1. Introduction
RNA viruses present a persistent and evolving threat to global public health. This was clearly exemplified by the recent devastating effects of the SARS-CoV-2 pandemic, causing greater than 700 million infections and 7 million deaths worldwide [
1]. The Center for Disease Control (CDC) has repeatedly reported its concern about the continuous threat of RNA viruses, specifically emphasizing viral families with low existing countermeasures and high pandemic potential [
2]. Virus groups that are of particular concern for future pandemics include bunyaviruses, which are responsible for hemorrhagic fevers and encephalitis, flaviviruses such as Zika virus (ZIKV), and the large and diverse paramyxovirus family [
2]. The CDC also highlights the importance of countermeasures against RNA viruses that are characterized by rapid mutation and evolution. An example of this includes the emergence of numerous strains of the coronavirus (a family of RNA viruses), presenting challenges to prevention and treatment measures. The high mutation rates, diverse transmission pathways, and propensity for emergence and re-emergence make RNA viruses a formidable threat to human health.
While antiviral drugs provide powerful countermeasures to certain RNA viruses, there are limitations. For example, most existing antivirals exhibit narrow specificity, targeting only a limited range of viral strains or families [
3]. This is an inherent limitation when pandemics or endemics emerge because the development of compounds effective at targeting specific strains of a virus only occurs after the virus has been identified and has already caused significant damage. In contrast to DNA viruses, most RNA viruses replicate exclusively in the cytoplasm of infected cells and use unique RNA-dependent-RNA polymerases for the amplification of their genomes. Importantly, the rapid mutation rates of RNA viruses can lead to the development of resistance to a given antiviral compound, necessitating the continuous development of new antiviral compounds [
4]. These very real limitations of current antivirals underscore the need for ongoing research and development into broad-spectrum antivirals that could be used to respond to future RNA virus outbreaks.
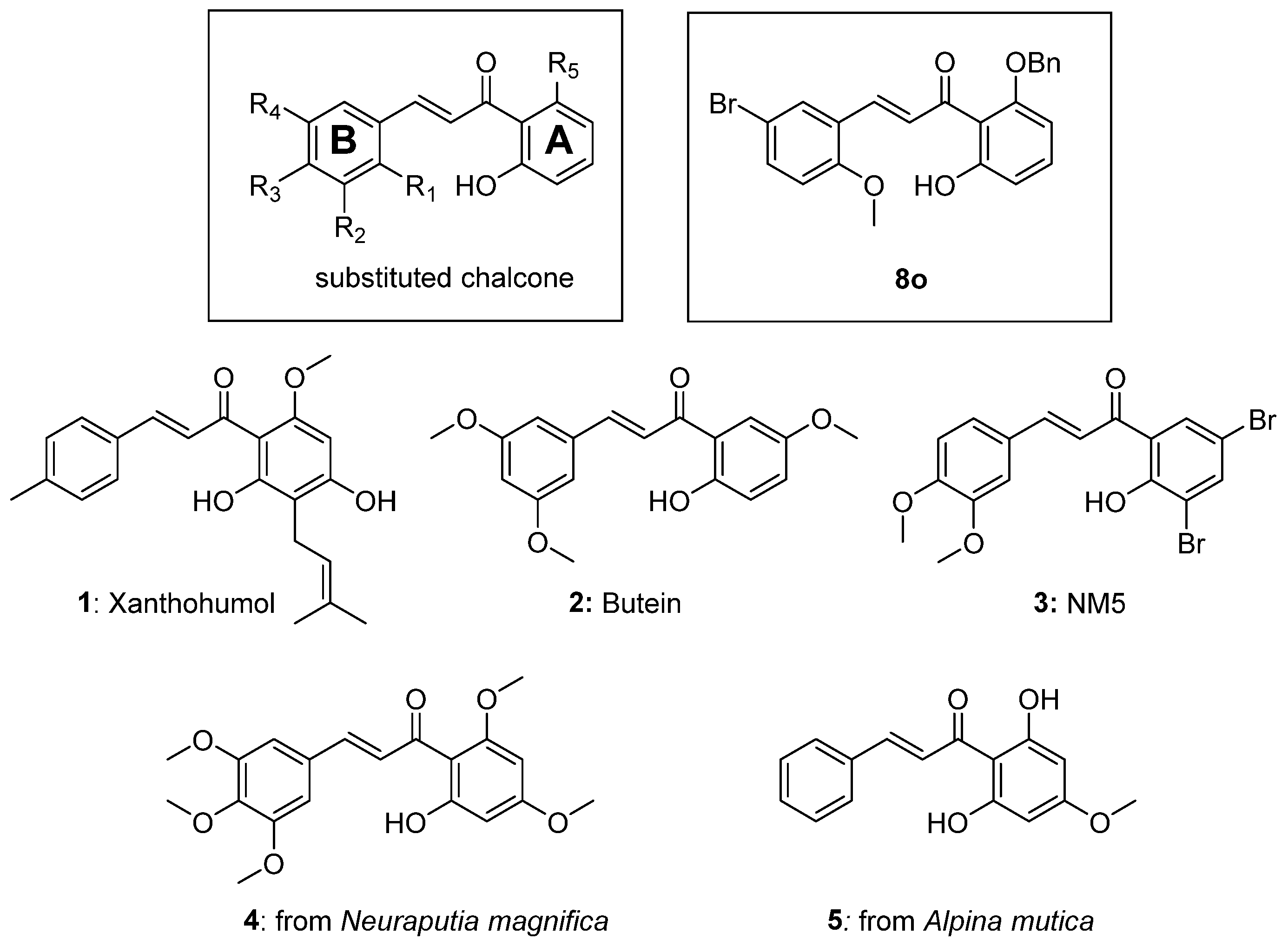
Chalcones are common precursors to flavones and flavonoid structures and have diverse biological effects [
5]. For example, chalcones are known to inhibit key enzymes such as 15-hydroxyprostaglandin dehydrogenase, 5-lipoxygenase, cyclooxygenase, and protein tyrosine phosphatase 1B [
5]. Chalcones were also reported to inhibit the phosphorylation of proteins involved in cell signaling cascades such as mammalian target of rapamycin (mTOR) [
6]. In addition, chalcones can serve in the treatment of asthma, inflammation, type 2 diabetes, and obesity [
7]. Not surprisingly, with such diverse medical applications, this propenone scaffold has received strong interest in the literature (
Figure 1) [
5].
Chalcones have also served as antiviral agents [
7,
10]. Prior work in our lab demonstrated that compound
8o had efficacy as an antiviral agent against both human cytomegalovirus (HCMV, a double stranded DNA virus) [
7] and human immunodeficiency virus (HIV, a positive sense, single-strand RNA virus) [
10].
Here, we tested the antiviral potential of chalcones against prototypic members of four diverse RNA virus families, with some members of each virus family being identified as potential candidates for new emerging pathogens. Briefly described, parainfluenza virus 5 (PIV5) is a prototype member of the paramyxovirus family of viruses, some of which are important animal and human pathogens, such as mumps virus, measles virus, respiratory syncytial virus, and Nipah virus [
11]. The negative-sense viral RNA genome is tightly bound into a ribo-nucleocapsid structure, which is enveloped by a lipid bilayer derived from the host cell. After binding to the cell surface, virions fuse with the plasma membrane to deposit the nucleocapsid into the cytoplasm. Similarly, bunyavirus La Crosse virus (LACV) also contains a negative sense RNA genome that is encapsidated by a lipid envelop, but here, the genome consists of three separate RNA segments, and infection of the host cell is initiated by the internalization of virus particles into cellular endosomes. LACV and other members of the bunyavirus family are important human pathogens, which are transmitted by insect bites [
12]. OC43 is a prototypic member of the coronavirus family of human pathogens responsible for a large number of seasonal respiratory tract infections [
13]. This enveloped positive stand RNA virus initiates infections by binding to the plasma membrane and the internalization of virions. Lastly, as member of the
Flaviviridae family, Zika Virus (ZIKV) has emerged in recent years as a public health concern due to its broad tropism for different organs and spread by mosquitoes to new regions [
14]. This enveloped positive-strand RNA virus initiates infections after binding to a range of potential receptors and internalization via clathrin-coated pits.
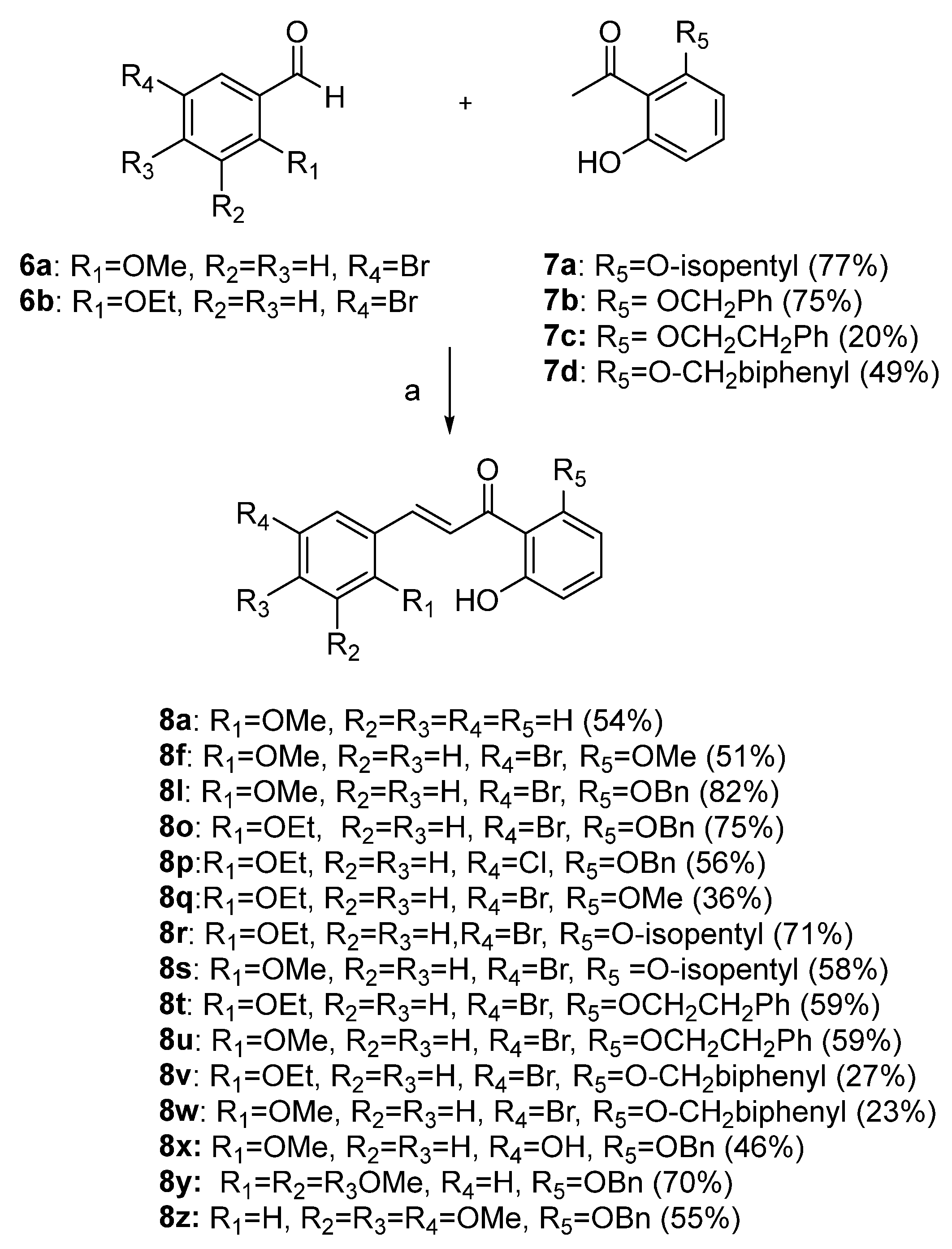
In search of general treatments for single-stranded RNA viruses, we investigated the antiviral activity of chalcones against PIV5, LACV, [
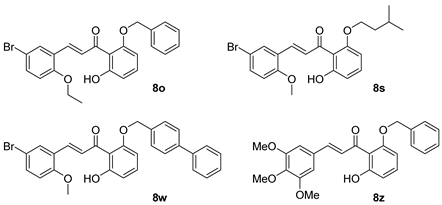
15] OC43, and ZIKV. We also evaluated mammalian target of rapamycin (mTOR) as a possible chalcone target. Our chalcone library was predicated upon the lead compound
8o and was synthesized through published methods using crossed aldol condensation and provided good yields of the target structures [
7,
10]. The modular synthetic approach allowed us to vary substituents on both the A and B rings (
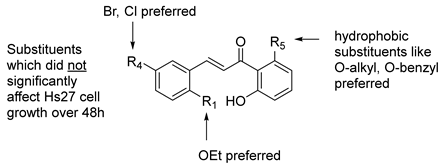
Figure 1) and expand the earlier structure–activity relationships (SAR) [
7,
10].
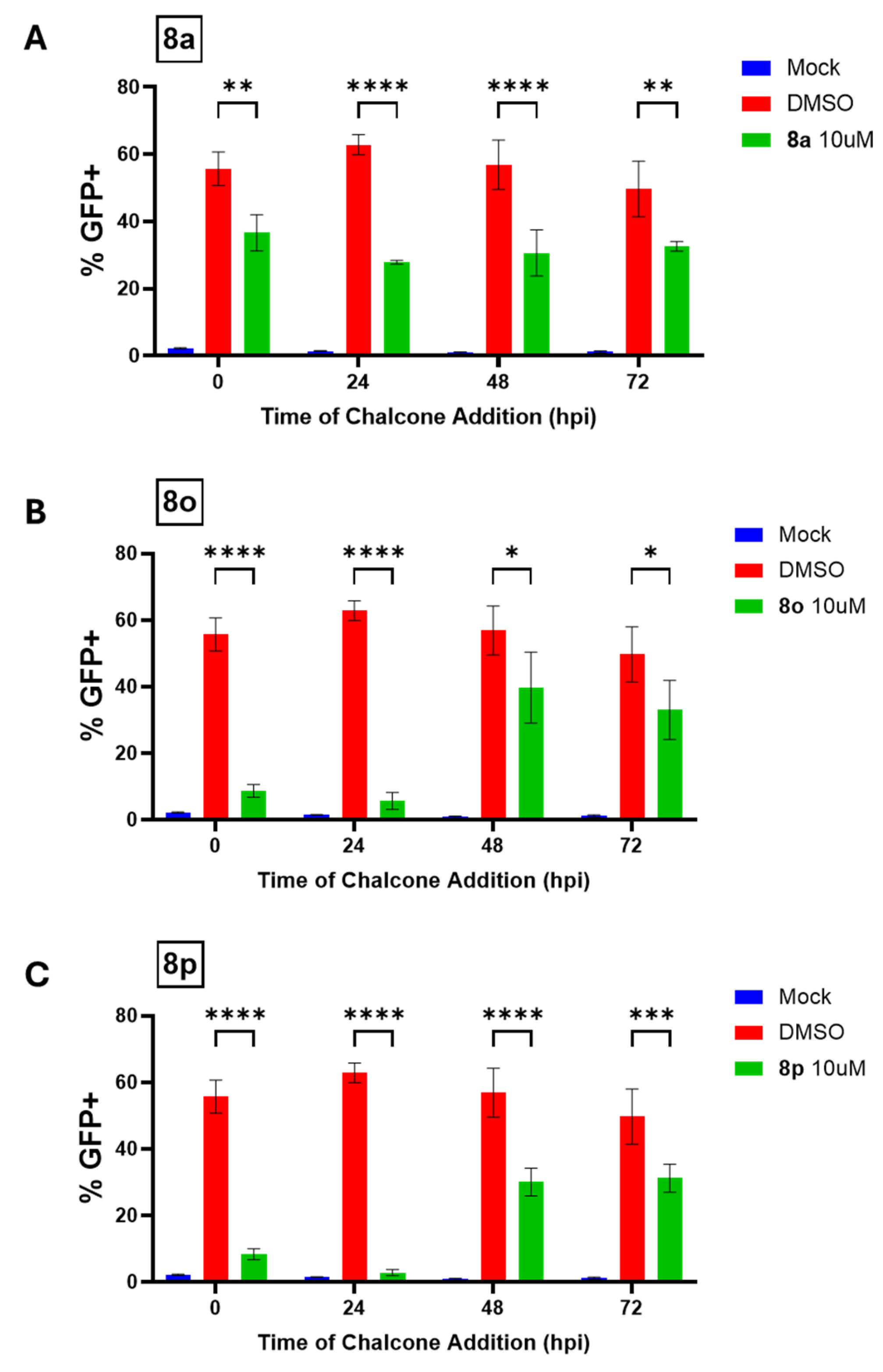
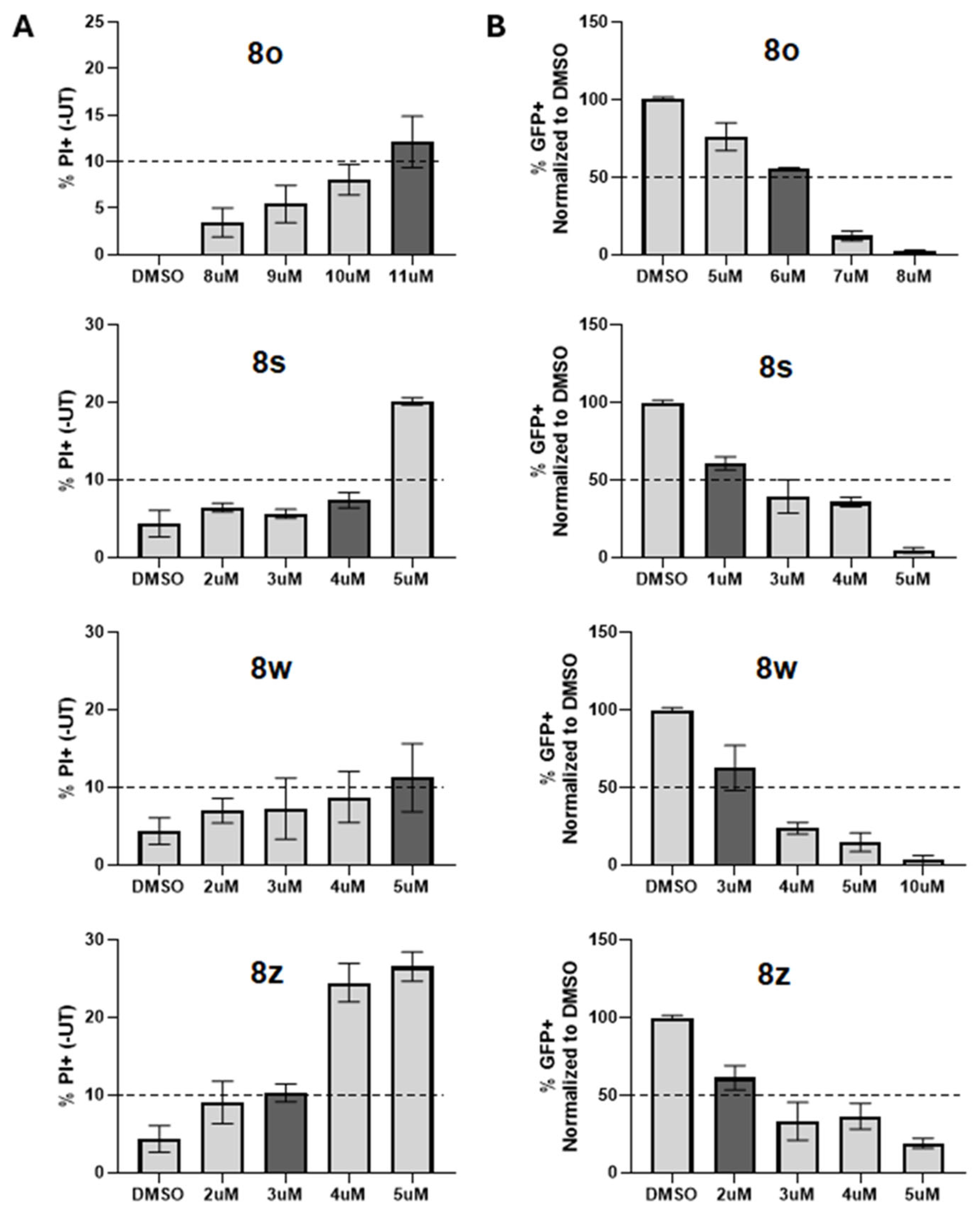
In this report, we show that chalcone treatment reduced the spread of PIV5 in fibroblast cell cultures when added to cultures as late as 24 h post-infection. The addition of select chalcones to cell cultures of PIV5-infected human fibroblasts resulted in a dose-dependent inhibition of PIV5 replication. Most importantly, we also demonstrate the inhibitory effects of chalcones in fibroblast cell cultures of the Bunyavirus La Crosse Virus (LACV, a negative sense single stranded RNA virus), as well as two other positive sense, single-stranded RNA viruses: the Coronavirus OC43 and the Flavivirus Zika Virus (ZIKV). These findings indicate the potential for broad spectrum anti-viral activity via specific chalcone designs. Collectively, these data provide a strong rationale for the further development of chalcone derivatives as potent, broad-spectrum antiviral treatments against diverse families of RNA viruses.
2. Materials and Methods
2.1. Cells Lines
Cultures of CHO-K1 (ATCC, catalog #CCL-61, Manassas, VA, USA) were grown at 37 °C in a humidified 5% CO2 atmosphere in Roswell Park Memorial Institute (RPMI 1640) medium in the presence of 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cultures of Hs27 (ATCC, catalog #CRL-1634) and Vero cells (ATCC, catalog #CCL-81) were grown at 37 °C in a humidified 5% CO2 atmosphere in Dulbecco Modified Eagle Medium (DMEM, catalog #11965118, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% heat-inactivated fetal bovine serum (HI FBS, Gibco, Thermo Fisher Scientific, Waltham, MA, USA). Cultures of PANC-1 (ATCC, catalog #CRL-1469) cells were grown at 37 °C in a humidified 5% CO2 atmosphere in DMEM with the addition of 10% fetal bovine serum and 1% penicillin/streptomycin.
2.2. Viruses and Infections
Parainfluenza virus 5 (PIV5) expressing green fluorescence protein (GFP) was derived from cDNA, grown at 37 °C in Madin–Darby bovine kidney (MDBK) cells and titered on CV-1 (African green monkey kidney) cells with a fibroblast morphology as previously described [
16]. MDBK and CV-1 cells were the kind gift of Dr. Robert Lamb (Northwestern University). Cells were infected with virus diluted in DMEM supplemented with 10% bovine serum albumin (BSA) for 1 h or mock infected with media alone. Following incubation, cells were washed with phosphate-buffered saline (PBS) and cultured in DMEM supplemented with 2% heat-inactivated (HI) FBS.
La Crosse Virus (LACV) was kindly provided by Andrew Pekosz (Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA), grown at 28 °C in C6/36 cells derived from the larvae of the Asian tiger mosquito (ATCC), and titered at 37 °C on Vero cells as previously described [
17]. Infections were carried out in DMEM containing 2% HI FBS for 1 h or mock infected with media alone. Cells were washed with PBS and cultured in DMEM containing 2% HI FBS.
The MR766 strain of Zika Virus (ZIKV) (ATCC-VR84, Manassas, VA, USA) was grown and titered at 37 °C using Vero cells as previously described [
18]. Infections were carried out in DMEM containing 10% BSA for 1 h. Cells were then washed with PBS and cultured in DMEM containing 10% HI FBS.
Human Coronavirus OC43 (ATCC, catalog number VR-1558) was grown in a human colorectal adenocarcinoma cell line (HCT-8; ATCC, Manassas, Virginia, catalog #CCL-244) at 33 °C as previously described [
19]. OC43 titers were determined at 33 °C via 50% Tissue Culture Infectious Dose assays (TCID
50) on confluent human rhabdomyosarcoma (RD; ATCC, Manassas, Virginia, catalog # CCL-136) cells in 96-well plates as previously described [
19]. Infections were carried out in DMEM containing 10% BSA for 1 h at 33 °C. Cells were then washed with PBS, cultured in DMEM supplemented with 2% HI FBS, and incubated at 33 °C.
The starting titers of stocks of PIV5, OC43, ZIKV, and LACV were 5.6 × 107 PFU/mL, 2 × 107 TCID50/mL, 1 × 106 PFU/mL, and 1.1 × 107 PFU/mL, respectively. Unless otherwise stated for a particular experiment, all high and low MOI virus infections were performed at 10 and 0.01 infectious units per cell, respectively.
2.3. Chalcone Treatments
Chalcone treatments were performed in DMEM supplemented with 10% HI FBS at the indicated concentrations and for indicated periods of time. Chalcone stocks were reconstituted in 100% dimethyl sulfoxide (DMSO) at a concentration of 10 mM and were serially diluted to working concentrations in DMSO. DMSO control treatments to test the effect of the vehicle were performed at volumes corresponding to the volume of chalcone added during each experiment (i.e., 1 µL chalcone DMSO solution/1 mL DMEM [10 µM] utilized alongside the DMSO control of 1 µL/1 mL DMEM). For cytotoxicity assays, untreated controls were subtracted from DMSO and chalcone sample values to account for the background signal.
2.4. Flow Cytometry
For cytotoxicity assays, cells were stained with propidium iodide (PI) at a dilution of 1:100. Cell viability was determined via flow cytometry using the CytoFLEX (Beckman Coulter, Brea, CA, USA). CytExpert software (Beckman Coulter, version 2.4) was used to analyze independent events. PIV5 infection was monitored using flow cytometry via the FITC channel by analyzing GFP expression of individual cells. ZIKV infection was monitored utilizing Flavivirus group antigen–antibody (D1-4G2-4-15; Novus Biologicals, Littleton, CO, USA) staining for the ZIKV envelope protein. Cells were fixed and permeabilized using eBioscience Intracellular Fixation and Permeabilization Buffer (Invitrogen, Thermo Fisher Scientific, MA, USA) according to the manufacturer’s instructions. Secondary staining was performed using an anti-Alexa Fluor 488 (AF488) antibody (Invitrogen, Thermo Fisher Scientific, MA, USA). OC43 infection was monitored utilizing a primary antibody targeted toward the OC43 nucleocapsid protein (NP, MAB9013, Sigma-Aldrich, St. Louis, MO, USA). Secondary staining was performed using an AF488 antibody. Cells were fixed and permeabilized as previously described. LACV infection was monitored utilizing an anti-LACV Gc 807.31ab antibody kindly provided by Andrew Pekosv. Secondary staining was performed using an AF488 antibody. Cells were fixed and permeabilized as previously described [
19].
2.5. RT-qPCR
Hs27 cells cultured in 6-well dishes were collected in TRIzol
® (Invitrogen) followed by RNA extraction. To produce cDNA, 1 μg of total RNA was used with TaqMan
® Reverse Transcription Reagents (Applied Biosystems, Foster City, CA, USA) as described in the manufacturer’s instructions. Quantitative real-time PCR was performed using Bio-Rad CFX Connect Real-Time (Bio-Rad, Hercules, CA, USA) and Fast SYBR
® FAST Green Master Mix (Applied Biosystems, Foster City, CA, USA). Relative gene expression was determined using CFX Manager Software (Bio-Rad, version 2.3) and the following primers (
Table 1).
2.6. Western Blotting
Hs27 (human skin fibroblast) cells cultured in 6-well dishes were lysed using protein lysis buffer. Cell lysates were resolved on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). After normalizing samples via western blotting for β-actin (1:20,000 dilution, catalog number A5316, Sigma-Aldrich, St. Louis, MO, USA), samples were probed with rabbit polyclonal antibodies for the PIV5 NP (1:2000) and P proteins (1:2000) [
20]. Blots were visualized using anti-mouse horseradish peroxidase (HRP)-conjugated antibodies (Sigma-Aldrich) and chemiluminescence (Thermo Fisher Scientific).
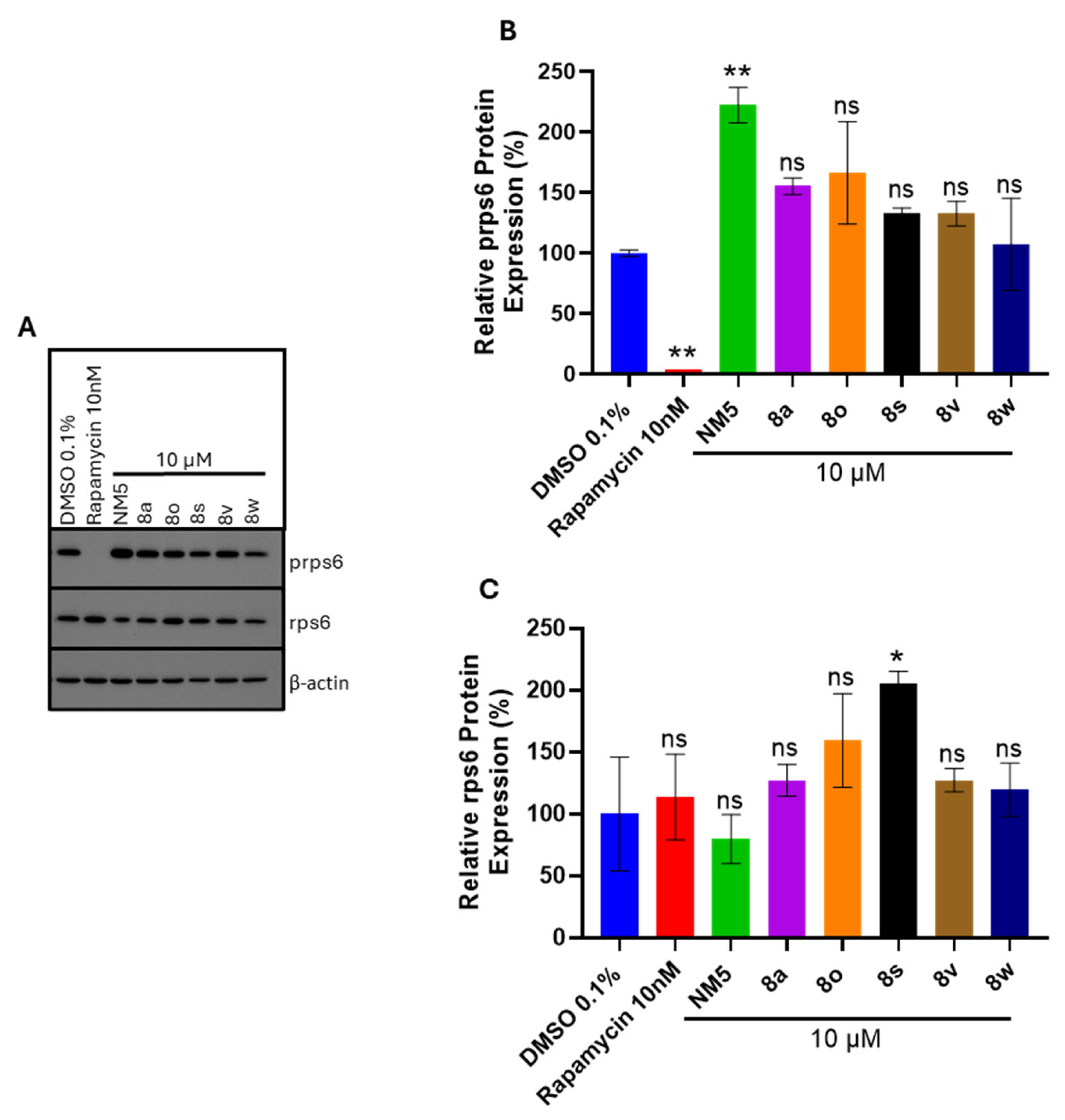
PANC-1 cells were cultured in 6-well dishes and were lysed using modified radioimmunoprecipitation assay (RIPA) buffer (20 mM HEPES, pH 7.0, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 1% deoxycholate, and 0.1% sodium dodecyl sulfate [SDS]) containing a complete protease inhibitor cocktail (Roche, Mannheim, Germany) and PhosSTOP phosphatase inhibitor cocktail (Roche, Mannheim, Germany). Protein concentrations were determined using the bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL, USA) according to manufacturer’s instructions. Cell lysates with 25 µg of protein were resolved on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and electro-transferred onto polyvinylidene difluoride (PVDF) membranes (Trans-Blot Turbo Transfer System, BioRad, Hercules, CA, USA). The samples were probed with rabbit monoclonal antibodies for the P-rps6 (1:1000) protein (Cell Signaling, Danvers, MA, USA) and mouse monoclonal antibodies for rps6 (1:1000) (Cell Signaling) and β-actin (1:5000) (Abcam, Waltham, MA, USA) proteins. Blots were visualized using anti-rabbit or anti-mouse horseradish peroxidase (HRP)-conjugated antibodies (Cell Signaling) and chemiluminescence reagent (Thermo Fisher Scientific, Waltham, MA, USA).
2.7. Growth Inhibition Assay
A cell growth assay using the MTS reagent was conducted to assess the general toxicity of each chalcone compound. Briefly, cell growth was assayed in sterile 96-well microtiter plates (Costar 3599, Corning Inc., Corning, NY, USA) in the presence of each chalcone. Experiments were conducted in triplicate. In a typical experiment, there are two plates. The first is the control plate, which allows one to assess the starting cell population on the day of drug addition (Day 0). The second plate is the test plate where cells are plated and tested with different drug concentrations on Day 0. For example, either CHO cells or Hs27 cells were plated in a 200 µL volume at 1000 cells/well on both the control and test plates and incubated overnight to allow the cells to adhere. On Day 0, 1 μL of PBS was added to the control plate wells so that their total volume was 201 μL. MTS reagent (Promega Cell Titer 96 Aqueous non-radioactive cell proliferation reagent, Promega, Madison, WI, USA) was added (20 μL) to each well on the control plate, and that plate was incubated for 4 h in a 5% CO2 atmosphere at 37 °C and then read on the plate reader. This data provided the starting absorbance for each well on Day 0.
On Day 0, 1 μL of a 200× stock solution of each chalcone was added to the respected well on the test plate, and each condition was tested in triplicate. Drug solutions were prepared in advance in 100% DMSO and dosed so that the final DMSO concentration was 0.5%. For example, 1 μL of chalcone solution was added to the cells plated in each well in 200 μL of media. Of note, the respective chalcone addition occurred after an initial overnight incubation of each cell line in each well to reach Day 0 (the drugging day). After the chalcone was added, the cells were incubated in 5% CO
2 for 48 h at 37 °C. After 48 h, the MTS reagent (Promega, Madison, WI, USA) was added (20 μL), and the test plates were incubated for an additional 4 h, and then, absorbance at 490 nm was measured on a BioTek Synergy MX plate reader. Controls run using ≤0.5% DMSO in the media showed no toxicity over the 48 h period compared to CHO or Hs27 cells grown in media only. IC
50 values were determined from the corresponding plot of relative absorbance at 490 nm vs. the drug concentration. The data is tabulated in
Table 2.
For highly colored materials like the chalcones, it is important to consider the contribution of the compound’s own absorbance to the MTS assay. To correct for the absorbance contribution of the chalcone itself, we measured each chalcone’s absorbance (Chalcone Abs) at 490 nm at the respective concentration (e.g., 10 µM) and subtracted the absorbance of the media background (blank Abs). This net value was then subtracted out of the Day 2 absorbance value to provide the % relative growth via the following equation:
2.8. Statistics
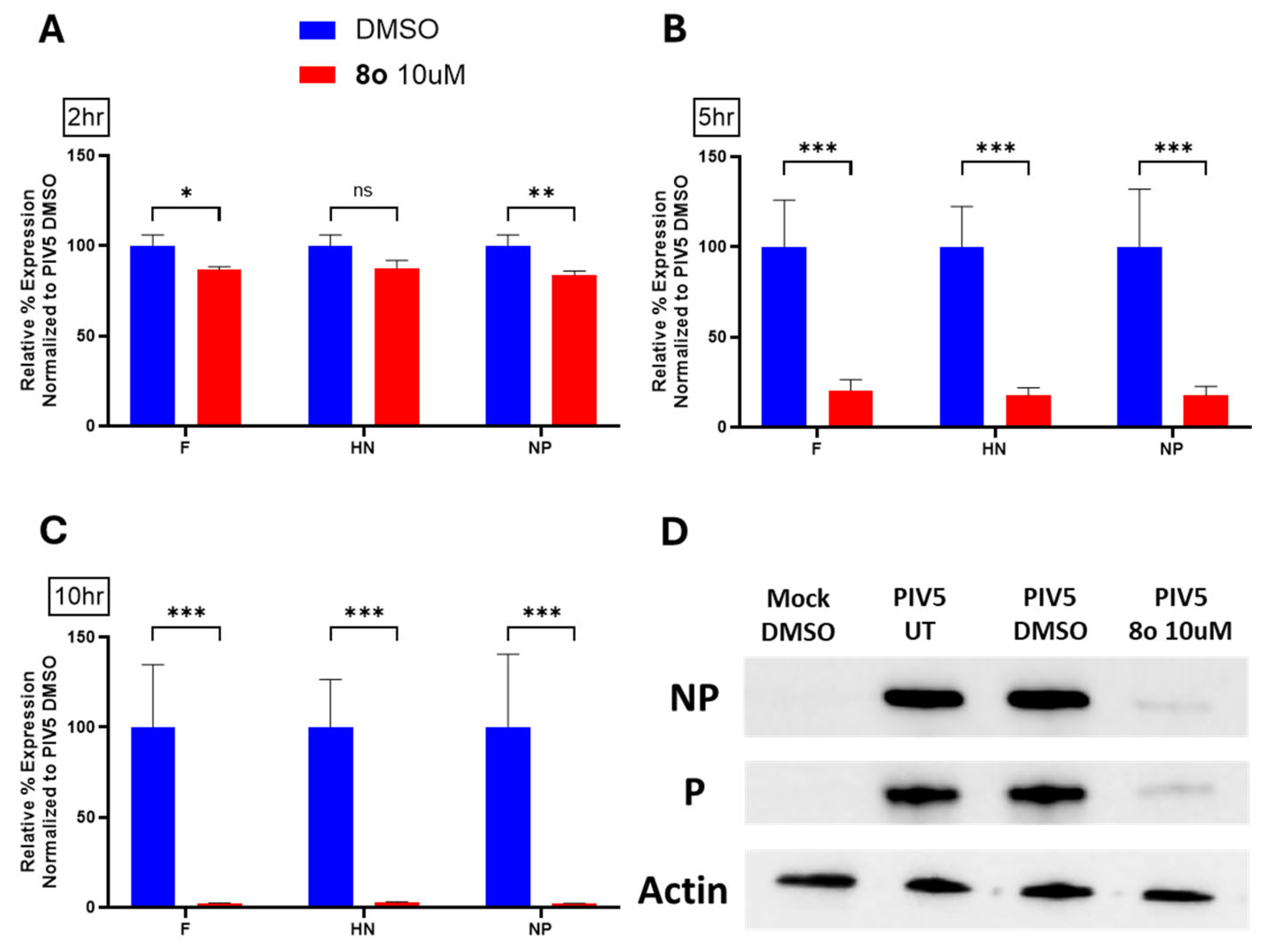
Statistical analysis was performed using GraphPad’s Student’s t-test and ANOVA. In all figures, * indicates a p-value < 0.05, ** indicates a p-value < 0.01, *** indicates a p-value < 0.001, and **** indicates a p-value < 0.0001.
2.9. Synthetic Methods
Reagents were ordered from commercial sources and used without further purification.
1H and
13C NMR spectra were obtained on a Bruker AVANCE III NMR instrument at 400 MHz and 125 MHz, respectively. Proof of purity was confirmed either via Shimadzu Prominence HPLC system or elemental analyses (Atlantic Microlabs, Norcross, GA, USA). The purity of each tested compound was ≥95% pure. High-resolution mass spectrometry was performed at the University of Florida Chemistry Department as a fee for service. Condensation reactions were conducted using a water condenser containing an attached drying tube filled with CaCl
2. Compounds
6a and
6b were reported previously [
7,
10]. For example, compound
6b was synthesized as follows.
2.10. 5-Bromo-2-Ethoxybenzaldehyde (6b)
A mixture of 5-bromo-2-hydroxybenzaldehyde (1.07 g, 5.3 mmol), ethyl bromide (1.09 g, d = 1.46 g/mL, 745 µL, 10 mmol), and solid anhydrous potassium carbonate (1.39 g, 10 mmol) in
N,
N-dimethylformamide (6.7 mL) was stirred at room temperature for 1 day. TLC (15% EtOAc/hexane) was used to monitor the reaction. The workup included the evaporation of DMF under reduced pressure. The resultant oil was dissolved in dichloromethane (DCM), and the organic phase was washed with 1 M aq. HCl. The bottom organic layer was separated, dried over anhydrous Na
2SO
4, filtered, and evaporated under reduced pressure to obtain a light-yellow oil. The oil was heated in hexane and cooled to rt over 1 h. The product recrystallized and was collected using a Buchner funnel under a vacuum to give
6b as a yellow solid. (0.92 g, 84%); R
f 0.46 (15% ethyl acetate:hexane). The
1H NMR matched the literature spectrum [
7].
1H NMR (CDCl
3): δ 10.42 (s, 1H), 7.93 (d, 1H,
J = 2.7 Hz), 7.61 (dd,1H,
J = 9, 2.7 Hz), 6.88 (d, 1H,
J = 9 Hz), 4.14 (q, 2H,
J = 6.9 Hz), 1.48 (t, 3H,
J = 7.1 Hz).
2.11. 1-[2-Hydroxy-6-(3-Methyl-Butoxy)-Phenyl]-Ethanone (7a)
To a solution containing 2,6-dihydroxyacetophenone (502 mg, 3.3 mmol) in acetone (5 mL), isopentyl bromide (465 mg, 394 µL, 3.0 mmol), KI (885 mg, 5.3 mmol), and solid anhydrous K
2CO
3 (1.46 g, 10.6 mmol) were added. The reaction mixture was heated to reflux overnight under anhydrous conditions and then cooled and filtered. The filtrate was concentrated under reduced pressure, and the residue was re-dissolved in ethyl acetate. The solution was acidified to pH 2 with 1 M HCl and washed with H
2O (50 mL). The water layer was washed three times with ethyl acetate. The organic layers were combined, dried over anhydrous Na
2SO
4, filtered, and concentrated under reduced pressure to give a crude yellow oil (709 mg). The solid was purified via flash chromatography (4% ethyl acetate/hexane) on a silica gel column (60 g silica). Elution with 4% ethyl acetate and hexane gave the recovered desired product
7a as a light-yellow oil (513 mg, 77%). R
f 0.34 (4% ethyl acetate/hexane). The
1H NMR matched the literature spectrum [
21].
1H NMR (CDCl
3): δ 13.26 (s, 1H), 7.31 (t, 1H,
J = 8.3 Hz), 6.54 (dd, 1H,
J = 8.4, 0.6 Hz), 6.37 (d, 1H,
J = 7.8 Hz), 4.06 (t, 2H,
J = 6.6 Hz), 2.69 (s, 3H), 1.83 (m, 1H), 1.76 (m, 2H), 0.99 (d, 6H,
J = 6.4 Hz).
13C NMR (CDCl
3): δ 205.1, 164.7, 161.0, 136.0, 111.3, 110.4, 101.7, 67.4, 37.8, 33.8, 25.2, 22.5.
2.12. 1-(2-Benzyloxy-6-Hydroxy-Phenyl)-Ethanone (7b)
To a solution containing 2,6-dihydroxy-acetophenone (1.51 g, 9.9 mmol) in acetone (15 mL), benzyl bromide (1.54 g, 1.07 mL, 9 mmol), KI (2.64 g, 15.9 mmol), and solid anhydrous K2CO3 (4.4 g, 31.8 mmol) were added. The reaction mixture was heated to reflux overnight under an N2 atmosphere and then cooled and filtered. The filtrate was concentrated under reduced pressure, and the residue was re-dissolved in ethyl acetate. The solution was acidified to pH 2 with 4 M HCl and washed with H2O (50 mL). The water layer was washed three times with ethyl acetate. The organic layers were combined, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give a crude yellow oil (2.51 g). The solid was purified via flash chromatography (10% ethyl acetate/hexane) on a silica gel column (260 g silica). Elution with 10% ethyl acetate and hexane gave the desired product 7b as a pale-yellow oil (2.17 g, 75%). Rf 0.4 (10% ethyl acetate/hexane). 1H NMR (CDCl3): δ 13.24 (s, 1H), 7.43-7.31 (m, 6H), 6.59 (dd, 1H, J = 8.3, 0.7 Hz), 6.47 (d, 1H, J = 8.1 Hz), 5.13 (s, 2H), 2.62 (s, 3H). 13C NMR (CDCl3): δ 205.2, 164.7, 160.6, 136.1, 135.8, 128.8, 128.5, 128.0, 111.6, 111.0, 102.23, 71.1, 34.1.
2.13. 1-(2-Hydroxy-6-Phenethyloxy-Phenyl)-Ethanone (7c)
To a solution containing 2,6-dihydroxyacetophenone (502 mg, 3.3 mmol) in acetone (5 mL), phenethyl bromide (555 mg, 410 µL, 3.0 mmol), KI (885 mg, 5.3 mmol), and solid anhydrous K
2CO
3 (1.46 g, 10.6 mmol) were added. The reaction mixture was heated to reflux overnight under anhydrous conditions and then cooled and filtered. The filtrate was concentrated under reduced pressure, and the residue was re-dissolved in ethyl acetate. The solution was acidified to pH 2 with 1 M HCl and washed with H
2O (50 mL). The water layer was washed three times with ethyl acetate. The organic layers were combined, dried over anhydrous Na
2SO
4, filtered, and concentrated under reduced pressure to give a crude orange solid (649 mg). The solid was purified via flash chromatography (4% ethyl acetate/hexane) on a silica gel column (52 g silica). Elution with 4% ethyl acetate and hexane gave the desired product
7c as a pale-yellow solid (157 mg, 20%). R
f 0.23 (4% ethyl acetate/hexane). The
1H NMR matched the literature spectrum [
22].
1H NMR (CDCl
3): δ 13.25 (s, 1H), 7.36 -7.23 (m, 6H), 6.56 (dd, 1H,
J = 8.3, 1Hz), 6.38 (d, 1H,
J = 8.3 Hz), 4.32 (t, 2H,
J = 7 Hz), 3.19 (t, 2H,
J = 6.8 Hz), 2.54 (s, 3H).
13C NMR (CDCl
3): δ 205.2, 164.7, 160.6, 137.6, 136.0, 128.7, 128.7, 126.8, 111.3, 110.8, 101.8, 69.4, 35.5, 33.9.
2.14. 1-[2(Biphenyl-4-Ylmethyoxy)6-Hydroxy)-Phenyl]-Ethanone (7d)
To a solution containing 2,6-dihydroxyacetophenone (508 mg, 3.3 mmol) in acetone (5 mL), 4-bromomethyl-biphenyl (745 mg, 3.0 mmol), KI (888 mg, 5.3 mmol), and solid anhydrous K2CO3 (1.46 g, 10.6 mmol) were added. The reaction mixture was heated to reflux overnight under anhydrous conditions and then cooled and filtered. The filtrate was concentrated under reduced pressure, and the residue was re-dissolved in ethyl acetate. The solution was acidified to pH 2 with 4 M HCl and washed with H2O (50 mL). The water layer was washed three times with ethyl acetate. The organic layers were combined, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give a crude orange solid (1.07 g). The solid was purified via flash chromatography (50% dichloromethane/hexane) on a silica gel column (107 g silica). Elution with (50% dichloromethane/hexane) gave the desired product 7d as a pale-yellow solid (282 mg, 27%). Rf 0.29 (50% dichloromethane/hexane). 1H NMR (CDCl3): δ 13.26 (s, 1H), 7.62 (m, 4H), 7.47 (m, 4H), 7.35 (m, 2H), 6.60 (d, 1H, J = 8.6 Hz), 6.68 (d, 1H, J = 8.3 Hz) 5.16 (s, 2H), 2.64 (s, 3H). 13C NMR (CDCl3): δ 205.2, 164.8, 160.7, 141.4, 140.5, 136.1, 134.8, 128.9, 128.4, 127.6, 127.5, 127.1, 111.6, 111.1, 102.3, 70.9, 34.2. HRMS for C21H18O3 (M+H): theory 319.1329, found 319.1326.
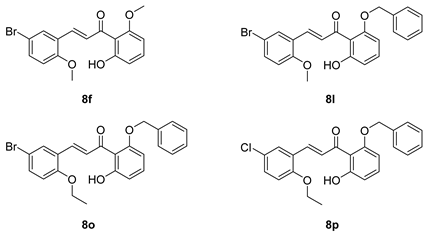
Compounds
8a,
8f,
8l,
8o, and
8p were synthesized previously [
7,
10].
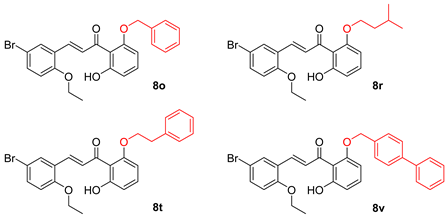
2.15. (2E)-3-(5-Bromo-2-Ethoxy-Phenyl)-1-(2-Hydroxy-6-Methoxy-Phenyl)-Propenone (8q)
5-Bromo-2-ethoxybenzaldehyde (6b) (227 mg, 1 mmol), 1-(2-hydroxy-6-methoxyphenyl) ethanone (167 mg, 1 mmol), and MeOH (5 mL) were combined, and KOH in methanol (40% weight/volume, 5 mL) was added at rt and then heated to 60 °C and stirred overnight. The workup included evaporation of the solvent under reduced pressure and the dropwise addition of 12 N HCl until the solution was at pH 1. The solution was then extracted with ethyl acetate (twice). Each organic layer was separated, pooled together, dried over anhydrous Na2SO4, filtered, and concentrated to give a crude yellow oil (470 mg). Column chromatography (10% ethyl acetate:hexane, Rf 0.24) was performed and provided the pure product 8q as an orange solid. (0.137 g, 36%). 1H NMR (CDCl3): δ 13.10 (s, 1H), 8.04 (d, 1H, J = 15.9 Hz), 7.82 (d, 1H, J = 15.6 Hz), 7.68 (d, 1H, J = 2.4 Hz), 7.39, (dd, 1H, J = 8.8, 2.4 Hz), 7.34 (t, 1H, J 8.4 Hz), 6.77 (d, 1H, J = 9 Hz), 6.60 (dd, 1H, J = 8.4, 0.9 Hz), 6.41 (d, 1H, J = 7.8 Hz), 4.07 (q, 2H, J = 6.8 Hz), 3.93 (s, 3H), 1.47 (t, 3H, J = 7 Hz). 13C NMR (CDCl3): δ 194.5, 164.7, 160.9, 157.0, 136.6, 135.8, 133.7, 130.9, 128.7, 126.3, 113.8, 112.7, 112.0, 110.8, 101.5, 64.3, 56.0, 14.7. HRMS for C18H17BrO4 (M+H): theory 377.0399, found 377.0388. mp 84–87 °C.
2.16. (2E)-3-(5-Bromo-2-Ethoxy-Phenyl)-1-[2-Hydroxy-6-(3-Methyl-Butoxy)-Phenyl]-Propenone (8r)
5-Bromo-2-ethoxybenzaldehyde (
6b) (115 mg, 0.5 mmol) and 1-[2-Hydroxy-6-(3-methyl-butoxy)-phenyl]-ethanone (
7a) [
21] (111 mg, 0.5 mmol), and MeOH (2.5 mL) were combined, and KOH in methanol (40% weight/volume, 2.5 mL) was added at rt and then heated to 60 °C. The reaction mixture was stirred at 60 °C overnight. Workup included evaporation of the solvent under reduced pressure, dropwise addition of 4N HCl until solution was at pH 1. The solution was then extracted with ethyl acetate (thrice). Each organic layer was separated, pooled together, dried over anhydrous Na
2SO
4, filtered and concentrated to give a crude orange solid (212 mg). Column chromatography (6% ethyl acetate:hexane, R
f 0.28) was performed and provided the pure product
8r as an orange solid. (141 mg, 71%).
1H NMR (CDCl
3): δ 13.10 (s, 1H), 8.11 (d, 1H,
J = 15.9 Hz), 7.87 (d, 1H,
J = 15.9 Hz), 7.74 (d, 1H,
J = 2.4 Hz), 7.42 (dd, 1H,
J = 8.8, 2.4 Hz), 7.34 (t, 1H), 6.80 (d, 1H,
J = 9 Hz), 6.60 (dd, 1H,
J = 8.3, 1 Hz), 6.42 (d, 1H,
J = 7.6 Hz), 4.09 (m, 4H), 1.79 (m, 3H), 1.48 (t, 3H,
J = 7.1 Hz), 0.92 (m, 6H).
13C NMR (CDCl
3): δ 194.6, 164.8, 160.6, 158.9, 136.0, 135.7, 133.8, 129.9, 128.8, 126.5, 114.0, 112.9, 112.1, 110.7, 102.2, 67.5, 64.5, 38.1, 25.1, 22.5, 14.7. HRMS for C
22H
25BrO
4 (M+H): theory 433.1014, found 433.0990. mp 84–86 °C.
2.17. (2E)-3-(5-Bromo-2-Methoxy-Phenyl)-1-[2-Hydroxy-6-(3-Methyl-Butoxy)-Phenyl]-Propenone (8s)
5-Bromo-2-methoxybenzaldehyde (108 mg, 0.5 mmol) and 1-[2-Hydroxy-6-(3-methyl-butoxy)-phenyl]-ethanone (
7a) [
21] (112 mg, 0.5 mmol), and MeOH (2.5 mL) were combined, and KOH in methanol (40% weight/volume, 2.5 mL) was added at rt and then heated to 60 °C. The reaction mixture was stirred at 60 °C overnight. Workup included evaporation of the solvent under reduced pressure, dropwise addition of 4N HCl until solution was at pH 2. The solution was then extracted with ethyl acetate (thrice). Each organic layer was separated, pooled together, dried over anhydrous Na
2SO
4, filtered and concentrated to give a crude orange solid (195 mg). Column chromatography (6% ethyl acetate:hexane, R
f 0.23) was performed and provided the pure product
8s as an orange solid (122 mg, 58%).
1H NMR (CDCl
3): δ 13.11 (s, 1H), 8.09 (d, 1H,
J = 15.6 Hz), 7.88 (d, 1H,
J = 15.7 Hz), 7.73 (d, 1H,
J = 2.4 Hz), 7.44 (dd,
J = 8.8, 2.4 Hz) 7.34 (t, 1H,
J = 8.3 Hz), 6.82 (d, 1H,
J = 8.8 Hz), 6.60 (d, 1H,
J = 8.3 Hz), 6.42 (d, 1H,
J = 6.42 Hz), 4.10 (t, 2H,
J = 6.4 Hz), 3.89 (s, 3H), 1.80 (m, 3H) 0.93 (d, 6H).
13C NMR (CDCl
3): δ 194.6, 160.6, 157.5, 136.0, 135.7, 133.8, 130.1, 129.0, 126.4, 113.1, 113.0, 112.1, 110.7, 102.2, 77.2, 67.5, 55.9, 38.1, 25.1, 22.5. HRMS for C
21H
23BrO
4 (M+H): theory 419.0689, found 419.0693. mp 94–98 °C.
2.18. (2E)-3-(5-Bromo-2-Ethoxy-Phenyl)-1-[2-Hydroxy-6-Phenethyloxy]-Propenone (8t)
5-Bromo-2-ethoxybenzaldehyde (
6b) (47 mg, 0.2 mmol) and 1-[2-Hydroxy-6-phenethyloxy-phenyl]-ethanone (
7c) [
22] (51 mg, 0.2 mmol), and MeOH (2.5 mL) were combined, and KOH in methanol (40% weight/volume, 2.5 mL) was added at rt and then heated to 60 °C. The reaction mixture was stirred at 60 °C overnight. Workup included evaporation of the solvent under reduced pressure, dropwise addition of 4N HCl until solution was at pH 1. The solution was then extracted with ethyl acetate (thrice). Each organic layer was separated, pooled together, dried over anhydrous Na
2SO
4, filtered and concentrated to give a crude orange solid (101 mg). Column chromatography (10% ethyl acetate:hexane, R
f 0.25) was performed and provided the pure product
8t as an orange solid. (55 mg, 59%).
1H NMR (CDCl
3): δ 13.03 (s, 1H), 8.07 (d, 1H,
J = 15.9 Hz), 7.84 (d, 1H,
J = 15.7 Hz), 7.69 (d, 1H, 2.4 Hz), 7.41 (dd, 1H,
J = 8.8, 2.4 Hz), 7.33 (t, 1H,
J = 8.4 Hz), 7.23 (m, 5H), 6.79 (d, 1H,
J = 8.8 Hz), 6.62 (dd, 1H,
J = 8.3, 0.7 Hz), 4.31 (t, 2H,
J = 7.2 Hz), 4.10 (m, 2H, 7.1 Hz), 3.20 (t, 2H, 7.2 Hz), 1.48 (t, 3H, 7 Hz).
13C NMR (CDCl
3): δ 193.6, 163.7, 159.1, 155.9, 136.2, 135.0, 134.9, 132.8, 129.3, 127.9, 127.8, 127.6, 125.7, 125.5, 112.9, 111.9, 111.2, 109.9, 101.5, 68.7, 63.4, 34.7, 13.7. HRMS for C
25H
23BrO
4 (M+H): theory 467.0858, found 467.0857. mp 105–110 °C
2.19. (2E)-3-(5-Bromo-2-Methoxy-Phenyl)-1-[2-Hydroxy-6-Phenethyloxy]-Propenone (8u)
5-Bromo-2-methoxy-benzaldehyde (38 mg, 0.18 mmol), 1-[2-hydroxy-6-phenethyloxy-phenyl]-ethanone (
7c) [
22] (46 mg, 0.18 mmol), and MeOH (2.5 mL) were combined, and KOH in methanol (40% weight/volume, 2.5 mL) was added at rt and then heated to 60 °C. The reaction mixture was stirred at 60 °C overnight. The workup included evaporation of the solvent under reduced pressure and the dropwise addition of 4 N HCl until the solution was at pH 1. The solution was then extracted with ethyl acetate (thrice). Each organic layer was separated, pooled together, dried over anhydrous Na
2SO
4, filtered and concentrated to give a crude orange solid (88 mg). Column chromatography (20% ethyl acetate:hexane, R
f 0.4) was performed and provided the pure product
8u as an orange solid. (47 mg, 59%).
1H NMR (CDCl
3): δ 13.05 (s, 1H), 8.04 (d, 1H,
J = 15.7 Hz), 7.84 (d, 1H,
J = 15.9 Hz), 7.67 (d, 1H,
J = 2.4 Hz), 7.43 (dd, 1H,
J = 8.8, 2.4 Hz), 7.32 (t, 1H,
J = 8.3 Hz), 7.23 (m, 5H), 6.80 (d, 1H,
J = 8.8 Hz), 6.60 (d, 1H,
J = 8.3 Hz), 6.41 (d, 1H,
J = 8.3 Hz), 4.31 (t, 2H,
J = 7.3), 3.33 (s, 3H), 3.20 (t, 2H,
J = 7.2 Hz).
13C NMR (CDCl
3): δ 194.6. 164.8, 160.1, 157.6, 137.3, 136.1, 135.9, 133.8, 130.6, 129.1, 128.8, 128.7, 126.8, 126.4, 113.1, 113.0, 112.2, 111.0, 102.5, 69.8, 55.9, 35.7. HRMS for C
24H
21BrO
4 (M+H): theory 453.0701, found 453.0704. mp 95–100 °C.
2.20. (2E)-1-[2-(Biphenyl-4-Ylmethoxy)-6-Hydroxy-Phenyl]3-(5-Bromo-2-Ethoxy-Phenyl)-Propenone (8v)
5-Bromo-2-ethoxybenzaldehyde (6b, 72 mg, 0.3 mmol), 1-[2(biphenyl-4-ylmethyoxy)6-hydroxy)-phenyl]-ethanone (7d, 100 mg, 0.3 mmol), and MeOH (2.5 mL) were combined, and KOH in methanol (40% weight/volume, 2.5 mL) was added at rt, and then, the reaction mixture was heated to 60 °C and stirred at 60 °C overnight. The workup included evaporation of the solvent under reduced pressure and the dropwise addition of 4 N HCl until the solution was at pH 1. The solution was then extracted with ethyl acetate (thrice). Each organic layer was separated, pooled together, dried over anhydrous Na2SO4, filtered, and concentrated to give a crude orange solid (187 mg). Column chromatography (1% acetone, 39% dichloromethane, 60% hexane, Rf 0.25) was performed and provided the pure product 8v as an orange solid. (45 mg, 27%). 1H NMR (CDCl3): δ 13.03 (s, 1H), 8.04 (d, 1H, J = 16 Hz), 7.85 (d, 1H, J = 16 Hz), 7.40 (m, 13H), 6.67 (m, 2H), 6.55 (d, 1H, J = 8.1 Hz), 5.18 (s, 2H), 3.96 (q, 2H) 1.40 (t, 3H). 13C NMR (CDCl3): δ 194.8, 164.7, 160.1, 156.9, 141.1, 140.5, 136.2, 135.9, 134.6, 133.8, 130.6, 129.2, 128.7, 127.9, 127.4, 127.1, 126.2, 113.7, 112.8, 112.4, 111.2, 102.8, 77.2, 71.1, 64.3, 14.7. HRMS for C30H25BrO4 (M+H): theory 531.0993, found 531.0979. mp 155–157 °C.
2.21. (2E)-1-[2-(Biphenyl-4-Ylmethoxy)-6-Hydroxy-Phenyl]3-(5-Bromo-2-Methoxy-Phenyl)-Propenone (8w)
5-Bromo-2-methoxybenzaldehyde (61 mg, 0.29 mmol), 1-[2(biphenyl-4-ylmethyoxy)6-hydroxy)-phenyl]ethanone (7d) (91 mg, 0.29 mmol), and MeOH (2.5 mL) were combined, and KOH in methanol (40% weight/volume, 2.5 mL) was added at rt and then heated to 60 °C. The reaction mixture was stirred at 60 °C overnight. The workup included evaporation of the solvent under reduced pressure and the dropwise addition of 4 N HCl until the solution was at pH 1. The solution was then extracted with ethyl acetate (thrice). Each organic layer was separated, pooled together, dried over anhydrous Na2SO4, filtered, and concentrated to give a crude orange solid (137 mg). Column chromatography (1% acetone, 49.5% dichloromethane, 50% hexane, Rf 0.31) was performed and provided the pure product 8w as an orange solid. (33 mg, 23%). 1H NMR (CDCl3): δ 13.04 (s, 1H), 8.03 (d, 1H, J = 16 Hz) 7.86 (d, 1H, J = 16 Hz), 7.43 (m, 13H), 6.70 (d, 1H, J = 8.8 Hz), 6.65 (d, 1H, J = 8.6 Hz), 6.55 (d, 1H, J = 8.3), 5.19 (s, 2H), 3.75 (s, 3H). 13C NMR (CDCl3): δ 194.7, 164.7, 160.1, 157.5, 141.1, 140.5, 136.2, 135.9, 134.6, 133.8, 130.7, 129.2, 128.7, 127.9, 127.4, 127.4, 127.1, 126.1, 112.9, 112.7, 112.4, 111.2, 102.7, 77.2, 71.0, 55.7. HRMS for C29H23BrO4 (M+H): theory 517.0837, found 517.0827. mp 164–167 °C.
2.22. (2E)-1-(2-Benzloxy-6-Hydroxy-Phenyl)-5-(5-Hydroxy-2-Methoxy-Phenyl)-Propenone (8x)
5-Hydroxy-2-methoxybenzaldehyde (81 mg, 0.53 mmol), 1-(2-benzyloxy-6-hydroxy-phenyl)-ethanone [
7] (
7b, 130 mg, 0.54 mmol), and MeOH (2.5 mL) were combined, and KOH in methanol (40% weight/volume, 2.5 mL) was added at rt, and the reaction mixture was stirred at 60 °C overnight. The workup included evaporation of the solvent under reduced pressure and the dropwise addition of 4 N HCl until the solution was at pH 2. The solution was then extracted with ethyl acetate (thrice). Each organic layer was separated, pooled together, dried over anhydrous Na
2SO
4, filtered, and concentrated to give a crude orange solid (252 mg). Column chromatography (20% ethyl acetate:hexane, R
f 0.3) was performed and provided the pure product
8x as an orange solid. (92 mg, 46%).
1H NMR (CDCl
3): δ 13.59 (s, 1H), 8.15 (d,1H,
J = 15.9 Hz), 7.82 (d, 1H,
J = 15.9 Hz), 7.55 (m, 2H), 7.43 (m, 3H), 7.38 (t, 1H,
J = 8.3 Hz), 6.77 (m 2H), 6.66 (dd, 2H,
J = 8.3, 0.7 Hz), 6.54 (d, 1H,
J = 8.1 Hz), 6.14 (d, 1H,
J = 2.9 Hz), 5.13 (s, 2H), 4.08 (s, 1H), 3.77 (s, 3H).
13C NMR (CDCl
3): δ 194.5, 165.5, 160.2, 153.1, 148.9, 137.7, 136.4, 135.9, 129.0,128.9, 128.8, 128.2, 127.5, 124.6, 118.5, 113.1, 112.7, 111.9, 111.5, 102.3, 71.2, 56.1. HRMS for C
23H
20O
3: (M+H): theory 377.1384, found 377.1391. mp 164–167 °C.
2.23. (2E)-1-(2-Benzloxy-6-Hydroxy-Phenyl)-3-(2,3,4-Trimethoxy-Phenyl)-Propenone (8y)
2,3,4-Trimethoxybenzyaldehyde (98 mg, 0.5 mmol), 1-(2-benzyloxy-6-hydroxy-phenyl)-ethanone [
7] (
7b, 121 mg, 0.5 mmol), and MeOH (2.5 mL) were combined, and KOH in methanol (40% weight/volume, 2.5 mL) was added at rt, and the reaction mixture was stirred at 60 °C overnight. The workup included evaporation of the solvent under reduced pressure and the dropwise addition of 4 N HCl until the solution was at pH 2. The solution was then extracted with ethyl acetate (thrice). Each organic layer was separated, pooled together, dried over anhydrous Na
2SO
4, filtered, and concentrated to give a crude orange solid (225 mg). Column chromatography (20% ethyl acetate:hexane, R
f 0.27) was performed and provided the pure product
8y as an orange solid. (148 mg, 70%).
1H NMR (CDCl
3): δ 13.49 (s, 1H), 8.06 (d, 1H,
J = 15.9 Hz), 7.81 (d, 1H,
J = 15.9 Hz), 7.48 (m, 2H), 7.36 (m, 4H), 6.66 (m, 2H), 6.52 (d, 1H,
J = 8.3 Hz), 6.43 (d, 1H,
J = 8.8 Hz), 5.14 (s, 2H), 3.89 (d, 6H,
J = 5.1 Hz), 3.85 (s, 3H).
13C NMR (CDCl
3): δ 194.5, 165.3, 160.1, 155.6, 153.7, 142.2, 138.1, 136.0, 135.6, 128.8, 128.3, 128.2, 126.4, 122.8, 122.3, 112.1, 111.4, 107.52, 102.5, 71.3, 61.7, 60.9, 56.1. HRMS for C
25H
24O
6 (M+H): theory 421.1646, found 421.1659. 130–138 °C.
2.24. (2E)-1-(2-Benzloxy-6-Hydroxy-Phenyl)-3-(3,4,5-Trimethoxy-Phenyl)-Propenone (8z)
3,4,5-Trimethoxybenzyaldehyde (98 mg, 0.5 mmol), 1-(2-benzyloxy-6-hydroxy-phenyl)ethanone [
7] (
7b, 121 mg, 0.5 mmol), and MeOH (2.5 mL) were combined, and KOH in methanol (40% weight/volume, 2.5 mL) was added at rt, and then, the reaction mixture was stirred at 60 °C overnight. The workup included evaporation of the solvent under reduced pressure and the dropwise addition of 4 N HCl until the solution was at pH 2 The solution was then extracted with ethyl acetate (thrice). Each organic layer was separated, pooled together, dried over anhydrous Na
2SO
4, filtered, and concentrated to give a crude orange oil (222 mg). Column chromatography (20% ethyl acetate:hexane, R
f 0.27) was performed and provided the pure product
8z as an orange solid. (116 mg, 55%).
1H NMR (CDCl
3): δ 13.09 (s, 1H), 7.79 (d, 1H,
J = 15.4 Hz), 7.72 (d, 1H,
J = 15.7 Hz), 7. 40 (m, 3H), 7.25 (m, 3H), 6.66 (dd, 2H,
J = 8.4 Hz,
J = 0.9 Hz), 6.62 (s, 2H,), 6.54 (d, 1H,
J = 7.3 Hz), 5.17 (s, 2H), 3.88 (s, 3H), 3.67 (s, 6H).
13C NMR (CDCl
3): δ 194.3, 164.8, 160.0, 153.2, 143.1, 140.2, 135.9, 135.9, 130.5, 128.7, 128.2, 127.1, 112.4, 111.4, 105.8, 102.8, 71.1, 60.9, 56.0. HRMS for C
25H
24O
6 (M+H): theory 421.1646, found 421.1652. mp 103-105 °C.
2.25. (2E)-1-(3,5-Dibromo-2-Hydroxyphenyl)-3-(3,4-Dimethoxyphenyl)-2-Propen-1-One (NM5, 3)
(2E)-1-(3,5-Dibromo-2-hydroxyphenyl)-3-(3,4-dimethoxyphenyl)-2-propen-1-one (
3:
NM5, see
Figure 1) was prepared as described by Mateeva et al. [
6] with a few changes. 3,4-Dimethoxybenzyaldehyde (166 mg, 1 mmol), 1-(3,5-dibromo-2-hydroxyphenyl)ethanone (294 mg, 1 mmol), and MeOH (5 mL) were combined, and KOH in methanol (40% weight/volume, 5 mL) was added at rt, and then, the mixture was heated to 60 °C and stirred at 60 °C for 3h. The workup included cooling the mixture to rt, evaporation of the solvent under reduced pressure, and the dropwise addition of 4 N HCl until the solution was at pH 2. The solution was then extracted with ethyl acetate (thrice). Each organic layer was separated, pooled together, dried over anhydrous Na
2SO
4, filtered, and concentrated to give a crude orange solid (478 mg). Column chromatography (15% ethyl acetate:hexane, R
f 0.23) was performed and provided the pure product
3 (
NM5) as an orange solid. (356 mg, 81%).
1H-NMR matched the literature spectrum [
23].
1H NMR (CDCl
3): δ 13.69 (s, 1H), 8.00–7.94 (m, 2H), 7.87 (d, 1H,
J = 2.2 Hz), 7.39 (d, 1H,
J = 15.2 Hz), 7.30 (dd, 1H,
J = 8.3, 2 Hz), 7.18 (d, 1H,
J = 2 Hz), 6.93 (d, 1H,
J = 8.3 Hz), 3.99 (s, 3H), 3.96 (s, 3H).
13C NMR (CDCl
3): δ 192.2, 159.3, 152.5, 149.5, 148.0, 141.1, 131.0, 127.1, 124.4, 121.7, 116.4, 113.4, 111.2, 110.5, 110.2, 56.2, 56.1. HRMS for C
17H
14Br
2O
4 (M-H)
−: theory 438.9186 found 438.9174.
4. Discussion
The most striking finding from our virology studies was the broad-spectrum antiviral effects that our chalcones showed on prototype members of four different virus families, each of which is considered a prime candidate family for an emerging pandemic virus: Paramyxo-, Bunya-, Flavi-, and Corona-viruses. This broad inhibition of multiple divergent virus families is the major discovery of this report.
The observed pan anti-viral activities of 8o and 8p are intriguing. Each of these four viral families employ different strategies for replication, indicating that chalcones may target conserved viral replication mechanisms. How then do these compounds work?
We speculate that the chalcone-mediated inhibition of viral entry is not likely because all four viruses tested here bind to different cellular receptors to initiate the infection: sialic acid for PIV5, DC-SIGN for LACV, AXL for ZIKV, and TMPRSS2 for OC43 [
26,
27,
28,
29]. Similarly, some of these viruses enter cells via fusion at the plasma membrane (e.g., PIV5), while others are internalized through clathrin-mediated (e.g., ZIKV) or caveolin-1-dependent (e.g., OC43) endocytosis and fusion from internal vesicles [
30,
31,
32]. However, as mentioned above, all four viruses share essential steps downstream of cell entry.
A common feature of these four viruses is that they are completely dependent on the production of a viral RNA-dependent-RNA polymerase (RDRP) from incoming genomes. Thus, a common mechanism of action of our chalcones may include inhibition of the viral RDRP. Our initial studies focused on the effect of chalcones on the replication of PIV5, a prototype member of the
Paramyxovirus family of negative-strand RNA viruses. The replication cycle of these viruses depends on initial transcription by the virion-associated RDRP to produce low levels of mRNA, followed by genome replication, and finally secondary transcription to produce detectable levels of the GFP protein encoded in the PIV5-GFP reporter virus used here [
24]. Time-of-addition studies showed that our chalcones inhibit viral RNA synthesis at an early stage of the virus growth cycle within 5-6 h post infection, which is within the timeframe when primary transcription is occurring. Since switching viral RDRP from carrying out primary transcription to genome replication requires the synthesis of new viral proteins, we speculate that the chalcones may target the production of viral genomes via the RDRP or act at the level of the translation of viral mRNAs.
Recent reports have suggested that chalcones can bind to the RDRP of SARS-CoV2, for example [
33,
34]. As such, RDRPs are excellent drug targets due to their typical low-level expression and specific enzymatic activities that are limited only to viral templates [
35]. Future work will determine if our chalcone derivatives are interacting with the RDRPs of various RNA viruses to explain their mechanism of action.
Another possible mechanism of action of our chalcones could be independent of the viral machinery. We speculate that host cells with inhibited growth are poor viral hosts. In one scenario, chalcone disruption of host cell growth and proliferation pathways may create unfavorable intracellular environments for viral replication. Some reports suggest that chalcones can induce cell cycle arrest [
36]. Our initial efforts to understand the mechanism of action of the lead chalcone
8o in host cell signaling pathways essentially ruled out mTOR inhibition as a mechanism of action. Surprisingly, the published mTOR inhibitor (chalcone NM5) was the only chalcone to affect the activation of mTOR, as evidenced by a significant increase in rps6 phosphorylation (see
Figure 12). Future work will analyze prps6 and rps6 protein expression in mock and infected Hs27 cells treated with the chalcone series to completely rule out mTOR signaling as the targeted pathway of infected cells.
We note that compound
8o meets several criteria for Lipinski’s rule of 5 [
37,
38]. It has an MW <500 (453 g/mol) and has <5 H bond donors and <10 H bond acceptors. However,
8o has a clog P of 5.84, which violates Lipinski’s Rule (clogP < 5). While there are documented violations to this rule of 5 [
38], future designs should aim to install more water-soluble substituents to improve the drug-like properties and oral availability of these constructs for clinical applications.
Our work represents an important contribution to this evolving research area by suggesting chalcones as a core scaffold for broad spectrum antivirals [
39,
40]. Other scaffolds have been suggested. For example, Patel et al. recognized structural similarity in the binding pocket of positive single stranded RNA viruses and the SARS-CoV2 3CL
pro viral protease and showed broad spectrum antiviral activity of SARS-CoV2 3CL
pro viral protease inhibitors [
39]. Luong et al. recently highlighted the role of viral attachment inhibitors, fusion inhibitors, viral biosynthesis inhibitors and viral assembly and release inhibitors as potential broad-spectrum antivirals [
40]. In addition, drug repurposing was recently used to develop pan-flavivirus compounds [
41]. These collective efforts bring us closer to pan antivirals. Moving forward, however, important caveats must be addressed including host cell toxicity and viral resistance.