Humanized Monoclonal Antibody Against Citrullinated Histone H3 Attenuates Myocardial Injury and Prevents Heart Failure in Rodent Models
Abstract
1. Introduction
2. Materials and Methods
2.1. hCitH3-mAb Generation
2.2. Acute Myocardial Ischemia/Reperfusion Injury
2.3. Post-Infarct Heart Failure Study
2.4. Surgical Procedures to Induce Myocardial Infarction in Mice
2.5. Determination of Myocardial Infarct Size
2.6. Echocardiography (ECHO) Image Acquisition and Longitudinal Evaluation of Cardiac Function
2.7. Western Blot
2.8. Collagen Deposition in Post-Mi Myocardium
2.9. Statistical Analysis
3. Results
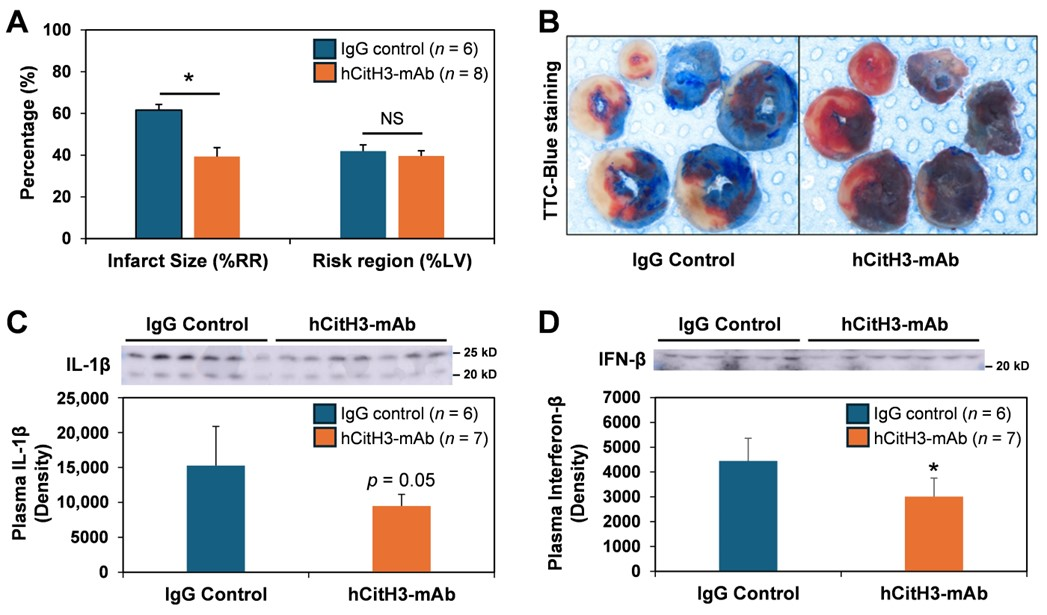
3.1. hCitH3-mAb Attenuates Acute Myocardial IRI Injury
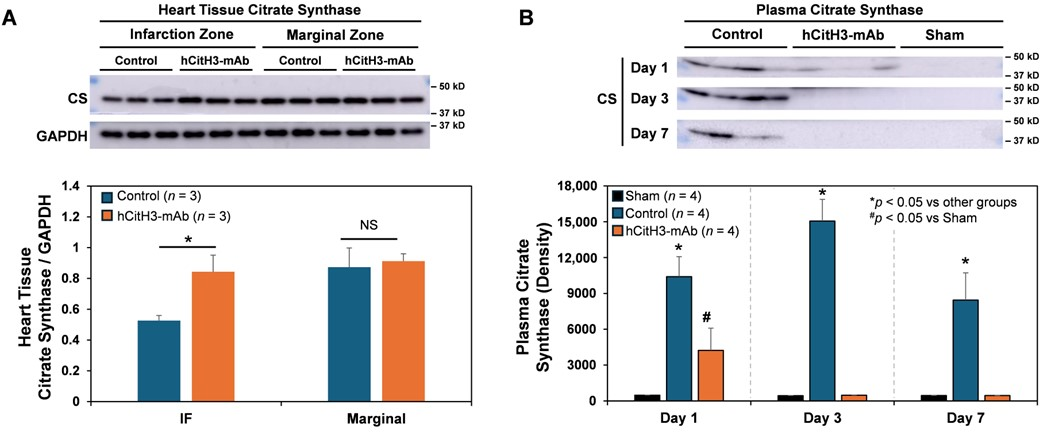
3.2. Mitochondrial Protection by hCitH3-mAb
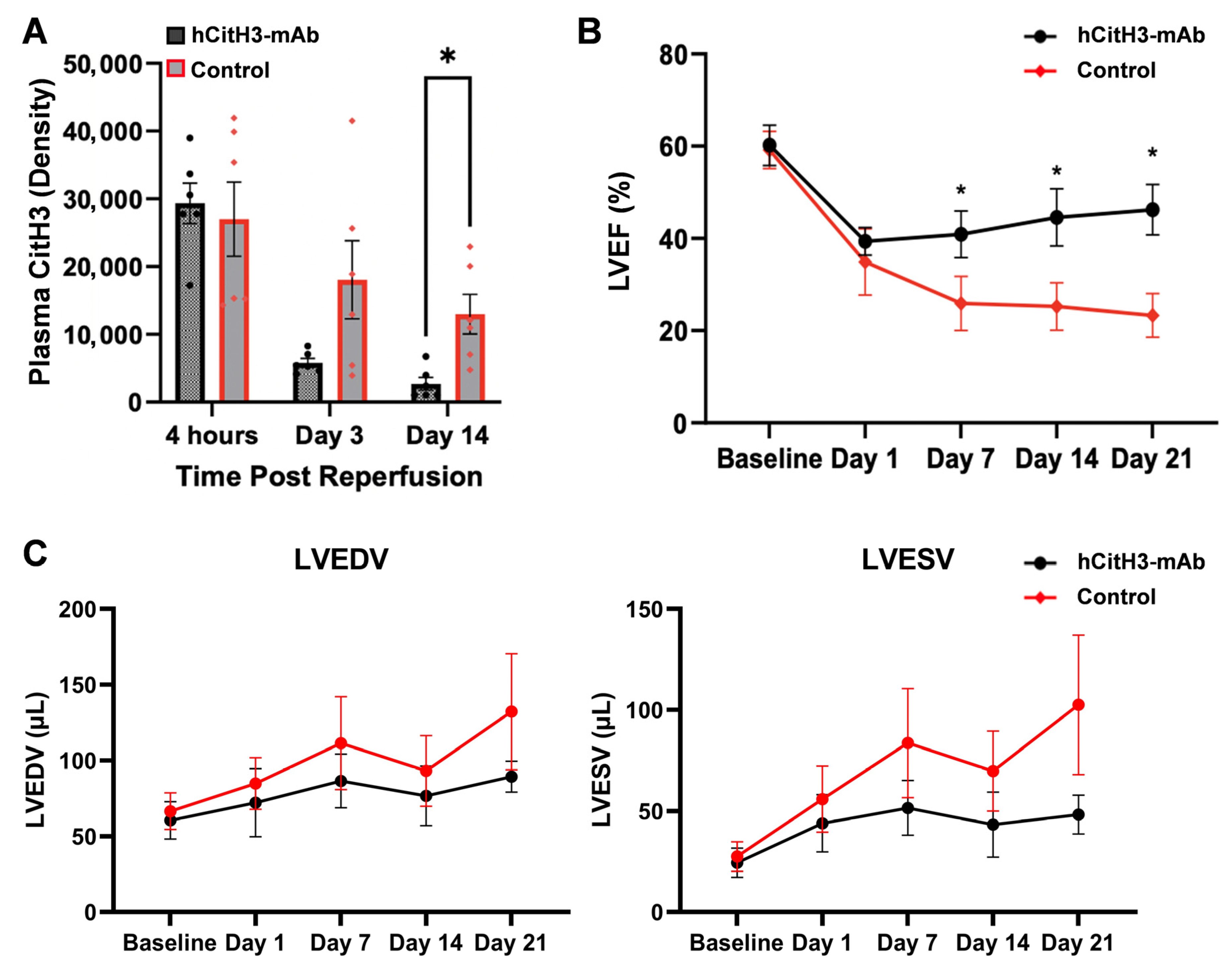
3.3. hCitH3-mAb Preserves Post-MI Cardiac Function
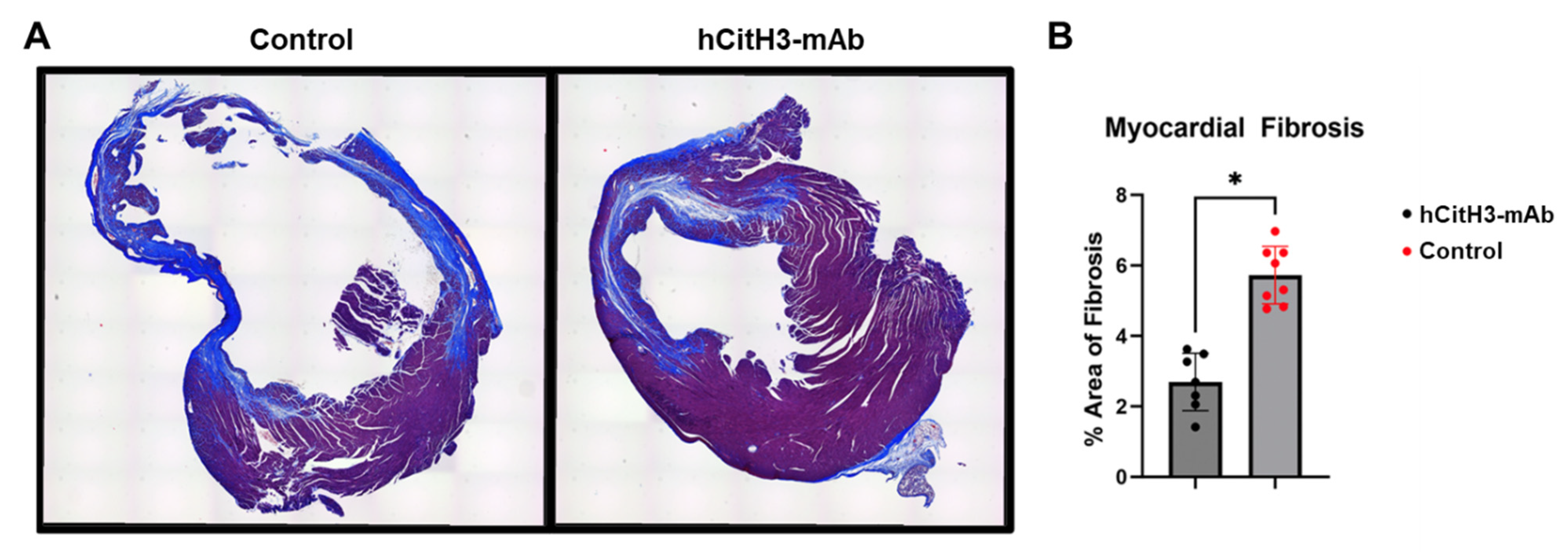
3.4. hCitH3-mAb Mitigates Adverse Cardiac Remodeling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data from the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef]
- Mulay, S.R.; Anders, H.J. Neutrophils and Neutrophil Extracellular Traps Regulate Immune Responses in Health and Disease. Cells 2020, 9, 2130. [Google Scholar] [CrossRef]
- Leliefeld, P.H.; Wessels, C.M.; Leenen, L.P.; Koenderman, L.; Pillay, J. The role of neutrophils in immune dysfunction during severe inflammation. Crit. Care 2016, 20, 73. [Google Scholar] [CrossRef]
- Sikora, J.P.; Karawani, J.; Sobczak, J. Neutrophils and the Systemic Inflammatory Response Syndrome (SIRS). Int. J. Mol. Sci. 2023, 24, 13469. [Google Scholar] [CrossRef] [PubMed]
- Margraf, A.; Lowell, C.A.; Zarbock, A. Neutrophils in acute inflammation: Current concepts and translational implications. Blood 2022, 139, 2130–2144. [Google Scholar] [CrossRef] [PubMed]
- Sreejit, G.; Abdel-Latif, A.; Athmanathan, B.; Annabathula, R.; Dhyani, A.; Noothi, S.K.; Quaife-Ryan, G.A.; Al-Sharea, A.; Pernes, G.; Dragoljevic, D.; et al. Neutrophil-Derived S100A8/A9 Amplify Granulopoiesis After Myocardial Infarction. Circulation 2020, 141, 1080–1094. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Steffens, S.; Hidalgo, A.; Weber, C. Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol. 2017, 17, 248–261. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Regulation of the inflammatory response in cardiac repair. Circ. Res. 2012, 110, 159–173. [Google Scholar] [CrossRef]
- Vinten-Johansen, J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc. Res. 2004, 61, 481–497. [Google Scholar] [CrossRef]
- Chen, Y.; Tetz, Z.A.; Zeng, X.; Go, S.J.; Ouyang, W.; Lee, K.E.; Dong, T.; Li, Y.; Ma, J. CitH3, a Druggable Biomarker for Human Diseases Associated with Acute NETosis and Chronic Immune Dysfunction. Pharmaceutics 2025, 17, 809. [Google Scholar] [CrossRef]
- Islam, M.M.; Takeyama, N. Role of Neutrophil Extracellular Traps in Health and Disease Pathophysiology: Recent Insights and Advances. Int. J. Mol. Sci. 2023, 24, 15805. [Google Scholar] [CrossRef]
- van der Linden, M.; Kumari, S.; Montizaan, D.; van Dalen, S.; Kip, A.; Foster, M.; Reinieren-Beeren, I.; Neubert, E.; Erpenbeck, L.; Waaijenberg, K.; et al. Anti-citrullinated histone monoclonal antibody CIT-013, a dual action therapeutic for neutrophil extracellular trap-associated autoimmune diseases. mAbs 2023, 15, 2281763. [Google Scholar] [CrossRef] [PubMed]
- Mutua, V.; Gershwin, L.J. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin. Rev. Allergy Immunol. 2021, 61, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Kostin, S.; Krizanic, F.; Kelesidis, T.; Pagonas, N. The role of NETosis in heart failure. Heart Fail. Rev. 2024, 29, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Kostin, S.; Richter, M.; Krizanic, F.; Sasko, B.; Kelesidis, T.; Pagonas, N. NETosis Is an Important Component of Chronic Myocardial Inflammation in Patients With Heart Failure. Circ. Heart Fail. 2025, 18, e012231. [Google Scholar] [CrossRef]
- Tian, Y.; Li, P.; Wu, Z.; Deng, Q.; Pan, B.; Stringer, K.A.; Alam, H.B.; Standiford, T.J.; Li, Y. Citrullinated Histone H3 Mediates Sepsis-Induced Lung Injury Through Activating Caspase-1 Dependent Inflammasome Pathway. Front. Immunol. 2021, 12, 761345. [Google Scholar] [CrossRef]
- Tomar, B.; Anders, H.J.; Desai, J.; Mulay, S.R. Neutrophils and Neutrophil Extracellular Traps Drive Necroinflammation in COVID-19. Cells 2020, 9, 1383. [Google Scholar] [CrossRef]
- Grilz, E.; Mauracher, L.M.; Posch, F.; Königsbrügge, O.; Zöchbauer-Müller, S.; Marosi, C.; Lang, I.; Pabinger, I.; Ay, C. Citrullinated histone H3, a biomarker for neutrophil extracellular trap formation, predicts the risk of mortality in patients with cancer. Br. J. Haematol. 2019, 186, 311–320. [Google Scholar] [CrossRef]
- Thålin, C.; Lundström, S.; Seignez, C.; Daleskog, M.; Lundström, A.; Henriksson, P.; Helleday, T.; Phillipson, M.; Wallén, H.; Demers, M. Citrullinated histone H3 as a novel prognostic blood marker in patients with advanced cancer. PLoS ONE 2018, 13, e0191231. [Google Scholar] [CrossRef]
- Saisorn, W.; Santiworakul, C.; Phuengmaung, P.; Siripen, N.; Rianthavorn, P.; Leelahavanichkul, A. Extracellular traps in peripheral blood mononuclear cell fraction in childhood-onset systemic lupus erythematosus. Sci. Rep. 2024, 14, 23177. [Google Scholar] [CrossRef]
- Vander Cruyssen, B.; Peene, I.; Cantaert, T.; Hoffman, I.; De Rycke, L.; Veys, E.; De Keyser, F. Anti-citrullinated protein/peptide antibodies (ACPA) in rheumatoid arthritis: Specificity and relation with rheumatoid factor. Autoimmun. Rev. 2005, 4, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.; Reimers, T.; Abdulahad, W.; Fierro, J.J.; Doornbos-van der Meer, B.; Bootsma, H.; Horvath, B.; de Leeuw, K.; Westra, J. Low-density granulocytes and neutrophil extracellular trap formation are increased in incomplete systemic lupus erythematosus. Rheumatology 2025, 64, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cao, Y.; Xiao, J.; Hong, Y.; Zhu, Y. The emerging role of neutrophil extracellular traps in the progression of rheumatoid arthritis. Front. Immunol. 2024, 15, 1438272. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, J.; Zhang, Y.; Huang, H.; Zhang, L.; Femel, J.; Dimberg, A.; Olsson, A.K. Neutrophil Extracellular Traps Accumulate in Peripheral Blood Vessels and Compromise Organ Function in Tumor-Bearing Animals. Cancer Res. 2015, 75, 2653–2662. [Google Scholar] [CrossRef]
- Zeng, F.; Zhang, Y.; Wang, Z.; Zhang, H.; Meng, X.; Wu, Y.; Qian, Z.; Ding, Y.; Li, J.; Ma, T. Neutrophil extracellular traps promote acetaminophen-induced acute liver injury in mice via AIM2. Acta Pharmacol. Sin. 2024, 45, 1660–1672. [Google Scholar] [CrossRef]
- Kim, S.-W.; Lee, H.; Lee, H.-K.; Kim, I.-D.; Lee, J.-K. Neutrophil extracellular trap induced by HMGB1 exacerbates damages in the ischemic brain. Acta Neuropathol. Commun. 2019, 7, 94. [Google Scholar] [CrossRef]
- Wu, Z.; Li, P.; Tian, Y.; Ouyang, W.; Ho, J.W.; Alam, H.B.; Li, Y. Peptidylarginine Deiminase 2 in Host Immunity: Current Insights and Perspectives. Front. Immunol. 2021, 12, 761946. [Google Scholar] [CrossRef]
- Deng, Q.; Pan, B.; Alam, H.B.; Liang, Y.; Wu, Z.; Liu, B.; Mor-Vaknin, N.; Duan, X.; Williams, A.M.; Tian, Y.; et al. Citrullinated Histone H3 as a Therapeutic Target for Endotoxic Shock in Mice. Front. Immunol. 2019, 10, 2957. [Google Scholar] [CrossRef]
- Dong, T.; Ouyang, W.; Yu, X.; Zhao, T.; Shao, L.; Quan, C.; Wang, S.; Ma, J.; Li, Y. Synergistic inhibition of CitH3 and S100A8/A9: A novel therapeutic strategy for mitigating sepsis-induced inflammation and lung injury. Int. J. Immunopathol. Pharmacol. 2025, 39, 3946320251338661. [Google Scholar] [CrossRef]
- Ouyang, W.; Chen, Y.; Tan, T.; Song, Y.; Dong, T.; Yu, X.; Lee, K.E.; Zhou, X.; Tets, Z.; Go, S.; et al. A Citrullinated Histone H3 Monoclonal Antibody for Immune Modulation in Sepsis. Nat. Commun. 2025, 16, 7435. [Google Scholar] [CrossRef]
- Tian, Y.; Charles, E.J.; Yan, Z.; Wu, D.; French, B.A.; Kron, I.L.; Yang, Z. The myocardial infarct-exacerbating effect of cell-free DNA is mediated by the high-mobility group box 1-receptor for advanced glycation end products-Toll-like receptor 9 pathway. J. Thorac. Cardiovasc. Surg. 2019, 157, 2256–2269 e2253. [Google Scholar] [CrossRef]
- Yang, Z.; Day, Y.-J.; Toufektsian, M.-C.; Xu, Y.; Ramos, S.I.; Marshall, M.A.; French, B.A.; Linden, J. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation 2006, 114, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.P.; Sommer, S.; Sinha, B.; Wiedemann, J.; Otto, C.; Aleksic, I.; Schimmer, C.; Leyh, R.G. Ischemia-reperfusion injury-induced pulmonary mitochondrial damage. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2011, 30, 811–818. [Google Scholar] [CrossRef]
- Yu, S.; Liu, J.; Yan, N. Endothelial Dysfunction Induced by Extracellular Neutrophil Traps Plays Important Role in the Occurrence and Treatment of Extracellular Neutrophil Traps-Related Disease. Int. J. Mol. Sci. 2022, 23, 5626. [Google Scholar] [CrossRef] [PubMed]
- Biermann, M.H.; Podolska, M.J.; Knopf, J.; Reinwald, C.; Weidner, D.; Maueroder, C.; Hahn, J.; Kienhofer, D.; Barras, A.; Boukherroub, R.; et al. Oxidative Burst-Dependent NETosis Is Implicated in the Resolution of Necrosis-Associated Sterile Inflammation. Front. Immunol. 2016, 7, 557. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yan, Z.; Fan, Y.; Fan, X.; Li, A.; Qi, Z.; Zhang, J. Cardiac repair after myocardial infarction: A two-sided role of inflammation-mediated. Front. Cardiovasc. Med. 2022, 9, 1077290. [Google Scholar] [CrossRef]
- Ma, Y. Role of Neutrophils in Cardiac Injury and Repair Following Myocardial Infarction. Cells 2021, 10, 1676. [Google Scholar] [CrossRef]
- Carbone, F.; Nencioni, A.; Mach, F.; Vuilleumier, N.; Montecucco, F. Pathophysiological role of neutrophils in acute myocardial infarction. Thromb. Haemost. 2013, 110, 501–514. [Google Scholar] [CrossRef]
- Dick, S.A.; Epelman, S. Chronic Heart Failure and Inflammation: What Do We Really Know? Circ. Res. 2016, 119, 159–176. [Google Scholar] [CrossRef]
- Ichimura, S.; Misaka, T.; Ogawara, R.; Tomita, Y.; Anzai, F.; Sato, Y.; Miura, S.; Yokokawa, T.; Sato, T.; Oikawa, M.; et al. Neutrophil Extracellular Traps in Myocardial Tissue Drive Cardiac Dysfunction and Adverse Outcomes in Patients with Heart Failure With Dilated Cardiomyopathy. Circ. Heart Fail. 2024, 17, e011057. [Google Scholar] [CrossRef]
- Varga, Z.V.; Ferdinandy, P.; Liaudet, L.; Pacher, P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am. J. physiology. Heart Circ. Physiol. 2015, 309, H1453–H1467. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, B.; Yang, X.; Zhang, C.; Jiao, Y.; Li, P.; Liu, Y.; Li, Z.; Qiao, B.; Bond Lau, W.; et al. S100a8/a9 Signaling Causes Mitochondrial Dysfunction and Cardiomyocyte Death in Response to Ischemic/Reperfusion Injury. Circulation 2019, 140, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Pathophysiology of Myocardial Infarction. Compr. Physiol. 2015, 5, 1841–1875. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Encina, M.; Booth, L.K.; Redgrave, R.E.; Folaranmi, O.; Spyridopoulos, I.; Richardson, G.D. Cellular Senescence, Mitochondrial Dysfunction, and Their Link to Cardiovascular Disease. Cells 2024, 13, 353. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Chiong, M.; Lavandero, S.; Klionsky, D.J.; Ren, J. Mitophagy in cardiovascular diseases: Molecular mechanisms, pathogenesis, and treatment. Trends Mol. Med. 2022, 28, 836–849. [Google Scholar] [CrossRef]
- Padhy, D.S.; Palit, P.; Ikbal, A.M.A.; Das, N.; Roy, D.K.; Banerjee, S. Selective inhibition of peptidyl-arginine deiminase (PAD): Can it control multiple inflammatory disorders as a promising therapeutic strategy? Inflammopharmacology 2023, 31, 731–744. [Google Scholar] [CrossRef]
- Holthaus, M.; Xiong, X.; Eghbalzadeh, K.; Großmann, C.; Geißen, S.; Piontek, F.; Mollenhauer, M.; Abdallah, A.T.; Kamphausen, T.; Rothschild, M.; et al. Loss of peptidylarginine deiminase 4 mitigates maladaptive cardiac remodeling after myocardial infarction through inhibition of inflammatory and profibrotic pathways. Transl. Res. J. Lab. Clin. Med. 2025, 280, 1–16. [Google Scholar] [CrossRef]
- Van Bruggen, S.; Kraisin, S.; Van Wauwe, J.; Bomhals, K.; Stroobants, M.; Carai, P.; Frederix, L.; Van De Bruaene, A.; Witsch, T.; Martinod, K. Neutrophil peptidylarginine deiminase 4 is essential for detrimental age-related cardiac remodelling and dysfunction in mice. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2023, 378, 20220475. [Google Scholar] [CrossRef]
- Yang, K.; Gao, R.; Chen, H.; Hu, J.; Zhang, P.; Wei, X.; Shi, J.; Chen, Y.; Zhang, L.; Chen, J.; et al. Myocardial reperfusion injury exacerbation due to ALDH2 deficiency is mediated by neutrophil extracellular traps and prevented by leukotriene C4 inhibition. Eur. Heart J. 2024, 45, 1662–1680. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, M.; Chen, Y.; Zhou, X.; Chun, H.; Wu, D.; Park, K.H.; Cai, C.; Li, Y.; Ma, J.; Yang, Z. Humanized Monoclonal Antibody Against Citrullinated Histone H3 Attenuates Myocardial Injury and Prevents Heart Failure in Rodent Models. Biomolecules 2025, 15, 1196. https://doi.org/10.3390/biom15081196
Weber M, Chen Y, Zhou X, Chun H, Wu D, Park KH, Cai C, Li Y, Ma J, Yang Z. Humanized Monoclonal Antibody Against Citrullinated Histone H3 Attenuates Myocardial Injury and Prevents Heart Failure in Rodent Models. Biomolecules. 2025; 15(8):1196. https://doi.org/10.3390/biom15081196
Chicago/Turabian StyleWeber, Matthew, Yuchen Chen, Xinyu Zhou, Heejae Chun, Di Wu, Ki Ho Park, Chuanxi Cai, Yongqing Li, Jianjie Ma, and Zequan Yang. 2025. "Humanized Monoclonal Antibody Against Citrullinated Histone H3 Attenuates Myocardial Injury and Prevents Heart Failure in Rodent Models" Biomolecules 15, no. 8: 1196. https://doi.org/10.3390/biom15081196
APA StyleWeber, M., Chen, Y., Zhou, X., Chun, H., Wu, D., Park, K. H., Cai, C., Li, Y., Ma, J., & Yang, Z. (2025). Humanized Monoclonal Antibody Against Citrullinated Histone H3 Attenuates Myocardial Injury and Prevents Heart Failure in Rodent Models. Biomolecules, 15(8), 1196. https://doi.org/10.3390/biom15081196






