Neuromyotonia and CASPR2 Antibodies: Electrophysiological Clues to Disease Pathophysiology
Abstract
1. Introduction
2. Functional Aspects of Voltage-Gated Potassium Channels and Related Proteins
3. Antibodies to Voltage-Gated Potassium Channels and Related Proteins
4. Neurophysiological Aspects of Peripheral Nerve Hyperexcitability in CASPR2 Autoimmunity
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAM | Cell adhesion molecules |
| CASPR2 | Contactin-associated protein-like 2 |
| LGI1 | Leucine-rich, Glioma Inactivated 1 |
| PNH | Peripheral nerve hyperexcitability |
| SPSD | Stiff person spectrum disorders |
| TAG-1 | Transient Axonal Glycoprotein 1 (TAG-1) |
| VGKC | Voltage-gated potassium channel |
References
- Dalmau, J.; Geis, C.; Graus, F. Autoantibodies to Synaptic Receptors and Neuronal Cell Surface Proteins in Autoimmune Diseases of the Central Nervous System. Physiol. Rev. 2017, 97, 839–887. [Google Scholar] [CrossRef]
- Prüss, H. Autoantibodies in neurological disease. Nat. Rev. Immunol. 2021, 21, 798–813. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.; Samões, R.; Cardoso, M.; Sousa, P.A.; Damásio, J.; Marinho, A.; Carneiro, P.; Neves, E.; Silva, M.A.; Santos, E. Distinct phenotypes in a cohort of anti-CASPR2 associated neurological syndromes. Clin. Neurol. Neurosurg. 2023, 234, 107994. [Google Scholar] [CrossRef] [PubMed]
- Sonderen, V.A.; Ariño, H.; Petit-Pedrol, M.; Leypoldt, F.; Körtvélyessy, P.; Wandinger, K.-P.; Lancaster, E.; Wirtz, W.P.; Schreurs, W.J.M.; Smitt, S.A.E.P.; et al. The clinical spectrum of Caspr2 antibody–associated disease. Neurology 2016, 87, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Küçükali, I.C.; Kürtüncü, M.; Akçay, İ.H.; Tüzün, E.; Öge, E.A. Peripheral nerve hyperexcitability syndromes. Rev. Neurosci. 2015, 26, 239–251. [Google Scholar] [CrossRef]
- Lance, W.J.; Burke, D.; Pollard, J. Hyperexcitability of motor and sensory neurons in neuromyotonia. Ann. Neurol. 1979, 5, 523–532. [Google Scholar] [CrossRef]
- Maddison, P.; Mills, R.K.; Newsom-Davis, J. Clinical electrophysiological characterization of the acquired neuromyotonia phenotype of autoimmune peripheral nerve hyperexcitability. Muscle Nerve 2006, 33, 801–808. [Google Scholar] [CrossRef]
- Bady, B.; Chauplannaz, G.; Vial, C.; Savet, J.-F. Autoimmune aetiology for acquired neuromyotonia. Lancet 1991, 338, 1330. [Google Scholar] [CrossRef]
- Burke, D. Excitability of motor axons in neuromyotonia. Muscle Nerve 1999, 22, 797–799. [Google Scholar] [CrossRef]
- Comperat, L.; Pegat, A.; Honnorat, J.; Joubert, B. Autoimmune neuromyotonia. Curr. Opin. Neurol. 2022, 35, 597–603. [Google Scholar] [CrossRef]
- Gutmann, L.; Gutmann, L. Myokymia and neuromyotonia 2004. J. Neurol. 2004, 251, 138–142. [Google Scholar] [CrossRef]
- Gutmann, L.; Libell, D.; Gutmann, L. When is myokymia neuromyotonia? Muscle Nerve 2001, 24, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.-X.; Ziskind-Conhaim, L. Development of Ionic Currents Underlying Changes in Action Potential Waveforms in Rat Spinal Motoneurons. J. Neurophysiol. 1998, 80, 3047–3061. [Google Scholar] [CrossRef] [PubMed]
- Poliak, S.; Salomon, D.; Elhanany, H.; Sabanay, H.; Kiernan, B.; Pevny, L.; Stewart, L.C.; Xu, X.; Chiu, S.-Y.; Shrager, P.; et al. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J. Cell Biol. 2003, 162, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Binks, M.N.S.; Klein, J.C.; Waters, P.; Pittock, J.S.; Irani, R.S. LGI1, CASPR2 and related antibodies: A molecular evolution of the phenotypes. J. Neurol. Neurosurg. Psychiatry 2018, 89, 526–534. [Google Scholar] [CrossRef]
- Bashford, J.; Chan, W.K.; Coutinho, E.; Norwood, F.; Mills, K.; Shaw, C.E. Demystifying the spontaneous phenomena of motor hyperexcitability. Clin. Neurophysiol. 2021, 132, 1830–1844. [Google Scholar] [CrossRef]
- Jenkins, M.P.; Bender, J.K. Axon initial segment structure and function in health and disease. Physiol. Rev. 2025, 105, 765–801. [Google Scholar] [CrossRef]
- Burke, D.; Kiernan, C.M.; Bostock, H. Excitability of human axons. Clin. Neurophysiol. 2001, 112, 1575–1585. [Google Scholar] [CrossRef]
- Ulbricht, W. Sodium Channel Inactivation: Molecular Determinants and Modulation. Physiol. Rev. 2005, 85, 1271–1301. [Google Scholar] [CrossRef]
- Shu, Y.; Yu, Y.; Yang, J.; Mccormick, A.D. Selective control of cortical axonal spikes by a slowly inactivating K+ current. Proc. Natl. Acad. Sci. USA 2007, 104, 11453–11458. [Google Scholar] [CrossRef]
- Yellen, G. The voltage-gated potassium channels and their relatives. Nature 2002, 419, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Kole, H.P.M.; Letzkus, J.J.; Stuart, J.G. Axon Initial Segment Kv1 Channels Control Axonal Action Potential Waveform and Synaptic Efficacy. Neuron 2007, 55, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.E.; Clark, D.B.; Zagha, E.; Nahmani, M.; Erisir, A.; Rudy, B. K+ Channels at the Axon Initial Segment Dampen Near-Threshold Excitability of Neocortical Fast-Spiking GABAergic Interneurons. Neuron 2008, 58, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Feria Pliego, J.A.; Pedroarena, C.M. Kv1 potassium channels control action potential firing of putative GABAergic deep cerebellar nuclear neurons. Sci. Rep. 2020, 10, 6954. [Google Scholar]
- Morgan, J.P.; Bourboulou, R.; Filippi, C.; Koenig-Gambini, J.; Epsztein, J. Kv1.1 contributes to a rapid homeostatic plasticity of intrinsic excitability in CA1 pyramidal neurons In Vivo. eLife 2019, 8, e49915. [Google Scholar] [CrossRef]
- Varanita, T.; Angi, B.; Scattolini, V.; Szabo, I. Kv1.3 K+ Channel Physiology Assessed by Genetic and Pharmacological Modulation. Physiology 2023, 38, 25–41. [Google Scholar] [CrossRef]
- Rasband, N.M.; Peles, E. The Nodes of Ranvier: Molecular Assembly and Maintenance. Cold Spring Harb. Perspect. Biol. 2016, 8, a020495. [Google Scholar] [CrossRef]
- Barrett, F.E.; Barrett, N.J. Intracellular recording from vertebrate myelinated axons: Mechanism of the depolarizing afterpotential. J. Physiol. 1982, 323, 117–144. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, C.-L.; Messing, A.; Chiu, Y.S. Temperature-Sensitive Neuromuscular Transmission in Kv1.1 Null Mice: Role of Potassium Channels under the Myelin Sheath in Young Nerves. J. Neurosci. 1998, 18, 7200–7215. [Google Scholar] [CrossRef]
- Poliak, S.; Gollan, L.; Martinez, R.; Custer, A.; Einheber, S.; Salzer, L.J.; Trimmer, S.J.; Shrager, P.; Peles, E. Caspr2, a New Member of the Neurexin Superfamily, Is Localized at the Juxtaparanodes of Myelinated Axons and Associates with K+ Channels. Neuron 1999, 24, 1037–1047. [Google Scholar] [CrossRef]
- Gordon, A.; Salomon, D.; Barak, N.; Pen, Y.; Tsoory, M.; Kimchi, T.; Peles, E. Expression of Cntnap2 (Caspr2) in multiple levels of sensory systems. Mol. Cell. Neurosci. 2016, 70, 42–53. [Google Scholar] [CrossRef]
- Traka, M.; Goutebroze, L.; Denisenko, N.; Bessa, M.; Nifli, A.; Havaki, S.; Iwakura, Y.; Fukamauchi, F.; Watanabe, K.; Soliven, B.; et al. Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers. J. Cell Biol. 2003, 162, 1161–1172. [Google Scholar] [CrossRef]
- Gu, C.; Gu, Y. Clustering and Activity Tuning of Kv1 Channels in Myelinated Hippocampal Axons. J. Biol. Chem. 2011, 286, 25835–25847. [Google Scholar] [CrossRef]
- Brownstein, A.C.; Beggs, H.A.; Rodan, L.; Shi, J.; Towne, C.M.; Pelletier, R.; Cao, S.; Rosenberg, A.P.; Urion, K.D.; Picker, J.; et al. Clinical heterogeneity associated with KCNA1 mutations include cataplexy and nonataxic presentations. Neurogenetics 2016, 17, 11–16. [Google Scholar] [CrossRef]
- Paulhus, K.; Ammerman, L.; Glasscock, E. Clinical Spectrum of KCNA1 Mutations: New Insights into Episodic Ataxia and Epilepsy Comorbidity. Int. J. Mol. Sci. 2020, 21, 2802. [Google Scholar] [CrossRef]
- Paulhus, K.; Glasscock, E. Novel Genetic Variants Expand the Functional, Molecular, and Pathological Diversity of KCNA1 Channelopathy. Int. J. Mol. Sci. 2023, 24, 8826. [Google Scholar] [CrossRef]
- Eunson, H.L.; Rea, R.; Zuberi, M.S.; Youroukos, S.; Panayiotopoulos, P.C.; Liguori, R.; Avoni, P.; Mcwilliam, C.R.; Stephenson, P.B.J.; Hanna, G.M.; et al. Clinical, genetic, and expression studies of mutations in the potassium channel gene KCNA1 reveal new phenotypic variability. Ann. Neurol. 2000, 48, 647–656. [Google Scholar] [CrossRef]
- Imbrici, P.; Accogli, A.; Blunck, R.; Altamura, C.; Iacomino, M.; D’Adamo, C.M.; Allegri, A.; Pedemonte, M.; Brolatti, N.; Vari, S.; et al. Musculoskeletal Features without Ataxia Associated with a Novel de novo Mutation in KCNA1 Impairing the Voltage Sensitivity of Kv1.1 Channel. Biomedicines 2021, 9, 75. [Google Scholar] [CrossRef]
- Falace, A.; Striano, P.; Manganelli, F.; Coppola, A.; Striano, S.; Minetti, C.; Zara, F. Inherited neuromyotonia: A clinical and genetic study of a family. Neuromuscul. Disord. 2007, 17, 23–27. [Google Scholar] [CrossRef]
- Peñagarikano, O.; Abrahams, S.B.; Herman, I.E.; Winden, D.K.; Gdalyahu, A.; Dong, H.; Sonnenblick, I.L.; Gruver, R.; Almajano, J.; Bragin, A.; et al. Absence of CNTNAP2 Leads to Epilepsy, Neuronal Migration Abnormalities, and Core Autism-Related Deficits. Cell 2011, 147, 235–246. [Google Scholar] [CrossRef]
- Poot, M.; Beyer, V.; Schwaab, I.; Damatova, N.; Slot, V.T.R.; Prothero, J.; Holder, E.S.; Haaf, T. Disruption of CNTNAP2 and additional structural genome changes in a boy with speech delay and autism spectrum disorder. Neurogenetics 2010, 11, 81–89. [Google Scholar] [CrossRef]
- Strauss, A.K.; Puffenberger, G.E.; Huentelman, J.M.; Gottlieb, S.; Dobrin, E.S.; Parod, M.J.; Stephan, A.D.; Morton, H.D. Recessive Symptomatic Focal Epilepsy and Mutant Contactin-Associated Protein-like 2. N. Engl. J. Med. 2006, 354, 1370–1377. [Google Scholar] [CrossRef]
- Tan, M.K.; Lennon, A.V.; Klein, J.C.; Boeve, F.B.; Pittock, J.S. Clinical spectrum of voltage-gated potassium channel autoimmunity. Neurology 2008, 70, 1883–1890. [Google Scholar] [CrossRef]
- Swayang, S.P.; Nalini, A.; Preethish-Kumar, V.; Udupa, K.; Yadav, R.; Vengalil, S.; Reshma, S.S.; Polavarapu, K.; Nashi, S.; Sathyaprabha, T.N.; et al. CASPR2-Related Morvan Syndrome. Neurol. Clin. Pract. 2021, 11, e267–e276. [Google Scholar] [CrossRef]
- Devine, F.M.; Louis, S.K.E. Sleep Disturbances Associated with Neurological Autoimmunity. Neurotherapeutics 2021, 18, 181–201. [Google Scholar] [CrossRef]
- Shillito, P.; Molenaar, C.P.; Vincent, A.; Leys, K.; Zheng, W.; Berg, D.V.J.R.; Plomp, J.J.; Kempen, V.H.T.G.; Chauplannaz, G.; Wintzen, R.A.; et al. Acquired neuromyotonia: Evidence for autoantibodies directed against K+channels of peripheral nerves. Ann. Neurol. 1995, 38, 714–722. [Google Scholar] [CrossRef]
- Bataller, L.; Kleopa, A.K.; Wu, F.G.; Rossi, E.J.; Rosenfeld, R.M.; Dalmau, J. Autoimmune limbic encephalitis in 39 patients: Immunophenotypes and outcomes. J. Neurol. Neurosurg. Psychiatry 2006, 78, 381–385. [Google Scholar] [CrossRef]
- Gadoth, A.; Pittock, J.S.; Dubey, D.; Mckeon, A.; Britton, W.J.; Schmeling, E.J.; Smith, A.; Kotsenas, L.A.; Watson, E.R.; Lachance, H.D.; et al. Expanded phenotypes and outcomes among 256 LGI1/CASPR2-IgG–positive patients. Ann. Neurol. 2017, 82, 79–92. [Google Scholar] [CrossRef]
- Gastaldi, M.; Rosa, D.A.; Maestri, M.; Zardini, E.; Scaranzin, S.; Guida, M.; Borrelli, P.; Ferraro, E.O.; Lampasona, V.; Furlan, R.; et al. Acquired neuromyotonia in thymoma-associated myasthenia gravis: A clinical and serological study. Eur. J. Neurol. 2019, 26, 992–999. [Google Scholar] [CrossRef]
- Newsom-Davis, J.; Mills, R.K. Immunological associations of acquired neuromyotonia (Isaacs’ syndrome). Brain 1993, 116, 453–469. [Google Scholar] [CrossRef]
- Newsom-Davis, J. Autoimmune Neuromyotonia (Isaacs’ Syndrome): An Antibody-mediated Potassium Channelopathy. Ann. N. Y. Acad. Sci. 1997, 835, 111–119. [Google Scholar] [CrossRef]
- Sinha, S.; Newsom-Davis, J.; Mills, K.; Byrne, N.; Lang, B.; Vincent, A. Autoimmune aetiology for acquired neuromyotonia (Isaacs’ syndrome). Lancet 1991, 338, 75–77. [Google Scholar] [CrossRef]
- Arimura, K.; Sonoda, Y.; Watanabe, O.; Nagado, T.; Kurono, A.; Tomimitsu, H.; Otsuka, R.; Kameyama, M.; Osame, M. Isaacs’ syndrome as a potassium channelopathy of the nerve. Muscle Nerve 2002, 999, S55–S58. [Google Scholar] [CrossRef] [PubMed]
- Nagado, T.; Arimura, K.; Sonoda, Y.; Kurono, A.; Horikiri, Y.; Kameyama, A.; Kameyama, M.; Pongs, O.; Osame, M. Potassium current suppression in patients with peripheral nerve hyperexcitability. Brain 1999, 122, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, Y.; Arimura, K.; Kurono, A.; Suehara, M.; Kameyama, M.; Minato, S.; Hayashi, A.; Osame, M. Serum of Isaacs’ syndrome suppresses potassium channels in PC-12 cell lines. Muscle Nerve 1996, 19, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Kleopa, A.K.; Elman, B.L.; Lang, B.; Vincent, A.; Scherer, S.S. Neuromyotonia and limbic encephalitis sera target mature Shaker-type K+ channels: Subunit specificity correlates with clinical manifestations. Brain 2006, 129, 1570–1584. [Google Scholar] [CrossRef]
- Lang, B.; Makuch, M.; Moloney, T.; Dettmann, I.; Mindorf, S.; Probst, C.; Stoecker, W.; Buckley, C.; Newton, R.C.; Leite, I.M.; et al. Intracellular and non-neuronal targets of voltage-gated potassium channel complex antibodies. J. Neurol. Neurosurg. Psychiatry 2017, 88, 353–361. [Google Scholar] [CrossRef]
- Irani, R.S.; Alexander, S.; Waters, P.; Kleopa, A.K.; Pettingill, P.; Zuliani, L.; Peles, E.; Buckley, C.; Lang, B.; Vincent, A. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain 2010, 133, 2734–2748. [Google Scholar] [CrossRef]
- Lancaster, E.; Huijbers, M.G.M.; Bar, V.; Boronat, A.; Wong, A.; Martinez-Hernandez, E.; Wilson, C.; Jacobs, D.; Lai, M.; Walker, W.R.; et al. Investigations of caspr2, an autoantigen of encephalitis and neuromyotonia. Ann. Neurol. 2011, 69, 303–311. [Google Scholar] [CrossRef]
- Dawes, M.J.; Weir, A.G.; Middleton, J.S.; Patel, R.; Chisholm, I.K.; Pettingill, P.; Peck, J.L.; Sheridan, J.; Shakir, A.; Jacobson, L.; et al. Immune or Genetic-Mediated Disruption of CASPR2 Causes Pain Hypersensitivity due to Enhanced Primary Afferent Excitability. Neuron 2018, 97, 806–822.e10. [Google Scholar] [CrossRef]
- Kortman, G.H.; Veldink, H.J.; Drost, G. Positive muscle phenomena—Diagnosis, pathogenesis and associated disorders. Nat. Rev. Neurol. 2012, 8, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Wel, D.B.; Claeys, G.K. Neuromuscular hyperexcitability syndromes. Curr. Opin. Neurol. 2021, 34, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Jing, S.; Quan, C.; Lu, J.; Qiao, X.; Qiao, K.; Lu, J.; Xi, J.; Zhao, C. Isaacs syndrome with CASPR2 antibody: A series of three cases. J. Clin. Neurosci. 2017, 41, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Sakamoto, Y.; Nishio, T.; Baulac, S.; Kuwamura, M.; Ohno, Y.; Takizawa, A.; Kaneko, S.; Serikawa, T.; Mashimo, T. Kcna1-mutant rats dominantly display myokymia, neuromyotonia and spontaneous epileptic seizures. Brain Res. 2012, 1435, 154–166. [Google Scholar] [CrossRef]
- Maddison, P.; Newsom-Davis, J.; Mills, R.K. Strength-duration properties of peripheral nerve in acquired neuromyotonia. Muscle Nerve 1999, 22, 823–830. [Google Scholar] [CrossRef]
- Benatar, M. Neurological potassium channelopathies. QJM 2000, 93, 787–797. [Google Scholar] [CrossRef][Green Version]
- Schwarz, R.J.; Reid, G.; Bostock, H. Action potentials and membrane currents in the human node of Ranvier. Pflügers Arch. Eur. J. Physiol. 1995, 430, 283–292. [Google Scholar] [CrossRef]
- Kleine, U.B.; Stegeman, F.D.; Drost, G.; Zwarts, J.M. Interspike interval analysis in a patient with peripheral nerve hyperexcitability and potassium channel antibodies. Muscle Nerve 2008, 37, 269–274. [Google Scholar] [CrossRef]
- Koshy, G.K.; Iype, T.; Panicker, P. Stimulus-Induced Motor Afterdischarges in CASPR2 (Contactin-Associated Protein-Like 2)-Positive Peripheral Nerve Hyperexcitability Syndrome. Cureus 2023, 15, e45643. [Google Scholar] [CrossRef]
- Niu, J.; Guan, H.; Cui, L.; Guan, Y.; Liu, M. Afterdischarges following M waves in patients with voltage-gated potassium channels antibodies. Clin. Neurophysiol. Pract. 2017, 2, 72–75. [Google Scholar] [CrossRef]
- Dhand, K.U. Isaacs’ syndrome: Clinical and electrophysiological response to gabapentin. Muscle Nerve 2006, 34, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Surana, S.; Kumar, R.; Pitt, M.; Hafner, P.; Mclellan, A.; Davidson, J.; Prabakhar, P.; Vincent, A.; Hacohen, Y.; Wright, S. Acquired neuromyotonia in children with CASPR2 and LGI1 antibodies. Dev. Med. Child Neurol. 2019, 61, 1344–1347. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ding, Q.; Feng, X.; Yimin, W.; Shen, D.; Shi, J.; Wu, S.; Cui, L.; Guan, Y. Characteristics of after-discharges following compound muscle action potential or F-wave in primary peripheral nerve hyperexcitability syndrome. Muscle Nerve 2024, 70, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Auger, G.R.; Daube, R.J.; Gomez, R.M.; Lambert, H.E. Hereditary form of sustained muscle activity of peripheral nerve origin causing generalized myokymia and muscle stiffness. Ann. Neurol. 1984, 15, 13–21. [Google Scholar] [CrossRef]
- Albers, W.J.; Allen, A.A.; Bastron, A.J.; Daube, R.J. Limb myokymia. Muscle Nerve 1981, 4, 494–504. [Google Scholar] [CrossRef]
- Smith, E.K.K.; Claussen, G.; Fesenmeier, T.J.; Oh, J.S. Myokymia–cramp syndrome: Evidence of hyperexcitable peripheral nerve. Muscle Nerve 1994, 17, 1065–1067. [Google Scholar] [CrossRef]
- Hosokawa, S.; Shinoda, H.; Sakai, T.; Kato, M.; Kuroiwa, Y. Electrophysiological study on limb myokymia in three women. J. Neurol. Neurosurg. Psychiatry 1987, 50, 877–881. [Google Scholar] [CrossRef]
- Tahmoush, J.A.; Alonso, J.R.; Tahmoush, P.G.; Heiman-Patterson, D.T. Cramp-fasciculation syndrome: A treatable hyperexcitable peripheral nerve disorder. Neurology 1991, 41, 1021. [Google Scholar] [CrossRef]
- Kiernan, C.M.; Lin, S.Y.C.; Burke, D. Differences in activity-dependent hyperpolarization in human sensory and motor axons. J. Physiol. 2004, 558, 341–349. [Google Scholar] [CrossRef]
- Kiernan, C.M.; Mogyoros, I.; Burke, D. Differences in the recovery of excitability in sensory and motor axons of human median nerve. Brain 1996, 119, 1099–1105. [Google Scholar] [CrossRef]
- Dijk, V.G.J.; Lammers, J.G.; Wintzen, R.A.; Molenaar, C.P. Repetitive CMAPs: Mechanisms of neural and synaptic genesis. Muscle Nerve 1996, 19, 1127–1133. [Google Scholar] [CrossRef]
- Deymeer, F.; Oge, E.A.; Serdaroglu, P.; Yazici, J.; Ozdemir, C.; Baslo, A. The use of botulinum toxin in localizing neuromyotonia to the terminal branches of the peripheral nerve. Muscle Nerve 1998, 21, 643–646. [Google Scholar] [CrossRef]
- Vucic, S.; Cheah, C.B.; Yiannikas, C.; Vincent, A.; Kiernan, C.M. Corticomotoneuronal function and hyperexcitability in acquired neuromyotonia. Brain 2010, 133, 2727–2733. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shi, J.; Gao, J.; Hu, Y.; Ren, H.; Guan, H.; Li, J.; Huang, Y.; Cui, L.; Guan, Y. Peripheral nerve hyperexcitability syndrome: A clinical, electrophysiological, and immunological study. Muscle Nerve 2021, 63, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Harrison, B.T.; Benatar, M. Accuracy of repetitive nerve stimulation for diagnosis of the cramp–fasciculation syndrome. Muscle Nerve 2007, 35, 776–780. [Google Scholar] [CrossRef]
- Parissis, D.; Ioannidis, P. After discharges following compound muscle action potential in CASPR2 antibody-related disease. Pract. Neurol. 2021, 21, 342–343. [Google Scholar] [CrossRef]
- Benatar, M.; Chapman, M.K.; Rutkove, B.S. Repetitive nerve stimulation for the evaluation of peripheral nerve hyperexcitability. J. Neurol. Sci. 2004, 221, 47–52. [Google Scholar] [CrossRef]
- Iwasaki, A. Immune Regulation of Antibody Access to Neuronal Tissues. Trends Mol. Med. 2017, 23, 227–245. [Google Scholar] [CrossRef]
- Hutto, K.S.; Harrison, B.T. Electrodiagnostic Assessment of Hyperexcitable Nerve Disorders. Neurol. Clin. 2021, 39, 1083–1096. [Google Scholar] [CrossRef]
- Santos, O.M.; Swash, M.; Carvalho, D.M. The generator site in acquired autoimmune neuromyotonia. Clin. Neurophysiol. 2017, 128, 643–646. [Google Scholar] [CrossRef]
- Irani, F.P.; Purohit, V.A.; Wadia, H.N. The syndrome of continuous muscle fiber activity. Evidence to suggest proximal neurogenic causation. Acta Neurol. Scand. 1977, 55, 273–288. [Google Scholar] [CrossRef]
- Jan, Y.L.; Jan, N.Y. Voltage-gated potassium channels and the diversity of electrical signalling. J. Physiol. 2012, 590, 2591–2599. [Google Scholar] [CrossRef]
- Hines, H.; Murray, M.N.; Ahmad, S.; Jaradeh, S.; Gold, A.C. Video NeuroImages: Paraneoplastic spinal myoclonus associated with Caspr2 antibodies. Neurology 2018, 90, 660–661. [Google Scholar] [CrossRef] [PubMed]
- Gövert, F.; Abrante, L.; Becktepe, J.; Balint, B.; Ganos, C.; Oy, H.-V.U.; Krogias, C.; Varley, J.; Irani, R.S.; Paneva, S.; et al. Distinct movement disorders in contactin-associated-protein-like-2 antibody-associated autoimmune encephalitis. Brain 2023, 146, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Termsarasab, P.; Thammongkolchai, T.; Frucht, J.S. Spinal-generated movement disorders: A clinical review. J. Clin. Mov. Disord. 2015, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Latorre, A.; Hale, B.; Rocchi, L. How Do I Find Clues About Where Myoclonus Is Originating? Mov. Disord. Clin. Pract. 2022, 9, 721–722. [Google Scholar] [CrossRef]
- Pinatel, D.; Hivert, B.; Boucraut, J.; Saint-Martin, M.; Rogemond, V.; Zoupi, L.; Karagogeos, D.; Honnorat, J.; Faivre-Sarrailh, C. Inhibitory axons are targeted in hippocampal cell culture by anti-Caspr2 autoantibodies associated with limbic encephalitis. Front. Cell. Neurosci. 2015, 9, 265. [Google Scholar] [CrossRef]
- Moura, J.; Rocchi, L.; Zandi, M.; Balint, B.; Bhatia, P.K.; Latorre, A. Neurophysiological Insights into the Pathophysiology of Stiff-Person Spectrum Disorders. Mov. Disord. Clin. Pract. 2025, 12, 409–417. [Google Scholar] [CrossRef]
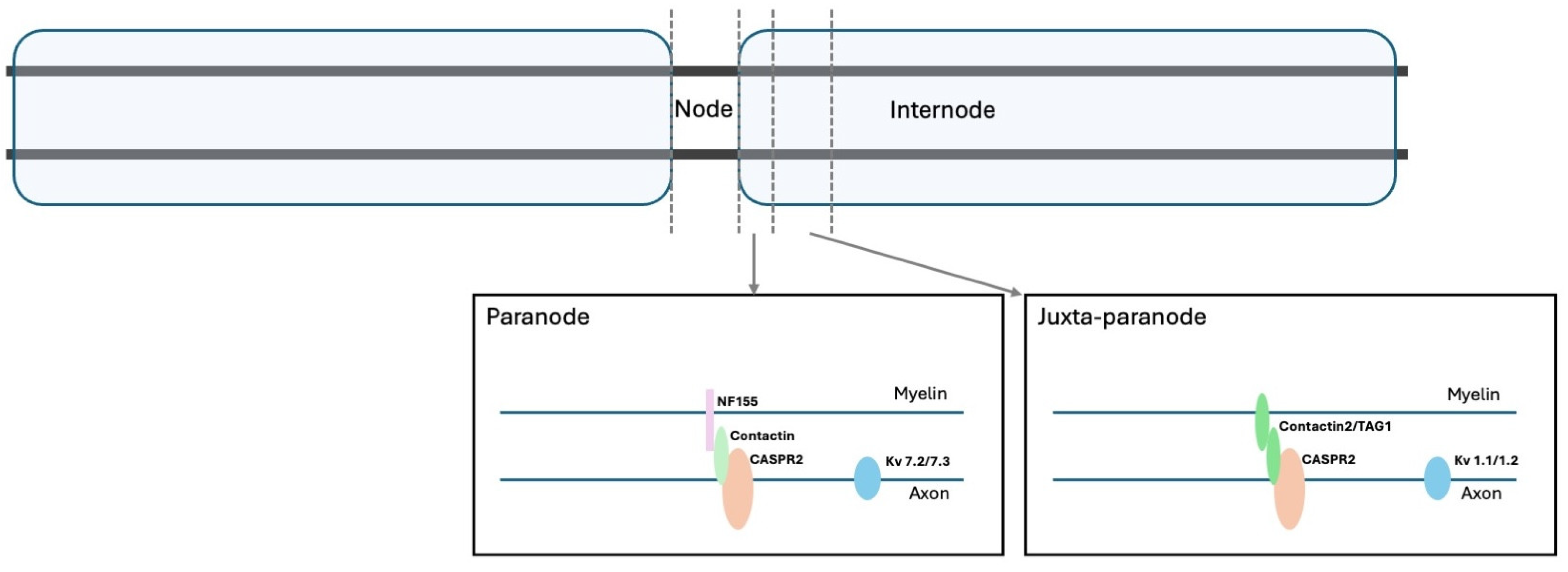
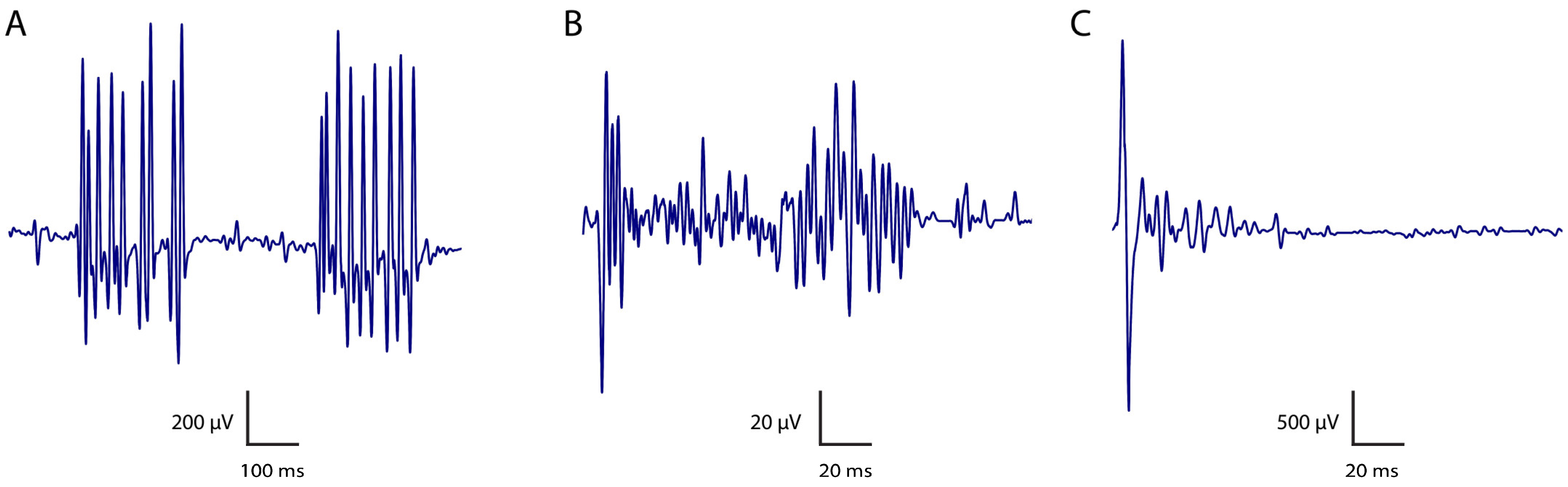
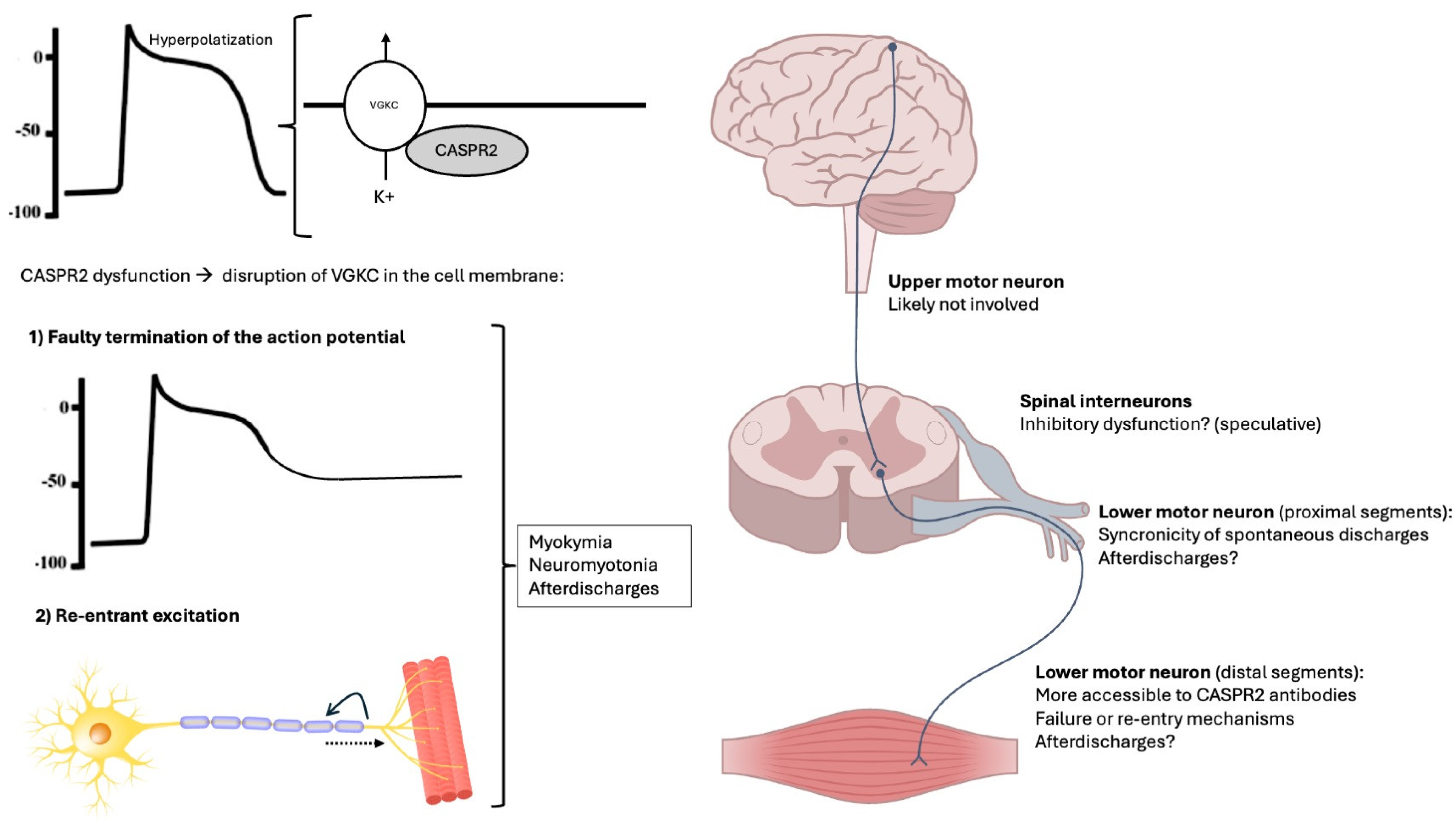
| PNH Feature | Definition | Electrophysiological Features |
|---|---|---|
| Myokymia | Random, undulating, rippling muscle movements (from the Greek kymia, which means wave). Typically recur rhythmically or semirhythmically and may range from focal (most commonly in facial muscles) to generalised. | Spontaneously generated bursts of single motor unit action potentials firing at 5–150 Hz rates. May occur as repetitive doublets, triplets, or multiplets, with interburst frequencies 1–5 Hz. Distinguished from fasciculations by its rhythmicity and involvement of the same motor units in each discharge. Characteristic “marching soldiers” sound in routine EMG, where changing to a longer sweep speed during recording makes it easier to recognise the bursting pattern of myokymic discharges. Freezing the screen often makes it easier to recognise the presence of the same motor unit potential firing repetitively in bursts. |
| Neuromyotonia | Also referred to as Isaac syndrome, pseudomyotonia, neurotonia and normo-calcemic tetany. Generalised muscle stiffness, delayed muscle relaxation and excessive sweating (hyperhidrosis). Muscle taping does not trigger a myotonic discharge as in myotonia. | Spontaneous, high-frequency and sustained motor unit discharge firing at 150–300 Hz, manifesting as prolonged bursts lasting up to a few seconds, with an abrupt onset and termination. Originates from motor neurons or their axons (in contrast with muscle fibres, as seen in myotonia). There is no evidence that myokymic and neuromyotonic discharges are distinct phenomena arising from different mechanisms. The higher frequency of neuromyotonic discharges may simply reflect the longer duration of their bursts. Characteristic “pinging” sound in routine EMG, where changing the sweep speed allows the identification of each potential as the same motor unit action potential. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moura, J.; Antenucci, P.; Coutinho, E.; Bhatia, K.P.; Rocchi, L.; Latorre, A. Neuromyotonia and CASPR2 Antibodies: Electrophysiological Clues to Disease Pathophysiology. Biomolecules 2025, 15, 1262. https://doi.org/10.3390/biom15091262
Moura J, Antenucci P, Coutinho E, Bhatia KP, Rocchi L, Latorre A. Neuromyotonia and CASPR2 Antibodies: Electrophysiological Clues to Disease Pathophysiology. Biomolecules. 2025; 15(9):1262. https://doi.org/10.3390/biom15091262
Chicago/Turabian StyleMoura, João, Pietro Antenucci, Ester Coutinho, Kailash P. Bhatia, Lorenzo Rocchi, and Anna Latorre. 2025. "Neuromyotonia and CASPR2 Antibodies: Electrophysiological Clues to Disease Pathophysiology" Biomolecules 15, no. 9: 1262. https://doi.org/10.3390/biom15091262
APA StyleMoura, J., Antenucci, P., Coutinho, E., Bhatia, K. P., Rocchi, L., & Latorre, A. (2025). Neuromyotonia and CASPR2 Antibodies: Electrophysiological Clues to Disease Pathophysiology. Biomolecules, 15(9), 1262. https://doi.org/10.3390/biom15091262







