Neuromuscular Defects in a Drosophila Model of the Congenital Disorder of Glycosylation SLC35A2-CDG
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey of Pathogenic Variants of SLC35A2 in Patients with Undiagnosed Disease
2.2. Fly Stocks and Mutant Generation
2.3. Real-Time PCR Analysis
2.4. Western Blot and Lectin Blot Analyses
2.5. Preparation of Tissue Lysates for Glycan Analysis
2.6. O-Glycan Preparation by Evaporative β-elimination with Pyrazolone
2.7. N-Glycan Preparation by Glycoblotting
2.8. Glycan Analysis by MALDI-TOF/TOF Mass Spectrometry
2.9. Immunostaining and Lectin Staining
2.10. Generation of the Rescue Construct
2.11. Statistical Analysis
3. Results
3.1. Neurological Abnormalities in a Patient with a Pathogenic Variant of the SLC35A2 Gene
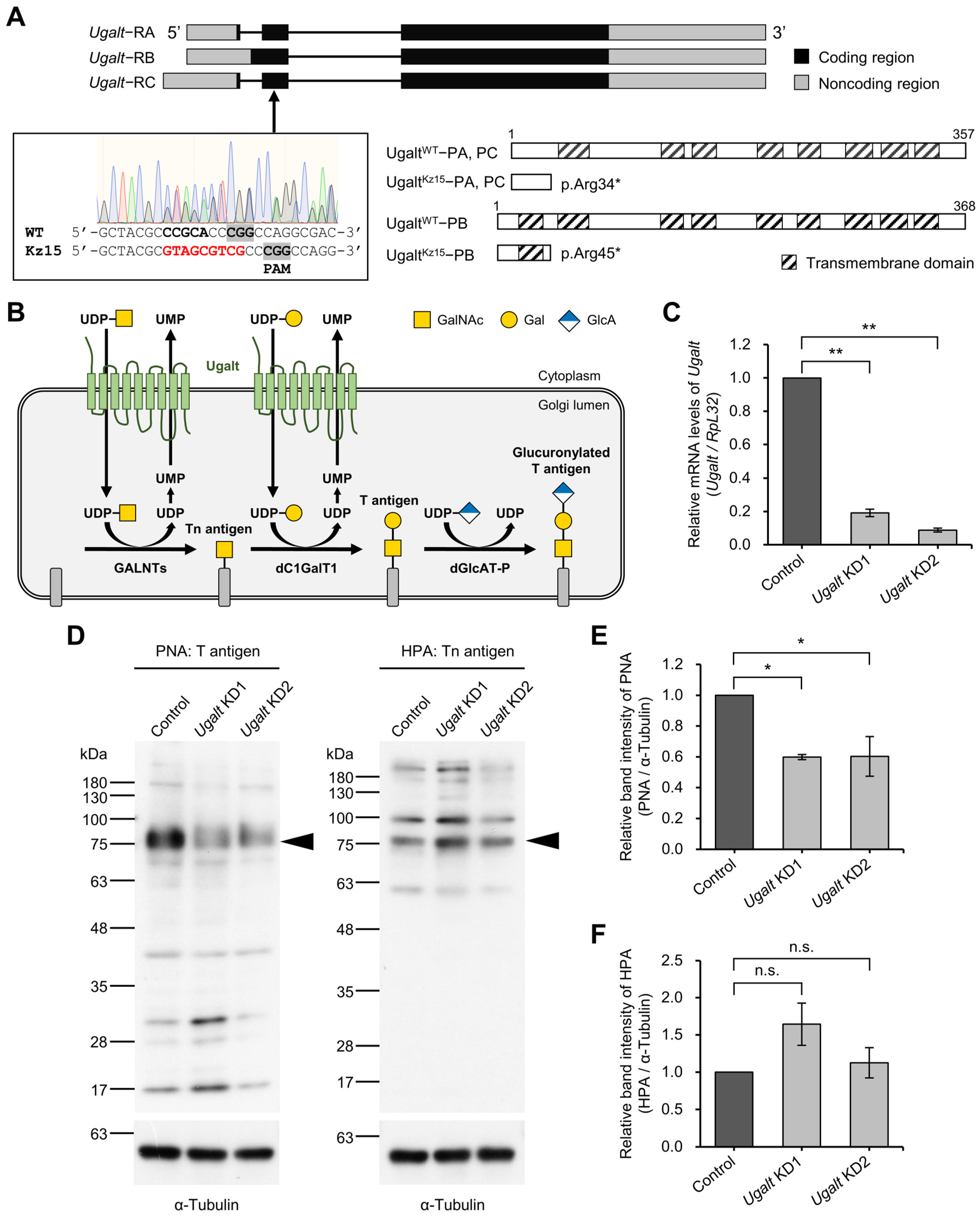
3.2. Drosophila Ortholog Ugalt Is Essential for Viability
3.3. Ugalt Is Required for the Synthesis of Mucin-Type O-Glycans
3.4. Ugalt Deficiency Selectively Reduces Mucin-Type O-Glycans But Not N-Glycans
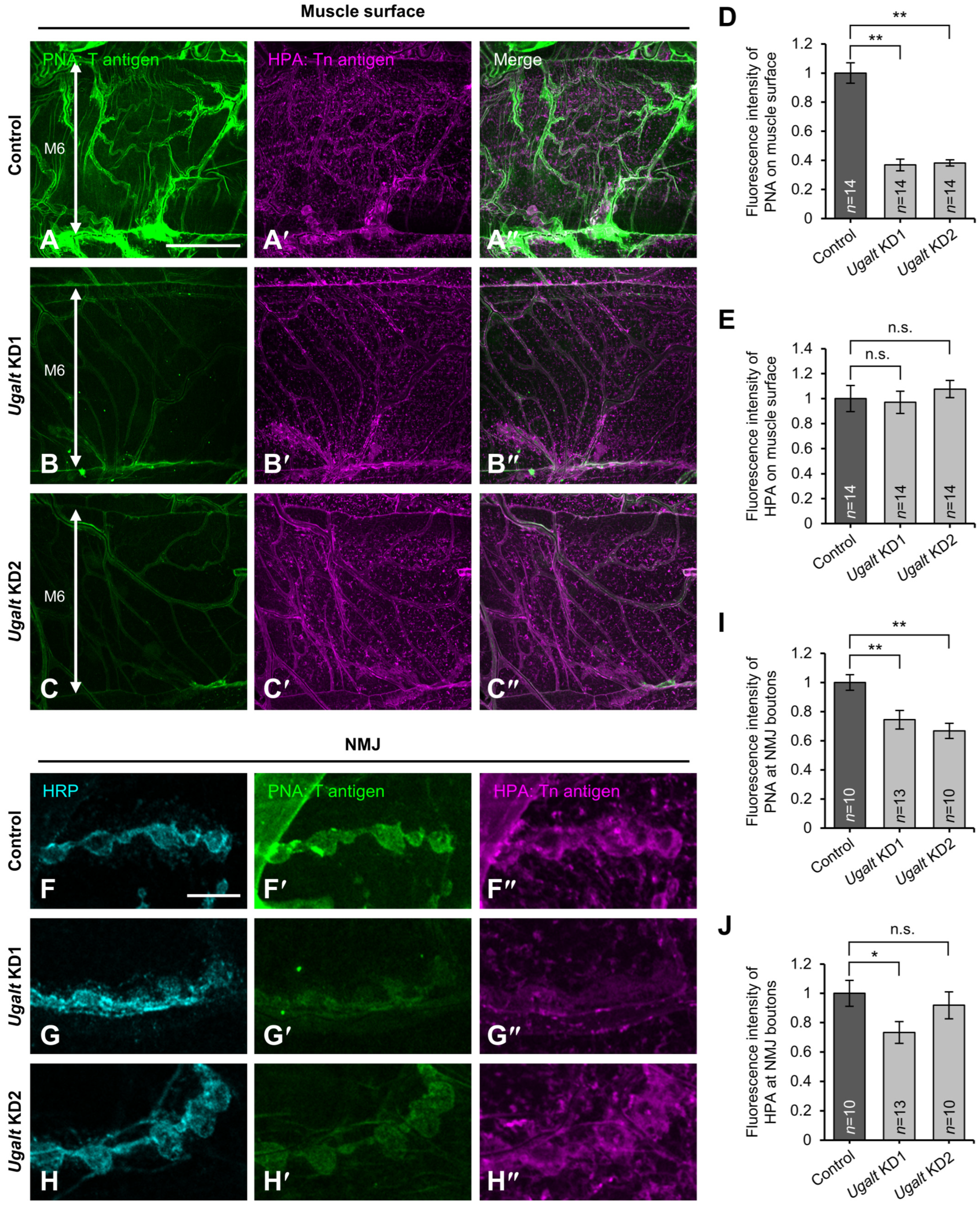
3.5. Loss of Ugalt Leads to Reduced T antigens on the Surfaces of Muscles and NMJs
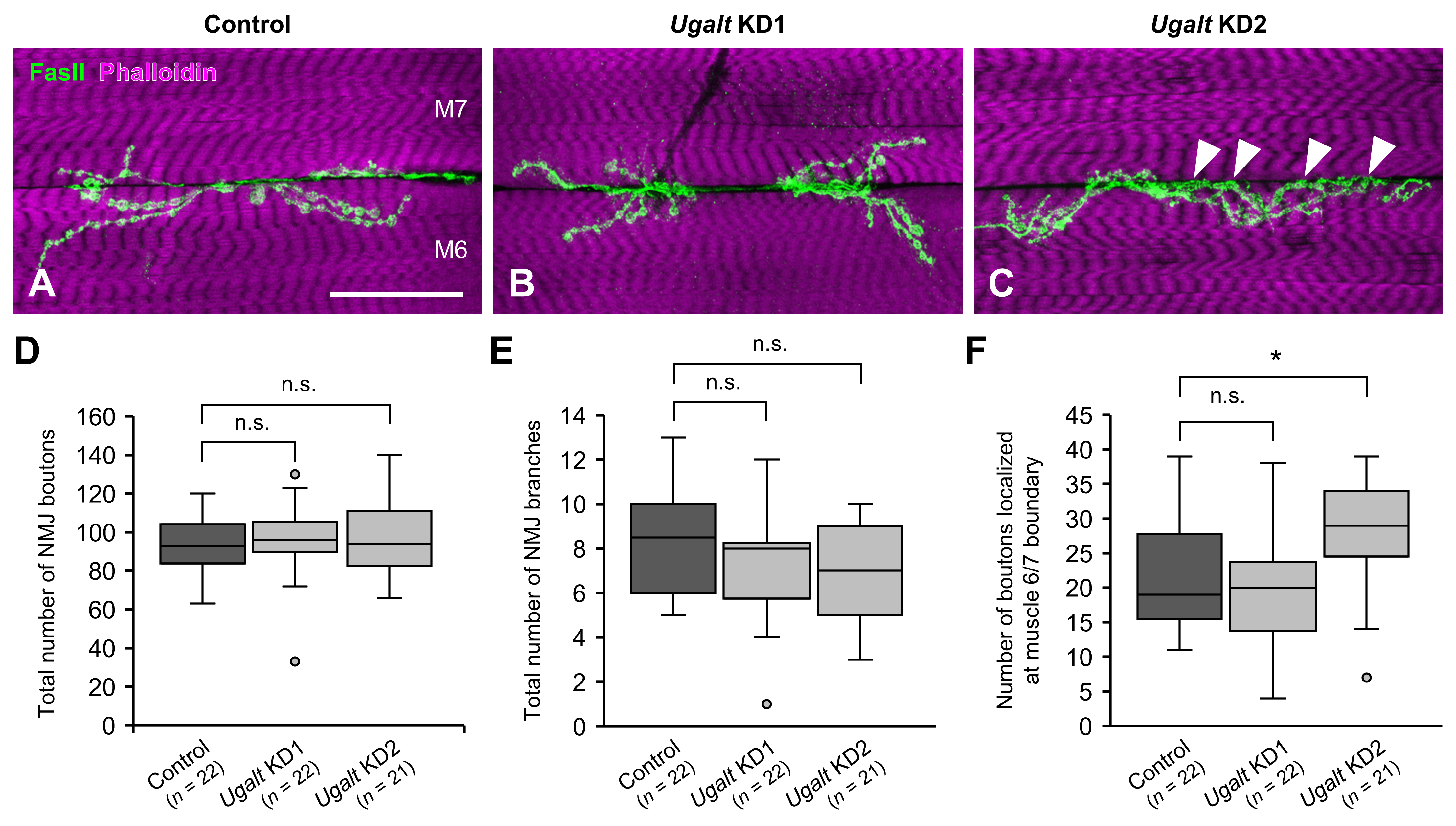
3.6. Mislocalization of NMJ Boutons at the Muscle 6/7 Boundary in Ugalt KD Larvae
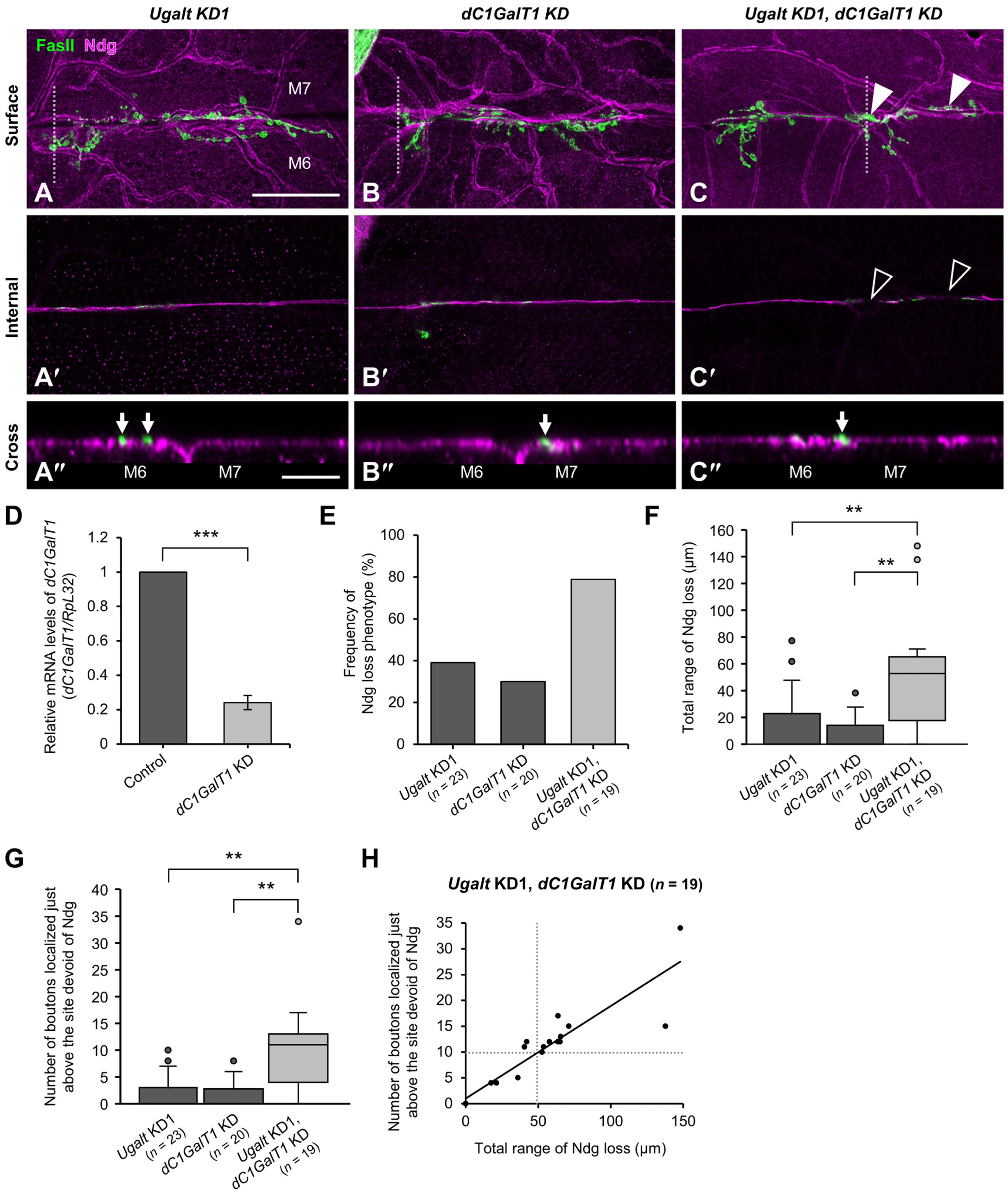
3.7. Loss of BM Components Underneath the Mislocalized NMJ Boutons in Ugalt KD Larvae
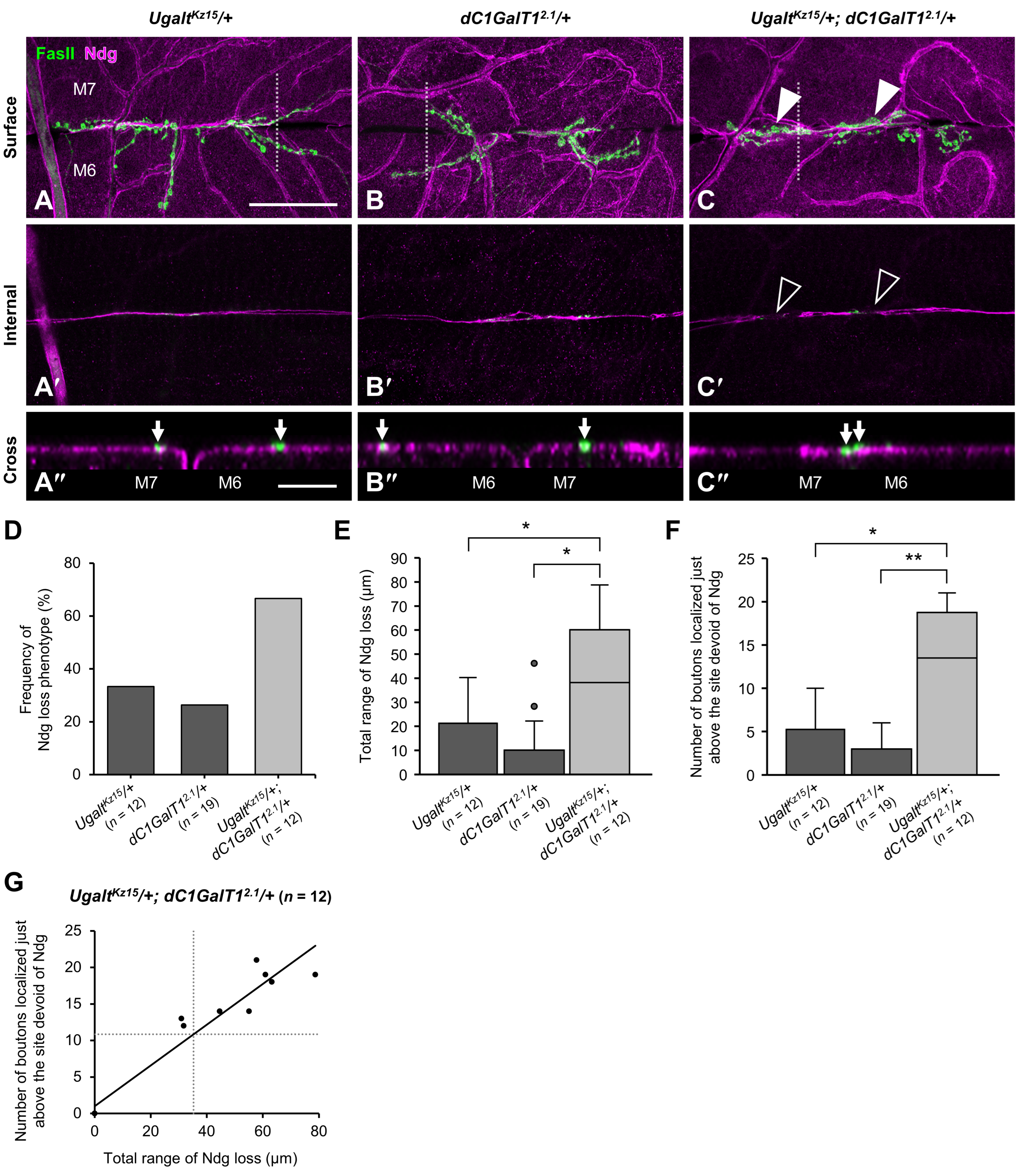
3.8. Ugalt Genetically Interacts with dC1GalT1
4. Discussion
4.1. Drosophila Model Unveils the Neurological Basis of SLC35A2-CDG
4.2. Neurological Features in a Newly Identified Case of SLC35A2-CDG
4.3. Essential Roles of SLC35A2 and Ugalt in Organismal Viability
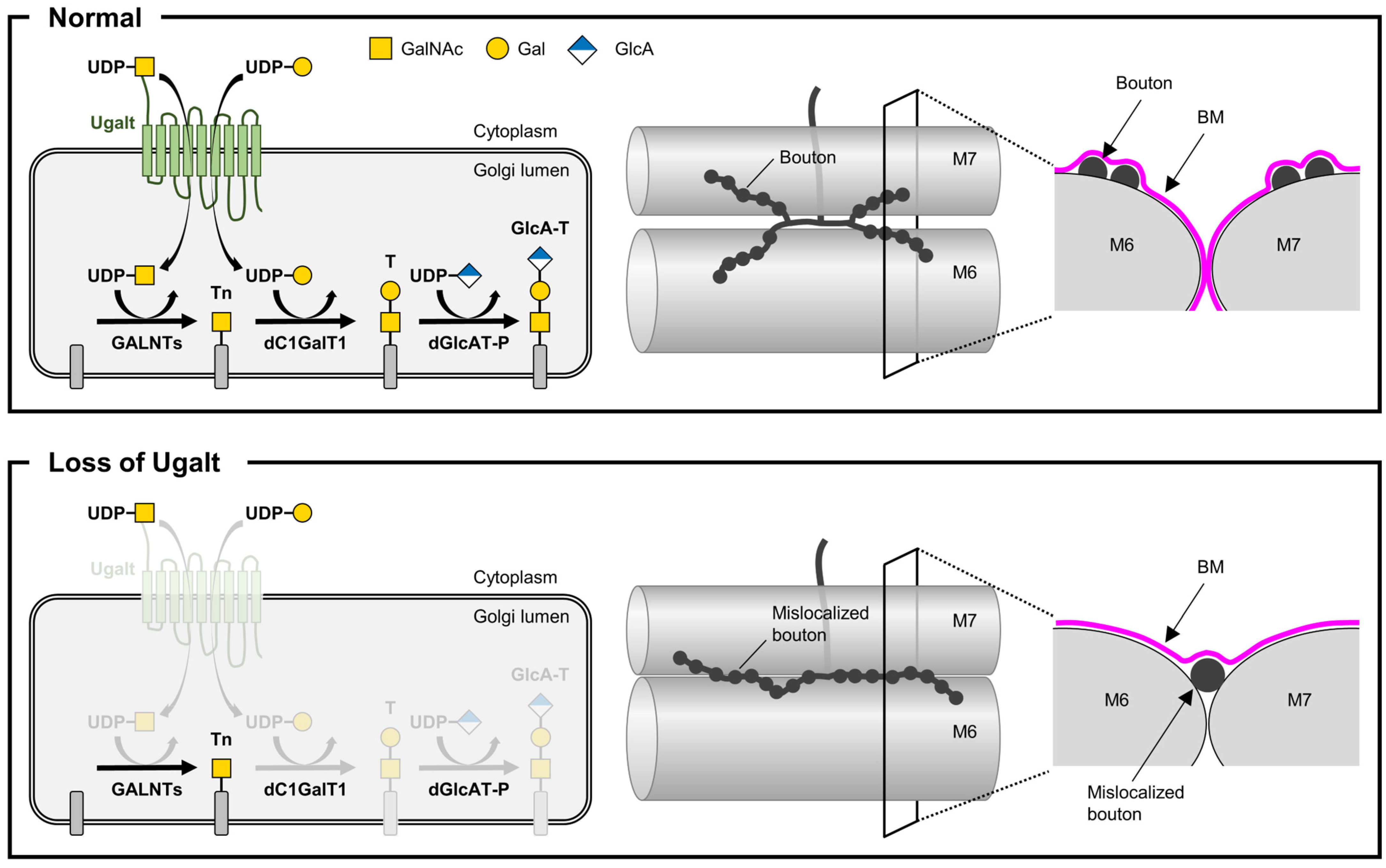
4.4. Impact of Ugalt Loss on Mucin-Type O-Glycan Synthesis and Compensation Mechanisms
4.5. Role of Mucin-Type O-Glycans in NMJ Bouton Localization and BM Integrity
4.6. Cooperative Roles of Ugalt and dC1GalT1 in T antigen Synthesis and NMJ Morphology
4.7. Proposed Mechanism of NMJ Bouton Mislocalization via Glycan-Mediated Cell Adhesion
4.8. Linking Synaptic Defects in Drosophila to Brain Abnormalities in SLC35A2-CDG
4.9. Potential Role of Mucin-Type O-Glycans in Neurological Symptoms of SLC35A2-CDG
4.10. A Drosophila CDG Model for Investigating Brain Disorders Caused by O-glycosylation Defects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quelhas, D.; Jaeken, J. Treatment of Congenital Disorders of Glycosylation: An Overview. Mol. Genet. Metab. 2024, 143, 108567. [Google Scholar] [CrossRef]
- Marques-da-Silva, D.; Francisco, R.; Webster, D.; Ferreira, V.d.R.; Jaeken, J.; Pulinilkunnil, T. Cardiac Complications of Congenital Disorders of Glycosylation (CDG): A Systematic Review of the Literature. J. Inherit. Metab. Dis. 2017, 40, 657–672. [Google Scholar] [CrossRef]
- Paprocka, J.; Jezela-Stanek, A.; Tylki-Szymańska, A.; Grunewald, S. Congenital Disorders of Glycosylation from a Neurological Perspective. Brain Sci. 2021, 11, 88. [Google Scholar] [CrossRef]
- Miura, N.; Ishida, N.; Hoshino, M.; Yamauchi, M.; Hara, T.; Ayusawa, D.; Kawakita, M. Human UDP-Galactose Translocator: Molecular Cloning of a Complementary DNA That Complements the Genetic Defect of a Mutant Cell Line Deficient in UDP-Galactose Translocator1. J. Biochem. 1996, 120, 236–241. [Google Scholar] [CrossRef]
- Ishida, N.; Miura, N.; Yoshioka, S.; Kawakita, M. Molecular Cloning and Characterization of a Novel Isoform of the Human UDP-Galactose Transporter, and of Related Complementary DNAs Belonging to the Nucleotide-Sugar Transporter Gene Family1. J. Biochem. 1996, 120, 1074–1078. [Google Scholar] [CrossRef]
- Segawa, H.; Kawakita, M.; Ishida, N. Human and Drosophila UDP-galactose Transporters Transport UDP-N-acetylgalactosamine in Addition to UDP-galactose. Eur. J. Biochem. 2002, 269, 128–138. [Google Scholar] [CrossRef]
- Ng, B.G.; Buckingham, K.J.; Raymond, K.; Kircher, M.; Turner, E.H.; He, M.; Smith, J.D.; Eroshkin, A.; Szybowska, M.; Losfeld, M.E.; et al. Mosaicism of the UDP-Galactose Transporter SLC35A2 Causes a Congenital Disorder of Glycosylation. Am. J. Hum. Genet. 2013, 92, 632–636. [Google Scholar] [CrossRef]
- Xia, B.; Zhang, W.; Li, X.; Jiang, R.; Harper, T.; Liu, R.; Cummings, R.D.; He, M. Serum N-Glycan and O-Glycan Analysis by Mass Spectrometry for Diagnosis of Congenital Disorders of Glycosylation. Anal. Biochem. 2013, 442, 178–185. [Google Scholar] [CrossRef]
- Kodera, H.; Nakamura, K.; Osaka, H.; Maegaki, Y.; Haginoya, K.; Mizumoto, S.; Kato, M.; Okamoto, N.; Iai, M.; Kondo, Y.; et al. De Novo Mutations in SLC35A2 Encoding a UDP-Galactose Transporter Cause Early-Onset Epileptic Encephalopathy. Hum. Mutat. 2013, 34, 1708–1714. [Google Scholar] [CrossRef]
- Bruneel, A.; Cholet, S.; Drouin-Garraud, V.; Jacquemont, M.; Cano, A.; Mégarbané, A.; Ruel, C.; Cheillan, D.; Dupré, T.; Vuillaumier-Barrot, S.; et al. Complementarity of Electrophoretic, Mass Spectrometric, and Gene Sequencing Techniques for the Diagnosis and Characterization of Congenital Disorders of Glycosylation. Electrophoresis 2018, 39, 3123–3132. [Google Scholar] [CrossRef]
- Vals, M.; Ashikov, A.; Ilves, P.; Loorits, D.; Zeng, Q.; Barone, R.; Huijben, K.; Sykut-Cegielska, J.; Diogo, L.; Elias, A.F.; et al. Clinical, Neuroradiological, and Biochemical Features of SLC35A2-CDG Patients. J. Inherit. Metab. Dis. 2019, 42, 553–564. [Google Scholar] [CrossRef]
- Witters, P.; Tahata, S.; Barone, R.; Õunap, K.; Salvarinova, R.; Grønborg, S.; Hoganson, G.; Scaglia, F.; Lewis, A.M.; Mori, M.; et al. Clinical and Biochemical Improvement with Galactose Supplementation in SLC35A2-CDG. Genet. Med. 2020, 22, 1102–1107. [Google Scholar] [CrossRef]
- Wada, Y. Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry to Detect Diagnostic Glycopeptide Markers of Congenital Disorders of Glycosylation. Mass. Spectrom. 2020, 9, A0084. [Google Scholar] [CrossRef]
- Kodríková, R.; Pakanová, Z.; Krchňák, M.; Šedivá, M.; Šesták, S.; Květoň, F.; Beke, G.; Šalingová, A.; Skalická, K.; Brennerová, K.; et al. N-Glycoprofiling of SLC35A2-CDG: Patient with a Novel Hemizygous Variant. Biomedicines 2023, 11, 580. [Google Scholar] [CrossRef]
- Bonduelle, T.; Hartlieb, T.; Baldassari, S.; Sim, N.S.; Kim, S.H.; Kang, H.-C.; Kobow, K.; Coras, R.; Chipaux, M.; Dorfmüller, G.; et al. Frequent SLC35A2 Brain Mosaicism in Mild Malformation of Cortical Development with Oligodendroglial Hyperplasia in Epilepsy (MOGHE). Acta Neuropathol. Commun. 2021, 9, 3. [Google Scholar] [CrossRef]
- Barba, C.; Blumcke, I.; Winawer, M.R.; Hartlieb, T.; Kang, H.-C.; Grisotto, L.; Chipaux, M.; Bien, C.G.; Heřmanovská, B.; Porter, B.E.; et al. Clinical Features, Neuropathology, and Surgical Outcome in Patients With Refractory Epilepsy and Brain Somatic Variants in the SLC35A2 Gene. Neurology 2023, 100, e528–e542. [Google Scholar] [CrossRef]
- Elziny, S.; Sran, S.; Yoon, H.; Corrigan, R.R.; Page, J.; Ringland, A.; Lanier, A.; Lapidus, S.; Foreman, J.; Heinzen, E.L.; et al. Loss of Slc35a2 Alters Development of the Mouse Cerebral Cortex. Neurosci. Lett. 2024, 836, 137881. [Google Scholar] [CrossRef]
- Spyrou, J.; Aung, K.P.; Vanyai, H.; Leventer, R.J.; Maljevic, S.; Lockhart, P.J.; Howell, K.B.; Reid, C.A. Slc35a2 Mosaic Knockout Impacts Cortical Development, Dendritic Arborisation, and Neuronal Firing. Neurobiol. Dis. 2024, 201, 106657. [Google Scholar] [CrossRef]
- Yoon, H.; Ringland, A.; Anderson, J.J.; Sran, S.; Elziny, S.; Huynh, C.; Shinagawa, N.; Badertscher, S.; Corrigan, R.R.; Mashburn-Warren, L.; et al. Mouse Models of Slc35a2 Brain Mosaicism Reveal Mechanisms of Mild Malformations of Cortical Development with Oligodendroglial Hyperplasia in Epilepsy. Epilepsia 2024, 65, 3717–3731. [Google Scholar] [CrossRef]
- Aumiller, J.J.; Jarvis, D.L. Expression and Functional Characterization of a Nucleotide Sugar Transporter from Drosophila Melanogaster: Relevance to Protein Glycosylation in Insect Cell Expression Systems. Protein Expr. Purif. 2002, 26, 438–448. [Google Scholar] [CrossRef]
- Yamamoto-Hino, M.; Abe, M.; Shibano, T.; Setoguchi, Y.; Awano, W.; Ueda, R.; Okano, H.; Goto, S. Cisterna-Specific Localization of Glycosylation-Related Proteins to the Golgi Apparatus. Cell Struct. Funct. 2012, 37, 55–63. [Google Scholar] [CrossRef]
- Yamamoto-Hino, M.; Yoshida, H.; Ichimiya, T.; Sakamura, S.; Maeda, M.; Kimura, Y.; Sasaki, N.; Aoki-Kinoshita, K.F.; Kinoshita-Toyoda, A.; Toyoda, H.; et al. Phenotype-based Clustering of Glycosylation-related Genes by RNAi-mediated Gene Silencing. Genes. Cells 2015, 20, 521–542. [Google Scholar] [CrossRef]
- Maszczak-Seneczko, D.; Sosicka, P.; Kaczmarek, B.; Majkowski, M.; Luzarowski, M.; Olczak, T.; Olczak, M. UDP-Galactose (SLC35A2) and UDP-N-Acetylglucosamine (SLC35A3) Transporters Form Glycosylation-Related Complexes with Mannoside Acetylglucosaminyltransferases (Mgats). J. Biol. Chem. 2015, 290, 15475–15486. [Google Scholar] [CrossRef]
- Khoder-Agha, F.; Sosicka, P.; Conde, M.E.; Hassinen, A.; Glumoff, T.; Olczak, M.; Kellokumpu, S. N-Acetylglucosaminyltransferases and Nucleotide Sugar Transporters Form Multi-Enzyme–Multi-Transporter Assemblies in Golgi Membranes In Vivo. Cell Mol. Life Sci. 2019, 76, 1821–1832. [Google Scholar] [CrossRef]
- Wiertelak, W.; Sosicka, P.; Olczak, M.; Maszczak-Seneczko, D. Analysis of Homologous and Heterologous Interactions between UDP-Galactose Transporter and Beta-1,4-Galactosyltransferase 1 Using NanoBiT. Anal. Biochem. 2020, 593, 113599. [Google Scholar] [CrossRef]
- Wiertelak, W.; Pavlovskyi, A.; Olczak, M.; Maszczak-Seneczko, D. Cytosolic UDP-Gal Biosynthetic Machinery Is Required for Dimerization of SLC35A2 in the Golgi Membrane and Its Interaction with B4GalT1. Front. Mol. Biosci. 2025, 12, 1563384. [Google Scholar] [CrossRef]
- Shauchuk, A.; Szulc, B.; Maszczak-Seneczko, D.; Wiertelak, W.; Skurska, E.; Olczak, M. N-Glycosylation of the Human Β1,4-Galactosyltransferase 4 Is Crucial for Its Activity and Golgi Localization. Glycoconj. J. 2020, 37, 577–588. [Google Scholar] [CrossRef]
- Sprong, H.; Degroote, S.; Nilsson, T.; Kawakita, M.; Ishida, N.; van der Sluijs, P.; van Meer, G. Association of the Golgi UDP-Galactose Transporter with UDP-Galactose:Ceramide Galactosyltransferase Allows UDP-Galactose Import in the Endoplasmic Reticulum. Mol. Biol. Cell 2003, 14, 3482–3493. [Google Scholar] [CrossRef]
- Wiertelak, W.; Chabowska, K.; Szulc, B.; Zadorozhna, Y.; Olczak, M.; Maszczak-Seneczko, D. SLC35A2 Deficiency Reduces Protein Levels of Core 1 β-1,3-Galactosyltransferase 1 (C1GalT1) and Its Chaperone Cosmc and Affects Their Subcellular Localization. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2023, 1870, 119462. [Google Scholar] [CrossRef] [PubMed]
- Påhlsson, P.; Blackall, D.P.; Ugorski, M.; Czerwinski, M.; Spitalnik, S.L. Biochemical Characterization of The O-Glycans on Recombinant Glycophorin A Expressed in Chinese Hamster Ovary Cells. Glycoconj. J. 1994, 11, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Szulc, B.; Sosicka, P.; Maszczak-Seneczko, D.; Skurska, E.; Shauchuk, A.; Olczak, T.; Freeze, H.H.; Olczak, M. Biosynthesis of GlcNAc-Rich N- and O-Glycans in the Golgi Apparatus Does Not Require the Nucleotide Sugar Transporter SLC35A3. J. Biol. Chem. 2020, 295, 16445–16463. [Google Scholar] [CrossRef]
- Bennett, E.P.; Mandel, U.; Clausen, H.; Gerken, T.A.; Fritz, T.A.; Tabak, L.A. Control of Mucin-Type O-Glycosylation: A Classification of the Polypeptide GalNAc-Transferase Gene Family. Glycobiology 2012, 22, 736–756. [Google Scholar] [CrossRef]
- Tran, D.T.; Hagen, K.G. Mucin-Type O-Glycosylation during Development. J. Biol. Chem. 2013, 288, 6921–6929. [Google Scholar] [CrossRef]
- Ju, T.; Cummings, R.D. A Unique Molecular Chaperone Cosmc Required for Activity of the Mammalian Core 1 β3-Galactosyltransferase. Proc. Natl. Acad. Sci. USA 2002, 99, 16613–16618. [Google Scholar] [CrossRef]
- Müller, R.; Hülsmeier, A.J.; Altmann, F.; Hagen, K.T.; Tiemeyer, M.; Hennet, T. Characterization of Mucin-Type Core-1 β1-3 Galactosyltransferase Homologous Enzymes in Drosophila Melanogaster. FEBS J. 2005, 272, 4295–4305. [Google Scholar] [CrossRef]
- Kim, B.-T.T.; Tsuchida, K.; Lincecum, J.; Kitagawa, H.; Bernfield, M.; Sugahara, K. Identification and Characterization of Three Drosophila Melanogaster Glucuronyltransferases Responsible for the Synthesis of the Conserved Glycosaminoglycan-Protein Linkage Region of Proteoglycans. Two Novel Homologs Exhibit Broad Specificity toward Oligosaccharides from Proteoglycans, Glycoproteins, and Glycosphingolipids. J. Biol. Chem. 2003, 278, 9116–9124. [Google Scholar] [CrossRef]
- Aoki, K.; Porterfield, M.; Lee, S.S.; Dong, B.; Nguyen, K.; McGlamry, K.H.; Tiemeyer, M. The Diversity of O-Linked Glycans Expressed during Drosophila Melanogaster Development Reflects Stage- and Tissue-Specific Requirements for Cell Signaling. J. Biol. Chem. 2008, 283, 30385–30400. [Google Scholar] [CrossRef]
- Breloy, I.; Schwientek, T.; Lehr, S.; Hanisch, F.-G.G. Glucuronic Acid Can Extend O-Linked Core 1 Glycans, but It Contributes Only Weakly to the Negative Surface Charge of Drosophila Melanogaster Schneider-2 Cells. FEBS Lett. 2008, 582, 1593–1598. [Google Scholar] [CrossRef]
- Breloy, I.; Schwientek, T.; Althoff, D.; Holz, M.; Koppen, T.; Krupa, A.; Hanisch, F.-G. Functional Analysis of the Glucuronyltransferases GlcAT-P and GlcAT-S of Drosophila Melanogaster: Distinct Activities towards the O-Linked T-Antigen. Biomolecules 2016, 6, 8. [Google Scholar] [CrossRef]
- Itoh, K.; Akimoto, Y.; Kondo, S.; Ichimiya, T.; Aoki, K.; Tiemeyer, M.; Nishihara, S. Glucuronylated Core 1 Glycans Are Required for Precise Localization of Neuromuscular Junctions and Normal Formation of Basement Membranes on Drosophila Muscles. Dev. Biol. 2018, 436, 108–124. [Google Scholar] [CrossRef]
- Itoh, K.; Nishihara, S. Mucin-Type O-Glycosylation in the Drosophila Nervous System. Front. Neuroanat. 2021, 15, 767126. [Google Scholar] [CrossRef]
- Berger, E.G. Tn-Syndrome. Biochim. Biophys. Acta 1999, 1455, 255–268. [Google Scholar] [CrossRef]
- Ju, T.; Cummings, R.D. Protein Glycosylation: Chaperone Mutation in Tn Syndrome. Nature 2005, 437, 1252. [Google Scholar] [CrossRef]
- Suzuki, H.; Moldoveanu, Z.; Hall, S.; Brown, R.; Vu, H.L.; Novak, L.; Julian, B.A.; Tomana, M.; Wyatt, R.J.; Edberg, J.C.; et al. IgA1-Secreting Cell Lines from Patients with IgA Nephropathy Produce Aberrantly Glycosylated IgA1. J. Clin. Investig. 2008, 118, 629–639. [Google Scholar] [CrossRef]
- Hiki, Y. O-Linked Oligosaccharides of the IgA1 Hinge Region: Roles of Its Aberrant Structure in the Occurrence and/or Progression of IgA Nephropathy. Clin. Exp. Nephrol. 2009, 13, 415–423. [Google Scholar] [CrossRef]
- Springer, G. T and Tn, General Carcinoma Autoantigens. Science 1984, 224, 1198–1206. [Google Scholar] [CrossRef]
- Ju, T.; Lanneau, G.S.; Gautam, T.; Wang, Y.; Xia, B.; Stowell, S.R.; Willard, M.T.; Wang, W.; Xia, J.Y.; Zuna, R.E.; et al. Human Tumor Antigens Tn and Sialyl Tn Arise from Mutations in Cosmc. Cancer Res. 2008, 68, 1636–1646. [Google Scholar] [CrossRef]
- Radhakrishnan, P.; Dabelsteen, S.; Madsen, F.B.; Francavilla, C.; Kopp, K.L.; Steentoft, C.; Vakhrushev, S.Y.; Olsen, J.V.; Hansen, L.; Bennett, E.P.; et al. Immature Truncated O-Glycophenotype of Cancer Directly Induces Oncogenic Features. Proc. Natl. Acad. Sci. USA 2014, 111, E4066–E4075. [Google Scholar] [CrossRef]
- Xia, L.; Ju, T.; Westmuckett, A.; An, G.; Ivanciu, L.; McDaniel, J.M.; Lupu, F.; Cummings, R.D.; McEver, R.P. Defective Angiogenesis and Fatal Embryonic Hemorrhage in Mice Lacking Core 1-Derived O-Glycans. J. Cell Biol. 2004, 164, 451–459. [Google Scholar] [CrossRef]
- Wang, Y.; Jobe, S.M.; Ding, X.; Choo, H.; Archer, D.R.; Mi, R.; Ju, T.; Cummings, R.D. Platelet Biogenesis and Functions Require Correct Protein O-Glycosylation. Proc. Natl. Acad. Sci. USA 2012, 109, 16143–16148. [Google Scholar] [CrossRef]
- Kudo, T.; Sato, T.; Hagiwara, K.; Kozuma, Y.; Yamaguchi, T.; Ikehara, Y.; Hamada, M.; Matsumoto, K.; Ema, M.; Murata, S.; et al. C1galt1-Deficient Mice Exhibit Thrombocytopenia Due to Abnormal Terminal Differentiation of Megakaryocytes. Blood 2013, 122, 1649–1657. [Google Scholar] [CrossRef]
- Fu, J.; Wei, B.; Wen, T.; Johansson, M.E.; Liu, X.; Bradford, E.; Thomsson, K.A.; McGee, S.; Mansour, L.; Tong, M.; et al. Loss of Intestinal Core 1-Derived O-Glycans Causes Spontaneous Colitis in Mice. J. Clin. Investig. 2011, 121, 1657–1666. [Google Scholar] [CrossRef]
- Bergstrom, K.; Liu, X.; Zhao, Y.; Gao, N.; Wu, Q.; Song, K.; Cui, Y.; Li, Y.; McDaniel, J.M.; McGee, S.; et al. Defective Intestinal Mucin-Type O-Glycosylation Causes Spontaneous Colitis-Associated Cancer in Mice. Gastroenterology 2016, 151, 152–164.e11. [Google Scholar] [CrossRef]
- Fuseya, S.; Suzuki, R.; Okada, R.; Hagiwara, K.; Sato, T.; Narimatsu, H.; Yokoi, H.; Kasahara, M.; Usui, T.; Morito, N.; et al. Mice Lacking Core 1-Derived O-Glycan in Podocytes Develop Transient Proteinuria, Resulting in Focal Segmental Glomerulosclerosis. Biochem. Biophys. Res. Commun. 2020, 523, 1007–1013. [Google Scholar] [CrossRef]
- Stotter, B.R.; Talbot, B.E.; Capen, D.E.; Artelt, N.; Zeng, J.; Matsumoto, Y.; Endlich, N.; Cummings, R.D.; Schlondorff, J.S. Cosmc-Dependent Mucin-Type O-Linked Glycosylation Is Essential for Podocyte Function. Am. J. Physiol.-Ren. 2020, 318, F518–F530. [Google Scholar] [CrossRef]
- Noel, M.; Suttapitugsakul, S.; Cummings, R.D.; Mealer, R.G. O-GalNAc Glycans Are Enriched in Neuronal Tracts and Regulate Nodes of Ranvier. Proc. Natl. Acad. Sci. USA 2025, 122, e2418949122. [Google Scholar] [CrossRef]
- Valoskova, K.; Biebl, J.; Roblek, M.; Emtenani, S.; Gyoergy, A.; Misova, M.; Ratheesh, A.; Reis-Rodrigues, P.; Shkarina, K.; Larsen, I.; et al. A Conserved Major Facilitator Superfamily Member Orchestrates a Subset of O-Glycosylation to Aid Macrophage Tissue Invasion. eLife 2019, 8, e41801. [Google Scholar] [CrossRef]
- Yoshida, H.; Fuwa, T.J.; Arima, M.; Hamamoto, H.; Sasaki, N.; Ichimiya, T.; Osawa, K.; Ueda, R.; Nishihara, S. Identification of the Drosophila Core 1 β1,3-Galactosyltransferase Gene That Synthesizes T Antigen in the Embryonic Central Nervous System and Hemocytes. Glycobiology 2008, 18, 1094–1104. [Google Scholar] [CrossRef][Green Version]
- Fuwa, T.J.; Kinoshita, T.; Nishida, H.; Nishihara, S. Reduction of T Antigen Causes Loss of Hematopoietic Progenitors in Drosophila through the Inhibition of Filopodial Extensions from the Hematopoietic Niche. Dev. Biol. 2015, 401, 206–219. [Google Scholar] [CrossRef]
- Lin, Y.; Reddy, B.V.V.G.; Irvine, K.D. Requirement for a Core 1 Galactosyltransferase in the Drosophila Nervous System. Dev. Dyn. 2008, 237, 3703–3714. [Google Scholar] [CrossRef]
- Lacin, H.; Zhu, Y.; DiPaola, J.T.; Wilson, B.A.; Zhu, Y.; Skeath, J.B. A Genetic Screen in Drosophila Uncovers a Role for Senseless-2 in Surface Glia in the Peripheral Nervous System to Regulate CNS Morphology. G3 Genes Genomes Genet. 2024, 14, jkae152. [Google Scholar] [CrossRef]
- Itoh, K.; Akimoto, Y.; Fuwa, T.J.; Sato, C.; Komatsu, A.; Nishihara, S. Mucin-Type Core 1 Glycans Regulate the Localization of Neuromuscular Junctions and Establishment of Muscle Cell Architecture in Drosophila. Dev. Biol. 2016, 412, 114–127. [Google Scholar] [CrossRef]
- Menon, K.P.; Carrillo, R.A.; Zinn, K. Development and Plasticity of the Drosophila Larval Neuromuscular Junction. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 647–670. [Google Scholar] [CrossRef]
- Aoki, E.; Manabe, N.; Ohno, S.; Aoki, T.; Furukawa, J.-I.; Togayachi, A.; Aoki-Kinoshita, K.; Inokuchi, J.-I.; Kurosawa, K.; Kaname, T.; et al. Predicting the Pathogenicity of Missense Variants Based on Protein Instability to Support Diagnosis of Patients with Novel Variants of ARSL. Mol. Genet. Metab. Rep. 2023, 37, 101016. [Google Scholar] [CrossRef]
- Ohno, S.; Ogura, C.; Yabuki, A.; Itoh, K.; Manabe, N.; Angata, K.; Togayachi, A.; Aoki-Kinoshita, K.; Furukawa, J.; Inamori, K.; et al. VarMeter2: An Enhanced Structure-Based Methodfmovi for Predicting Pathogenic Missense Variants through Mahalanobis Distance. Comput. Struct. Biotechnol. J. 2025, 27, 1034–1047. [Google Scholar] [CrossRef]
- Kondo, S.; Ueda, R. Highly Improved Gene Targeting by Germline-Specific Cas9 Expression in Drosophila. Genetics 2013, 195, 715–721. [Google Scholar] [CrossRef]
- Hanamatsu, H.; Yokota, I.; Kurogochi, M.; Akasaka-Manya, K.; Miura, N.; Manya, H.; Endo, T.; Nishikaze, T.; Furukawa, J.; Tanaka, K. Direct Derivatization of Sialic Acids and Mild β-Elimination for Linkage-Specific Sialyl O-Glycan Analysis. Anal. Chim. Acta 2024, 1318, 342945. [Google Scholar] [CrossRef]
- Furukawa, J.; Shinohara, Y.; Kuramoto, H.; Miura, Y.; Shimaoka, H.; Kurogochi, M.; Nakano, M.; Nishimura, S.-I. Comprehensive Approach to Structural and Functional Glycomics Based on Chemoselective Glycoblotting and Sequential Tag Conversion. Anal. Chem. 2008, 80, 1094–1101. [Google Scholar] [CrossRef]
- Hanamatsu, H.; Nishikaze, T.; Miura, N.; Piao, J.; Okada, K.; Sekiya, S.; Iwamoto, S.; Sakamoto, N.; Tanaka, K.; Furukawa, J. Sialic Acid Linkage Specific Derivatization of Glycosphingolipid Glycans by Ring-Opening Aminolysis of Lactones. Anal. Chem. 2018, 90, 13193–13199. [Google Scholar] [CrossRef]
- Ueyama, M.; Akimoto, Y.; Ichimiya, T.; Ueda, R.; Kawakami, H.; Aigaki, T.; Nishihara, S. Increased Apoptosis of Myoblasts in Drosophila Model for the Walker-Warburg Syndrome. PLoS ONE 2010, 5, e11557. [Google Scholar] [CrossRef]
- Aoki, K.; Perlman, M.; Lim, J.-M.; Cantu, R.; Wells, L.; Tiemeyer, M. Dynamic Developmental Elaboration of N-Linked Glycan Complexity in the Drosophila Melanogaster Embryo. J. Biol. Chem. 2007, 282, 9127–9142. [Google Scholar] [CrossRef]
- Quelhas, D.; Correia, J.; Jaeken, J.; Azevedo, L.; Lopes-Marques, M.; Bandeira, A.; Keldermans, L.; Matthijs, G.; Sturiale, L.; Martins, E. SLC35A2-CDG: Novel Variant and Review. Mol. Genet. Metab. Rep. 2021, 26, 100717. [Google Scholar] [CrossRef]
- Yüksel, M.F.; Doğulu, N.; Yıldırım, M.; Köse, E.; Bektaş, Ö.; Eminoğlu, F.T.; Teber, S. Metabolic Etiologies in Children with Infantile Epileptic Spasm Syndrome: Experience at a Tertiary Pediatric Neurology Center. Brain Dev. 2024, 46, 213–218. [Google Scholar] [CrossRef]
- Kabuß, R.; Ashikov, A.; Oelmann, S.; Gerardy-Schahn, R.; Bakker, H. Endoplasmic Reticulum Retention of the Large Splice Variant of the UDP-Galactose Transporter Is Caused by a Dilysine Motif. Glycobiology 2005, 15, 905–911. [Google Scholar] [CrossRef]
- Westenfield, K.; Sarafoglou, K.; Speltz, L.C.; Pierpont, E.I.; Steyermark, J.; Nascene, D.; Bower, M.; Pierpont, M.E. Mosaicism of the UDP-Galactose Transporter SLC35A2 in a Female Causing a Congenital Disorder of Glycosylation: A Case Report. BMC Med. Genet. 2018, 19, 100. [Google Scholar] [CrossRef]
- Goto, S.; Taniguchi, M.; Muraoka, M.; Toyoda, H.; Sado, Y.; Kawakita, M.; Hayashi, S. UDP–Sugar Transporter Implicated in Glycosylation and Processing of Notch. Nat. Cell Biol. 2001, 3, 816–822. [Google Scholar] [CrossRef]
- Pastor-Pareja, J.C.; Xu, T. Shaping Cells and Organs in Drosophila by Opposing Roles of Fat Body-Secreted Collagen IV and Perlecan. Dev. Cell 2011, 21, 245–256. [Google Scholar] [CrossRef]
- Zhong, Y.; Shanley, J. Altered Nerve Terminal Arborization and Synaptic Transmission in Drosophila Mutants of Cell Adhesion Molecule Fasciclin I. J. Neurosci. 1995, 15, 6679–6687. [Google Scholar] [CrossRef]
- Takeda, T.; Go, W.; Orlando, R.; Farquhar, M. Expression of Podocalyxin Inhibits Cell-Cell Adhesion and Modifies Junctional Properties in Madin-Darby Canine Kidney Cells. Mol. Biol. Cell 2000, 11, 3219–3232. [Google Scholar] [CrossRef]
- Doyonnas, R.; Kershaw, D.B.; Duhme, C.; Merkens, H.; Chelliah, S.; Graf, T.; McNagny, K.M. Anuria, Omphalocele, and Perinatal Lethality in Mice Lacking the CD34-Related Protein Podocalyxin. J. Exp. Med. 2001, 194, 13–27. [Google Scholar] [CrossRef]
- Wada, Y.; Okamoto, N. Apolipoprotein C-III O-glycoform Profiling of 500 Serum Samples by Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry for Diagnosis of Congenital Disorders of Glycosylation. J. Mass. Spectrom. 2020, 56, e4597. [Google Scholar] [CrossRef]
- Wilkinson, H.; Thomsson, K.A.; Rebelo, A.L.; Hilliard, M.; Pandit, A.; Rudd, P.M.; Karlsson, N.G.; Saldova, R. The O-Glycome of Human Nigrostriatal Tissue and Its Alteration in Parkinson’s Disease. J. Proteome Res. 2021, 20, 3913–3924. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.E.; Noel, M.; Lehoux, S.; Cetinbas, M.; Xavier, R.J.; Sadreyev, R.I.; Scolnick, E.M.; Smoller, J.W.; Cummings, R.D.; Mealer, R.G. Mammalian Brain Glycoproteins Exhibit Diminished Glycan Complexity Compared to Other Tissues. Nat. Commun. 2022, 13, 275. [Google Scholar] [CrossRef] [PubMed]
- Erger, F.; Aryal, R.P.; Reusch, B.; Matsumoto, Y.; Meyer, R.; Zeng, J.; Knopp, C.; Noel, M.; Muerner, L.; Wenzel, A.; et al. Germline C1GALT1C1 Mutation Causes a Multisystem Chaperonopathy. Proc. Natl. Acad. Sci. USA 2023, 120, e2211087120. [Google Scholar] [CrossRef] [PubMed]
- Zilmer, M.; Edmondson, A.C.; Khetarpal, S.A.; Alesi, V.; Zaki, M.S.; Rostasy, K.; Madsen, C.G.; Lepri, F.R.; Sinibaldi, L.; Cusmai, R.; et al. Novel Congenital Disorder of O-Linked Glycosylation Caused by GALNT2 Loss of Function. Brain 2020, 143, 1114–1126. [Google Scholar] [CrossRef]
- Ng, B.G.; Sosicka, P.; Agadi, S.; Almannai, M.; Bacino, C.A.; Barone, R.; Botto, L.D.; Burton, J.E.; Carlston, C.; Chung, B.H.-Y.; et al. SLC35A2-CDG: Functional Characterization, Expanded Molecular, Clinical, and Biochemical Phenotypes of 30 Unreported Individuals. Hum. Mutat. 2019, 40, 908–925. [Google Scholar] [CrossRef]
- Haines, N.; Irvine, K.D. Functional Analysis of Drosophila β1,4-N-Acetlygalactosaminyltransferases. Glycobiology 2005, 15, 335–346. [Google Scholar] [CrossRef]
- Sasaki, N.; Yoshida, H.; Fuwa, T.J.; Kinoshita-Toyoda, A.; Toyoda, H.; Hirabayashi, Y.; Ishida, H.; Ueda, R.; Nishihara, S. Drosophila β1,4-N-Acetylgalactosaminyltransferase-A Synthesizes the LacdiNAc Structures on Several Glycoproteins and Glycosphingolipids. Biochem. Biophys. Res. Commun. 2006, 354, 522–527. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itoh, K.; Kurogochi, M.; Kaname, T.; Furukawa, J.-i.; Nishihara, S. Neuromuscular Defects in a Drosophila Model of the Congenital Disorder of Glycosylation SLC35A2-CDG. Biomolecules 2025, 15, 1256. https://doi.org/10.3390/biom15091256
Itoh K, Kurogochi M, Kaname T, Furukawa J-i, Nishihara S. Neuromuscular Defects in a Drosophila Model of the Congenital Disorder of Glycosylation SLC35A2-CDG. Biomolecules. 2025; 15(9):1256. https://doi.org/10.3390/biom15091256
Chicago/Turabian StyleItoh, Kazuyoshi, Masaki Kurogochi, Tadashi Kaname, Jun-ichi Furukawa, and Shoko Nishihara. 2025. "Neuromuscular Defects in a Drosophila Model of the Congenital Disorder of Glycosylation SLC35A2-CDG" Biomolecules 15, no. 9: 1256. https://doi.org/10.3390/biom15091256
APA StyleItoh, K., Kurogochi, M., Kaname, T., Furukawa, J.-i., & Nishihara, S. (2025). Neuromuscular Defects in a Drosophila Model of the Congenital Disorder of Glycosylation SLC35A2-CDG. Biomolecules, 15(9), 1256. https://doi.org/10.3390/biom15091256






