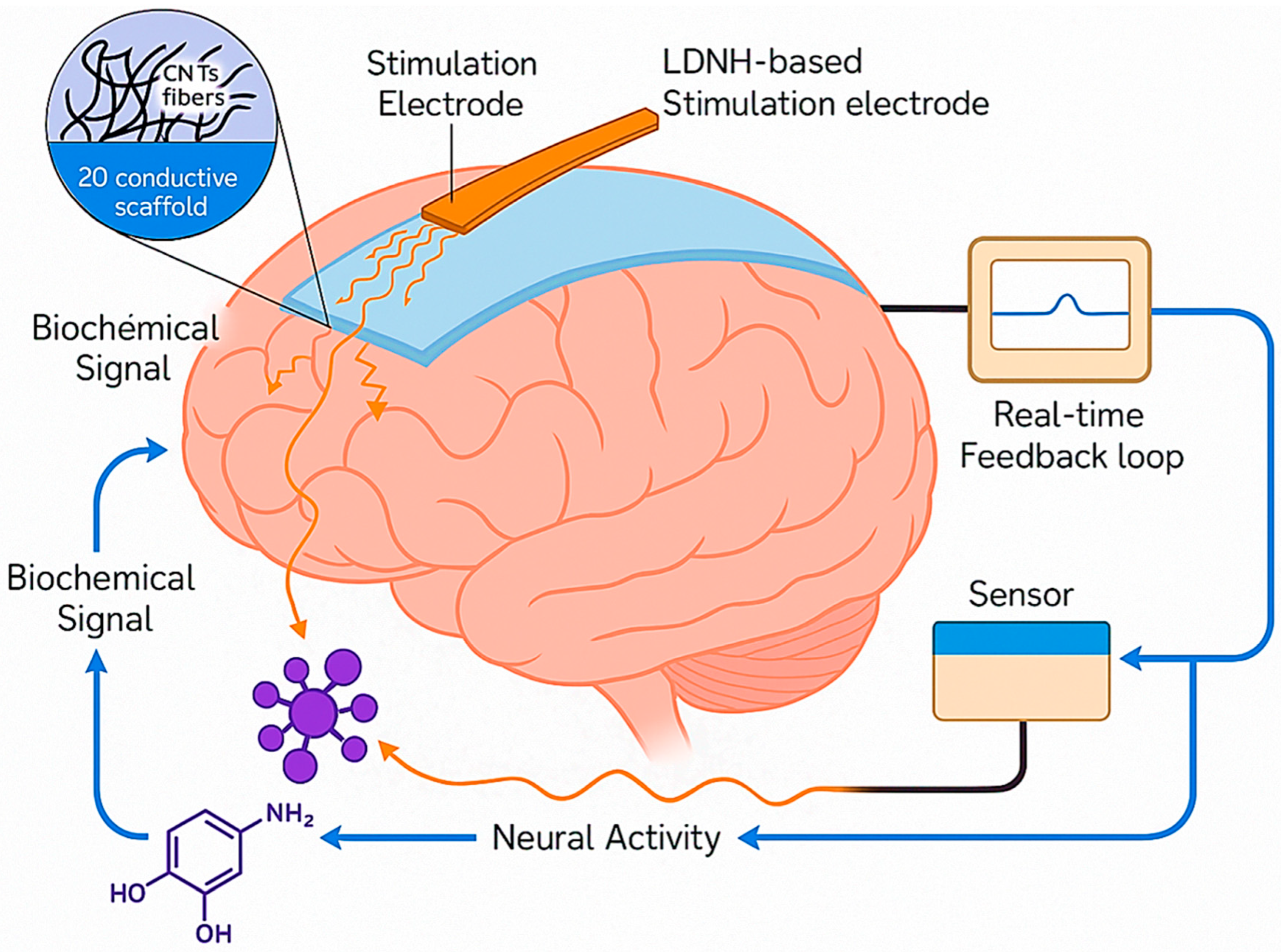

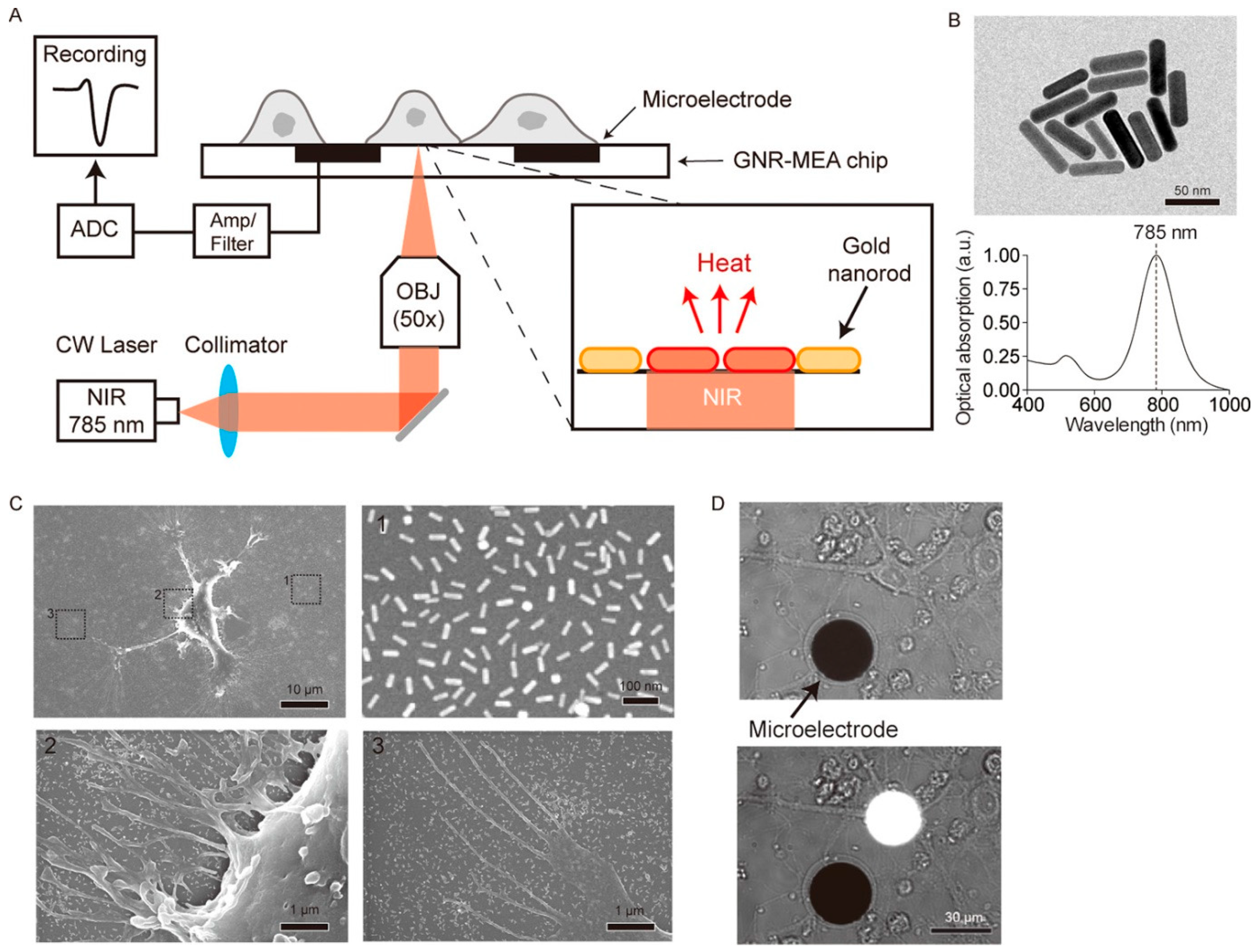
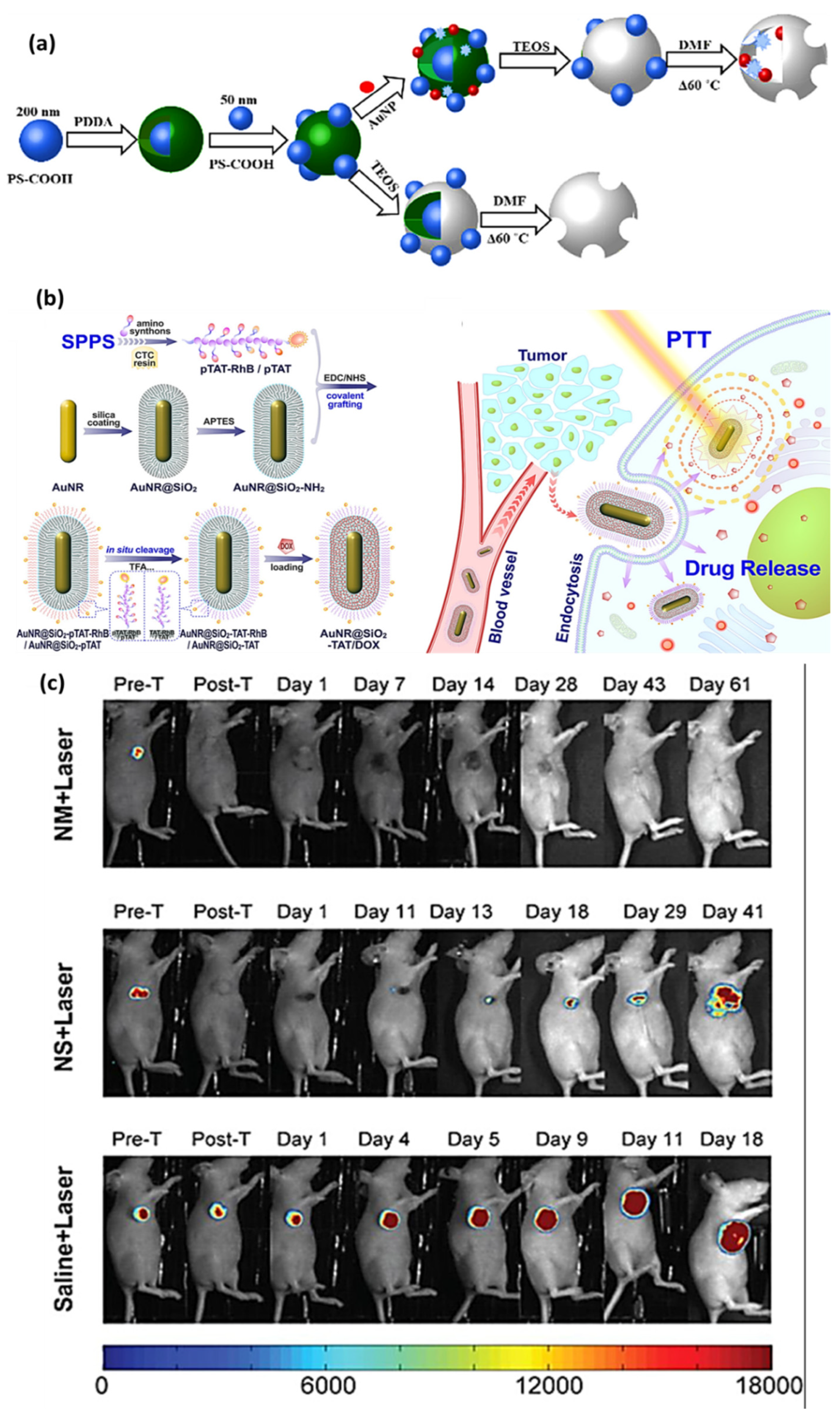
| Neurotransmitter | LDNH System | Detection Method | LOD | Key Application | Ref. |
|---|---|---|---|---|---|
| Dopamine (DA) | MXene-CNT hybrid | Amperometry | 50 pM | Parkinson’s disease monitoring | [108] |
| Glutamate | MoS2-graphene FET | FET-based sensing | 10 fM | Epilepsy research | [109] |
| Serotonin (5-HT) | Graphene-Au nanorods | DPV | 0.2 nM | Depression biomarker detection | [25] |
| Norepinephrine (NE) | PPy-MIP-GO | Impedimetry | 5 nM | Stress monitoring in sweat | [111] |
| GABA | Prussian blue-QD-aptamer | Redox cycling | 0.3 nM | Epilepsy diagnostics | [112] |
| Dopamine | PEDOT:PSS-MoS2 | CV | 0.8 nM | Neurodegenerative disease studies | [113] |
| Serotonin | CNT-PEDOT | Amperometry | 0.5 nM | Psychiatric disorder analysis | [114] |
| Glutamate | MXene-PtNP | Chronoamperometry | 50 pM | Brain injury monitoring | [115] |
| Dopamine | CdTe QD-tyrosinase | Photoelectrochemistry | 0.1 nM | Neuropharmacology research | [116] |
| Norepinephrine | ZnO-CuO nanofibers | EIS | 5 nM | Wearable stress sensors | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabish, M.; Malik, I.; Akhtar, A.; Afzal, M. A Review on Low-Dimensional Nanoarchitectonics for Neurochemical Sensing and Modulation in Responsive Neurological Outcomes. Biomolecules 2025, 15, 1405. https://doi.org/10.3390/biom15101405
Tabish M, Malik I, Akhtar A, Afzal M. A Review on Low-Dimensional Nanoarchitectonics for Neurochemical Sensing and Modulation in Responsive Neurological Outcomes. Biomolecules. 2025; 15(10):1405. https://doi.org/10.3390/biom15101405
Chicago/Turabian StyleTabish, Mohammad, Iram Malik, Ali Akhtar, and Mohd Afzal. 2025. "A Review on Low-Dimensional Nanoarchitectonics for Neurochemical Sensing and Modulation in Responsive Neurological Outcomes" Biomolecules 15, no. 10: 1405. https://doi.org/10.3390/biom15101405
APA StyleTabish, M., Malik, I., Akhtar, A., & Afzal, M. (2025). A Review on Low-Dimensional Nanoarchitectonics for Neurochemical Sensing and Modulation in Responsive Neurological Outcomes. Biomolecules, 15(10), 1405. https://doi.org/10.3390/biom15101405







