Regulatory Ouabain Action on Excitatory Transmission in Rat Hippocampus: Facilitation of Synaptic Responses and Weakening of LTP
Abstract
1. Introduction
2. Materials and Methods
2.1. Hippocampal Slice Preparation
2.2. Recording of Evoked Postsynaptic Responses
2.3. LTP Induction
2.4. Data Analysis
2.5. Drugs
3. Results
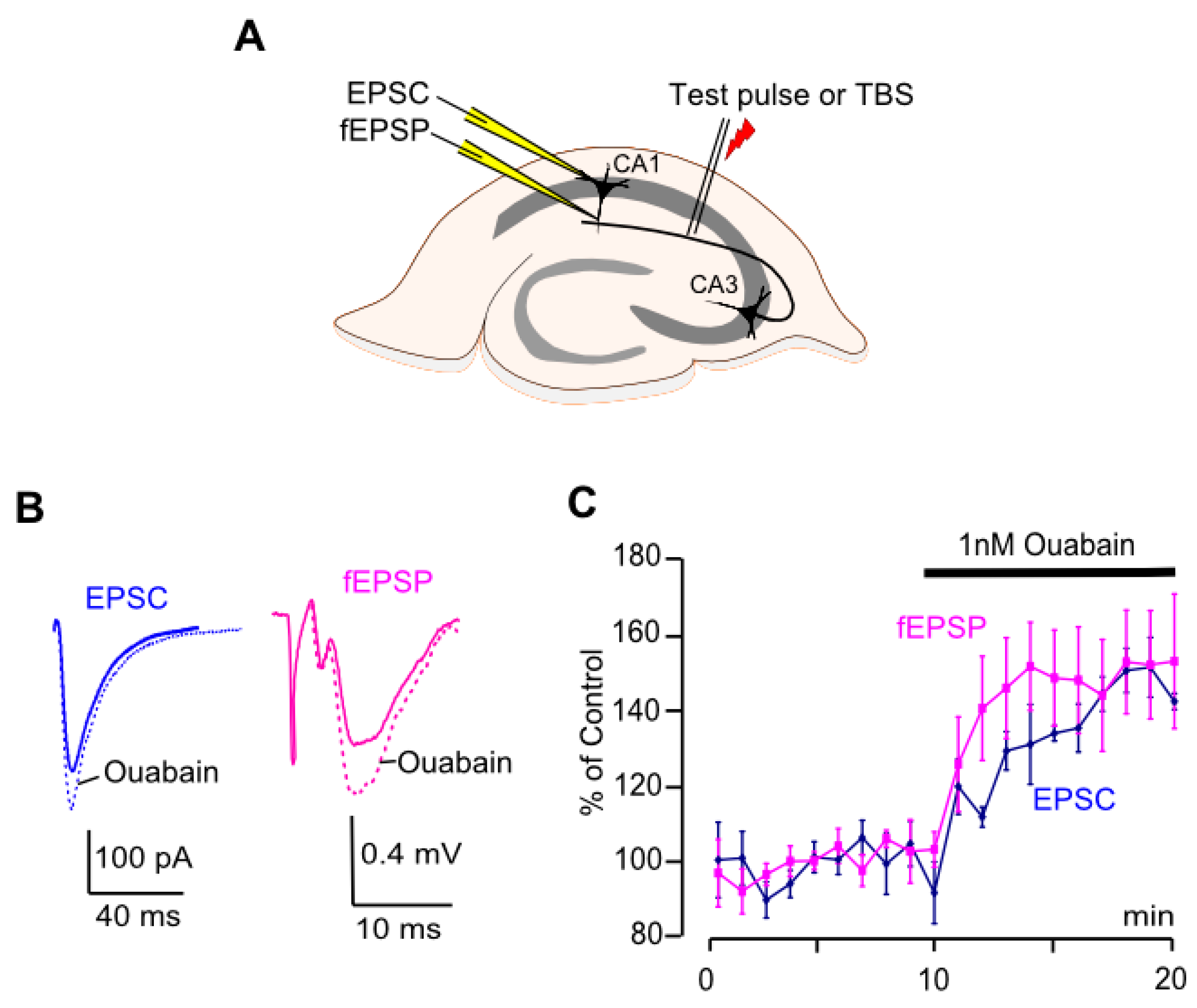
3.1. Ouabain Increases EPSC Amplitude and fEPSP Slope in CA1
3.2. Ouabain-Induced Rise of fEPSP Slope Is NMDAR-Independent
3.3. Ouabain Reduces LTP
3.4. Ouabain Increases fEPSP Slope After LTP Induction
4. Discussion
4.1. Effects of Ouabain on Evoked Synaptic Responses
4.2. LTP Inhibition by Ouabain
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACSF | Artificial cerebrospinal fluid |
| AMPAR | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
| AP5 | (2R)-amino-5-phosphonovaleric acid |
| CaMKII | Calcium/calmodulin-dependent protein kinase II |
| CTS | Cardiotonic steroids |
| EPSC | Excitatory postsynaptic current |
| fEPSP | Field excitatory postsynaptic potential |
| LTP | Long-term potentiation |
| NMDAR | N-methyl-D-aspartate receptor |
| NKA | Na+/K+-ATPase |
| PKA | Proteinkinase A |
| PKC | Proteinkinase C |
| SEM | Standard error of mean |
| TBS | Theta-burst stimulation |
References
- O’Brien, W.J.; Lingrel, J.B.; Wallick, E.T. Ouabain binding kinetics of the rat alpha two and alpha three isoforms of the sodium-potassium adenosine triphosphate. Arch. Biochem. Biophys. 1994, 310, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, M.P.; Hamlyn, J.M. Sensational site: The sodium pump ouabain-binding site and its ligands. Am. J. Physiol. Cell Physiol. 2024, 326, C1120–C1177. [Google Scholar] [CrossRef] [PubMed]
- Veldhuis, W.B.; van der Stelt, M.; Delmas, F.; Gillet, B.; Veldink, G.A.; Vliegenthart, J.F.; Nicolay, K.; Bär, P.R. In vivo excitotoxicity induced by ouabain, a Na+/K+-ATPase inhibitor. J. Cereb. Blood Flow Metab. 2003, 23, 62–74. [Google Scholar] [CrossRef]
- Sibarov, D.A.; Bolshakov, A.E.; Abushik, P.A.; Krivoi, I.I.; Antonov, S.M. Na+,K+-ATPase functionally interacts with the plasma membrane Na+,Ca2+ exchanger to prevent Ca2+ overload and neuronal apoptosis in excitotoxic stress. J. Pharmacol. Exp. Ther. 2012, 343, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O.V.; Bagrov, A.Y. Inhibition of Na/K ATPase from rat aorta by two Na/K pump inhibitors, ouabain and marinobufagenin: Evidence of interaction with different alpha-subunit isoforms. Am. J. Hypertens. 1997, 10, 929–935. [Google Scholar] [CrossRef]
- Dobretsov, M.; Stimers, J.R. Neuronal function and alpha3 isoform of the Na/K-ATPase. Front. Biosci. 2005, 2005 10, 2373–2396. [Google Scholar] [CrossRef]
- Schoner, W.; Scheiner-Bobis, G. Endogenous and exogenous cardiac glycosides: Their roles in hypertension, salt metabolism, and cell growth. Am. J. Physiol. Cell Physiol. 2007, 293, C509–C536. [Google Scholar] [CrossRef]
- Kravtsova, V.V.; Bouzinova, E.V.; Matchkov, V.V.; Krivoi, I.I. Skeletal muscle Na,K-ATPase as a target for circulating ouabain. Int. J. Mol. Sci. 2020, 21, 2875. [Google Scholar] [CrossRef]
- Ivanova, M.A.; Kokorina, A.D.; Timofeeva, P.D.; Karelina, T.V.; Abushik, P.A.; Stepanenko, J.D.; Sibarov, D.A.; Antonov, S.M. Calcium export from neurons and multi-kinase signaling cascades contribute to ouabain neuroprotection in hyperhomocysteinemia. Biomolecules 2020, 10, 1104. [Google Scholar] [CrossRef]
- Sibarov, D.A.; Zhuravleva, Z.D.; Ilina, M.A.; Boikov, S.I.; Stepanenko, Y.D.; Karelina, T.V.; Antonov, S.M. Unveiling the role of cholesterol in subnanomolar ouabain rescue of cortical neurons from calcium overload caused by excitotoxic insults. Cells 2023, 12, 2011. [Google Scholar] [CrossRef]
- Akkuratov, E.E.; Westin, L.; Vazquez-Juarez, E.; de Marothy, M.; Melnikova, A.K.; Blom, H.; Lindskog, M.; Brismar, H.; Aperia, A. Ouabain modulates the functional interaction between Na,K-ATPase and NMDA receptor. Mol. Neurobiol. 2020, 57, 4018–4030. [Google Scholar] [CrossRef]
- Malenka, R.C. The role of postsynaptic calcium in the induction of long-term potentiation. Mol. Neurobiol. 1991, 5, 289–295. [Google Scholar] [CrossRef]
- Narayanan, R.; Johnston, D. Long-term potentiation in rat hippocampal neurons is accompanied by spatially widespread changes in intrinsic oscillatory dynamics and excitability. Neuron 2007, 56, 1061–1075. [Google Scholar] [CrossRef]
- Kinoshita, P.F.; Orellana, A.M.; Andreotti, D.Z.; de Souza, G.A.; de Mello, N.P.; de Sá Lima, L.; Kawamoto, E.M.; Scavone, C. Consequences of the lack of TNFR1 in ouabain response in the hippocampus of C57BL/6J mice. Biomedicines 2022, 10, 2937. [Google Scholar] [CrossRef]
- Zhang, D.; Hou, Q.; Wang, M.; Lin, A.; Jarzylo, L.; Navis, A.; Raissi, A.; Liu, F.; Man, H.Y. Na,K-ATPase activity regulates AMPA receptor turnover through proteasome-mediated proteolysis. J. Neurosci. 2009, 29, 4498–4511. [Google Scholar] [CrossRef]
- Nosyreva, E.; Szabla, K.; Autry, A.E.; Ryazanov, A.G.; Monteggia, L.M.; Kavalali, E.T. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J. Neurosci. 2013, 33, 6990–7002. [Google Scholar] [CrossRef]
- Kang-Park, M.H.; Sarda, M.A.; Jones, K.H.; Moore, S.D.; Shenolikar, S.; Clark, S.; Wilson, W.A. Protein phosphatases mediate depotentiation induced by high-intensity theta-burst stimulation. J. Neurophysiol. 2003, 89, 684–690. [Google Scholar] [CrossRef]
- Volianskis, A.; France, G.; Jensen, M.S.; Bortolotto, Z.A.; Jane, D.E.; Collingridge, G.L. Long-term potentiation and the role of N-methyl-D-aspartate receptors. Brain Res. 2015, 1621, 5–16. [Google Scholar] [CrossRef]
- Rodrigues, N.C.; Silva-Cruz, A.; Caulino-Rocha, A.; Bento-Oliveira, A.; Ribeiro, J.A.; Cunha-Reis, D. Hippocampal CA1 theta burst-induced LTP from weaning to adulthood: Cellular and molecular mechanisms in young male rats revisited. Eur. J. Neurosci. 2021, 54, 5272–5292. [Google Scholar] [CrossRef]
- Mayer, M.L.; Westbrook, G.L.; Guthrie, P.B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 1984, 309, 261–263. [Google Scholar] [CrossRef]
- Nowak, L.; Bregestovski, P.; Ascher, P.; Herbet, A.; Prochiantz, A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 1984, 307, 462–465. [Google Scholar] [CrossRef]
- Kang, M.G.; Chen, C.C.; Wakamori, M.; Hara, Y.; Mori, Y.; Campbell, K.P. A functional AMPA receptor-calcium channel complex in the postsynaptic membrane. Proc. Natl. Acad. Sci. USA 2006, 103, 5561–5566. [Google Scholar] [CrossRef]
- Bowie, D.; Mayer, M.L. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 1995, 15, 453–462. [Google Scholar] [CrossRef]
- Anggono, V.; Huganir, R.L. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr. Opin. Neurobiol. 2012, 22, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Plant, K.; Pelkey, K.A.; Bortolotto, Z.A.; Morita, D.; Terashima, A.; McBain, C.J.; Collingridge, G.L.; Isaac, J.T. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat. Neurosci. 2006, 9, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Bayazitov, I.T.; Richardson, R.J.; Fricke, R.G.; Zakharenko, S.S. Slow presynaptic and fast postsynaptic components of compound long-term potentiation. J. Neurosci. 2007, 27, 11510–11521. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Thompson, S.M.; Blaustein, M.P. Nanomolar ouabain augments Ca2+ signalling in rat hippocampal neurones and glia. J. Physiol. 2013, 591, 1671–1689. [Google Scholar] [CrossRef]
- Grigoryev, P.N.; Zefirov, A.L. The same synaptic vesicles originate synchronous and asynchronous transmitter release. Acta Naturae 2015, 7, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Rozov, A.; Bolshakov, A.P.; Valiullina-Rakhmatullina, F. The ever-growing puzzle of asynchronous release. Front. Cell. Neurosci. 2019, 13, 28. [Google Scholar] [CrossRef]
- Sibarov, D.A.; Abushik, P.A.; Poguzhelskaya, E.E.; Bolshakov, K.V.; Antonov, S.M. Inhibition of plasma membrane Na/Ca-exchanger by KB-R7943 or lithium reveals its role in Ca-dependent N-methyl-D-aspartate receptor inactivation. J. Pharmacol. Exp. Ther. 2015, 355, 484–495. [Google Scholar] [CrossRef]
- Sibarov, D.A.; Poguzhelskaya, E.E.; Antonov, S.M. Downregulation of calcium-dependent NMDA receptor desensitization by sodium-calcium exchangers: A role of membrane cholesterol. BMC Neurosci. 2018, 19, 73. [Google Scholar] [CrossRef] [PubMed]
- Glushchenko, T.S.; Izvarina, N.L. Na+, K+-ATPase activity in neurons and glial cells of the olfactory cortex of the rat brain during the development of long-term potentiation. Neurosci. Behav. Physiol. 1997, 27, 49–52. [Google Scholar] [CrossRef]
- Kim, Y.; Hsu, C.L.; Cembrowski, M.S.; Mensh, B.D.; Spruston, N. Dendritic sodium spikes are required for long-term potentiation at distal synapses on hippocampal pyramidal neurons. eLife 2015, 4, e06414. [Google Scholar] [CrossRef]
- Megwa, O.F.; Pascual, L.M.; Günay, C.; Pulver, S.R.; Prinz, A.A. Temporal dynamics of Na/K pump mediated memory traces: Insights from conductance-based models of Drosophila neurons. Front. Neurosci. 2023, 17, 1154549. [Google Scholar] [CrossRef]
- Clausen, T. Acute stimulation of Na/K pump by cardiac glycosides in the nanomolar range. J. Gen. Physiol. 2002, 119, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wymore, R.S.; Wang, Y.; Gaudette, G.R.; Krukenkamp, I.B.; Cohen, I.S.; Mathias, R.T. Isoform-specific stimulation of cardiac Na/K pumps by nanomolar concentrations of glycosides. J. Gen. Physiol. 2002, 119, 297–312. [Google Scholar] [CrossRef]
- Lopachev, A.V.; Abaimov, D.A.; Fedorova, T.N.; Lopacheva, O.M.; Akkuratova, N.V.; Akkuratov, E.E. Cardiotonic steroids as potential endogenous regulators in the nervous system. Neurochem. J. 2018, 12, 1–8. [Google Scholar] [CrossRef]
- Yoshika, M.; Komiyama, Y.; Takahashi, H. An ouabain-like factor is secreted from immortalized hypothalamic cells in an aldosterone-dependent manner. Neurochem. Int. 2011, 59, 104–108. [Google Scholar] [CrossRef]
- Kniffin, A.R.; Briand, L.A. Sex differences in glutamate transmission and plasticity in reward related regions. Front. Behav. Neurosci. 2024, 18, 1455478. [Google Scholar] [CrossRef]
- Narattil, N.R.; Maroun, M. Differential role of NMDA receptors in hippocampal-dependent spatial memory and plasticity in juvenile male and female rats. Hippocampus 2024, 34, 564–574. [Google Scholar] [CrossRef]
- Jacobs, B.E.; Liu, Y.; Pulina, M.V.; Golovina, V.A.; Hamlyn, J.M. Normal pregnancy: Mechanisms underlying the paradox of a ouabain-resistant state with elevated endogenous ouabain, suppressed arterial sodium calcium exchange, and low blood pressure. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1317–H1329. [Google Scholar] [CrossRef]
- Vlkovicová, J.; Javorková, V.; Pechánová, O.; Vrbjar, N. Gender difference in functional properties of Na,K-ATPase in the heart of spontaneously hypertensive rats. Life Sci. 2005, 76, 971–982. [Google Scholar] [CrossRef]
- Manunta, P.; Rogowski, A.C.; Hamilton, B.P.; Hamlyn, J.M. Ouabain-induced hypertension in the rat: Relationships among plasma and tissue ouabain and blood pressure. J. Hypertens. 1994, 12, 549–560. [Google Scholar] [CrossRef]




| Section in Results | Slices (n) | Animals | Sex | Normality | |
|---|---|---|---|---|---|
| fEPSP | 3.1 | 7 | 5 | 3 ♂ + 2 ♀ | yes |
| EPSC | 3.1 | 5 | 4 | 1 ♂ + 3 ♀ | no |
| fEPSP, AP5 | 3.2 | 6 | 4 | 3 ♂ + 1 ♀ | yes |
| fEPSP, Control | 3.3 | 7 | 3 | 1 ♂ + 2 ♀ | yes |
| fEPSP, with AP5 | 3.3 | 8 | 4 | 2 ♂ + 2 ♀ | no |
| fEPSC, Ouabain pre-treat | 3.3 | 5 | 3 | 3 ♂ | yes |
| fEPSP, Ouabain pre-treat | 3.3 | 7 | 3 | 2 ♂ + 1 ♀ | yes |
| fEPSP, Control | 3.4 | 5 | 3 | 2 ♂ + 1 ♀ | yes |
| fEPSP, Ouabain post-treat | 3.4 | 7 | 3 | 1 ♂ + 2 ♀ | no |
| Total | 57 | 32 | 20 ♂ + 12 ♀ |
| Intact (fEPSP) | Intact (EPSC) | +AP5 (fEPSP) | After LTP Onset (fEPSP) | |
|---|---|---|---|---|
| Control | 99 ± 0.2 (n = 7) | 98 ± 0.4 (n = 5) | 102 ± 2 (n = 6) | 95 ± 11 (n = 5) |
| Ouabain | 150 ± 13 (n = 7) ##, p < 0.01 | 135 ± 0. 9 (n = 5) ****, p < 0.0001 | 134 ± 6 (n = 6) **, p < 0.002 | 133 ± 11 (n = 7) *, p < 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanenko, Y.D.; Sibarov, D.A.; Antonov, S.M. Regulatory Ouabain Action on Excitatory Transmission in Rat Hippocampus: Facilitation of Synaptic Responses and Weakening of LTP. Biomolecules 2025, 15, 1236. https://doi.org/10.3390/biom15091236
Stepanenko YD, Sibarov DA, Antonov SM. Regulatory Ouabain Action on Excitatory Transmission in Rat Hippocampus: Facilitation of Synaptic Responses and Weakening of LTP. Biomolecules. 2025; 15(9):1236. https://doi.org/10.3390/biom15091236
Chicago/Turabian StyleStepanenko, Yulia D., Dmitry A. Sibarov, and Sergei M. Antonov. 2025. "Regulatory Ouabain Action on Excitatory Transmission in Rat Hippocampus: Facilitation of Synaptic Responses and Weakening of LTP" Biomolecules 15, no. 9: 1236. https://doi.org/10.3390/biom15091236
APA StyleStepanenko, Y. D., Sibarov, D. A., & Antonov, S. M. (2025). Regulatory Ouabain Action on Excitatory Transmission in Rat Hippocampus: Facilitation of Synaptic Responses and Weakening of LTP. Biomolecules, 15(9), 1236. https://doi.org/10.3390/biom15091236








