Nicotinamide N-Methyltransferase in Cardiovascular Diseases: Metabolic Regulator and Emerging Therapeutic Target
Abstract
1. Introduction
2. Metabolic Link Between NNMT and CVDs
3. Crosstalk Between NNMT and the SIRT Signaling Pathway
4. NNMT in Oxidative Stress and Inflammation
5. Role of NNMT in CVDs
5.1. NNMT and Hyperlipidemia
5.2. NNMT and Atherosclerosis
5.3. NNMT and Hypertension
5.4. NNMT and Myocardial Ischemia
6. Therapeutic Potential of NNMT in CVDs
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chong, B.; Jayabaskaran, J.; Jauhari, S.M.; Chan, S.P.; Goh, R.; Kueh, M.T.W.; Li, H.; Chin, Y.H.; Kong, G.; Anand, V.V.; et al. Global burden of cardiovascular diseases: Projections from 2025 to 2050. Eur. J. Prev. Cardiol. 2024, zwae281. [Google Scholar] [CrossRef]
- Sun, W.D.; Zhu, X.J.; Li, J.J.; Mei, Y.Z.; Li, W.S.; Li, J.H. Nicotinamide N-methyltransferase (NNMT): A novel therapeutic target for metabolic syndrome. Front. Pharmacol. 2024, 15, 1410479. [Google Scholar] [CrossRef]
- Mateuszuk, Ł.; Khomich, T.I.; Słomińska, E.; Gajda, M.; Wójcik, L.; Łomnicka, M.; Gwóźdź, P.; Chłopicki, S. Activation of nicotinamide N-methyltrasferase and increased formation of 1-methylnicotinamide (MNA) in atherosclerosis. Pharmacol. Rep. 2009, 61, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Giuliante, R.; Sartini, D.; Bacchetti, T.; Rocchetti, R.; Klöting, I.; Polidori, C.; Ferretti, G.; Emanuelli, M. Potential involvement of nicotinamide N-methyltransferase in the pathogenesis of metabolic syndrome. Metab. Syndr. Relat. Disord. 2015, 13, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Rotllan, N.; Camacho, M.; Tondo, M.; Diarte-Añazco, E.M.G.; Canyelles, M.; Méndez-Lara, K.A.; Benitez, S.; Alonso, N.; Mauricio, D.; Escolà-Gil, J.C.; et al. Therapeutic Potential of Emerging NAD+-Increasing Strategies for Cardiovascular Diseases. Antioxidants 2021, 10, 1939. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Martin, N.I.; van Haren, M.J. Nicotinamide N-methyl transferase (NNMT): An emerging therapeutic target. Drug Discov. Today 2021, 26, 2699–2706. [Google Scholar] [CrossRef]
- Roberti, A.; Fernández, A.F.; Fraga, M.F. Nicotinamide N-methyltransferase: At the crossroads between cellular metabolism and epigenetic regulation. Mol. Metab. 2021, 45, 101165. [Google Scholar] [CrossRef]
- Veloso, C.D.; Belew, G.D.; Ferreira, L.L.; Grilo, L.F.; Jones, J.G.; Portincasa, P.; Sardão, V.A.; Oliveira, P.J. A Mitochondrial Approach to Cardiovascular Risk and Disease. Curr. Pharm. Des. 2019, 25, 3175–3194. [Google Scholar] [CrossRef]
- Liu, M.; Chu, J.; Gu, Y.; Shi, H.; Zhang, R.; Wang, L.; Chen, J.; Shen, L.; Yu, P.; Chen, X.; et al. Serum N1-Methylnicotinamide is Associated With Coronary Artery Disease in Chinese Patients. J. Am. Heart Assoc. 2017, 6, e004328. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhu, X.J.; Li, J.H. Association between Nicotinamide N-Methyltransferase Gene Polymorphisms and Obesity in Chinese Han Male College Students. BioMed Res. Int. 2017, 2017, 2984826. [Google Scholar] [CrossRef]
- Li, J.H.; Wang, Y.; Zhu, X.; Zhou, Q.; Xie, Z.; Yao, T.F. Metabolomics study on the association between nicotinamide N-methyltransferase gene polymorphisms and type 2 diabetes. Int. J. Diabetes Dev. Ctries. 2018, 38, 409–416. [Google Scholar] [CrossRef]
- Zhu, X.J.; Lin, Y.J.; Chen, W.; Wang, Y.H.; Qiu, L.Q.; Cai, C.X.; Xiong, Q.; Chen, F.; Chen, L.H.; Zhou, Q.; et al. Physiological Study on Association between Nicotinamide N-Methyltransferase Gene Polymorphisms and Hyperlipidemia. BioMed Res. Int. 2016, 2016, 7521942. [Google Scholar] [CrossRef]
- Guan, X.X.; Zhu, X.J.; Deng, Z.H.; Zeng, Y.R.; Liu, J.R.; Li, J.H. The association between nicotinamide N-methyltransferase gene polymorphisms and primary hypertension in Chinese Han Population. AIMS Bioeng. 2021, 8, 130–139. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.; Li, Q.; Trammell, S.A.; Schmidt, M.S.; Pires, K.M.; Cai, J.; Zhang, Y.; Kenny, H.; Boudina, S.; et al. Control of NAD+ homeostasis by autophagic flux modulates mitochondrial and cardiac function. EMBO J. 2024, 43, 362–390. [Google Scholar] [CrossRef]
- Li, J.J.; Sun, W.D.; Zhu, X.J.; Mei, Y.Z.; Li, W.S.; Li, J.H. Nicotinamide N-Methyltransferase (NNMT): A New Hope for Treating Aging and Age-Related Conditions. Metabolites 2024, 14, 343. [Google Scholar] [CrossRef] [PubMed]
- Trammell, S.A.; Brenner, C. NNMT: A Bad Actor in Fat Makes Good in Liver. Cell Metab. 2015, 22, 200–201. [Google Scholar] [CrossRef]
- Cavaliere, G.; Cimmino, F.; Trinchese, G.; Catapano, A.; Petrella, L.; D’Angelo, M.; Lucchin, L.; Mollica, M.P. From Obesity-Induced Low-Grade Inflammation to Lipotoxicity and Mitochondrial Dysfunction: Altered Multi-Crosstalk between Adipose Tissue and Metabolically Active Organs. Antioxidants 2023, 12, 1172. [Google Scholar] [CrossRef] [PubMed]
- Kraus, D.; Yang, Q.; Kong, D.; Banks, A.S.; Zhang, L.; Rodgers, J.T.; Pirinen, E.; Pulinilkunnil, T.C.; Gong, F.; Wang, Y.C.; et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature 2014, 508, 258–262. [Google Scholar] [CrossRef]
- Kaplan, P.; Tatarkova, Z.; Sivonova, M.K.; Racay, P.; Lehotsky, J. Homocysteine and Mitochondria in Cardiovascular and Cerebrovascular Systems. Int. J. Mol. Sci. 2020, 21, 7698. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, S.; Sun, Y.; Chen, C.; Yang, S.; Lin, M.; Long, J.; Yao, J.; Lin, Y.; Yi, F.; et al. Targeting oxidative stress as a preventive and therapeutic approach for cardiovascular disease. J. Transl. Med. 2023, 21, 519. [Google Scholar] [CrossRef]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Kannt, A.; Pfenninger, A.; Teichert, L.; Tönjes, A.; Dietrich, A.; Schön, M.R.; Klöting, N.; Blüher, M. Association of nicotinamide-N-methyltransferase mRNA expression in human adipose tissue and the plasma concentration of its product, 1-methylnicotinamide, with insulin resistance. Diabetologia 2015, 58, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Kannt, A.; Rajagopal, S.; Kadnur, S.V.; Suresh, J.; Bhamidipati, R.K.; Swaminathan, S.; Hallur, M.S.; Kristam, R.; Elvert, R.; Czech, J.; et al. A small molecule inhibitor of Nicotinamide N-methyltransferase for the treatment of metabolic disorders. Sci. Rep. 2018, 8, 3660. [Google Scholar] [CrossRef]
- Hong, S.; Moreno-Navarrete, J.M.; Wei, X.; Kikukawa, Y.; Tzameli, I.; Prasad, D.; Lee, Y.; Asara, J.M.; Fernandez-Real, J.M.; Maratos-Flier, E.; et al. Nicotinamide N-methyltransferase regulates hepatic nutrient metabolism through Sirt1 protein stabilization. Nat. Med. 2015, 21, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.E.; Sinclair, D.A. Sirtuins and NAD+ in the Development and Treatment of Metabolic and Cardiovascular Diseases. Circ. Res. 2018, 123, 868–885. [Google Scholar] [CrossRef]
- Dang, W. The controversial world of sirtuins. Drug Discov. Today Technol. 2014, 12, e9–e17. [Google Scholar] [CrossRef]
- Liu, Y.P.; Wen, R.; Liu, C.F.; Zhang, T.N.; Yang, N. Cellular and molecular biology of sirtuins in cardiovascular disease. Biomed. Pharmacother. 2023, 164, 114931. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Wang, Y.X.; Zhou, K.; Xie, H.L.; Ren, Z.; Liu, H.T.; Ou, Y.S.; Zhou, Z.X.; Jiang, Z.S. Biological functions of CRTC2 and its role in metabolism-related diseases. J. Cell Commun. Signal. 2023, 17, 495–506. [Google Scholar] [CrossRef]
- Han, H.S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef]
- Sun, Y.; Rawish, E.; Nording, H.M.; Langer, H.F. Inflammation in Metabolic and Cardiovascular Disorders-Role of Oxidative Stress. Life 2021, 11, 672. [Google Scholar] [CrossRef]
- Song, Z.; Zhong, X.; Li, M.; Gao, P.; Ning, Z.; Sun, Z.; Song, X. 1-MNA Ameliorates High Fat Diet-Induced Heart Injury by Upregulating Nrf2 Expression and Inhibiting NF-κB in vivo and in vitro. Front. Cardiovasc. Med. 2021, 8, 721814. [Google Scholar] [CrossRef] [PubMed]
- Campagna, R.; Mateuszuk, Ł.; Wojnar-Lason, K.; Kaczara, P.; Tworzydło, A.; Kij, A.; Bujok, R.; Mlynarski, J.; Wang, Y.; Sartini, D.; et al. Nicotinamide N-methyltransferase in endothelium protects against oxidant stress-induced endothelial injury. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119082. [Google Scholar] [CrossRef]
- Oppedisano, F.; Nesci, S.; Spagnoletta, A. Mitochondrial sirtuin 3 and role of natural compounds: The effect of post-translational modifications on cellular metabolism. Crit. Rev. Biochem. Mol. Biol. 2024, 59, 199–220. [Google Scholar] [CrossRef]
- Riederer, M.; Erwa, W.; Zimmermann, R.; Frank, S.; Zechner, R. Adipose tissue as a source of nicotinamide N-methyltransferase and homocysteine. Atherosclerosis 2009, 204, 412–417. [Google Scholar] [CrossRef]
- Familtseva, A.; Chaturvedi, P.; Kalani, A.; Jeremic, N.; Metreveli, N.; Kunkel, G.H.; Tyagi, S.C. Toll-like receptor 4 mutation suppresses hyperhomocysteinemia-induced hypertension. Am. J. Physiol. Cell Physiol. 2016, 311, C596–C606. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Liu, J.; Zhao, J.; Mao, J.; Zhang, X.; Feng, L.; Han, C.; Li, M.; Wang, S.; Wu, D. Homocysteine induces the expression of C-reactive protein via NMDAr-ROS-MAPK-NF-κB signal pathway in rat vascular smooth muscle cells. Atherosclerosis 2014, 236, 73–81. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J. Clinical implication of homocysteine in premature acute coronary syndrome female patients: Its distribution and association with clinical characteristics and major adverse cardiovascular events risk. Medicine 2021, 100, e25677. [Google Scholar] [CrossRef]
- Sternak, M.; Khomich, T.I.; Jakubowski, A.; Szafarz, M.; Szczepański, W.; Białas, M.; Stojak, M.; Szymura-Oleksiak, J.; Chłopicki, S. Nicotinamide N-methyltransferase (NNMT) and 1-methylnicotinamide (MNA) in experimental hepatitis induced by concanavalin A in the mouse. Pharmacol. Rep. 2010, 62, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Fedorowicz, A.; Mateuszuk, Ł.; Kopec, G.; Skórka, T.; Kutryb-Zając, B.; Zakrzewska, A.; Walczak, M.; Jakubowski, A.; Łomnicka, M.; Słomińska, E.; et al. Activation of the nicotinamide N-methyltransferase (NNMT)-1-methylnicotinamide (MNA) pathway in pulmonary hypertension. Respir. Res. 2016, 17, 108. [Google Scholar] [CrossRef]
- Kim, H.C.; Mofarrahi, M.; Vassilakopoulos, T.; Maltais, F.; Sigala, I.; Debigare, R.; Bellenis, I.; Hussain, S.N. Expression and functional significance of nicotinamide N-methyl transferase in skeletal muscles of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 181, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Pissios, P. Nicotinamide N-Methyltransferase: More Than a Vitamin B3 Clearance Enzyme. Trends Endocrinol. Metab. 2017, 28, 340–353. [Google Scholar] [CrossRef]
- Jakubowski, A.; Sternak, M.; Jablonski, K.; Ciszek-Lenda, M.; Marcinkiewicz, J.; Chlopicki, S. 1-Methylnicotinamide protects against liver injury induced by concanavalin A via a prostacyclin-dependent mechanism: A possible involvement of IL-4 and TNF-α. Int. Immunopharmacol. 2016, 31, 98–104. [Google Scholar] [CrossRef]
- Yang, C.; Wang, T.; Zhu, S.; Zong, Z.; Luo, C.; Zhao, Y.; Liu, J.; Li, T.; Liu, X.; Liu, C.; et al. Nicotinamide N-Methyltransferase Remodeled Cell Metabolism and Aggravated Proinflammatory Responses by Activating STAT3/IL1β/PGE2 Pathway. ACS Omega 2022, 7, 37509–37519. [Google Scholar] [CrossRef]
- Stewart, J.; McCallin, T.; Martinez, J.; Chacko, S.; Yusuf, S. Hyperlipidemia. Pediatr. Rev. 2020, 41, 393–402. [Google Scholar] [CrossRef]
- Sreckovic, B.; Sreckovic, V.D.; Soldatovic, I.; Colak, E.; Sumarac-Dumanovic, M.; Janeski, H.; Janeski, N.; Gacic, J.; Mrdovic, I. Homocysteine is a marker for metabolic syndrome and atherosclerosis. Diabetes Metab. Syndr. 2017, 11, 179–182. [Google Scholar] [CrossRef]
- Wei, J.; Yang, Q.; Wang, X.; He, X.; Zhu, W.; Lin, L.; Liu, C.; Zhu, C.; Zhang, M. Association between homocysteine levels and hyperlipidemia prevalence as well as all-cause mortality of hyperlipidemia patients in the US population: Results from NHANES database. Front. Cardiovasc. Med. 2024, 11, 1419579. [Google Scholar] [CrossRef]
- Mondal, K.; Chakraborty, P.; Kabir, S.N. Hyperhomocysteinemia and hyperandrogenemia share PCSK9-LDLR pathway to disrupt lipid homeostasis in PCOS. Biochem. Biophys. Res. Commun. 2018, 503, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Yoda, M.; Mizuno, R.; Izumi, Y.; Takahashi, M.; Bamba, T.; Kawaoka, S. Nicotinamide-N-methyltransferase regulates lipid metabolism via SAM and 1-methylnicotinamide in the AML12 hepatocyte cell line. J. Biochem. 2023, 174, 89–98. [Google Scholar] [CrossRef]
- Xu, W.; Hou, L.; Li, P.; Li, L. Effect of nicotinamide N-methyltransferase on lipid accumulation in 3T3-L1 adipocytes. Bioengineered 2022, 13, 12421–12434. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Chen, Y.; Wang, J.; Hao, L.; Huang, C.; Griffiths, A.; Sun, Z.; Zhou, Z.; Song, Z. ER stress-induced upregulation of NNMT contributes to alcohol-related fatty liver development. J. Hepatol. 2020, 73, 783–793. [Google Scholar] [CrossRef]
- Oikonomou, E.; Leopoulou, M.; Theofilis, P.; Antonopoulos, A.S.; Siasos, G.; Latsios, G.; Mystakidi, V.C.; Antoniades, C.; Tousoulis, D. A link between inflammation and thrombosis in atherosclerotic cardiovascular diseases: Clinical and therapeutic implications. Atherosclerosis 2020, 309, 16–26. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Shi, W.T.; Zhang, J.; Zhao, W.R.; Xiao, Y.; Zhang, K.Y.; Ma, J.; Tang, J.Y.; Wang, Y. Sodium tanshinone IIA sulfonate protects against hyperhomocysteine-induced vascular endothelial injury via activation of NNMT/SIRT1-mediated NRF2/HO-1 and AKT/MAPKs signaling in human umbilical vascular endothelial cells. Biomed. Pharmacother. 2023, 158, 114137. [Google Scholar] [CrossRef]
- Shi, L.; Liu, X.Y.; Huang, Z.G.; Ma, Z.Y.; Xi, Y.; Wang, L.Y.; Sun, N.L. Endogenous hydrogen sulfide and ERK1/2-STAT3 signaling pathway may participate in the association between homocysteine and hypertension. J. Geriatr. Cardiol. 2019, 16, 822–834. [Google Scholar]
- Wu, D.F.; Yin, R.X.; Deng, J.L. Homocysteine, hyperhomocysteinemia, and H-type hypertension. Eur. J. Prev. Cardiol. 2024, 31, 1092–1103. [Google Scholar] [CrossRef]
- Loring, H.S.; Thompson, P.R. Kinetic Mechanism of Nicotinamide N-Methyltransferase. Biochemistry 2018, 57, 5524–5532. [Google Scholar] [CrossRef]
- Zhang, S.F.; Mao, X.J.; Jiang, W.M.; Fang, Z.Y. Qian Yang Yu Yin Granule protects against hypertension-induced renal injury by epigenetic mechanism linked to Nicotinamide N-Methyltransferase (NNMT) expression. J. Ethnopharmacol. 2020, 255, 112738. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Niu, H.; Zhang, J. Homocysteine induces mitochondrial dysfunction and oxidative stress in myocardial ischemia/reperfusion injury through stimulating ROS production and the ERK1/2 signaling pathway. Exp. Ther. Med. 2020, 20, 938–944. [Google Scholar] [CrossRef]
- Zhao, K.; Tang, J.; Xie, H.; Liu, L.; Qin, Q.; Sun, B.; Qin, Z.H.; Sheng, R.; Zhu, J. Nicotinamide riboside attenuates myocardial ischemia-reperfusion injury via regulating SIRT3/SOD2 signaling pathway. Biomed. Pharmacother. 2024, 175, 116689. [Google Scholar] [CrossRef] [PubMed]
- Jafari-Azad, A.; Hosseini, L.; Rajabi, M.; Høilund-Carlsen, P.F.; Vafaee, M.S.; Feyzizadeh, S.; Badalzadeh, R. Nicotinamide mononucleotide and melatonin counteract myocardial ischemia-reperfusion injury by activating SIRT3/FOXO1 and reducing apoptosis in aged male rats. Mol. Biol. Rep. 2021, 48, 3089–3096. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Kanda, T.; Komatsu, M.; Itoh, T.; Minakuchi, H.; Urai, H.; Kuroita, T.; Shigaki, S.; Tsukamoto, T.; Higuchi, N.; et al. The significance of NAD + metabolites and nicotinamide N-methyltransferase in chronic kidney disease. Sci. Rep. 2022, 12, 6398. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Shin, E.J.; Kim, T.H.; Yang, J.H.; Ki, S.H.; Kang, K.W.; Kim, K.M. Exploring NNMT: From metabolic pathways to therapeutic targets. Arch. Pharmacal Res. 2024, 47, 893–913. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Li, Y.; Lin, Y.; Yang, X.; Yang, J.; Hu, S.; Liu, A. Nicotinamide N-methyltransferase and liver diseases. Genes Dis. 2022, 10, 1883–1893. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Badimon, L.; Vilahur, G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 2014, 276, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Chlopicki, S.; Swies, J.; Mogielnicki, A.; Buczko, W.; Bartus, M.; Lomnicka, M.; Adamus, J.; Gebicki, J. 1-Methylnicotinamide (MNA), a primary metabolite of nicotinamide, exerts anti-thrombotic activity mediated by a cyclooxygenase-2/prostacyclin pathway. Br. J. Pharmacol. 2007, 152, 230–239. [Google Scholar] [CrossRef]
- Gargiulo, S.; Rossin, D.; Testa, G.; Gamba, P.; Staurenghi, E.; Biasi, F.; Poli, G.; Leonarduzzi, G. Up-regulation of COX-2 and mPGES-1 by 27-hydroxycholesterol and 4-hydroxynonenal: A crucial role in atherosclerotic plaque instability. Free Radic. Biol. Med. 2018, 129, 354–363. [Google Scholar] [CrossRef]
- Gomez, I.; Foudi, N.; Longrois, D.; Norel, X. The role of prostaglandin E2 in human vascular inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 55–63. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Liu, M.; He, A.; Chu, J.; Chen, C.; Zhang, S.; He, Y.; Tao, W.; Lu, M.; Hua, M.; Ju, W.; et al. Serum N1-methylnicotinamide is Associated with Left Ventricular Systolic Dysfunction in Chinese. Sci. Rep. 2018, 8, 8581. [Google Scholar] [CrossRef]
- Alefishat, E.; Alexander, S.P.; Ralevic, V. Effects of NAD at purine receptors in isolated blood vessels. Purinergic Signal. 2015, 11, 47–57. [Google Scholar] [CrossRef]
- Iqbal, M.K.; Ambreen, A.; Mujahid, M.; Zarlashat, Y.; Abid, M.; Yasin, A.; Ullah, M.N.; Shahzad, R.; Harlina, P.W.; Khan, S.U.; et al. Cardiomegaly: Navigating the uncharted territories of heart failure—A multimodal radiological journey through advanced imaging, pathophysiological landscapes, and innovative therapeutic frontiers. Curr. Probl. Cardiol. 2024, 49, 102748. [Google Scholar] [CrossRef]
- Theofilis, P.; Antonopoulos, A.S.; Sagris, M.; Papanikolaou, A.; Oikonomou, E.; Tsioufis, K.; Tousoulis, D. Silent Myocardial Ischemia: From Pathophysiology to Diagnosis and Treatment. Biomedicines 2024, 12, 259. [Google Scholar] [CrossRef]
- Shi, C.; Wen, Z.; Yang, Y.; Shi, L.; Liu, D. NAD+ metabolism and therapeutic strategies in cardiovascular diseases. Atheroscler. Plus 2024, 57, 1–12. [Google Scholar] [CrossRef]
- Song, Q.; Zhou, X.; Xu, K.; Liu, S.; Zhu, X.; Yang, J. The Safety and Antiaging Effects of Nicotinamide Mononucleotide in Human Clinical Trials: An Update. Adv. Nutr. 2023, 14, 1416–1435. [Google Scholar] [CrossRef]
- Ruf, S.; Rajagopal, S.; Kadnur, S.V.; Hallur, M.S.; Rani, S.; Kristam, R.; Swaminathan, S.; Zope, B.R.; Gondrala, P.K.; Swamy, I.; et al. Novel tricyclic small molecule inhibitors of Nicotinamide N-methyltransferase for the treatment of metabolic disorders. Sci. Rep. 2022, 12, 15440. [Google Scholar] [CrossRef] [PubMed]
- Brachs, S.; Polack, J.; Brachs, M.; Jahn-Hofmann, K.; Elvert, R.; Pfenninger, A.; Bärenz, F.; Margerie, D.; Mai, K.; Spranger, J.; et al. Genetic Nicotinamide N-Methyltransferase (Nnmt) Deficiency in Male Mice Improves Insulin Sensitivity in Diet-Induced Obesity but Does Not Affect Glucose Tolerance. Diabetes 2019, 68, 527–542. [Google Scholar] [CrossRef]
- Li, S.; Rodrigues, P.G.; Chakraborty, A.D.; Correia, C.; Schouten, E.M.; Strömstedt, M.; Löfgren, L.; Persson, M.; Fredlund, L.; Rohman, M.; et al. Nicotinamide-N-methyltransferase inhibition improves cardiac function and structure in a heart failure with preserved ejection fraction mouse model. Pharmacol. Res. 2025, 217, 107820. [Google Scholar] [CrossRef]
- Wang, Y.C.; Koay, Y.C.; Pan, C.; Zhou, Z.; Tang, W.; Wilcox, J.; Li, X.S.; Zagouras, A.; Marques, F.; Allayee, H.; et al. Indole-3-Propionic Acid Protects Against Heart Failure With Preserved Ejection Fraction. Circ. Res. 2024, 134, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.; Tran, N. miRNA interplay: Mechanisms and consequences in cancer. Dis. Model. Mech. 2021, 14, dmm047662. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xiao, M.; Ren, G. Long non-coding RNA XIST promotes osteoporosis by inhibiting the differentiation of bone marrow mesenchymal stem cell by sponging miR-29b-3p that suppresses nicotinamide N-methyltransferase. Bioengineered 2021, 12, 6057–6069. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, T.; Feng, S. Knockdown of long non-coding RNA HOTAIR promotes bone marrow mesenchymal stem cell differentiation by sponging microRNA miR-378g that inhibits nicotinamide N-methyltransferase. Bioengineered 2021, 12, 12482–12497. [Google Scholar] [CrossRef]
- van Haren, M.J.; Sastre Toraño, J.; Sartini, D.; Emanuelli, M.; Parsons, R.B.; Martin, N.I. A Rapid and Efficient Assay for the Characterization of Substrates and Inhibitors of Nicotinamide N-Methyltransferase. Biochemistry 2016, 55, 5307–5315. [Google Scholar] [CrossRef]
- Parsons, R.B.; Facey, P.D. Nicotinamide N-Methyltransferase: An Emerging Protagonist in Cancer Macro(r)evolution. Biomolecules 2021, 11, 1418. [Google Scholar] [CrossRef]
- Swaminathan, S.; Birudukota, S.; Thakur, M.K.; Parveen, R.; Kandan, S.; Juluri, S.; Shaik, S.; Anand, N.N.; Burri, R.R.; Kristam, R.; et al. Crystal structures of monkey and mouse nicotinamide N-methyltransferase (NNMT) bound with end product, 1-methyl nicotinamide. Biochem. Biophys. Res. Commun. 2017, 491, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Przyborowski, K.; Wojewoda, M.; Sitek, B.; Zakrzewska, A.; Kij, A.; Wandzel, K.; Zoladz, J.A.; Chlopicki, S. Effects of 1-Methylnicotinamide (MNA) on Exercise Capacity and Endothelial Response in Diabetic Mice. PLoS ONE 2015, 10, e0130908. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, H.; Vance, V.; Wetzel, M.D.; Wang, H.L.; McHardy, S.F.; Finnerty, C.C.; Hommel, J.D.; Watowich, S.J. Selective and membrane-permeable small molecule inhibitors of nicotinamide N-methyltransferase reverse high fat diet-induced obesity in mice. Biochem. Pharmacol. 2018, 147, 141–152. [Google Scholar] [CrossRef]
- Ruf, S.; Hallur, M.S.; Anchan, N.K.; Swamy, I.N.; Murugesan, K.R.; Sarkar, S.; Narasimhulu, L.K.; Putta, V.P.R.K.; Shaik, S.; Chandrasekar, D.V.; et al. Novel nicotinamide analog as inhibitor of nicotinamide N-methyltransferase. Bioorganic Med. Chem. Lett. 2018, 28, 922–925. [Google Scholar] [CrossRef]
- Liu, M.; Li, L.; Chu, J.; Zhu, B.; Zhang, Q.; Yin, X.; Jiang, W.; Dai, G.; Ju, W.; Wang, Z.; et al. Serum N(1)-Methylnicotinamide Is Associated With Obesity and Diabetes in Chinese. J. Clin. Endocrinol. Metab. 2015, 100, 3112–3117. [Google Scholar] [CrossRef]
- Chen, D.; Li, L.; Diaz, K.; Iyamu, I.D.; Yadav, R.; Noinaj, N.; Huang, R. Novel Propargyl-Linked Bisubstrate Analogues as Tight-Binding Inhibitors for Nicotinamide N-Methyltransferase. J. Med. Chem. 2019, 62, 10783–10797. [Google Scholar] [CrossRef]
- Gao, Y.; van Haren, M.J.; Moret, E.E.; Rood, J.J.M.; Sartini, D.; Salvucci, A.; Emanuelli, M.; Craveur, P.; Babault, N.; Jin, J.; et al. Bisubstrate Inhibitors of Nicotinamide N-Methyltransferase (NNMT) with Enhanced Activity. J. Med. Chem. 2019, 62, 6597–6614. [Google Scholar] [CrossRef]
- Babault, N.; Allali-Hassani, A.; Li, F.; Fan, J.; Yue, A.; Ju, K.; Liu, F.; Vedadi, M.; Liu, J.; Jin, J. Discovery of Bisubstrate Inhibitors of Nicotinamide N-Methyltransferase (NNMT). J. Med. Chem. 2018, 61, 1541–1551, Erratum in J. Med. Chem. 2018, 61, 5771–5772. [Google Scholar] [CrossRef]
- Roberti, A.; Tejedor, J.R.; Díaz-Moreno, I.; López, V.; Santamarina-Ojeda, P.; Pérez, R.F.; Urdinguio, R.G.; Concellón, C.; Martínez-Chantar, M.L.; Fernández-Morera, J.L.; et al. Nicotinamide N-methyltransferase (NNMT) regulates the glucocorticoid signaling pathway during the early phase of adipogenesis. Sci. Rep. 2023, 13, 8293. [Google Scholar] [CrossRef] [PubMed]
- van Haren, M.J.; Zhang, Y.; Thijssen, V.; Buijs, N.; Gao, Y.; Mateuszuk, L.; Fedak, F.A.; Kij, A.; Campagna, R.; Sartini, D.; et al. Macrocyclic peptides as allosteric inhibitors of nicotinamide N-methyltransferase (NNMT). RSC Chem. Biol. 2021, 2, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- van Haren, M.J.; Taig, R.; Kuppens, J.; Sastre Toraño, J.; Moret, E.E.; Parsons, R.B.; Sartini, D.; Emanuelli, M.; Martin, N.I. Inhibitors of nicotinamide N-methyltransferase designed to mimic the methylation reaction transition state. Org. Biomol. Chem. 2017, 15, 6656–6667. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Qian, B.; Yu, H.; Ke, S.; Li, Z.; Hua, Y.; Lu, S.; Wang, C.; Li, M.; Guo, S.; et al. NNMT/1-MNA protects against hepatic ischemia-reperfusion injury through the AKT/FOXO1/ANGPT2/JNK axis. Nat. Commun. 2025, 16, 4779. [Google Scholar] [CrossRef]
- Neelakantan, H.; Brightwell, C.R.; Graber, T.G.; Maroto, R.; Wang, H.L.; McHardy, S.F.; Papaconstantinou, J.; Fry, C.S.; Watowich, S.J. Small molecule nicotinamide N-methyltransferase inhibitor activates senescent muscle stem cells and improves regenerative capacity of aged skeletal muscle. Biochem. Pharmacol. 2019, 163, 481–492. [Google Scholar] [CrossRef]
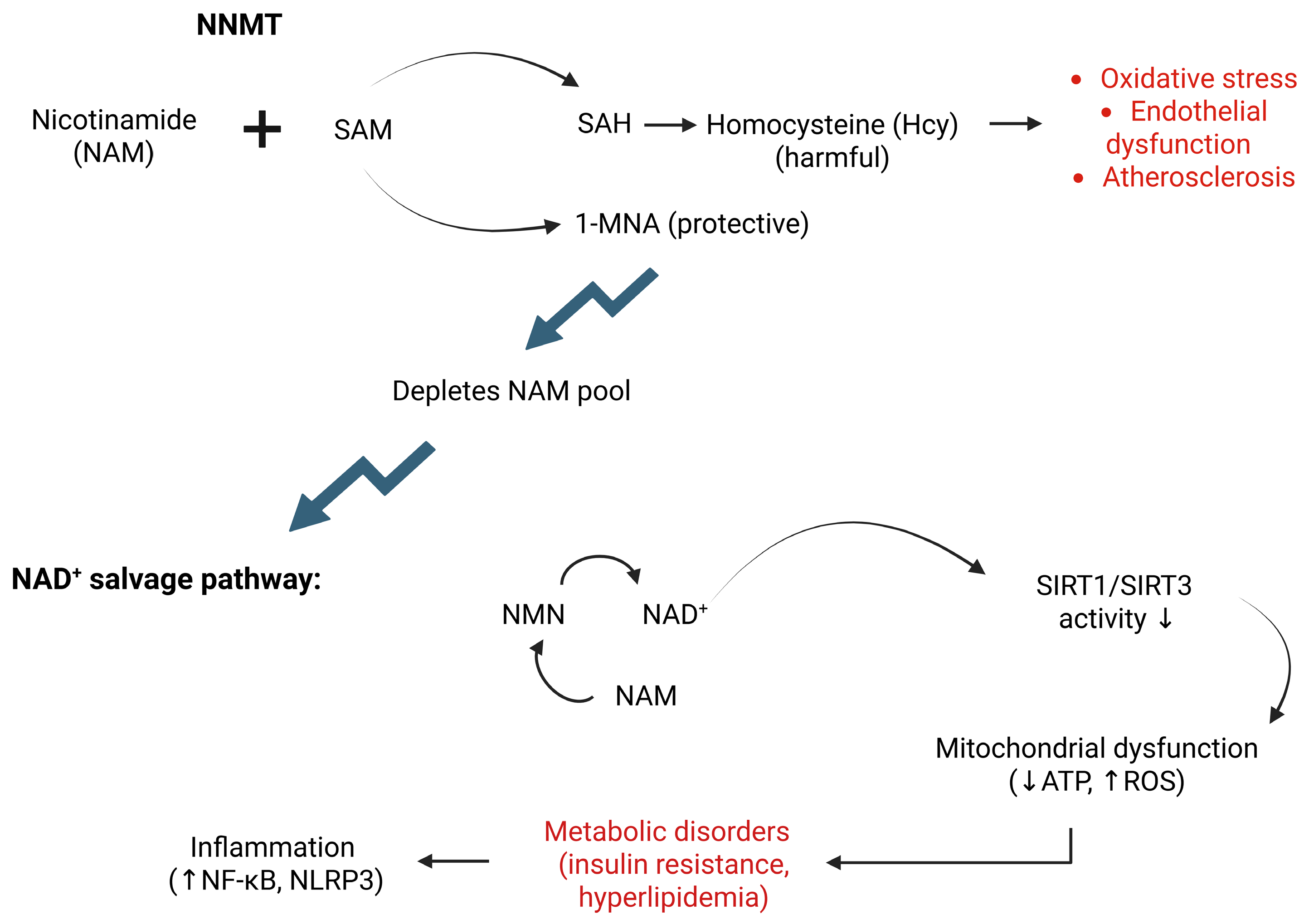
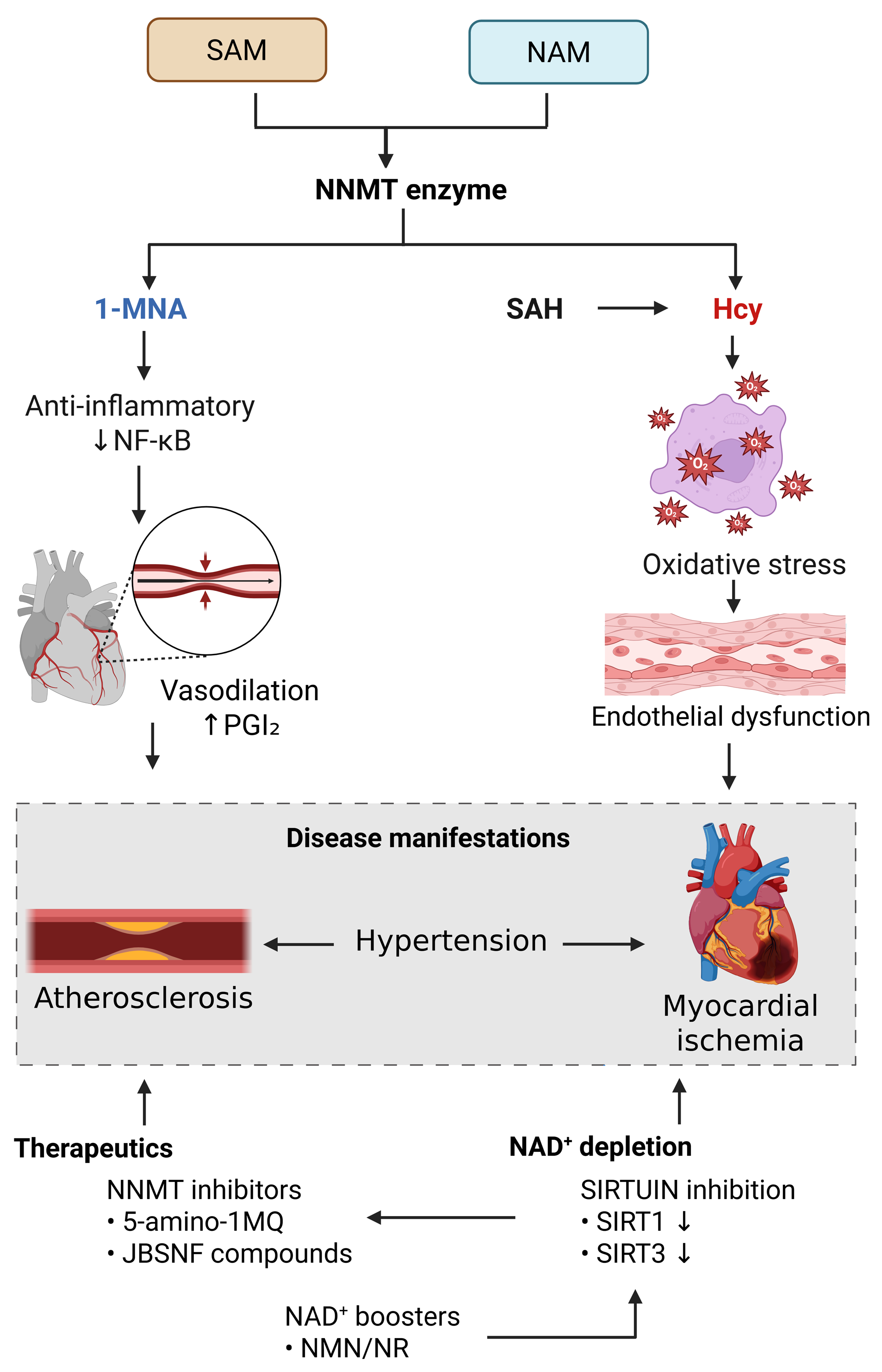
| Disease/Condition | Pathogenic Role of NNMT | Key Mechanisms Influenced by NNMT | Regulatory Effects |
|---|---|---|---|
| Hyperlipidemia | Dysregulated lipid metabolism [49]; fat accumulation [16,50] | Fat metabolism [18]; resting energy expenditure [12]; elevated plasma Hcy [49]; de novo lipogenesis [50] | NNMT knockdown downregulates Srebf1 [48] and suppresses transcriptional activity of Lpl, Slc27a1, Fasn, and Fabp4 [51] |
| Atherosclerosis | Plaque instability; vascular inflammation [43]; endothelial dysfunction [21]; oxidative stress [15,19] | Hcy metabolism [52]; STAT3–IL-1β–PGE2 pathway [43]; NNMT–1-MNA pathway [52] | NNMT upregulation increases expression of COX-2 and PGE2 [43] |
| Hypertension | Endothelial dysfunction [21]; HHcy [53] | NNMT–1-MNA pathway [39]; elevated plasma Hcy [54]; reduced NAD+ biosynthesis [55] | Epigenetic modulation [56] |
| Myocardial Ischemia | Mitochondrial dysfunction [57]; oxidative damage [58]; ischemia–reperfusion injury [59] | NAD+ metabolism [2,43,60]; NMN depletion [59]; energy-related and inflammatory pathways; elevated plasma Hcy [61] | NNMT overactivation reduces Sirt3 expression [62]; NNMT knockdown increases Nampt expression [18] |
| Category | Inhibitor | IC50 (μM) | Role of Inhibitor | Targeted Condition(s) |
|---|---|---|---|---|
| SAM-Competitive | SAH [6] | 35.3 ± 5.5 [90] | Reduces NAM methylation; preserves NAD+ levels [15,60] | N/A |
| NAM-Competitive | 1-MNA [18,24] | 24.6 ± 3.2 [94] | Reduces inflammation, oxidative stress, vascular injury [31,95] | Obesity-related cardiac injury [31]; atherosclerosis [3,91]; cardiac fibrosis [31]; hepatic ischemia–reperfusion injury [95] |
| 5-amino-1MQ (NNMTi) [86,96] | 1.2 ± 0.1 [6] | Improves metabolic parameters; enhances insulin sensitivity [86] | Obesity-related metabolic syndrome [86] | |
| JBSNF-000088 [23] | 2.4 ± 0.1 [6] | Restores glucose tolerance; reduces body weight [23] | Diet-induced obesity [23] | |
| Dual-Substrate | CC-410 [92] | N/A | Inhibits pre-adipocyte differentiation; modulates glucocorticoid signaling [92] | Glucocorticoid-induced obesity [92] |
| Allosteric | Macrocyclic peptides [93] | 0.229 ± 0.007 [6] | Non-competitive inhibition; downregulates 1-MNA production [93] | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jawaria; Zarlashat, Y.; Philippovich, M.; Dósa, E. Nicotinamide N-Methyltransferase in Cardiovascular Diseases: Metabolic Regulator and Emerging Therapeutic Target. Biomolecules 2025, 15, 1281. https://doi.org/10.3390/biom15091281
Jawaria, Zarlashat Y, Philippovich M, Dósa E. Nicotinamide N-Methyltransferase in Cardiovascular Diseases: Metabolic Regulator and Emerging Therapeutic Target. Biomolecules. 2025; 15(9):1281. https://doi.org/10.3390/biom15091281
Chicago/Turabian StyleJawaria, Yusra Zarlashat, Márton Philippovich, and Edit Dósa. 2025. "Nicotinamide N-Methyltransferase in Cardiovascular Diseases: Metabolic Regulator and Emerging Therapeutic Target" Biomolecules 15, no. 9: 1281. https://doi.org/10.3390/biom15091281
APA StyleJawaria, Zarlashat, Y., Philippovich, M., & Dósa, E. (2025). Nicotinamide N-Methyltransferase in Cardiovascular Diseases: Metabolic Regulator and Emerging Therapeutic Target. Biomolecules, 15(9), 1281. https://doi.org/10.3390/biom15091281






