Optogenetics: A Novel Therapeutic Avenue for Age-Related Macular Degeneration
Abstract
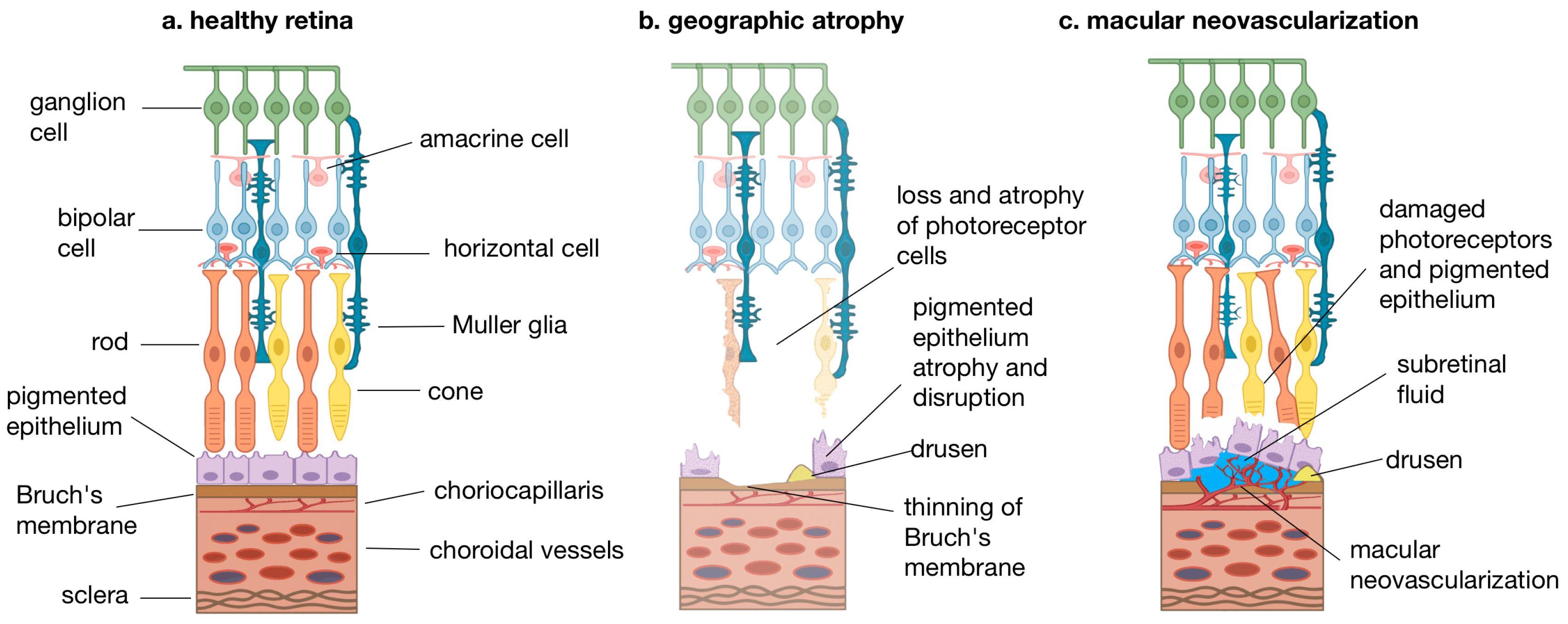
1. Introduction
2. Principles of Optogenetics
2.1. AAV Serotypes and Delivery Mechanisms
2.2. Optogenetic Light Sources
2.3. Opsin Variants for AMD Therapy
- -
- Sensitivity: Highly sensitive opsins allow activation with low light intensities, minimizing phototoxicity and maximizing compatibility with ambient light conditions. Next-generation opsins engineered for low light intensity thresholds are continuously being developed.
- -
- Kinetics: The speed of opsin activation and inactivation influences the temporal resolution of the restored vision. Faster kinetics may be crucial for processing rapidly changing visual information.
- -
- Spectral Properties: Different opsins are activated by different wavelengths of light. Choosing opsins with activation spectra that are well transmitted through ocular tissues and can be safely delivered by external light sources is important. Red-shifted opsins reduce phototoxicity, the thermal effect, and oxidative stress associated with high-intensity illumination. These properties help minimize the risk of retinal damage related to light stimulation [38,88].
- -
- Expression Stability: Robust and stable expression ensures long-term therapeutic efficacy.
2.4. Cellular Targets
2.5. Clinical Evidence
3. Preclinical Applications of Optogenetics in AMD Models
Clinical Considerations for Optogenetic Therapy in AMD
4. Challenges and Future Directions
5. Future Research Focus
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMD | Age-related Macular Degeneration |
| AAV | Adeno-Associated Virus |
| AAV2 | Adeno-Associated Virus Serotype 2 |
| BCs | Bipolar Cells |
| ChR2 | Channelrhodopsin-2 |
| ChRmine | Channelrhodopsin Mine |
| CNV | Choroidal Neovascularization |
| FDA | U.S. Food and Drug Administration |
| GA | Geographic Atrophy |
| GHCR | Gleobacter–Human Chimeric Rhodopsin |
| ILM | Inner Limiting Membrane |
| IVT | Intravitreal Injection |
| LED | Light-Emitting Diode |
| MCO1/MCO-010 | Multicharacteristic Opsin 1/010 |
| MNV | Macular Neovascularization |
| MW-opsin | Medium-Wavelength Cone Opsin |
| ONL | Outer Nuclear Layer |
| OPN4 | Melanopsin |
| PPV | Pars Plana Vitrectomy |
| PRs | Photoreceptors |
| RGCs | Retinal Ganglion Cells |
| Rho | Rhodopsin |
| RPE | Retinal Pigment Epithelium |
| RPD | Reticular Pseudodrusen |
| SC | Suprachoroidal Injection |
| SD-OCT | Spectral Domain Optical Coherence Tomography |
| SDD | Subretinal Drusenoid Deposits |
| SNAG-mGluR2 | Synthetic Opsin–Metabotropic Glutamate Receptor 2 Fusion Protein |
| SR | Subretinal Injection |
| VEGF | Vascular Endothelial Growth Factor |
References
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- de Jong, E.K.; Geerlings, M.J.; den Hollander, A.I. Age-related macular degeneration. In Genetics and Genomics of Eye Disease; Academic Press: Cambridge, MA, USA, 2020; pp. 155–180. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef]
- Steinmetz, J.D.; Bourne, R.R.; Briant, P.S.; Flaxman, S.R.; Talor, H.R.; Jonas, J.B.; Abdoli, A.A.; Abrha, W.A.; Abualhasan, A.; Abu-Gharbieh, E.G.; et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Ferris, F.L.; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R. Clinical Classification of Age-related Macular Degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef]
- Faatz, H.; Lommatzsch, A.P. Altersbedingte Makuladegeneration [Age-related macular degeneration–Part 1: Pathophysiology, classification and diagnostic]. Klin Monbl Augenheilkd. 2024. (In Germany) [Google Scholar] [CrossRef] [PubMed]
- Davis, M.D.; Gangnon, R.E.; Lee, L.Y.; Hubbard, L.D.; Klein, B.E.; Klein, R.; Ferris, F.L.; Bressler, S.B.; Milton, R.C.; Age-Related Eye Disease Study, G. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch. Ophthalmol. 2005, 123, 1484–1498. [Google Scholar] [CrossRef]
- Ferris, F.L.; Davis, M.D.; Clemons, T.E.; Lee, L.Y.; Chew, E.Y.; Lindblad, A.S.; Milton, R.C.; Bressler, S.B.; Klein, R. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch. Ophthalmol. 2005, 123, 1570–1574. [Google Scholar] [CrossRef]
- Ashique, S.; Kumar, S.; Hussain, A.; Farid, A.; Mishra, N.; Garg, A. Age-associated macular degeneration: Epidemiologic features, complications, and potential therapeutic approaches. In Targeting Angiogenesis, Inflammation, and Oxidative Stress in Chronic Diseases; Academic Press: Cambridge, MA, USA, 2024; pp. 381–429. [Google Scholar] [CrossRef]
- Wong, T.Y.; Chakravarthy, U.; Klein, R.; Mitchell, P.; Zlateva, G.; Buggage, R.; Fahrbach, K.; Probst, C.; Sledge, I. The natural history and prognosis of neovascular age-related macular degeneration: A systematic review of the literature and meta-analysis. Ophthalmology 2008, 115, 116–126. [Google Scholar] [CrossRef]
- Hernández-Zimbrón, L.F.; Zamora-Alvarado, R.; Ochoa-De la Paz, L.; Velez-Montoya, R.; Zenteno, E.; Gulias-Cañizo, R.; Quiroz-Mercado, H.; Gonzalez-Salinas, R. Age-Related Macular Degeneration: New Paradigms for Treatment and Management of AMD. Oxidative Med. Cell. Longev. 2018, 2018, 8374647. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, P.; Kolekar, K.; Vishwas, S.; Shetti, P.; Kumbar, V.; Andreoli Pinto, T.d.J.; Paiva-Santos, A.C.; Veiga, F.; Gupta, G.; Singh, S.K.; et al. Treatment avenues for age-related macular degeneration: Breakthroughs and bottlenecks. Ageing Res. Rev. 2024, 98, 102322. [Google Scholar] [CrossRef] [PubMed]
- Fairbanks, A.M.; Husain, D. Controversies and Disparities in the Management of Age-Related Macular Degeneration. Semin. Ophthalmol. 2023, 38, 134–142. [Google Scholar] [CrossRef]
- Heier, J.S.; Lad, E.M.; Holz, F.G.; Rosenfeld, P.J.; Guymer, R.H.; Boyer, D.; Grossi, F.; Baumal, C.R.; Korobelnik, J.F.; Slakter, J.S.; et al. Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): Two multicentre, randomised, double-masked, sham-controlled, phase 3 trials. Lancet 2023, 402, 1434–1448. [Google Scholar] [CrossRef]
- Nadeem, A.; Malik, I.A.; Shariq, F.; Afridi, E.K.; Taha, M.; Raufi, N.; Naveed, A.K.; Iqbal, J.; Habte, A. Advancements in the treatment of geographic atrophy: Focus on pegcetacoplan in age-related macular degeneration. Ann. Med. Surg. 2023, 85, 6067–6077. [Google Scholar] [CrossRef]
- Shakeel, L.; Khan, A.; Akilimali, A. Izervay (avacincaptad pegol): Paving the way for vision preservation in geographic atrophy. Ann. Med. Surg. 2024, 86, 2413–2416. [Google Scholar] [CrossRef]
- Kang, C. Avacincaptad Pegol: First Approval. Drugs 2023, 83, 1447–1453. [Google Scholar] [CrossRef]
- European Medicines Agency. Syfovre (Pegcetacoplan). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/syfovre (accessed on 24 August 2025).
- Saraf, S.S.; Olmos de Koo, L.C. Vision Restoration in Outer Retinal Degenerations: Current Approaches and Future Directions. Int. Ophthalmol. Clin. 2019, 59, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Parnami, K.; Bhattacharyya, A. Current approaches to vision restoration using optogenetic therapy. Front. Cell. Neurosci. 2023, 17, 1236826. [Google Scholar] [CrossRef] [PubMed]
- Maguire, A.M.; Russell, S.; Wellman, J.A.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; Marshall, K.A.; et al. Efficacy, Safety, and Durability of Voretigene Neparvovec-rzyl in RPE65 Mutation-Associated Inherited Retinal Dystrophy: Results of Phase 1 and 3 Trials. Ophthalmology 2019, 126, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Gamlin, P.D.; Alexander, J.J.; Boye, S.L.; Witherspoon, C.D.; Boye, S.E. SubILM Injection of AAV for Gene Delivery to the Retina. Methods Mol. Biol. 2019, 1950, 249–262. [Google Scholar] [CrossRef]
- Ahuja, A.K.; Dorn, J.D.; Caspi, A.; McMahon, M.J.; Dagnelie, G.; Dacruz, L.; Stanga, P.; Humayun, M.S.; Greenberg, R.J. Blind subjects implanted with the Argus II retinal prosthesis are able to improve performance in a spatial-motor task. Br. J. Ophthalmol. 2011, 95, 539–543. [Google Scholar] [CrossRef]
- Dagnelie, G.; Christopher, P.; Arditi, A.; da Cruz, L.; Duncan, J.L.; Ho, A.C.; Olmos de Koo, L.C.; Sahel, J.A.; Stanga, P.E.; Thumann, G.; et al. Performance of real-world functional vision tasks by blind subjects improves after implantation with the Argus® II retinal prosthesis system. Clin. Exp. Ophthalmol. 2017, 45, 152–159. [Google Scholar] [CrossRef]
- Ghani, N.; Bansal, J.; Naidu, A.; Chaudhary, K.M. Long term positional stability of the Argus II retinal prosthesis epiretinal implant. BMC Ophthalmol. 2023, 23, 70. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.C.; Humayun, M.S.; Dorn, J.D.; da Cruz, L.; Dagnelie, G.; Handa, J.; Barale, P.O.; Sahel, J.A.; Stanga, P.E.; Hafezi, F.; et al. Long-Term Results from an Epiretinal Prosthesis to Restore Sight to the Blind. Ophthalmology 2015, 122, 1547–1554. [Google Scholar] [CrossRef]
- Polosukhina, A.; Litt, J.; Tochitsky, I.; Nemargut, J.; Sychev, Y.; De Kouchkovsky, I.; Huang, T.; Borges, K.; Trauner, D.; Van Gelder, R.N.; et al. Photochemical restoration of visual responses in blind mice. Neuron 2012, 75, 271–282. [Google Scholar] [CrossRef]
- Tochitsky, I.; Helft, Z.; Meseguer, V.; Fletcher, R.B.; Vessey, K.A.; Telias, M.; Denlinger, B.; Malis, J.; Fletcher, E.L.; Kramer, R.H. How Azobenzene Photoswitches Restore Visual Responses to the Blind Retina. Neuron 2016, 92, 100–113. [Google Scholar] [CrossRef]
- Tochitsky, I.; Polosukhina, A.; Degtyar, V.E.; Gallerani, N.; Smith, C.M.; Friedman, A.; Van Gelder, R.N.; Trauner, D.; Kaufer, D.; Kramer, R.H. Restoring visual function to blind mice with a photoswitch that exploits electrophysiological remodeling of retinal ganglion cells. Neuron 2014, 81, 800–813. [Google Scholar] [CrossRef]
- Van Gelder, R.N.; Chiang, M.F.; Dyer, M.A.; Greenwell, T.N.; Levin, L.A.; Wong, R.O.; Svendsen, C.N. Regenerative and restorative medicine for eye disease. Nat. Med. 2022, 28, 1149–1156. [Google Scholar] [CrossRef]
- Yang, J.; Lewis, G.P.; Hsiang, C.H.; Menges, S.; Luna, G.; Cho, W.; Turovets, N.; Fisher, S.K.; Klassen, H. Amelioration of Photoreceptor Degeneration by Intravitreal Transplantation of Retinal Progenitor Cells in Rats. Int. J. Mol. Sci. 2024, 25, 8060. [Google Scholar] [CrossRef] [PubMed]
- Lingam, S.; Liu, Z.; Yang, B.; Wong, W.; Parikh, B.H.; Ong, J.Y.; Goh, D.; Wong, D.S.L.; Tan, Q.S.W.; Tan, G.S.W.; et al. cGMP-grade human iPSC-derived retinal photoreceptor precursor cells rescue cone photoreceptor damage in non-human primates. Stem. Cell Res. Ther. 2021, 12, 464. [Google Scholar] [CrossRef]
- Sakai, D.; Tomita, H.; Maeda, A. Optogenetic Therapy for Visual Restoration. Int. J. Mol. Sci. 2022, 23, 15041. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Pan, Z.-H. Optogenetic Strategies for Vision Restoration. Adv. Exp. Med. Biol. 2021, 1293, 545–555. [Google Scholar] [CrossRef]
- Lindner, M.; Gilhooley, M.J.; Hughes, S.; Hankins, M.W. Optogenetics for visual restoration: From proof of principle to translational challenges. Prog. Retin. Eye Res. 2022, 91, 101089. [Google Scholar] [CrossRef]
- Prosseda, P.P.; Tran, M.; Kowal, T.; Wang, B.; Sun, Y. Advances in Ophthalmic Optogenetics: Approaches and Applications. Biomolecules 2022, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Poboży, K.; Poboży, T.; Domański, P.; Derczyński, M.; Konarski, W.; Domańska-Poboża, J. Evolution of Light-Sensitive Proteins in Optogenetic Approaches for Vision Restoration: A Comprehensive Review. Biomedicines 2025, 13, 429. [Google Scholar] [CrossRef]
- Provansal, M.; Marazova, K.; Sahel, J.A.; Picaud, S. Vision Restoration by Optogenetic Therapy and Developments Toward Sonogenetic Therapy. Transl. Vis. Sci. Technol. 2022, 11, 18. [Google Scholar] [CrossRef]
- Maguire, A.M.; Simonelli, F.; Pierce, E.A.; Pugh, E.N., Jr.; Mingozzi, F.; Bennicelli, J.; Banfi, S.; Marshall, K.A.; Testa, F.; Surace, E.M.; et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008, 358, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.G.; Cideciyan, A.V.; Roman, A.J.; Sumaroka, A.; Schwartz, S.B.; Heon, E.; Hauswirth, W.W. Improvement and decline in vision with gene therapy in childhood blindness. N. Engl. J. Med. 2015, 372, 1920–1926. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Mahapatra, S.; Batabyal, S.; Carlson, M.; Kanungo, G.; Ayyagari, A.; Tchedre, K.; Franco, J.A.; Singer, M.; Barone, S.B.; et al. A synthetic opsin restores vision in patients with severe retinal degeneration. Mol. Ther. 2025, 33, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Biber, J.; Gandor, C.; Becirovic, E.; Michalakis, S. Retina-directed gene therapy: Achievements and remaining challenges. Pharmacol. Ther. 2025, 271, 108862. [Google Scholar] [CrossRef]
- de Melo, J.; Blackshaw, S. In Vivo Electroporation of Developing Mouse Retina. Methods Mol. Biol. 2018, 1715, 101–111. [Google Scholar] [CrossRef]
- Baker, C.K.; Flannery, J.G. Innovative Optogenetic Strategies for Vision Restoration. Front. Cell Neurosci. 2018, 12, 316. [Google Scholar] [CrossRef]
- Han, I.C.; Cheng, J.L.; Burnight, E.R.; Ralston, C.L.; Fick, J.L.; Thomsen, G.J.; Tovar, E.F.; Russell, S.R.; Sohn, E.H.; Mullins, R.F.; et al. Retinal Tropism and Transduction of Adeno-Associated Virus Varies by Serotype and Route of Delivery (Intravitreal, Subretinal, or Suprachoroidal) in Rats. Hum. Gene Ther. 2020, 31, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Auricchio, A.; Kobinger, G.; Anand, V.; Hildinger, M.; O’Connor, E.; Maguire, A.M.; Wilson, J.M.; Bennett, J. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: The retina as a model. Hum. Mol. Genet. 2001, 10, 3075–3081. [Google Scholar] [CrossRef] [PubMed]
- Natkunarajah, M.; Trittibach, P.; McIntosh, J.; Duran, Y.; Barker, S.E.; Smith, A.J.; Nathwani, A.C.; Ali, R.R. Assessment of ocular transduction using single-stranded and self-complementary recombinant adeno-associated virus serotype 2/8. Gene Ther. 2008, 15, 463–467. [Google Scholar] [CrossRef]
- Zincarelli, C.; Soltys, S.; Rengo, G.; Rabinowitz, J.E. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 2008, 16, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Dalkara, D.; Byrne, L.C.; Klimczak, R.R.; Visel, M.; Yin, L.; Merigan, W.H.; Flannery, J.G.; Schaffer, D.V. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 2013, 5, 189ra176. [Google Scholar] [CrossRef]
- Klimczak, R.R.; Koerber, J.T.; Dalkara, D.; Flannery, J.G.; Schaffer, D.V. A novel adeno-associated viral variant for efficient and selective intravitreal transduction of rat Müller cells. PLoS ONE 2009, 4, e7467. [Google Scholar] [CrossRef]
- Zinn, E.; Pacouret, S.; Khaychuk, V.; Turunen, H.T.; Carvalho, L.S.; Andres-Mateos, E.; Shah, S.; Shelke, R.; Maurer, A.C.; Plovie, E.; et al. In Silico Reconstruction of the Viral Evolutionary Lineage Yields a Potent Gene Therapy Vector. Cell Rep. 2015, 12, 1056–1068. [Google Scholar] [CrossRef]
- Khabou, H.; Garita-Hernandez, M.; Chaffiol, A.; Reichman, S.; Jaillard, C.; Brazhnikova, E.; Bertin, S.; Forster, V.; Desrosiers, M.; Winckler, C.; et al. Noninvasive gene delivery to foveal cones for vision restoration. JCI Insight 2018, 3, e96029. [Google Scholar] [CrossRef]
- Maeder, M.L.; Stefanidakis, M.; Wilson, C.J.; Baral, R.; Barrera, L.A.; Bounoutas, G.S.; Bumcrot, D.; Chao, H.; Ciulla, D.M.; DaSilva, J.A.; et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat. Med. 2019, 25, 229–233. [Google Scholar] [CrossRef]
- Petrs-Silva, H.; Dinculescu, A.; Li, Q.; Deng, W.T.; Pang, J.J.; Min, S.H.; Chiodo, V.; Neeley, A.W.; Govindasamy, L.; Bennett, A.; et al. Novel properties of tyrosine-mutant AAV2 vectors in the mouse retina. Mol. Ther. 2011, 19, 293–301. [Google Scholar] [CrossRef]
- Deverman, B.E.; Pravdo, P.L.; Simpson, B.P.; Kumar, S.R.; Chan, K.Y.; Banerjee, A.; Wu, W.L.; Yang, B.; Huber, N.; Pasca, S.P.; et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 2016, 34, 204–209. [Google Scholar] [CrossRef]
- McClements, M.E.; Staurenghi, F.; Visel, M.; Flannery, J.G.; MacLaren, R.E.; Cehajic-Kapetanovic, J. AAV Induced Expression of Human Rod and Cone Opsin in Bipolar Cells of a Mouse Model of Retinal Degeneration. BioMed Res. Int. 2021, 2021, 4014797. [Google Scholar] [CrossRef]
- Sauter, M.M.; Noel, H.R.; Sinha, D.; Nelson, E.C.; Xiong, M.N.; Gamm, D.M.; Brandt, C.R. AAV2.7m8 transduction of stage 2 human retinal organoids induces highly variable responses in innate and inflammatory gene expression and cytokine secretion. Exp. Eye Res. 2025, 258, 110478. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Jiang, H.; Li, Q.; Qin, Y.; Yang, S.; Li, J.; Xu, L.; Gou, Y.; Zhang, Y.; Liu, F.; et al. An adeno-associated virus variant enabling efficient ocular-directed gene delivery across species. Nat. Commun. 2024, 15, 3780. [Google Scholar] [CrossRef]
- Huang, J.; Li, J.; Xu, X.; Li, K. Adeno-Associated Virus Vectors in Retinal Gene Therapy: Challenges, Innovations, and Future Directions. Biomolecules 2025, 15, 940. [Google Scholar] [CrossRef] [PubMed]
- Charbel Issa, P.; De Silva, S.R.; Lipinski, D.M.; Singh, M.S.; Mouravlev, A.; You, Q.; Barnard, A.R.; Hankins, M.W.; During, M.J.; Maclaren, R.E. Assessment of tropism and effectiveness of new primate-derived hybrid recombinant AAV serotypes in the mouse and primate retina. PLoS ONE 2013, 8, e60361. [Google Scholar] [CrossRef]
- Alsalloum, A.; Gornostal, E.; Mingaleva, N.; Pavlov, R.; Kuznetsova, E.; Antonova, E.; Nadzhafova, A.; Kolotova, D.; Kadyshev, V.; Mityaeva, O.; et al. A Comparative Analysis of Models for AAV-Mediated Gene Therapy for Inherited Retinal Diseases. Cells 2024, 13, 1706. [Google Scholar] [CrossRef]
- Tso, A.; da Costa, B.L.; Fehnel, A.; Levi, S.R.; Jenny, L.A.; Ragi, S.D.; Li, Y.; Quinn, P.M.J. Generation of Human iPSC-Derived Retinal Organoids for Assessment of AAV-Mediated Gene Delivery. Methods Mol. Biol. 2023, 2560, 287–302. [Google Scholar] [CrossRef]
- Dalkara, D.; Kolstad, K.D.; Caporale, N.; Visel, M.; Klimczak, R.R.; Schaffer, D.V.; Flannery, J.G. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol. Ther. 2009, 17, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- McClements, M.E.; Staurenghi, F.; MacLaren, R.E.; Cehajic-Kapetanovic, J. Optogenetic Gene Therapy for the Degenerate Retina: Recent Advances. Front. Neurosci. 2020, 14, 570909. [Google Scholar] [CrossRef] [PubMed]
- De Silva, S.R.; Charbel Issa, P.; Singh, M.S.; Lipinski, D.M.; Barnea-Cramer, A.O.; Walker, N.J.; Barnard, A.R.; Hankins, M.W.; MacLaren, R.E. Single residue AAV capsid mutation improves transduction of photoreceptors in the Abca4(−/−) mouse and bipolar cells in the rd1 mouse and human retina ex vivo. Gene Ther. 2016, 23, 767–774. [Google Scholar] [CrossRef]
- Gaub, B.M.; Berry, M.H.; Holt, A.E.; Isacoff, E.Y.; Flannery, J.G. Optogenetic Vision Restoration Using Rhodopsin for Enhanced Sensitivity. Mol. Ther. 2015, 23, 1562–1571. [Google Scholar] [CrossRef]
- Kaiser, P.K.; Giani, A.; Fuchs, H.; Chong, V.; Heier, J.S. Factors That Can Prolong Ocular Treatment Duration in Age-Related Macular Degeneration. Ophthalmic Res. 2023, 66, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Gao, A.; Giunta, M.; Tran, S.D. What’s New in Ocular Drug Delivery: Advances in Suprachoroidal Injection since 2023. Pharmaceuticals 2024, 17, 1007. [Google Scholar] [CrossRef]
- Wang, J.H.; Cui, M.; Liu, H.; Guo, P.; McGowan, J.; Cheng, S.Y.; Gessler, D.J.; Xie, J.; Punzo, C.; Tai, P.W.L.; et al. Cell-penetrating peptide-grafted AAV2 capsids for improved retinal delivery via intravitreal injection. Mol. Ther. Methods Clin. Dev. 2025, 33, 101426. [Google Scholar] [CrossRef]
- Song, H.; Zeng, Y.; Sardar Pasha, S.P.B.; Bush, R.A.; Vijayasarathy, C.; Qian, H.; Wei, L.; Wiley, H.E.; Wu, Z.; Sieving, P.A. Trans-Ocular Electric Current In Vivo Enhances AAV-Mediated Retinal Transduction in Large Animal Eye After Intravitreal Vector Administration. Transl. Vis. Sci. Technol. 2020, 9, 28. [Google Scholar] [CrossRef]
- Woodard, K.T.; Liang, K.J.; Bennett, W.C.; Samulski, R.J. Heparan Sulfate Binding Promotes Accumulation of Intravitreally Delivered Adeno-associated Viral Vectors at the Retina for Enhanced Transduction but Weakly Influences Tropism. J. Virol. 2016, 90, 9878–9888. [Google Scholar] [CrossRef] [PubMed]
- Rajala, A.; Wang, Y.; Zhu, Y.; Ranjo-Bishop, M.; Ma, J.X.; Mao, C.; Rajala, R.V. Nanoparticle-assisted targeted delivery of eye-specific genes to eyes significantly improves the vision of blind mice in vivo. Nano Lett. 2014, 14, 5257–5263. [Google Scholar] [CrossRef]
- Cronin, T.; Vandenberghe, L.H.; Hantz, P.; Juttner, J.; Reimann, A.; Kacsó, A.E.; Huckfeldt, R.M.; Busskamp, V.; Kohler, H.; Lagali, P.S.; et al. Efficient transduction and optogenetic stimulation of retinal bipolar cells by a synthetic adeno-associated virus capsid and promoter. EMBO Mol. Med. 2014, 6, 1175–1190. [Google Scholar] [CrossRef]
- Balaggan, K.S.; Ali, R.R. Ocular gene delivery using lentiviral vectors. Gene Ther. 2012, 19, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Puppo, A.; Cesi, G.; Marrocco, E.; Piccolo, P.; Jacca, S.; Shayakhmetov, D.M.; Parks, R.J.; Davidson, B.L.; Colloca, S.; Brunetti-Pierri, N.; et al. Retinal transduction profiles by high-capacity viral vectors. Gene Ther. 2014, 21, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Nash, Z.; Conley, S.M.; Fliesler, S.J.; Cooper, M.J.; Naash, M.I. A partial structural and functional rescue of a retinitis pigmentosa model with compacted DNA nanoparticles. PLoS ONE 2009, 4, e5290. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Kim, J.; Tzeng, S.Y.; Ding, K.; Hafiz, Z.; Long, D.; Wang, J.; Green, J.J.; Campochiaro, P.A. Suprachoroidal gene transfer with nonviral nanoparticles. Sci. Adv. 2020, 6, eaba1606. [Google Scholar] [CrossRef]
- Han, Z.; Koirala, A.; Makkia, R.; Cooper, M.J.; Naash, M.I. Direct gene transfer with compacted DNA nanoparticles in retinal pigment epithelial cells: Expression, repeat delivery and lack of toxicity. Nanomedicine 2012, 7, 521–539. [Google Scholar] [CrossRef]
- Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 2015, 18, 1213–1225. [Google Scholar] [CrossRef]
- Drag, S.; Dotiwala, F.; Upadhyay, A.K. Gene Therapy for Retinal Degenerative Diseases: Progress, Challenges, and Future Directions. Investig. Ophthalmol. Vis. Sci. 2023, 64, 39. [Google Scholar] [CrossRef]
- Shang, X.; Ling, W.; Chen, Y.; Li, C.; Huang, X. Construction of a Flexible Optogenetic Device for Multisite and Multiregional Optical Stimulation Through Flexible micro-LED Displays on the Cerebral Cortex. Small 2023, 19, e2302241. [Google Scholar] [CrossRef]
- Batabyal, S.; Kim, S.; Carlson, M.; Narcisse, D.; Tchedre, K.; Dibas, A.; Sharif, N.A.; Mohanty, S. Multi-Characteristic Opsin Therapy to Functionalize Retina, Attenuate Retinal Degeneration, and Restore Vision in Mouse Models of Retinitis Pigmentosa. Transl. Vis. Sci. Technol. 2024, 13, 25. [Google Scholar] [CrossRef]
- Berry, M.H.; Holt, A.; Salari, A.; Veit, J.; Visel, M.; Levitz, J.; Aghi, K.; Gaub, B.M.; Sivyer, B.; Flannery, J.G.; et al. Restoration of high-sensitivity and adapting vision with a cone opsin. Nat. Commun. 2019, 10, 1221. [Google Scholar] [CrossRef] [PubMed]
- De Silva, S.R.; Barnard, A.R.; Hughes, S.; Tam, S.K.E.; Martin, C.; Singh, M.S.; Barnea-Cramer, A.O.; McClements, M.E.; During, M.J.; Peirson, S.N.; et al. Long-term restoration of visual function in end-stage retinal degeneration using subretinal human melanopsin gene therapy. Proc. Natl. Acad. Sci. USA 2017, 114, 11211–11216. [Google Scholar] [CrossRef]
- Gauvain, G.; Akolkar, H.; Chaffiol, A.; Arcizet, F.; Khoei, M.A.; Desrosiers, M.; Jaillard, C.; Caplette, R.; Marre, O.; Bertin, S.; et al. Optogenetic therapy: High spatiotemporal resolution and pattern discrimination compatible with vision restoration in non-human primates. Commun. Biol. 2021, 4, 125. [Google Scholar] [CrossRef]
- Munch, M.; Kawasaki, A. Intrinsically photosensitive retinal ganglion cells: Classification, function and clinical implications. Curr. Opin. Neurol. 2013, 26, 45–51. [Google Scholar] [CrossRef]
- Roecklein, K.A.; Rohan, K.J.; Duncan, W.C.; Rollag, M.D.; Rosenthal, N.E.; Lipsky, R.H.; Provencio, I. A missense variant (P10L) of the melanopsin (OPN4) gene in seasonal affective disorder. J. Affect. Disord. 2009, 114, 279–285. [Google Scholar] [CrossRef]
- Wright, W.W.; Gajjeraman, S.; Batabyal, S.; Pradhan, S.; Bhattacharya, S.; Mahapatra, V.; Tripathy, A.; Mohanty, S.K. Restoring vision in mice with retinal degeneration using multicharacteristic opsin. Neurophotonics 2017, 4, 041505. [Google Scholar] [CrossRef] [PubMed]
- Bressler, N.M. Antiangiogenic approaches to age-related macular degeneration today. Ophthalmology 2009, 116, S15–S23. [Google Scholar] [CrossRef] [PubMed]
- Fragiotta, S.; Bassis, L.; Abdolrahimzadeh, B.; Marino, A.; Sepe, M.; Abdolrahimzadeh, S. Exploring Current Molecular Targets in the Treatment of Neovascular Age-Related Macular Degeneration toward the Perspective of Long-Term Agents. Int. J. Mol. Sci. 2024, 25, 4433. [Google Scholar] [CrossRef]
- Lim, J.H.; Wickremasinghe, S.S.; Xie, J.; Chauhan, D.S.; Baird, P.N.; Robman, L.D.; Hageman, G.; Guymer, R.H. Delay to treatment and visual outcomes in patients treated with anti-vascular endothelial growth factor for age-related macular degeneration. Am. J. Ophthalmol. 2012, 153, 678–686.e6862. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.; Ross, J.S.; Sangaralingham, L.R.; Adelman, R.A.; Shah, N.D.; Barkmeier, A.J. Trends of Anti-Vascular Endothelial Growth Factor Use in Ophthalmology Among Privately Insured and Medicare Advantage Patients. Ophthalmology 2017, 124, 352–358. [Google Scholar] [CrossRef]
- Avery, R.L.; Castellarin, A.A.; Steinle, N.C.; Dhoot, D.S.; Pieramici, D.J.; See, R.; Couvillion, S.; Nasir, M.A.; Rabena, M.D.; Le, K.; et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br. J. Ophthalmol. 2014, 98, 1636–1641. [Google Scholar] [CrossRef]
- Solomon, S.D.; Lindsley, K.; Vedula, S.S.; Krzystolik, M.G.; Hawkins, B.S. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst. Rev. 2019, 3, CD005139. [Google Scholar] [CrossRef]
- Li, W.; Chen, L.; Gu, Z.; Chen, Z.; Li, H.; Cheng, Z.; Li, H.; Zou, L. Co-delivery of microRNA-150 and quercetin by lipid nanoparticles (LNPs) for the targeted treatment of age-related macular degeneration (AMD). J. Control. Release 2023, 355, 358–370. [Google Scholar] [CrossRef]
- Cehajic-Kapetanovic, J.; Eleftheriou, C.; Allen, A.E.; Milosavljevic, N.; Pienaar, A.; Bedford, R.; Davis, K.E.; Bishop, P.N.; Lucas, R.J. Restoration of Vision with Ectopic Expression of Human Rod Opsin. Curr. Biol. 2015, 25, 2111–2122. [Google Scholar] [CrossRef]
- Doroudchi, M.M.; Greenberg, K.P.; Liu, J.; Silka, K.A.; Boyden, E.S.; Lockridge, J.A.; Arman, A.C.; Janani, R.; Boye, S.E.; Boye, S.L.; et al. Virally delivered channelrhodopsin-2 safely and effectively restores visual function in multiple mouse models of blindness. Mol. Ther. 2011, 19, 1220–1229. [Google Scholar] [CrossRef]
- Kralik, J.; van Wyk, M.; Stocker, N.; Kleinlogel, S. Bipolar cell targeted optogenetic gene therapy restores parallel retinal signaling and high-level vision in the degenerated retina. Commun. Biol. 2022, 5, 1116. [Google Scholar] [CrossRef] [PubMed]
- Bi, A.; Cui, J.; Ma, Y.P.; Olshevskaya, E.; Pu, M.; Dizhoor, A.M.; Pan, Z.H. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron 2006, 50, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Gilhooley, M.J.; Lindner, M.; Palumaa, T.; Hughes, S.; Peirson, S.N.; Hankins, M.W. A systematic comparison of optogenetic approaches to visual restoration. Mol. Ther. Methods Clin. Dev. 2022, 25, 111–123. [Google Scholar] [CrossRef]
- Sahel, J.A.; Boulanger-Scemama, E.; Pagot, C.; Arleo, A.; Galluppi, F.; Martel, J.N.; Esposti, S.D.; Delaux, A.; de Saint Aubert, J.B.; de Montleau, C.; et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nat. Med. 2021, 27, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Boyer, D.S.; Bergstrom, L.; Emanuelli, A.; Gonzalez, V.H.; Wykoff, C.C.; Gupta, S.; Liao, D.S.; Zak, V.; Chavala, S.H.; Mohanty, S.; et al. Efficacy and safety of MCO-010 optogenetic therapy for vision restoration in patients with severe vision loss due to retinitis pigmentosa: A phase 2b randomized, sham-controlled, multi-center, multi-dose, double-masked clinical trial (RESTORE). Investig. Ophthalmol. Vis. Sci. 2023, 64, 5443. [Google Scholar]
- Gonzalez, V.H.; Lam, B.L.; Zak, V.; Mohanty, S.; Bataybal, S.; Chang, J.; Ayyagari, A.; Chavala, S.H.; Piltz-Seymour, J.; Koester, J.; et al. MCO-010 intravitreal optogenetic therapy in Stargardt disease. 6-month outcomes from the Phase 2 STARLIGHT trial. Investig. Ophthalmol. Vis. Sci. 2023, 64, 3546. [Google Scholar]
- Ho, A. Longitudinal BCVA analysis of low- or high-dose MCO-010 mutation agnostic optogenetic therapy for retinitis pigmentosa: 12-month results from a Phase 2b/3 randomized, sham-controlled, patient- and assessor-masked clinical trial (RESTORE). Investig. Ophthalmol. Vis. Sci. 2024, 65, 2137. [Google Scholar]
- Mahajan, V.B. Longitudinal BCVA Analysis of Patients with Stargardt Disease and Macular Degeneration Treated with MCO-010, a Mutation-Agnostic Optogenetic Therapy: 48-Week Results From a Phase 2a Clinical Trial (STARLIGHT). Investig. Ophthalmol. Vis. Sci. 2024, 65, 5266. [Google Scholar]
- Liu, M.M.; Dai, J.M.; Liu, W.Y.; Zhao, C.J.; Lin, B.; Yin, Z.Q. Human melanopsin-AAV2/8 transfection to retina transiently restores visual function in rd1 mice. Int. J. Ophthalmol. 2016, 9, 655–661. [Google Scholar] [CrossRef]
- Lin, B.; Koizumi, A.; Tanaka, N.; Panda, S.; Masland, R.H. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc. Natl. Acad. Sci. USA 2008, 105, 16009–16014. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, M.; Pielecka-Fortuna, J.; Löwel, S.; Kleinlogel, S. Restoring the ON Switch in Blind Retinas: Opto-mGluR6, a Next-Generation, Cell-Tailored Optogenetic Tool. PLoS Biol. 2015, 13, e1002143. [Google Scholar] [CrossRef]
- Berry, M.H.; Holt, A.; Levitz, J.; Broichhagen, J.; Gaub, B.M.; Visel, M.; Stanley, C.; Aghi, K.; Kim, Y.J.; Cao, K.; et al. Restoration of patterned vision with an engineered photoactivatable G protein-coupled receptor. Nat. Commun. 2017, 8, 1862. [Google Scholar] [CrossRef] [PubMed]
- Katada, Y.; Yoshida, K.; Serizawa, N.; Lee, D.; Kobayashi, K.; Negishi, K.; Okano, H.; Kandori, H.; Tsubota, K.; Kurihara, T. Highly sensitive visual restoration and protection via ectopic expression of chimeric rhodopsin in mice. iScience 2023, 26, 107716. [Google Scholar] [CrossRef]
- Wang, T.; Liu, S.; Huang, Y.; Zhou, Y. Red-shifted optogenetics comes to the spotlight. Clin. Transl. Med. 2022, 12, e807. [Google Scholar] [CrossRef] [PubMed]
- Farber, D.B.; Flannery, J.G.; Bowes-Rickman, C. The rd mouse story: Seventy years of research on an animal model of inherited retinal degeneration. Prog. Retin. Eye Res. 1994, 13, 31–64. [Google Scholar] [CrossRef]
- Gaub, B.M.; Berry, M.H.; Holt, A.E.; Reiner, A.; Kienzler, M.A.; Dolgova, N.; Nikonov, S.; Aguirre, G.D.; Beltran, W.A.; Flannery, J.G.; et al. Restoration of visual function by expression of a light-gated mammalian ion channel in retinal ganglion cells or ON-bipolar cells. Proc. Natl. Acad. Sci. USA 2014, 111, E5574–E5583. [Google Scholar] [CrossRef]
- Barrett, J.M.; Degenaar, P.; Sernagor, E. Blockade of pathological retinal ganglion cell hyperactivity improves optogenetically evoked light responses in rd1 mice. Front. Cell. Neurosci. 2015, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Kralik, J.; Kleinlogel, S. Functional Availability of ON-Bipolar Cells in the Degenerated Retina: Timing and Longevity of an Optogenetic Gene Therapy. Int. J. Mol. Sci. 2021, 22, 11515. [Google Scholar] [CrossRef]
- Hulliger, E.C.; Hostettler, S.M.; Kleinlogel, S. Empowering Retinal Gene Therapy with a Specific Promoter for Human Rod and Cone ON-Bipolar Cells. Mol. Ther.-Methods Clin. Dev. 2020, 17, 505–519. [Google Scholar] [CrossRef]
- van Wyk, M.; Hulliger, E.C.; Girod, L.; Ebneter, A.; Kleinlogel, S. Present Molecular Limitations of ON-Bipolar Cell Targeted Gene Therapy. Front. Neurosci. 2017, 11, 161. [Google Scholar] [CrossRef]
- Rodgers, J.; Hughes, S.; Lindner, M.; Allen, A.E.; Ebrahimi, A.S.; Storchi, R.; Peirson, S.N.; Lucas, R.J.; Hankins, M.W. Functional integrity of visual coding following advanced photoreceptor degeneration. Curr. Biol. 2023, 33, 474–486.e475. [Google Scholar] [CrossRef]
- Rodgers, J.; Wright, P.; Ballister, E.R.; Hughes, R.B.; Storchi, R.; Wynne, J.; Martial, F.P.; Lucas, R.J. Modulating signalling lifetime to optimise a prototypical animal opsin for optogenetic applications. Pflügers Arch.-Eur. J. Physiol. 2023, 475, 1387–1407. [Google Scholar] [CrossRef]
- Fujii, R.; Matsushita, M.; Itani, Y.; Hama, A.; Natsume, T.; Takamatsu, H. Intravitreal Administration of Avacincaptad Pegol in a Nonhuman Primate Model of Dry Age-Related Macular Degeneration. Pharmacol. Res. Perspect. 2025, 13, e70052. [Google Scholar] [CrossRef]
- Ahn, S.M.; Ahn, J.; Cha, S.; Yun, C.; Park, T.K.; Kim, Y.-J.; Goo, Y.S.; Kim, S.-W. Morphologic and electrophysiologic findings of retinal degeneration after intravitreal sodium iodate injection following vitrectomy in canines. Sci. Rep. 2020, 10, 3588. [Google Scholar] [CrossRef]
- Ou, Q.; Zhu, T.; Li, P.; Li, Z.; Wang, L.; Lian, C.; Xu, H.; Jin, C.; Gao, F.; Xu, J.-Y.; et al. Establishment of Retinal Degeneration Model in Rat and Monkey by Intravitreal Injection of Sodium Iodate. Curr. Mol. Med. 2019, 18, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hao, Y.; Pan, H.; Zhang, Y.; Cheng, G.; Liu, B.; Chang, J.; Wang, H. CRISPR-dcas9 Optogenetic Nanosystem for the Blue Light-Mediated Treatment of Neovascular Lesions. ACS Appl. Bio Mater. 2021, 4, 2502–2513. [Google Scholar] [CrossRef] [PubMed]
- Marc, R.E.; Jones, B.W.; Watt, C.B.; Vazquez-Chona, F.; Vaughan, D.K.; Organisciak, D.T. Extreme retinal remodeling triggered by light damage: Implications for age related macular degeneration. Mol. Vis. 2008, 14, 782–806. [Google Scholar] [PubMed]
- Jones, B.W.; Watt, C.B.; Frederick, J.M.; Baehr, W.; Chen, C.K.; Levine, E.M.; Milam, A.H.; Lavail, M.M.; Marc, R.E. Retinal remodeling triggered by photoreceptor degenerations. J. Comp. Neurol. 2003, 464, 1–16. [Google Scholar] [CrossRef]
- Sullivan, R.; Penfold, P.; Pow, D.V. Neuronal migration and glial remodeling in degenerating retinas of aged rats and in nonneovascular AMD. Investig. Ophthalmol. Vis. Sci. 2003, 44, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Sassmannshausen, M.; Pfau, M.; Thiele, S.; Fimmers, R.; Steinberg, J.S.; Fleckenstein, M.; Holz, F.G.; Schmitz-Valckenberg, S. Longitudinal Analysis of Structural and Functional Changes in Presence of Reticular Pseudodrusen Associated with Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2020, 61, 19. [Google Scholar] [CrossRef]
- Cicinelli, M.V.; Rabiolo, A.; Sacconi, R.; Lamanna, F.; Querques, L.; Bandello, F.; Querques, G. Retinal vascular alterations in reticular pseudodrusen with and without outer retinal atrophy assessed by optical coherence tomography angiography. Br. J. Ophthalmol. 2018, 102, 1192–1198. [Google Scholar] [CrossRef]
- Spaide, R.F. Outer retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age-related macular degeneration. Retina 2013, 33, 1800–1808. [Google Scholar] [CrossRef]
- Li, M.; Dolz-Marco, R.; Huisingh, C.; Messinger, J.D.; Feist, R.M.; Ferrara, D.; Freund, K.B.; Curcio, C.A. Clinicopathologic Correlation of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Retina 2019, 39, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Pfau, M.; von der Emde, L.; Dysli, C.; Moller, P.T.; Thiele, S.; Lindner, M.; Schmid, M.; Rubin, D.L.; Fleckenstein, M.; Holz, F.G.; et al. Determinants of Cone and Rod Functions in Geographic Atrophy: AI-Based Structure-Function Correlation. Am. J. Ophthalmol. 2020, 217, 162–173. [Google Scholar] [CrossRef]
- Ramkumar, H.L.; Nguyen, B.; Bartsch, D.U.; Saunders, L.J.; Muftuoglu, I.K.; You, Q.; Freeman, W.R. Reduced Ganglion Cell Volume on Optical Coherence Tomography in Patients with Geographic Atrophy. Retina 2018, 38, 2159–2167. [Google Scholar] [CrossRef]
- Mantopoulos, D.; Ray, H.; Sanchez, G.; Pokroy, R.; Roth, D.B. Ganglion Cell Layer Thickness Change in Neovascular Age-Related Macular Degeneration Treated with Anti-VEGF Injections. J. Vitr. Dis. 2022, 6, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Buyukavsar, C.; Sonmez, M.; Sagdic, S.K.; Unal, M.H. Relationship between ganglion cell complex thickness and vision in age-related macular degeneration treated with aflibercept. Eur. J. Ophthalmol. 2023, 33, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, G.; Fragiotta, S.; Scuderi, L.; Iodice, C.M.; Perdicchi, A. Ganglion Cell Complex Analysis in Glaucoma Patients: What Can It Tell Us? Eye Brain 2020, 12, 33–44. [Google Scholar] [CrossRef] [PubMed]
- De Silva, S.R.; Moore, A.T. Optogenetic approaches to therapy for inherited retinal degenerations. J. Physiol. 2022, 600, 4623–4632. [Google Scholar] [CrossRef] [PubMed]
- Khabou, H.; Orendorff, E.; Trapani, F.; Rucli, M.; Desrosiers, M.; Yger, P.; Dalkara, D.; Marre, O. Optogenetic targeting of AII amacrine cells restores retinal computations performed by the inner retina. Mol. Ther. Methods Clin. Dev. 2023, 31, 101107. [Google Scholar] [CrossRef]
- Ferrari, U.; Deny, S.; Sengupta, A.; Caplette, R.; Trapani, F.; Sahel, J.A.; Dalkara, D.; Picaud, S.; Duebel, J.; Marre, O. Towards optogenetic vision restoration with high resolution. PLoS Comput. Biol. 2020, 16, e1007857. [Google Scholar] [CrossRef]
| AAV Serotype/Variant | Key Features | Delivery Route | Reference |
|---|---|---|---|
| AAV2 | Most widely used in retinal gene therapy; limited penetration across ILM | Subretinal/Intravitreal | Maguire et al., 2008 [39] |
| AAV2-RPE65 (Luxturna) | First FDA-approved ocular gene therapy; durable expression | Subretinal | Jacobson et al., 2015 [40] |
| AAV5 | Altered tropism through capsid modifications | Subretinal | Auricchio et al., 2001 [46] |
| AAV8 | Efficient transduction; tested in ocular models | Subretinal | Natkunarajah et al., 2008 [47] |
| AAV1–9 | Systemic injection shows broad tissue tropism | Systemic | Zincarelli et al., 2008 [48] |
| AAV2.7m8 | Directed evolution variant optimized for intravitreal delivery | Intravitreal | Dalkara et al., 2013 [49] |
| AAV variant (Müller selective) | Efficient intravitreal transduction of Müller cells | Intravitreal | Klimczak et al., 2009 [50] |
| Anc80 | Potent synthetic capsid with broad tropism | Systemic/Intravitreal | Zinn et al., 2015 [51] |
| Engineered capsid | Noninvasive delivery to foveal cones | Intravitreal | Khabou et al., 2018 [52] |
| Gene-editing AAV approach | CRISPR-based therapy for LCA10 | Subretinal | Maeder et al., 2019 [53] |
| Tyrosine-mutant AAV2 | Improved transduction efficiency in mouse retina | Subretinal | Petrs-Silva et al., 2011 [54] |
| Cre-dependent AAV variants | Designed for widespread CNS delivery, tested in eye models | Intravitreal/Systemic | Deverman et al., 2016 [55] |
| Injection Route | Target Cells | Advantages | Limitations and Risks |
|---|---|---|---|
| Intravitreal (IVT) | Inner retina (RGCs, Müller cells) | Safest and office based | Low outer retina transduction; ILM/vitreous barriers; potential neutralization/inflammation |
| Subretinal (SR) | Photoreceptors, RPE | Highest efficiency targeting RPE and PRs | Requires PPV and detachment; surgical complexity; risk of retinal damage |
| Suprachoroidal (SC) | Outer retina (ONL, RPE) | Less invasive; avoids ILM and PPV | Proteasomal degradation; blood–retinal barrier; variable efficiency; potential mild immune response |
| Cellular Target | Opsins | Notes |
|---|---|---|
| Microbial (channel opsins) | ||
| RGCs, ON BC | Channelrhodopsin-2 (ChR2) | First-generation microbial opsin |
| RGCs | ChrimsonR, ChRmine | Red-shifted variants with higher light sensitivity, lower phototoxicity |
| BC | MCO1 | Multicharacteristic opsin, tested in RESTORE and STARLIGHT trials |
| Mammalian (GCPR opsins) | ||
| RGCs ON BC | Human rod opsin (RHO) | High intrinsic light sensitivity |
| RGCs | Melanopsin (OPN4) | Endogenous opsin; reduced immunogenicity risk |
| RGCs | Medium-wavelength cone opsin (MW-opsin) | Restores adapting vision under natural light conditions, high light sensitivity, and fast response kinetics |
| Chimeric/Engineered | ||
| RGCs | GHCR | Chimeric opsin (Gloeobacter–Human Chimeric Rhodopsin) with enhanced sensitivity and improved kinetics |
| RGCs | SNAG-mGluR2 | Engineered opsin based on metabotropic glutamate receptor; modulates inhibitory signaling pathways |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grenga, P.L.; Ciancimino, C.; Meduri, A.; Fragiotta, S. Optogenetics: A Novel Therapeutic Avenue for Age-Related Macular Degeneration. Biomolecules 2025, 15, 1286. https://doi.org/10.3390/biom15091286
Grenga PL, Ciancimino C, Meduri A, Fragiotta S. Optogenetics: A Novel Therapeutic Avenue for Age-Related Macular Degeneration. Biomolecules. 2025; 15(9):1286. https://doi.org/10.3390/biom15091286
Chicago/Turabian StyleGrenga, Pier Luigi, Chiara Ciancimino, Alessandro Meduri, and Serena Fragiotta. 2025. "Optogenetics: A Novel Therapeutic Avenue for Age-Related Macular Degeneration" Biomolecules 15, no. 9: 1286. https://doi.org/10.3390/biom15091286
APA StyleGrenga, P. L., Ciancimino, C., Meduri, A., & Fragiotta, S. (2025). Optogenetics: A Novel Therapeutic Avenue for Age-Related Macular Degeneration. Biomolecules, 15(9), 1286. https://doi.org/10.3390/biom15091286






