Glycerol Kinase 2 as a Metabolic Sentinel for Human Sperm Motility and Male Fertility
Abstract
1. Introduction
2. The Landscape of Assisted Reproductive Technologies (ARTs)
3. Spermatozoa: Structure, Function, and Motility
3.1. Structural Basis of Sperm Function
3.2. Molecular Events for Spermatozoa Motility
3.3. Spermatozoa Abnormalities
4. Metabolic Pathways in Sperm Function
4.1. Metabolic Adaptations for Sperm Propulsion
4.2. Metabolic Significance of Glycerol in Spermatozoa
5. Glycerol Kinases and Male Fertility
5.1. Role of Glycerol Kinase 2 on Spermatozoa (and Spermatogenesis) and Its Influence on Male Fertility
| Organism | Matrix | Results | Outcomes/Findings | Reference |
|---|---|---|---|---|
| Mouse | Sperm |
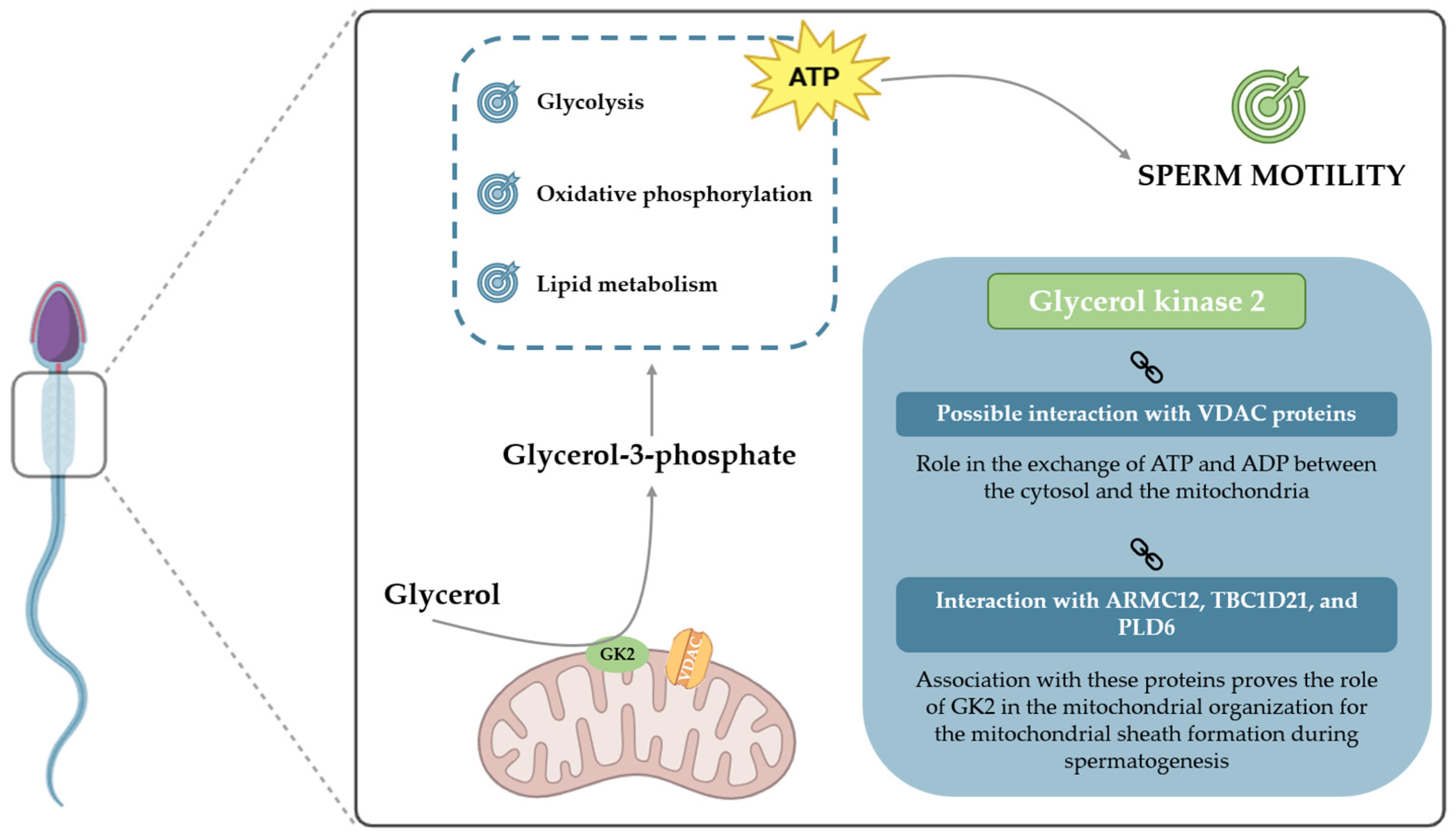
| GK2 is essential for the arrangement of crescent-like mitochondria to form the mitochondrial sheath during spermatogenesis. | Shimada et al. [88] |
| Mouse | Sperm |
| GK2 interaction with ARMC12 shows that it is involved in mitochondrial dynamics to form the mitochondrial sheath. | Shimada et al. [89] |
| Mouse | Sperm |
| Association with TBC1D21 proves the involvement of GK2 in the formation of the mitochondrial sheath. | Chen et al. [90] |
| Bovine | Testis |
| GK2 association with PLD6 suggests that, as PLD6 has a pivotal role in the development of male germ cells, this enzyme may also be involved in this process. | Yang et al. [91] |
| Mouse | Sperm |
| Presents a putative interaction between GK2 and VDAC proteins and a consequent role of GK2 in ATP exchanges in the mitochondrial membrane necessary to sustain motility. | Mise et al. [92] |
| Human | Sperm |
| Increased GK2 expression in asthenozoospermic individuals may be caused by incomplete maturation, causing more redundant cytoplasm and, therefore, more cytosolic proteins. | Siva et al. [71] |
5.2. Methodologies for Evaluating GK2 as a Fertility Biomarker
6. Conclusions and Future Prospects
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| AKAPs | A-kinase anchor proteins |
| AQP7 | Aquaglyceroporin-7 |
| ARTs | Assisted reproductive technologies |
| ARMC12 | Armadillo repeat-containing 12 |
| ATP BTB | Adenosine triphosphate Blood–testis barrier |
| cAMP | Cyclic adenosine monophosphatase |
| CDC | Centers for Disease Control |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| DC | Distal centriole |
| DHAP | Dihydroxyacetone phosphate |
| ETC | Electron transport chain |
| FAD | Flavin adenine dinucleotide |
| FADH2 | Flavin adenine dinucleotide |
| FFAs | Free fatty acids |
| FSs | Fibrous sheets |
| FSH | Follicle-stimulating hormone |
| G3P | Glycerol-3-phosphate |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GK | Glycerol kinase |
| GK1 | Glycerol kinase 1 |
| GK2 | Glycerol kinase 2 |
| GnRH | Gonadotropin-releasing hormone |
| GSK3 | Glycogen synthase kinase 3 |
| ICSI | Intracytoplasmic sperm injection |
| IVF | In vitro fertilization |
| LH | Luteinizing hormone |
| LRP6 | Lipoprotein receptor-related protein 6 receptor |
| NAD+ | Nicotinamide adenine dinucleotide |
| NADH | Reduce nicotinamide adenine dinucleotide |
| PA | Phosphatidic acid |
| PC | Proximal centriole |
| PDPK1 | 3-phosphoinositide-dependent protein kinase 1 |
| PGT | Preimplantation genetic testing |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PIP3 | Phosphatidylinositol 3,4,5-trisphosphate |
| PLA | Proximity-ligation assay |
| PLD6 | Phospholipase D family member 6 |
| PPME1 | Protein phosphatase methylesterase 1 |
| PPP | Pentose phosphate pathway |
| PPP1 | Phosphoprotein phosphatase 1 |
| PPP1R2 | PPP1 regulatory subunit 2 |
| PPP1R2P3 | PPP1 regulatory subunit 2 isoform |
| PPP2A | Phosphoprotein phosphatase 2A |
| PPP2CA | Phosphoprotein phosphatase 2A isoform alpha |
| PRKA | Protein kinase A |
| ROS | Reactive oxygen species |
| sAC | Soluble adenylyl cyclase |
| TBC1D21 | TBC1 domain family member 21 |
| TCA | Tricarboxylic acid |
| VDAC | Voltage-dependent anion channel |
| WHO | World Health Organization |
References
- Clavijo, R.I.; Hsiao, W. Update on male reproductive endocrinology. Transl. Androl. Urol. 2018, 7 (Suppl. 3), S367–S372. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wang, Z.; Kong, Y.; Jin, M.; Ma, L. Global, regional and national burden of male infertility in 204 countries and territories between 1990 and 2019: An analysis of global burden of disease study. BMC Public Health 2023, 23, 2195. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Ghuman, N.; Ramalingam, M. Male infertility. Obstet. Gynaecol. Reprod. Med. 2018, 28, 7–14. [Google Scholar] [CrossRef]
- Kose, S.I. Imaging in Male Infertility. Curr. Probl. Diagn. Radiol. 2023, 52, 439–447. (In English) [Google Scholar] [CrossRef]
- Merchant, R.; Gandhi, G.; Allahbadia, G.N. In vitro fertilization/intracytoplasmic sperm injection for male infertility. Indian J. Urol. 2011, 27, 121–132. [Google Scholar] [CrossRef]
- Begum, M. Assisted Reproductive Technology: Techniques and Limitations. J. Bangladesh Coll. Physicians Surg. 2010, 26, 135–141. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Lobo, N.; Satchi, M. The diagnosis and management of men with low sperm motility. Trends Urol. Men’s Health 2019, 10, 24–27. [Google Scholar] [CrossRef]
- Moreira, R.J.; Oliveira, P.F.; Spadella, M.A.; Ferreira, R.; Alves, M.G. Do Lifestyle Interventions Mitigate the Oxidative Damage and Inflammation Induced by Obesity in the Testis? Antioxidants 2025, 14, 150. [Google Scholar] [CrossRef]
- Esteves, S.C. Evolution of the World Health Organization semen analysis manual: Where are we? Nat. Rev. Urol. 2022, 19, 439–446. [Google Scholar] [CrossRef]
- Perrone, P.; Lettieri, G.; Marinaro, C.; Longo, V.; Capone, S.; Forleo, A.; Pappalardo, S.; Montano, L.; Piscopo, M. Molecular Alterations and Severe Abnormalities in Spermatozoa of Young Men Living in the “Valley of Sacco River” (Latium, Italy): A Preliminary Study. Int. J. Environ. Res. Public Health 2022, 19, 11023. [Google Scholar] [CrossRef] [PubMed]
- Crisóstomo, L.; Alves, M.G.; Calamita, G.; Sousa, M.; Oliveira, P.F. Glycerol and testicular activity: The good, the bad and the ugly. Mol. Hum. Reprod. 2017, 23, 725–737. [Google Scholar] [CrossRef]
- Sargent, C.A.; Young, C.; Marsh, S.; Ferguson-Smith, M.A.; Affara, N.A. The glycerol kinase gene family: Structure of the Xp gene, and related intronless retroposons. Hum. Mol. Genet. 1994, 3, 1317–1324. [Google Scholar] [CrossRef]
- Peroni, O.; Large, V.; Beylot, M. Measuring gluconeogenesis with [2–13C]glycerol and mass isotopomer distribution analysis of glucose. Am. J. Physiol. 1995, 269 Pt 1, E516–E523. [Google Scholar] [CrossRef]
- Assisted Reproductive Technology (ART). Centers for Disease Control and Prevention (CDC). Available online: https://www.cdc.gov/art/about/index.html (accessed on 8 August 2025).
- Liberman, R.F.; Getz, K.D.; Heinke, D.; Luke, B.; Stern, J.E.; Declercq, E.R.; Chen, X.; Lin, A.E.; Anderka, M. Assisted Reproductive Technology and Birth Defects: Effects of Subfertility and Multiple Births. Birth Defects Res. 2017, 109, 1144–1153. [Google Scholar] [CrossRef]
- Davidovitch, M.; Chodick, G.; Shalev, V.; Eisenberg, V.H.; Dan, U.; Reichenberg, A.; Sandin, S.; Levine, S.Z. Infertility treatments during pregnancy and the risk of autism spectrum disorder in the offspring. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 86, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Hattori, H.; Hiura, H.; Kitamura, A.; Miyauchi, N.; Kobayashi, N.; Takahashi, S.; Okae, H.; Kyono, K.; Kagami, M.; Ogata, T.; et al. Association of four imprinting disorders and ART. Clin. Epigenetics 2019, 11, 21. [Google Scholar] [CrossRef]
- Njagi, P.; Groot, W.; Arsenijevic, J.; Dyer, S.; Mburu, G.; Kiarie, J. Financial costs of assisted reproductive technology for patients in low- and middle-income countries: A systematic review. Hum. Reprod. Open 2023, 2023, hoad007. [Google Scholar] [CrossRef] [PubMed]
- Sallam, H.N.; Sallam, N.H. Religious aspects of assisted reproduction. Facts Views Vis. ObGyn 2016, 8, 33–48. [Google Scholar]
- Grin, L.; Girsh, E.; Harlev, A. Male fertility preservation-Methods, indications and challenges. Andrologia 2021, 53, e13635. [Google Scholar] [CrossRef]
- Albamonte, M.I.; Vitullo, A.D. Preservation of fertility in female and male prepubertal patients diagnosed with cancer. J. Assist. Reprod. Genet. 2023, 40, 2755–2767. [Google Scholar] [CrossRef]
- Fesahat, F.; Montazeri, F.; Hoseini, S.M. Preimplantation genetic testing in assisted reproduction technology. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101723. [Google Scholar] [CrossRef] [PubMed]
- Greco, E.; Litwicka, K.; Minasi, M.G.; Cursio, E.; Greco, P.F.; Barillari, P. Preimplantation Genetic Testing: Where We Are Today. Int. J. Mol. Sci. 2020, 21, 4381. [Google Scholar] [CrossRef] [PubMed]
- Raja, N.S.; Russell, C.B.; Moravek, M.B. Assisted reproductive technology: Considerations for the nonheterosexual population and single parents. Fertil. Steril. 2022, 118, 47–53. [Google Scholar] [CrossRef]
- Sharma, R.; Agarwal, A. Spermatogenesis: An overview. Sperm Chromatin: Biological and Clinical Applications in Male Infertility and Assisted Reproduction; Springer Science and Business Media: New York, NY, USA, 2011; pp. 19–44. [Google Scholar]
- de Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum. Reprod. 1998, 13 (Suppl. 1), 1–8. [Google Scholar] [CrossRef]
- Hermo, L.; Lalli, M.; Clermont, Y. Arrangement of connective tissue components in the walls of seminiferous tubules of man and monkey. Am. J. Anat. 1977, 148, 433–445. [Google Scholar] [CrossRef]
- Berezney, R.; Coffey, D.S. Nuclear matrix. Isolation and characterization of a framework structure from rat liver nuclei. J. Cell Biol. 1977, 73, 616–637. [Google Scholar] [CrossRef] [PubMed]
- Neto, F.T.L.; Bach, P.V.; Najari, B.B.; Li, P.S.; Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 2016, 59, 10–26. [Google Scholar] [CrossRef]
- Freitas, M.J.; Vijayaraghavan, S.; Fardilha, M. Signaling mechanisms in mammalian sperm motility. Biol. Reprod. 2017, 96, 2–12. [Google Scholar] [CrossRef]
- Barratt, C.L.; Aitken, R.J.; Björndahl, L.; Carrell, D.T.; de Boer, P.; Kvist, U.; Lewis, S.E.; Perreault, S.D.; Perry, M.J.; Ramos, L.; et al. Sperm DNA: Organization, protection and vulnerability: From basic science to clinical applications--a position report. Hum. Reprod. 2010, 25, 824–838. [Google Scholar] [CrossRef]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef]
- Lehti, M.S.; Sironen, A. Formation and function of sperm tail structures in association with sperm motility defects. Biol. Reprod. 2017, 97, 522–536. [Google Scholar] [CrossRef]
- Eddy, E.M. The scaffold role of the fibrous sheath. Soc. Reprod. Fertil. Suppl. 2007, 65, 45–62. [Google Scholar]
- Amaro, A.; Bernardo, C. Reprodução Humana Masculina—Princípios Fundamentais; ARC Publishing: Lisbon, Portugal, 2015. [Google Scholar]
- Korrodi-Gregório, L.; Ferreira, M.; Vintém, A.P.; Wu, W.; Muller, T.; Marcus, K.; Vijayaraghavan, S.; Brautigan, D.L.; da Cruz, E.S.O.A.; Fardilha, M.; et al. Identification and characterization of two distinct PPP1R2 isoforms in human spermatozoa. BMC Cell Biol. 2013, 14, 15. [Google Scholar] [CrossRef]
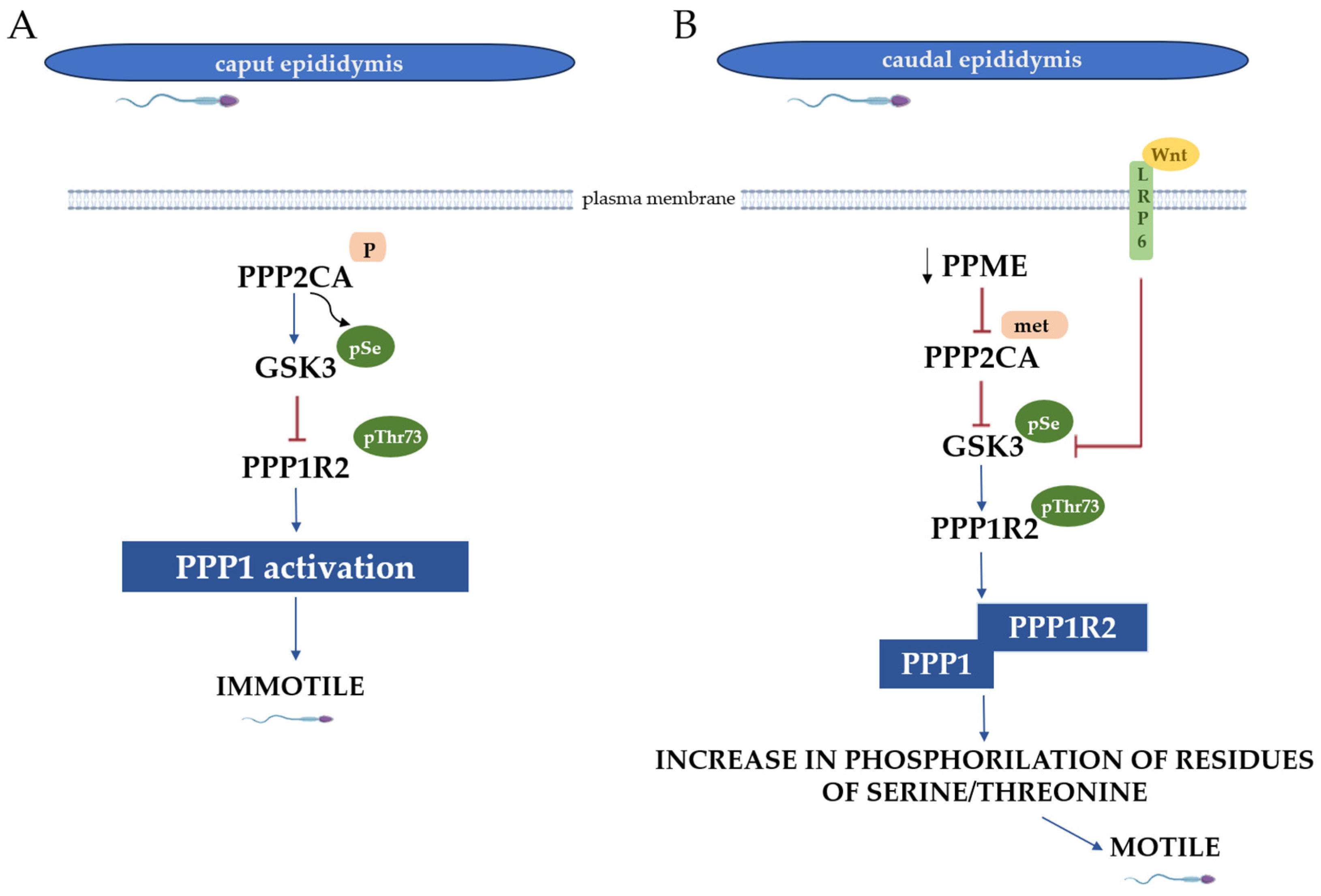
- Vijayaraghavan, S.; Stephens, D.T.; Trautman, K.; Smith, G.D.; Khatra, B.; da Cruz e Silva, E.F.; Greengard, P. Sperm motility development in the epididymis is associated with decreased glycogen synthase kinase-3 and protein phosphatase 1 activity. Biol. Reprod. 1996, 54, 709–718. [Google Scholar] [CrossRef]
- Koch, S.; Acebron, S.P.; Herbst, J.; Hatiboglu, G.; Niehrs, C. Post-transcriptional Wnt Signaling Governs Epididymal Sperm Maturation. Cell 2015, 163, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.M.; Park, I.K.; Fiol, C.J.; Roach, P.J.; DePaoli-Roach, A.A. Isoform differences in substrate recognition by glycogen synthase kinases 3 alpha and 3 beta in the phosphorylation of phosphatase inhibitor 2. Biochemistry 1994, 33, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Dudiki, T.; Kadunganattil, S.; Ferrara, J.K.; Kline, D.W.; Vijayaraghavan, S. Changes in Carboxy Methylation and Tyrosine Phosphorylation of Protein Phosphatase PP2A Are Associated with Epididymal Sperm Maturation and Motility. PLoS ONE 2015, 10, e0141961. [Google Scholar] [CrossRef]
- Mortimer, D.; Mortimer, S. Laboratory Investigation of the Infertile Male; Cambridge University Press: Cambridge, UK, 2005; pp. 61–91. [Google Scholar]
- Chemes, H.; Olmedo, S.B.; Carrere, C.; Oses, R.; Carizza, C.; Leisner, M.; Blaquier, J. Ultrastructural pathology of the sperm flagellum: Association between flagellar pathology and fertility prognosis in severely asthenozoospermic men. Hum. Reprod. 1998, 13, 2521–2526. [Google Scholar] [CrossRef] [PubMed]
- Damsgaard, J.; Joensen, U.N.; Carlsen, E.; Erenpreiss, J.; Blomberg Jensen, M.; Matulevicius, V.; Zilaitiene, B.; Olesen, I.A.; Perheentupa, A.; Punab, M.; et al. Varicocele Is Associated with Impaired Semen Quality and Reproductive Hormone Levels: A Study of 7035 Healthy Young Men from Six European Countries. Eur. Urol. 2016, 70, 1019–1029. [Google Scholar] [CrossRef]
- Diemer, T.; Huwe, P.; Ludwig, M.; Hauck, E.; Weidner, W. Urogenital infection and sperm motility. Andrologia 2003, 35, 283–287. [Google Scholar] [CrossRef]
- Pattinson, H.A.; Mortimer, D. Prevalence of sperm surface antibodies in the male partners of infertile couples as determined by immunobead screening. Fertil. Steril. 1987, 48, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Buckingham, D.W.; Brindle, J.; Gomez, E.; Baker, H.W.; Irvine, D.S. Analysis of sperm movement in relation to the oxidative stress created by leukocytes in washed sperm preparations and seminal plasma. Hum. Reprod. 1995, 10, 2061–2071. [Google Scholar] [CrossRef]
- Lettieri, G.; D’Agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimmino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, S.; Notari, T.; et al. Discovery of the Involvement in DNA Oxidative Damage of Human Sperm Nuclear Basic Proteins of Healthy Young Men Living in Polluted Areas. Int. J. Mol. Sci. 2020, 21, 4198. [Google Scholar] [CrossRef]
- Nunzio, A.D.; Giarra, A.; Toscanesi, M.; Amoresano, A.; Piscopo, M.; Ceretti, E.; Zani, C.; Lorenzetti, S.; Trifuoggi, M.; Montano, L. Comparison between Macro and Trace Element Concentrations in Human Semen and Blood Serum in Highly Polluted Areas in Italy. Int. J. Environ. Res. Public Health 2022, 19, 11635. [Google Scholar] [CrossRef] [PubMed]
- Rotimi, D.E.; Singh, S.K. Implications of lifestyle factors on male reproductive health. JBRA Assist. Reprod. 2024, 28, 320–330. [Google Scholar] [CrossRef]
- Turner, R.M. Tales from the tail: What do we really know about sperm motility? J. Androl. 2003, 24, 790–803. [Google Scholar] [CrossRef] [PubMed]
- du Plessis, S.S.; Agarwal, A.; Mohanty, G.; van der Linde, M. Oxidative phosphorylation versus glycolysis: What fuel do spermatozoa use? Asian J. Androl. 2015, 17, 230–235. [Google Scholar] [CrossRef]
- Hereng, T.H.; Elgstøen, K.B.; Cederkvist, F.H.; Eide, L.; Jahnsen, T.; Skålhegg, B.S.; Rosendal, K.R. Exogenous pyruvate accelerates glycolysis and promotes capacitation in human spermatozoa. Hum. Reprod. 2011, 26, 3249–3263. [Google Scholar] [CrossRef]
- Mukai, C.; Okuno, M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol. Reprod. 2004, 71, 540–547. [Google Scholar] [CrossRef]
- Williams, A.C.; Ford, W.C. The role of glucose in supporting motility and capacitation in human spermatozoa. J. Androl. 2001, 22, 680–695. [Google Scholar] [CrossRef]
- Ferramosca, A.; Provenzano, S.P.; Coppola, L.; Zara, V. Mitochondrial respiratory efficiency is positively correlated with human sperm motility. Urology 2012, 79, 809–814. [Google Scholar] [CrossRef]
- Paoli, D.; Gallo, M.; Rizzo, F.; Baldi, E.; Francavilla, S.; Lenzi, A.; Lombardo, F.; Gandini, L. Mitochondrial membrane potential profile and its correlation with increasing sperm motility. Fertil. Steril. 2011, 95, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Ford, W.C. Glycolysis and sperm motility: Does a spoonful of sugar help the flagellum go round? Hum. Reprod. Update 2006, 12, 269–274. [Google Scholar] [CrossRef]
- Dias, T.R.; Alves, M.G.; Silva, B.M.; Oliveira, P.F. Sperm glucose transport and metabolism in diabetic individuals. Mol. Cell. Endocrinol. 2014, 396, 37–45. [Google Scholar] [CrossRef]
- Amaral, A. Energy metabolism in mammalian sperm motility. WIREs Mech. Dis. 2022, 14, e1569. (In English) [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Castillo, J.; Estanyol, J.M.; Ballescà, J.L.; Ramalho-Santos, J.; Oliva, R. Human sperm tail proteome suggests new endogenous metabolic pathways. Mol. Cell. Proteom. 2013, 12, 330–342. [Google Scholar] [CrossRef]
- Amaral, A.; Paiva, C.; Baptista, M.; Sousa, A.P.; Ramalho-Santos, J. Exogenous glucose improves long-standing human sperm motility, viability, and mitochondrial function. Fertil. Steril. 2011, 96, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Carrageta, D.F.; Alves, M.G.; Oliveira, P.F. Testicular Glycogen Metabolism: An Overlooked Source of Energy for Spermatogenesis? BioChem 2022, 2, 198–214. [Google Scholar] [CrossRef]
- Miki, K. Energy metabolism and sperm function. Soc. Reprod. Fertil. Suppl. 2007, 65, 309–325. [Google Scholar]
- Chaves, V.E.; Frasson, D.; Garófalo, M.A.; Navegantes, L.C.; Migliorini, R.H.; Kettelhut, I.C. Increased glyceride-glycerol synthesis in liver and brown adipose tissue of rat: In-vivo contribution of glycolysis and glyceroneogenesis. Lipids 2012, 47, 773–780. [Google Scholar] [CrossRef]
- Christoffersen, C.; Bollano, E.; Lindegaard, M.L.; Bartels, E.D.; Goetze, J.P.; Andersen, C.B.; Nielsen, L.B. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology 2003, 144, 3483–3490. [Google Scholar] [CrossRef]
- Jin, E.S.; Sherry, A.D.; Malloy, C.R. Interaction between the pentose phosphate pathway and gluconeogenesis from glycerol in the liver. J. Biol. Chem. 2014, 289, 32593–32603. [Google Scholar] [CrossRef]
- Mugabo, Y.; Zhao, S.; Seifried, A.; Gezzar, S.; Al-Mass, A.; Zhang, D.; Lamontagne, J.; Attane, C.; Poursharifi, P.; Iglesias, J.; et al. Identification of a mammalian glycerol-3-phosphate phosphatase: Role in metabolism and signaling in pancreatic β-cells and hepatocytes. Proc. Natl. Acad. Sci. USA 2016, 113, E430–E439. [Google Scholar] [CrossRef]
- Ratner, P.L.; Fisher, M.; Burkart, D.; Cook, J.R.; Kozak, L.P. The role of mRNA levels and cellular localization in controlling sn-glycerol-3-phosphate dehydrogenase expression in tissues of the mouse. J. Biol. Chem. 1981, 256, 3576–3579. [Google Scholar] [CrossRef]
- Siva, A.B.; Kameshwari, D.B.; Singh, V.; Pavani, K.; Sundaram, C.S.; Rangaraj, N.; Deenadayal, M.; Shivaji, S. Proteomics-based study on asthenozoospermia: Differential expression of proteasome alpha complex. Mol. Hum. Reprod. 2010, 16, 452–462. [Google Scholar] [CrossRef]
- Kosuga, M.; Henderson-MacLennan, N.K.; Zhang, Y.H.; Huang, B.L.; Dipple, K.M.; McCabe, E.R. Glycerol homeostasis and metabolism in glycerol kinase carrier mice. Mol. Genet. Metab. 2011, 103, 297–299. [Google Scholar] [CrossRef]
- Wikiera, B.; Jakubiak, A.; Zimowski, J.; Noczyńska, A.; Smigiel, R. Complex glycerol kinase deficiency—X-linked contiguous gene syndrome involving congenital adrenal hypoplasia, glycerol kinase deficiency, muscular Duchenne dystrophy and intellectual disability (IL1RAPL gene deletion). Pediatr. Endocrinol. Diabetes Metab. 2012, 18, 153–157. [Google Scholar]
- Green, D.E. Alpha-Glycerophosphate dehydrogenase. Biochem. J. 1936, 30, 629–644. [Google Scholar] [CrossRef]
- Houstĕk, J.; Drahota, Z. The regulation of glycerol 3-phosphate oxidase of rate brownadipose tissue mitochondria by long-chain free fatty acids. Mol. Cell. Biochem. 1975, 7, 45–50. [Google Scholar] [CrossRef]
- Wu, J.W.; Yang, H.; Wang, S.P.; Soni, K.G.; Brunel-Guitton, C.; Mitchell, G.A. Inborn errors of cytoplasmic triglyceride metabolism. J. Inherit. Metab. Dis. 2015, 38, 85–98. [Google Scholar] [CrossRef]
- Hibuse, T.; Maeda, N.; Nakatsuji, H.; Tochino, Y.; Fujita, K.; Kihara, S.; Funahashi, T.; Shimomura, I. The heart requires glycerol as an energy substrate through aquaporin 7, a glycerol facilitator. Cardiovasc. Res. 2009, 83, 34–41. [Google Scholar] [CrossRef]
- Mohammad, M.A.; Maningat, P.; Sunehag, A.L.; Haymond, M.W. Precursors of hexoneogenesis within the human mammary gland. Am. J. Physiol.-Endocrinol. Metab. 2015, 308, E680–E687. [Google Scholar] [CrossRef]
- Nye, C.K.; Hanson, R.W.; Kalhan, S.C. Glyceroneogenesis is the dominant pathway for triglyceride glycerol synthesis in vivo in the rat. J. Biol. Chem. 2008, 283, 27565–27574. [Google Scholar] [CrossRef]
- Bukowiecki, L.J.; Lindberg, O. Control of sn-glycerol 3-phosphate oxidation in brown adipose tissue mitochondria by calcium and acyl-CoA. Biochim. Biophys. Acta 1974, 348, 115–125. [Google Scholar] [CrossRef]
- Garrib, A.; McMurray, W.C. Purification and characterization of glycerol-3-phosphate dehydrogenase (flavin-linked) from rat liver mitochondria. J. Biol. Chem. 1986, 261, 8042–8048. [Google Scholar] [CrossRef]
- Mráček, T.; Holzerová, E.; Drahota, Z.; Kovářová, N.; Vrbacký, M.; Ješina, P.; Houštěk, J. ROS generation and multiple forms of mammalian mitochondrial glycerol-3-phosphate dehydrogenase. Biochim. Biophys. Acta 2014, 1837, 98–111. [Google Scholar] [CrossRef]
- Mráček, T.; Drahota, Z.; Houštěk, J. The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues. Biochim. Biophys. Acta 2013, 1827, 401–410. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Bernardino, R.L.; Gonçalves, A.; Barros, A.; Calamita, G.; Alves, M.G.; Oliveira, P.F. Aquaporin-7-Mediated Glycerol Permeability Is Linked to Human Sperm Motility in Asthenozoospermia and during Sperm Capacitation. Cells 2023, 12, 2003. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Bernardino, R.L.; Carrageta, D.F.; Soveral, G.; Calamita, G.; Alves, M.G.; Oliveira, P.F. CFTR modulates aquaporin-mediated glycerol permeability in mouse Sertoli cells. Cell. Mol. Life Sci. 2022, 79, 592. [Google Scholar] [CrossRef]
- Kota, V.; Dhople, V.M.; Shivaji, S. Tyrosine phosphoproteome of hamster spermatozoa: Role of glycerol-3-phosphate dehydrogenase 2 in sperm capacitation. Proteomics 2009, 9, 1809–1826. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, P.; Huang, Y.; Li, M.; Zhang, X.; Ding, C.; Feng, J.; Zhang, Z.; Zhang, X.; Gao, Y.; et al. Glycerol kinase-like proteins cooperate with Pld6 in regulating sperm mitochondrial sheath formation and male fertility. Cell Discov. 2017, 3, 17030. [Google Scholar] [CrossRef]
- Shimada, K.; Kato, H.; Miyata, H.; Ikawa, M. Glycerol kinase 2 is essential for proper arrangement of crescent-like mitochondria to form the mitochondrial sheath during mouse spermatogenesis. J. Reprod. Dev. 2019, 65, 155–162. [Google Scholar] [CrossRef]
- Shimada, K.; Park, S.; Miyata, H.; Yu, Z.; Morohoshi, A.; Oura, S.; Matzuk, M.M.; Ikawa, M. ARMC12 regulates spatiotemporal mitochondrial dynamics during spermiogenesis and is required for male fertility. Proc. Natl. Acad. Sci. USA 2021, 118, e2018355118. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Zhang, H.; Sha, Y.; Meng, R.; Shao, T.; Yang, X.; Jin, P.; Zhuang, Y.; Min, W.; et al. TBC1D21 is an essential factor for sperm mitochondrial sheath assembly and male fertility. Biol. Reprod. 2022, 107, 619–634. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, B.; Zhu, W.; Zhu, C.; Chen, L.; Zhao, Y.; Wang, Y.; Zhang, Y.; Riaz, A.; Tang, B.; et al. Expression of Phospholipase D Family Member 6 in Bovine Testes and Its Molecular Characteristics. Int. J. Mol. Sci. 2023, 24, 12172. [Google Scholar] [CrossRef]
- Mise, S.; Matsumoto, A.; Shimada, K.; Hosaka, T.; Takahashi, M.; Ichihara, K.; Shimizu, H.; Shiraishi, C.; Saito, D.; Suyama, M.; et al. Kastor and Polluks polypeptides encoded by a single gene locus cooperatively regulate VDAC and spermatogenesis. Nat. Commun. 2022, 13, 1071. [Google Scholar] [CrossRef]
- Fiek, C.; Benz, R.; Roos, N.; Brdiczka, D. Evidence for identity between the hexokinase-binding protein and the mitochondrial porin in the outer membrane of rat liver mitochondria. Biochim. Biophys. Acta (BBA)—Biomembr. 1982, 688, 429–440. [Google Scholar] [CrossRef]
- Kwon, W.-S.; Park, Y.-J.; Mohamed, E.-S.A.; Pang, M.-G. Voltage-dependent anion channels are a key factor of male fertility. Fertil. Steril. 2013, 99, 354–361. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, W.; Wang, Z. Voltage-dependent anion channel in mammalian spermatozoa. Biochem. Biophys. Res. Commun. 2010, 397, 633–636. [Google Scholar] [CrossRef]
- McCabe, E.R. Human glycerol kinase deficiency: An inborn error of compartmental metabolism. Biochem. Med. 1983, 30, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Patsouris, D.; Mandard, S.; Voshol, P.J.; Escher, P.; Tan, N.S.; Havekes, L.M.; Koenig, W.; März, W.; Tafuri, S.; Wahli, W.; et al. PPARalpha governs glycerol metabolism. J. Clin. Investig. 2004, 114, 94–103. [Google Scholar] [CrossRef]
- Seidler, N.W. GAPDH and intermediary metabolism. Adv. Exp. Med. Biol. 2013, 985, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, J.P.; Kowalik, A.; Gallardi, R.L.; Egeler, O.; Clubb, B.H. Glycerol disrupts tight junction-associated actin microfilaments, occludin, and microtubules in Sertoli cells. J. Androl. 2000, 21, 625–635. [Google Scholar] [CrossRef]
- Lettieri, G.; Marinaro, C.; Brogna, C.; Montano, L.; Lombardi, M.; Trotta, A.; Troisi, J.; Piscopo, M. A Metabolomic Analysis to Assess the Responses of the Male Gonads of Mytilus galloprovincialis after Heavy Metal Exposure. Metabolites 2023, 13, 1168. [Google Scholar] [CrossRef]
- Bucci, D.; Spinaci, M.; Galeati, G.; Tamanini, C. Different approaches for assessing sperm function. Anim. Reprod. 2020, 16, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Cui, Y.; Zhang, X.; Lou, J.; Zhou, J.; Bei, H.; Wei, R. Proteomic profile of human spermatozoa in healthy and asthenozoospermic individuals. Reprod. Biol. Endocrinol. 2018, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Glycerol Kinase—Assay. Available online: https://www.worthington-biochem.com/products/glycerol-kinase/assay (accessed on 8 August 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, J.S.; Moreira, R.J.; Martins, A.D.; Alves, M.G.; Oliveira, P.F. Glycerol Kinase 2 as a Metabolic Sentinel for Human Sperm Motility and Male Fertility. Biomolecules 2025, 15, 1249. https://doi.org/10.3390/biom15091249
Oliveira JS, Moreira RJ, Martins AD, Alves MG, Oliveira PF. Glycerol Kinase 2 as a Metabolic Sentinel for Human Sperm Motility and Male Fertility. Biomolecules. 2025; 15(9):1249. https://doi.org/10.3390/biom15091249
Chicago/Turabian StyleOliveira, João S., Rúben J. Moreira, Ana D. Martins, Marco G. Alves, and Pedro F. Oliveira. 2025. "Glycerol Kinase 2 as a Metabolic Sentinel for Human Sperm Motility and Male Fertility" Biomolecules 15, no. 9: 1249. https://doi.org/10.3390/biom15091249
APA StyleOliveira, J. S., Moreira, R. J., Martins, A. D., Alves, M. G., & Oliveira, P. F. (2025). Glycerol Kinase 2 as a Metabolic Sentinel for Human Sperm Motility and Male Fertility. Biomolecules, 15(9), 1249. https://doi.org/10.3390/biom15091249










