Extracellular Vesicles Derived from Breast Cancer Cells: Emerging Biomarkers of Tumor Progression and Metastasis
Abstract
1. Introduction
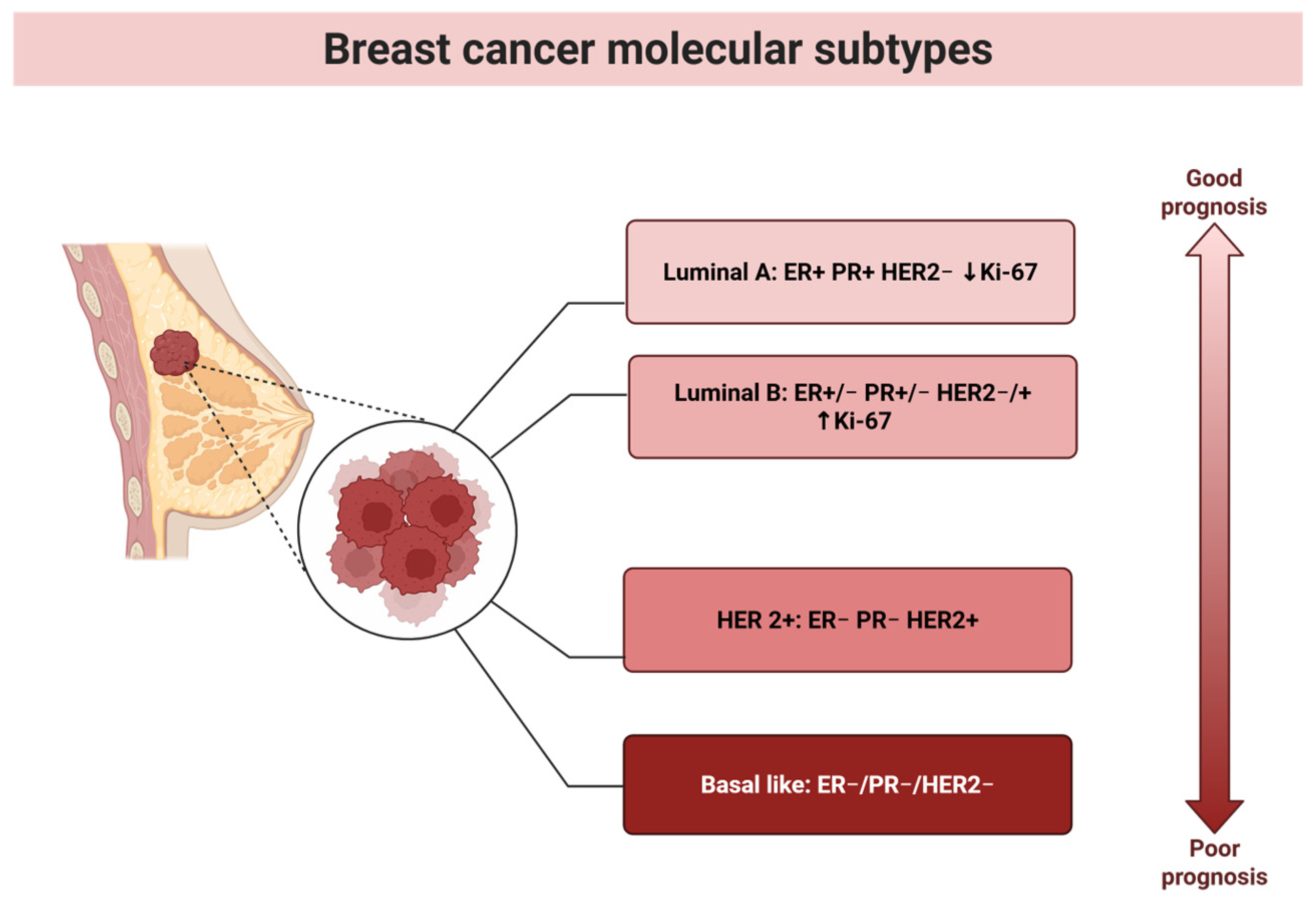
2. Breast Cancer
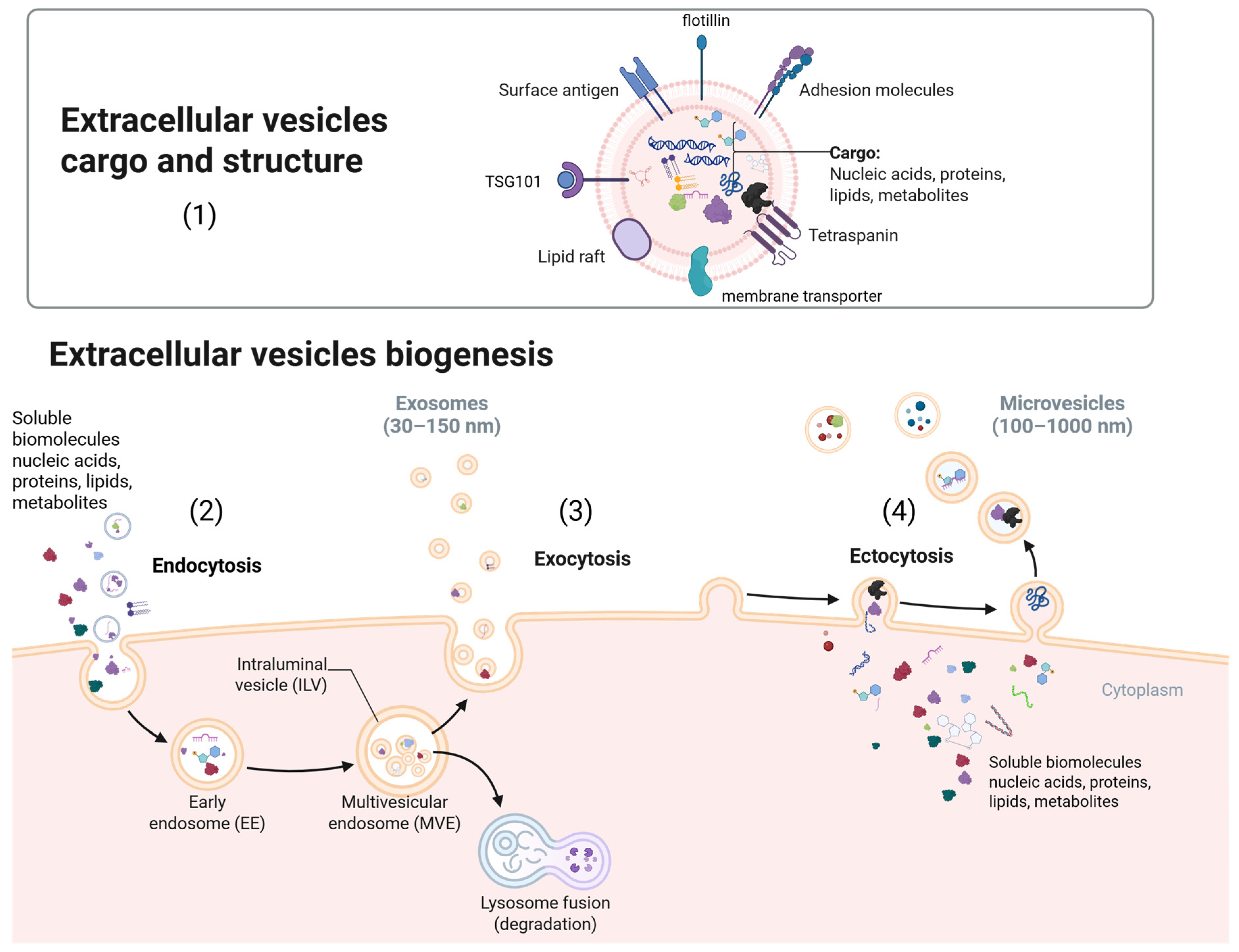
3. Extracellular Vesicles
4. Extracellular Vesicles and Cancer
4.1. RNA EV Cargo Regulating Cancer Progression and Metastasis
4.2. DNA EV Cargo and Its Presence in Cancer
4.3. Protein EV Cargo in Cancer
4.4. Lipid EV Cargo in Cancer
4.5. Metabolite EV Cargo in Cancer
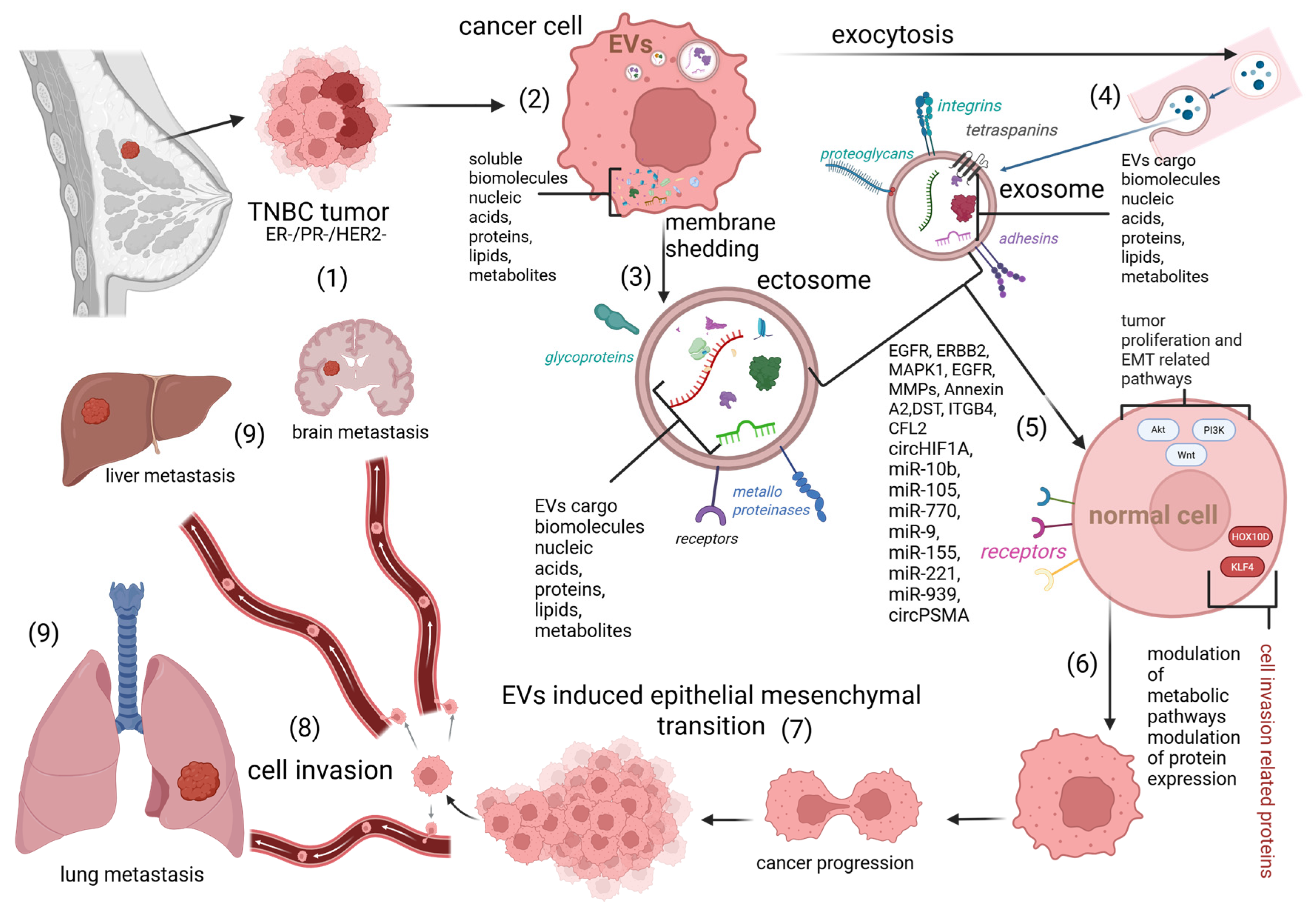
5. EVs Derived from BC Cells
5.1. TNBC
5.1.1. RNAs in TNBC EVs
| EV Cargo | TNBC | Function | References |
|---|---|---|---|
| RNA | miR-9-5p | CHL metabolism | [132] |
| RNA | miR-155-5p | Cancer initiation, WNT signaling pathway activation, tumor progression, drug resistance | [133,134,135,136] |
| RNA | miR-4516 | Inhibition of cell proliferation | [139] |
| RNA | miR-10b | Promotion of cell invasion | [141] |
| RNA | miR-105 | Metastatic and vascularization processes | [142] |
| RNA | miR-142-5p | Diagnostic potential for BC subtype differentiation | [143] |
| RNA | miR-150-5p | Diagnostic potential, downregulated in TNBC EVs compared to Luminal A | [143] |
| RNA | miR-576-3p | Prognostic marker for recurrence in TNBC | [144] |
| RNA | miR-4665-5p | Prognostic marker for recurrence in TNBC | [144] |
| RNA | miR-421-5p | Discrimination between TNBC and Luminal A patients | [143] |
| RNA | miR-100-5p, miR-21-5p, let-7f-5p, let-7i-5p, miR-486-5p, let-7a-5p, miR-92a-3p, let-7g-5p, miR-451a, miR-27b-3p | Significant expression in MDA-MB-231 cell line EVs, glucocorticoid receptor overexpression | [138] |
| RNA | let-7f-2-3p, miR-103b, miR-4742-3p, let-7a-3p, miR-505-3p, let-7f-5p, let-7i-3p, miR-22-5p, let-7b-3p, miR-196b-5p | Significant expression in MDA-MB-468 cell line EVs, glucocorticoid receptor overexpression | [138] |
| RNA | miR-770, miR-9, miR-155, miR-221, miR-939 | Metastatic processes | [140] |
| RNA | circHIF1A | Growth and metastasis | [137] |
| RNA | circPSMA | Metastatic processes | [140] |
5.1.2. DNA in TNBC EVs
- -
- Complex I: MT-ND1, ND2, ND3, ND4, ND4L, ND5, ND6;
- -
- Complex III: MT-CYTB;
- -
- Complex IV: MT-CO1, CO2, CO3;
- -
- Complex V: MT-ATP6, ATP8.
5.1.3. Proteins in TNBC EVs
5.1.4. Lipids in TNBC EVs
5.1.5. Metabolites in TNBC EVs
5.2. Luminal A
5.2.1. RNAs in Luminal A Subtype EVs
5.2.2. Proteins in Luminal A Subtype EVs
5.3. Luminal B
Proteins in Luminal B Subtype EVs
5.4. HER-2+
5.4.1. RNAs in HER-2+ Subtype EVs
5.4.2. Proteins in HER-2+ Subtype EVs
6. Conclusions
- EVs derived from BC cells have emerged as key players in tumor progression and metastasis, offering promising potential in modern diagnostics and therapeutic strategies. These vesicles, which carry diverse bioactive molecules such as RNA, DNA, proteins, lipids, and metabolites, are integral to intercellular communication and the modulation of the tumor microenvironment. EVs facilitate processes like immune evasion, angiogenesis, and metastatic niche formation, underscoring their significance in BC pathophysiology. Notably, EV cargo varies across BC subtypes, including TNBC, Luminal A, Luminal B, and HER-2+, reflecting unique molecular profiles that can be exploited for precision diagnostics and targeted therapies.
- Despite their transformative potential, EV-based liquid biopsy diagnostics for BC remain experimental and unstandardized. Current findings highlight the relevance of EV cargo in subtype-specific BC characterization, yet comprehensive references for all bioactive molecules in each subtype are limited. Advances in EV research could pave the way for non-invasive diagnostic tools, enabling early detection, subtype differentiation, and real-time monitoring of disease progression. Continued exploration of EVs and their molecular cargo holds promise for revolutionizing BC management, offering hope for improved patient outcomes through precision oncology.
- As per the current understanding of BC and extracellular vesicle EV-based potential clinical applications, we recommend the following future directions for improved diagnostics, prognostics, and therapeutics:
- (1)
- Standardized EV isolation and profiling: Developing robust and reproducible protocols for EV isolation, such as advanced microfluidic systems and immunoaffinity techniques, will enhance the consistency and scalability of EV-based diagnostics. Improved characterization methods using next-generation sequencing, mass spectrometry, and metabolomics can uncover novel subtype-specific biomarkers.
- (2)
- Integration of artificial intelligence: Employing AI and machine learning algorithms to analyze EV molecular data could accelerate biomarker discovery, improve subtype classification, and predict patient outcomes with high precision. This approach will enable the identification of complex diagnostic patterns in EV cargo.
- (3)
- Multiplex panels for subtype differentiation: Comprehensive profiling of EV cargo across BC subtypes can facilitate the creation of multiplex panels for early detection, subtype classification, and therapy monitoring. These panels could combine RNA, DNA, protein, lipid, and metabolite biomarkers to improve diagnostic accuracy.
- (4)
- Therapeutic modulation of EV biogenesis: Investigating methods to modulate EV production and cargo content could lead to novel therapeutic strategies. For example, engineering EVs for targeted drug delivery or disrupting EV-mediated oncogenic signaling pathways could reduce tumor aggressiveness and metastasis.
- (5)
- Clinical validation and trials: Expanding large-scale clinical trials to validate EV-based biomarkers and diagnostic tools is critical for translating experimental findings into clinical practice. Collaborations between research institutions, industry, and healthcare providers can accelerate the development of EV-based technologies.
- (6)
- Subtype-specific therapeutic approaches: Tailoring therapies based on the molecular cargo of EVs in specific BC subtypes, such as TNBC or HER-2+, could improve treatment efficacy. For example, targeting EVs enriched with oncogenic RNAs or proteins in TNBC could offer new therapeutic avenues.
- By addressing these research and development priorities, EVs could become central to precision oncology, transforming the landscape of BC management and improving patient outcomes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAM10 | Metalloproteinase domain-containing protein 10 |
| BC | Breast cancer |
| CAFs | Cancer-associated fibroblasts |
| CDCP1 | CUB domain-containing protein 1 |
| Cer | Ceramide |
| cfDNA | Freely circulating DNA |
| cf-mRNA | Cell-free mRNA |
| circRNA | Circular RNA |
| CRC | Colorectal cancer |
| dsDNA | Double-stranded DNA |
| ECM-receptor | Extracellular matrix-associated receptor |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial–mesenchymal transition |
| ER | Estrogen receptor |
| EVs | Extracellular vesicles |
| HER2 | Epidermal growth factor receptor 2 |
| HPV | Human papillomavirus |
| CHL | Cholesterol |
| lncRNA | Long non-coding RNA |
| LysoPC | Lysophosphatidylcholine |
| MET | Mesenchymal–epithelial transition |
| miRNA | MicroRNA |
| MISEV | Minimal information for studies of extracellular vesicles |
| MMPs | Matrix metalloproteinases |
| mRNA | Messenger RNA |
| mtDNA | Mitochondrial DNA |
| MVEs | Multivesicular endosome |
| PC | Phosphatidylcholine |
| PR | Progesterone receptor |
| PS | Phosphatidylserine |
| RCC | Renal cell carcinoma |
| sEVs | Small extracellular vesicles |
| sncRNA | Small non-coding RNA |
| THSD7A | Thrombospondin type 1 domain-containing 7A |
| TNBC | Triple negative breast cancer |
| TP53INP1 | p53-inducible nuclear protein 1 |
| tRFs | tRNA-derived fragments |
| WHO | World Health Organization |
References
- Piombino, C.; Mastrolia, I.; Omarini, C.; Candini, O.; Dominici, M.; Piacentini, F.; Toss, A. The Role of Exosomes in Breast Cancer Diagnosis. Biomedicines 2021, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, C.; Yu, J.; Bu, J.; Ai, F.; Wang, Y.; Lin, J.; Zhu, X. Extracellular Vesicles in the Treatment and Diagnosis of Breast Cancer: A Status Update. Front. Endocrinol. 2023, 14, 1202493. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, Q.; Sumrin, A.; Saleem, Y.; Wajid, A.; Mahnoor, M. Exosomes in Cancer: Diagnostic and Therapeutic Applications. Clin. Med. Insights Oncol. 2024, 18, 11795549231215966. [Google Scholar] [CrossRef]
- Loric, S.; Denis, J.A.; Desbene, C.; Sabbah, M.; Conti, M. Extracellular Vesicles in Breast Cancer: From Biology and Function to Clinical Diagnosis and Therapeutic Management. Int. J. Mol. Sci. 2023, 24, 7208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andre, M.; Caobi, A.; Miles, J.S.; Vashist, A.; Ruiz, M.A.; Raymond, A.D. Diagnostic potential of exosomal extracellular vesicles in oncology. BMC Cancer 2024, 24, 322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saadh, M.J.; Al-Rihaymee, A.M.A.; Kaur, M.; Kumar, A.; Mutee, A.F.; Ismaeel, G.L.; Shomurotova, S.; Alubiady, M.H.S.; Hamzah, H.F.; Alhassan, Z.A.A.; et al. Advancements in Exosome Proteins for Breast Cancer Diagnosis and Detection: With a Focus on Nanotechnology. AAPS PharmSciTech 2024, 25, 276. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.; Qin, F.; Chen, J. Exosomes: A Promising Avenue for Cancer Diagnosis beyond Treatment. Front. Cell Dev. Biol. 2024, 12, 1344705. [Google Scholar] [CrossRef]
- Nakase, I.; Takatani-Nakase, T. Exosomes: Breast Cancer-Derived Extracellular Vesicles; Recent Key Findings and Technologies in Disease Progression, Diagnostics, and Cancer Targeting. Drug Metab. Pharmacokinet. 2022, 42, 100435. [Google Scholar] [CrossRef]
- Chen, I.-P.; Henning, S.; Bender, M.; Degenhardt, S.; Mhamdi Ghodbani, M.; Bergmann, A.K.; Volkmer, B.; Brockhoff, G.; Wege, A.K.; Greinert, R. Detection of Human Circulating and Extracellular Vesicle-Derived miRNAs in Serum of Humanized Mice Transplanted with Human Breast Cancer (HER2+ and TNBC) Cells—A Proof of Principle Investigation. Int. J. Mol. Sci. 2025, 26, 3629. [Google Scholar] [CrossRef]
- Zayakin, P.; Sadovska, L.; Eglītis, K.; Romanchikova, N.; Radoviča-Spalviņa, I.; Endzeliņš, E.; Liepniece-Karele, I.; Eglītis, J.; Linē, A. Extracellular Vesicles—A Source of RNA Biomarkers for the Detection of Breast Cancer in Liquid Biopsies. Cancers 2023, 15, 4329. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Houghton, S.C.; Hankinson, S.E. Cancer Progress and Priorities: Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2021, 30, 822–844. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Kim, J.; Harper, A.; McCormack, V.; Sung, H.; Houssami, N.; Morgan, E.; Mutebi, M.; Garvey, G.; Soerjomataram, I.; Fidler-Benaoudia, M.M. Global Patterns and Trends in Breast Cancer Incidence and Mortality across 185 Countries. Nat. Med. 2025, 31, 1154–1162. [Google Scholar] [CrossRef]
- OECD. European Commission EU Country Cancer Profile: Slovak Republic 2025; EU Country Cancer Profiles; OECD Publishing: Paris, France, 2025. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Simpson, P.T.; Reis-Filho, J.S.; Gale, T.; Lakhani, S.R. Molecular Evolution of Breast Cancer. J. Pathol. 2005, 205, 248–254. [Google Scholar] [CrossRef]
- Reis-Filho, J.S.; Simpson, P.T.; Gale, T.; Lakhani, S.R. The Molecular Genetics of Breast Cancer: The Contribution of Com-parative Genomic Hybridization. Pathol.—Res. Pract. 2005, 201, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and Current Knowledge of Breast Cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef]
- Sun, Y.-S.; Zhao, Z.; Yang, Z.-N.; Xu, F.; Lu, H.-J.; Zhu, Z.-Y.; Shi, W.; Jiang, J.; Yao, P.-P.; Zhu, H.-P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Swanton, C.; Bernard, E.; Abbosh, C.; André, F.; Auwerx, J.; Balmain, A.; Bar-Sagi, D.; Bernards, R.; Bullman, S.; DeGregori, J.; et al. Embracing Cancer Complexity: Hallmarks of Systemic Disease. Cell 2024, 187, 1589–1616. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular Portraits of Human Breast Tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; et al. Gene Expression Patterns of Breast Carcinomas Distinguish Tumor Subclasses with Clinical Implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef]
- Parker, J.S.; Mullins, M.; Cheang, M.C.U.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised Risk Predictor of Breast Cancer Based on Intrinsic Subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.O.; Hsu, F.D.; Jensen, K.; Cheang, M.; Karaca, G.; Hu, Z.; Hernandez-Boussard, T.; Livasy, C.; Cowan, D.; Dressler, L.; et al. Immunohistochemical and Clinical Characterization of the Basal-Like Subtype of Invasive Breast Carcinoma. Clin. Cancer Res. 2004, 10, 5367–5374. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Parker, J.S.; Karginova, O.; Fan, C.; Livasy, C.; Herschkowitz, J.I.; He, X.; Perou, C.M. Phenotypic and Molecular Characterization of the Claudin-Low Intrinsic Subtype of Breast Cancer. Breast Cancer Res. 2010, 12, R68. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.H.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R.; et al. The 2019 World Health Organization Classification of Tumours of the Breast. Histopathology 2020, 77, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, M.; Vughs, J.; Noseda, M.; Emanueli, C. Exosomes: Basic Biology and Technological Advancements Suggesting Their Potential as Ischemic Heart Disease Therapeutics. Front. Physiol. 2018, 9, 1159. [Google Scholar] [CrossRef]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A Comparison of Methods for the Isolation and Separation of Extracellular Vesicles from Protein and Lipid Particles in Human Serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. BioScience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Di Vizio, D.; Morello, M.; Dudley, A.C.; Schow, P.W.; Adam, R.M.; Morley, S.; Mulholland, D.; Rotinen, M.; Hager, M.H.; Insabato, L.; et al. Large Oncosomes in Human Prostate Cancer Tissues and in the Circulation of Mice with Metastatic Disease. Am. J. Pathol. 2012, 181, 1573–1584. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicle 2024, 13, e12404. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B Lymphocytes Secrete Antigen-Presenting Vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Stegmayr, B.; Ronquist, G. Promotive Effect on Human Sperm Progressive Motility by Prostasomes. Urol. Res. 1982, 10, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicle 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicle 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current Knowledge of Their Composition, Biological Functions, and Diagnostic and Therapeutic Potentials. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2012, 1820, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Wee, I.; Syn, N.; Sethi, G.; Goh, B.C.; Wang, L. Role of Tumor-Derived Exosomes in Cancer Metastasis. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2019, 1871, 12–19. [Google Scholar] [CrossRef]
- Cocucci, E.; Meldolesi, J. Ectosomes. Curr. Biol. 2011, 21, R940–R941. [Google Scholar] [CrossRef]
- Heijnen, H.F.G.; Schiel, A.E.; Fijnheer, R.; Geuze, H.J.; Sixma, J.J. Activated Platelets Release Two Types of Membrane Vesicles: Microvesicles by Surface Shedding and Exosomes Derived From Exocytosis of Multivesicular Bodies and-Granules. Blood 1999, 94, 3791–3799. [Google Scholar] [CrossRef]
- Morello, M.; Minciacchi, V.; De Candia, P.; Yang, J.; Posadas, E.; Kim, H.; Griffiths, D.; Bhowmick, N.; Chung, L.; Gan-dellini, P.; et al. Large Oncosomes Mediate Intercellular Transfer of Functional microRNA. Cell Cycle 2013, 12, 3526–3536. [Google Scholar] [CrossRef]
- El Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular Vesicles: Biology and Emerging Therapeutic Opportunities. Nat. Rev. Drug. Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef]
- Théry, C.; Boussac, M.; Véron, P.; Ricciardi-Castagnoli, P.; Raposo, G.; Garin, J.; Amigorena, S. Proteomic Analysis of Dendritic Cell-Derived Exosomes: A Secreted Subcellular Compartment Distinct from Apoptotic Vesicles. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef]
- Greco, V.; Hannus, M.; Eaton, S. Argosomes: A potential vehicle for the spread of morphogens through epithelia. Cell 2001, 106, 633–645. [Google Scholar] [CrossRef]
- Borges, F.T.; Reis, L.A.; Schor, N. Extracellular Vesicles: Structure, Function, and Potential Clinical Uses in Renal Diseases. Braz. J. Med. Biol. Res. 2013, 46, 824–830. [Google Scholar] [CrossRef]
- Wickman, G.; Julian, L.; Olson, M.F. How Apoptotic Cells Aid in the Removal of Their Own Cold Dead Bodies. Cell Death Differ. 2012, 19, 735–742. [Google Scholar] [CrossRef]
- Karlsson, M.; Lundin, S.; Dahlgren, U.; Kahu, H.; Pettersson, I.; Telemo, E. “Tolerosomes” Are Produced by Intestinal Epithelial Cells. Eur. J. Immunol. 2001, 31, 2892–2900. [Google Scholar] [CrossRef] [PubMed]
- Rivett, A.J. Proteasomes: Multicatalytic Proteinase Complexes. Biochem. J. 1993, 291, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Etlinger, J.D.; Goldberg, A.L. A Soluble ATP-Dependent Proteolytic System Responsible for the Degradation of Abnormal Proteins in Reticulocytes. Proc. Natl. Acad. Sci. USA 1977, 74, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Karbanová, J.; Thamm, K.; Fargeas, C.A.; Deniz, I.A.; Lorico, A.; Corbeil, D. Prominosomes—A Particular Class of Extracellular Vesicles Containing Prominin-1/CD133? J. Nanobiotechnol. 2025, 23, 61. [Google Scholar] [CrossRef]
- Huttner, H.B.; Janich, P.; Köhrmann, M.; Jászai, J.; Siebzehnrubl, F.; Blümcke, I.; Suttorp, M.; Gahr, M.; Kuhnt, D.; Nimsky, C.; et al. The Stem Cell Marker Prominin-1/CD133 on Membrane Particles in Human Cerebrospinal Fluid Offers Novel Approaches for Studying Central Nervous System Disease. Stem Cells 2008, 26, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.M.; Luzio, J.P. Ectocytosis Caused by Sublytic Autologous Complement Attack on Human Neutrophils. The Sorting of Endogenous Plasma-Membrane Proteins and Lipids into Shed Vesicles. Biochem. J. 1991, 274, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Cocucci, E.; Meldolesi, J. Ectosomes and Exosomes: Shedding the Confusion between Extracellular Vesicles. Trends Cell Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular Organelles Important in Intercellular Communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Kooijmans, S.A.A.; De Jong, O.G.; Schiffelers, R.M. Exploring Interactions between Extracellular Vesicles and Cells for Innovative Drug Delivery System Design. Adv. Drug Deliv. Rev. 2021, 173, 252–278. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiao, Z.; Zhu, W. The Roles of Small Extracellular Vesicles as Prognostic Biomarkers and Treatment Approaches in Triple-Negative Breast Cancer. Front. Oncol. 2022, 12, 998964. [Google Scholar] [CrossRef]
- H. Rashed, M.; Bayraktar, E.; Helal, G.K.; Abd-Ellah, M.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 538. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracel-lular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Meldolesi, J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef]
- Chang, W.-H.; Cerione, R.A.; Antonyak, M.A. Extracellular Vesicles and Their Roles in Cancer Progression. In Cancer Cell Signaling; Robles-Flores, M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; Volume 2174, pp. 143–170. ISBN 978-1-0716-0758-9. [Google Scholar]
- Kalluri, R.; McAndrews, K.M. The Role of Extracellular Vesicles in Cancer. Cell 2023, 186, 1610–1626. [Google Scholar] [CrossRef]
- Lopez, K.; Lai, S.W.T.; Lopez Gonzalez, E.D.J.; Dávila, R.G.; Shuck, S.C. Extracellular Vesicles: A Dive into Their Role in the Tumor Microenvironment and Cancer Progression. Front. Cell Dev. Biol. 2023, 11, 1154576. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Li, J.; Li, L.; Sun, J.; Fabbri, M.; Wayne, A.S.; Seeger, R.C.; Jong, A.Y. Extracellular Vesicles Derived from Natural Killer Cells Use Multiple Cytotoxic Proteins and Killing Mechanisms to Target Cancer Cells. J. Extracell. Vesicle 2019, 8, 1588538. [Google Scholar] [CrossRef]
- Cianciaruso, C.; Phelps, E.A.; Pasquier, M.; Hamelin, R.; Demurtas, D.; Alibashe Ahmed, M.; Piemonti, L.; Hirosue, S.; Swartz, M.A.; De Palma, M.; et al. Primary Human and Rat β-Cells Release the Intracellular Autoantigens GAD65, IA-2, and Proinsulin in Exosomes Together With Cytokine-Induced Enhancers of Immunity. Diabetes 2017, 66, 460–473. [Google Scholar] [CrossRef]
- Hánělová, K.; Raudenská, M.; Masařík, M.; Balvan, J. Protein Cargo in Extracellular Vesicles as the Key Mediator in the Progression of Cancer. Cell Commun. Signal. 2024, 22, 25. [Google Scholar] [CrossRef]
- Escola, J.-M.; Kleijmeer, M.J.; Stoorvogel, W.; Griffith, J.M.; Yoshie, O.; Geuze, H.J. Selective Enrichment of Tetraspan Proteins on the Internal Vesicles of Multivesicular Endosomes and on Exosomes Secreted by Human B-Lymphocytes. J. Biol. Chem. 1998, 273, 20121–20127. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of mRNAs and mi-croRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic Stem Cell-Derived Mi-crovesicles Reprogram Hematopoietic Progenitors: Evidence for Horizontal Transfer of mRNA and Protein Delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef]
- Fyfe, J.; Casari, I.; Manfredi, M.; Falasca, M. Role of Lipid Signalling in Extracellular Vesicles-Mediated Cell-to-Cell Com-munication. Cytokine Growth Factor Rev. 2023, 73, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Guo, W.; Chen, B.; Chen, L.; Gong, J.; Li, W. Tumor-released lncRNA H19 Promotes Gefitinib Resistance via Packaging into Exosomes in Non-small Cell Lung Cancer. Oncol. Rep. 2018, 40, 3034–3446. [Google Scholar] [CrossRef]
- La Camera, G.; Gelsomino, L.; Caruso, A.; Panza, S.; Barone, I.; Bonofiglio, D.; Andò, S.; Giordano, C.; Catalano, S. The Emerging Role of Extracellular Vesicles in Endocrine Resistant Breast Cancer. Cancers 2021, 13, 1160. [Google Scholar] [CrossRef]
- Wang, Z.; Kim, S.Y.; Tu, W.; Kim, J.; Xu, A.; Yang, Y.M.; Matsuda, M.; Reolizo, L.; Tsuchiya, T.; Billet, S.; et al. Extracellular Vesicles in Fatty Liver Promote a Metastatic Tumor Microenvironment. Cell Metab. 2023, 35, 1209–1226.e13. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Zhang, Q.; Xu, P.; Tao, W.; Lin, F.; Liu, R.; Li, M.; Duan, X.; Cai, C.; Gu, D.; et al. Extracellular Vesicle-circEHD2 Promotes the Progression of Renal Cell Carcinoma by Activating Cancer-Associated Fibroblasts. Mol. Cancer 2023, 22, 117. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A. Inflammation by Remote Control. Nature 2005, 435, 752–753. [Google Scholar] [CrossRef]
- Bonjoch, L.; Casas, V.; Carrascal, M.; Closa, D. Involvement of Exosomes in Lung Inflammation Associated with Experimental Acute Pancreatitis: Exosomes in Acute Pancreatitis. J. Pathol. 2016, 240, 235–245. [Google Scholar] [CrossRef]
- Choi, H.-I.; Choi, J.-P.; Seo, J.; Kim, B.J.; Rho, M.; Han, J.K.; Kim, J.G. Helicobacter Pylori-Derived Extracellular Vesicles Increased in the Gastric Juices of Gastric Adenocarcinoma Patients and Induced Inflammation Mainly via Specific Targeting of Gastric Epithelial Cells. Exp. Mol. Med. 2017, 49, e330. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, H.; Luo, Y.; Lin, Y.; An, M.; Kong, Y.; Zhao, Y.; Yin, Y.; Ai, L.; Huang, J.; et al. An HGF-Dependent Positive Feedback Loop between Bladder Cancer Cells and Fibroblasts Mediates Lymphangiogenesis and Lymphatic Metastasis. Cancer Commun. 2023, 43, 1289–1311. [Google Scholar] [CrossRef]
- Zhao, S.; Mi, Y.; Zheng, B.; Wei, P.; Gu, Y.; Zhang, Z.; Xu, Y.; Cai, S.; Li, X.; Li, D. Highly-metastatic Colorectal Cancer Cell Released miR-181a-5p-rich Extracellular Vesicles Promote Liver Metastasis by Activating Hepatic Stellate Cells and Remodelling the Tumour Microenvironment. J. Extracell. Vesicle 2022, 11, e12186. [Google Scholar] [CrossRef]
- Luo, Z.; Mei, J.; Wang, X.; Wang, R.; He, Z.; Geffen, Y.; Sun, X.; Zhang, X.; Xu, J.; Wan, R.; et al. Voluntary Exercise Sensi-tizes Cancer Immunotherapy via the Collagen Inhibition-Orchestrated Inflammatory Tumor Immune Microenvironment. Cell Rep. 2024, 43, 114697. [Google Scholar] [CrossRef]
- Kim, H.J.; Rames, M.J.; Goncalves, F.; Kirschbaum, C.W.; Roskams-Hieter, B.; Spiliotopoulos, E.; Briand, J.; Doe, A.; Es-tabrook, J.; Wagner, J.T.; et al. Selective Enrichment of Plasma Cell-Free Messenger RNA in Cancer-Associated Extracellular Vesicles. Commun. Biol. 2023, 6, 885. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Nakayama, J.; Urabe, F.; Ito, K.; Nishida-Aoki, N.; Kitagawa, M.; Yokoi, A.; Kuroda, M.; Hattori, Y.; Yamamoto, Y.; et al. Aberrant Regulation of Serine Metabolism Drives Extracellular Vesicle Release and Cancer Progression. Cell Rep. 2024, 43, 114517. [Google Scholar] [CrossRef]
- Zhang, P.; Lim, S.B.; Jiang, K.; Chew, T.W.; Low, B.C.; Lim, C.T. Distinct mRNAs in Cancer Extracellular Vesicles Activate Angiogenesis and Alter Transcriptome of Vascular Endothelial Cells. Cancers 2021, 13, 2009. [Google Scholar] [CrossRef]
- Zhu, L.; Li, J.; Gong, Y.; Wu, Q.; Tan, S.; Sun, D.; Xu, X.; Zuo, Y.; Zhao, Y.; Wei, Y.-Q.; et al. Exosomal tRNA-Derived Small RNA as a Promising Biomarker for Cancer Diagnosis. Mol. Cancer 2019, 18, 74. [Google Scholar] [CrossRef]
- Kumar, S.R.; Kimchi, E.T.; Manjunath, Y.; Gajagowni, S.; Stuckel, A.J.; Kaifi, J.T. RNA Cargos in Extracellular Vesicles Derived from Blood Serum in Pancreas Associated Conditions. Sci. Rep. 2020, 10, 2800. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, Y.; Han, Y.; Zhang, Q.; Jiang, Z.; Zhang, X.; Huang, B.; Xu, X.; Zheng, J.; Cao, X. Tumor Exosomal RNAs Promote Lung Pre-Metastatic Niche Formation by Activating Alveolar Epithelial TLR3 to Recruit Neutrophils. Cancer Cell 2016, 30, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Driedonks, T.A.P.; Van Der Grein, S.G.; Ariyurek, Y.; Buermans, H.P.J.; Jekel, H.; Chow, F.W.N.; Wauben, M.H.M.; Buck, A.H.; Hoen, P.A.C.‘t.; Nolte-‘t Hoen, E.N.M. Immune Stimuli Shape the Small Non-Coding Transcriptome of Extracellular Vesi-cles Released by Dendritic Cells. Cell. Mol. Life Sci. 2018, 75, 3857–3875. [Google Scholar] [CrossRef] [PubMed]
- Sansone, P.; Savini, C.; Kurelac, I.; Chang, Q.; Amato, L.B.; Strillacci, A.; Stepanova, A.; Iommarini, L.; Mastroleo, C.; Daly, L.; et al. Packaging and Transfer of Mitochondrial DNA via Exosomes Regulate Escape from Dormancy in Hormonal Thera-py-Resistant Breast Cancer. Proc. Natl. Acad. Sci. USA 2017, 114, E9066–E9075. [Google Scholar] [CrossRef]
- Rabas, N.; Palmer, S.; Mitchell, L.; Ismail, S.; Gohlke, A.; Riley, J.S.; Tait, S.W.G.; Gammage, P.; Soares, L.L.; Macpherson, I.R.; et al. PINK1 Drives Production of mtDNA-Containing Extracellular Vesicles to Promote Invasiveness. J. Cell Biol. 2021, 220, e202006049. [Google Scholar] [CrossRef]
- Peng, Q.; Chiu, P.K.-F.; Wong, C.Y.-P.; Cheng, C.K.-L.; Teoh, J.Y.-C.; Ng, C.-F. Identification of piRNA Targets in Urinary Extracellular Vesicles for the Diagnosis of Prostate Cancer. Diagnostics 2021, 11, 1828. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Y.; Zhao, S.; Gao, J.; Hao, X.; Wang, Z.; Li, M.; Wang, M.; Liu, Y.; Yu, X.; et al. Serum-Derived piR-Hsa-164586 of Extracellular Vesicles as a Novel Biomarker for Early Diagnosis of Non-Small Cell Lung Cancer. Front. Oncol. 2022, 12, 850363. [Google Scholar] [CrossRef] [PubMed]
- Chakrabortty, S.K.; Prakash, A.; Nechooshtan, G.; Hearn, S.; Gingeras, T.R. Extracellular Vesicle-Mediated Transfer of Processed and Functional RNY5 RNA. RNA 2015, 21, 1966–1979. [Google Scholar] [CrossRef] [PubMed]
- Balaj, L.; Lessard, R.; Dai, L.; Cho, Y.-J.; Pomeroy, S.L.; Breakefield, X.O.; Skog, J. Tumour Microvesicles Contain Re-trotransposon Elements and Amplified Oncogene Sequences. Nat. Commun. 2011, 2, 180. [Google Scholar] [CrossRef]
- Adashek, J.J.; Janku, F.; Kurzrock, R. Signed in Blood: Circulating Tumor DNA in Cancer Diagnosis, Treatment and Screening. Cancers 2021, 13, 3600. [Google Scholar] [CrossRef]
- Zocco, D.; Bernardi, S.; Novelli, M.; Astrua, C.; Fava, P.; Zarovni, N.; Carpi, F.M.; Bianciardi, L.; Malavenda, O.; Quaglino, P.; et al. Isolation of Extracellular Vesicles Improves the Detection of Mutant DNA from Plasma of Metastatic Melanoma Patients. Sci. Rep. 2020, 10, 15745. [Google Scholar] [CrossRef]
- Castellanos-Rizaldos, E.; Grimm, D.G.; Tadigotla, V.; Hurley, J.; Healy, J.; Neal, P.L.; Sher, M.; Venkatesan, R.; Karlovich, C.; Raponi, M.; et al. Exosome-Based Detection of EGFR T790M in Plasma from Non–Small Cell Lung Cancer Patients. Clin. Cancer Res. 2018, 24, 2944–2950. [Google Scholar] [CrossRef]
- Tutanov, O.; Shtam, T.; Grigor’eva, A.; Tupikin, A.; Tsentalovich, Y.; Tamkovich, S. Blood Plasma Exosomes Contain Cir-culating DNA in Their Crown. Diagnostics 2022, 12, 854. [Google Scholar] [CrossRef]
- Cui, S.; Cheng, Z.; Qin, W.; Jiang, L. Exosomes as a Liquid Biopsy for Lung Cancer. Lung Cancer 2018, 116, 46–54. [Google Scholar] [CrossRef]
- Clancy, J.W.; Sheehan, C.S.; Boomgarden, A.C.; D’Souza-Schorey, C. Recruitment of DNA to Tumor-Derived Microvesicles. Cell Rep. 2022, 38, 110443. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Chennakrishnaiah, S.; Audemard, E.; Montermini, L.; Meehan, B.; Rak, J. Oncogenic Ras-Driven Cancer Cell Vesiculation Leads to Emission of Double-Stranded DNA Capable of Interacting with Target Cells. Biochem. Biophys. Res. Commun. 2014, 451, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, C.; Melo, S.A.; Protopopov, A.; Tang, J.; Seth, S.; Koch, M.; Zhang, J.; Weitz, J.; Chin, L.; Futreal, A.; et al. Identi-fication of Double-Stranded Genomic DNA Spanning All Chromosomes with Mutated KRAS and P53 DNA in the Serum Exo-somes of Patients with Pancreatic Cancer. J. Biol. Chem. 2014, 289, 3869–3875. [Google Scholar] [CrossRef] [PubMed]
- Guescini, M.; Genedani, S.; Stocchi, V.; Agnati, L.F. Astrocytes and Glioblastoma Cells Release Exosomes Carrying mtDNA. J. Neural Transm. 2010, 117, 1–4. [Google Scholar] [CrossRef]
- Urabe, F.; Kosaka, N.; Yamamoto, Y.; Ito, K.; Otsuka, K.; Soekmadji, C.; Egawa, S.; Kimura, T.; Ochiya, T. Metastatic Prostate Cancer-derived Extracellular Vesicles Facilitate Osteoclastogenesis by Transferring the CDCP1 Protein. J. Extracell. Vesicle 2023, 12, 12312. [Google Scholar] [CrossRef]
- Hüser, L.; Chhabra, Y.; Gololobova, O.; Wang, V.; Liu, G.; Dixit, A.; Rocha, M.R.; Harper, E.I.; Fane, M.E.; Marino-Bravante, G.E.; et al. Aged Fibroblast-Derived Extracellular Vesicles Promote Angiogenesis in Melanoma. Cell Rep. 2024, 43, 114721. [Google Scholar] [CrossRef] [PubMed]
- McAtee, C.; Patel, M.; Hoshino, D.; Sung, B.H.; Von Lersner, A.; Shi, M.; Hong, N.H.; Young, A.; Krystofiak, E.; Zijlstra, A.; et al. Secreted Exosomes Induce Filopodia Formation. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.; et al. Melanoma Exosomes Educate Bone Marrow Progenitor Cells toward a Pro-Metastatic Phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Mears, R.; Craven, R.A.; Hanrahan, S.; Totty, N.; Upton, C.; Young, S.L.; Patel, P.; Selby, P.J.; Banks, R.E. Proteomic Analysis of Melanoma-derived Exosomes by Two-dimensional Polyacrylamide Gel Electrophoresis and Mass Spectrometry. Proteomics 2004, 4, 4019–4031. [Google Scholar] [CrossRef]
- Staubach, S.; Razawi, H.; Hanisch, F. Proteomics of MUC1-containing Lipid Rafts from Plasma Membranes and Exosomes of Human Breast Carcinoma Cells MCF-7. Proteomics 2009, 9, 2820–2835. [Google Scholar] [CrossRef]
- Ghadami, S.; Dellinger, K. The Lipid Composition of Extracellular Vesicles: Applications in Diagnostics and Therapeutic Delivery. Front. Mol. Biosci. 2023, 10, 1198044. [Google Scholar] [CrossRef]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An Emerging Focus on Lipids in Extracellular Vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Subra, C.; Grand, D.; Laulagnier, K.; Stella, A.; Lambeau, G.; Paillasse, M.; De Medina, P.; Monsarrat, B.; Perret, B.; Sil-vente-Poirot, S.; et al. Exosomes Account for Vesicle-Mediated Transcellular Transport of Activatable Phospholipases and Pros-taglandins. J. Lipid Res. 2010, 51, 2105–2120. [Google Scholar] [CrossRef]
- Record, M.; Carayon, K.; Poirot, M.; Silvente-Poirot, S. Exosomes as New Vesicular Lipid Transporters Involved in Cell–Cell Communication and Various Pathophysiologies. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2014, 1841, 108–120. [Google Scholar] [CrossRef]
- Keller, S.; Ridinger, J.; Rupp, A.-K.; Janssen, J.W.; Altevogt, P. Body Fluid Derived Exosomes as a Novel Template for Clinical Diagnostics. J. Transl. Med. 2011, 9, 86. [Google Scholar] [CrossRef]
- Zakharova, L.; Svetlova, M.; Fomina, A.F. T Cell Exosomes Induce Cholesterol Accumulation in Human Monocytes via Phosphatidylserine Receptor. J. Cell. Physiol. 2007, 212, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Donoso-Quezada, J.; Ayala-Mar, S.; González-Valdez, J. The Role of Lipids in Exosome Biology and Intercellular Commu-nication: Function, Analytics and Applications. Traffic 2021, 22, 204–220. [Google Scholar] [CrossRef]
- Skotland, T.; Ekroos, K.; Kauhanen, D.; Simolin, H.; Seierstad, T.; Berge, V.; Sandvig, K.; Llorente, A. Molecular Lipid Species in Urinary Exosomes as Potential Prostate Cancer Biomarkers. Eur. J. Cancer 2017, 70, 122–132. [Google Scholar] [CrossRef]
- Hamed, M.A.; Wasinger, V.; Wang, Q.; Graham, P.; Malouf, D.; Bucci, J.; Li, Y. Prostate Cancer-Derived Extracellular Vesicles Metabolic Biomarkers: Emerging Roles for Diagnosis and Prognosis. J. Control. Release 2024, 371, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, M.; Kurczyk, A.; Jelonek, K.; Żyła, J.; Mielańczyk, Ł.; Sitkiewicz, M.; Pietrowska, M.; Polańska, J.; Rzyman, W.; Widłak, P. The Lipid Composition of Serum-Derived Small Extracellular Vesicles in Participants of a Lung Cancer Screening Study. Cancers 2021, 13, 3414. [Google Scholar] [CrossRef]
- Del Boccio, P.; Raimondo, F.; Pieragostino, D.; Morosi, L.; Cozzi, G.; Sacchetta, P.; Magni, F.; Pitto, M.; Urbani, A. A Hyphenated microLC-Q-TOF-MS Platform for Exosomal Lipidomics Investigations: Application to RCC Urinary Exosomes. Electrophoresis 2012, 33, 689–696. [Google Scholar] [CrossRef]
- Palviainen, M.; Laukkanen, K.; Tavukcuoglu, Z.; Velagapudi, V.; Kärkkäinen, O.; Hanhineva, K.; Auriola, S.; Ranki, A.; Siljander, P. Cancer Alters the Metabolic Fingerprint of Extracellular Vesicles. Cancers 2020, 12, 3292. [Google Scholar] [CrossRef]
- Tadokoro, H.; Hirayama, A.; Kudo, R.; Hasebe, M.; Yoshioka, Y.; Matsuzaki, J.; Yamamoto, Y.; Sugimoto, M.; Soga, T.; Ochiya, T. Adenosine Leakage from Perforin-Burst Extracellular Vesicles Inhibits Perforin Secretion by Cytotoxic T-Lymphocytes. PLoS ONE 2020, 15, e0231430. [Google Scholar] [CrossRef]
- Hamed, M.A.; Wasinger, V.; Wang, Q.; Biazik, J.; Graham, P.; Malouf, D.; Bucci, J.; Li, Y. Optimising Extracellular Vesicle Metabolomic Methodology for Prostate Cancer Biomarker Discovery. Metabolites 2024, 14, 367. [Google Scholar] [CrossRef] [PubMed]
- Romani, R.; Talesa, V.N.; Antognelli, C. The Glyoxalase System Is a Novel Cargo of Amniotic Fluid Stem-Cell-Derived Extracellular Vesicles. Antioxidants 2022, 11, 1524. [Google Scholar] [CrossRef]
- Mc Cluskey, M.; Dubouchaud, H.; Nicot, A.-S.; Saudou, F. A Vesicular Warburg Effect: Aerobic Glycolysis Occurs on Axonal Vesicles for Local NAD+ Recycling and Transport. Traffic 2024, 25, e12926. [Google Scholar] [CrossRef]
- Xu, K.Y.; Becker, L.C. Ultrastructural Localization of Glycolytic Enzymes on Sarcoplasmic Reticulum Vesticles. J. Histochem. Cytochem. 1998, 46, 419–427. [Google Scholar] [CrossRef]
- Clos-Garcia, M.; Loizaga-Iriarte, A.; Zuñiga-Garcia, P.; Sánchez-Mosquera, P.; Rosa Cortazar, A.; González, E.; Torrano, V.; Alonso, C.; Pérez-Cormenzana, M.; Ugalde-Olano, A.; et al. Metabolic Alterations in Urine Extracellular Vesicles Are Associated to Prostate Cancer Pathogenesis and Progression. J. Extracell. Vesicle 2018, 7, 1470442. [Google Scholar] [CrossRef]
- Ludwig, N.; Gillespie, D.G.; Reichert, T.E.; Jackson, E.K.; Whiteside, T.L. Purine Metabolites in Tumor-Derived Exosomes May Facilitate Immune Escape of Head and Neck Squamous Cell Carcinoma. Cancers 2020, 12, 1602. [Google Scholar] [CrossRef]
- Ying, X.; Zheng, X.; Zhang, X.; Yin, Y.; Wang, X. Kynurenine in IDO1high Cancer Cell-Derived Extracellular Vesicles Promotes Angiogenesis by Inducing Endothelial Mitophagy in Ovarian Cancer. J. Transl. Med. 2024, 22, 267. [Google Scholar] [CrossRef]
- Su, Y.; Li, Y.; Guo, R.; Zhao, J.; Chi, W.; Lai, H.; Wang, J.; Wang, Z.; Li, L.; Sang, Y.; et al. Plasma Extracellular Vesicle Long RNA Profiles in the Diagnosis and Prediction of Treatment Response for Breast Cancer. npj Breast Cancer 2021, 7, 154. [Google Scholar] [CrossRef]
- Bhullar, A.S.; Jin, K.; Shi, H.; Jones, A.; Hironaka, D.; Xiong, G.; Xu, R.; Guo, P.; Binzel, D.W.; Shu, D. Engineered Extra-cellular Vesicles for Combinatorial TNBC Therapy: SR-SIM-Guided Design Achieves Substantial Drug Dosage Reduction. Mol. Ther. 2024, 32, 4467–4481. [Google Scholar] [CrossRef]
- Eroles, P.; Bosch, A.; Alejandro Pérez-Fidalgo, J.; Lluch, A. Molecular Biology in Breast Cancer: Intrinsic Subtypes and Signaling Pathways. Cancer Treat. Rev. 2012, 38, 698–707. [Google Scholar] [CrossRef]
- Li, M.-X.; Hu, S.; Lei, H.-H.; Yuan, M.; Li, X.; Hou, W.-K.; Huang, X.-J.; Xiao, B.-W.; Yu, T.-X.; Zhang, X.-H.; et al. Tu-mor-Derived miR-9-5p-Loaded EVs Regulate Cholesterol Homeostasis to Promote Breast Cancer Liver Metastasis in Mice. Nat. Commun. 2024, 15, 10539. [Google Scholar] [CrossRef]
- Yi, Y.; Wu, M.; Zeng, H.; Hu, W.; Zhao, C.; Xiong, M.; Lv, W.; Deng, P.; Zhang, Q.; Wu, Y. Tumor-Derived Exosomal Non-Coding RNAs: The Emerging Mechanisms and Potential Clinical Applications in Breast Cancer. Front. Oncol. 2021, 11, 738945. [Google Scholar] [CrossRef]
- Ozawa, P.M.M.; Alkhilaiwi, F.; Cavalli, I.J.; Malheiros, D.; de Souza Fonseca Ribeiro, E.M.; Cavalli, L.R. Extracellular Vesicles from Triple-Negative Breast Cancer Cells Promote Proliferation and Drug Resistance in Non-Tumorigenic Breast Cells. Breast Cancer Res. Treat. 2018, 172, 713–723. [Google Scholar] [CrossRef]
- Seillier, M.; Peuget, S.; Gayet, O.; Gauthier, C.; N’Guessan, P.; Monte, M.; Carrier, A.; Iovanna, J.L.; Dusetti, N.J. TP53INP1, a Tumor Suppressor, Interacts with LC3 and ATG8-Family Proteins through the LC3-Interacting Region (LIR) and Promotes Autophagy-Dependent Cell Death. Cell Death Differ. 2012, 19, 1525–1535. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Dong, Z.; Xu, H.; Yan, L.; Wang, W.; Yang, Q.; Chen, C. MicroRNA-155-5p Promotes Tumor Progression and Contributes to Paclitaxel Resistance via TP53INP1 in Human Breast Cancer. Pathol.—Res. Pract. 2021, 220, 153405. [Google Scholar] [CrossRef]
- Chen, T.; Wang, X.; Li, C.; Zhang, H.; Liu, Y.; Han, D.; Li, Y.; Li, Z.; Luo, D.; Zhang, N.; et al. CircHIF1A Regulated by FUS Accelerates Triple-Negative Breast Cancer Progression by Modulating NFIB Expression and Translocation. Oncogene 2021, 40, 2756–2771. [Google Scholar] [CrossRef]
- Buschmann, D.; González, R.; Kirchner, B.; Mazzone, C.; Pfaffl, M.W.; Schelling, G.; Steinlein, O.; Reithmair, M. Glucocor-ticoid Receptor Overexpression Slightly Shifts microRNA Expression Patterns in Triple-Negative Breast Cancer. Int. J. Oncol. 2018, 52, 1765–1776. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, B.G.; Jang, Y.; Kang, S.; Lee, J.H.; Cho, N.H. The Stromal Loss of miR-4516 Promotes the FOSL1-Dependent Proliferation and Malignancy of Triple Negative Breast Cancer. Cancer Lett. 2020, 469, 256–265. [Google Scholar] [CrossRef]
- Dong, M.; Liu, Q.; Xu, Y.; Zhang, Q. Extracellular Vesicles: The Landscape in the Progression, Diagnosis, and Treatment of Triple-Negative Breast Cancer. Front. Cell Dev. Biol. 2022, 10, 842898. [Google Scholar] [CrossRef]
- Singh, R.; Pochampally, R.; Watabe, K.; Lu, Z.; Mo, Y.-Y. Exosome-Mediated Transfer of miR-10b Promotes Cell Invasion in Breast Cancer. Mol. Cancer 2014, 13, 256. [Google Scholar] [CrossRef]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.F.; Chin, A.R.; et al. Cancer-Secreted miR-105 Destroys Vascular Endothelial Barriers to Promote Metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef]
- Ozawa, P.M.M.; Vieira, E.; Lemos, D.S.; Souza, I.L.M.; Zanata, S.M.; Pankievicz, V.C.; Tuleski, T.R.; Souza, E.M.; Wowk, P.F.; Urban, C.D.A.; et al. Identification of miRNAs Enriched in Extracellular Vesicles Derived from Serum Samples of Breast Cancer Patients. Biomolecules 2020, 10, 150. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Q.; Zhong, H.; Li, L.; Zhang, Q.; Huang, Q.; Yu, Z. Differentially Expressed microRNAs in Exosomes of Patients with Breast Cancer Revealed by Next-generation Sequencing. Oncol. Rep. 2019, 43, 240–250. [Google Scholar] [CrossRef]
- Vikramdeo, K.S.; Anand, S.; Sudan, S.K.; Pramanik, P.; Singh, S.; Godwin, A.K.; Singh, A.P.; Dasgupta, S. Profiling Mitochondrial DNA Mutations in Tumors and Circulating Extracellular Vesicles of Triple-negative Breast Cancer Patients for Potential Biomarker Development. FASEB BioAdvances 2023, 5, 412–426. [Google Scholar] [CrossRef]
- Abad, E.; Lyakhovich, A. Movement of Mitochondria with Mutant DNA through Extracellular Vesicles Helps Cancer Cells Acquire Chemoresistance. ChemMedChem 2022, 17, e202100642. [Google Scholar] [CrossRef]
- De Carolis, S.; Storci, G.; Ceccarelli, C.; Savini, C.; Gallucci, L.; Sansone, P.; Santini, D.; Seracchioli, R.; Taffurelli, M.; Fabbri, F.; et al. HPV DNA Associates With Breast Cancer Malignancy and It Is Transferred to Breast Cancer Stromal Cells by Extracellular Vesicles. Front. Oncol. 2019, 9, 860. [Google Scholar] [CrossRef]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, Biogenesis, and Mechanisms in Cancer Metastasis and Drug Resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef]
- Raiter, A.; Lipovetsky, J.; Stenbac, A.; Lubin, I.; Yerushalmi, R. TNBC-Derived Gal3BP/Gal3 Complex Induces Immuno-suppression through CD45 Receptor. Oncoimmunology 2023, 12, 2246322. [Google Scholar] [CrossRef]
- Desai, P.P.; Narra, K.; James, J.D.; Jones, H.P.; Tripathi, A.K.; Vishwanatha, J.K. Combination of Small Extracellular Vesi-cle-Derived Annexin A2 Protein and mRNA as a Potential Predictive Biomarker for Chemotherapy Responsiveness in Aggressive Triple-Negative Breast Cancer. Cancers 2022, 15, 212. [Google Scholar] [CrossRef]
- Kang, S.Y.; Lee, E.J.; Byun, J.W.; Han, D.; Choi, Y.; Hwang, D.W.; Lee, D.S. Extracellular Vesicles Induce an Aggressive Phenotype in Luminal Breast Cancer Cells Via PKM2 Phosphorylation. Front. Oncol. 2021, 11, 785450. [Google Scholar] [CrossRef]
- Risha, Y.; Minic, Z.; Ghobadloo, S.M.; Berezovski, M.V. The Proteomic Analysis of Breast Cell Line Exosomes Reveals Disease Patterns and Potential Biomarkers. Sci. Rep. 2020, 10, 13572. [Google Scholar] [CrossRef]
- Xu, G.; Huang, R.; Wumaier, R.; Lyu, J.; Huang, M.; Zhang, Y.; Chen, Q.; Liu, W.; Tao, M.; Li, J.; et al. Proteomic Profiling of Serum Extracellular Vesicles Identifies Diagnostic Signatures and Therapeutic Targets in Breast Cancer. Cancer Res. 2024, 84, 3267–3285. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liebenberg, K.; Shen, Y.; Liu, F.; Xu, Z.; Hao, X.; Wu, L.; Zhang, W.; Chan, H.L.; Wei, B.; et al. Tumor-Derived Arachidonic Acid Reprograms Neutrophils to Promote Immune Suppression and Therapy Resistance in Triple-Negative Breast Cancer. Immunity 2025, 58, 909–925.e7. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, R.; Hüttmann, N.; Minic, Z.; Berezovski, M.V. Untargeted Metabolomic Profiling of Small Extracellular Vesicles Reveals Potential New Biomarkers for Triple Negative Breast Cancer. Metabolomics 2024, 20, 123. [Google Scholar] [CrossRef] [PubMed]
| EV Cargo Type | Examples | Mechanisms/Impact | Cancer Types |
|---|---|---|---|
| RNA | lncRNA H19, miR-181a-5p, circEHD2, miR-29a-3p | Regulation of metastasis, drug resistance, tumor-promoting inflammation | Lung, CRC, RCC, bladder |
| DNA | dsDNA (KRAS, TP53), mtDNA, c-Myc | Stable detection of mutations, tumor-specific DNA for liquid biopsy | Melanoma, pancreatic, colorectal |
| Proteins | CDCP1, CD9, TGF-β co-receptors, TYRP2, HSP70 | Modulation of angiogenesis, metastasis, immune evasion | Prostate, melanoma, breast, HNSCC |
| Lipids | Phosphatidylserine, ceramide, glycosphingolipids | Biomarker potential, metabolic regulation, cancer-specific lipid signatures | Prostate, RCC, lung |
| Metabolites | Proline, adenosine, L-kynurenine, MG-H1, glyoxalase 1/2 | Immunosuppression, metabolic reprogramming, angiogenesis | Prostate, breast, ovarian, colon, HNSCC |
| EV Cargo | TNBC | Function | References |
|---|---|---|---|
| DNA | mtDNA | Mutated mtDNA associated with early-stage cancer diagnostics and preventive care | [145] |
| DNA | mtDNA (RNR1, RNR2) | Regulatory region mutations detected in 15% of patient samples | [145] |
| DNA | mtDNA (tRNA genes) | Mutations specific to tRNA genes, impacting tumorigenesis and chemoresistance | [145] |
| DNA | mtDNA (G1888A mutation) | Mutation detected in 6% of patient samples | [145] |
| DNA | mtDNA (C5720T mutation) | Mutation present in 16% of patient samples | [145] |
| DNA | mtDNA (T4434G mutation) | Mutation present in 20% of patient samples | [145] |
| DNA | mtDNA (Respiratory Complex I) | Mutations in MT-ND1, ND2, ND3, ND4, ND4L, ND5, ND6 regions associated with mitochondrial respiratory chain | [145] |
| DNA | mtDNA (Respiratory Complex III) | Mutations in MT-CYTB, specifically T14894G mutation accounting for 20% | [145] |
| DNA | mtDNA (Respiratory Complex IV) | Mutations in MT-CO1, CO2, CO3 regions, with T7953G mutation exhibiting 38% prevalence | [145] |
| DNA | mtDNA (Respiratory Complex V) | Mutations in MT-ATP6 and ATP8 subunits | [145] |
| DNA | mtND4 gene | Increased mtDNA levels mutated in mtND4 gene transported in EVs responsible for tumorigenesis and chemoresistance | [146] |
| DNA | HPV DNA | Prevalence of HPV DNA in EVs linked to stromal cell activation and mammosphere formation | [147] |
| EV Cargo | TNBC | Function | References |
|---|---|---|---|
| Proteins | EGFR, MMPs | Tumor-promoting proteins that enhance invasion and migration | [148] |
| Proteins | Gal3BP/Gal3 complex | Induces immunosuppression via the CD45 receptor | [149] |
| Proteins | Annexin A2 | Involved in TNBC metastasis | [150] |
| Proteins | EGFR, ERBB2, MAPK1 | Promotes PI3K/Akt signaling pathway, metabolism, proliferation, cell survival, EMT activation | [151] |
| Proteins | Glypican-1, glucose transporter-1, ADAM10 | Potential EV membrane/surface markers for TNBC | [152] |
| Proteins | TLN2, VASP, GNAS | Platelet activation-related proteins | [153] |
| Proteins | B2M, PSMB9 | Antigen processing and presentation-related proteins | [153] |
| Proteins | CFL2, ITGB4, GIT1 | Regulation of actin cytoskeleton-related proteins | [153] |
| Proteins | CEACAM1, COL4A2, CALD1 | Angiogenesis-related proteins | [153] |
| Proteins | DST, ITGB4, CFL2 | Cell motility-related proteins | [153] |
| EV Cargo | TNBC | Function Specification | References |
|---|---|---|---|
| Lipids | Cardiolipin | Elevated levels associated with TNBC patient samples | [145] |
| Lipids | Arachidonic acid | Reprograms neutrophils, leading to lipid droplet accumulation and fostering an immunosuppressive tumor microenvironment | [155] |
| Lipids | LysoPC 22:6/0:0 | Proposed as a new biomarker for TNBC diagnostics | [155] |
| EV Cargo | Luminal A | Function | References |
|---|---|---|---|
| Proteins | SDCBP, COLEC11, LTF | Proteolysis-related proteins | [153] |
| Proteins | CCT2, HSP90AA1, PPIG | Protein folding-related proteins | [153] |
| Proteins | HSP90AA1, SDCBP | Regulation of necroptotic cell death | [153] |
| Proteins | HSBP1, IL6, HSP90AA1 | Cellular stress response and responses to external stimuli | [153] |
| EV Cargo | Luminal B | Function | References |
|---|---|---|---|
| Proteins | MYL6, AFDN | Tight junction-associated proteins | [153] |
| Proteins | RELN, GP1BB | ECM–receptor interaction-related proteins | [153] |
| Proteins | SLC2A14, SLC2A3 | Fructose and mannose metabolism-related proteins | [153] |
| Proteins | SLC2A14, SLC2A3 | Glucose metabolism-related proteins | [153] |
| Proteins | PKLR, PYGM | Insulin signaling pathway-associated proteins | [153] |
| EV Cargo | HER-2+ | Function | References |
|---|---|---|---|
| Proteins | SRC, ARG1, MAPK13 | Cellular response to hydrogen peroxide | [153] |
| Proteins | DOCK7, SRC | ErbB2/ErbB3 signaling event-related proteins | [153] |
| Proteins | CDSN, KRT78, KRT23 | Keratinization-related proteins | [153] |
| Proteins | FAH, ADHFE1 | Tyrosine metabolism-related proteins | [153] |
| Proteins | ARG1, RARS1 | Arginine and proline metabolism-related proteins | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernatova, S.; Nicodemou, A.; Cehakova, M.; Danisovic, L.; Bohac, M. Extracellular Vesicles Derived from Breast Cancer Cells: Emerging Biomarkers of Tumor Progression and Metastasis. Biomolecules 2025, 15, 1195. https://doi.org/10.3390/biom15081195
Bernatova S, Nicodemou A, Cehakova M, Danisovic L, Bohac M. Extracellular Vesicles Derived from Breast Cancer Cells: Emerging Biomarkers of Tumor Progression and Metastasis. Biomolecules. 2025; 15(8):1195. https://doi.org/10.3390/biom15081195
Chicago/Turabian StyleBernatova, Sona, Andreas Nicodemou, Michaela Cehakova, Lubos Danisovic, and Martin Bohac. 2025. "Extracellular Vesicles Derived from Breast Cancer Cells: Emerging Biomarkers of Tumor Progression and Metastasis" Biomolecules 15, no. 8: 1195. https://doi.org/10.3390/biom15081195
APA StyleBernatova, S., Nicodemou, A., Cehakova, M., Danisovic, L., & Bohac, M. (2025). Extracellular Vesicles Derived from Breast Cancer Cells: Emerging Biomarkers of Tumor Progression and Metastasis. Biomolecules, 15(8), 1195. https://doi.org/10.3390/biom15081195






