Dynamic Interplay Between miR-124-3p and EGF in the Regulation of Overgrowth via RNA Signaling
Abstract
1. Introduction
2. Material Methods
2.1. Animals
2.2. RNA Microinjection in Fertilised Eggs
2.3. Three-Dimensional Hippocampal Cell Culture
2.4. RNA Extraction, RT-PCR, Pre-Amplification, and qPCR
2.5. miRNA Expression by qPCR
2.6. Behavioural Tests
2.7. Statistical Analyses
3. Results
3.1. Recapitulation of the “Giant” Phenotype in the Balb/C Background Following Microinjection of miR-124-3p RNA into Fertilised Mouse Eggs
3.2. Behavioural Findings
3.3. miR-124-3p Microinjection Does Not Significantly Alter miR-124 Expression Levels in Early Embryos or Adult Hippocampus
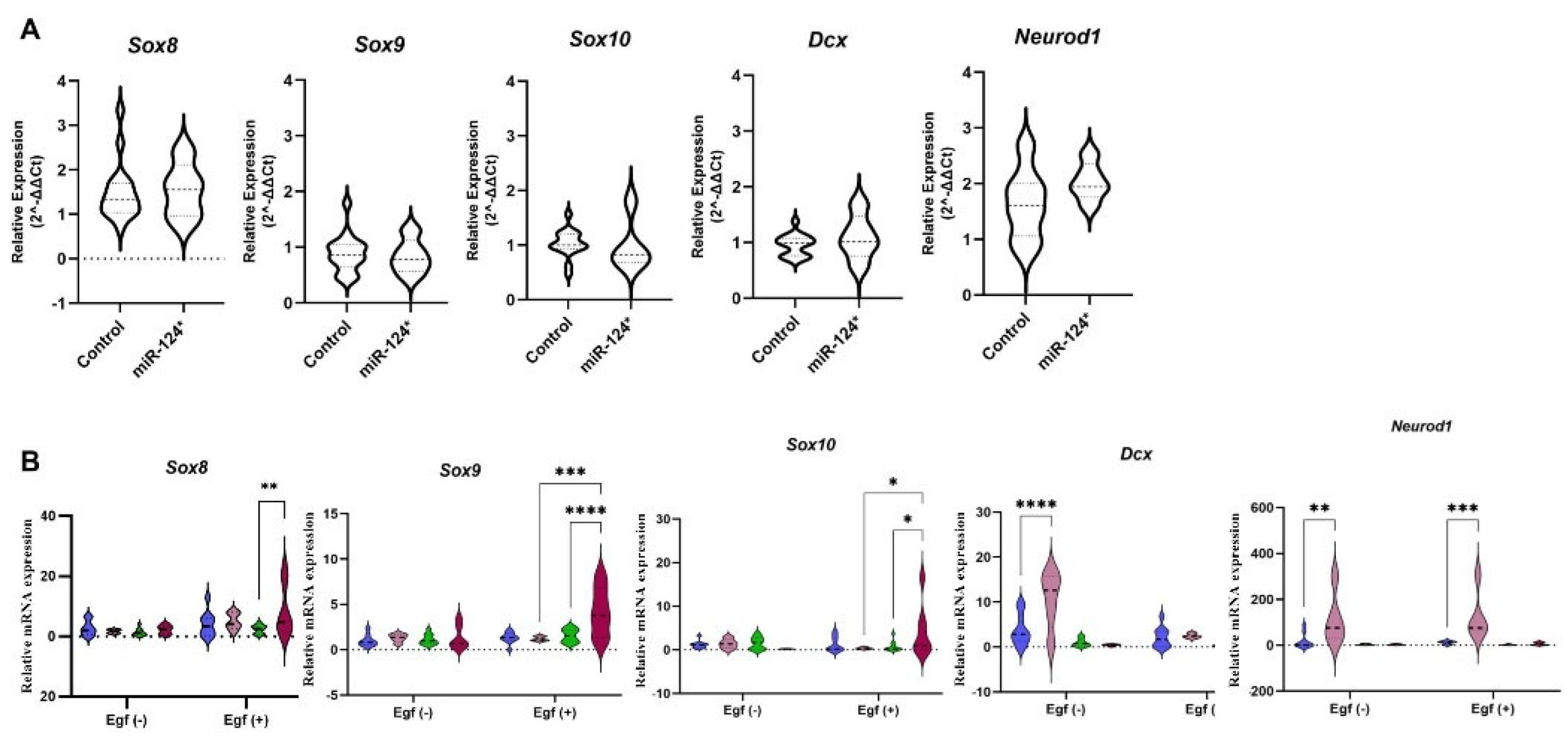
3.4. Neuronal Differentiation Could Be Promoted in miR-124-3p* Embryos During the Early Stage
3.5. The Role of miR-124-3p* in Regulating Body Growth Pathway During Early Embryonic Stage
3.6. miR-124-3p* Embryos Are Sensitive to Growth Factors Such as EGF During the Late Embryonic Period

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vasudevan, S.N.; Pooja, S.K.; Raju, T.J.; Damini, C.S. Cisgenics and intragenics: Boon or bane for crop improvement. Front. Plant Sci. 2023, 14, 1275145. [Google Scholar] [CrossRef]
- Grandjean, V.; Gounon, P.; Wagner, N.; Martin, L.; Wagner, K.D.; Bernex, F.; Cuzin, F.; Rassoulzadegan, M. The miR-124-Sox9 paramutation: RNA-mediated epigenetic control of embryonic and adult growth. Development 2009, 136, 3647–3655. [Google Scholar] [CrossRef]
- Karabulut, S.; Bayramov, K.K.; Bayramov, R.; Ozdemir, F.; Topaloglu, T.; Ergen, E.; Yazgan, K.; Taskiran, A.S.; Golgeli, A. Effects of post-learning REM sleep deprivation on hippocampal plasticity-related genes and microRNA in mice. Behav. Brain Res. 2019, 361, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Zhang, X.; Su, X.; Kong, C.; Yang, Y.; Ye, Y.; Fan, Z.; He, X. Activation of miR-124-3p/Notch pathway promotes proliferation and differentiation of rat neural stem cells after traumatic brain injury. Chin. J. Cell. Mol. Immunol. 2020, 36, 49–55. [Google Scholar]
- Esteves, M.; Abreu, R.; Fernandes, H.; Serra-Almeida, C.; Martins, P.A.; Barão, M.; Cristóvão, A.C.; Saraiva, C.; Ferreira, R.; Ferreira, L.; et al. MicroRNA-124-3p-enriched small extracellular vesicles as a therapeutic approach for Parkinson’s disease. Mol. Ther. 2022, 30, 3176–3192. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, S.J.; Carew, T.J. MicroRNAs in Memory Processing. Neuron 2009, 63, 714–716. [Google Scholar] [CrossRef]
- Huang, F.; Shen, Q.; Zhao, J.T. Growth and differentiation of neural stem cells in a three-dimensional collagen gel scaffold. Neural Regen. Res. 2013, 8, 313–319. [Google Scholar]
- Sock, E.; Schmidt, K.; Hermanns-Borgmeyer, I.; Bösl, M.R.; Wegner, M. Idiopathic Weight Reduction in Mice Deficient in the High-Mobility-Group Transcription Factor Sox8. Mol. Cell. Biol. 2001, 21, 6951–6959. [Google Scholar] [CrossRef] [PubMed]
- Pingault, V.; Bondurand, N.; Kuhlbrodt, K.; Goerich, D.E.; Préhu, M.O.; Puliti, A.; Herbarth, B.; Hermans-Borgmeyer, I.; Legius, E.; Matthijs, G.; et al. SOX10 mutations in patients with Waardenburg-Hirschsprung disease. Nat. Genet. 1998, 18, 171–173. [Google Scholar] [CrossRef]
- Nosten-Bertrand, M.; Kappeler, C.; Dinocourt, C.; Denis, C.; Germain, J.; Tuy, F.P.D.; Verstraeten, S.; Alvarez, C.; Métin, C.; Chelly, J.; et al. Epilepsy in Dcx Knockout Mice Associated with Discrete Lamination Defects and Enhanced Excitability in the Hippocampus. PLoS ONE 2008, 3, e2473. [Google Scholar] [CrossRef]
- Yilmaz Sukranli, Z.; Korkmaz Bayram, K.; Taheri, S.; Cuzin, F.; Ozkul, Y.; Rassoulzadegan, M. Experimentally altering microRNA levels in embryos alters adult phenotypes. Sci. Rep. 2024, 14, 19014. [Google Scholar] [CrossRef] [PubMed]
- Sukranli, Z.Y.; Bayram, K.K.; Mehmetbeyoglu, E.; Doganyigit, Z.; Beyaz, F.; Sener, E.F.; Taheri, S.; Ozkul, Y.; Rassoulzadegan, M. Trans Species RNA Activity: Sperm RNA of the Father of an Autistic Child Programs Glial Cells and Behavioral Disorders in Mice. Biomolecules 2024, 14, 201. [Google Scholar] [CrossRef]
- Rassoulzadegan, M.; Grandjean, V.; Gounon, P.; Vincent, S.; Gillot, I.; Cuzin, F. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature 2006, 441, 469–474. [Google Scholar] [CrossRef]
- Bayram, K.K.; Fitremann, J.; Bayram, A.; Yılmaz, Z.; Mehmetbeyoğlu, E.; Özkul, Y.; Rassoulzadegan, M. Gene Expression of Mouse Hippocampal Stem Cells Grown in a Galactose-Derived Molecular Gel Compared to In Vivo and Neurospheres. Processes 2021, 9, 716. [Google Scholar] [CrossRef]
- Ozkul, Y.; Taheri, S.; Bayram, K.K.; Sener, E.F.; Mehmetbeyoglu, E.; Öztop, D.B.; Aybuga, F.; Tufan, E.; Bayram, A.; Dolu, N.; et al. A heritable profile of six miRNAs in autistic patients and mouse models. Sci. Rep. 2020, 10, 9011. [Google Scholar] [CrossRef] [PubMed Central]
- Ahmadu, P.U.; Victor, E.; Ameh, F.S. Studies on some neuropharmacological properties of Nevirapine in mice. IBRO Neurosci. Rep. 2022, 12, 12–19. [Google Scholar] [CrossRef] [PubMed Central]
- Haj-Mirzaian, A.; Nikbakhsh, R.; Ramezanzadeh, K.; Rezaee, M.; Amini-Khoei, H.; Ghesmati, M.; Afshari, K.; Haddadi, N.-S.; Dehpour, A.R. Involvement of opioid system in behavioral despair induced by social isolation stress in mice. Biomed. Pharmacother. 2019, 109, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Ayabe, T.; Ohya, R.; Ano, Y. Matured hop bitter acids improve spatial working and object recognition memory via nicotinic acetylcholine receptors. Psychopharmacology 2019, 236, 2847–2854. [Google Scholar] [CrossRef] [PubMed]
- Grayson, B.; Leger, M.; Piercy, C.; Adamson, L.; Harte, M.; Neill, J.C. Assessment of disease-related cognitive impairments using the novel object recognition (NOR) task in rodents. Behav. Brain Res. 2015, 285, 176–193. [Google Scholar] [CrossRef]
- Ogurtsov, S.V.; Antipov, V.A.; Permyakov, M.G. Sex differences in exploratory behaviour of the common toad, Bufo bufo. Ethol. Ecol. Evol. 2018, 30, 543–568. [Google Scholar] [CrossRef]
- Ter Horst, J.P.; De Kloet, E.R.; Schächinger, H.; Oitzl, M.S. Relevance of stress and female sex hormones for emotion and cognition. Cell. Mol. Neurobiol. 2012, 32, 725–735. [Google Scholar] [CrossRef]
- Oliveira-Pinto, A.V.; Santos, R.M.; Coutinho, R.A.; Oliveira, L.M.; Santos, G.B.; Alho, A.T.L.; Leite, R.E.P.; Farfel, J.M.; Suemoto, C.K.; Grinberg, L.T.; et al. Sexual dimorphism in the human olfactory bulb: Females have more neurons and glial cells than males. PLoS ONE 2014, 9, e111733. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2019. [Google Scholar]
- Singh, S.; Botvinnik, A.; Shahar, O.; Wolf, G.; Yakobi, C.; Saban, M.; Salama, A.; Lotan, A.; Lerer, B.; Lifschytz, T. Effect of psilocybin on marble burying in ICR mice: Role of 5-HT1A receptors and implications for the treatment of obsessive-compulsive disorder. Transl. Psychiatry 2023, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Dringenberg, H.C.; Levine, Y.; Menard, J.L. Electrical stimulation of dorsal, but not ventral hippocampus reduces behavioral defense in the elevated plus maze and shock-probe burying test in rats. Behav. Brain Res. 2008, 186, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, R.; Strazielle, C. The Hole-Board Test in Mutant Mice. Behav. Genet. 2022, 52, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Benaroya-Milshtein, N.; Hollander, N.; Apter, A.; Kukulansky, T.; Raz, N.; Wilf, A.; Yaniv, I.; Pick, C. Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. Eur. J. Neurosci. 2004, 20, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Lovick, T.A.; Zangrossi, H. Effect of Estrous Cycle on Behavior of Females in Rodent Tests of Anxiety. Front. Psychiatry 2021, 12, 711065. [Google Scholar] [CrossRef]
- Visvanathan, J.; Lee, S.; Lee, B.; Lee, J.W.; Lee, S.K. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 2007, 21, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Mokabber, H.; Najafzadeh, N.; Mohammadzadeh Vardin, M. miR-124 promotes neural differentiation in mouse bulge stem cells by repressing Ptbp1 and Sox9. J. Cell. Physiol. 2019, 234, 8941–8950. [Google Scholar] [CrossRef]
- Grimberg, A.; Cohen, P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J. Cell. Physiol. 2000, 183, 1–9. [Google Scholar] [CrossRef]
- Guan, J.; Borenäs, M.; Xiong, J.; Lai, W.Y.; Palmer, R.H.; Hallberg, B. IGF1R Contributes to Cell Proliferation in ALK-Mutated Neuroblastoma with Preference for Activating the PI3K-AKT Signaling Pathway. Cancers 2023, 15, 4252. [Google Scholar] [CrossRef]
- Lanjewar, S.N.; Sloan, S.A. Growing Glia: Cultivating Human Stem Cell Models of Gliogenesis in Health and Disease. Front. Cell Dev. Biol. 2021, 9, 649538. [Google Scholar] [CrossRef]
- Gleeson, J.G.; Lin, P.T.; Flanagan, L.A.; Walsh, C.A. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 1999, 23, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Germain, J.; Bruel-Jungerman, E.; Grannec, G.; Denis, C.; Lepousez, G.; Giros, B.; Francis, F.; Nosten-Bertrand, M.; Burne, T. Doublecortin Knockout Mice Show Normal Hippocampal-Dependent Memory Despite CA3 Lamination Defects. PLoS ONE 2013, 8, e74992. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ninomiya, M.; Yamashita, T.; Araki, N.; Okano, H.; Sawamoto, K. Enhanced neurogenesis in the ischemic striatum following EGF-induced expansion of transit-amplifying cells in the subventricular zone. Neurosci. Lett. 2006, 403, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, Y.; Mo, W.; Qiu, R.; Wang, X.; Wu, J.Y.; He, R. MiR-124 regulates early neurogenesis in the optic vesicle and forebrain, targeting NeuroD1. Nucleic Acids Res. 2011, 39, 2869–2879. [Google Scholar] [CrossRef]
- Papadimitriou, E.; Koutsoudaki, P.N.; Thanou, I.; Karagkouni, D.; Karamitros, T.; Chroni-Tzartou, D.; Gaitanou, M.; Gkemisis, C.; Margariti, M.; Xingi, E.; et al. A miR-124-mediated post-transcriptional mechanism controlling the cell fate switch of astrocytes to induced neurons. Stem Cell Rep. 2023, 18, 915–935. [Google Scholar] [CrossRef]
- Xue, Y.; Ouyang, K.; Huang, J.; Zhou, Y.; Ouyang, H.; Li, H.; Wang, G.; Wu, Q.; Wei, C.; Bi, Y.; et al. Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated MicroRNA circuits. Cell 2013, 152, 82–96. [Google Scholar] [CrossRef]
- Mtango, N.R.; Varisanga, M.D.; Suzuki, T. Effects of Growth Hormone and Growth Factors on the Improvement of Culture Conditions of In vitro Produced Bovine Embryos. Pak. J. Biol. Sci. 2002, 5, 604–606. [Google Scholar] [CrossRef]
- Humphrey, R.G.; Sonnenberg-Hirche, C.; Smith, S.D.; Hu, C.; Barton, A.; Sadovsky, Y.; Nelson, D.M. Epidermal growth factor abrogates hypoxia-induced apoptosis in cultured human trophoblasts through phosphorylation of BAD serine 112. Endocrinology 2008, 149, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Balciuniene, J.; Bardwell, V.J.; Zarkower, D. Mice Mutant in the DM Domain Gene Dmrt4 Are Viable and Fertile but Have Polyovular Follicles. Mol. Cell. Biol. 2006, 26, 8984–8991. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, W.O.; Wasinski, F.; Tavares, M.R.; Campos, A.M.; Elias, C.F.; List, E.O.; Kopchick, J.J.; Szawka, R.E.; Donato, J., Jr. Ablation of Growth Hormone Receptor in GABAergic Neurons Leads to Increased Pulsatile Growth Hormone Secretion. Endocrinology 2022, 163, bqac103. [Google Scholar] [CrossRef] [PubMed Central]
- Pilitsi, E.; Peradze, N.; Perakakis, N.; Mantzoros, C.S. Circulating levels of the components of the GH/IGF-1/IGFBPs axis total and intact IGF-binding proteins (IGFBP) 3 and IGFBP 4 and total IGFBP 5, as well as PAPPA, PAPPA2 and Stanniocalcin-2 levels are not altered in response to energy deprivation and/or metreleptin administration in humans. Metabolism 2019, 97, 32–39. [Google Scholar]
- Yang, L.; Zhu, Y.; Kong, D.; Gong, J.; Yu, W.; Liang, Y.; Nie, Y.; Teng, C.-B. EGF suppresses the expression of miR-124a in pancreatic β cell lines via ETS2 activation through the MEK and PI3K signaling pathways. Int. J. Biol. Sci. 2019, 15, 2561–2575. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korkmaz Bayram, K.; Bayram, A.; Yilmaz Sukranli, Z.; Mehmetbeyoglu Duman, E.; Aybuga, F.; Tufan Benli, E.; Taheri, S.; Ozkul, Y.; Rassoulzadegan, M. Dynamic Interplay Between miR-124-3p and EGF in the Regulation of Overgrowth via RNA Signaling. Biomolecules 2025, 15, 1186. https://doi.org/10.3390/biom15081186
Korkmaz Bayram K, Bayram A, Yilmaz Sukranli Z, Mehmetbeyoglu Duman E, Aybuga F, Tufan Benli E, Taheri S, Ozkul Y, Rassoulzadegan M. Dynamic Interplay Between miR-124-3p and EGF in the Regulation of Overgrowth via RNA Signaling. Biomolecules. 2025; 15(8):1186. https://doi.org/10.3390/biom15081186
Chicago/Turabian StyleKorkmaz Bayram, Keziban, Arslan Bayram, Zeynep Yilmaz Sukranli, Ecmel Mehmetbeyoglu Duman, Fatma Aybuga, Esra Tufan Benli, Serpil Taheri, Yusuf Ozkul, and Minoo Rassoulzadegan. 2025. "Dynamic Interplay Between miR-124-3p and EGF in the Regulation of Overgrowth via RNA Signaling" Biomolecules 15, no. 8: 1186. https://doi.org/10.3390/biom15081186
APA StyleKorkmaz Bayram, K., Bayram, A., Yilmaz Sukranli, Z., Mehmetbeyoglu Duman, E., Aybuga, F., Tufan Benli, E., Taheri, S., Ozkul, Y., & Rassoulzadegan, M. (2025). Dynamic Interplay Between miR-124-3p and EGF in the Regulation of Overgrowth via RNA Signaling. Biomolecules, 15(8), 1186. https://doi.org/10.3390/biom15081186






