Angiotensin-Converting Enzyme Inhibitors and Metabolic Aging: A Drosophila Perspective
Abstract
1. Introduction
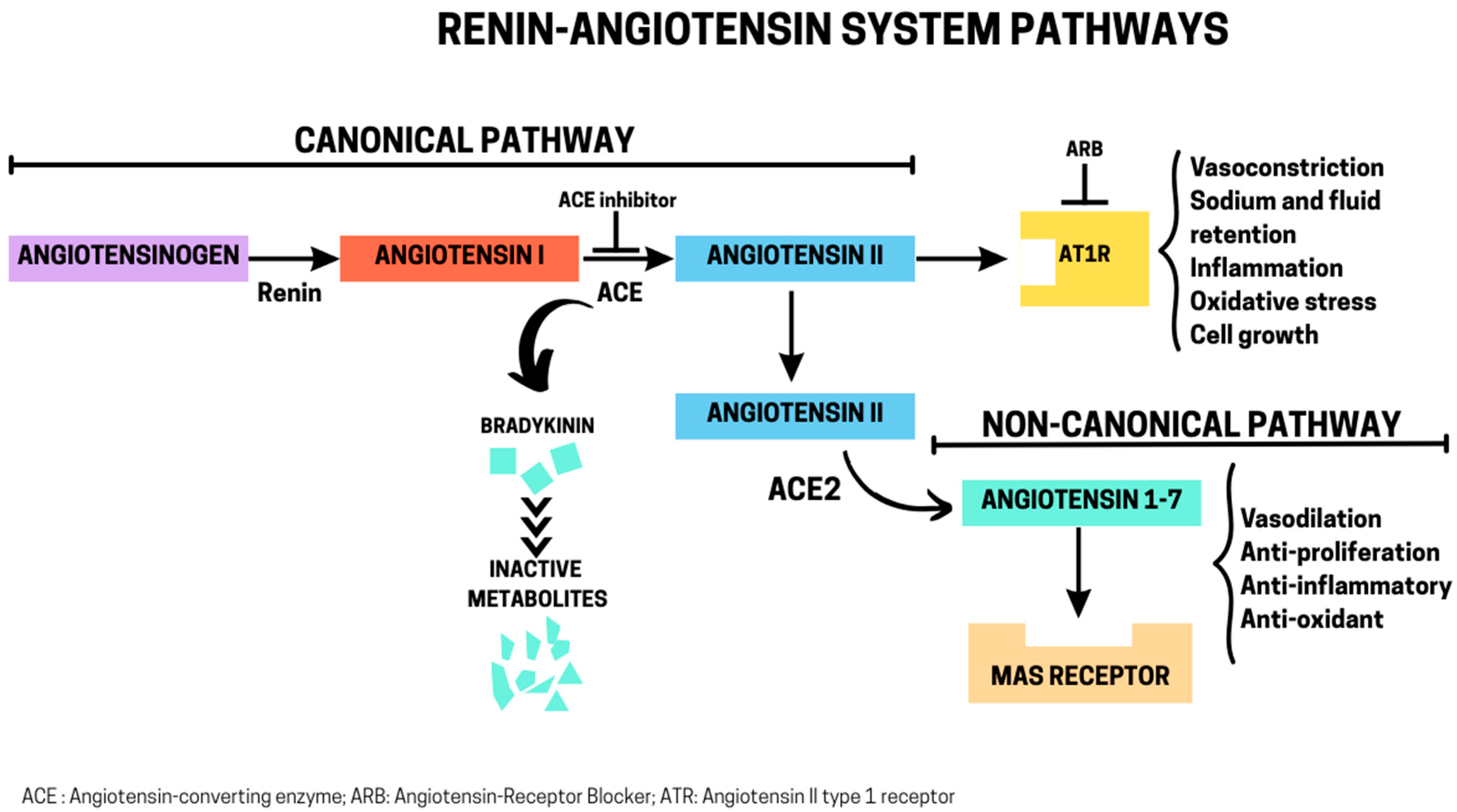
2. RAS: Canonical and Non-Canonical Pathways
3. Pharmacological Inhibition of RAS: Implications for Metabolic Aging in Humans
3.1. Aging and Metabolism
3.2. RAS Blockade and Metabolic Aging
4. ACE-like Enzymes in D. melanogaster: Pharmacological Inhibition and Effects on Metabolic Aging
4.1. Functional Roles of ACE-like Enzymes in Drosophila
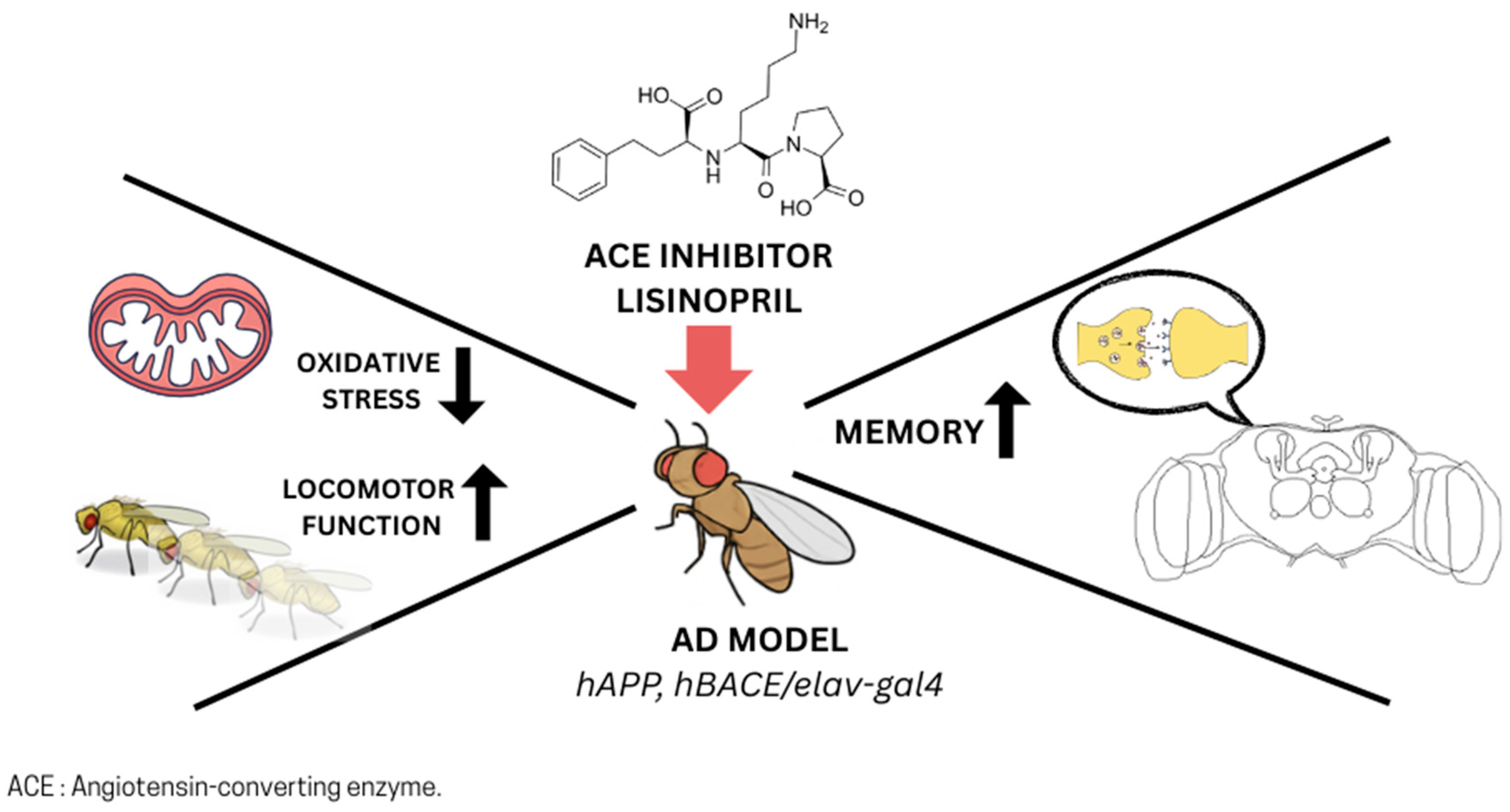
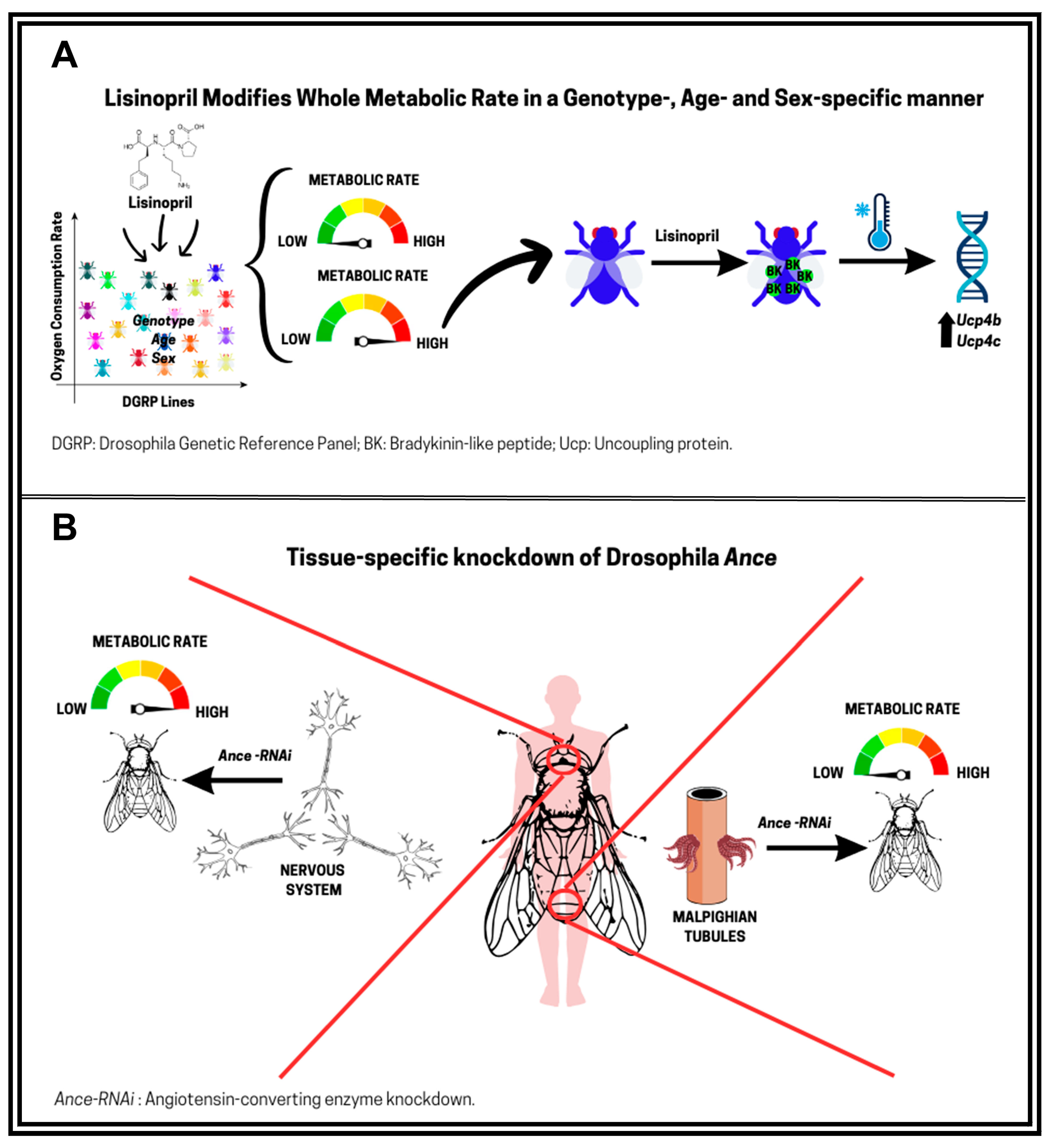
4.2. ACE Inhibitors, Metabolism, and Aging in D. melanogaster
4.3. Effects of Lisinopril on Thermoregulation in D. melanogaster
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harper, S. Economic and social implications of aging societies. Science 2014, 346, 587–591. [Google Scholar] [CrossRef]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef]
- Li, S.; Vazquez, J.M.; Sudmant, P.H. The evolution of aging and lifespan. Trends Genet. 2023, 39, 830–843. [Google Scholar] [CrossRef]
- Mc Auley, M.T. The evolution of ageing: Classic theories and emerging ideas. Biogerontology 2024, 26, 6. [Google Scholar] [CrossRef] [PubMed]
- Medawar, P.B. An Unsolved Problem of Biology: An Inaugural Lecture Delivered at University College, London, 6 December, 1951; H.K. Lewis and Company: London, UK, 1952; p. 24. [Google Scholar]
- Williams, G.C. Pleiotrophy, Natural Selection, and the Evolution of Senescence. Evolution 1957, 11, 398–411. [Google Scholar] [CrossRef]
- Kirkwood, T.B. Evolution of ageing. Nature 1977, 270, 301–304. [Google Scholar] [CrossRef]
- Kirkwood, T.B.; Austad, S.N. Why do we age? Nature 2000, 408, 233–238. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Wang, A.M.; Bennett, C.F.; Kaeberlein, M. Biochemical Genetic Pathways that Modulate Aging in Multiple Species. Cold Spring Harb. Perspect. Med. 2015, 5, a025114. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From discoveries in ageing research to therapeutics for healthy ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef]
- Sierra, F. The Emergence of Geroscience as an Interdisciplinary Approach to the Enhancement of Health Span and Life Span. Cold Spring Harb. Perspect. Med. 2016, 6, a025163. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking aging to chronic disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef]
- GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- Seals, D.R.; Justice, J.N.; LaRocca, T.J. Physiological geroscience: Targeting function to increase healthspan and achieve optimal longevity. J. Physiol. 2016, 594, 2001–2024. [Google Scholar] [CrossRef]
- Crimmins, E.M. Lifespan and Healthspan: Past, Present, and Promise. Gerontologist 2015, 55, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Fries, J.F.; Bruce, B.; Chakravarty, E. Compression of morbidity 1980-2011: A focused review of paradigms and progress. J. Aging Res. 2011, 2011, 261702. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Freire, M.; Diaz-Ruiz, A.; Hauser, D.; Martinez-Romero, J.; Ferrucci, L.; Bernier, M.; de Cabo, R. The road ahead for health and lifespan interventions. Ageing Res. Rev. 2020, 59, 101037. [Google Scholar] [CrossRef] [PubMed]
- Warner, H.R. NIA’s Intervention Testing Program at 10 years of age. Age 2015, 37, 22. [Google Scholar] [CrossRef][Green Version]
- Miller, R.A.; Harrison, D.E.; Astle, C.M.; Floyd, R.A.; Flurkey, K.; Hensley, K.L.; Javors, M.A.; Leeuwenburgh, C.; Nelson, J.F.; Ongini, E.; et al. An Aging Interventions Testing Program: Study design and interim report. Aging Cell 2007, 6, 565–575. [Google Scholar] [CrossRef]
- Driscoll, M.; Sedore, C.A.; Onken, B.; Coleman-Hulbert, A.L.; Johnson, E.; Phillips, P.C.; Lithgow, G. NIA Caenorhabditis Intervention Testing Program: Identification of robust and reproducible pharmacological interventions that promote longevity across experimentally accessible, genetically diverse populations. Geroscience 2025, 47, 2791–2816. [Google Scholar] [CrossRef]
- Yang, Y.; Mayo, A.; Levy, T.; Raz, N.; Shenhar, B.; Jarosz, D.F.; Alon, U. Compression of morbidity by interventions that steepen the survival curve. Nat. Commun. 2025, 16, 3340. [Google Scholar] [CrossRef]
- de Cavanagh, E.M.V.; Inserra, F.; Ferder, L. Renin-angiotensin system inhibitors positively impact on multiple aging regulatory pathways: Could they be used to protect against human aging? Physiol. Rep. 2024, 12, e16094. [Google Scholar] [CrossRef]
- Egan, B.M.; Scharf, A.; Pohl, F.; Kornfeld, K. Control of aging by the renin-angiotensin system: A review of C. elegans, Drosophila, and mammals. Front. Pharmacol. 2022, 13, 938650. [Google Scholar] [CrossRef]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef]
- Skeggs, L.T., Jr.; Kahn, J.R.; Shumway, N.P. The preparation and function of the hypertensin-converting enzyme. J. Exp. Med. 1956, 103, 295–299. [Google Scholar] [CrossRef]
- Rojas, A.M.; Fuentes, G.; Rausell, A.; Valencia, A. The Ras protein superfamily: Evolutionary tree and role of conserved amino acids. J. Cell Biol. 2012, 196, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Pandey, U.B.; Nichols, C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef] [PubMed]
- Bader, M. Tissue renin-angiotensin-aldosterone systems: Targets for pharmacological therapy. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 439–465. [Google Scholar] [CrossRef] [PubMed]
- Atlas, S.A. The renin-angiotensin aldosterone system: Pathophysiological role and pharmacologic inhibition. J. Manag. Care Pharm. 2007, 13, 9–20. [Google Scholar] [CrossRef]
- Kawai, T.; Forrester, S.J.; O’Brien, S.; Baggett, A.; Rizzo, V.; Eguchi, S. AT1 receptor signaling pathways in the cardiovascular system. Pharmacol. Res. 2017, 125, 4–13. [Google Scholar] [CrossRef]
- Padia, S.H.; Carey, R.M. AT2 receptors: Beneficial counter-regulatory role in cardiovascular and renal function. Pflug. Arch. 2013, 465, 99–110. [Google Scholar] [CrossRef]
- Paz Ocaranza, M.; Riquelme, J.A.; Garcia, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.S.; Lavandero, S. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 116–129. [Google Scholar] [CrossRef]
- Leung, P.S. Local RAS. Adv. Exp. Med. Biol. 2010, 690, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Saleem, T.S.; Bharani, K.; Gauthaman, K. ACE inhibitors—Angiotensin II receptor antagonists: A useful combination therapy for ischemic heart disease. Open Access Emerg. Med. 2010, 2, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.K.; Jensen, M.D. Metabolic changes in aging humans: Current evidence and therapeutic strategies. J. Clin. Investig. 2022, 132, e158451. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.W.; Egan, J.M.; Ferrucci, L. Age-Related Changes in Glucose Metabolism, Hyperglycemia, and Cardiovascular Risk. Circ. Res. 2018, 123, 886–904. [Google Scholar] [CrossRef]
- Khalaf, F.; Barayan, D.; Saldanha, S.; Jeschke, M.G. Metabolaging: A new geroscience perspective linking aging pathologies and metabolic dysfunction. Metabolism 2025, 166, 156158. [Google Scholar] [CrossRef]
- Kuk, J.L.; Saunders, T.J.; Davidson, L.E.; Ross, R. Age-related changes in total and regional fat distribution. Ageing Res. Rev. 2009, 8, 339–348. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Gallagher, D.; Poehlman, E.T.; Wolper, C.; Nonas, K.; Nelson, D.; Wang, Z.M. Menopausal changes in body composition and energy expenditure. Exp. Gerontol. 1994, 29, 377–389. [Google Scholar] [CrossRef]
- Von Bank, H.; Kirsh, C.; Simcox, J. Aging adipose: Depot location dictates age-associated expansion and dysfunction. Ageing Res. Rev. 2021, 67, 101259. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, M.; Palmer, J.; Valtos, J.; Iasiello, C.; Sowers, J. Metabolic syndrome in the elderly. Curr. Diab Rep. 2006, 6, 64–71. [Google Scholar] [CrossRef]
- McCormick, R.; Vasilaki, A. Age-related changes in skeletal muscle: Changes to life-style as a therapy. Biogerontology 2018, 19, 519–536. [Google Scholar] [CrossRef]
- Elia, M. Organ and Tissue Contribution to Metabolic Rate. In Energy Metabolism: Tissue Determinants and Cellular Corollaries; Kinney, J.M., Tucker, H.N., Eds.; Raven Press: New York, NY, USA, 1992; pp. 61–79. [Google Scholar]
- Pontzer, H.; Yamada, Y.; Sagayama, H.; Ainslie, P.N.; Andersen, L.F.; Anderson, L.J.; Arab, L.; Baddou, I.; Bedu-Addo, K.; Blaak, E.E.; et al. Daily energy expenditure through the human life course. Science 2021, 373, 808–812. [Google Scholar] [CrossRef]
- Manini, T.M. Energy expenditure and aging. Ageing Res. Rev. 2010, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, F.; Zhu, W.L. Evidence for the ‘rate-of-living’ hypothesis between mammals and lizards, but not in birds, with field metabolic rate. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2021, 253, 110867. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Pahlavani, M.; Kalupahana, N.S.; Ramalingam, L.; Moustaid-Moussa, N. Regulation and Functions of the Renin-Angiotensin System in White and Brown Adipose Tissue. Compr. Physiol. 2017, 7, 1137–1150. [Google Scholar] [CrossRef]
- Luther, J.M.; Brown, N.J. The renin-angiotensin-aldosterone system and glucose homeostasis. Trends Pharmacol. Sci. 2011, 32, 734–739. [Google Scholar] [CrossRef]
- Werner, C.; Baumhakel, M.; Teo, K.K.; Schmieder, R.; Mann, J.; Unger, T.; Yusuf, S.; Bohm, M. RAS blockade with ARB and ACE inhibitors: Current perspective on rationale and patient selection. Clin. Res. Cardiol. 2008, 97, 418–431. [Google Scholar] [CrossRef]
- Bhandari, S.; Mehta, S.; Khwaja, A.; Cleland, J.G.F.; Ives, N.; Brettell, E.; Chadburn, M.; Cockwell, P.; Investigators, S.A.T. Renin-Angiotensin System Inhibition in Advanced Chronic Kidney Disease. N. Engl. J. Med. 2022, 387, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Sumukadas, D.; Witham, M.D.; Struthers, A.D.; McMurdo, M.E. Effect of perindopril on physical function in elderly people with functional impairment: A randomized controlled trial. CMAJ 2007, 177, 867–874. [Google Scholar] [CrossRef]
- Onder, G.; Penninx, B.W.; Balkrishnan, R.; Fried, L.P.; Chaves, P.H.; Williamson, J.; Carter, C.; Di Bari, M.; Guralnik, J.M.; Pahor, M. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: An observational study. Lancet 2002, 359, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Di Bari, M.; van de Poll-Franse, L.V.; Onder, G.; Kritchevsky, S.B.; Newman, A.; Harris, T.B.; Williamson, J.D.; Marchionni, N.; Pahor, M.; Health, A.; et al. Antihypertensive medications and differences in muscle mass in older persons: The Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2004, 52, 961–966. [Google Scholar] [CrossRef]
- Buford, T.W.; Manini, T.M.; Hsu, F.C.; Cesari, M.; Anton, S.D.; Nayfield, S.; Stafford, R.S.; Church, T.S.; Pahor, M.; Carter, C.S. Angiotensin-converting enzyme inhibitor use by older adults is associated with greater functional responses to exercise. J. Am. Geriatr. Soc. 2012, 60, 1244–1252. [Google Scholar] [CrossRef]
- Caulfield, L.; Heslop, P.; Walesby, K.E.; Sumukadas, D.; Sayer, A.A.; Witham, M.D. Effect of Angiotensin System Inhibitors on Physical Performance in Older People—A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2021, 22, 1215–1221. e2. [Google Scholar] [CrossRef]
- The LACE study group; Achison, M.; Adamson, S.; Akpan, A.; Aspray, T.; Avenell, A.; Band, M.M.; Bashir, T.; Burton, L.A.; Cvoro, V.; et al. Effect of perindopril or leucine on physical performance in older people with sarcopenia: The LACE randomized controlled trial. J. Cachexia Sarcopenia Muscle 2022, 13, 858–871. [Google Scholar] [CrossRef]
- Houard, X.; Williams, T.A.; Michaud, A.; Dani, P.; Isaac, R.E.; Shirras, A.D.; Coates, D.; Corvol, P. The Drosophila melanogaster-related angiotensin-I-converting enzymes Acer and Ance--distinct enzymic characteristics and alternative expression during pupal development. Eur. J. Biochem. 1998, 257, 599–606. [Google Scholar] [CrossRef]
- Herrera, P.; Cauchi, R.J. Functional characterisation of the ACE2 orthologues in Drosophila provides insights into the neuromuscular complications of COVID-19. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166818. [Google Scholar] [CrossRef]
- Cornell, M.J.; Williams, T.A.; Lamango, N.S.; Coates, D.; Corvol, P.; Soubrier, F.; Hoheisel, J.; Lehrach, H.; Isaac, R.E. Cloning and expression of an evolutionary conserved single-domain angiotensin converting enzyme from Drosophila melanogaster. J. Biol. Chem. 1995, 270, 13613–13619. [Google Scholar] [CrossRef] [PubMed]
- Bingham, R.J.; Dive, V.; Phillips, S.E.; Shirras, A.D.; Isaac, R.E. Structural diversity of angiotensin-converting enzyme. FEBS J. 2006, 273, 362–373. [Google Scholar] [CrossRef]
- Boggs, C.L. Understanding insect life histories and senescence through a resource allocation lens. Funct. Ecol. 2009, 23, 27–37. [Google Scholar] [CrossRef]
- Hurst, D.; Rylett, C.M.; Isaac, R.E.; Shirras, A.D. The drosophila angiotensin-converting enzyme homologue Ance is required for spermiogenesis. Dev. Biol. 2003, 254, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Gabrawy, M.M.; Campbell, S.; Carbone, M.A.; Morozova, T.V.; Arya, G.H.; Turlapati, L.B.; Walston, J.D.; Starz-Gaiano, M.; Everett, L.; Mackay, T.F.C.; et al. Lisinopril Preserves Physical Resilience and Extends Life Span in a Genotype-Specific Manner in Drosophila melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1844–1852. [Google Scholar] [CrossRef]
- Karlsen, T.; Nauman, J.; Dalen, H.; Langhammer, A.; Wisloff, U. The Combined Association of Skeletal Muscle Strength and Physical Activity on Mortality in Older Women: The HUNT2 Study. Mayo Clin. Proc. 2017, 92, 710–718. [Google Scholar] [CrossRef]
- Arvandi, M.; Strasser, B.; Meisinger, C.; Volaklis, K.; Gothe, R.M.; Siebert, U.; Ladwig, K.H.; Grill, E.; Horsch, A.; Laxy, M.; et al. Gender differences in the association between grip strength and mortality in older adults: Results from the KORA-age study. BMC Geriatr. 2016, 16, 201. [Google Scholar] [CrossRef]
- Demontis, F.; Perrimon, N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell 2010, 143, 813–825. [Google Scholar] [CrossRef]
- Vrailas-Mortimer, A.; del Rivero, T.; Mukherjee, S.; Nag, S.; Gaitanidis, A.; Kadas, D.; Consoulas, C.; Duttaroy, A.; Sanyal, S. A muscle-specific p38 MAPK/Mef2/MnSOD pathway regulates stress, motor function, and life span in Drosophila. Dev. Cell 2011, 21, 783–795. [Google Scholar] [CrossRef]
- Bowden Davies, K.A.; Pickles, S.; Sprung, V.S.; Kemp, G.J.; Alam, U.; Moore, D.R.; Tahrani, A.A.; Cuthbertson, D.J. Reduced physical activity in young and older adults: Metabolic and musculoskeletal implications. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819888824. [Google Scholar] [CrossRef] [PubMed]
- Kerr, N.R.; Booth, F.W. Contributions of physical inactivity and sedentary behavior to metabolic and endocrine diseases. Trends Endocrinol. Metab. 2022, 33, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Protasiewicz, J.; Snider, S.; Khan, M.; Tao, L.; Wessells, R.J.; Sujkowski, A. A new Drosophila model of prolonged inactivity shortens lifespan and impairs muscle function. Sci. Rep. 2025, 15, 27908. [Google Scholar] [CrossRef]
- Liao, F.T.; Chang, C.Y.; Su, M.T.; Kuo, W.C. Necessity of angiotensin-converting enzyme-related gene for cardiac functions and longevity of Drosophila melanogaster assessed by optical coherence tomography. J. Biomed. Opt. 2014, 19, 011014. [Google Scholar] [CrossRef]
- Glover, Z.; Hodges, M.D.; Dravecz, N.; Cameron, J.; Askwith, H.; Shirras, A.; Broughton, S.J. Loss of angiotensin-converting enzyme-related (ACER) peptidase disrupts behavioural and metabolic responses to diet in Drosophila melanogaster. J. Exp. Biol. 2019, 222, jeb194332. [Google Scholar] [CrossRef]
- Mackay, T.F.; Richards, S.; Stone, E.A.; Barbadilla, A.; Ayroles, J.F.; Zhu, D.; Casillas, S.; Han, Y.; Magwire, M.M.; Cridland, J.M.; et al. The Drosophila melanogaster Genetic Reference Panel. Nature 2012, 482, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Massouras, A.; Inoue, Y.; Peiffer, J.; Ramia, M.; Tarone, A.M.; Turlapati, L.; Zichner, T.; Zhu, D.; Lyman, R.F.; et al. Natural variation in genome architecture among 205 Drosophila melanogaster Genetic Reference Panel lines. Genome Res. 2014, 24, 1193–1208. [Google Scholar] [CrossRef] [PubMed]
- Ghalayini, J.; Boulianne, G.L. Deciphering mechanisms of action of ACE inhibitors in neurodegeneration using Drosophila models of Alzheimer’s disease. Front. Neurosci. 2023, 17, 1166973. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Smith, H.; Smith, C.A.; Coward, L.; Gorman, G.; De Luca, M.; Jumbo-Lucioni, P. The Angiotensin-Converting Enzyme Inhibitor Lisinopril Mitigates Memory and Motor Deficits in a Drosophila Model of Alzheimer’s Disease. Pathophysiology 2021, 28, 307–319. [Google Scholar] [CrossRef]
- Cole, S.L.; Vassar, R. The role of amyloid precursor protein processing by BACE1, the beta-secretase, in Alzheimer disease pathophysiology. J. Biol. Chem. 2008, 283, 29621–29625. [Google Scholar] [CrossRef]
- Lee, S.H.; Gomes, S.M.; Ghalayini, J.; Iliadi, K.G.; Boulianne, G.L. Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Rescue Memory Defects in Drosophila-Expressing Alzheimer’s Disease-Related Transgenes Independently of the Canonical Renin Angiotensin System. eNeuro 2020, 7, ENEURO.0235-20.2020. [Google Scholar] [CrossRef]
- Ederer, K.A.; Jin, K.; Bouslog, S.; Wang, L.; Gorman, G.S.; Rowe, G.C.; Abadir, P.; Raftery, D.; Moellering, D.; Promislow, D.; et al. Age- and Genotype-Specific Effects of the Angiotensin-Converting Enzyme Inhibitor Lisinopril on Mitochondrial and Metabolic Parameters in Drosophila melanogaster. Int. J. Mol. Sci. 2018, 19, 3351. [Google Scholar] [CrossRef]
- de Cavanagh, E.M.; Inserra, F.; Ferder, L. Angiotensin II blockade: A strategy to slow ageing by protecting mitochondria? Cardiovasc. Res. 2011, 89, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Vajapey, R.; Rini, D.; Walston, J.; Abadir, P. The impact of age-related dysregulation of the angiotensin system on mitochondrial redox balance. Front. Physiol. 2014, 5, 439. [Google Scholar] [CrossRef] [PubMed]
- Vecchie, D.; Wolter, J.M.; Perry, J.; Jumbo-Lucioni, P.; De Luca, M. The Impact of the Angiotensin-Converting Enzyme Inhibitor Lisinopril on Metabolic Rate in Drosophila melanogaster. Int. J. Mol. Sci. 2024, 25, 10103. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, L.; Saville, M.E.; Young, J.B. Sympathoadrenal system and regulation of thermogenesis. Am. J. Physiol. 1984, 247, E181–E189. [Google Scholar] [CrossRef]
- Alba, B.K.; Castellani, J.W.; Charkoudian, N. Cold-induced cutaneous vasoconstriction in humans: Function, dysfunction and the distinctly counterproductive. Exp. Physiol. 2019, 104, 1202–1214. [Google Scholar] [CrossRef]
- Xiao, F.; Jiang, H.; Li, Z.; Jiang, X.; Chen, S.; Niu, Y.; Yin, H.; Shu, Y.; Peng, B.; Lu, W.; et al. Reduced hepatic bradykinin degradation accounts for cold-induced BAT thermogenesis and WAT browning in male mice. Nat. Commun. 2023, 14, 2523. [Google Scholar] [CrossRef]
- Geneva, I.I.; Cuzzo, B.; Fazili, T.; Javaid, W. Normal Body Temperature: A Systematic Review. Open Forum Infect. Dis. 2019, 6, ofz032. [Google Scholar] [CrossRef]
- Rogers, N.H. Brown adipose tissue during puberty and with aging. Ann. Med. 2015, 47, 142–149. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vecchie’, D.; Faber, V.G.; Jumbo-Lucioni, P.; Anholt, R.R.H.; Mackay, T.F.C.; De Luca, M. Angiotensin-Converting Enzyme Inhibitors and Metabolic Aging: A Drosophila Perspective. Biomolecules 2025, 15, 1378. https://doi.org/10.3390/biom15101378
Vecchie’ D, Faber VG, Jumbo-Lucioni P, Anholt RRH, Mackay TFC, De Luca M. Angiotensin-Converting Enzyme Inhibitors and Metabolic Aging: A Drosophila Perspective. Biomolecules. 2025; 15(10):1378. https://doi.org/10.3390/biom15101378
Chicago/Turabian StyleVecchie’, Denise, Victoria G. Faber, Patricia Jumbo-Lucioni, Robert R. H. Anholt, Trudy F. C. Mackay, and Maria De Luca. 2025. "Angiotensin-Converting Enzyme Inhibitors and Metabolic Aging: A Drosophila Perspective" Biomolecules 15, no. 10: 1378. https://doi.org/10.3390/biom15101378
APA StyleVecchie’, D., Faber, V. G., Jumbo-Lucioni, P., Anholt, R. R. H., Mackay, T. F. C., & De Luca, M. (2025). Angiotensin-Converting Enzyme Inhibitors and Metabolic Aging: A Drosophila Perspective. Biomolecules, 15(10), 1378. https://doi.org/10.3390/biom15101378






