Neutrophil Extracellular Traps in Cardiovascular Diseases: Pathological Roles and Therapeutic Implications
Abstract
1. Introduction
2. Characteristics and Functions of NETs
3. NET Signaling Pathway
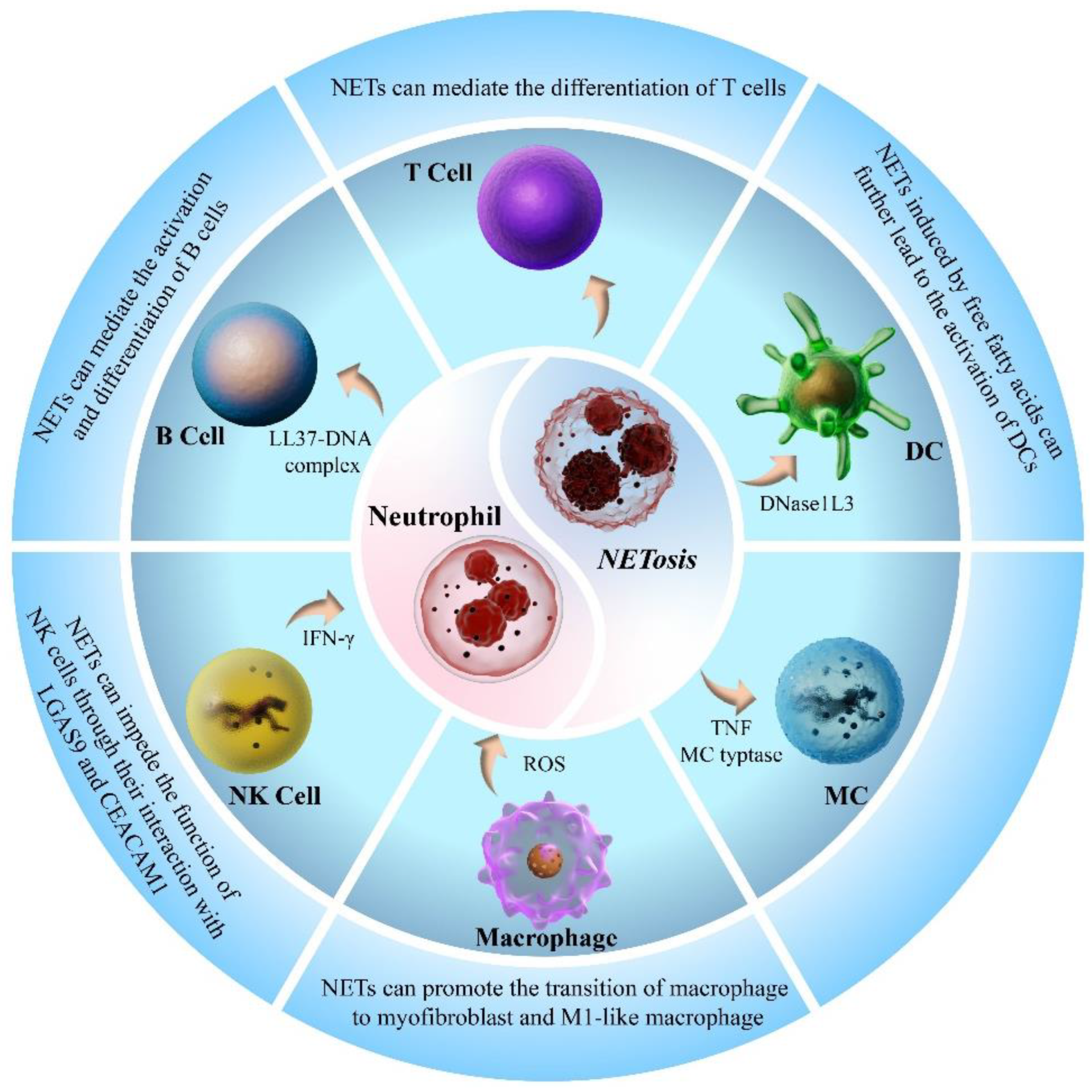
4. Interaction of NETs with Other Immune Cells
5. Distinction Between NETosis and Other Cell Deaths
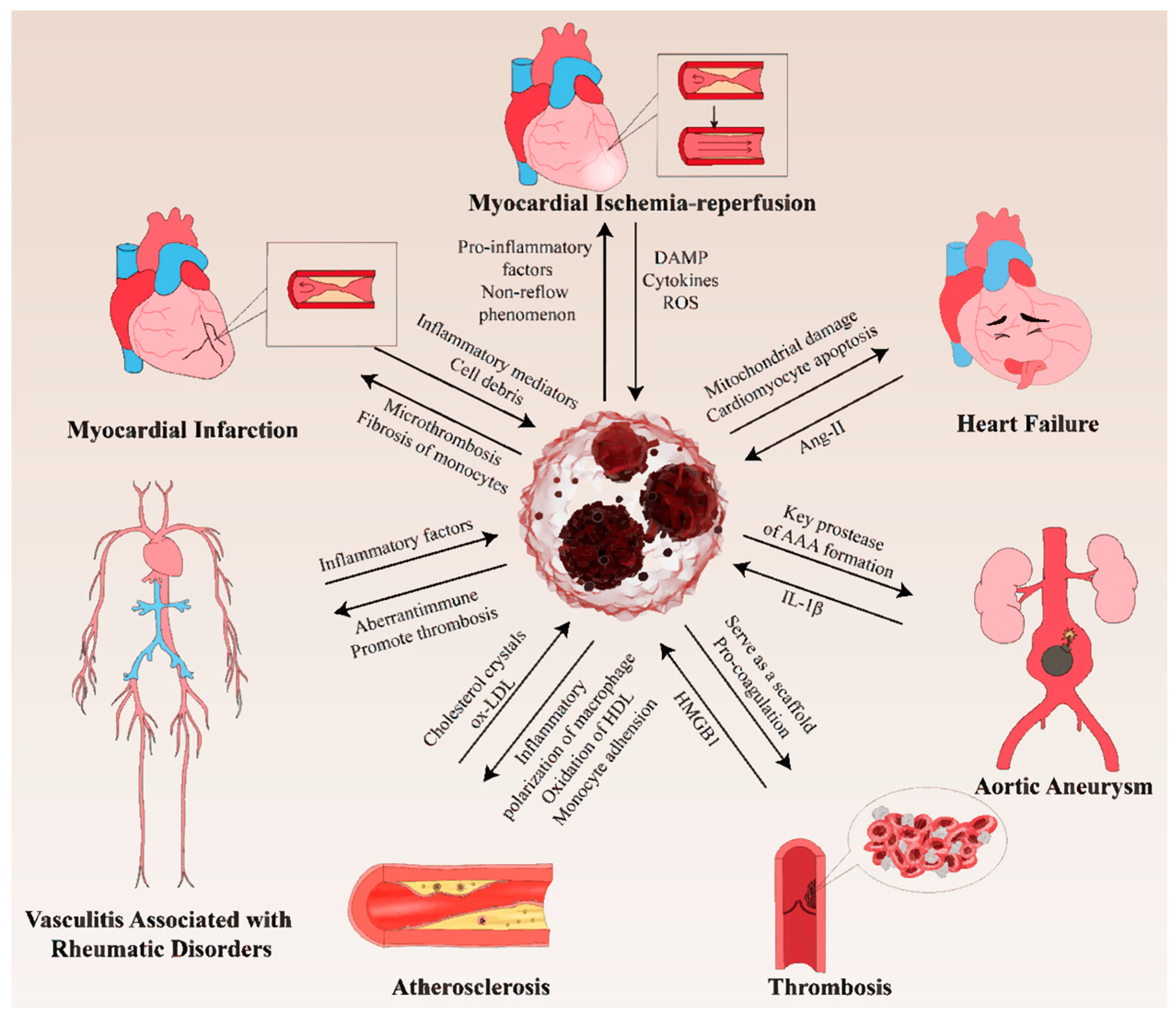
6. The Roles of NETs in CVDs
6.1. NETs in Thrombosis
6.2. NETs in Atherosclerosis
6.3. NETs in MI
6.4. NETs in Myocardial Ischemia–Reperfusion (MI/R)
6.5. NETs in Heart Failure (HF)
6.6. NETs in Vasculitis-Associated Rheumatic Disorders
6.7. NETs in Aortic Aneurysm
7. Potential Therapy Targeting NETs
8. Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAA | abdominal aortic aneurysm |
| AV | antineutrophil cytoplasmic antibody-associated vasculitis |
| AKT | protein Kinase B |
| AMI | acute myocardial infarction |
| ANCA | antineutrophil cytoplasmic antibody |
| AP | alternative complement pathway |
| Apo A-I | apolipoprotein A-I |
| apoVs | apoptotic vesicles |
| APS | antiphospholipid syndrome |
| Ca2+ | calcium ion |
| CAD | coronary artery disease |
| CC | cholesterol crystal |
| CitH3 | citrullinated histone H3 |
| CVD | cardiovascular disease |
| DADA2 | deficiency of adenosine deaminase 2 |
| DC | dendritic cell |
| DNase I | deoxyribonuclease I |
| EAM | experimental autoimmune myocarditis |
| GPX4 | glutathione peroxidase 4 |
| GSDMD | gasdermin D |
| GSDME | gasdermin E |
| HDL | high density lipoprotein |
| HF | heart failure |
| HFmrEF | heart failure with mid-range ejection fraction |
| HFpEF | heart failure with preserved ejection fraction |
| HFrEF | heart failure with reduced ejection fraction |
| HMDM | human monocyte-derived macrophage |
| HMGB1 | high mobility group box 1 protein |
| IFN-γ | interferon γ |
| IL-1β | Interleukin-1β |
| IL-8 | interleukin-8 |
| IRE1a | inositol-requiring enzyme-1 alpha |
| JAK | Janus kinase |
| LC3 | Light-chain 3 |
| LPS | lipopolysaccharides |
| LVAD | left ventricular assist devices |
| MAPK | mitogen-activated protein kinase |
| MC | mast cell |
| MSC-EVs | mesenchymal stem cell-derived extracellular vesicles |
| MEK | mitogen-activated extracellular signal-regulated kinase |
| MI/R | myocardial ischemia–reperfusion |
| MLKL | mixed lineage kinase domain–like |
| MPO | myeloperoxidase |
| mTORC | rapamycin complex |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NE | neutrophil elastase |
| NET | neutrophil extracellular trap |
| NF-κB | nuclear factor kappa-B |
| NK cell | natural killer cell |
| NLRP | nucleotide-binding oligomerization domain-like receptor pyrin domain-containing |
| NOX | NADPH oxidase |
| ox-LDL | oxidized low-density lipoprotein |
| PAD4 | peptidylarginine deiminase 4 |
| PAK2 | p21-activated kinase 2 |
| PCI | percutaneous coronary intervention |
| PD-L1 | programmed cell death 1 ligand 1 |
| PI3K | phosphatidylinositol-3-kinase |
| PKC | protein kinase C |
| PMA | phorbol myristate acetate |
| PMN | polymorphic mononuclear neutrophils |
| RA | rheumatoid arthritis; RBC, red blood cell |
| RIP3 | receptor-interacting protein 3 |
| RIPK | receptor-interacting protein kinase |
| ROS | reactive oxygen species |
| SK | small conductance potassium |
| SLE | systemic lupus erythematosus |
| STAM | nonalcoholic steatohepatitis induced by neonatal streptozotocin and high-fat diet |
| STAT3 | signal transducer and activator of transcription 3 |
| STEMI | ST-elevation myocardial infarction |
| STING | stimulator of interferon genes |
| SYK | spleen tyrosine kinase |
| TAC | transverse aortic constriction |
| TLR2 | toll-like receptor 2 |
| TLR-9 | toll-like receptor 9 |
| TNF | tumor necrosis factor |
| tPA | tissue plasminogen activator |
| VTE | venous thromboembolism |
References
- Andreadou, I.; Cabrera-Fuentes, H.A.; Devaux, Y.; Frangogiannis, N.G.; Frantz, S.; Guzik, T.; Liehn, E.A.; Gomes, C.P.C.; Schulz, R.; Hausenloy, D.J.; et al. Immune cells as targets for cardioprotection: New players and novel therapeutic opportunities. Cardiovasc. Res. 2019, 115, 1117–1130. [Google Scholar] [CrossRef]
- Dong, Y.L.; Kang, Z.Y.; Zhang, Z.L.; Zhang, Y.Q.; Zhou, H.F.; Liu, Y.F.; Shuai, X.X.; Li, J.Y.; Yin, L.Q.Q.; Wang, X.X.; et al. Single-cell profile reveals the landscape of cardiac immunity and identifies a cardio-protective Ym-1hi neutrophil in myocardial ischemia-reperfusion injury. Sci. Bull. 2024, 69, 949–967. [Google Scholar] [CrossRef]
- Zhang, F.; Xia, Y.; Su, J.; Quan, F.; Zhou, H.; Li, Q.; Feng, Q.; Lin, C.; Wang, D.; Jiang, Z. Neutrophil diversity and function in health and disease. Signal Transduct. Target. Ther. 2024, 9, 343. [Google Scholar] [CrossRef]
- Burn, G.L.; Foti, A.; Marsman, G.; Patel, D.F.; Zychlinsky, A. The Neutrophil. Immunity 2021, 54, 1377–1391. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Du, X.Y.; Ren, B.Y.; Li, C.; Li, Q.; Kan, S.; Wang, X.; Bai, W.J.; Wu, C.Y.; Kassegne, K.; Yan, H.B.; et al. PRL2 regulates neutrophil extracellular trap formation which contributes to severe malaria and acute lung injury. Nat. Commun. 2024, 15, 881. [Google Scholar] [CrossRef] [PubMed]
- Herre, M.; Cedervall, J.; Mackman, N.; Olsson, A.K. Neutrophil extracellular traps in the pathology of cancer and other inflammatory diseases. Physiol. Rev. 2023, 103, 277–312. [Google Scholar] [CrossRef]
- Guillotin, F.; Fortier, M.; Portes, M.; Demattei, C.; Mousty, E.; Nouvellon, E.; Mercier, E.; Chea, M.; Letouzey, V.; Gris, J.C.; et al. Vital NETosis vs. suicidal NETosis during normal pregnancy and preeclampsia. Front. Cell Dev. Biol. 2023, 10, 1099038. [Google Scholar] [CrossRef]
- Lavillegrand, J.R.; Al-Rifai, R.; Thietart, S.; Guyon, T.; Vandestienne, M.; Cohen, R.; Duval, V.; Zhong, X.; Yen, D.; Ozturk, M.; et al. Alternating high-fat diet enhances atherosclerosis by neutrophil reprogramming. Nature 2024, 634, 447–456. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, X.; Li, J.; Liu, C.; Li, W.; Zhao, J.; Li, Z.; Wang, N.; Wang, F.; Dong, J.; et al. Neutrophil extracellular traps mediated by platelet microvesicles promote thrombosis and brain injury in acute ischemic stroke. Cell Commun. Signal. 2024, 22, 50. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, S.; Tan, N.N.; Yan, Y.; Zhu, R.X.; Liu, J.J.; Mao, Q.; Wang, K.Y.; Zhang, W.H.; Wang, G.; et al. 13-methylpalmatine alleviates myocardial ischemia/reperfusion injury by potentially targeting the C5a-C5aR1 axis to inhibit neutrophil extracellular trap formation. Redox Biol. 2025, 86, 103802. [Google Scholar] [CrossRef]
- Wang, Y.M.; Li, M.; Stadler, S.; Correll, S.; Li, P.X.; Wang, D.C.; Hayama, R.; Leonelli, L.; Han, H.; Grigoryev, S.A.; et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 2009, 184, 205–213. [Google Scholar] [CrossRef]
- Li, P.X.; Li, M.; Lindberg, M.R.; Kennett, M.J.; Xiong, N.; Wang, Y.M. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010, 207, 1853–1862. [Google Scholar] [CrossRef]
- Arita, K.; Hashimoto, H.; Shimizu, T.; Nakashima, K.; Yamada, M.; Sato, M. Structural basis for Ca2+-induced activation of human PAD4. Nat. Struct. Mol. Biol. 2004, 11, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Hann, J.; Bueb, J.L.; Tolle, F.; Bréchard, S. Calcium signaling and regulation of neutrophil functions: Still a long way to go. J. Leukoc. Biol. 2020, 107, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Hong, W.Q.; Wan, M.H.; Zheng, L.M. Molecular mechanisms and therapeutic target of NETosis in diseases. MedComm 2022, 3, e162. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Gao, R.F.; Chen, H.C.; Hu, J.J.; Zhang, P.; Wei, X.; Shi, J.R.; Chen, Y.Y.; Zhang, L.W.; Chen, J.T.; et al. Myocardial reperfusion injury exacerbation due to ALDH2 deficiency is mediated by neutrophil extracellular traps and prevented by leukotriene C4 inhibition. Eur. Heart J. 2024, 45, 1662–1680. [Google Scholar] [CrossRef]
- Zhu, Y.F.P.P.; Speir, M.; Tan, Z.H.; Lee, J.C.; Nowell, C.J.; Chen, A.A.; Amatullah, H.; Salinger, A.J.; Huang, C.J.; Wu, G.; et al. NET formation is a default epigenetic program controlled by PAD4 in apoptotic neutrophils. Sci. Adv. 2023, 9, eadj1397. [Google Scholar] [CrossRef]
- Douda, D.N.; Khan, M.A.; Grasemann, H.; Palaniyar, N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. USA 2015, 112, 2817–2822. [Google Scholar] [CrossRef]
- D'Cruz, A.A.; Speir, M.; Bliss-Moreau, M.; Dietrich, S.; Wang, S.; Chen, A.A.; Gavillet, M.; Al-Obeidi, A.; Lawlor, K.E.; Vince, J.E.; et al. The pseudokinase MLKL activates PAD4-dependent NET formation in necroptotic neutrophils. Sci. Signal 2018, 11, eaao1716. [Google Scholar] [CrossRef]
- Chen, X.; He, W.T.; Hu, L.C.; Li, J.X.; Fang, Y.; Wang, X.; Xu, X.Z.; Wang, Z.; Huang, K.; Han, J.H. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016, 26, 1007–1020. [Google Scholar] [CrossRef]
- Gong, Y.N.; Guy, C.; Olauson, H.; Becker, J.U.; Yang, M.; Fitzgerald, P.; Linkermann, A.; Green, D.R. ESCRT-III Acts Downstream of MLKL to Regulate Necroptotic Cell Death and Its Consequences. Cell 2017, 169, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.W.; Monteleone, M.; Boucher, D.; Sollberger, G.; Ramnath, D.; Condon, N.D.; von Pein, J.B.; Broz, P.; Sweet, M.J.; Schroder, K. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci. Immunol. 2018, 3, eaar6676. [Google Scholar] [CrossRef] [PubMed]
- Sollberger, G.; Choidas, A.; Burn, G.L.; Habenberger, P.; Di Lucrezia, R.; Kordes, S.; Menninger, S.; Eickhoff, J.; Nussbaumer, P.; Klebl, B.; et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci. Immunol. 2018, 3, eaar6689. [Google Scholar] [CrossRef] [PubMed]
- Monteith, A.J.; Miller, J.M.; Beavers, W.N.; Maloney, K.N.; Seifert, E.L.; Hajnoczky, G.; Skaar, E.P. Mitochondrial Calcium Uniporter Affects Neutrophil Bactericidal Activity during Infection. Infect. Immun. 2022, 90, e00551-21. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef]
- Remijsen, Q.; Vanden Berghe, T.; Wirawan, E.; Asselbergh, B.; Parthoens, E.; De Rycke, R.; Noppen, S.; Delforge, M.; Willems, J.; Vandenabeele, P. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011, 21, 290–304. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef]
- Yipp, B.G.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.V.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.Y.; Meijndert, H.C.; et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking. Nat. Med. 2012, 18, 1386–1393. [Google Scholar] [CrossRef]
- Melbouci, D.; Ahmad, A.H.; Decker, P. Neutrophil extracellular traps (NET): Not only antimicrobial but also modulators of innate and adaptive immunities in inflammatory autoimmune diseases. RMD Open 2023, 9, e003104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2011, 7, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Wolach, O.; Sellar, R.S.; Martinod, K.; Cherpokova, D.; McConkey, M.; Chappell, R.J.; Silver, A.J.; Adams, D.; Castellano, C.A.; Schneider, R.K.; et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci. Transl. Med. 2018, 10, eaan8292. [Google Scholar] [CrossRef]
- van der Linden, M.; Westerlaken, G.H.A.; van der Vlist, M.; van Montfrans, J.; Meyaard, L. Differential Signalling and Kinetics of Neutrophil Extracellular Trap Release Revealed by Quantitative Live Imaging. Sci. Rep. 2017, 7, 6529. [Google Scholar] [CrossRef]
- DeSouza-Vieira, T.; Guimaraes-Costa, A.; Rochael, N.C.; Lira, M.N.; Nascimento, M.T.; Lima-Gomez, P.D.; Mariante, R.M.; Persechini, P.M.; Saraiva, E.M. Neutrophil extracellular traps release induced by Leishmania: Role of PI3Kγ, ERK, PI3Kσ, PKC, and [Ca2+]. J. Leukoc. Biol. 2016, 100, 801–810. [Google Scholar] [CrossRef]
- Douda, D.N.; Yip, L.; Khan, M.A.; Grasemann, H.; Palaniyar, N. Akt is essential to induce NADPH-dependent NETosis and to switch the neutrophil death to apoptosis. Blood 2014, 123, 597–600. [Google Scholar] [CrossRef]
- Lapponi, M.J.; Carestia, A.; Landoni, V.I.; Rivadeneyra, L.; Etulain, J.; Negrotto, S.; Pozner, R.G.; Schattner, M. Regulation of Neutrophil Extracellular Trap Formation by Anti-Inflammatory Drugs. J. Pharmacol. Exp. Ther. 2013, 345, 430–437. [Google Scholar] [CrossRef]
- Shi, G.H.; Liu, L.; Cao, Y.Y.; Ma, G.S.; Zhu, Y.L.; Xu, J.Y.; Zhang, X.; Li, T.; Mi, L.; Jia, H.R.; et al. Inhibition of neutrophil extracellular trap formation ameliorates neuroinflammation and neuronal apoptosis via STING-dependent IRE1α/ASK1/JNK signaling pathway in mice with traumatic brain injury. J. Neuroinflamm. 2023, 20, 222. [Google Scholar] [CrossRef]
- Wang, R.R.; Zhu, Y.B.; Liu, Z.W.; Chang, L.P.; Bai, X.F.; Kang, L.J.; Cao, Y.L.; Yang, X.; Yu, H.L.; Shi, M.J.; et al. Neutrophil extracellular traps promote tPA-induced brain hemorrhage via cGAS in mice with stroke. Blood 2021, 138, 91–103. [Google Scholar] [CrossRef]
- Yang, S.; Wang, S.; Chen, L.; Wang, Z.; Chen, J.; Ni, Q.; Guo, X.; Zhang, L.; Xue, G. Neutrophil Extracellular Traps Delay Diabetic Wound Healing by Inducing Endothelial-to-Mesenchymal Transition via the Hippo pathway. Int. J. Biol. Sci. 2023, 19, 347–361. [Google Scholar] [CrossRef]
- An, Z.; Li, J.; Yu, J.; Wang, X.; Gao, H.; Zhang, W.; Wei, Z.; Zhang, J.; Zhang, Y.; Zhao, J.; et al. Neutrophil extracellular traps induced by IL-8 aggravate atherosclerosis via activation NF-kappaB signaling in macrophages. Cell Cycle 2019, 18, 2928–2938. [Google Scholar] [CrossRef]
- Wilson, A.S.; Randall, K.L.; Pettitt, J.A.; Ellyard, J.I.; Blumenthal, A.; Enders, A.; Quah, B.J.; Bopp, T.; Parish, C.R.; Brüstle, A. Neutrophil extracellular traps and their histones promote Th17 cell differentiation directly via TLR2. Nat. Commun. 2022, 13, 528. [Google Scholar] [CrossRef]
- Taifour, T.; Attalla, S.S.; Zuo, D.M.; Gu, Y.; Sanguin-Gendreau, V.; Proud, H.; Solymoss, E.; Bui, T.; Kuasne, H.; Papavasiliou, V.; et al. The tumor-derived cytokine Chi3l1 induces neutrophil extracellular traps that promote T cell exclusion in triple-negative breast cancer. Immunity 2023, 56, 2755–2772. [Google Scholar] [CrossRef]
- Kaltenmeier, C.; Yazdani, H.O.; Morder, K.; Geller, D.A.; Simmons, R.L.; Tohme, S. Neutrophil Extracellular Traps Promote T Cell Exhaustion in the Tumor Microenvironment. Front. Immunol. 2021, 12, 785222. [Google Scholar] [CrossRef]
- Chen, W.; Chen, H.; Yang, Z.T.; Mao, E.Q.; Chen, Y.; Chen, E.Z. Free fatty acids-induced neutrophil extracellular traps lead to dendritic cells activation and T cell differentiation in acute lung injury. Aging 2021, 13, 26148–26160. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.J.; Wang, Y.; Brown, Z.J.; Xia, Y.J.; Huang, Z.; Shen, C.L.; Hu, Z.W.; Beane, J.; Ansa-Addo, E.A.; et al. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J. Hepatol. 2021, 75, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.Y.; Bai, Z.Y.; Yan, S.X.; Li, J.P. Collagen-induced DDR1 upregulates CXCL5 to promote neutrophil extracellular traps formation and Treg infiltration in breast cancer. Int. Immunopharmacol. 2023, 120, 110235. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Vasconcellos, A.; Marken, J.; Skopelja-Gardner, S.; Lood, C.; Giltiay, N.V. Immune complex-driven neutrophil activation and BAFF release: A link to B cell responses in SLE. Lupus Sci. Med. 2022, 9, e000709. [Google Scholar] [CrossRef]
- Corsiero, E.; Bombardieri, M.; Carlotti, E.; Pratesi, F.; Robinson, W.; Migliorini, P.; Pitzalis, C. Single cell cloning and recombinant monoclonal antibodies generation from RA synovial B cells reveal frequent targeting of citrullinated histones of NETs. Ann. Rheum. Dis. 2016, 75, 1866–1875. [Google Scholar] [CrossRef]
- Gestermann, N.; Di Domizio, J.; Lande, R.; Demaria, O.; Frasca, L.; Feldmeyer, L.; Di Lucca, J.; Gilliet, M. Netting Neutrophils Activate Autoreactive B Cells in Lupus. J. Immunol. 2018, 200, 3364–3371. [Google Scholar] [CrossRef]
- Fang, H.; Shao, S.; Xue, K.; Yuan, X.; Qiao, P.; Zhang, J.Y.; Cao, T.Y.; Luo, Y.X.; Bai, X.C.; Li, W.J.; et al. Neutrophil extracellular traps contribute to immune dysregulation in bullous pemphigoid via inducing B-cell differentiation and antibody production. Faseb J. 2021, 35, e21746. [Google Scholar] [CrossRef]
- Bertin, F.R.; Rys, R.N.; Mathieu, C.; Laurance, S.; Lemarié, C.A.; Blostein, M.D. Natural killer cells induce neutrophil extracellular trap formation in venous thrombosis. J. Thromb. Haemost. 2019, 17, 403–414. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Yin, Y.M.; Zhang, Y.Y.; Cao, Y.Y.; Lin, X.M.; Huang, L.H.; Hoffmann, D.; Lu, M.J.; Qiu, Y.W. Excessive Neutrophils and Neutrophil Extracellular Traps in COVID-19. Front. Immunol. 2020, 11, 2063. [Google Scholar] [CrossRef]
- Zhai, R.D.; Gong, Z.Z.; Wang, M.Q.; Ni, Z.H.; Zhang, J.Y.; Wang, M.Y.; Zhang, Y.; Zeng, F.R.; Gu, Z.Y.; Chen, X.Y.; et al. Neutrophil extracellular traps promote invasion and metastasis via NLRP3-mediated oral squamous cell carcinoma pyroptosis inhibition. Cell Death Discov. 2024, 10, 214. [Google Scholar] [CrossRef]
- Valayer, A.; Brea, D.; Lajoie, L.; Avezard, L.; Combes-Soia, L.; Labas, V.; Korkmaz, B.; Thibault, G.; Baranek, T.; Si-Tahar, M. Neutrophils can disarm NK cell response through cleavage of NKp46. J. Leukoc. Biol. 2017, 101, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Lazzaretto, B.; Fadeel, B. Intra- and Extracellular Degradation of Neutrophil Extracellular Traps by Macrophages and Dendritic Cells. J. Immunol. 2019, 203, 2276–2290. [Google Scholar] [CrossRef] [PubMed]
- Dudeck, J.; Kotrba, J.; Immler, R.; Hoffmann, A.; Voss, M.; Alexaki, V.I.; Morton, L.; Jahn, S.R.; Katsoulis-Dimitriou, K.; Winzer, S.; et al. Directional mast cell degranulation of tumor necrosis factor into blood vessels primes neutrophil extravasation. Immunity 2021, 54, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Pejler, G.; Alanazi, S.; Grujic, M.; Adler, J.; Olsson, A.K.; Sommerhoff, C.P.; Melo, F.R. Mast Cell Tryptase Potentiates Neutrophil Extracellular Trap Formation. J. Innate Immun. 2022, 14, 433–446. [Google Scholar] [CrossRef]
- Miralda, I.; Uriarte, S.M.; McLeish, K.R. Multiple Phenotypic Changes Define Neutrophil Priming. Front. Cell. Infect. Microbiol. 2017, 7, 217. [Google Scholar] [CrossRef]
- Dinallo, V.; Marafini, I.; Di Fusco, D.; Laudisi, F.; Franzè, E.; Di Grazia, A.; Figliuzzi, M.M.; Caprioli, F.; Stolfi, C.; Monteleone, I.; et al. Neutrophil Extracellular Traps Sustain Inflammatory Signals in Ulcerative Colitis. J. Crohns Colitis 2019, 13, 772–784. [Google Scholar] [CrossRef]
- Kuang, L.J.; Wu, Y.J.; Shu, J.X.; Yang, J.W.; Zhou, H.B.; Huang, X. Pyroptotic Macrophage-Derived Microvesicles Accelerate Formation of Neutrophil Extracellular Traps GSDMD-N-expressing Mitochondrial Transfer during Sepsis. Int. J. Biol. Sci. 2024, 20, 733–750. [Google Scholar] [CrossRef]
- Josefs, T.; Barrett, T.J.; Brown, E.J.; Quezada, A.; Wu, X.Y.; Voisin, M.; Amengual, J.; Fisher, E.A. Neutrophil extracellular traps promote macrophage inflammation and impair atherosclerosis resolution in diabetic mice. JCI Insight 2020, 5, e134796. [Google Scholar] [CrossRef]
- Wei, X.Q.; Zou, S.; Xie, Z.H.; Wang, Z.; Huang, N.Y.; Cen, Z.F.; Hao, Y.; Zhang, C.X.; Chen, Z.Y.; Zhao, F.L.; et al. EDIL3 deficiency ameliorates adverse cardiac remodelling by neutrophil extracellular traps (NET)-mediated macrophage polarization. Cardiovasc. Res. 2022, 118, 2179–2195. [Google Scholar] [CrossRef]
- Farrera, C.; Fadeel, B. Macrophage Clearance of Neutrophil Extracellular Traps Is a Silent Process. J. Immunol. 2013, 191, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Haider, P.; Kral-Pointner, J.B.; Mayer, J.; Richter, M.; Kaun, C.; Brostjan, C.; Eilenberg, W.; Fischer, M.B.; Speidl, W.S.; Hengstenberg, C.; et al. Neutrophil Extracellular Trap Degradation by Differently Polarized Macrophage Subsets. Arter. Throm Vas. 2020, 40, 2265–2278. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.B.; Mehrotra, P.; Arandjelovic, S.; Perrys, J.S.A.; Guo, Y.Z.; Morioka, S.; Barron, B.; Walk, S.F.; Ghesquière, B.; Lorenz, U.; et al. Metabolites released from apoptotic cells act as tissue messengers. Nature 2020, 580, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.M.; Ju, Y.H.; Dai, X.C.; Ni, N.; Liu, Y.; Zhang, D.D.; Gao, H.Q.; Sun, H.; Zhang, J.; Gu, P. HO-1-mediated ferroptosis as a target for protection against retinal pigment epithelium degeneration. Redox Biol. 2021, 43, 101971. [Google Scholar] [CrossRef]
- Teng, J.F.; Mei, Q.B.; Zhou, X.G.; Tang, Y.; Xiong, R.; Qiu, W.Q.; Pan, R.; Law, B.Y.K.; Wong, V.K.W.; Yu, C.L.; et al. Polyphyllin VI Induces Caspase-1-Mediated Pyroptosis via the Induction of ROS/NF-κB/NLRP3/GSDMD Signal Axis in Non-Small Cell Lung Cancer. Cancers 2020, 12, 193. [Google Scholar] [CrossRef]
- Sumida, Y.; Yoneda, M. Current and future pharmacological therapies for NAFLD/NASH. J. Gastroenterol. 2018, 53, 362–376. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.R.; Wang, X.Y.; Sun, N.; Gong, Y.H. Pathway network of pyroptosis and its potential inhibitors in acute kidney injury. Pharmacol. Res. 2022, 175, 106033. [Google Scholar] [CrossRef]
- Kung, Y.A.; Chiang, H.J.; Li, M.L.; Gong, Y.N.; Chiu, H.P.; Hung, C.T.; Huang, P.N.; Huang, S.Y.; Wang, P.Y.; Hsu, T.A.; et al. Acyl-Coenzyme A Synthetase Long-Chain Family Member 4 Is Involved in Viral Replication Organelle Formation and Facilitates Virus Replication via Ferroptosis. Mbio 2022, 13, e02717-21. [Google Scholar] [CrossRef]
- Rohn, T.T. The role of caspases in Alzheimer's disease; potential novel therapeutic opportunities. Apoptosis 2010, 15, 1403–1409. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Bredesen, D.E. Neural apoptosis. Ann. Neurol. 1995, 38, 839–851. [Google Scholar] [CrossRef]
- Walker, P.R.; Leblanc, J.; Smith, B.; Pandey, S.; Sikorska, A. Detection of DNA fragmentation and endonucleases in apoptosis. Methods 1999, 17, 329–338. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Chen, L.S.; Zhao, Y.F.; Lai, D.M.; Zhang, P.; Yang, Y.; Li, Y.H.; Fei, K.; Jiang, G.N.; Fan, J. Neutrophil extracellular traps promote macrophage pyroptosis in sepsis. Cell Death Dis. 2018, 9, 597. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Shi, M.M.; Liu, L.; Zuo, Y.; Jia, H.R.; Min, X.B.; Liu, X.L.; Chen, Z.J.; Zhou, Y.; Li, S.H.; et al. Inhibition of neutrophil extracellular trap formation attenuates NLRP1-dependent neuronal pyroptosis STING/IRE1α pathway after traumatic brain injury in mice. Front. Immunol. 2023, 14, 1125759. [Google Scholar] [CrossRef]
- Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar]
- Perdomo, J.; Leung, H.H.L.; Ahmadi, Z.; Yan, F.; Chong, J.J.H.; Passam, F.H.; Chong, B.H. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat. Commun. 2019, 10, 1322. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.P.; Mackman, N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arter. Throm Vas. 2018, 38, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Stakos, D.A.; Kambas, K.; Konstantinidis, T.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Tsironidou, V.; Giatromanolaki, A.; Skendros, P.; Konstantinides, S.; et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur. Heart J. 2015, 36, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- von Brühl, M.L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Köllnberger, M.; et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef]
- Guglietta, S.; Chiavelli, A.; Zagato, E.; Krieg, C.; Gandini, S.; Ravenda, P.S.; Bazolli, B.; Lu, B.; Penna, G.; Rescigno, M. Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. Nat. Commun. 2016, 7, 11037. [Google Scholar] [CrossRef]
- Ortiz-Espinosa, S.; Morales, X.; Senent, Y.; Alignani, D.; Tavira, B.; Macaya, I.; Ruiz, B.; Moreno, H.; Remírez, A.; Sainz, C.; et al. Complement C5a induces the formation of neutrophil extracellular traps by myeloid-derived suppressor cells to promote metastasis. Cancer Lett. 2022, 529, 70–84. [Google Scholar] [CrossRef]
- Yuen, J.; Pluthero, F.G.; Douda, D.N.; Riedl, M.; Cherry, A.; Ulanova, M.; Kahr, W.H.A.; Palaniyar, N.; Licht, C. NETosing Neutrophils Activate Complement Both on Their Own NETs and Bacteria Alternative and Non-alternative Pathways. Front. Immunol. 2016, 7, 137. [Google Scholar] [CrossRef]
- Carestia, A.; Kaufman, T.; Schattner, M. Platelets: New Bricks in the Building of Neutrophil Extracellular Traps. Front. Immunol. 2016, 7, 271. [Google Scholar] [CrossRef]
- Ma, A.C.; Kubes, P. Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J. Thromb. Haemost. 2008, 6, 415–420. [Google Scholar] [CrossRef]
- Maugeri, N.; Campana, L.; Gavina, M.; Covino, C.; De Metrio, M.; Panciroli, C.; Maiuri, L.; Maseri, A.; D'Angelo, A.; Bianchi, M.E.; et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 2014, 12, 2074–2088. [Google Scholar] [CrossRef]
- Chilingaryan, Z.; Deshmukh, T.; Leung, H.H.L.; Perdomo, J.; Emerson, P.; Kurup, R.; Chong, B.H.; Chong, J.J.H. Erythrocyte interaction with neutrophil extracellular traps in coronary artery thrombosis following myocardial infarction. Pathology 2022, 54, 87–94. [Google Scholar] [CrossRef]
- Megens, R.T.A.; Vijayan, S.; Lievens, D.; Döring, Y.; van Zandvoort, M.A.M.J.; Grommes, J.; Weber, C.; Soehnlein, O. Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb. Haemost. 2012, 107, 597–598. [Google Scholar] [CrossRef]
- Bonaventura, A.; Montecucco, F.; Dallegri, F.; Carbone, F.; Lüscher, T.F.; Camici, G.G.; Liberale, L. Novel findings in neutrophil biology and their impact on cardiovascular disease. Cardiovasc. Res. 2019, 115, 1266–1285. [Google Scholar] [CrossRef] [PubMed]
- Warnatsch, A.; Ioannou, M.; Wang, Q.; Papayannopoulos, V. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015, 349, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Baumer, Y.; Mehta, N.N.; Dey, A.K.; Powell-Wiley, T.M.; Boisvert, W.A. Cholesterol crystals and atherosclerosis. Eur. Heart J. 2020, 41, 2236–2239. [Google Scholar] [CrossRef] [PubMed]
- Tektonidou, M.G. Cardiovascular disease risk in antiphospholipid syndrome: Thrombo-inflammation and atherothrombosis. J. Autoimmun. 2022, 128, 102813. [Google Scholar] [CrossRef]
- Awasthi, D.; Nagarkoti, S.; Kumar, A.; Dubey, M.; Singh, A.K.; Pathak, P.; Chandra, T.; Barthwal, M.K.; Dikshit, M. Oxidized LDL induced extracellular trap formation in human neutrophils via TLR-PKC-IRAK-MAPK and NADPH-oxidase activation. Free Radic. Biol. Med. 2016, 93, 190–203. [Google Scholar] [CrossRef]
- Obama, T.; Ohinata, H.; Takaki, T.; Iwamoto, S.; Sawada, N.; Aiuchi, T.; Kato, R.; Itabe, H. Cooperative Action of Oxidized Low-Density Lipoproteins and Neutrophils on Endothelial Inflammatory Responses Through Neutrophil Extracellular Trap Formation. Front. Immunol. 2019, 10, 1899. [Google Scholar] [CrossRef]
- Han, H.; Liu, C.; Li, M.; Wang, J.; Liu, Y.S.; Zhou, Y.; Li, Z.C.; Hu, R.; Li, Z.H.; Wang, R.M.; et al. Increased intracellular Cl concentration mediates neutrophil extracellular traps formation in atherosclerotic cardiovascular diseases. Acta Pharmacol. Sin. 2022, 43, 2848–2861. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.K.; Vivekanandan-Giri, A.; Tang, C.; Knight, J.S.; Mathew, A.; Padilla, R.L.; Gillespie, B.W.; Carmona-Rivera, C.; Liu, X.; Subramanian, V.; et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: An additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. 2014, 66, 2532–2544. [Google Scholar] [CrossRef] [PubMed]
- Schumski, A.; Ortega-Gómez, A.; Wichapong, K.; Winter, C.; Lemnitzer, P.; Viola, J.R.; Pinilla-Vera, M.; Folco, E.; Solis-Mezarino, V.; Völker-Albert, M.; et al. Endotoxinemia Accelerates Atherosclerosis Through Electrostatic Charge-Mediated Monocyte Adhesion. Circulation 2021, 143, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Rupa-Matysek, J.; Urbanowicz, T. High-intensity statin therapy and its anti-inflammatory and anti-thrombogenic properties related to neutrophil extracellular trap formation. Pol. Arch. Intern. Med. 2024, 134, 16871. [Google Scholar] [CrossRef]
- Shao, L.; Wu, D.Y.; Zhang, P.; Li, W.Z.; Wang, J.; Su, G.H.; Liao, Y.H.; Wang, Z.H.; Liu, K. The Significance of Microthrombosis and fgl2 in No-Reflow Phenomenon of Rats with Acute Myocardial Ischemia/Reperfusion. Clin. Appl. Thromb.-Hem. 2013, 19, 19–28. [Google Scholar] [CrossRef]
- Chrysanthopoulou, A.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Mikroulis, D.; Konstantinidis, T.; Sivridis, E.; Koffa, M.; Giatromanolaki, A.; Boumpas, D.T.; et al. Neutrophil extracellular traps promote differentiation and function of fibroblasts. J. Pathol. 2014, 233, 294–307. [Google Scholar] [CrossRef]
- Liu, J.; Yang, D.D.; Wang, X.Q.; Zhu, Z.H.; Wang, T.Z.; Ma, A.Q.; Liu, P. Neutrophil extracellular traps and dsDNA predict outcomes among patients with ST-elevation myocardial infarction. Sci. Rep. 2019, 9, 11599. [Google Scholar] [CrossRef]
- Hofbauer, T.M.; Mangold, A.; Scherz, T.; Seidl, V.; Panzenböck, A.; Ondracek, A.S.; Müller, J.; Schneider, M.; Binder, T.; Hell, L.; et al. Neutrophil extracellular traps and fibrocytes in ST-segment elevation myocardial infarction. Basic Res. Cardiol. 2019, 114, 33. [Google Scholar] [CrossRef]
- Du, M.J.; Yang, W.G.; Schmull, S.; Gu, J.M.; Xue, S. Inhibition of peptidyl arginine deiminase-4 protects against myocardial infarction induced cardiac dysfunction. Int. Immunopharmacol. 2020, 78, 106055. [Google Scholar] [CrossRef]
- Eghbalzadeh, K.; Georgi, L.; Louis, T.; Zhao, H.; Keser, U.; Weber, C.; Mollenhauer, M.; Conforti, A.; Wahlers, T.; Paunel-Gorgulu, A. Compromised Anti-inflammatory Action of Neutrophil Extracellular Traps in PAD4-Deficient Mice Contributes to Aggravated Acute Inflammation After Myocardial Infarction. Front. Immunol. 2019, 10, 2313. [Google Scholar] [CrossRef]
- Cai, W.B.; Liu, L.; Shi, X.L.; Liu, Y.A.; Wang, J.; Fang, X.; Chen, Z.P.; Ai, D.; Zhu, Y.; Zhang, X. Alox15/15-HpETE Aggravates Myocardial Ischemia-Reperfusion Injury by Promoting Cardiomyocyte Ferroptosis. Circulation 2023, 147, 1444–1460. [Google Scholar] [CrossRef]
- Ge, L.; Zhou, X.; Ji, W.J.; Lu, R.Y.; Zhang, Y.; Zhang, Y.D.; Ma, Y.Q.; Zhao, J.H.; Li, Y.M. Neutrophil extracellular traps in ischemia-reperfusion injury-induced myocardial no-reflow: Therapeutic potential of DNase-based reperfusion strategy. Am. J. Physiol.-Heart C 2015, 308, H500–H509. [Google Scholar] [CrossRef]
- Pashevin, D.O.; Nagibin, V.S.; Tumanovska, L.V.; Moibenko, A.A.; Dosenko, V.E. Proteasome Inhibition Diminishes the Formation of Neutrophil Extracellular Traps and Prevents the Death of Cardiomyocytes in Coculture with Activated Neutrophils during Anoxia-Reoxygenation. Pathobiology 2015, 82, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Silk, E.; Zhao, H.L.; Weng, H.; Ma, D.Q. The role of extracellular histone in organ injury. Cell Death Dis. 2017, 8, e2812. [Google Scholar] [CrossRef]
- Fousert, E.; Toes, R.; Desai, J. Neutrophil Extracellular Traps (NETs) Take the Central Stage in Driving Autoimmune Responses. Cells 2020, 9, 915. [Google Scholar] [CrossRef]
- Baumann Kreuziger, L.; Slaughter, M.S.; Sundareswaran, K.; Mast, A.E. Clinical Relevance of Histopathologic Analysis of HeartMate II Thrombi. ASAIO J. 2018, 64, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Tanai, E.; Frantz, S. Pathophysiology of Heart Failure. Compr. Physiol. 2015, 6, 187–214. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, T.; Olasinska-Wisniewska, A.; Wojtasinska, E.; Filipiak, K.J.; Tomaszewska, M.; Sikora, J.; Krama, M.; Radek, Z.; Grodecki, K.; Krasinska-Plachta, A.; et al. Neutrophil Extracellular Trap Formation in Advanced Heart Failure Patients-Preliminary Report. Int. J. Mol. Sci. 2024, 25, 9633. [Google Scholar] [CrossRef]
- Patel, S.; Raman, V.K.; Faselis, C.; Fonarow, G.C.; Lam, P.H.; Ahmed, A.A.; Heidenreich, P.A.; Anker, S.D.; Deedwania, P.; Morgan, C.J.; et al. Outcomes of KDIGO-Defined CKD in U.S. Veterans with HFpEF, HFmrEF, and HFrEF. JACC Heart Fail. 2025, 13, 467–479. [Google Scholar] [CrossRef]
- Dumont, B.L.; Neagoe, P.E.; Charles, E.; Villeneuve, L.; Ninni, S.; Tardif, J.C.; Rakel, A.; White, M.; Sirois, M.G. Low-Density Neutrophils and Neutrophil Extracellular Traps (NETs) Are New Inflammatory Players in Heart Failure. Can. J. Cardiol. 2024, 40, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Hage, C.; Michaelsson, E.; Kull, B.; Miliotis, T.; Svedlund, S.; Linde, C.; Donal, E.; Daubert, J.C.; Gan, L.M.; Lund, L.H. Myeloperoxidase and related biomarkers are suggestive footprints of endothelial microvascular inflammation in HFpEF patients. ESC Heart Fail. 2020, 7, 1534–1546. [Google Scholar] [CrossRef] [PubMed]
- van Essen, B.J.; Tromp, J.; Gevaert, A.B.; De Jong, T.V.; Ouwerkerk, W.; Koekemoer, A.; Djordjevic, D.; Baumhove, L.; Tharshana, G.N.; Conde-Knape, K.; et al. Activation of Neutrophil Extracellular Trap Formation in Patients with Heart Failure and a Preserved Ejection Fraction. J. Card. Fail. 2025. [Google Scholar] [CrossRef] [PubMed]
- Bratseth, V.; Nendl, A.; Raju, S.C.; Holm, K.; Broch, K.; Hov, J.R.; Seljeflot, I.; Troseid, M.; Awoyemi, A. Gut dysbiosis and neutrophil extracellular traps in chronic heart failure. Int. J. Cardiol. 2025, 419, 132689. [Google Scholar] [CrossRef]
- Tang, W.H.; Tong, W.; Troughton, R.W.; Martin, M.G.; Shrestha, K.; Borowski, A.; Jasper, S.; Hazen, S.L.; Klein, A.L. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J. Am. Coll. Cardiol. 2007, 49, 2364–2370. [Google Scholar] [CrossRef]
- Ichimura, S.; Misaka, T.; Ogawara, R.; Tomita, Y.; Anzai, F.; Sato, Y.; Miura, S.; Yokokawa, T.; Sato, T.; Oikawa, M.; et al. Neutrophil Extracellular Traps in Myocardial Tissue Drive Cardiac Dysfunction and Adverse Outcomes in Patients with Heart Failure with Dilated Cardiomyopathy. Circ. Heart Fail. 2024, 17, e011057. [Google Scholar] [CrossRef]
- Li, X.; Xu, C.; Li, Q.; Shen, Q.; Zeng, L. Exploring key genes associated with neutrophil function and neutrophil extracellular traps in heart failure: A comprehensive analysis of single-cell and bulk sequencing data. Front. Cell Dev. Biol. 2023, 11, 1258959. [Google Scholar] [CrossRef]
- Mang, G.; Chen, J.F.; Sun, P.; Ma, R.S.; Du, J.W.; Wang, X.Q.; Cui, J.X.; Yang, M.; Tong, Z.H.; Yan, X.Y.; et al. Von Willebrand factor exacerbates heart failure through formation of neutrophil extracellular traps. Eur. Heart J. 2024, 45, 3853–3867. [Google Scholar] [CrossRef]
- Zhao, M.; Zheng, Z.; Yin, Z.; Zhang, J.; Peng, S.; Liu, J.; Pan, W.; Wei, C.; Xu, Y.; Qin, J.J.; et al. DEL-1 deficiency aggravates pressure overload-induced heart failure by promoting neutrophil infiltration and neutrophil extracellular traps formation. Biochem. Pharmacol. 2023, 218, 115912. [Google Scholar] [CrossRef]
- Mahabeleshwar, G.H.; Kawanami, D.; Sharma, N.; Takami, Y.; Zhou, G.; Shi, H.; Nayak, L.; Jeyaraj, D.; Grealy, R.; White, M.; et al. The myeloid transcription factor KLF2 regulates the host response to polymicrobial infection and endotoxic shock. Immunity 2011, 34, 715–728. [Google Scholar] [CrossRef]
- Tang, X.; Wang, P.; Zhang, R.; Watanabe, I.; Chang, E.; Vinayachandran, V.; Nayak, L.; Lapping, S.; Liao, S.; Madera, A.; et al. KLF2 regulates neutrophil activation and thrombosis in cardiac hypertrophy and heart failure progression. J. Clin. Investig. 2022, 132, e147191. [Google Scholar] [CrossRef]
- Urbanowicz, T.; Wojtasinska, E.; Olasinska-Wisniewska, A.; Filipiak, K.J.; Ladzinska, M.; Sikora, J.; Straburzynska-Migaj, E.; Tykarski, A.; Jemielity, M.; Rupa-Matysek, J. Neutrophil to extracellular traps (NETs) as an early marker of right ventricular dilatation in patients with left ventricular assist devices (LVAD). Pol. Heart J. 2024, 82, 777–779. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Krumbholz, M.; Schönermarck, U.; Back, W.; Gross, W.L.; Werb, Z.; Gröne, H.J.; Brinkmann, V.; Jenne, D.E. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 2009, 15, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.; Rousselle, A.; Becker, J.U.; von Mässenhausen, A.; Linkermann, A.; Kettritz, R. Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc. Natl. Acad. Sci. USA 2017, 114, E9618–E9625. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Rivera, C.; Khaznadar, S.S.; Shwin, K.W.; Irizarry-Caro, J.A.; O'Neil, L.J.; Liu, Y.D.; Jacobson, K.A.; Ombrello, A.K.; Stone, D.L.; Tsai, W.X.L.; et al. Deficiency of adenosine deaminase 2 triggers adenosine-mediated NETosis and TNF production in patients with DADA2. Blood 2019, 134, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Weckbach, L.T.; Grabmaier, U.; Uhl, A.; Gess, S.; Boehm, F.; Zehrer, A.; Pick, R.; Salvermoser, M.; Czermak, T.; Pircher, J.; et al. Midkine drives cardiac inflammation by promoting neutrophil trafficking and NETosis in myocarditis. J. Exp. Med. 2019, 216, 350–368. [Google Scholar] [CrossRef]
- Yalavarthi, S.; Gould, T.J.; Rao, A.N.; Mazza, L.F.; Morris, A.E.; Núñez-Alvarez, C.; Hernández-Ramírez, D.; Bockenstedt, P.L.; Liaw, P.C.; Cabral, A.R.; et al. Release of Neutrophil Extracellular Traps by Neutrophils Stimulated with Antiphospholipid Antibodies: A Newly Identified Mechanism of Thrombosis in the Antiphospholipid Syndrome. Arthritis Rheumatol. 2015, 67, 2990–3003. [Google Scholar] [CrossRef]
- Meng, H.; Yalavarthi, S.; Kanthi, Y.; Mazza, L.F.; Elfline, M.A.; Luke, C.E.; Pinsky, D.J.; Henke, P.K.; Knight, J.S. In Vivo Role of Neutrophil Extracellular Traps in Antiphospholipid Antibody-Mediated Venous Thrombosis. Arthritis Rheumatol. 2017, 69, 655–667. [Google Scholar] [CrossRef]
- Ali, R.A.; Estes, S.K.; Gandhi, A.A.; Yalavarthi, S.; Hoy, C.K.; Shi, H.; Zuo, Y.; Erkan, D.; Knight, J.S. Defibrotide Inhibits Antiphospholipid Antibody-Mediated Neutrophil Extracellular Trap Formation and Venous Thrombosis. Arthritis Rheumatol. 2022, 74, 902–907. [Google Scholar] [CrossRef]
- Smith, C.K.; Kaplan, M.J. The role of neutrophils in the pathogenesis of systemic lupus erythematosus. Curr. Opin. Rheumatol. 2015, 27, 448–453. [Google Scholar] [CrossRef]
- Dieker, J.; Tel, J.; Pieterse, E.; Thielen, A.; Rother, N.; Bakker, M.; Fransen, J.; Dijkman, H.B.P.M.; Berden, J.H.; de Vries, J.M.; et al. Circulating Apoptotic Microparticles in Systemic Lupus Erythematosus Patients Drive the Activation of Dendritic Cell Subsets and Prime Neutrophils for NETosis. Arthritis Rheumatol. 2016, 68, 462–472. [Google Scholar] [CrossRef]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 2013, 5, 178ra140. [Google Scholar] [CrossRef]
- Nygaard, G.; Firestein, G.S. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 2020, 16, 316–333. [Google Scholar] [CrossRef] [PubMed]
- Eilenberg, W.; Zagrapan, B.; Bleichert, S.; Ibrahim, N.; Knöbl, V.; Brandau, A.; Martelanz, L.; Grasl, M.T.; Hayden, H.; Nawrozi, P.; et al. Histone citrullination as a novel biomarker and target to inhibit progression of abdominal aortic aneurysms. Transl. Res. 2021, 233, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.M.; Zhou, H.F.; Akk, A.; Hu, Y.; Springer, L.E.; Ennis, T.L.; Pham, C.T.N. Neutrophil Proteases Promote Experimental Abdominal Aortic Aneurysm via Extracellular Trap Release and Plasmacytoid Dendritic Cell Activation. Arter. Throm Vas. 2016, 36, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Meher, A.K.; Spinosa, M.; Davis, J.P.; Pope, N.; Laubach, V.E.; Su, G.; Serbulea, V.; Leitinger, N.; Ailawadi, G.; Upchurch, G.R. Novel Role of IL (Interleukin)-1β in Neutrophil Extracellular Trap Formation and Abdominal Aortic Aneurysms. Arter. Throm Vas. 2018, 38, 843–853. [Google Scholar] [CrossRef]
- Brandau, A.; Ibrahim, N.; Klopf, J.; Hayden, H.; Ozsvar-Kozma, M.; Afonyushkin, T.; Bleichert, S.; Fuchs, L.; Watzinger, V.; Nairz, V.; et al. Association of Lipoproteins with Neutrophil Extracellular Traps in Patients with Abdominal Aortic Aneurysm. Biomedicines 2022, 10, 217. [Google Scholar] [CrossRef]
- Wei, M.; Wang, X.; Song, Y.; Zhu, D.; Qi, D.; Jiao, S.; Xie, G.; Liu, Y.; Yu, B.; Du, J.; et al. Inhibition of Peptidyl Arginine Deiminase 4-Dependent Neutrophil Extracellular Trap Formation Reduces Angiotensin II-Induced Abdominal Aortic Aneurysm Rupture in Mice. Front. Cardiovasc. Med. 2021, 8, 676612. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.T.; Wang, Z.Y.; Zhang, L.Y.; Xu, Y.; Li, Y.N.; Zhang, L.; Wang, G.M.; Yang, S.F.; Xue, G.H. Mesenchymal stem cell-derived extracellular vesicles protect against abdominal aortic aneurysm formation by inhibiting NET-induced ferroptosis. Exp. Mol. Med. 2023, 55, 939–951. [Google Scholar] [CrossRef]
- Yang, S.F.; Chen, L.; Wang, Z.Y.; Chen, J.Q.; Ni, Q.H.; Guo, X.J.; Liu, W.F.; Lv, L.; Xue, G.H. Neutrophil extracellular traps induce abdominal aortic aneurysm formation by promoting the synthetic and proinflammatory smooth muscle cell phenotype via Hippo-YAP pathway. Transl. Res. 2023, 255, 85–96. [Google Scholar] [CrossRef]
- Spinosa, M.; Su, G.; Salmon, M.D.; Lu, G.Y.; Cullen, J.M.; Fashandi, A.Z.; Hawkins, R.B.; Montgomery, W.; Meher, A.K.; Conte, M.S.; et al. Resolvin D1 decreases abdominal aortic aneurysm formation by inhibiting NETosis in a mouse model. J. Vasc. Surg. 2018, 68, 93s–103s. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, S.L.; Tang, Q.Y.; Zhou, X.; Zhang, J.Q.; He, Z.Y.; Bai, J.; Li, M.H.; Deng, J.M.; Liang, Y.; et al. Erythromycin suppresses neutrophil extracellular traps in smoking-related chronic pulmonary inflammation. Cell Death Dis. 2019, 10, 678. [Google Scholar] [CrossRef]
- Kraaij, T.; Kamerling, S.W.A.; de Rooij, E.N.M.; van Daele, P.L.A.; Bredewold, O.W.; Bakker, J.A.; Bajema, I.M.; Scherer, H.U.; Toes, R.E.M.; Huizinga, T.J.W.; et al. The NET-effect of combining rituximab with belimumab in severe systemic lupus erythematosus. J. Autoimmun. 2018, 91, 45–54. [Google Scholar] [CrossRef]
- Pieterse, E.; Rother, N.; Garsen, M.; Hofstra, J.M.; Satchell, S.C.; Hoffmann, M.; Loeven, M.A.; Knaapen, H.K.; van der Heijden, O.W.H.; Berden, J.H.M.; et al. Neutrophil Extracellular Traps Drive Endothelial-to-Mesenchymal Transition. Arter. Throm Vas. 2017, 37, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Mengozzi, L.; Barison, I.; Maly, M.; Lorenzoni, G.; Fedrigo, M.; Castellani, C.; Gregori, D.; Maly, P.; Matej, R.; Tousek, P.; et al. Neutrophil Extracellular Traps and Thrombolysis Resistance: New Insights for Targeting Therapies. Stroke 2024, 55, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Eilenberg, W.; Neumayer, C.; Brostjan, C. Neutrophil Extracellular Traps in Cardiovascular and Aortic Disease: A Narrative Review on Molecular Mechanisms and Therapeutic Targeting. Int. J. Mol. Sci. 2024, 25, 3983. [Google Scholar] [CrossRef] [PubMed]
- Hayden, H.; Ibrahim, N.; Klopf, J.; Zagrapan, B.; Mauracher, L.M.; Hell, L.; Hofbauer, T.M.; Ondracek, A.S.; Schoergenhofer, C.; Jilma, B.; et al. ELISA detection of MPO-DNA complexes in human plasma is error-prone and yields limited information on neutrophil extracellular traps formed. PLoS ONE 2021, 16, e0250265. [Google Scholar] [CrossRef]
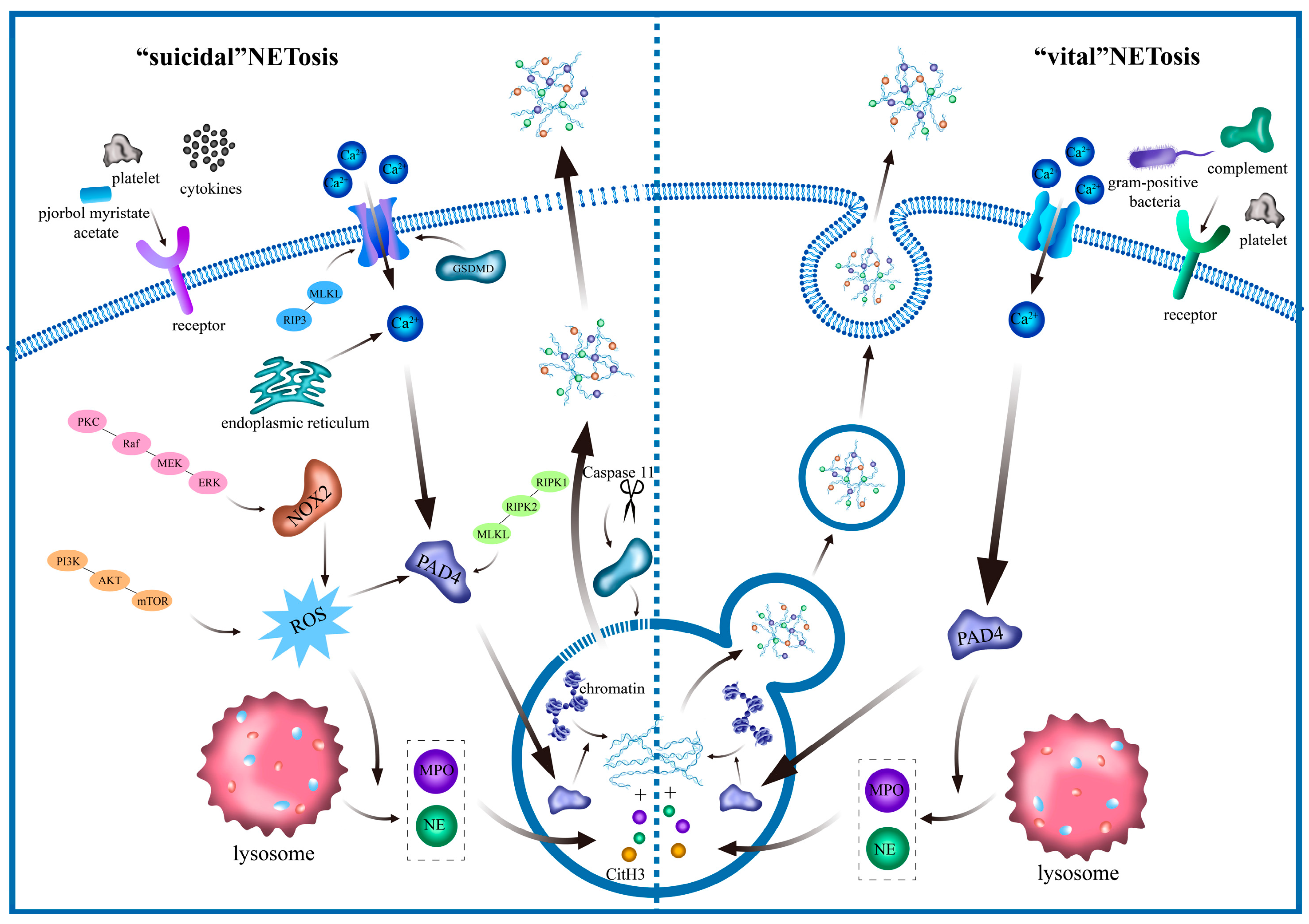
| Type | Morphological Features | Biochemical Features | Biomarker | Pharmacological Modulators | Regulator | Ref. |
|---|---|---|---|---|---|---|
| NETosis | The plasma membrane ruptures; NET with reticulate structure are released into the extracellular space | Intracellular calcium concentration; ROS production; Deconstruction of chromatin; Rupture of plasma membrane (suicide NETosis) | CitH3, MPO, NE, PAD4 | activator: PMA inhibitor: Cl-amidine | positive: IL-8, LPS, activated platelets, TNF-α negative: metabolites released from apoptotic cells act as tissue | [39,42] |
| Apoptosis | Membrane blebbing; Decreased cell size; Formation of apoptotic body; Maintain integrity of organelle | Fragmentation of DNA; Activation of caspase cascade; Release of cytochrome c from mitochondria; Phosphatidylserine is everted | caspase-3, Bcl2, Bax, PARP | activator: Dexamethasone inhibitor: Emricasan, Z-VAD-FMK | positive: p53, Bax, TGF-β negative: Bcl-2, Bcd-XL, IL-4 | [67,70,71,74] |
| Ferroptosis | Shrinking of mitochondria with compact membrane; Fewer mitochondrial ridges and outer mitochondrial membrane rupture | Depletion of glutathione; Lipid peroxidation; Increase in ROS | PTGS2, GPX4, ATG, ACSL4 | activator: Sorafenib, Erastin inhibitor: Ferrostatin-1, Liproxstatin-1, Troglitazone, Rosiglitazone, Pioglitazone | positive: RSL3, RAS, p53 negative: GPX4, SLC7A11, FSP1, NRF2, DFO | [68,72,75] |
| Pyroptosis | Cells are swollen; Formation of ballooning bubbles; Perforation of cell membrane | Formation of inflammasomes; Activation of Caspase1 and GSDMD; Intense inflammation | caspase-1, caspase-4/5/11, GSDMD, IL-1β | activator: Polyphyllin VI inhibitor: Z-VAD-FMK, Q-VD-Oph | positive: GSDMD, NLRP3, Caspase-1, Caspase-11 negative: - | [69,71,73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Zhang, J.; Qi, Y.; Lu, Y.; Dong, Y.; Hu, D. Neutrophil Extracellular Traps in Cardiovascular Diseases: Pathological Roles and Therapeutic Implications. Biomolecules 2025, 15, 1263. https://doi.org/10.3390/biom15091263
Ma Y, Zhang J, Qi Y, Lu Y, Dong Y, Hu D. Neutrophil Extracellular Traps in Cardiovascular Diseases: Pathological Roles and Therapeutic Implications. Biomolecules. 2025; 15(9):1263. https://doi.org/10.3390/biom15091263
Chicago/Turabian StyleMa, Yan, Jun Zhang, Yaxuan Qi, Yating Lu, Yalan Dong, and Desheng Hu. 2025. "Neutrophil Extracellular Traps in Cardiovascular Diseases: Pathological Roles and Therapeutic Implications" Biomolecules 15, no. 9: 1263. https://doi.org/10.3390/biom15091263
APA StyleMa, Y., Zhang, J., Qi, Y., Lu, Y., Dong, Y., & Hu, D. (2025). Neutrophil Extracellular Traps in Cardiovascular Diseases: Pathological Roles and Therapeutic Implications. Biomolecules, 15(9), 1263. https://doi.org/10.3390/biom15091263






