Ca2+ Signaling in Striated Muscle Cells During Intracellular Acidosis
Abstract
1. Introduction
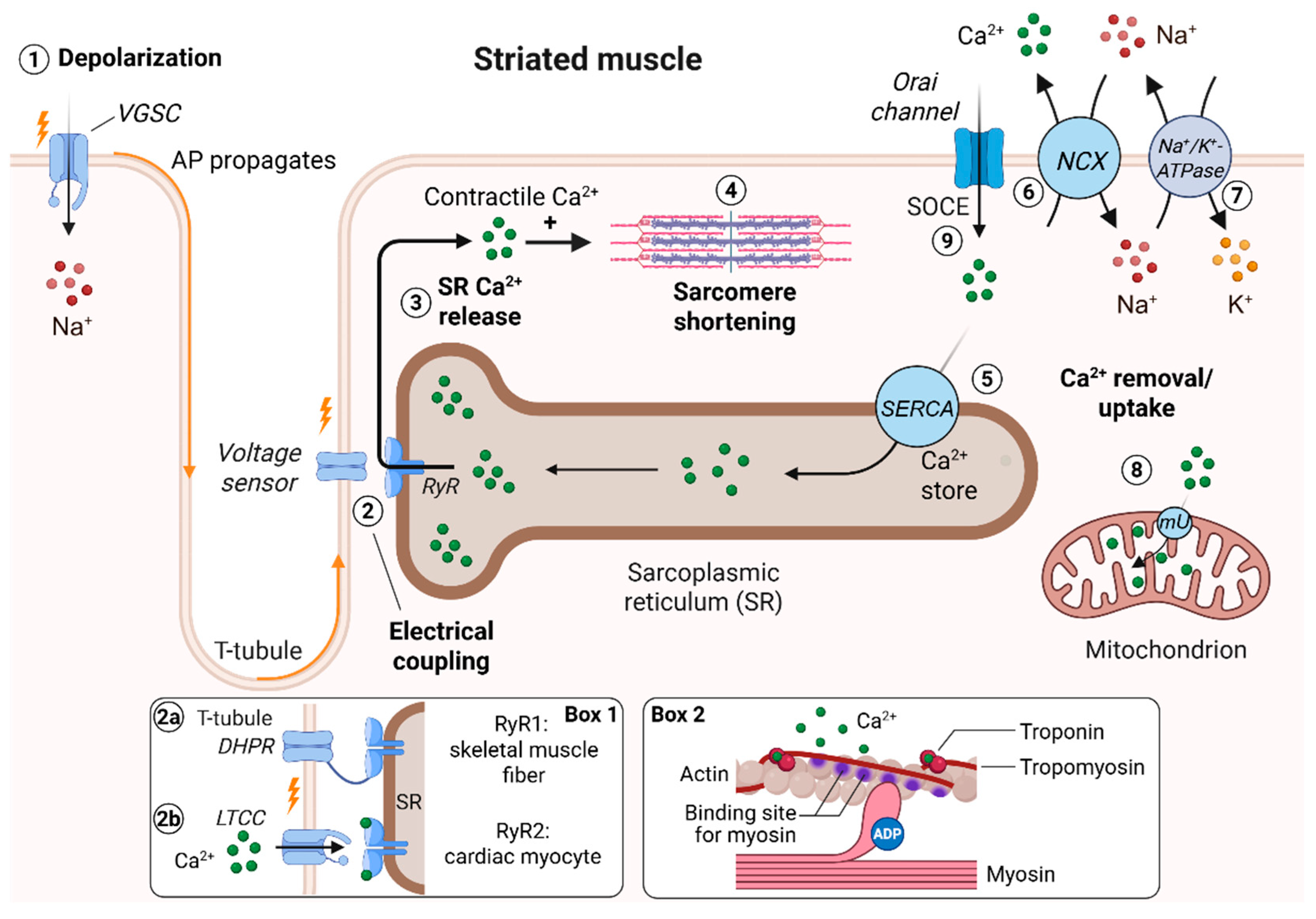
2. Excitation–Contraction Coupling (ECC) in Striated Muscle Cells
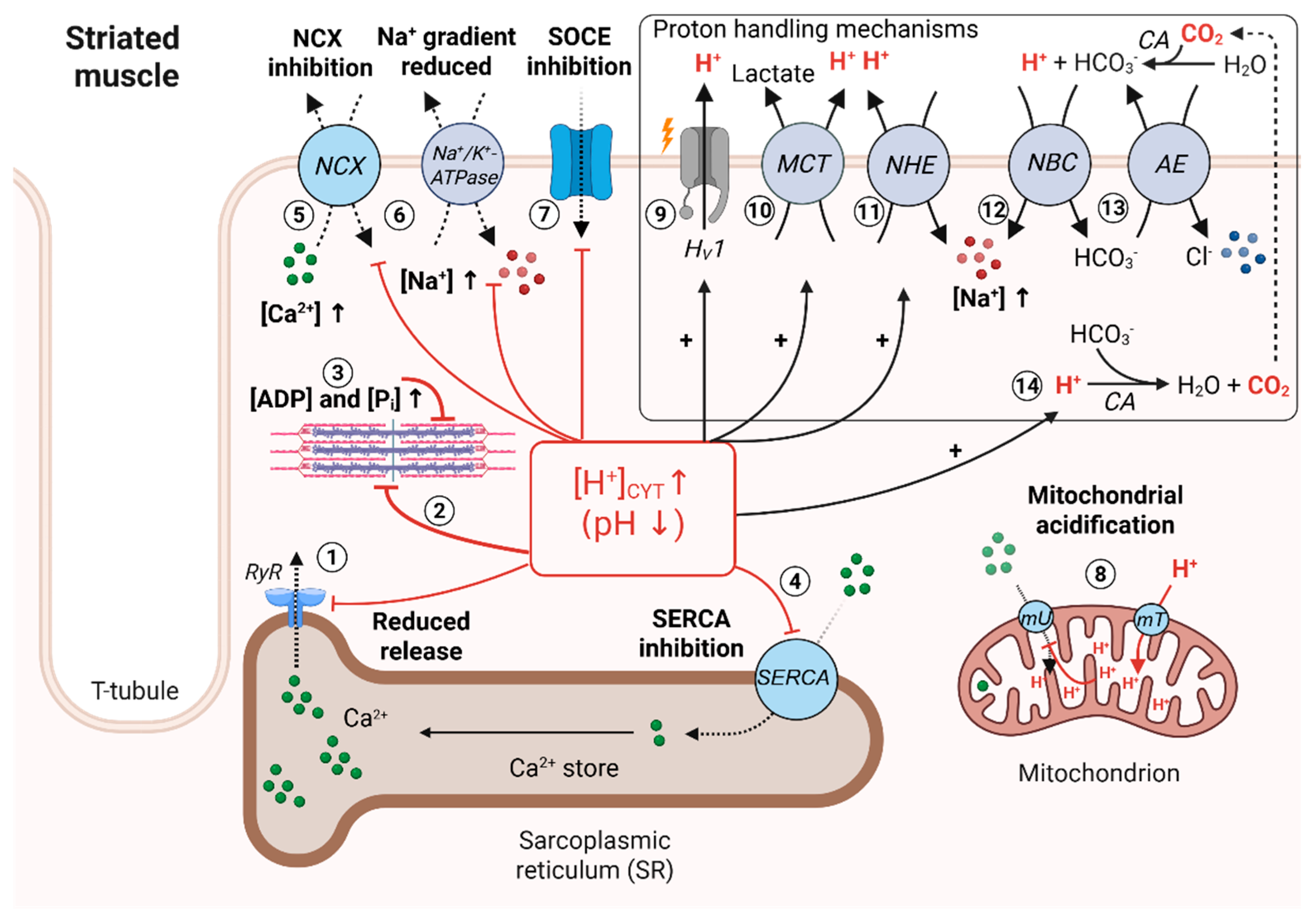
3. Mechanisms Inducing Intracellular Acidosis in Striated Muscle Cells
4. Effect of Low pHi on Striated Muscle Contractility
5. Effect of Low pHi on Ca2+ Buffers and Ca2+-Storing Organelles
6. Optical Methods to Measure Ca2+CYT and pHi in Living Cells
7. Discussion and Outlook
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| AE | (Cl−/bicarbonate) anion exchanger |
| ATP | Adenosine triphosphate |
| ASIC | Acid-sensing ion channel |
| BCECF | 2″,7″-Bis-(2-carboxyethyl)-5-(and-6-) carboxyfluorescein |
| Ca2+ | Calcium ion |
| [Ca2+]CYT | Intracellular (cytosolic) Ca2+ concentration |
| CO2 | Carbon dioxide |
| CRAC | Ca2+ release-activated current |
| CICR | Ca2+-induced Ca2+ release |
| DHPR | Dihydropyridine receptor |
| ECC | Excitation-contraction coupling |
| ER | Endoplasmic reticulum |
| GECO | Genetically encoded calcium indicator for optical imaging |
| Gq | Gq alpha subunit of heterotrimeric G proteins |
| GPCR | G-protein-coupled receptor |
| GPR68 | G-protein-coupled receptor 68 |
| H+ | Proton |
| [H+]CYT | Intracellular (cytosolic) proton concentration |
| HV1 | Hydrogen voltage-gated channel 1 |
| IP3 | Inositol trisphosphate |
| IP3R | IP3 receptor |
| Kd | Dissociation constant |
| LTCC | L-type Ca2+ channel |
| MCT | Monocarboxylate transporter |
| mT | Mitochondrial transport |
| mU | Mitochondrial uniporter |
| nAChR | Nicotinic acetylcholine receptor |
| [NA+]CYT | Intracellular (cytosolic) sodium concentration |
| NBC | Sodium-bicarbonate cotransporter |
| NCX | Sodium-calcium exchanger |
| NHE | Sodium-proton exchanger |
| pCa50 | Negative logarithm of the Ca2+ concentration at 50% force development |
| PCO2 | Partial pressure of CO2 |
| pH | Negative logarithm of the proton concentration [H+] |
| pHi | Intracellular (cytosolic) pH |
| pHo | Extracellular pH |
| Pi | Inorganic phosphate |
| PIP2 | Phosphatidylinositol bisphosphate |
| pKa | Negative logarithm of the acid dissociation constant Ka |
| PKA | Protein kinase A |
| PLC | Phospholipase C |
| ROS | Reactive oxygen species |
| RyR | Ryanodine receptor |
| SERCA | Sarcoplasmic/endoplasmic Ca2+ ATPase |
| SNARF | Seminaphtorhodafluor |
| SOCE | Store-operated Ca2+ entry |
| SR | Sarcoplasmic reticulum |
| TRP | Transient receptor potential |
| V-ATPase | Vacuolar-type ATPase |
| VGSC | Voltage-gated sodium channel |
| YFP | Yellow fluorescent protein |
References
- Myers, R.J. One-hundred years of pH. J. Chem. Educ. 2010, 87, 30–32. [Google Scholar] [CrossRef]
- Sørensen, S.P.L. Über die Messung und die Bedeutung der Wasserstoffionenkonzentration bei enzymatischen Prozessen. Biochem. Z. 1909, 21, 131. [Google Scholar]
- Putnam, R.W. 22—Intracellular pH regulation. In Cell Physiology Source Book, 3rd ed.; Sperelakis, N., Ed.; Academic Press: San Diego, CA, USA, 2001; pp. 357–372. [Google Scholar]
- Boron, W.F. Regulation of intracellular pH. Adv. Physiol. Educ. 2004, 28, 160–179. [Google Scholar] [CrossRef]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.; Thomas, R. Direct measurement of the intracellular pH of mammalian cardiac muscle. J. Physiol. 1976, 262, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Fitts, R.H. Cellular mechanisms of muscle fatigue. Physiol. Rev. 1994, 74, 49–94. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.J.; Brandes, R.; Weiner, M.W. Effects of intracellular acidosis on Ca2+ activation, contraction, and relaxation of frog skeletal muscle. Am. J. Physiol. Cell Physiol. 1995, 268, C55–C63. [Google Scholar] [CrossRef]
- Westerblad, H.; Allen, D.G. The influence of intracellular pH on contraction, relaxation and [Ca2+]i in intact single fibres from mouse muscle. J. Physiol. 1993, 466, 611–628. [Google Scholar] [CrossRef]
- Williamson, J.R.; Safer, B.; Rich, T.; Schaffer, S.; Kobayashi, K. Effects of acidosis on myocardial contractility and metabolism. Acta Medica Scand. 1976, 199, 95–112. [Google Scholar] [CrossRef]
- Williamson, J.R.; Schaffer, S.W.; Ford, C.; Safer, B. Contribution of tissue acidosis to ischemic injury in the perfused rat heart. Circulation 1976, 53, I3–I14. [Google Scholar]
- Gonzalez, A.; Pariente, J.A.; Salido, G.M.; Camello, P.J. Intracellular pH and calcium signalling in rat pancreatic acinar cells. Pflügers Arch. 1997, 434, 609–614. [Google Scholar] [CrossRef]
- Kuo, I.Y.; Ehrlich, B.E. Signaling in muscle contraction. Cold Spring Harb. Perspect. Biol. 2015, 7, a006023. [Google Scholar] [CrossRef]
- Hill-Eubanks, D.C.; Werner, M.E.; Heppner, T.J.; Nelson, M.T. Calcium signaling in smooth muscle. Cold Spring Harb. Perspect. Biol. 2011, 3, a004549. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Yang, C.-F.; Tsai, W.-C. Calmodulin: The switch button of calcium signaling. Tzu Chi Med. J. 2022, 34, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Katrukha, I.A. Human cardiac troponin complex. Structure and functions. Biochemistry. Biokhimiia 2013, 78, 1447–1465. [Google Scholar] [CrossRef]
- Palmer, S.; Kentish, J.C. The role of troponin C in modulating the Ca2+ sensitivity of mammalian skinned cardiac and skeletal muscle fibres. J. Physiol. 1994, 480, 45–60. [Google Scholar] [CrossRef]
- Putney, J.W. Capacitative calcium entry: From concept to molecules. Immunol. Rev. 2009, 231, 10–22. [Google Scholar] [CrossRef]
- Bootman, M.D. Calcium signaling. Cold Spring Harb. Perspect. Biol. 2012, 4, a011171. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Calcium microdomains: Organization and function. Cell Calcium 2006, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Schwaller, B. Cytosolic Ca2+ buffers are inherently Ca2+ signal modulators. Cold Spring Harb. Perspect. Biol. 2020, 12, a035543. [Google Scholar] [CrossRef] [PubMed]
- Eisner, D.; Neher, E.; Taschenberger, H.; Smith, G. Physiology of intracellular calcium buffering. Physiol. Rev. 2023, 103, 2767–2845. [Google Scholar] [CrossRef]
- Schönichen, A.; Webb, B.A.; Jacobson, M.P.; Barber, D.L. Considering protonation as a posttranslational modification regulating protein structure and function. Annu. Rev. Biophys. 2013, 42, 289–314. [Google Scholar] [CrossRef]
- Molinari, G.; Nervo, E. Role of protons in calcium signaling. Biochem. J. 2021, 478, 895–910. [Google Scholar] [CrossRef]
- Owen, J.A.; Robson, J.S. The nomenclature of acid-base balance. Scott. Med. J. 1956, 1, 294–296. [Google Scholar] [CrossRef]
- Adrogué, H.J.; Gennari, F.J.; Galla, J.H.; Madias, N.E. Assessing acid–base disorders. Kidney Int. 2009, 76, 1239–1247. [Google Scholar] [CrossRef]
- Roos, A.; Boron, W.F. Intracellular pH. Physiol. Rev. 1981, 61, 296–434. [Google Scholar] [CrossRef]
- Vanheel, B.; de Hemptinne, A.; Leusen, I. Influence of surface pH on intracellular pH regulation in cardiac and skeletal muscle. Am. J. Physiol. Cell Physiol. 1986, 250, C748–C760. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-L.; Jensen, P.E.; Nilsson, H.; Aalkjær, C. Effect of acidosis on tension and [Ca2+]i in rat cerebral arteries: Is there a role for membrane potential? Am. J. Physiol.Heart Circ. Physiol. 1998, 274, H655–H662. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Excitation-Contraction Coupling. In Excitation-Contraction Coupling and Cardiac Contractile Force; Developments in Cardiovascular Medicine; Springer: Dordrecht, The Netherlands, 2001; Volume 237, pp. 203–244. [Google Scholar]
- Bers, D.M. Cardiac excitation–contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef]
- Calderón, J.C.; Bolaños, P.; Caputo, C. The excitation–contraction coupling mechanism in skeletal muscle. Biophys. Rev. 2014, 6, 133–160. [Google Scholar] [CrossRef]
- Anderson, K.; Meissner, G. T-tubule depolarization-induced SR Ca2+ release is controlled by dihydropyridine receptor- and Ca2+-dependent mechanisms in cell homogenates from rabbit skeletal muscle. J. Gen. Physiol. 1995, 105, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Mann, G.; Meissner, G. Regulation of cardiac Ca2+ release channel (ryanodine receptor) by Ca2+, H+, Mg2+, and adenine nucleotides under normal and simulated ischemic conditions. Circ. Res. 1996, 79, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Fill, M.; Knudson, C.M.; Campbell, K.P.; Coronado, R. Ryanodine receptor of skeletal muscle is a gap junction-type channel. Science 1988, 242, 99–102. [Google Scholar] [CrossRef]
- Dirksen, R.T.; Eisner, D.A.; Ríos, E.; Sipido, K.R. Excitation—Contraction coupling in cardiac, skeletal, and smooth muscle. J. Gen. Physiol. 2022, 154, e202213244. [Google Scholar] [CrossRef]
- Craig, R.; Padrón, R. Molecular structure of the sarcomere. Myology 2004, 3, 129–144. [Google Scholar]
- Hitchcock-DeGregori, S.E.; Barua, B. Tropomyosin Structure, Function, and Interactions: A Dynamic Regulator. In Fibrous Proteins: Structures and Mechanisms; Parry, D., Squire, J., Eds.; Springer: Cham, Switzerland, 2017; Volume 82, pp. 253–284. [Google Scholar]
- Sevrieva, I.R.; Kampourakis, T.; Irving, M. Structural changes in troponin during activation of skeletal and heart muscle determined in situ by polarised fluorescence. Biophys. Rev. 2024, 16, 753–772. [Google Scholar] [CrossRef] [PubMed]
- Herzog, W.; Schappacher-Tilp, G. Molecular mechanisms of muscle contraction: A historical perspective. J. Biomech. 2023, 155, 111659. [Google Scholar] [CrossRef]
- Huxley, H.E. The crossbridge mechanism of muscular contraction and its implications. J. Exp. Biol. 1985, 115, 17–30. [Google Scholar] [CrossRef]
- Fuchs, F.; Smith, S.H. Calcium, cross-bridges, and the frank-starling relationship. Physiology 2001, 16, 5–10. [Google Scholar] [CrossRef]
- Sweeney, H.L.; Houdusse, A. The motor mechanism of myosin v: Insights for muscle contraction. Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 1829. [Google Scholar]
- Rayment, I.; Holden, H.M.; Whittaker, M.; Yohn, C.B.; Lorenz, M.; Holmes, K.C.; Milligan, R.A. Structure of the actin-myosin complex and its implications for muscle contraction. Science 1993, 261, 58–65. [Google Scholar] [CrossRef]
- Capitanio, M.; Canepari, M.; Cacciafesta, P.; Lombardi, V.; Cicchi, R.; Maffei, M.; Pavone, F.S.; Bottinelli, R. Two independent mechanical events in the interaction cycle of skeletal muscle myosin with actin. Proc. Natl. Acad. Sci. USA 2006, 103, 87–92. [Google Scholar] [CrossRef]
- Baker, J.E.; Brosseau, C.; Joel, P.B.; Warshaw, D.M. The biochemical kinetics underlying actin movement generated by one and many skeletal muscle myosin molecules. Biophys. J. 2002, 82, 2134–2147. [Google Scholar] [CrossRef]
- Lanner, J.T.; Georgiou, D.K.; Joshi, A.D.; Hamilton, S.L. Ryanodine receptors: Structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Perspect. Biol. 2010, 2, a003996. [Google Scholar] [CrossRef]
- Bolaños, P.; Calderón, J.C. Excitation-contraction coupling in mammalian skeletal muscle: Blending old and last-decade research. Front. Physiol. 2022, 13, 989796. [Google Scholar] [CrossRef] [PubMed]
- Sztretye, M.; Geyer, N.; Vincze, J.; Al-Gaadi, D.; Oláh, T.; Szentesi, P.; Kis, G.; Antal, M.; Balatoni, I.; Csernoch, L.; et al. SOCE is important for maintaining sarcoplasmic calcium content and release in skeletal muscle fibers. Biophys. J. 2017, 113, 2496–2507. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, I.M.; Belevych, A.E.; Baine, S.; Stepanov, A.; Mezache, L.; Bodnar, T.; Liu, B.; Volpe, P.; Priori, S.; Weisleder, N.; et al. Enhancement of cardiac store operated calcium entry (SOCE) within novel intercalated disk microdomains in arrhythmic disease. Sci. Rep. 2019, 9, 10179. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, A.Y.; Kim, J.J.; Graham, V.; Finch, E.A.; Nepliouev, I.; Zhao, G.; Li, T.; Lederer, W.; Stiber, J.A. Stim1–Ca2+ signaling modulates automaticity of the mouse sinoatrial node. Proc. Natl. Acad. Sci. USA 2015, 112, E5618–E5627. [Google Scholar] [CrossRef]
- Hulot, J.-S.; Fauconnier, J.; Ramanujam, D.; Chaanine, A.; Aubart, F.; Sassi, Y.; Merkle, S.; Cazorla, O.; Ouillé, A.; Dupuis, M. Critical role for stromal interaction molecule 1 in cardiac hypertrophy. Circulation 2011, 124, 796–805. [Google Scholar] [CrossRef]
- Correll, R.N.; Goonasekera, S.A.; van Berlo, J.H.; Burr, A.R.; Accornero, F.; Zhang, H.; Makarewich, C.A.; York, A.J.; Sargent, M.A.; Chen, X. Stim1 elevation in the heart results in aberrant Ca2+ handling and cardiomyopathy. J. Mol. Cell. Cardiol. 2015, 87, 38–47. [Google Scholar] [CrossRef]
- Perocchi, F.; Gohil, V.M.; Girgis, H.S.; Bao, X.R.; McCombs, J.E.; Palmer, A.E.; Mootha, V.K. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature 2010, 467, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sanz, C.; De la Fuente, S.; Sheu, S.S. Mitochondrial Ca2+ concentrations in live cells: Quantification methods and discrepancies. FEBS Lett. 2019, 593, 1528–1541. [Google Scholar] [CrossRef]
- Wüst, R.C.; Helmes, M.; Martin, J.L.; van der Wardt, T.J.; Musters, R.J.; van der Velden, J.; Stienen, G.J. Rapid frequency-dependent changes in free mitochondrial calcium concentration in rat cardiac myocytes. J. Physiol. 2017, 595, 2001–2019. [Google Scholar] [CrossRef]
- Rossini, M.; Filadi, R. Sarcoplasmic reticulum-mitochondria kissing in cardiomyocytes: Ca2+, ATP, and undisclosed secrets. Front. Cell Dev. Biol. 2020, 8, 532. [Google Scholar] [CrossRef]
- Poburko, D.; Demaurex, N. Regulation of the mitochondrial proton gradient by cytosolic Ca2+ signals. Pflügers Arch. Eur. J. Physiol. 2012, 464, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Reggiani, C.; Marcucci, L. A controversial issue: Can mitochondria modulate cytosolic calcium and contraction of skeletal muscle fibers? J. Gen. Physiol. 2022, 154, e202213167. [Google Scholar]
- Pesta, D. Mitochondrial density in skeletal and cardiac muscle. Mitochondrion 2024, 75, 101838. [Google Scholar] [CrossRef]
- D’Angelo, D.; Vecellio Reane, D.; Raffaello, A. Neither too much nor too little: Mitochondrial calcium concentration as a balance between physiological and pathological conditions. Front. Mol. Biosci. 2023, 10, 1336416. [Google Scholar] [CrossRef]
- Kent-Braun, J.A.; Fitts, R.H.; Christie, A. Skeletal muscle fatigue. Compr. Physiol. 2012, 2, 997–1044. [Google Scholar] [CrossRef] [PubMed]
- Lännergren, J.; Westerblad, H. Force decline due to fatigue and intracellular acidification in isolated fibres from mouse skeletal muscle. J. Physiol. 1991, 434, 307–322. [Google Scholar] [CrossRef]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef]
- Pollack, G.H. The cross-bridge theory. Physiol. Rev. 1983, 63, 1049–1113. [Google Scholar] [CrossRef]
- Sundberg, C.W.; Fitts, R.H. Bioenergetic basis of skeletal muscle fatigue. Curr. Opin. Physiol. 2019, 10, 118–127. [Google Scholar] [CrossRef]
- Debold, E.P.; Beck, S.E.; Warshaw, D.M. Effect of low pH on single skeletal muscle myosin mechanics and kinetics. Am. J. Physiol. Cell Physiol. 2008, 295, C173–C179. [Google Scholar] [CrossRef] [PubMed]
- Spriet, L.; Lindinger, M.; McKelvie, R.; Heigenhauser, G.; Jones, N. Muscle glycogenolysis and H+ concentration during maximal intermittent cycling. J. Appl. Physiol. 1989, 66, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R502–R516. [Google Scholar] [CrossRef]
- Sahlin, K.; Harris, R.; Nylind, B.; Hultman, E. Lactate content and pH in muscle samples obtained after dynamic exercise. Pflügers Arch. 1976, 367, 143–149. [Google Scholar] [CrossRef]
- Böning, D.; Strobel, G.N.; Beneke, R.; Maassen, N. Lactic acid still remains the real cause of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R902–R903. [Google Scholar] [CrossRef]
- Proia, P.; Di Liegro, C.M.; Schiera, G.; Fricano, A.; Di Liegro, I. Lactate as a metabolite and a regulator in the central nervous system. Int. J. Mol. Sci. 2016, 17, 1450. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Juel, C. Lactate-proton cotransport in skeletal muscle. Physiol. Rev. 1997, 77, 321–358. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 5th ed.; W.H. Freeman & Co.: New York, NY, USA, 2002. [Google Scholar]
- Brooks, G.A. The science and translation of lactate shuttle theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Robergs, R.A.; Opeyemi, O.; Torrens, S. How to be a better scientist: Lessons from scientific philosophy, the historical development of science, and past errors within exercise physiology. Sports Med. Health Sci. 2022, 4, 140–146. [Google Scholar] [CrossRef]
- Lindinger, M.I.; Kowalchuk, J.M.; Heigenhauser, G.J.F. Applying physicochemical principles to skeletal muscle acid-base status. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R891–R894. [Google Scholar] [CrossRef]
- Pesi, R.; Balestri, F.; Ipata, P.L. Anaerobic glycolysis and glycogenolysis do not release protons and do not cause acidosis. Curr. Metabolomics Syst. Biol. (Discontin.) 2020, 7, 6–10. [Google Scholar] [CrossRef]
- Bountra, C.; Kaila, K.; Vaughan-Jones, R.D. Effect of repetitive activity upon intracellular pH, sodium and contraction in sheep cardiac Purkinje fibres. J. Physiol. 1988, 398, 341–360. [Google Scholar] [CrossRef]
- DeCoursey, T.E. Voltage-gated proton channels: Molecular biology, physiology, and pathophysiology of the Hv family. Physiol. Rev. 2013, 93, 599–652. [Google Scholar] [CrossRef] [PubMed]
- Song, C.W.; Griffin, R.; Park, H.J. Influence of Tumor pH on Therapeutic Response. In Cancer Drug Resistance. Cancer Drug Discovery and Development; Teicher, B.A., Ed.; Humana Press: Totowa, NJ, USA, 2006. [Google Scholar]
- Feuvray, D. The regulation of intracellular pH in the diabetic myocardium. Cardiovasc. Res. 1997, 34, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Orchard, C.H. The effects of changes of pH on intracellular calcium transients in mammalian cardiac muscle. J. Physiol. 1983, 335, 555–567. [Google Scholar] [CrossRef]
- Senneff, S.; Lowery, M.M. Effects of extracellular potassium on calcium handling and force generation in a model of excitation-contraction coupling in skeletal muscle. J. Theor. Biol. 2021, 519, 110656. [Google Scholar] [CrossRef] [PubMed]
- Dulhunty, A.F. Heterogeneity of T-tubule geometry in vertebrate skeletal muscle fibres. J. Muscle Res. Cell Motil. 1984, 5, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Sejersted, O.M.; Sjøgaard, G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol. Rev. 2000, 80, 1411–1481. [Google Scholar] [CrossRef]
- Knuth, S.T.; Dave, H.; Peters, J.R.; Fitts, R. Low cell pH depresses peak power in rat skeletal muscle fibres at both 30 °C and 15 °C: Implications for muscle fatigue. J. Physiol. 2006, 575, 887–899. [Google Scholar] [CrossRef]
- Parsons, B.; Szczesna, D.; Zhao, J.; Van Slooten, G.; Kerrick, W.G.L.; Putkey, J.A.; Potter, J.D. The effect of pH on the Ca2+ affinity of the Ca2+ regulatory sites of skeletal and cardiac troponin c in skinned muscle fibres. J. Muscle Res. Cell Motil. 1997, 18, 599–609. [Google Scholar] [CrossRef]
- Fabiato, A.; Fabiato, F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiac and skeletal muscles. J. Physiol. 1978, 276, 233–255. [Google Scholar] [CrossRef]
- Ball, K.L.; Johnson, M.D.; Solaro, R.J. Isoform specific interactions of troponin I and troponin C determine pH sensitivity of myofibrillar Ca2+ activation. Biochemistry 1994, 33, 8464–8471. [Google Scholar] [CrossRef] [PubMed]
- Metzger, J.; Moss, R. Effects of tension and stiffness due to reduced pH in mammalian fast- and slow-twitch skinned skeletal muscle fibres. J. Physiol. 1990, 428, 737–750. [Google Scholar] [CrossRef]
- Metzger, J.M.; Moss, R.L. pH modulation of the kinetics of a Ca2+-sensitive cross-bridge state transition in mammalian single skeletal muscle fibres. J. Physiol. 1990, 428, 751–764. [Google Scholar] [CrossRef]
- Metzger, J.M.; Moss, R.L. Greater hydrogen ion-induced depression of tension and velocity in skinned single fibres of rat fast than slow muscles. J. Physiol. 1987, 393, 727–742. [Google Scholar] [CrossRef]
- Cooke, R.; Franks, K.; Luciani, G.B.; Pate, E. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J. Physiol. 1988, 395, 77–97. [Google Scholar] [CrossRef]
- Debold, E.P.; Romatowski, J.; Fitts, R.H. The depressive effect of Pi on the force-PCa relationship in skinned single muscle fibers is temperature dependent. Am. J. Physiol. Cell Physiol. 2006, 290, C1041–C1050. [Google Scholar] [CrossRef]
- Fitts, R.H. The cross-bridge cycle and skeletal muscle fatigue. J. Appl. Physiol. 2008, 104, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Nosek, T.M.; Fender, K.Y.; Godt, R.E. It is diprotonated inorganic phosphate that depresses force in skinned skeletal muscle fibers. Science 1987, 236, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Wolosker, H.; Rocha, J.B.; Engelender, S.; Panizzutti, R.; Miranda, J.D.; Meis, L.D. Sarco/endoplasmic reticulum Ca2+-ATPase isoforms: Diverse responses to acidosis. Biochem. J. 1997, 321, 545–550. [Google Scholar] [CrossRef]
- Allen, D.G. Skeletal muscle function: Role of ionic changes in fatigue, damage and disease. Clin. Exp. Pharmacol. Physiol. 2004, 31, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Favero, T.G. Sarcoplasmic reticulum Ca2+ release and muscle fatigue. J. Appl. Physiol. 1999, 87, 471–483. [Google Scholar] [CrossRef]
- Philipson, K.D.; Bersohn, M.M.; Nishimoto, A. Effects of pH on Na+-Ca2+ exchange in canine cardiac sarcolemmal vesicles. Circ. Res. 1982, 50, 287–293. [Google Scholar] [CrossRef]
- Scranton, K.; John, S.; Escobar, A.; Goldhaber, J.I.; Ottolia, M. Modulation of the cardiac Na+-Ca2+ exchanger by cytoplasmic protons: Molecular mechanisms and physiological implications. Cell Calcium 2020, 87, 102140. [Google Scholar] [CrossRef]
- Linck, B.; Qiu, Z.; He, Z.; Tong, Q.; Hilgemann, D.W.; Philipson, K.D. Functional comparison of the three isoforms of the Na+/Ca2+ exchanger (NCX1, NCX2, NCX3). Am. J. Physiol. Cell Physiol. 1998, 274, C415–C423. [Google Scholar] [CrossRef]
- Donoso Laurent, P.; Hidalgo Tapia, M.C. Sodium-calcium exchange in transverse tubules isolated from frog skeletal muscle. Biochim. Biophys. Acta Biomembr. 1989, 987, 8–16. [Google Scholar] [CrossRef]
- Garcia, M.; Diaz, A.; Godinez, R.; Sanchez, J. Effect of sodium deprivation on contraction and charge movement in frog skeletal muscle fibres. J. Muscle Res. Cell Motil. 1992, 13, 354–365. [Google Scholar] [CrossRef]
- Gavriliouk, D.; Scrimgeour, N.R.; Grigoryev, S.; Ma, L.; Zhou, F.H.; Barritt, G.J.; Rychkov, G.Y. Regulation of Orai1/Stim1 mediated ICRAC by intracellular pH. Sci. Rep. 2017, 7, 9829. [Google Scholar] [CrossRef]
- Yu, A.S.; Yue, Z.; Feng, J.; Yue, L. Regulation of Orai/Stim channels by pH. In Calcium Entry Channels in Non-Excitable Cells, 1st ed.; Kozak, J.A., Putney, J.W., Jr., Eds.; CRC Press: Boca Raton, FL, USA, 2017; Chapter 9. [Google Scholar]
- Cairns, S.P.; Lindinger, M.I. Lactic acidosis: Implications for human exercise performance. Eur. J. Appl. Physiol. 2025, 125, 1761–1795. [Google Scholar] [CrossRef]
- Bouviere, J.; Fortunato, R.S.; Dupuy, C.; Werneck-de-Castro, J.P.; Carvalho, D.P.; Louzada, R.A. Exercise-stimulated ROS sensitive signaling pathways in skeletal muscle. Antioxidants 2021, 10, 537. [Google Scholar] [CrossRef]
- Drust, B.; Rasmussen, P.; Mohr, M.; Nielsen, B.; Nybo, L. Elevations in core and muscle temperature impairs repeated sprint performance. Acta Physiol. Scand. 2005, 183, 181–190. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The role of oxidative stress in cardiac disease: From physiological response to injury factor. Oxidative Med. Cell. Longev. 2020, 2020, 5732956. [Google Scholar] [CrossRef]
- Aggarwal, N.T.; Makielski, J.C. Redox control of cardiac excitability. Antioxid. Redox Signal. 2013, 18, 432–468. [Google Scholar] [CrossRef] [PubMed]
- Slavin, M.B.; Khemraj, P.; Hood, D.A. Exercise, mitochondrial dysfunction and inflammasomes in skeletal muscle. Biomed. J. 2024, 47, 100636. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Gao, H.; Liu, X.; Zhao, Z.; Gao, B.; Zhang, L. Establishment and identification of an animal model of long-term exercise-induced fatigue. Front. Endocrinol. 2022, 13, 915937. [Google Scholar] [CrossRef]
- Ho, J.Q.; Abramowitz, M.K. Clinical consequences of metabolic acidosis-muscle. Adv. Chronic Kidney Dis. 2022, 29, 395–405. [Google Scholar] [CrossRef]
- Vaughan-Jones, R.D. Regulation of intracellular pH in cardiac muscle. In Ciba Foundation Symposium 139—Proton Passage Across Cell Membranes; Bock, G., Marsh, J., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2007; pp. 23–46. [Google Scholar]
- Vandenberg, J.I.; Carter, N.D.; Bethell, H.W.; Nogradi, A.; Ridderstrale, Y.; Metcalfe, J.C.; Grace, A.A. Carbonic anhydrase and cardiac pH regulation. Am. J. Physiol. Cell Physiol. 1996, 271, C1838–C1846. [Google Scholar] [CrossRef]
- Orlowski, A.; Di Mattia, R.A.; Aiello, E.A. Intracellular pH regulation in ventricular myocytes: Implications for cardiac health and disease. Circ. Res. 2025, 136, 1636–1656. [Google Scholar] [CrossRef]
- Vaughan-Jones, R.; Eisner, D.; Lederer, W. Effects of changes of intracellular pH on contraction in sheep cardiac Purkinje fibers. J. Gen. Physiol. 1987, 89, 1015–1032. [Google Scholar] [CrossRef]
- Jacobus, W.E.; Pores, I.H.; Lucas, S.K.; Weisfeldt, M.L.; Flaherty, J.T. Intracellular acidosis and contractility in the normal and ischemic heart as examined by 31P NMR. J. Mol. Cell. Cardiol. 1982, 14, 13–20. [Google Scholar] [CrossRef]
- Choi, H.S.; Trafford, A.W.; Orchard, C.H.; Eisner, D.A. The effect of acidosis on systolic Ca2+ and sarcoplasmic reticulum calcium content in isolated rat ventricular myocytes. J. Physiol 2000, 529, 661–668. [Google Scholar] [CrossRef]
- Orchard, C.; Kentish, J.C. Effects of changes of pH on the contractile function of cardiac muscle. Am. J. Physiol. Cell Physiol. 1990, 258, C967–C981. [Google Scholar] [CrossRef] [PubMed]
- Kaibara, M.; Kameyama, M. Inhibition of the calcium channel by intracellular protons in single ventricular myocytes of the guinea pig. J. Physiol. 1988, 403, 621–640. [Google Scholar] [CrossRef] [PubMed]
- Balnave, C.; Vaughan-Jones, R. Effect of intracellular pH on spontaneous Ca2+ sparks in rat ventricular myocytes. J. Physiol. 2000, 528, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Rozanski, G.J.; Witt, R.C. Acidosis masks beta-adrenergic control of cardiac L-type calcium current. J. Mol. Cell. Cardiol. 1995, 27, 1781–1788. [Google Scholar] [CrossRef]
- Brodde, O.-E. The functional importance of beta1 and beta2 adrenoceptors in the human heart. Am. J. Cardiol. 1988, 62, 24C–29C. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, E.; Pinkos, J. pH modulates conducting and gating behaviour of single calcium release channels. Pflügers Arch. 1990, 415, 645–647. [Google Scholar] [CrossRef]
- Hulme, J.; Orchard, C. Effect of acidosis on Ca2+ uptake and release by sarcoplasmic reticulum of intact rat ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 1998, 275, H977–H987. [Google Scholar] [CrossRef]
- Fabiato, A. Use of aequorin for the appraisal of the hypothesis of the release of calcium from the sarcoplasmic reticulum induced by a change of pH in skinned cardiac cells. Cell Calcium 1985, 6, 95–108. [Google Scholar] [CrossRef]
- Kentish, J.C.; Xiang, J.-Z. Ca2+- and caffeine-induced Ca2+ release from the sarcoplasmic reticulum in rat skinned trabeculae: Effects of pH and Pi. Cardiovasc. Res. 1997, 33, 314–323. [Google Scholar] [CrossRef]
- Orchard, C. The role of the sarcoplasmic reticulum in the response of ferret and rat heart muscle to acidosis. J. Physiol. 1987, 384, 431–449. [Google Scholar] [CrossRef]
- Bers, D.M.; Ellis, D. Intracellular calcium and sodium activity in sheep heart Purkinje fibres: Effect of changes of external sodium and intracellular pH. Pflügers Arch. 1982, 393, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.; MacLeod, K. Sodium-dependent control of intracellular pH in Purkinje fibres of sheep heart. J. Physiol. 1985, 359, 81–105. [Google Scholar] [CrossRef]
- Aronson, P.S.; Giebisch, G. Effects of pH on potassium: New explanations for old observations. J. Am. Soc. Nephrol. 2011, 22, 1981–1989. [Google Scholar] [CrossRef]
- Hilgemann, D.W.; Matsuoka, S.; Nagel, G.A.; Collins, A. Steady-state and dynamic properties of cardiac sodium-calcium exchange. Sodium-dependent inactivation. J. Gen. Physiol. 1992, 100, 905–932. [Google Scholar] [PubMed]
- Scranton, K.; John, S.; Angelini, M.; Steccanella, F.; Umar, S.; Zhang, R.; Goldhaber, J.I.; Olcese, R.; Ottolia, M. Cardiac function is regulated by the sodium-dependent inhibition of the sodium-calcium exchanger NCX1. Nat. Commun. 2024, 15, 3831. [Google Scholar] [CrossRef]
- Doering, A.E.; Lederer, W. The action of Na+ as a cofactor in the inhibition by cytoplasmic protons of the cardiac Na+-Ca2+ exchanger in the guinea pig. J. Physiol. 1994, 480, 9–20. [Google Scholar] [CrossRef]
- Allen, D.G.; Xiao, X.-H. Role of the cardiac Na+/H+ exchanger during ischemia and reperfusion. Cardiovasc. Res. 2003, 57, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, S. The Na+/Ca2+ exchanger in cardiac ischemia/reperfusion injury. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2012, 18, RA161. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, X.; Kim, S.; Kim, B.; Xie, C.; Gonzalez, D.; Norris, R.; Chin, N.; Li, L.; John, S. Regulation of Na+/Ca2+ exchange by cytoplasmic protons modifies intracellular calcium dynamics and the cardiac response to ischemia. Proc. Natl. Acad. Sci. USA 2025, 122, e2423203122. [Google Scholar] [CrossRef]
- Gursahani, H.I.; Schaefer, S. Acidification reduces mitochondrial calcium uptake in rat cardiac mitochondria. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2659–H2665. [Google Scholar] [CrossRef]
- Silverstein, T.P. The proton in biochemistry: Impacts on bioenergetics, biophysical chemistry, and bioorganic chemistry. Front. Mol. Biosci. 2021, 8, 764099. [Google Scholar] [CrossRef]
- Spitzer, K.W.; Ershler, P.R.; Skolnick, R.L.; Vaughan-Jones, R.D. Generation of intracellular pH gradients in single cardiac myocytes with a microperfusion system. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H1371–H1382. [Google Scholar] [CrossRef] [PubMed]
- Swietach, P.; Leem, C.-H.; Spitzer, K.W.; Vaughan-Jones, R.D. Experimental generation and computational modeling of intracellular pH gradients in cardiac myocytes. Biophys. J. 2005, 88, 3018–3037. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Swietach, P.; Youm, J.-B.; Saegusa, N.; Leem, C.-H.; Spitzer, K.W.; Vaughan-Jones, R.D. Coupled Ca2+/H+ transport by cytoplasmic buffers regulates local Ca2+/H+ ion signaling. Proc. Natl. Acad. Sci. USA 2013, 110, E2064–E2073. [Google Scholar] [CrossRef] [PubMed]
- Zaniboni, M.; Swietach, P.; Rossini, A.; Yamamoto, T.; Spitzer, K.W.; Vaughan-Jones, R.D. Intracellular proton mobility and buffering power in cardiac ventricular myocytes from rat, rabbit, and guinea pig. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H1236–H1246. [Google Scholar] [CrossRef]
- Kraut, J.A.; Madias, N.E. Lactic acidosis: Current treatments and future directions. Am. J. Kidney Dis. 2016, 68, 473–482. [Google Scholar] [CrossRef]
- Rodríguez-Villar, S.; Kraut, J.; Arévalo-Serrano, J.; Sakka, S.; Harris, C.; Awad, I.; Toolan, M.; Vanapalli, S.; Collins, A.; Spataru, A. Systemic acidemia impairs cardiac function in critically ill patients. eClinicalMedicine 2021, 37, 100956. [Google Scholar] [CrossRef]
- Jung, B.; Rimmele, T.; Goff, C.L.; Chanques, G.; Corne, P.; Jonquet, O.; Muller, L.; Lefrant, J.-Y.; Guervilly, C.; Papazian, L.; et al. Severe metabolic or mixed acidemia on intensive care unit admission: Incidence, prognosis and administration of buffer therapy. A prospective, multiple-center study. Crit. Care 2011, 15, R238. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Gao, X.; Li, Y.; DeCoursey, T.E.; Shull, G.E.; Wang, H.-S. The HVCN1 voltage-gated proton channel contributes to pH regulation in canine ventricular myocytes. J. Physiol. 2022, 600, 2089–2103. [Google Scholar] [CrossRef] [PubMed]
- Vaughan-Jones, R.D.; Spitzer, K.W. Role of bicarbonate in the regulation of intracellular pH in the mammalian ventricular myocyte. Biochem. Cell Biol. 2002, 80, 579–596. [Google Scholar] [CrossRef]
- Leem, C.H.; Lagadic-Gossmann, D.; Vaughan-Jones, R.D. Characterization of intracellular pH regulation in the guinea pig ventricular myocyte. J. Physiol. 1999, 517, 159–180. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrase versatility: From pH regulation to CO2 sensing and metabolism. Front. Mol. Biosci. 2023, 10, 1326633. [Google Scholar] [CrossRef]
- Wang, H.-S.; Chen, Y.; Vairamani, K.; Shull, G.E. Critical role of bicarbonate and bicarbonate transporters in cardiac function. World J. Biol. Chem. 2014, 5, 334. [Google Scholar] [CrossRef]
- JUEL, C. Lactate/proton co-transport in skeletal muscle: Regulation and importance for pH homeostasis. Acta Physiol. Scand. 1996, 156, 369–374. [Google Scholar] [CrossRef]
- Geers, C.; Gros, G. Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol. Rev. 2000, 80, 681–715. [Google Scholar] [CrossRef]
- Michenkova, M.; Taki, S.; Blosser, M.C.; Hwang, H.J.; Kowatz, T.; Moss, F.J.; Occhipinti, R.; Qin, X.; Sen, S.; Shinn, E. Carbon dioxide transport across membranes. Interface Focus 2021, 11, 20200090. [Google Scholar] [CrossRef]
- Sahlin, K.; Alvestrand, A.; Brandt, R.; Hultman, E. Intracellular pH and bicarbonate concentration in human muscle during recovery from exercise. J. Appl. Physiol. 1978, 45, 474–480. [Google Scholar] [CrossRef]
- Clancy, R.; EB, B. In vivo CO2 buffer curves of skeletal and cardiac muscle. Am. J. Physiol. Leg. Content 1966, 211, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Greiser, M.; Karbowski, M.; Kaplan, A.D.; Coleman, A.K.; Verhoeven, N.; Mannella, C.A.; Lederer, W.J.; Boyman, L. Calcium and bicarbonate signaling pathways have pivotal, resonating roles in matching ATP production to demand. Elife 2023, 12, e84204. [Google Scholar] [CrossRef]
- Torella, D.; Ellison, G.M.; Torella, M.; Vicinanza, C.; Aquila, I.; Iaconetti, C.; Scalise, M.; Marino, F.; Henning, B.J.; Lewis, F.C.; et al. Carbonic anhydrase activation is associated with worsened pathological remodeling in human ischemic diabetic cardiomyopathy. J. Am. Heart Assoc. 2014, 3, e000434. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, B.V.; Quon, A.L.; Mullen, J.; Casey, J.R. Quantification of carbonic anhydrase gene expression in ventricle of hypertrophic and failing human heart. BMC Cardiovasc. Disord. 2013, 13, 2. [Google Scholar] [CrossRef]
- Yamamoto, T.; Shirayama, T.; Sakatani, T.; Takahashi, T.; Tanaka, H.; Takamatsu, T.; Spitzer, K.W.; Matsubara, H. Enhanced activity of ventricular Na+-HCO3− cotransport in pressure overload hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1254–H1264. [Google Scholar] [CrossRef]
- Djojosugito, A.M.; Folkow, B.; Lisander, B.; Sparks, H. Mechanism of escape of skeletal muscle resistance vessels from the influence of sympathetic cholinergic vasodilator fibre activity. Acta Physiol. Scand. 1968, 72, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Wirth, K.J.; Scheibenbogen, C. Pathophysiology of skeletal muscle disturbances in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2021, 19, 162. [Google Scholar] [CrossRef]
- Iwata, Y.; Katanosaka, Y.; Hisamitsu, T.; Wakabayashi, S. Enhanced Na+/H+ exchange activity contributes to the pathogenesis of muscular dystrophy via involvement of P2 receptors. Am. J. Pathol. 2007, 171, 1576–1587. [Google Scholar] [CrossRef]
- Wu, D.; Kraut, J.A. Role of NHE1 in the cellular dysfunction of acute metabolic acidosis. Am. J. Nephrol. 2014, 40, 36–42. [Google Scholar] [CrossRef]
- Heizmann, C. Parvalbumin, and intracellular calcium-binding protein; Distribution, properties and possible roles in mammalian cells. Experientia 1984, 40, 910–921. [Google Scholar] [CrossRef]
- Haiech, J.; Derancourt, J.; Pechere, J.F.; Demaille, J.G. Magnesium and calcium binding to parvalbumins: Evidence for differences between parvalbumins and an explanation of their relaxing function. Biochemistry 1979, 18, 2752–2758. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, M.; Erne, P. Calcium and magnesium binding to rat parvalbumin. Eur. J. Biochem. 1994, 222, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Chin, E.R.; Grange, R.W.; Viau, F.; Simard, A.R.; Humphries, C.; Shelton, J.; Bassel-Duby, R.; Williams, R.S.; Michel, R.N. Alterations in slow-twitch muscle phenotype in transgenic mice overexpressing the Ca2+ buffering protein parvalbumin. J. Physiol. 2003, 547, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Vongvatcharanon, S.; Vongvatcharanon, U.; Boonyoung, P. Immunohistochemical localization of parvalbumin calcium-binding protein in the heart tissues of various species. Acta Histochem. 2008, 110, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Heizmann, C.W.; Berchtold, M.W.; Rowlerson, A.M. Correlation of parvalbumin concentration with relaxation speed in mammalian muscles. Proc. Natl. Acad. Sci. USA 1982, 79, 7243–7247. [Google Scholar] [CrossRef]
- Gao, W.D.; Backx, P.H.; Azan-Backx, M.; Marban, E. Myofilament Ca2+ sensitivity in intact versus skinned rat ventricular muscle. Circ. Res. 1994, 74, 408–415. [Google Scholar] [CrossRef]
- Higgins, E.R.; Cannell, M.B.; Sneyd, J. A buffering SERCA pump in models of calcium dynamics. Biophys. J. 2006, 91, 151–163. [Google Scholar] [CrossRef]
- Smith, G.L.; Eisner, D.A. Calcium buffering in the heart in health and disease. Circulation 2019, 139, 2358–2371. [Google Scholar] [CrossRef]
- Cheng, Y.-R.; Chi, C.-H.; Lee, C.-H.; Lin, S.-H.; Min, M.-Y.; Chen, C.-C. Probing the effect of acidosis on tether-mode mechanotransduction of proprioceptors. Int. J. Mol. Sci. 2023, 24, 12783. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, E.M.; Solaro, R.J. Inhibition of the activation and troponin calcium binding of dog cardiac myofibrils by acidic pH. Circ. Res. 1984, 55, 382–391. [Google Scholar] [CrossRef]
- Nicholls, D.G. Mitochondria and calcium signaling. Cell Calcium 2005, 38, 311–317. [Google Scholar] [CrossRef]
- Dedkova, E.N.; Blatter, L.A. Calcium signaling in cardiac mitochondria. J. Mol. Cell. Cardiol. 2013, 58, 125–133. [Google Scholar] [CrossRef]
- Gunter, K.K.; Gunter, T.E. Transport of calcium by mitochondria. J. Bioenerg. Biomembr. 1994, 26, 471–485. [Google Scholar] [CrossRef]
- O’Rourke, B.; Blatter, L.A. Mitochondrial Ca2+ uptake: Tortoise or hare? J. Mol. Cell. Cardiol. 2009, 46, 767–774. [Google Scholar] [CrossRef]
- Pallafacchina, G.; Zanin, S.; Rizzuto, R. Recent advances in the molecular mechanism of mitochondrial calcium uptake. F1000Research 2018, 7, 1858. [Google Scholar] [CrossRef] [PubMed]
- Glancy, B.; Balaban, R.S. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry 2012, 51, 2959–2973. [Google Scholar] [CrossRef] [PubMed]
- Santo-Domingo, J.; Demaurex, N. The renaissance of mitochondrial pH. J. Gen. Physiol. 2012, 139, 415–423. [Google Scholar] [CrossRef]
- Wei, A.-C.; Aon, M.A.; O’Rourke, B.; Winslow, R.L.; Cortassa, S. Mitochondrial energetics, pH regulation, and ion dynamics: A computational-experimental approach. Biophys. J. 2011, 100, 2894–2903. [Google Scholar] [CrossRef]
- Zotova, L.; Aleschko, M.; Sponder, G.; Baumgartner, R.; Reipert, S.; Prinz, M.; Schweyen, R.J.; Nowikovsky, K. Novel components of an active mitochondrial K+/H+ exchange. J. Biol. Chem. 2010, 285, 14399–14414. [Google Scholar] [CrossRef]
- Jiang, D.; Zhao, L.; Clapham, D.E. Genome-wide RNAi screen identifies LETM1 as a mitochondrial Ca2+/H+ antiporter. Science 2009, 326, 144–147. [Google Scholar] [CrossRef]
- Lyu, Y.; Thai, P.N.; Ren, L.; Timofeyev, V.; Jian, Z.; Park, S.; Ginsburg, K.S.; Overton, J.; Bossuyt, J.; Bers, D.M.; et al. Beat-to-beat dynamic regulation of intracellular pH in cardiomyocytes. iScience 2022, 25, 103624. [Google Scholar] [CrossRef]
- Abou Sawan, S.; Mazzulla, M.; Moore, D.R.; Hodson, N. More than just a garbage can: Emerging roles of the lysosome as an anabolic organelle in skeletal muscle. Am. J. Physiology. Cell Physiol. 2020, 319, C561–C568. [Google Scholar] [CrossRef] [PubMed]
- Terman, A.; Kurz, T.; Gustafsson, B.; Brunk, U.T. The involvement of lysosomes in myocardial aging and disease. Curr. Cardiol. Rev. 2008, 4, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Bootman, M.D.; Bultynck, G. Fundamentals of cellular calcium signaling: A primer. Cold Spring Harb. Perspect. Biol. 2020, 12, a038802. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Fuchs, R.; Helenius, A. Acidification of the endocytic and exocytic pathways. Annu. Rev. Biochem. 1986, 55, 663–700. [Google Scholar] [CrossRef]
- Settembre, C.; Fraldi, A.; Medina, D.L.; Ballabio, A. Signals from the lysosome: A control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 2013, 14, 283–296. [Google Scholar] [CrossRef]
- Raffaello, A.; Mammucari, C.; Gherardi, G.; Rizzuto, R. Calcium at the center of cell signaling: Interplay between endoplasmic reticulum, mitochondria, and lysosomes. Trends Biochem. Sci. 2016, 41, 1035–1049. [Google Scholar] [CrossRef]
- Breton, S.; Brown, D. Regulation of luminal acidification by the V-ATPase. Physiology 2013, 28, 318–329. [Google Scholar] [CrossRef]
- Mindell, J.A. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 2012, 74, 69–86. [Google Scholar] [CrossRef]
- Xiong, J.; Zhu, M.X. Regulation of lysosomal ion homeostasis by channels and transporters. Sci. China Life Sci. 2016, 59, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Galione, A. A primer of NAADP-mediated Ca2+ signalling: From sea urchin eggs to mammalian cells. Cell Calcium 2015, 58, 27–47. [Google Scholar] [CrossRef]
- Capel, R.A.; Bolton, E.L.; Lin, W.K.; Aston, D.; Wang, Y.; Liu, W.; Wang, X.; Burton, R.-A.B.; Bloor-Young, D.; Shade, K.-T. Two-pore channels (TPC2s) and nicotinic acid adenine dinucleotide phosphate (NAADP) at lysosomal-sarcoplasmic reticular junctions contribute to acute and chronic β-adrenoceptor signaling in the heart. J. Biol. Chem. 2015, 290, 30087–30098. [Google Scholar] [CrossRef]
- Serano, M.; Perni, S.; Pierantozzi, E.; Laurino, A.; Sorrentino, V.; Rossi, D. Intracellular membrane contact sites in skeletal muscle cells. Membranes 2025, 15, 29. [Google Scholar] [CrossRef]
- Espinoza-Fonseca, L.M. The Ca2+-ATPase pump facilitates bidirectional proton transport across the sarco/endoplasmic reticulum. Mol. Biosyst. 2017, 13, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Belavek, K.J.; Miller, E.W. Origins of Ca2+ imaging with fluorescent indicators. Biochemistry 2021, 60, 3547–3554. [Google Scholar] [CrossRef]
- Takahashi, A.; Camacho, P.; Lechleiter, J.D.; Herman, B. Measurement of intracellular calcium. Physiol. Rev. 1999, 79, 1089–1125. [Google Scholar] [CrossRef]
- Tsien, R.Y. New calcium indicators and buffers with high selectivity against magnesium and protons: Design, synthesis, and properties of prototype structures. Biochemistry 1980, 19, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Paredes, R.M.; Etzler, J.C.; Watts, L.T.; Zheng, W.; Lechleiter, J.D. Chemical calcium indicators. Methods 2008, 46, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Prole, D.L.; Shen, Y.; Lin, Z.; Gnanasekaran, A.; Liu, Y.; Chen, L.; Zhou, H.; Chen, S.W.; Usachev, Y.M. Red fluorescent genetically encoded Ca2+ indicators for use in mitochondria and endoplasmic reticulum. Biochem. J. 2014, 464, 13–22. [Google Scholar] [CrossRef]
- Carlson, H.J.; Campbell, R.E. Circular permutated red fluorescent proteins and calcium ion indicators based on mCherry. Protein Eng. Des. Sel. 2013, 26, 763–772. [Google Scholar] [CrossRef]
- Zhao, Y.; Araki, S.; Wu, J.; Teramoto, T.; Chang, Y.-F.; Nakano, M.; Abdelfattah, A.S.; Fujiwara, M.; Ishihara, T.; Nagai, T.; et al. An expanded palette of genetically encoded Ca2+ indicators. Science 2011, 333, 1888–1891. [Google Scholar] [CrossRef]
- Davis, L.C.; Morgan, A.J.; Galione, A. NAADP-regulated two-pore channels drive phagocytosis through endo- and lysosomal Ca2+ nanodomains, calcineurin and dynamin. EMBO J. 2020, 39, e104058. [Google Scholar] [CrossRef] [PubMed]
- Patterson, G.H.; Knobel, S.M.; Sharif, W.D.; Kain, S.R.; Piston, D.W. Use of the green fluorescent protein and its mutants in quantitative fluorescence microscopy. Biophys. J. 1997, 73, 2782–2790. [Google Scholar] [CrossRef]
- Li, S.-A.; Meng, X.-Y.; Zhang, Y.-J.; Chen, C.-L.; Jiao, Y.-X.; Zhu, Y.-Q.; Liu, P.-P.; Sun, W. Progress in pH-sensitive sensors: Essential tools for organelle pH detection, spotlighting mitochondrion and diverse applications. Front. Pharmacol. 2024, 14, 1339518. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, T.; Zhao, Y.; Nguyen, T.H.; Campbell, R.E.; Johnson, J.D. Fluorescent biosensors illuminate calcium levels within defined beta-cell endosome subpopulations. Cell Calcium 2015, 57, 263–274. [Google Scholar] [CrossRef]
- Mank, M.; Griesbeck, O. Genetically encoded calcium indicators. Chem. Rev. 2008, 108, 1550–1564. [Google Scholar] [CrossRef]
- Llopis, J.; McCaffery, J.M.; Miyawaki, A.; Farquhar, M.G.; Tsien, R.Y. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc. Natl. Acad. Sci. USA 1998, 95, 6803–6808. [Google Scholar] [CrossRef]
- Han, J.; Burgess, K. Fluorescent indicators for intracellular pH. Chem. Rev. 2010, 110, 2709–2728. [Google Scholar] [CrossRef]
- Martinez-Zaguilan, R.; Tompkins, L.S.; Gillies, R.J.; Lynch, R.M. Simultaneous analysis of intracellular pH and Ca2+ from cell populations. In Calcium Signaling Protocols; Lambert, D., Rainbow, R., Eds.; Humana Press: Totowa, NJ, USA, 2013; Volume 937, pp. 253–271. [Google Scholar]
- Austin, C.; Dilly, K.; Eisner, D.; Wray, S. Simultaneous measurement of intracellular pH, calcium, and tension in rat mesenteric vessels: Effects of extracellular pH. Biochem. Biophys. Res. Commun. 1996, 222, 537–540. [Google Scholar] [CrossRef]
- Westerblad, H. Acidosis is not a significant cause of skeletal muscle fatigue. Med. Sci. Sports Exerc. 2016, 48, 2339–2342. [Google Scholar] [CrossRef] [PubMed]
- Stackhouse, S.K.; Reisman, D.S.; Binder-Macleod, S.A. Challenging the role of pH in skeletal muscle fatigue. Phys. Ther. 2001, 81, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-W.; Kolb, J.; Farman, G.P.; Gohlke, J.; Granzier, H.L. Glycerol storage increases passive stiffness of muscle fibers through effects on titin extensibility. J. Gen. Physiol. 2025, 157, e202413729. [Google Scholar] [CrossRef]
- Ranatunga, K. Effects of acidosis on tension development in mammalian skeletal muscle. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 1987, 10, 439–445. [Google Scholar] [CrossRef]
- Bernheim, L.; Krause, R.M.; Baroffio, A.; Hamann, M.; Kaelin, A.; Bader, C.R. A voltage-dependent proton current in cultured human skeletal muscle myotubes. J. Physiol. 1993, 470, 313–333. [Google Scholar] [CrossRef]
- Krause, R.M.; Bernheim, L.; Bader, C.R. Human skeletal muscle has a voltage-gated proton current. Neuromuscul. Disord. NMD 1993, 3, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Vairamani, K.; Wang, H.-S.; Medvedovic, M.; Lorenz, J.N.; Shull, G.E. RNAseq analysis indicates that the AE3 Cl−/HCO3− exchanger contributes to active transport-mediated CO2 disposal in heart. Sci. Rep. 2017, 7, 7264. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G.; Jacques, D. Na+–H+ exchanger and proton channel in heart failure associated with Becker and Duchenne muscular dystrophies. Can. J. Physiol. Pharmacol. 2017, 95, 1213–1223. [Google Scholar] [CrossRef]
- Wakabayashi, S.; Hisamitsu, T.; Nakamura, T.Y. Regulation of the cardiac Na+/H+ exchanger in health and disease. J. Mol. Cell. Cardiol. 2013, 61, 68–76. [Google Scholar] [CrossRef]
- Swietach, P.; Despa, S. Channelling protons out of the heart. J. Physiol. 2022, 600, 2551–2552. [Google Scholar] [CrossRef] [PubMed]
- Decoursey, T.E. Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 2003, 83, 475–579. [Google Scholar] [CrossRef]
- Cherny, V.V.; Markin, V.S.; DeCoursey, T.E. The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J. Gen. Physiol. 1995, 105, 861–896. [Google Scholar] [CrossRef] [PubMed]
- Chaves, G.; Jardin, C.; Derst, C.; Musset, B. Voltage-gated proton channels in the tree of life. Biomolecules 2023, 13, 1035. [Google Scholar] [CrossRef]
- Musset, B.; Smith, S.M.; Rajan, S.; Morgan, D.; Cherny, V.V.; DeCoursey, T.E. Aspartate 112 is the selectivity filter of the human voltage-gated proton channel. Nature 2011, 480, 273–277. [Google Scholar] [CrossRef]
- Meech, R.; Thomas, R. Effect of measured calcium chloride injections on the membrane potential and internal pH of snail neurones. J. Physiol. 1980, 298, 111–129. [Google Scholar] [CrossRef]
- Hu, Y.-L.; Mi, X.; Huang, C.; Wang, H.-F.; Song, J.-R.; Shu, Q.; Ni, L.; Chen, J.-G.; Wang, F.; Hu, Z.-L. Multiple H+ sensors mediate the extracellular acidification-induced [Ca2+]i elevation in cultured rat ventricular cardiomyocytes. Sci. Rep. 2017, 7, 44951. [Google Scholar] [CrossRef]
- Sisignano, M.; Fischer, M.J.M.; Geisslinger, G. Proton-sensing GPCRs in health and disease. Cells 2021, 10, 2050. [Google Scholar] [CrossRef]
- Jaimovich, E.; Carrasco, M.A. Ip3 dependent Ca2+ signals in muscle cells are involved in regulation of gene expression. Biol. Res. 2002, 35, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Ca2+-calmodulin-dependent protein kinase II regulation of cardiac excitation-transcription coupling. Heart Rhythm. 2011, 8, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Jean-Baptiste, G.; Yang, Z.; Khoury, C.; Gaudio, S.; Greenwood, M.T. Peptide and non-peptide G-protein coupled receptors (GPCRs) in skeletal muscle. Peptides 2005, 26, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.L.; Goetsch, S.C.; Aguilar, H.R.; Coe, H.; Luo, X.; Liu, N.; van Rooij, E.; Frantz, D.E.; Schneider, J.W. Regulated expression of pH sensing G protein-coupled receptor-68 identified through chemical biology defines a new drug target for ischemic heart disease. ACS Chem. Biol. 2012, 7, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pluteanu, F.; Musset, B.; Rinne, A. Ca2+ Signaling in Striated Muscle Cells During Intracellular Acidosis. Biomolecules 2025, 15, 1244. https://doi.org/10.3390/biom15091244
Pluteanu F, Musset B, Rinne A. Ca2+ Signaling in Striated Muscle Cells During Intracellular Acidosis. Biomolecules. 2025; 15(9):1244. https://doi.org/10.3390/biom15091244
Chicago/Turabian StylePluteanu, Florentina, Boris Musset, and Andreas Rinne. 2025. "Ca2+ Signaling in Striated Muscle Cells During Intracellular Acidosis" Biomolecules 15, no. 9: 1244. https://doi.org/10.3390/biom15091244
APA StylePluteanu, F., Musset, B., & Rinne, A. (2025). Ca2+ Signaling in Striated Muscle Cells During Intracellular Acidosis. Biomolecules, 15(9), 1244. https://doi.org/10.3390/biom15091244





