Targeting Integrin α2 to Overcome Imatinib Resistance in Chronic Myeloid Leukemia Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability Assay
2.3. Analysis of ITGA2 Protein Expression
2.4. Flow Cytometry for Annexin V
2.5. Caspase 3/7 Activity
2.6. Real-Time Quantitative RT-qPCR
2.7. P-Glycoprotein Activity Measurement in Cell Lines
2.8. Bioinformatic Analysis
2.9. Statistical Analysis
3. Results
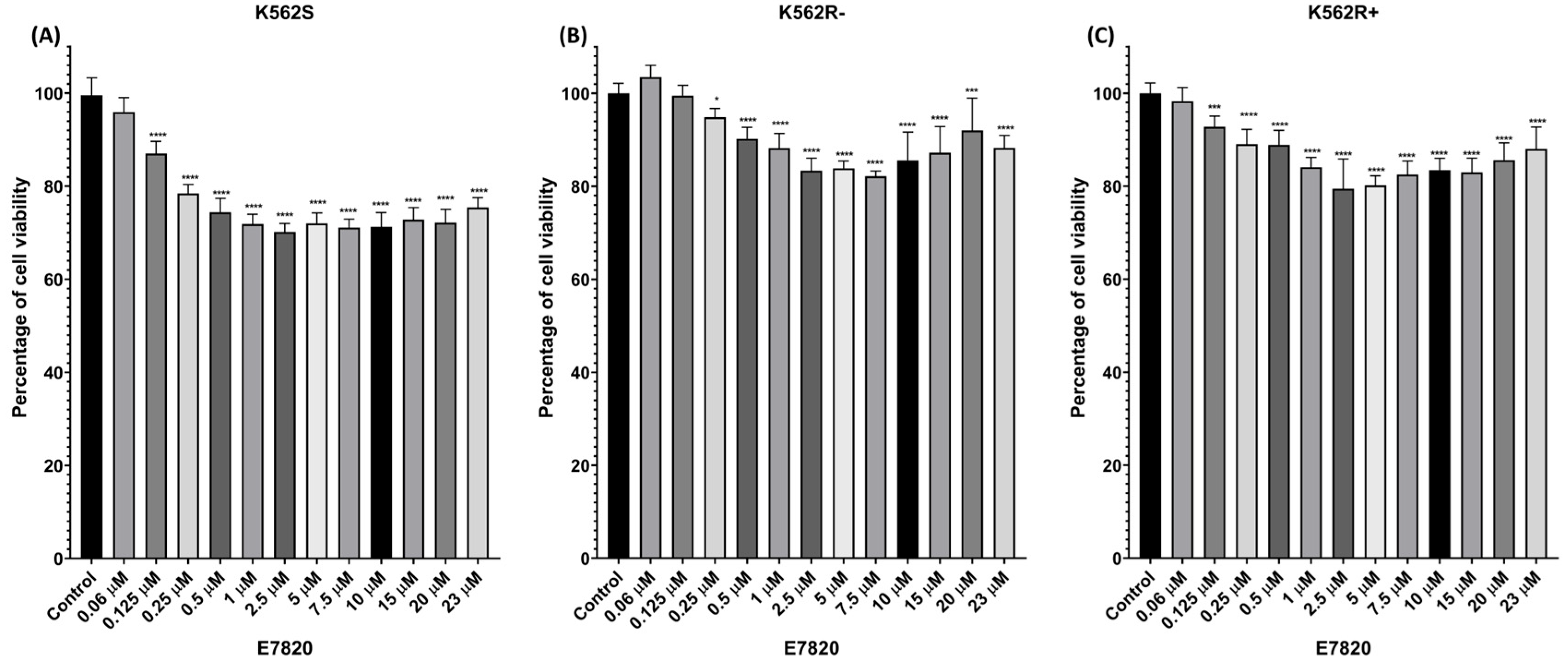
3.1. Cytotoxic Effects of E7820 on CML Cell Lines
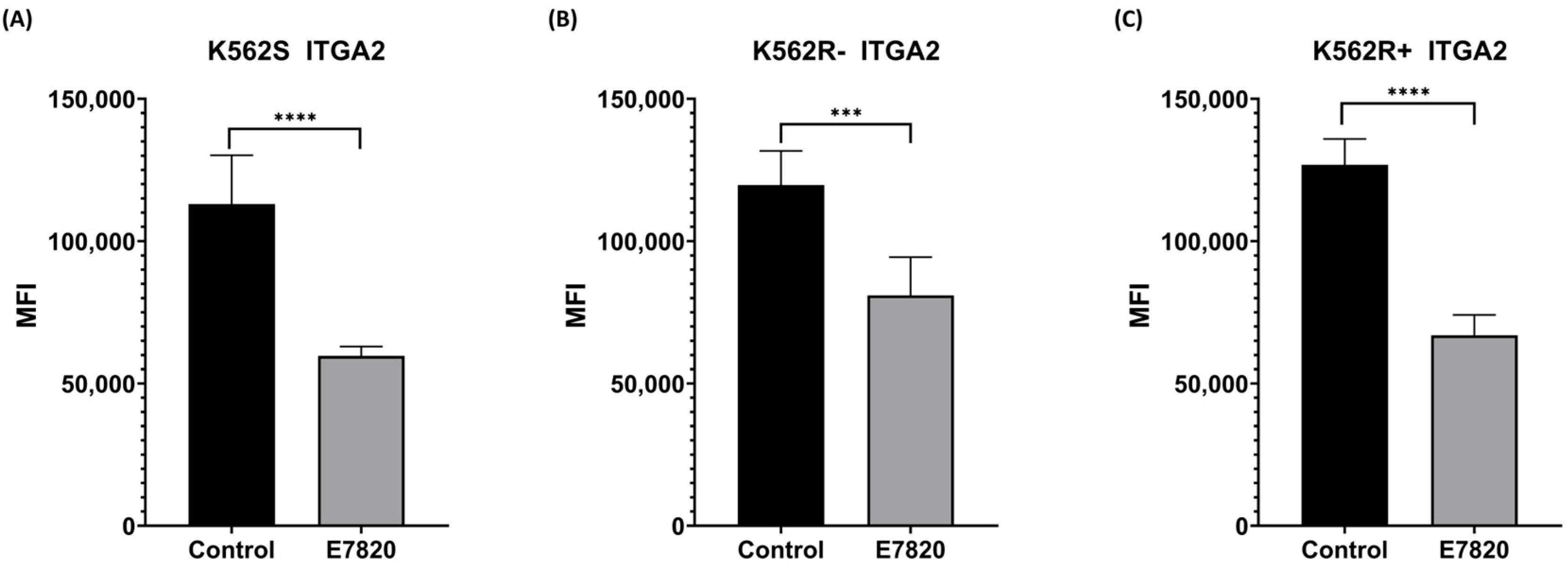
3.2. Effect of E7820 on ITRGA2 Protein Level in CML Cells
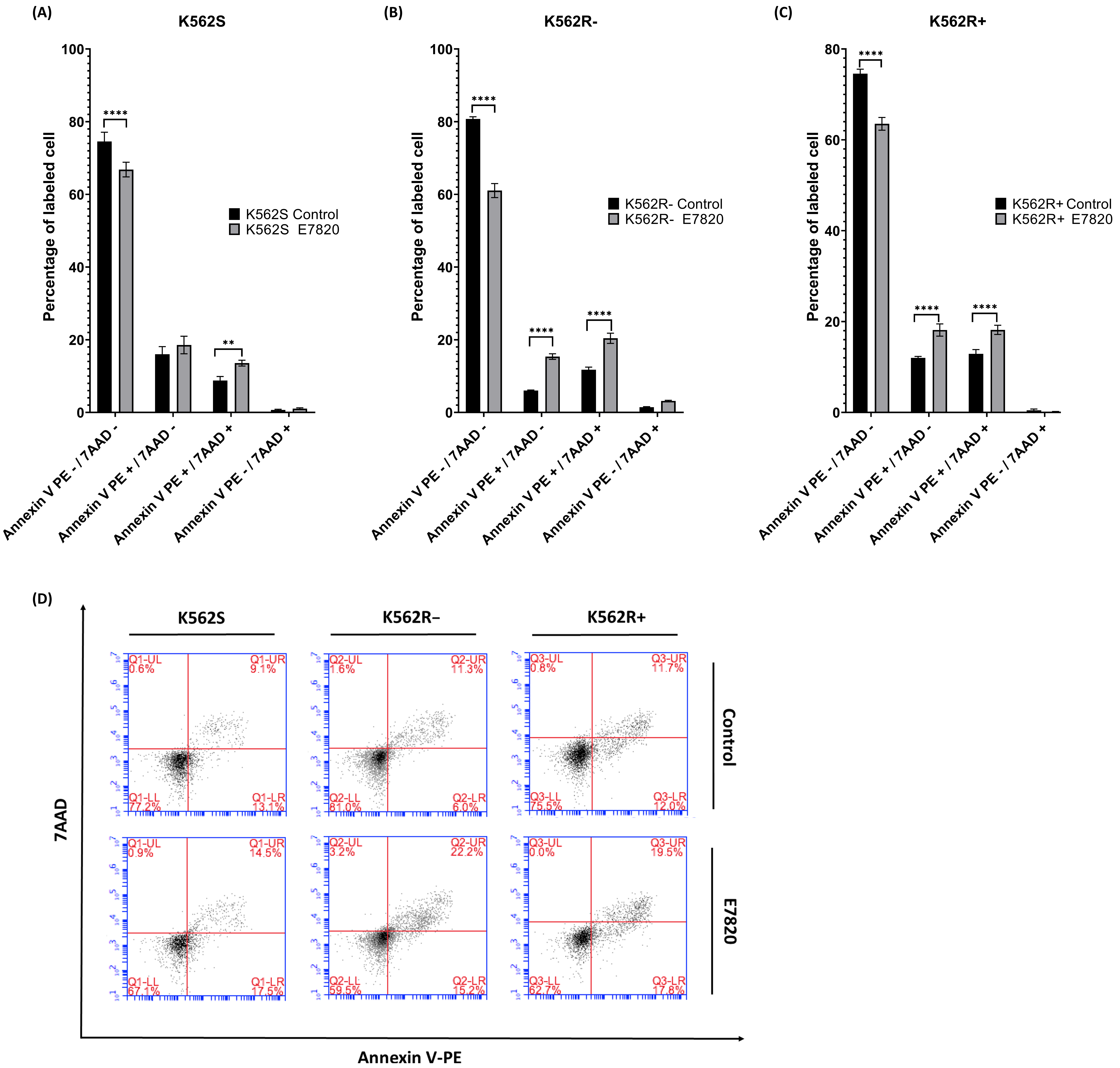
3.3. Effect of E7820 in Apoptosis Induction of CML Cells
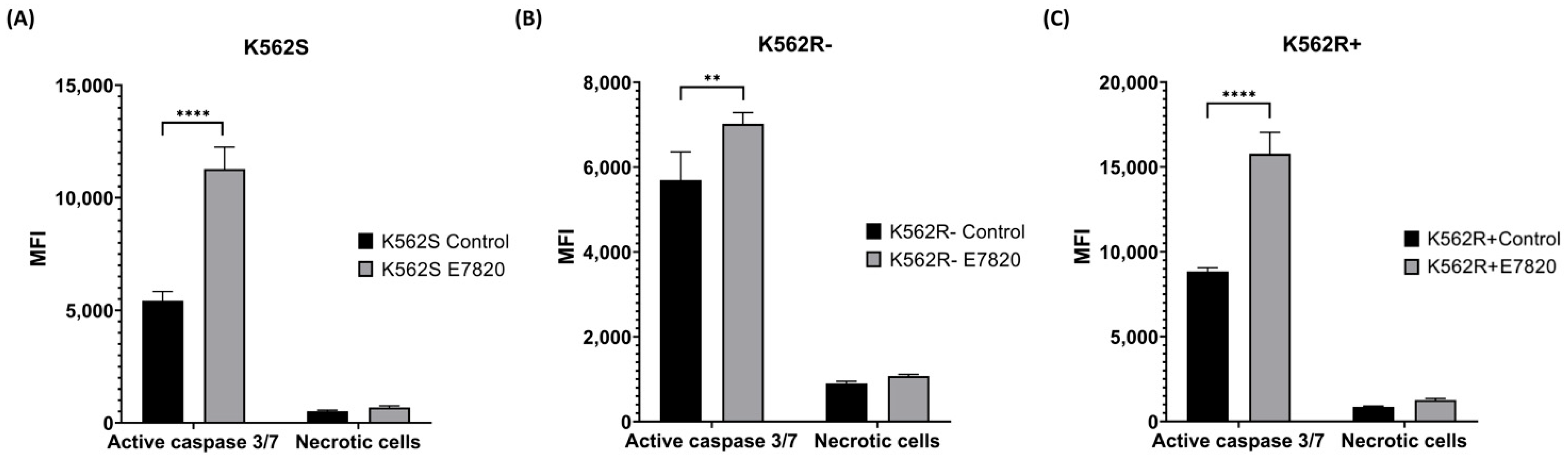
3.4. Effect of E7820 on Caspase 3/7 Activation in CML Cells
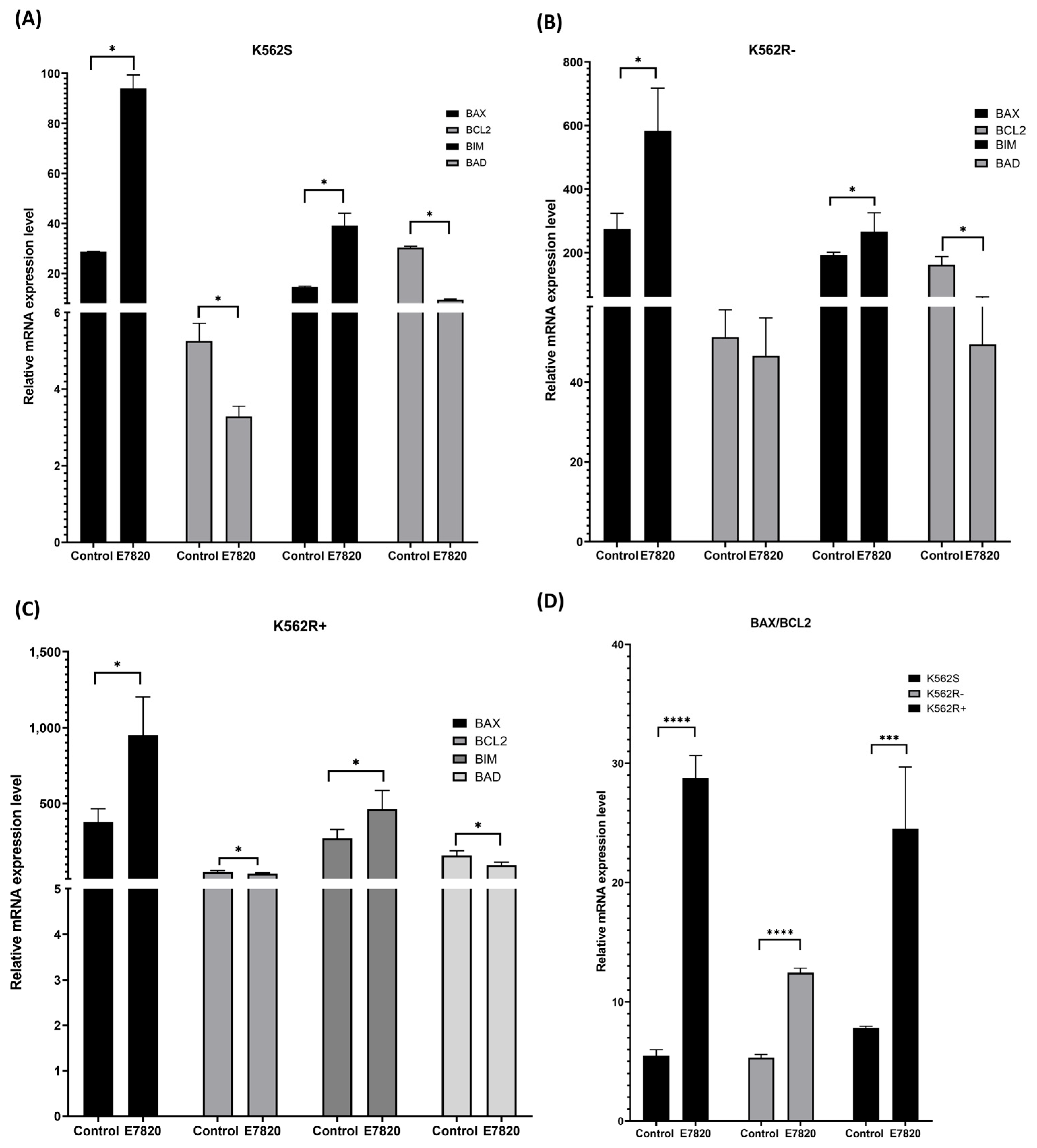
3.5. Effect of E7820 on Expression Profiling of Apoptotic Genes in CML Cells
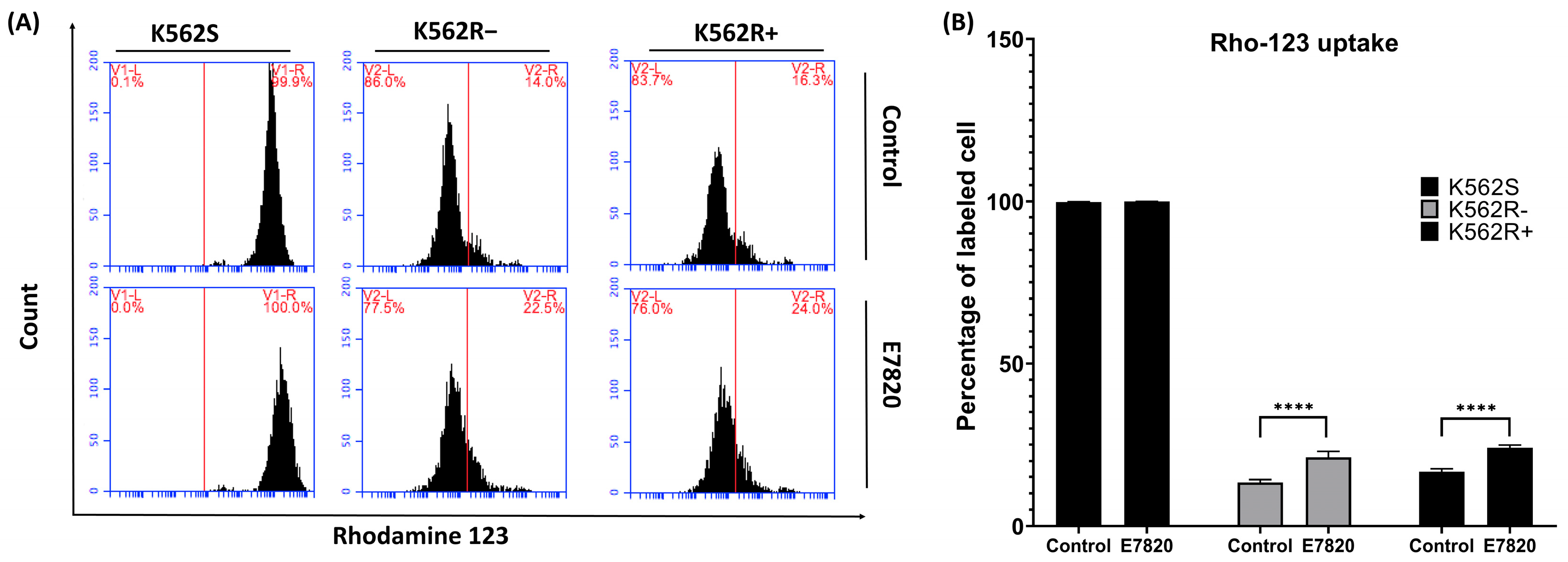
3.6. Effect of E7820 on Intracellular Accumulation of Rho-123 in CML Cells
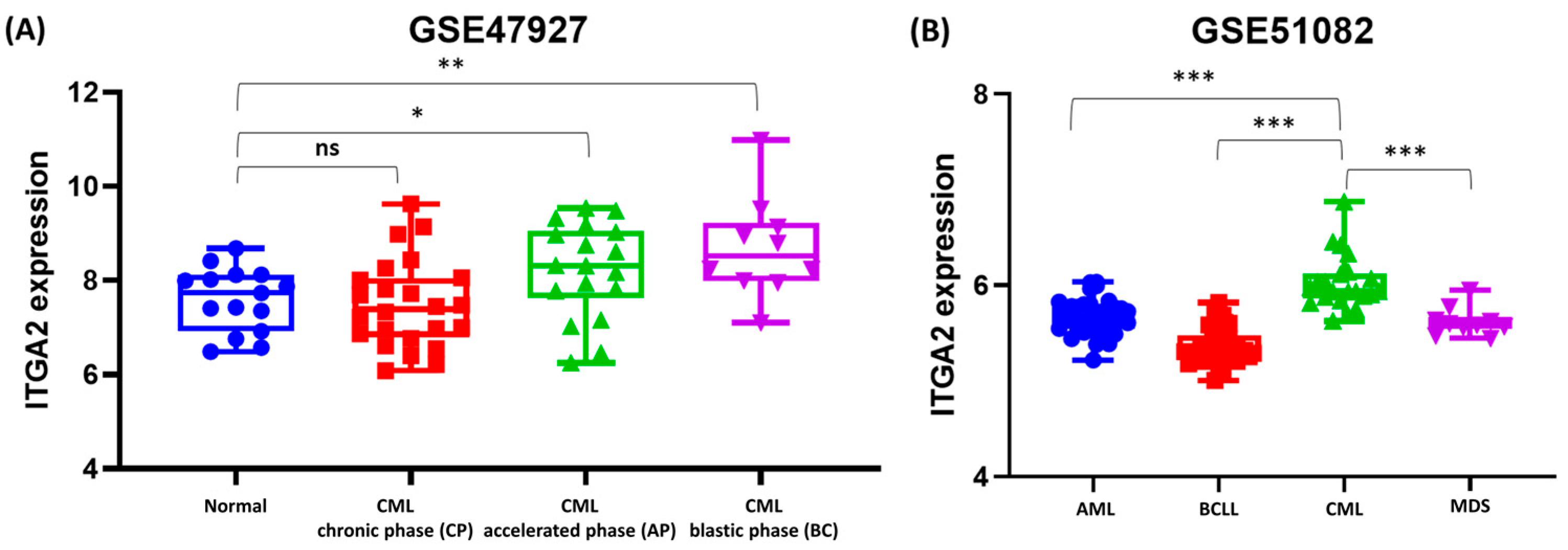
3.7. Differential ITGA2 Expression Across CML Stages and Leukemia Subtypes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CML | chronic myeloid leukemia |
| IMA | imatinib |
| ITGA2 | integrin A2 |
| K562S | IMA-sensitive K562 |
| K562R | IMA-resistant K562 |
| µM | micromolar |
| ECM | extracellular matrix |
| SNPs | single nucleotide polymorphisms |
| ALL | acute lymphoblastic leukemia |
| AML | acute myeloid leukemia |
| B-ALL | B-cell acute lymphoblastic leukemia |
| T-ALL | T-cell acute lymphoblastic leukemia |
| CLL | chronic lymphocytic leukemia |
| B-CLL | B-cell chronic lymphocytic leukemia |
| CMML | chronic myelomonocytic leukemia |
| MDS | myelodysplastic syndromes |
| P-gp | P-glycoprotein |
| FBS | fetal bovine serum |
| MFI | mean fluorescence intensity |
| BB | binding buffer |
| Rho-123 | Rhodamin 123 |
| CP | chronic phase |
| AP | accelerated phase |
| BC | blast crisis |
| GEO | Gene Expression Omnibus |
References
- Kaehler, M.; Litterst, M.; Kolarova, J.; Bohm, R.; Bruckmueller, H.; Ammerpohl, O.; Cascorbi, I.; Nagel, I. Genome-wide expression and methylation analyses reveal aberrant cell adhesion signaling in tyrosine kinase inhibitor-resistant CML cells. Oncol. Rep. 2022, 48, 144. [Google Scholar] [CrossRef]
- Amarante-Mendes, G.P.; Rana, A.; Datoguia, T.S.; Hamerschlak, N.; Brumatti, G. BCR-ABL1 Tyrosine Kinase Complex Signaling Transduction: Challenges to Overcome Resistance in Chronic Myeloid Leukemia. Pharmaceutics 2022, 14, 215. [Google Scholar] [CrossRef]
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2025 update on diagnosis, therapy, and monitoring. Am. J. Hematol. 2024, 99, 2191–2212. [Google Scholar] [CrossRef]
- Krenn, P.W.; Koschmieder, S.; Fassler, R. Kindlin-3 loss curbs chronic myeloid leukemia in mice by mobilizing leukemic stem cells from protective bone marrow niches. Proc. Natl. Acad. Sci. USA 2020, 117, 24326–24335. [Google Scholar] [CrossRef]
- Hekmatshoar, Y.; Ozkan, T.; Altinok Gunes, B.; Bozkurt, S.; Karadag, A.; Karabay, A.Z.; Sunguroglu, A. Characterization of imatinib-resistant K562 cell line displaying resistance mechanisms. Cell. Mol. Biol. 2018, 64, 23–30. [Google Scholar] [CrossRef]
- Hekmatshoar, Y.; Karadag Gurel, A.; Ozkan, T.; Rahbar Saadat, Y.; Koc, A.; Karabay, A.Z.; Bozkurt, S.; Sunguroglu, A. Phenotypic and functional characterization of subpopulation of Imatinib resistant chronic myeloid leukemia cell line. Adv. Med. Sci. 2023, 68, 238–248. [Google Scholar] [CrossRef]
- Shishido, S.; Bonig, H.; Kim, Y.M. Role of integrin alpha4 in drug resistance of leukemia. Front. Oncol. 2014, 4, 99. [Google Scholar] [CrossRef] [PubMed]
- Scharff, B.; Modvig, S.; Marquart, H.V.; Christensen, C. Integrin-Mediated Adhesion and Chemoresistance of Acute Lymphoblastic Leukemia Cells Residing in the Bone Marrow or the Central Nervous System. Front. Oncol. 2020, 10, 775. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.; Brennan, M.; Moran, N. Integrins as therapeutic targets: Lessons and opportunities. Nat. Rev. Drug Discov. 2010, 9, 804–820. [Google Scholar] [CrossRef] [PubMed]
- Adorno-Cruz, V.; Liu, H. Regulation and functions of integrin alpha2 in cell adhesion and disease. Genes. Dis. 2019, 6, 16–24. [Google Scholar] [CrossRef]
- Elices, M.J. The integrin alpha 4 beta 1 (VLA-4) as a therapeutic target. Ciba Found. Symp. 1995, 189, 79–85; discussion 85–90, 174–176. [Google Scholar]
- Ayala, F.; Dewar, R.; Kieran, M.; Kalluri, R. Contribution of bone microenvironment to leukemogenesis and leukemia progression. Leukemia 2009, 23, 2233–2241. [Google Scholar] [CrossRef]
- Di Paola, J.; Jugessur, A.; Goldman, T.; Reiland, J.; Tallman, D.; Sayago, C.; Murray, J.C. Platelet glycoprotein I(b)alpha and integrin alpha2 beta1 polymorphisms: Gene frequencies and linkage disequilibrium in a population diversity panel. J. Thromb. Haemost. 2005, 3, 1511–1521. [Google Scholar] [CrossRef]
- Zutter, M.M.; Santoro, S.A. Widespread histologic distribution of the alpha 2 beta 1 integrin cell-surface collagen receptor. Am. J. Pathol. 1990, 137, 113–120. [Google Scholar]
- Zutter, M.M.; Painter, A.A.; Staatz, W.D.; Tsung, Y.L. Regulation of alpha 2 integrin gene expression in cells with megakaryocytic features: A common theme of three necessary elements. Blood 1995, 86, 3006–3014. [Google Scholar] [CrossRef] [PubMed]
- Naci, D.; Aoudjit, F. Alpha2beta1 integrin promotes T cell survival and migration through the concomitant activation of ERK/Mcl-1 and p38 MAPK pathways. Cell. Signal. 2014, 26, 2008–2015. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.Y.; Zhang, W.; Wu, D.H.; Ma, J.C.; Zhou, J.D.; Zhang, Z.H.; Wen, X.M.; Xu, Z.J.; Lin, J.; Qian, J. Methylation-independent ITGA2 overexpression is associated with poor prognosis in de novo acute myeloid leukemia. J. Cell Physiol. 2018, 233, 9584–9593. [Google Scholar] [CrossRef]
- Yoshimura, K.; Meckel, K.F.; Laird, L.S.; Chia, C.Y.; Park, J.J.; Olino, K.L.; Tsunedomi, R.; Harada, T.; Iizuka, N.; Hazama, S.; et al. Integrin alpha2 mediates selective metastasis to the liver. Cancer Res. 2009, 69, 7320–7328. [Google Scholar] [CrossRef]
- Goodman, S.L.; Picard, M. Integrins as therapeutic targets. Trends Pharmacol. Sci. 2012, 33, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Oda, T.; Takizawa, M.; Ishizaki, T.; Tsukamoto, N.; Yokohama, A.; Takei, H.; Saitoh, T.; Shimizu, H.; Honma, K.; et al. Integrin alpha7 and Extracellular Matrix Laminin 211 Interaction Promotes Proliferation of Acute Myeloid Leukemia Cells and Is Associated with Granulocytic Sarcoma. Cancers 2020, 12, 363. [Google Scholar] [CrossRef]
- de la Fuente, M.T.; Casanova, B.; Cantero, E.; Hernandez del Cerro, M.; Garcia-Marco, J.; Silva, A.; Garcia-Pardo, A. Involvement of p53 in alpha4beta1 integrin-mediated resistance of B-CLL cells to fludarabine. Biochem. Biophys. Res. Commun. 2003, 311, 708–712. [Google Scholar] [CrossRef][Green Version]
- Hsieh, Y.T.; Gang, E.J.; Geng, H.; Park, E.; Huantes, S.; Chudziak, D.; Dauber, K.; Schaefer, P.; Scharman, C.; Shimada, H.; et al. Integrin alpha4 blockade sensitizes drug resistant pre-B acute lymphoblastic leukemia to chemotherapy. Blood 2013, 121, 1814–1818. [Google Scholar] [CrossRef]
- Dal Bo, M.; Bulian, P.; Bomben, R.; Zucchetto, A.; Rossi, F.M.; Pozzo, F.; Tissino, E.; Benedetti, D.; Bittolo, T.; Nanni, P.; et al. CD49d prevails over the novel recurrent mutations as independent prognosticator of overall survival in chronic lymphocytic leukemia. Leukemia 2016, 30, 2011–2018. [Google Scholar] [CrossRef]
- Matsunaga, T.; Takemoto, N.; Sato, T.; Takimoto, R.; Tanaka, I.; Fujimi, A.; Akiyama, T.; Kuroda, H.; Kawano, Y.; Kobune, M.; et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat. Med. 2003, 9, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Damiano, J.S.; Cress, A.E.; Hazlehurst, L.A.; Shtil, A.A.; Dalton, W.S. Cell adhesion mediated drug resistance (CAM-DR): Role of integrins and resistance to apoptosis in human myeloma cell lines. Blood 1999, 93, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Gu, Y.; Zhang, Y.; Li, Y. Integrin alpha2 in the microenvironment and the tumor compartment of digestive (gastrointestinal) cancers: Emerging regulators and therapeutic opportunities. Front. Oncol. 2024, 14, 1439709. [Google Scholar]
- Cai, H.; Guo, F.; Wen, S.; Jin, X.; Wu, H.; Ren, D. Overexpressed integrin alpha 2 inhibits the activation of the transforming growth factor beta pathway in pancreatic cancer via the TFCP2-SMAD2 axis. J. Exp. Clin. Cancer Res. 2022, 41, 73. [Google Scholar] [CrossRef]
- Kawamura, T.; Endo, Y.; Yonemura, Y.; Nojima, N.; Fujita, H.; Fujimura, T.; Obata, T.; Yamaguchi, T.; Sasaki, T. Significance of integrin alpha2/beta1 in peritoneal dissemination of a human gastric cancer xenograft model. Int. J. Oncol. 2001, 18, 809–815. [Google Scholar] [PubMed]
- Korovina, I.; Vehlow, A.; Temme, A.; Cordes, N. Targeting integrin alpha2 as potential strategy for radiochemosensitization of glioblastoma. Neuro Oncol. 2023, 25, 648–661. [Google Scholar] [CrossRef]
- Rattanasinchai, C.; Navasumrit, P.; Ruchirawat, M. Elevated ITGA2 expression promotes collagen type I-induced clonogenic growth of intrahepatic cholangiocarcinoma. Sci. Rep. 2022, 12, 22429. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, T.; Guo, K.; Zhou, Y.; Liu, H.; Pan, Y.; Hou, Q.; Nie, Y.; Fan, D.; Lu, Y.; et al. Regulation of Integrin Subunit Alpha 2 by miR-135b-5p Modulates Chemoresistance in Gastric Cancer. Front. Oncol. 2020, 10, 308. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, R.; Ren, G.; Li, X.; Wang, J.; Sun, Y.; Liang, J.; Nie, Y.; Wu, K.; Feng, B.; et al. HMGA2-FOXL2 Axis Regulates Metastases and Epithelial-to-Mesenchymal Transition of Chemoresistant Gastric Cancer. Clin. Cancer Res. 2017, 23, 3461–3473. [Google Scholar] [CrossRef] [PubMed]
- Guha, D.; Saha, T.; Bose, S.; Chakraborty, S.; Dhar, S.; Khan, P.; Adhikary, A.; Das, T.; Sa, G. Integrin-EGFR interaction regulates anoikis resistance in colon cancer cells. Apoptosis 2019, 24, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Naci, D.; El Azreq, M.A.; Chetoui, N.; Lauden, L.; Sigaux, F.; Charron, D.; Al-Daccak, R.; Aoudjit, F. alpha2beta1 integrin promotes chemoresistance against doxorubicin in cancer cells through extracellular signal-regulated kinase (ERK). J Biol Chem 2012, 287, 17065–17076. [Google Scholar] [CrossRef]
- Naci, D.; Berrazouane, S.; Barabe, F.; Aoudjit, F. Cell adhesion to collagen promotes leukemia resistance to doxorubicin by reducing DNA damage through the inhibition of Rac1 activation. Sci. Rep. 2019, 9, 19455. [Google Scholar] [CrossRef]
- Ito, K.; Semba, T.; Uenaka, T.; Wakabayashi, T.; Asada, M.; Funahashi, Y. Enhanced anti-angiogenic effect of E7820 in combination with erlotinib in epidermal growth factor receptor-tyrosine kinase inhibitor-resistant non-small-cell lung cancer xenograft models. Cancer Sci. 2014, 105, 1023–1031. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Kage, H.; Morota, M.; Zokumasu, K.; Ando, T.; Maemura, K.; Watanabe, K.; Kawakami, M.; Hinata, M.; Ushiku, T.; et al. Integrin alpha 2 is associated with tumor progression and postoperative recurrence in non-small cell lung cancer. Jpn. J. Clin. Oncol. 2023, 53, 63–73. [Google Scholar] [CrossRef]
- Kawami, M.; Ojima, T.; Yumoto, R.; Takano, M. Role of integrin alpha2 in methotrexate-induced epithelial-mesenchymal transition in alveolar epithelial A549 cells. Toxicol. Res. 2022, 38, 449–458. [Google Scholar] [CrossRef]
- Uehara, T.; Minoshima, Y.; Sagane, K.; Sugi, N.H.; Mitsuhashi, K.O.; Yamamoto, N.; Kamiyama, H.; Takahashi, K.; Kotake, Y.; Uesugi, M.; et al. Selective degradation of splicing factor CAPERalpha by anticancer sulfonamides. Nat. Chem. Biol. 2017, 13, 675–680. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Gao, Z.F.; Dong, G.H.; Li, X.; Wu, Y.; Li, G.; Wang, A.L.; Li, H.L.; Yin, D.L. Suppression of αvβ6 downregulates P-glycoprotein and sensitizes multidrug-resistant breast cancer cells to anticancer drugs. Neoplasma 2020, 67, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Liu, X.; Li, T.; Zhang, Y.; Zhao, X.; Sun, W.; Li, Z. Silencing PLOD2 attenuates cancer stem cell-like characteristics and cisplatin-resistant through Integrin β1 in laryngeal cancer. Transl. Oncol. 2022, 22, 101460. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, I.S.; Huang, W.H.; Liou, H.C.; Chuang, W.J.; Yang, R.S.; Fu, W.M. Upregulation of drug transporter expression by osteopontin in prostate cancer cells. Mol. Pharmacol. 2013, 83, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Bewersdorf, J.P.; Stahl, M.; Taylor, J.; Mi, X.; Chandhok, N.S.; Watts, J.; Derkach, A.; Wysocki, M.; Lu, S.X.; Bourcier, J.; et al. E7820, an anti-cancer sulfonamide, degrades RBM39 in patients with splicing factor mutant myeloid malignancies: A phase II clinical trial. Leukemia 2023, 37, 2512–2516. [Google Scholar] [CrossRef]
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| BAX | GACGCAACTTCAACTGGG | AGGAGTCTCACCCAA CAC |
| BIM | ATCTCACAATGGCTTCC | CATAGTAAGCGTTAAACTCGTCTCC |
| BAD | GATGAGTGACGAGTTTGTGGA | CAAGTTCCGATCCCACCAG |
| BCL2 | CGCCCTGTGGATGACTGAGT | GGGCCGTACAGTTCCACAA |
| HPRT | TGACACTGCAAAACAATGCA | GGTCCTTTTCACCAGCAAGCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hekmatshoar, Y.; Ozkan, T.; Karabay, A.Z.; Bozkurt, S.; Gurel, A.K.; Gomleksiz, O.K.; Fisgin, T.; Sunguroglu, A. Targeting Integrin α2 to Overcome Imatinib Resistance in Chronic Myeloid Leukemia Cells. Biomolecules 2025, 15, 1245. https://doi.org/10.3390/biom15091245
Hekmatshoar Y, Ozkan T, Karabay AZ, Bozkurt S, Gurel AK, Gomleksiz OK, Fisgin T, Sunguroglu A. Targeting Integrin α2 to Overcome Imatinib Resistance in Chronic Myeloid Leukemia Cells. Biomolecules. 2025; 15(9):1245. https://doi.org/10.3390/biom15091245
Chicago/Turabian StyleHekmatshoar, Yalda, Tulin Ozkan, Arzu Zeynep Karabay, Sureyya Bozkurt, Aynur Karadag Gurel, Ozlem Kurnaz Gomleksiz, Tunc Fisgin, and Asuman Sunguroglu. 2025. "Targeting Integrin α2 to Overcome Imatinib Resistance in Chronic Myeloid Leukemia Cells" Biomolecules 15, no. 9: 1245. https://doi.org/10.3390/biom15091245
APA StyleHekmatshoar, Y., Ozkan, T., Karabay, A. Z., Bozkurt, S., Gurel, A. K., Gomleksiz, O. K., Fisgin, T., & Sunguroglu, A. (2025). Targeting Integrin α2 to Overcome Imatinib Resistance in Chronic Myeloid Leukemia Cells. Biomolecules, 15(9), 1245. https://doi.org/10.3390/biom15091245






