Antibiofilm Inhibitor Ferulic Acid as an Antibacterial Synergist Against Escherichia coli
Abstract
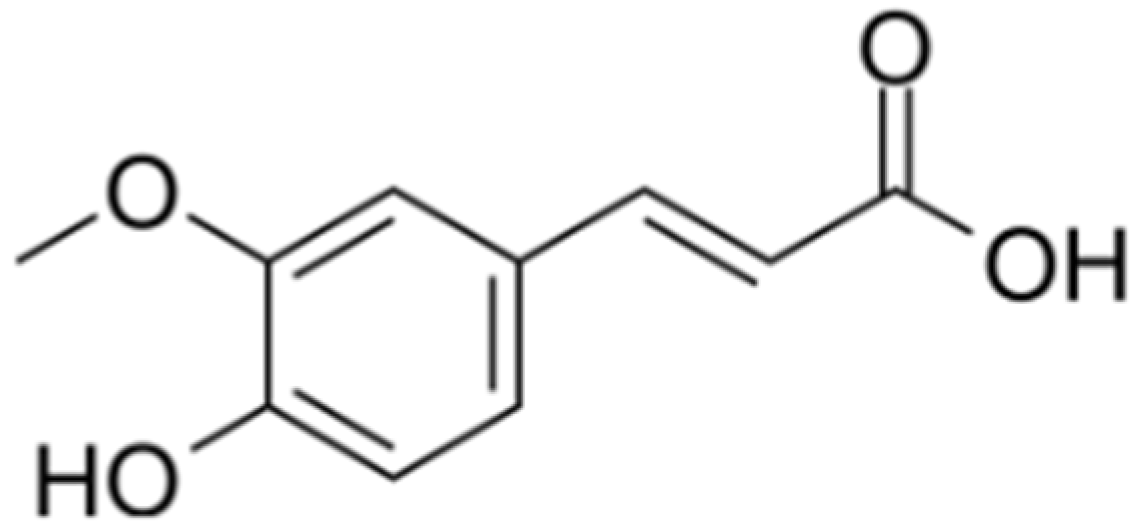
1. Introduction
2. Materials and Methods
2.1. Materials and Bacterial Strains
2.2. Growth and Metabolic Activity
- Eoxi(OD570) = 80,586: Molar extinction coefficient of oxidized AB at 570 nm;
- Eoxi(OD600) = 117,216: Molar extinction coefficient of oxidized AB at 600 nm;
- Ered(OD570) = 155,677: Molar extinction coefficient of reduced AB at 570 nm;
- Ered(OD600) = 14,652: Molar extinction coefficient of reduced AB at 600 nm;
- T: Test sample; B: Blank control.
2.3. Cytotoxicity
2.4. Biofilm Assay
2.4.1. Biofilm Inhibition Assay
2.4.2. Scanning Electron Microscopy (SEM) Analysis
2.5. EPS Production
2.6. Motility Assay
2.7. qRT-PCR
2.8. Synergistic Antibacterial Activity of FA Combined with Antibiotics
2.9. Statistical Analysis
3. Results
3.1. Effects of FA on Growth and Metabolic Activity of E. coli
3.2. Cytotoxicity of FA on Caco-2 Cells
3.3. The Effect of FA on E. coli Biofilm
3.4. Effects of FA on EPS Production of E. coli
3.5. Effects of FA on the Motility of E. coli
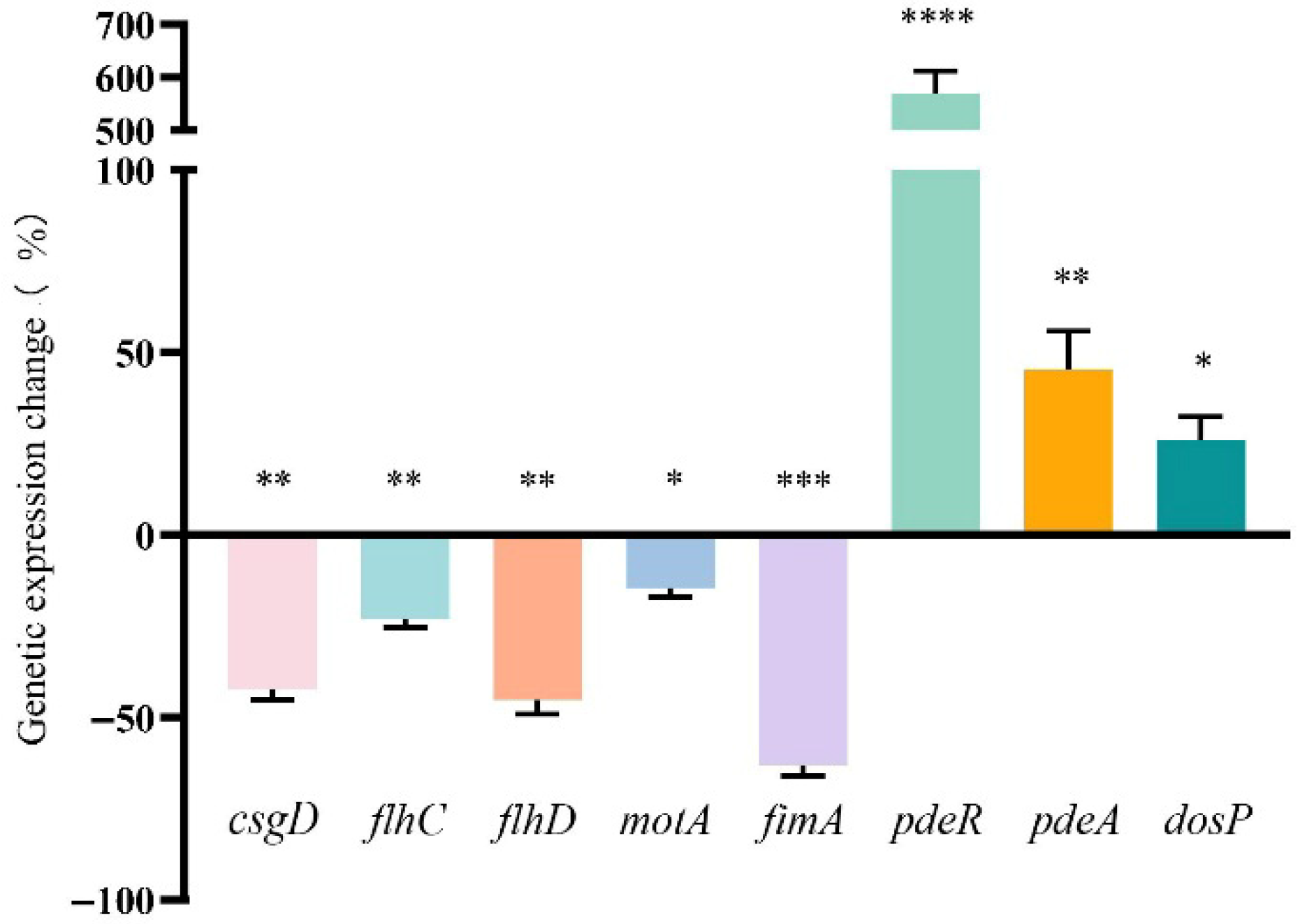
3.6. Effect of FA on the Transcription of Biofilm-Regulated Genes of E. coli
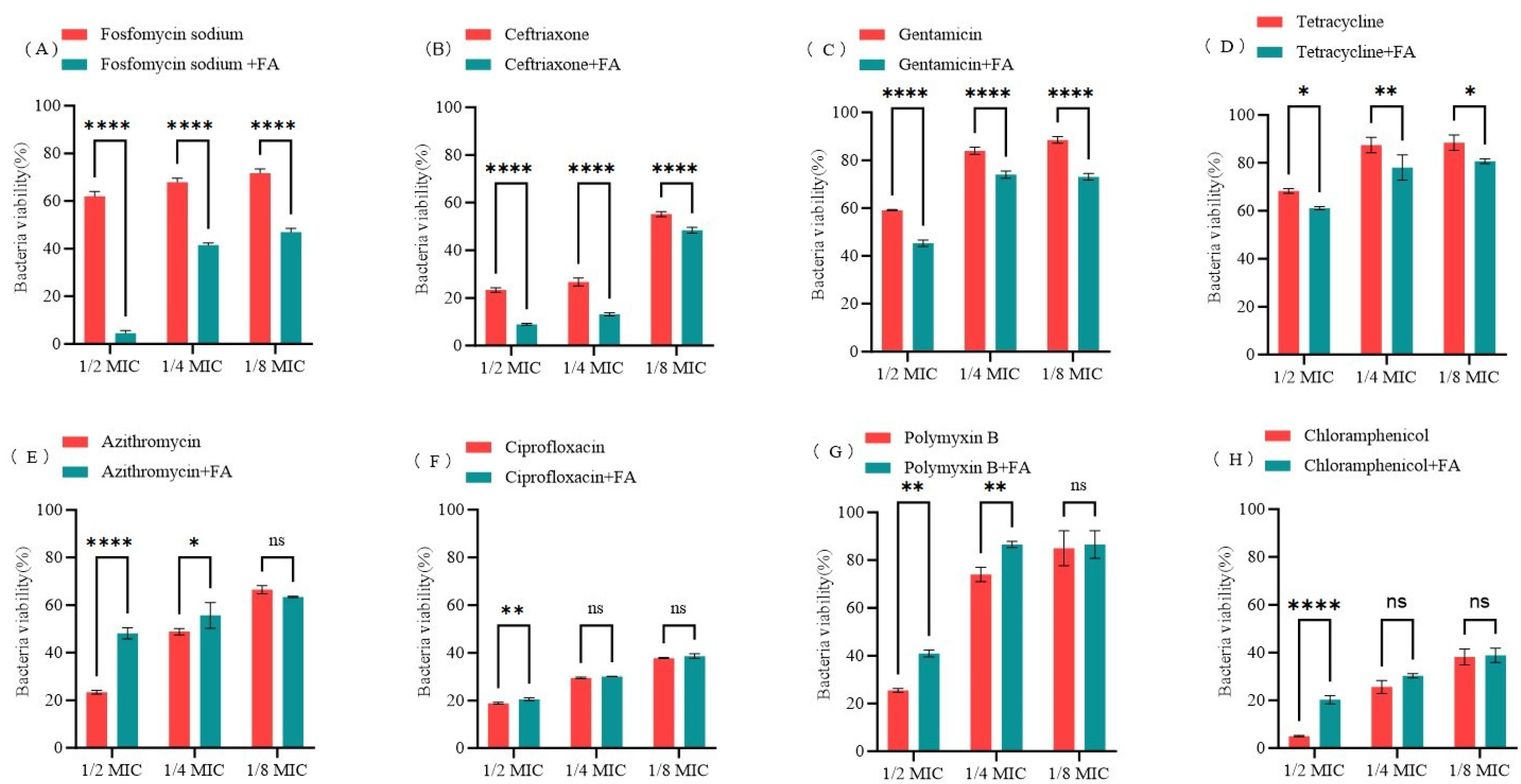
3.7. Synergistic Effects of FA in Combination with Antibiotics Against E. coli
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miquel, S.; Lagrafeuille, R.; Souweine, B.; Forestier, C. Anti-Biofilm Activity as a Health Issue. Front. Microbiol. 2016, 7, 592. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Tarar, S.M.; Gul, I.; Nawaz, U.; Arshad, M. Challenges of antibiotic resistance biofilms and potential combating strategies: A review. 3 Biotech 2021, 11, 169. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for Combating Bacterial Biofilms: A Focus on Anti-Biofilm Agents and Their Mechanisms of Action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Tascini, C.; Sozio, E.; Corte, L.; Sbrana, F.; Scarparo, C.; Ripoli, A.; Bertolino, G.; Merelli, M.; Tagliaferri, E.; Corcione, A.; et al. The Role of Biofilm Forming on Mortality in Patients with Candidemia: A Study Derived from Real World Data. Infect. Dis. 2018, 50, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, X.; Yu, C.; Wang, Y. Promising Therapeutic Strategies Against Microbial Biofilm Challenges. Front. Cell. Infect. Microbiol. 2020, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liang, X.; Gadd, G.M.; Zhao, Q. Superhydrophobic Coatings for Urinary Catheters to Delay Bacterial Biofilm Formation and Catheter-Associated Urinary Tract Infection. ACS Appl. Bio Mater. 2020, 3, 282–291. [Google Scholar] [CrossRef]
- Berra, L.; De Marchi, L.; Yu, Z.-X.; Laquerriere, P.; Baccarelli, A.; Kolobow, T. Endotracheal Tubes Coated with Antiseptics Decrease Bacterial Colonization of the Ventilator Circuits, Lungs, and Endotracheal Tube. Anesthesiology 2004, 100, 1446–1456. [Google Scholar] [CrossRef]
- Ramos, S.; Silva, V.; Dapkevicius, M.L.E.; Caniça, M.; Tejedor-Junco, M.T.; Igrejas, G.; Poeta, P. Escherichia coli as Commensal and Pathogenic Bacteria Among Food-Producing Animals: Health Implications of Extended Spectrum β-Lactamase (ESBL) Production. Animals 2020, 10, 2239. [Google Scholar] [CrossRef]
- Newell, D.G.; La Ragione, R.M. Enterohaemorrhagic and Other Shiga Toxin-Producing Escherichia coli (STEC): Where Are We Now Regarding Diagnostics and Control Strategies? Transbound. Emerg. Dis. 2018, 65, 49–71. [Google Scholar] [CrossRef]
- Poirel, L.; Madec, J.Y.; Lupo, A.; Schink, A.K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 10-1128. [Google Scholar] [CrossRef]
- Danese, P.N.; Pratt, L.A.; Kolter, R. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J. Bacteriol. 2000, 182, 3593–3596. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Yin, F.; Wang, S.; Zhao, A.; Li, Y.; Liu, Y. Helicobacter Pylori Biofilm-Related Drug Resistance and New Developments in Its Anti-Biofilm Agents. Infect. Drug Resist. 2022, 15, 1561–1571. [Google Scholar] [CrossRef]
- Nahum, Y.; Muhvich, J.; Morones-Ramirez, J.R.; Casillas-Vega, N.G.; Zaman, M.H. Biofilms as Potential Reservoirs of Antimicrobial Resistance in Vulnerable Settings. Front. Public Health 2025, 13, 1568463. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar]
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on Major Food-Borne Zoonotic Bacterial Pathogens. J. Trop. Med. 2020, 2020, 4674235. [Google Scholar] [CrossRef]
- Flemming, H.C.; Neu, T.R.; Wozniak, D.J. The EPS Matrix: The “House of Biofilm Cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef]
- Branda, S.S.; Vik, Å.; Friedman, L.; Kolter, R. Biofilms: The Matrix Revisited. Trends Microbiol. 2005, 13, 20–26. [Google Scholar] [CrossRef]
- Chusri, S.; Na Phatthalung, P.; Voravuthikunchai, S. Anti-Biofilm Activity of Quercus infectoria G. Olivier against Methicillin-Resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2012, 54, 511–517. [Google Scholar] [CrossRef]
- Mirzaei, A.; Esfahani, B.N.; Ghanadian, M.; Moghim, S. Alhagi Maurorum Extract Modulates Quorum Sensing Genes and Biofilm Formation in Proteus Mirabilis. Sci. Rep. 2022, 12, 13992. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.M.F.; Winans, S.C.; Pinto, U.M. Quorum Sensing Interference by Phenolic Compounds—A Matter of Bacterial Misunderstanding. Heliyon 2023, 9, e17657. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, W.; Shi, M.; Wei, X.; Zhou, X.; Li, B.; Zhang, J. Novel Antibiofilm Inhibitor Ginkgetin as an Antibacterial Synergist against Escherichia coli. Int. J. Mol. Sci. 2022, 23, 8809. [Google Scholar] [CrossRef]
- Zhou, J.-W.; Chen, T.-T.; Tan, X.-J.; Sheng, J.-Y.; Jia, A.-Q. Can the Quorum Sensing Inhibitor Resveratrol Function as an Aminoglycoside Antibiotic Accelerant against Pseudomonas Aeruginosa? Int. J. Antimicrob. Agents 2018, 52, 35–41. [Google Scholar] [CrossRef]
- Stompor-Gorący, M.; Machaczka, M. Recent Advances in Biological Activity, New Formulations and Prodrugs of Ferulic Acid. Int. J. Mol. Sci. 2021, 22, 12889. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, J.R.; Rizwanullah, M. Ferulic Acid: A Comprehensive Review. Cureus 2024, 16, e68063. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Choi, J.; Park, J.; Kim, K.; Lee, H.; Kim, S. In vitro and in vivo antithrombotic and cytotoxicity effects of ferulic acid. J. Biochem. Mol. Toxicol. 2018, 32, e22004. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Tirawanchai, N.; Komindr, S. Ferulic Acid Supplementation Improves Lipid Profiles, Oxidative Stress, and Inflammatory Status in Hyperlipidemic Subjects: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2018, 10, 713. [Google Scholar] [CrossRef]
- Chaikijurajai, T.; Tang, W.H.W. Myeloperoxidase: A potential therapeutic target for coronary artery disease. Expert Opin. Ther. Targets 2020, 24, 695–705. [Google Scholar] [CrossRef]
- Wu, X.; Hu, Z.; Zhou, J.; Liu, J.; Ren, P.; Huang, X. Ferulic Acid Alleviates Atherosclerotic Plaques by Inhibiting VSMC Proliferation Through the NO/p21 Signaling pathway. J. Cardiovasc. Transl. Res. 2022, 15, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Mastoor, S.; Nazim, F.; Rizwan-Ul-Hasan, S.; Ahmed, K.; Khan, S.; Ali, S.N.; Abidi, S.H. Analysis of the Antimicrobial and Anti-Biofilm Activity of Natural Compounds and Their Analogues against Staphylococcus aureus Isolates. Molecules 2022, 27, 6874. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, S.; Barik, S.; Muralitharan, G.; Busi, S. Ferulic acid encapsulated chitosan-tripolyphosphate nanoparticles attenuate quorum sensing regulated virulence and biofilm formation in Pseudomonas aeruginosa PAO1. IET Nanobiotechnol. 2018, 12, 1056–1061. [Google Scholar] [CrossRef]
- Hassan, M.M.; Albogami, B.; Mwabvu, T.; Awad, M.F.; Alorabi, J.A.A.; Hassan, M.M.; Al-Harthi, H.F.; Kadi, R.H.; Almohaimeed, H.M. Molecular characterization of virulence genes and influence of Xanthium strumarium extract against two Enterobacter species isolated from some soil invertebrates. Cell. Mol. Biol. 2024, 70, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Xu, M.; McCanna, D.J.; Sivak, J.G. Use of the Viability Reagent PrestoBlue in Comparison with alamarBlue and MTT to Assess the Viability of Human Corneal Epithelial Cells. J. Pharmacol. Toxicol. Methods 2015, 71, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-G.; Gao, Y.; He, J.-G.; Xu, W.-F.; Jiang, M.; Jin, H.-S. Effects of azithromycin on Pseudomonas aeruginosa isolates from catheter-associated urinary tract infection. Exp. Ther. Med. 2015, 9, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Rudin, L.; Roth, N.; Kneubühler, J.; Dubey, B.N.; Bornstein, M.M.; Shyp, V. Inhibitory Effect of Natural Flavone Luteolin on Streptococcus Mutans Biofilm Formation. Microbiol. Spectr. 2023, 11, e0522322. [Google Scholar] [CrossRef]
- Braet, F.; De Zanger, R.; Wisse, E. Drying Cells for SEM, AFM and TEM by Hexamethyldisilazane: A Study on Hepatic Endothelial Cells. J. Microsc. 1997, 186, 84–87. [Google Scholar] [CrossRef]
- Nair, M.S.; Upadhyay, A.; Fancher, S.; Upadhyaya, I.; Dey, S.; Kollanoor-Johny, A.; Zhao, J.; Venkitanarayanan, K. Inhibition and Inactivation of Escherichia coli O157:H7 Biofilms by Selenium. J. Food Prot. 2018, 81, 926–933. [Google Scholar] [CrossRef]
- Vikram, A.; Jayaprakasha, G.K.; Uckoo, R.M.; Patil, B.S. Inhibition of Escherichia coli O157:H7 Motility and Biofilm by β-Sitosterol Glucoside. Biochim. Biophys. Acta 2013, 1830, 5219–5228. [Google Scholar] [CrossRef]
- Pan, Y.; Zhao, Y.; Zeng, H.-R.; Wu, J.-Q.; Song, Y.-Y.; Rao, Y.-H.; Li, G.-Q.; Jin, L. Reference Genes for Expression Analyses by qRT-PCR in Enterobacter cancerogenus. Microorganisms 2024, 12, 1024. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, S.; Sharma, P.; Chandola, D.; Dang, S.; Gupta, S.; Gabrani, R. Escherichia coli Biofilm: Development and Therapeutic Strategies. J. Appl. Microbiol. 2016, 121, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Cugini, C.; Shanmugam, M.; Landge, N.; Ramasubbu, N. The Role of Exopolysaccharides in Oral Biofilms. J. Dent. Res. 2019, 98, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, J.; Zhou, X.; Li, Y. Inhibition of Streptococcus Mutans Biofilm Formation by Strategies Targeting the Metabolism of Exopolysaccharides. Crit. Rev. Microbiol. 2021, 47, 667–677. [Google Scholar] [CrossRef]
- Vaikkathillam, P.; Mini, M.; Mohan, A.; Jayakumar, D.; Rajan, P.P.; Asha, S.; Kumar, P. Anti-biofilm effect of ferulic acid against Enterobacter hormaechei and Klebsiella pneumoniae: In vitro and in silico investigation. Biofouling 2025, 41, 157–170. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, J.; Liu, N.; Sun, W.; Yu, B.; Niu, H.; Liu, D.; Ouyang, P.; Ying, H.; Chen, Y.; et al. Type I Fimbriae Subunit fimA Enhances Escherichia coli Biofilm Formation but Affects L-Threonine Carbon Distribution. Front. Bioeng. Biotechnol. 2022, 10, 904636. [Google Scholar] [CrossRef]
- Miller, A.L.; Bessho, S.; Grando, K.; Tükel, Ç. Microbiome or Infections: Amyloid-Containing Biofilms as a Trigger for Complex Human Diseases. Front. Immunol. 2021, 12, 638867. [Google Scholar] [CrossRef]
- Kuboniwa, M.; Amano, A.; Inaba, H.; Hashino, E.; Shizukuishi, S. Homotypic biofilm structure of Porphyromonas gingivalis is affected by FimA type variations. Oral Microbiol. Immunol. 2009, 24, 260–263. [Google Scholar] [CrossRef]
- Suzuki, I.; Shimizu, T.; Senpuku, H. Role of SCFAs for Fimbrillin-Dependent Biofilm Formation of Actinomyces oris. Microorganisms 2018, 6, 114. [Google Scholar] [CrossRef]
- Song, Y.J.; Yu, H.H.; Kim, Y.J.; Lee, N.-K.; Paik, H.-D. Anti-Biofilm Activity of Grapefruit Seed Extract against Staphylococcus Aureus and Escherichia coli. J. Microbiol. Biotechnol. 2019, 29, 1177–1183. [Google Scholar] [CrossRef]
- He, X.; Ding, H.; Gao, Z.; Zhang, X.; Wu, R.; Li, K. Variations in the Motility and Biofilm Formation Abilities of Escherichia coli O157:H7 during Noodle Processing. Food Res. Int. 2023, 168, 112670. [Google Scholar] [CrossRef] [PubMed]
- Benyoussef, W.; Deforet, M.; Monmeyran, A.; Henry, N. Flagellar Motility During E. coli Biofilm Formation Provides a Competitive Disadvantage Which Recedes in the Presence of Co-Colonizers. Front. Cell. Infect. Microbiol. 2022, 12, 896898. [Google Scholar]
- Haiko, J.; Westerlund-Wikström, B. The role of the bacterial flagellum in adhesion and virulence. Biology 2013, 2, 1242–1267. [Google Scholar] [CrossRef]
- Friedlander, R.S.; Vogel, N.; Aizenberg, J. Role of Flagella in Adhesion of Escherichia coli to Abiotic Surfaces. Langmuir 2015, 31, 6137–6144. [Google Scholar] [CrossRef] [PubMed]
- Opoku-Temeng, C.; Sintim, H.O. Targeting C-Di-GMP Signaling, Biofilm Formation, and Bacterial Motility with Small Molecules. Methods Mol. Biol. 2017, 1657, 419–430. [Google Scholar] [PubMed]
- He, Y.; Jia, W.; Chi, S.; Meng, Q.; Chen, Y.; Wang, X. Research progress of c-di-GMP in the regulation of Escherichia coli biofilm. Sheng Wu Gong Cheng Xue Bao 2022, 38, 2811–2820. [Google Scholar]
- Banerjee, P.; Sahoo, P.K.; Sheenu; Adhikary, A.; Ruhal, R.; Jain, D. Molecular and structural facets of c-di-GMP signalling associated with biofilm formation in Pseudomonas aeruginosa. Mol. Asp. Med. 2021, 81, 101001. [Google Scholar] [CrossRef]
- Prentice, J.A.; Bridges, A.A.; Bassler, B.L. Synergy between c-di-GMP and Quorum-Sensing Signaling in Vibrio cholerae Biofilm Morphogenesis. J. Bacteriol. 2022, 204, e0024922. [Google Scholar] [CrossRef]
- Xue, X.F.; Zhang, M.M.; Sun, J.F.; Li, X.; Wu, Q.M.; Yin, Z.; Yang, W.H.; Ni, B.; Hu, L.F.; Zhou, D.S.; et al. H-NS Represses Biofilm Formation and c-di-GMP Synthesis in Vibrio parahaemolyticus. Biomed. Environ. Sci. 2022, 35, 821–829. [Google Scholar]
- Chua, S.L.; Sivakumar, K.; Rybtke, M.; Yuan, M.; Andersen, J.B.; Nielsen, T.E.; Givskov, M.; Tolker-Nielsen, T.; Cao, B.; Kjelleberg, S.; et al. C-di-GMP regulates Pseudomonas aeruginosa stress response to tellurite during both planktonic and biofilm modes of growth. Sci. Rep. 2015, 5, 10052. [Google Scholar] [CrossRef]
- Sheppard, D.C.; Howell, P.L. Biofilm Exopolysaccharides of Pathogenic Fungi: Lessons from Bacteria. J. Biol. Chem. 2016, 291, 12529–12537. [Google Scholar] [CrossRef] [PubMed]
- Steiner, S.; Lori, C.; Boehm, A.; Jenal, U. Allosteric activation of exopolysaccharide synthesis through cyclic di-GMP-stimulated protein-protein interaction. EMBO J. 2013, 32, 354–368. [Google Scholar] [CrossRef]
- Zhang, Y.; Sass, A.; Van Acker, H.; Wille, J.; Verhasselt, B.; Van Nieuwerburgh, F.; Kaever, V.; Crabbé, A.; Coenye, T. Coumarin Reduces Virulence and Biofilm Formation in Pseudomonas aeruginosa by Affecting Quorum Sensing, Type III Secretion and C-di-GMP Levels. Front. Microbiol. 2018, 9, 1952. [Google Scholar] [CrossRef]
- Lamprecht, O.; Ratnikava, M.; Jacek, P.; Kaganovitch, E.; Buettner, N.; Fritz, K.; Biazruchka, I.; Köhler, R.; Pietsch, J.; Sourjik, V.; et al. Regulation by Cyclic Di-GMP Attenuates Dynamics and Enhances Robustness of Bimodal Curli Gene Activation in Escherichia coli. PLOS Genet. 2023, 19, e1010750. [Google Scholar] [CrossRef] [PubMed]
- Gilles-Gonzalez, M.-A.; Sousa, E.H.S. Escherichia coli DosC and DosP: A Role of c-Di-GMP in Compartmentalized Sensing by Degradosomes. Adv. Microb. Physiol. 2019, 75, 53–67. [Google Scholar] [PubMed]
- Venkatesan, N.; Perumal, G.; Doble, M. Bacterial Resistance in Biofilm-Associated Bacteria. Futur. Microbiol. 2015, 10, 1743–1750. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Coppo, E.; Barbieri, R.; Barreca, D.; Chebaibi, S.; Sobarzo-Sanchez, E.; Nabavi, S.F.; Nabavi, S.M.; Daglia, M. The Natural Plant Compound Carvacrol as an Antimicrobial and Anti-Biofilm Agent: Mechanisms, Synergies and Bio-Inspired Anti-Infective Materials. Biofouling 2018, 34, 630–656. [Google Scholar] [CrossRef]
- Gilbert-Girard, S.; Reigada, I.; Savijoki, K.; Yli-Kauhaluoma, J.; Fallarero, A. Screening of natural compounds identifies ferutinin as an antibacterial and anti-biofilm compound. Biofouling 2021, 37, 791–807. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, P.; Lv, H.; Deng, X.; Wang, J. A Natural Dietary Flavone Myricetin as an α-Hemolysin Inhibitor for Controlling Staphylococcus Aureus Infection. Front. Cell. Infect. Microbiol. 2020, 10, 330. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Huang, C.-H.; Huang, Y.-C.; Yen, C.-L.; Hsu, C.-R.; Ferran, A.A. Anti-Biofilm Activities and Antibiotic Synergy of Naturally Occurring Compounds against Drug-Resistant Rapidly Growing Mycobacteria. Microbiol. Spectr. 2024, 12, e0019924. [Google Scholar] [CrossRef]
- Samreen; Siddiqui, S.A.; Ahmad, I. Harnessing Anti-Infective Efficacy of Cinnamomum Verum in Synergy with β-Lactam and Fluoroquinolones Drugs to Combat Virulence and Biofilms of Pseudomonas Aeruginosa PAO1. Microb. Pathog. 2024, 197, 107097. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.D.; Paterna, N.J.; Senetra, A.S.; Casey, K.R.; Trieu, P.D.; Caputo, G.A.; Vaden, T.D.; Carone, B.R. Synergistic interactions of ionic liquids and antimicrobials improve drug efficacy. iScience 2020, 24, 101853. [Google Scholar] [CrossRef] [PubMed]
- Florio, W.; Rizzato, C.; Becherini, S.; Guazzelli, L.; D’aNdrea, F.; Lupetti, A. Synergistic activity between colistin and the ionic liquids 1-methyl-3-dodecylimidazolium bromide, 1-dodecyl-1-methylpyrrolidinium bromide, or 1-dodecyl-1-methylpiperidinium bromide against Gram-negative bacteria. J. Glob. Antimicrob. Resist. 2020, 21, 99–104. [Google Scholar] [CrossRef]
- Ma, Y.; Kang, X.; Wang, G.; Luo, S.; Luo, X.; Wang, G. Inhibition of Staphylococcus aureus biofilm by quercetin combined with antibiotics. Biofouling 2024, 40, 996–1011. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Luo, J.; Du, Z.; Chen, Y.; Liu, T. Synergistic Effects of Baicalin and Levofloxacin Against Hypervirulent Klebsiella pneumoniae Biofilm In Vitro. Curr. Microbiol. 2023, 80, 126. [Google Scholar] [CrossRef]

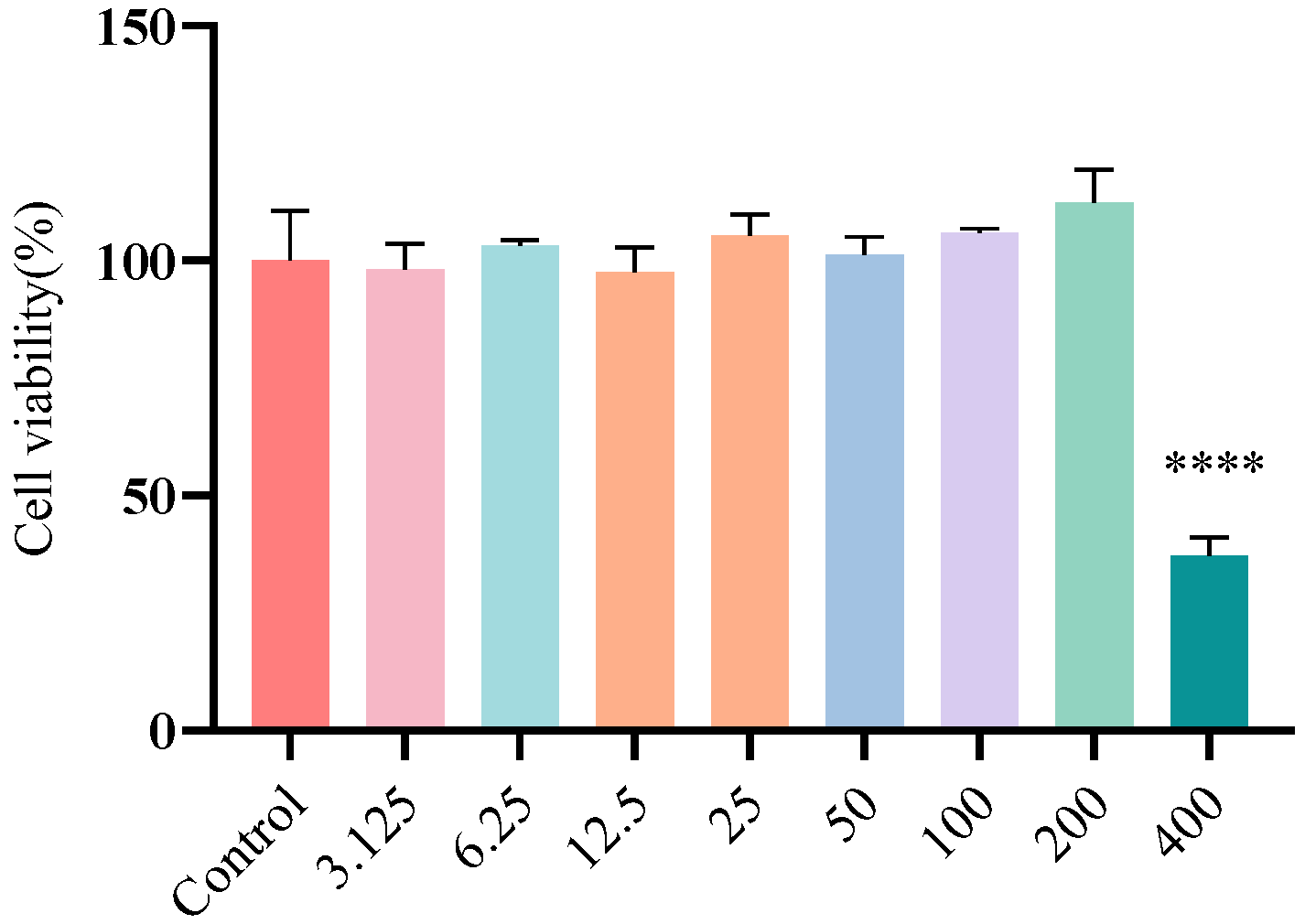



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Xu, J.; Wei, X.; Hu, R.; Zhu, Z.; Shang, Z.; Wang, W.; Li, B.; Bai, Y.; Zhang, J. Antibiofilm Inhibitor Ferulic Acid as an Antibacterial Synergist Against Escherichia coli. Biomolecules 2025, 15, 1253. https://doi.org/10.3390/biom15091253
Zhang Z, Xu J, Wei X, Hu R, Zhu Z, Shang Z, Wang W, Li B, Bai Y, Zhang J. Antibiofilm Inhibitor Ferulic Acid as an Antibacterial Synergist Against Escherichia coli. Biomolecules. 2025; 15(9):1253. https://doi.org/10.3390/biom15091253
Chicago/Turabian StyleZhang, Zhijin, Jing Xu, Xiaojuan Wei, Rongbin Hu, Zhen Zhu, Zixuan Shang, Weiwei Wang, Bing Li, Yubin Bai, and Jiyu Zhang. 2025. "Antibiofilm Inhibitor Ferulic Acid as an Antibacterial Synergist Against Escherichia coli" Biomolecules 15, no. 9: 1253. https://doi.org/10.3390/biom15091253
APA StyleZhang, Z., Xu, J., Wei, X., Hu, R., Zhu, Z., Shang, Z., Wang, W., Li, B., Bai, Y., & Zhang, J. (2025). Antibiofilm Inhibitor Ferulic Acid as an Antibacterial Synergist Against Escherichia coli. Biomolecules, 15(9), 1253. https://doi.org/10.3390/biom15091253




