TMCO1 as an Endoplasmic Reticulum Calcium Load-Activated Channel: Mechanisms and Disease Implications
Abstract
1. Introduction
2. The Protein Structure Characteristics of TMCO1
2.1. Two Different Subtypes of TMCO1
2.2. TMCO1 Belongs to the YidC/Alb3/Oxa1 Family
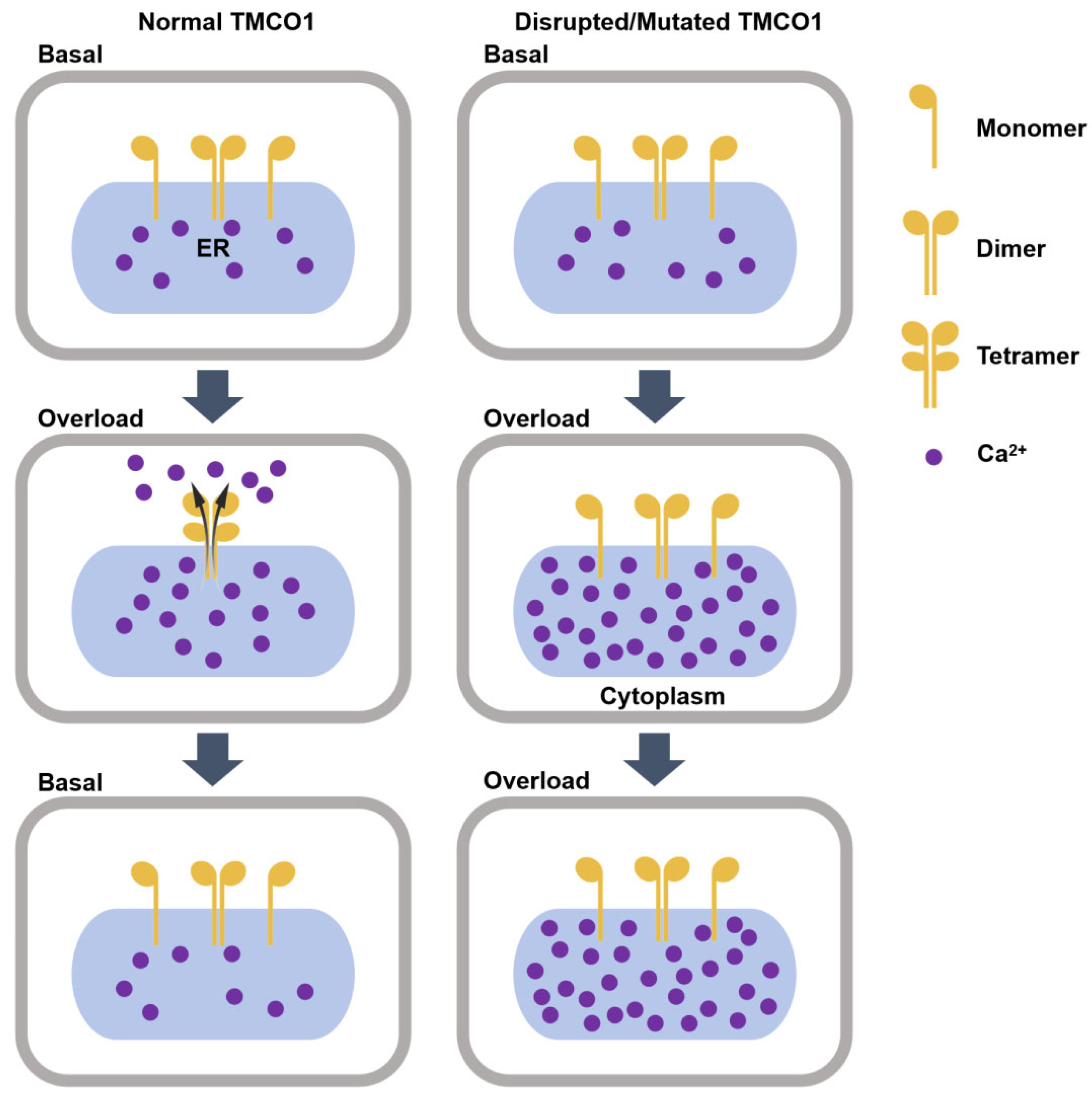
3. TMCO1 Is a Ca2+ Channel Activated by Ca2+ Overloading in the ER
3.1. TMCO1-Mediated ER Ca2+ Efflux
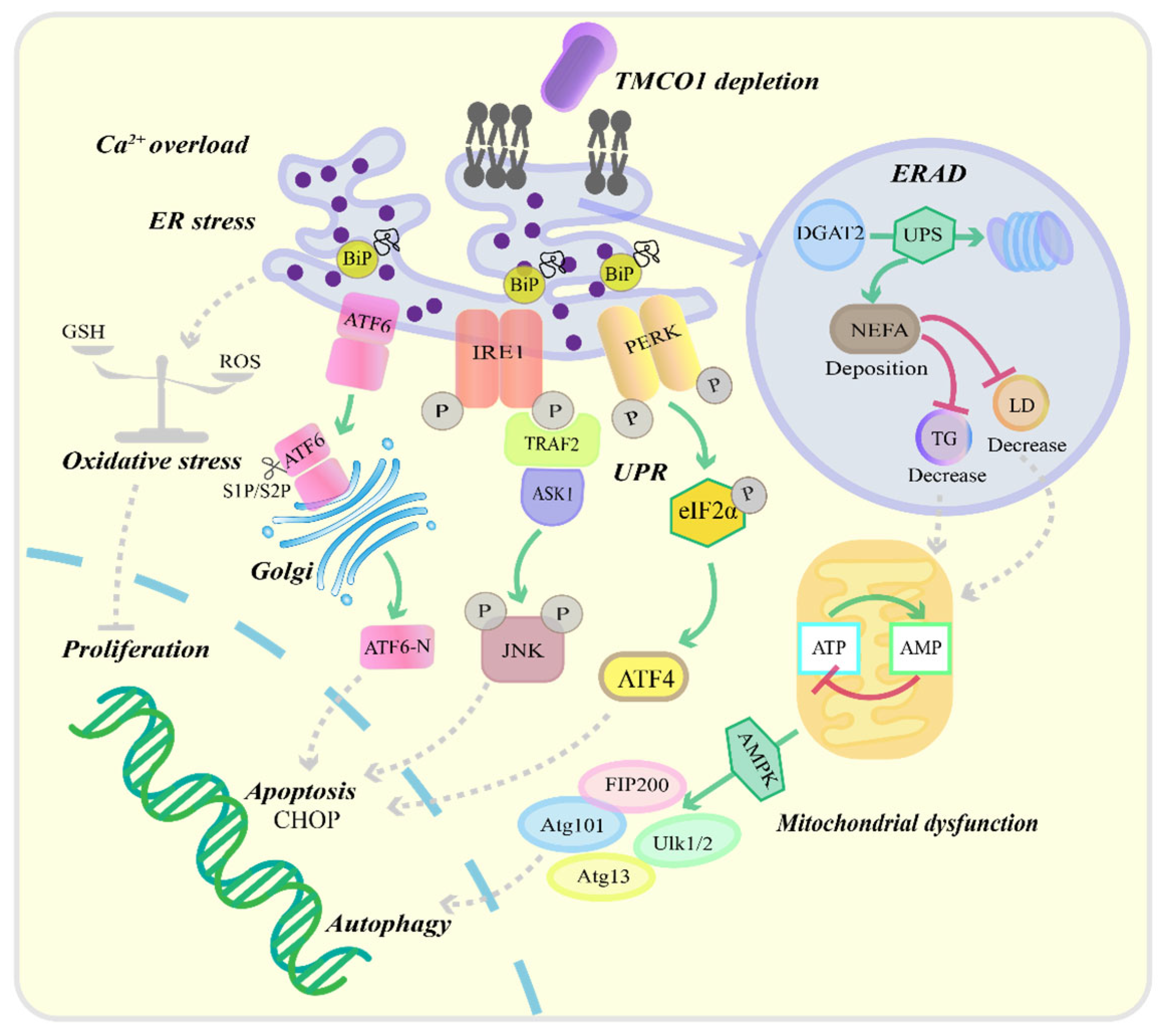
3.2. Effects of TMCO1 Deficiency on Cellular Functions
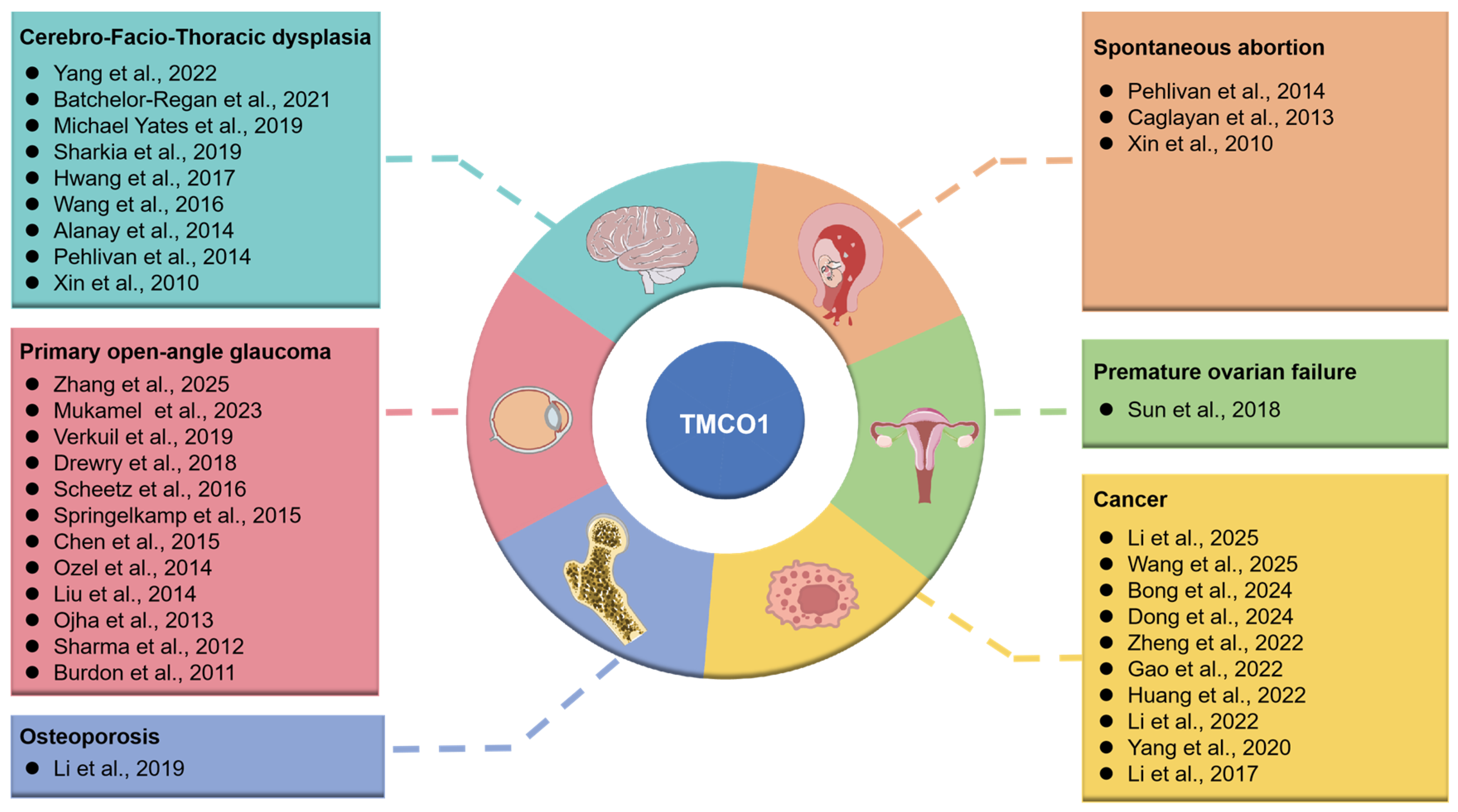
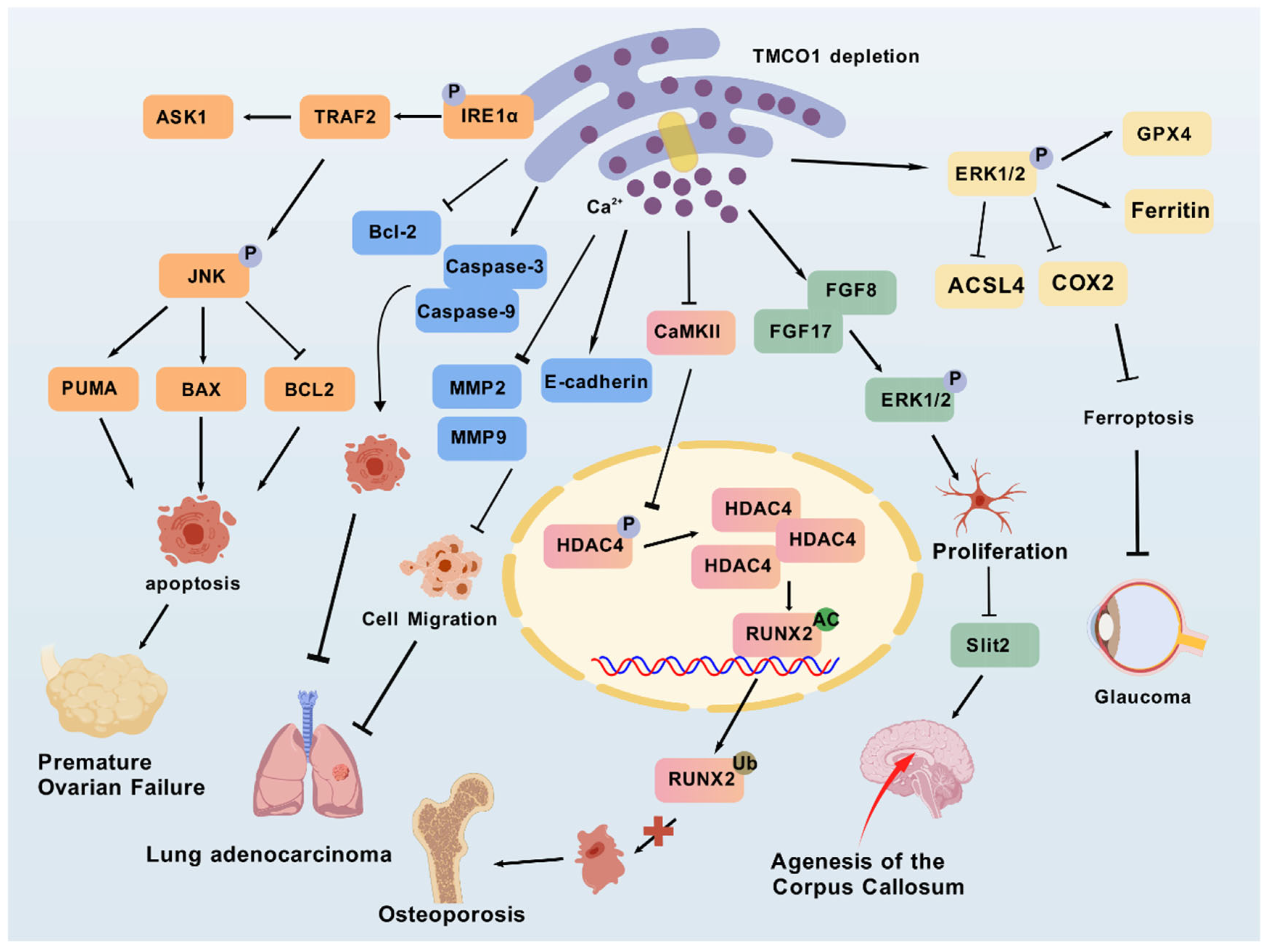
4. TMCO1 Mutation and Diseases
4.1. Cerebro-Facio-Thoracic Dysplasia
4.2. Glaucoma
4.3. Osteoporosis
4.4. Cancer
4.5. Premature Ovarian Failure
4.6. Spontaneous Abortion
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rubaiy, H.N. ORAI Calcium Channels: Regulation, Function, Pharmacology, and Therapeutic Targets. Pharmaceuticals 2023, 16, 162. [Google Scholar] [CrossRef] [PubMed]
- Makio, T.; Chen, J.; Simmen, T. ER stress as a sentinel mechanism for ER Ca2+ homeostasis. Cell Calcium 2024, 124, 102961. [Google Scholar] [CrossRef] [PubMed]
- Danowska, M.; Strączkowski, M. The Ca2+/Calmodulin-dependent Calcineurin/NFAT Signaling Pathway in the Pathogenesis of Insulin Resistance in Skeletal Muscle. Exp. Clin. Endocrinol. Diabetes 2023, 131, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Ta, N.; Liu, L.; Shi, G.; Kang, T.; Zheng, Z. Activation of CaMKII via ER-stress mediates coxsackievirus B3-induced cardiomyocyte apoptosis. Cell Biol. Int. 2020, 44, 488–498. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, X.; Zhao, D.; Liu, H.; Hu, Y. Calcium homeostasis and cancer: Insights from endoplasmic reticulum-centered organelle communications. Trends Cell Biol. 2023, 33, 312–323. [Google Scholar] [CrossRef]
- Albalawi, S.S.; Aljabri, A.; Alshibani, M.; Al-Gayyar, M.M. The Involvement of Calcium Channels in the Endoplasmic Reticulum Membrane in Nonalcoholic Fatty Liver Disease Pathogenesis. Cureus 2023, 15, e49150. [Google Scholar] [CrossRef]
- Li, X.; Zhao, X.; Qin, Z.; Li, J.; Sun, B.; Liu, L. Regulation of calcium homeostasis in endoplasmic reticulum-mitochondria crosstalk: Implications for skeletal muscle atrophy. Cell Commun. Signal 2025, 23, 17. [Google Scholar] [CrossRef]
- Bednarski, T.K.; Rahim, M.; Hasenour, C.M.; Banerjee, D.R.; Trenary, I.A.; Wasserman, D.H.; Young, J.D. Pharmacological SERCA activation limits diet-induced steatohepatitis and restores liver metabolic function in mice. J. Lipid Res. 2024, 65, 100558. [Google Scholar] [CrossRef]
- Rao, Z.; Zheng, Y.; Xu, L.; Wang, Z.; Zhou, Y.; Chen, M.; Dong, N.; Cai, Z.; Li, F. Endoplasmic Reticulum Stress and Pathogenesis of Vascular Calcification. Front. Cardiovasc. Med. 2022, 9, 918056. [Google Scholar] [CrossRef]
- Yu, Y.; Xia, F.; Liu, R.; Yan, Y.; Yin, L. Effect of Calcium Supplementation and TMEM16A Inhibition on Endoplasmic Reticulum Stress Induced by Dental Fluorosis in Mice. Discov. Med. 2024, 36, 753–764. [Google Scholar] [CrossRef]
- Carreras-Sureda, A.; Zhang, X.; Laubry, L.; Brunetti, J.; Koenig, S.; Wang, X.; Castelbou, C.; Hetz, C.; Liu, Y.; Frieden, M.; et al. The ER stress sensor IRE1 interacts with STIM1 to promote store-operated calcium entry, T cell activation, and muscular differentiation. Cell Rep. 2023, 42, 113540. [Google Scholar] [CrossRef]
- Shi, X.; Yao, J.; Huang, Y.; Wang, Y.; Jiang, X.; Wang, Z.; Zhang, M.; Zhang, Y.; Liu, X. Hhatl ameliorates endoplasmic reticulum stress through autophagy by associating with LC3. J. Biol. Chem. 2024, 300, 107335. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Jiang, Y.; Wang, Y.; Huo, R.; Ma, N.; Shen, X.; Chang, G. β-carotene targets IP3R/GRP75/VDAC1-MCU axis to renovate LPS-induced mitochondrial oxidative damage by regulating STIM1. Free Radic. Biol. Med. 2023, 205, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Li, W.; Zhang, X.; Yokoyama, T.; Sakamoto, M.; Wang, Y. The A-kinase anchoring protein Yotiao decrease the ER calcium content by inhibiting the store operated calcium entry. Cell Calcium 2024, 121, 102906. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.; Puffenberger, E.G.; Turben, S.; Tan, H.; Zhou, A.; Wang, H. Homozygous frameshift mutation in TMCO1 causes a syndrome with craniofacial dysmorphism, skeletal anomalies, and mental retardation. Proc. Natl. Acad. Sci. USA 2010, 107, 258–263. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, Q.; Tan, H.; Zhang, B.; Li, X.; Yang, Y.; Yu, J.; Liu, Y.; Chai, H.; Wang, X.; et al. TMCO1 Is an ER Ca(2+) Load-Activated Ca(2+) Channel. Cell 2016, 165, 1454–1466. [Google Scholar] [CrossRef]
- Wang, G.; Liu, D.; Leng, J.; Jin, D.; Wang, Q.; Wang, H.; Bu, Y.; Wang, F.; Hui, Y. TMCO1 regulates energy metabolism and mitochondrial function of hepatocellular carcinoma cells through TOMM20, affecting the growth of subcutaneous graft tumors and infiltration of CAFs. Biochem. Cell Biol. 2025, 103, 1–15. [Google Scholar] [CrossRef]
- Bong, A.H.L.; Robitaille, M.; Lin, S.; Mccart-Reed, A.; Milevskiy, M.; Angers, S.; Roberts-Thomson, S.J.; Monteith, G.R. TMCO1 is upregulated in breast cancer and regulates the response to pro-apoptotic agents in breast cancer cells. Cell Death Discov. 2024, 10, 421. [Google Scholar] [CrossRef]
- Abdelrazek, I.M.; Holling, T.; Harms, F.L.; Alawi, M.; Omar, T.; Abdalla, E.; Kutsche, K. Craniofacial dysmorphism, skeletal anomalies, and impaired intellectual development syndrome-1 in two new patients with the same homozygous TMCO1 variant and review of the literature. Eur. J. Med. Genet. 2023, 66, 104715. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, R.; Xu, S.; Shen, B.; Yu, H.; Chen, J.; Yao, H.; Huang, S.; Zhong, Y. TMCO1 promotes ferroptosis and ECM deposition in glaucomatous trabecular meshwork via ERK1/2 signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167530. [Google Scholar] [CrossRef]
- Yang, K.-Y.; Zhao, S.; Feng, H.; Shen, J.; Chen, Y.; Wang, S.-T.; Wang, S.-J.; Zhang, Y.-X.; Wang, Y.; Guo, C.; et al. Ca2+ homeostasis maintained by TMCO1 underlies corpus callosum development via ERK signaling. Cell Death Dis. 2022, 13, 674. [Google Scholar] [CrossRef]
- Batchelor-Regan, H.; Xin, B.; Zhou, A.; Wang, H. From Disease Description and Gene Discovery to Functional Cell Pathway: A Decade-Long Journey for TMCO1. Front. Genet. 2021, 12, 652400. [Google Scholar] [CrossRef] [PubMed]
- Michael Yates, T.; Ng, O.-H.; Offiah, A.C.; Willoughby, J.; Berg, J.N.; Johnson, D.S. Cerebrofaciothoracic dysplasia: Four new patients with a recurrent TMCO1 pathogenic variant. Am. J. Med. Genet. A 2019, 179, 43–49. [Google Scholar] [CrossRef]
- Sharkia, R.; Zalan, A.; Jabareen-Masri, A.; Hengel, H.; Schöls, L.; Kessel, A.; Azem, A.; Mahajnah, M. A novel biallelic loss-of-function mutation in TMCO1 gene confirming and expanding the phenotype spectrum of cerebro-facio-thoracic dysplasia. Am. J. Med. Genet. A 2019, 179, 1338–1345. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, Y.L.; Kang, S.; Kim, S.; Kim, S.O.; Lee, J.H.; Han, D.H. Genetic analysis of hereditary gingival fibromatosis using whole exome sequencing and bioinformatics. Oral Dis. 2017, 23, 102–109. [Google Scholar] [CrossRef]
- Alanay, Y.; Ergüner, B.; Utine, E.; Haçariz, O.; Kiper, P.O.S.; Taşkıran, E.Z.; Perçin, F.; Uz, E.; Sağiroğlu, M.Ş.; Yuksel, B.; et al. TMCO1 deficiency causes autosomal recessive cerebrofaciothoracic dysplasia. Am. J. Med. Genet. A 2014, 164A, 291–304. [Google Scholar] [CrossRef]
- Pehlivan, D.; Karaca, E.; Aydin, H.; Beck, C.R.; Gambin, T.; Muzny, D.M.; Bilge Geckinli, B.; Karaman, A.; Jhangiani, S.N.; Gibbs, R.A.; et al. Whole-exome sequencing links TMCO1 defect syndrome with cerebro-facio-thoracic dysplasia. Eur. J. Hum. Genet. 2014, 22, 1145–1148. [Google Scholar] [CrossRef]
- Mukamel, R.E.; Handsaker, R.E.; Sherman, M.A.; Barton, A.R.; Hujoel, M.L.A.; Mccarroll, S.A.; Loh, P.-R. Repeat polymorphisms underlie top genetic risk loci for glaucoma and colorectal cancer. Cell 2023, 186, 3659–3673. [Google Scholar] [CrossRef]
- Verkuil, L.; Danford, I.; Pistilli, M.; Collins, D.W.; Gudiseva, H.V.; Trachtman, B.T.; He, J.; Rathi, S.; Haider, N.; Ying, G.-S.; et al. SNP located in an AluJb repeat downstream of TMCO1, rs4657473, is protective for POAG in African Americans. Br. J. Ophthalmol. 2019, 103, 1530–1536. [Google Scholar] [CrossRef]
- Drewry, M.D.; Challa, P.; Kuchtey, J.G.; Navarro, I.; Helwa, I.; Hu, Y.; Mu, H.; Stamer, W.D.; Kuchtey, R.W.; Liu, Y. Differentially expressed microRNAs in the aqueous humor of patients with exfoliation glaucoma or primary open-angle glaucoma. Hum. Mol. Genet. 2018, 27, 1263–1275. [Google Scholar] [CrossRef]
- Scheetz, T.E.; Faga, B.; Ortega, L.; Roos, B.R.; Gordon, M.O.; Kass, M.A.; Wang, K.; Fingert, J.H. Glaucoma Risk Alleles in the Ocular Hypertension Treatment Study. Ophthalmology 2016, 123, 2527–2536. [Google Scholar] [CrossRef]
- Springelkamp, H.; Iglesias, A.I.; Cuellar-Partida, G.; Amin, N.; Burdon, K.P.; Van Leeuwen, E.M.; Gharahkhani, P.; Mishra, A.; Van Der Lee, S.J.; Hewitt, A.W.; et al. ARHGEF12 influences the risk of glaucoma by increasing intraocular pressure. Hum. Mol. Genet. 2015, 24, 2689–2699. [Google Scholar] [CrossRef]
- Chen, Y.; Hughes, G.; Chen, X.; Qian, S.; Cao, W.; Wang, L.; Wang, M.; Sun, X. Genetic Variants Associated With Different Risks for High Tension Glaucoma and Normal Tension Glaucoma in a Chinese Population. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2595–2600. [Google Scholar] [CrossRef]
- Ozel, A.B.; Moroi, S.E.; Reed, D.M.; Nika, M.; Schmidt, C.M.; Akbari, S.; Scott, K.; Rozsa, F.; Pawar, H.; Musch, D.C.; et al. Genome-wide association study and meta-analysis of intraocular pressure. Hum. Genet. 2014, 133, 41–57. [Google Scholar] [CrossRef]
- Liu, Y.; Garrett, M.E.; Yaspan, B.L.; Bailey, J.C.; Loomis, S.J.; Brilliant, M.; Budenz, D.L.; Christen, W.G.; Fingert, J.H.; Gaasterland, D.; et al. DNA copy number variants of known glaucoma genes in relation to primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8251–8258. [Google Scholar] [CrossRef]
- Ojha, P.; Wiggs, J.L.; Pasquale, L.R. The genetics of intraocular pressure. Semin. Ophthalmol. 2013, 28, 301–305. [Google Scholar] [CrossRef]
- Sharma, S.; Burdon, K.P.; Chidlow, G.; Klebe, S.; Crawford, A.; Dimasi, D.P.; Dave, A.; Martin, S.; Javadiyan, S.; Wood, J.P.M.; et al. Association of genetic variants in the TMCO1 gene with clinical parameters related to glaucoma and characterization of the protein in the eye. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4917–4925. [Google Scholar] [CrossRef]
- Burdon, K.P.; Macgregor, S.; Hewitt, A.W.; Sharma, S.; Chidlow, G.; Mills, R.A.; Danoy, P.; Casson, R.; Viswanathan, A.C.; Liu, J.Z.; et al. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat. Genet. 2011, 43, 574–578. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.; Li, Y.; Zheng, Q.; Xu, Y.; Liu, B.; Sun, W.; Li, Y.; Ji, S.; Liu, M.; et al. TMCO1-mediated Ca2+ leak underlies osteoblast functions via CaMKII signaling. Nat. Commun. 2019, 10, 1589. [Google Scholar] [CrossRef]
- Caglayan, A.O.; Per, H.; Akgumus, G.; Gumus, H.; Baranoski, J.; Canpolat, M.; Calik, M.; Yikilmaz, A.; Bilguvar, K.; Kumandas, S.; et al. Whole-exome sequencing identified a patient with TMCO1 defect syndrome and expands the phenotic spectrum. Clin. Genet. 2013, 84, 394–395. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, H.; Wang, X.; Wang, Q.-C.; Zhang, C.; Wang, J.-Q.; Wang, Y.-H.; An, C.-Q.; Yang, K.-Y.; Wang, Y.; et al. TMCO1 is essential for ovarian follicle development by regulating ER Ca2+ store of granulosa cells. Cell Death Differ. 2018, 25, 1686–1701. [Google Scholar] [CrossRef]
- Li, T.; Chen, L.; Zhou, G.; Deng, Y.; Zhou, M.; Yan, M.; Dong, S.; Xing, K.; Yu, S.; He, H. TMCO1, as a potential biomarker of prognosis and immunotherapy response, regulates head and neck squamous cell carcinoma proliferation and migration. Discov. Oncol. 2025, 16, 652. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Kang, S.; Cao, F.; Chen, X.; Wang, X.; Wang, L.; Wang, Q.; Zhai, Y. The relationship between TMCO1 and CALR in the pathological characteristics of prostate cancer and its effect on the metastasis of prostate cancer cells. Open Life Sci. 2024, 19, 20220972. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhao, D.; Hou, G.; Zhao, S.; Zhang, W.; Wang, X.; Li, L.; Lin, L.; Tang, T.-S.; Hu, Y. iASPP suppresses Gp78-mediated TMCO1 degradation to maintain Ca2+ homeostasis and control tumor growth and drug resistance. Proc. Natl. Acad. Sci. USA 2022, 119, e2111380119. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Ye, Z.; Liu, J.-H.; Yang, J.-A.; Li, Y.; Cai, J.-Y.; Wang, Y.-X.; Tong, S.-A.; Deng, G.; Zhang, S.; et al. TMCO1 expression promotes cell proliferation and induces epithelial-mesenchymal transformation in human gliomas. Med. Oncol. 2022, 39, 90. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, S.; Zhang, K.; Tan, W.; Chen, Y.; Shang, C. High Expression of Long Non-Coding RNA TMCO1-AS1 is Associated With Poor Prognosis of Hepatocellular Carcinoma. Front. Mol. Biosci. 2022, 9, 814058. [Google Scholar] [CrossRef]
- Li, H.-X.; Liu, T.-R.; Tu, Z.-X.; Xie, C.-B.; Wen, W.-P.; Sun, W. Screening of Tumor Antigens and Construction of Immune Subtypes for mRNA Vaccine Development in Head and Neck Squamous Cell Carcinoma. Biomolecules 2022, 13, 90. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Bai, J.-Q.; Zhang, J.-R.; Hu, P.-Y.; Zhu, Y.; Ouyang, Q.; Su, H.-M.; Li, Q.-Y.; Zhang, P. Mechanism of transmembrane and coiled-coil domain 1 in the regulation of proliferation and migration of A549 cells. Oncol. Lett. 2020, 20, 159. [Google Scholar] [CrossRef]
- Li, C.-F.; Wu, W.-R.; Chan, T.-C.; Wang, Y.-H.; Chen, L.-R.; Wu, W.-J.; Yeh, B.-W.; Liang, S.-S.; Shiue, Y.-L. Transmembrane and Coiled-Coil Domain 1 Impairs the AKT Signaling Pathway in Urinary Bladder Urothelial Carcinoma: A Characterization of a Tumor Suppressor. Clin. Cancer Res. 2017, 23, 7650–7663. [Google Scholar] [CrossRef]
- Zhang, Z.; Mo, D.; Cong, P.; He, Z.; Ling, F.; Li, A.; Niu, Y.; Zhao, X.; Zhou, C.; Chen, Y. Molecular cloning, expression patterns and subcellular localization of porcine TMCO1 gene. Mol. Biol. Rep. 2010, 37, 1611–1618. [Google Scholar] [CrossRef]
- Li, J.; Hu, J. The Expression Analysis of Two Different Isoforms of TMCO1. J. Nankai Univ. (Nat. Sci.) 2018, 51, 44–47+59. [Google Scholar]
- Gemmer, M.; Förster, F. A clearer picture of the ER translocon complex. J. Cell Sci. 2020, 133, jcs231340. [Google Scholar] [CrossRef] [PubMed]
- Mcgilvray, P.T.; Anghel, S.A.; Sundaram, A.; Zhong, F.; Trnka, M.J.; Fuller, J.R.; Hu, H.; Burlingame, A.L.; Keenan, R.J. An ER translocon for multi-pass membrane protein biogenesis. eLife 2020, 9, e56889. [Google Scholar] [CrossRef] [PubMed]
- Anghel, S.A.; Mcgilvray, P.T.; Hegde, R.S.; Keenan, R.J. Identification of Oxa1 Homologs Operating in the Eukaryotic Endoplasmic Reticulum. Cell Rep. 2017, 21, 3708–3716. [Google Scholar] [CrossRef]
- Olsen, J.V.; Vermeulen, M.; Santamaria, A.; Kumar, C.; Miller, M.L.; Jensen, L.J.; Gnad, F.; Cox, J.; Jensen, T.S.; Nigg, E.A.; et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci. Signal 2010, 3, ra3. [Google Scholar] [CrossRef]
- Saint-Martin Willer, A.; Montani, D.; Capuano, V.; Antigny, F. Orai1/STIMs modulators in pulmonary vascular diseases. Cell Calcium 2024, 121, 102892. [Google Scholar] [CrossRef]
- Diao, F.; Jiang, C.; Sun, Y.; Gao, Y.; Bai, J.; Nauwynck, H.; Wang, X.; Yang, Y.; Jiang, P.; Liu, X. Porcine reproductive and respiratory syndrome virus infection triggers autophagy via ER stress-induced calcium signaling to facilitate virus replication. PLoS Pathog. 2023, 19, e1011295. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, C.; He, L. ORAI Ca2+ Channels in Cancers and Therapeutic Interventions. Biomolecules 2024, 14, 417. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Shi, C.; Zhang, D.; Zhang, Q.; Wang, L.; Gong, Z. Mitochondrial calcium uniporter complex: Unveiling the interplay between its regulators and calcium homeostasis. Cell. Signal. 2024, 121, 111284. [Google Scholar] [CrossRef]
- Santulli, G.; Nakashima, R.; Yuan, Q.; Marks, A.R. Intracellular calcium release channels: An update. J. Physiol. 2017, 595, 3041–3051. [Google Scholar] [CrossRef]
- Atakpa, P.; Van Marrewijk, L.M.; Apta-Smith, M.; Chakraborty, S.; Taylor, C.W. GPN does not release lysosomal Ca2+ but evokes Ca2+ release from the ER by increasing the cytosolic pH independently of cathepsin C. J. Cell Sci. 2019, 132, jcs223883. [Google Scholar] [CrossRef]
- Fan, R.-F.; Chen, X.-W.; Cui, H.; Fu, H.-Y.; Xu, W.-X.; Li, J.-Z.; Lin, H. Selenoprotein K knockdown induces apoptosis in skeletal muscle satellite cells via calcium dyshomeostasis-mediated endoplasmic reticulum stress. Poult. Sci. 2023, 102, 103053. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.-C.; Sun, Z.; Li, T.; Yang, K.; An, C.; Guo, C.; Tang, T.-S. ER stress mediated degradation of diacylglycerol acyltransferase impairs mitochondrial functions in TMCO1 deficient cells. Biochem. Biophys. Res. Commun. 2019, 512, 914–920. [Google Scholar] [CrossRef]
- Kaur, S.; Sehrawat, A.; Mastana, S.S.; Kandimalla, R.; Sharma, P.K.; Bhatti, G.K.; Bhatti, J.S. Targeting calcium homeostasis and impaired inter-organelle crosstalk as a potential therapeutic approach in Parkinson’s disease. Life Sci. 2023, 330, 121995. [Google Scholar] [CrossRef]
- Wang, M.; Kaufman, R.J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 2016, 529, 326–335. [Google Scholar] [CrossRef]
- Mekahli, D.; Bultynck, G.; Parys, J.B.; De Smedt, H.; Missiaen, L. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a004317. [Google Scholar] [CrossRef]
- Khanna, M.; Agrawal, N.; Chandra, R.; Dhawan, G. Targeting unfolded protein response: A new horizon for disease control. Expert Rev. Mol. Med. 2021, 23, e1. [Google Scholar] [CrossRef]
- Fung, T.S.; Liao, Y.; Liu, D.X. The endoplasmic reticulum stress sensor IRE1α protects cells from apoptosis induced by the coronavirus infectious bronchitis virus. J. Virol. 2014, 88, 12752–12764. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, H.; Jiang, D.; Yang, K.; Wang, S.-T.; Zhang, Y.-X.; Wang, Y.; Liu, H.; Guo, C.; Tang, T.-S. ER Ca2+ overload activates the IRE1α signaling and promotes cell survival. Cell Biosci. 2023, 13, 123. [Google Scholar] [CrossRef]
- Logue, S.E.; Cleary, P.; Saveljeva, S.; Samali, A. New directions in ER stress-induced cell death. Apoptosis 2013, 18, 537–546. [Google Scholar] [CrossRef]
- Badawy, A.a.B. The role of nonesterified fatty acids in cancer biology: Focus on tryptophan and related metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2024, 1869, 159531. [Google Scholar] [CrossRef]
- Hwang, H.-Y.; Shim, J.S.; Kim, D.; Kwon, H.J. Antidepressant drug sertraline modulates AMPK-MTOR signaling-mediated autophagy via targeting mitochondrial VDAC1 protein. Autophagy 2021, 17, 2783–2799. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, B.; Li, Z.; Zhong, Y.; Wang, B.; Zhang, B.; Du, J.; Ye, R.; Xian, H.; Min, W.; et al. Polystyrene nanoplastic exposure induces excessive mitophagy by activating AMPK/ULK1 pathway in differentiated SH-SY5Y cells and dopaminergic neurons in vivo. Part. Fibre Toxicol. 2023, 20, 44. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, Y.; Saredy, J.; Wang, X.; Drummer Iv, C.; Shao, Y.; Saaoud, F.; Xu, K.; Liu, M.; Yang, W.Y.; et al. ROS systems are a new integrated network for sensing homeostasis and alarming stresses in organelle metabolic processes. Redox Biol. 2020, 37, 101696. [Google Scholar] [CrossRef]
- Pascual-Castroviejo, I.; Santolaya, J.M.; Martin, V.L.; Rodriguez-Costa, T.; Tendero, A.; Mulas, F. Cerebro-facio-thoracic dysplasia: Report of three cases. Dev. Med. Child Neurol. 1975, 17, 343–351. [Google Scholar] [CrossRef]
- Tender, J.a.F.; Ferreira, C.R. Cerebro-facio-thoracic dysplasia (Pascual-Castroviejo syndrome): Identification of a novel mutation, use of facial recognition analysis, and review of the literature. Transl. Sci. Rare Dis. 2018, 3, 37–43. [Google Scholar] [CrossRef]
- Rensvold, J.W.; Shishkova, E.; Sverchkov, Y.; Miller, I.J.; Cetinkaya, A.; Pyle, A.; Manicki, M.; Brademan, D.R.; Alanay, Y.; Raiman, J.; et al. Defining mitochondrial protein functions through deep multiomic profiling. Nature 2022, 606, 382–388. [Google Scholar] [CrossRef]
- Jiang, S.; Li, H.; Zhang, L.; Mu, W.; Zhang, Y.; Chen, T.; Wu, J.; Tang, H.; Zheng, S.; Liu, Y.; et al. Generic Diagramming Platform (GDP): A comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2025, 53, D1670–D1676. [Google Scholar] [CrossRef]
- Irkec, M.; Konstas, A.G.; Holló, G.; Dikmetaş, Ö.; Algedik Tokyürek, M.Ö.; Bozkurt, B. Investigational drugs for glaucoma: Novel mechanistic approaches of preclinical agents. Expert Opin. Investig. Drugs 2025, 34, 231–243. [Google Scholar] [CrossRef]
- Lo Faro, V.; Bhattacharya, A.; Zhou, W.; Zhou, D.; Wang, Y.; Läll, K.; Kanai, M.; Lopera-Maya, E.; Straub, P.; Pawar, P.; et al. Novel ancestry-specific primary open-angle glaucoma loci and shared biology with vascular mechanisms and cell proliferation. Cell Rep. Med. 2024, 5, 101430. [Google Scholar] [CrossRef]
- Sakurada, Y.; Mabuchi, F.; Kashiwagi, K. Genetics of primary open-angle glaucoma and its endophenotypes. Prog. Brain Res. 2020, 256, 31–47. [Google Scholar]
- Xiong, K.; Zhang, Q.A.; Mao, H.; Congdon, N.; Liang, Y. Assessment of Causality Between Diet-Derived Antioxidants and Primary Open-Angle Glaucoma: A Mendelian Randomization Study. Transl. Vis. Sci. Technol. 2024, 13, 20. [Google Scholar] [CrossRef]
- Rognon, G.T.; Liao, A.Y.-A.; Pasteurin, R.P.; Soundararajan, A.; Pattabiraman, P.P. Lipids and lipid regulators in intraocular pressure homeostasis. Curr. Opin. Pharmacol. 2025, 82, 102523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ma, Q.; Dai, Z.; Hong, J.; Gu, J.; Shi, R.; Xu, J.; Ma, Y.; Sun, X.; Sun, J. Sustained release of brimonidine from conjunctival sac insert to reduce intraocular pressure for glaucoma treatment. Expert Opin. Drug Deliv. 2024, 21, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Zukerman, R.; Harris, A.; Vercellin, A.V.; Siesky, B.; Pasquale, L.R.; Ciulla, T.A. Molecular Genetics of Glaucoma: Subtype and Ethnicity Considerations. Genes 2020, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; You, M.; Fan, C.; Rong, R.; Li, H.; Xia, X. Pathologically high intraocular pressure induces mitochondrial dysfunction through Drp1 and leads to retinal ganglion cell PANoptosis in glaucoma. Redox Biol. 2023, 62, 102687. [Google Scholar] [CrossRef]
- Kim, Y.W.; Lee, Y.H.; Kim, J.-S.; Lee, J.; Kim, Y.J.; Cheong, H.S.; Kim, S.H.; Park, K.H.; Kim, D.M.; Choi, H.J.; et al. Genetic analysis of primary open-angle glaucoma-related risk alleles in a Korean population: The GLAU-GENDISK study. Br. J. Ophthalmol. 2021, 105, 1307–1312. [Google Scholar] [CrossRef]
- Burdon, K.P.; Mitchell, P.; Lee, A.; Healey, P.R.; White, A.J.R.; Rochtchina, E.; Thomas, P.B.M.; Wang, J.J.; Craig, J.E. Association of open-angle glaucoma loci with incident glaucoma in the Blue Mountains Eye Study. Am. J. Ophthalmol. 2015, 159, 31–36. [Google Scholar] [CrossRef]
- Van Koolwijk, L.M.E.; Ramdas, W.D.; Ikram, M.K.; Jansonius, N.M.; Pasutto, F.; Hysi, P.G.; Macgregor, S.; Janssen, S.F.; Hewitt, A.W.; Viswanathan, A.C.; et al. Common genetic determinants of intraocular pressure and primary open-angle glaucoma. PLoS Genet. 2012, 8, e1002611. [Google Scholar] [CrossRef]
- Formosa, M.M.; Christou, M.A.; Mäkitie, O. Bone fragility and osteoporosis in children and young adults. J. Endocrinol. Investig. 2024, 47, 285–298. [Google Scholar] [CrossRef]
- Silverstein, W.K.; Cantor, N.; Cheung, A.M. Postmenopausal Osteoporosis. N. Engl. J. Med. 2024, 390, 673–674. [Google Scholar] [PubMed]
- Ao, Q.; Hu, H.; Huang, Y. Ferroptosis and endoplasmic reticulum stress in rheumatoid arthritis. Front. Immunol. 2024, 15, 1438803. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xie, R.; Song, W.; Ouyang, K.; Ren, L. Biomimetic Hyaluronic Acid-Based Brush Polymers Modulate Chondrocyte Homeostasis via ROS/Ca2+/TRPV4. Biomacromolecules 2023, 24, 4240–4252. [Google Scholar] [CrossRef] [PubMed]
- Chacar, S.; Abdi, A.; Almansoori, K.; Alshamsi, J.; Al Hageh, C.; Zalloua, P.; Khraibi, A.A.; Holt, S.G.; Nader, M. Role of CaMKII in diabetes induced vascular injury and its interaction with anti-diabetes therapy. Rev. Endocr. Metab. Disord. 2024, 25, 369–382. [Google Scholar] [CrossRef]
- Wu, Y.; Jia, C.; Liu, W.; Zhan, W.; Chen, Y.; Lu, J.; Bao, Y.; Wang, S.; Yu, C.; Zheng, L.; et al. Sodium citrate targeting Ca2+/CAMKK2 pathway exhibits anti-tumor activity through inducing apoptosis and ferroptosis in ovarian cancer. J. Adv. Res. 2024, 65, 89–104. [Google Scholar] [CrossRef]
- Pouladvand, N.; Azarnia, M.; Zeinali, H.; Fathi, R.; Tavana, S. An overview of different methods to establish a murine premature ovarian failure model. Animal Model. Exp. Med. 2024, 7, 835–852. [Google Scholar] [CrossRef]
- Liu, D.; Guan, X.; Liu, W.; Jia, Y.; Zhou, H.; Xi, C.; Zhao, M.; Fang, Y.; Wu, L.; Li, K. Identification of transcriptome characteristics of granulosa cells and the possible role of UBE2C in the pathogenesis of premature ovarian insufficiency. J. Ovarian Res. 2023, 16, 203. [Google Scholar] [CrossRef]
- Xiang, S.; You, Q.; Mu, F.; Zhang, N. Spontaneous Abortion and Myocardial Infarction: A Mendelian Randomization Investigation and Transcriptomic Analysis. Glob. Heart 2025, 20, 12. [Google Scholar] [CrossRef]
- Capatina, N.; Hemberger, M.; Burton, G.J.; Watson, E.D.; Yung, H.W. Excessive endoplasmic reticulum stress drives aberrant mouse trophoblast differentiation and placental development leading to pregnancy loss. J. Physiol. 2021, 599, 4153–4181. [Google Scholar] [CrossRef]
- Wan, S.; Wang, X.; Chen, W.; Wang, M.; Zhao, J.; Xu, Z.; Wang, R.; Mi, C.; Zheng, Z.; Zhang, H. Exposure to high dose of polystyrene nanoplastics causes trophoblast cell apoptosis and induces miscarriage. Part. Fibre Toxicol. 2024, 21, 13. [Google Scholar] [CrossRef]
| Diseases | Cells/Tissues | Mutation/Disruption | Regulation of Proteins/Pathways | Effects | Symptom | Ref. |
|---|---|---|---|---|---|---|
| Cerebro-facio-thoracic dysplasia | adult and fetal tissues of humans | TMCO1 (C.139–140 delAG) | Premature cessation of Ser47 of the protein | A highly truncated protein (p.Ser47Ter), only a quarter of its original length | Craniofacial deformity, spinal abnormalities, mental retardation, epilepsy, hyper-gingival growth, etc. | [16,25,39] |
| Primary open-angle glaucoma | Ocular tissues | rs4656461/rs7555523 TMCO1 risk allele | MYOC expression | Intraocular pressure (IOP) changes | Characteristic bending of the optic nerve head concurrent with diminished peripheral visual perception | [20,33] |
| Osteoporosis | Osteoblast | TMCO1 deletion/loss-of-function mutation or downregulation | Decreasing the CAMKII expression level and promoting CaMKII-HDAC4-mediated RUNX2 degradation | Inhibition of osteoblastic differentiation and bone formation | Decreasing bone mass and degrading microstructure of the bone tissue | [39,48] |
| Cancer | Cancer cells | TMCO1 gene overexpression | Calcium signaling regulation/Inhibiting the AKT signaling pathway | Promotion/inhibition of cancer cell growth and migration | Mass effects, organ obstruction/destruction, invasive growth, dissemination, overcrowding of normal tissue, metastasis formation, pain, metabolic effects (cachexia), paraneoplastic syndromes, etc . | [17,43] |
| Premature ovarian failure | Granulosa cells (GCs) | TMCO1 deletion or loss-of-function mutation | As a Ca2+ channel triggered by Ca2+ loads | Increased amounts of ROS and apoptosis caused by ERS | Amenorrhea before age 40 with elevated gonadotropin and luteinizing hormone levels | [41] |
| Spontaneous abortion | Human adult and fetal tissues | TMCO1 functional defect | Unclear | Unclear | Termination of pregnancy for less than 28 weeks of pregnancy and less than 1000 g of fetal weight | [15,27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhu, P.; Li, Z.; Su, X.; Qi, M.; Zhou, A.; Kong, X. TMCO1 as an Endoplasmic Reticulum Calcium Load-Activated Channel: Mechanisms and Disease Implications. Biomolecules 2025, 15, 1200. https://doi.org/10.3390/biom15081200
Wang J, Zhu P, Li Z, Su X, Qi M, Zhou A, Kong X. TMCO1 as an Endoplasmic Reticulum Calcium Load-Activated Channel: Mechanisms and Disease Implications. Biomolecules. 2025; 15(8):1200. https://doi.org/10.3390/biom15081200
Chicago/Turabian StyleWang, Jingbo, Panpan Zhu, Zhuohang Li, Xiaohui Su, Mingzhu Qi, Aimin Zhou, and Xiangying Kong. 2025. "TMCO1 as an Endoplasmic Reticulum Calcium Load-Activated Channel: Mechanisms and Disease Implications" Biomolecules 15, no. 8: 1200. https://doi.org/10.3390/biom15081200
APA StyleWang, J., Zhu, P., Li, Z., Su, X., Qi, M., Zhou, A., & Kong, X. (2025). TMCO1 as an Endoplasmic Reticulum Calcium Load-Activated Channel: Mechanisms and Disease Implications. Biomolecules, 15(8), 1200. https://doi.org/10.3390/biom15081200





