Obesity-Mediated Inflammation and Its Influence on Inflammatory Bowel Disease: Pathophysiology, Clinical Impact, and Therapeutic Implications
Abstract
1. Introduction
2. Shared Pathophysiological Mechanisms Between Obesity and IBD
2.1. Intestinal Dysbiosis
2.2. Chronic Immune Activation and Pro-Inflammatory Cytokines
2.3. The Role of Adipokines
2.4. Intestinal Barrier Integrity
3. Obesity as a Risk Factor for IBD Development
4. The Impact of Obesity on the Clinical Course and Outcomes of IBD
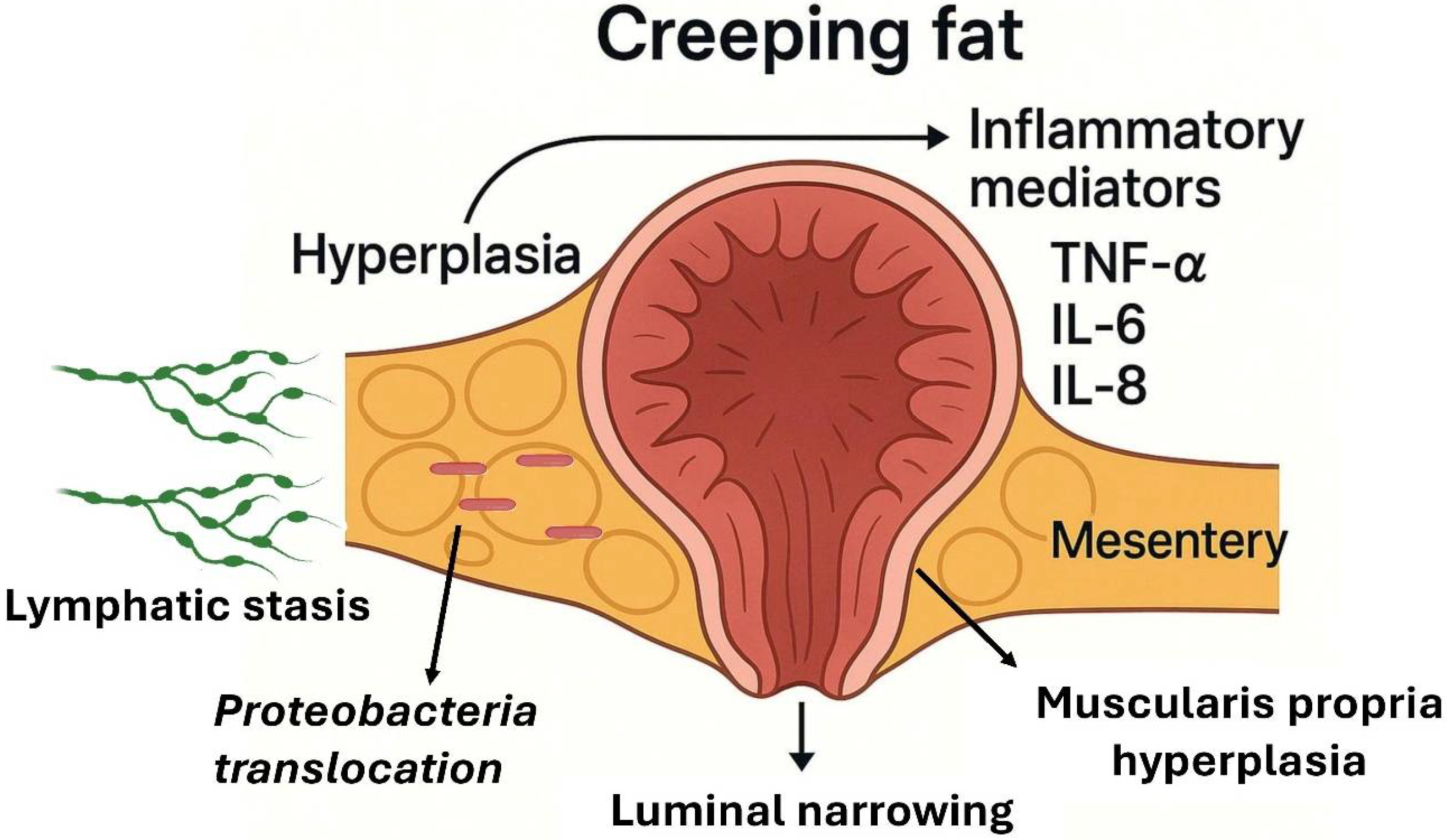
5. Visceral Adipose Tissue, Creeping Fat, and IBD
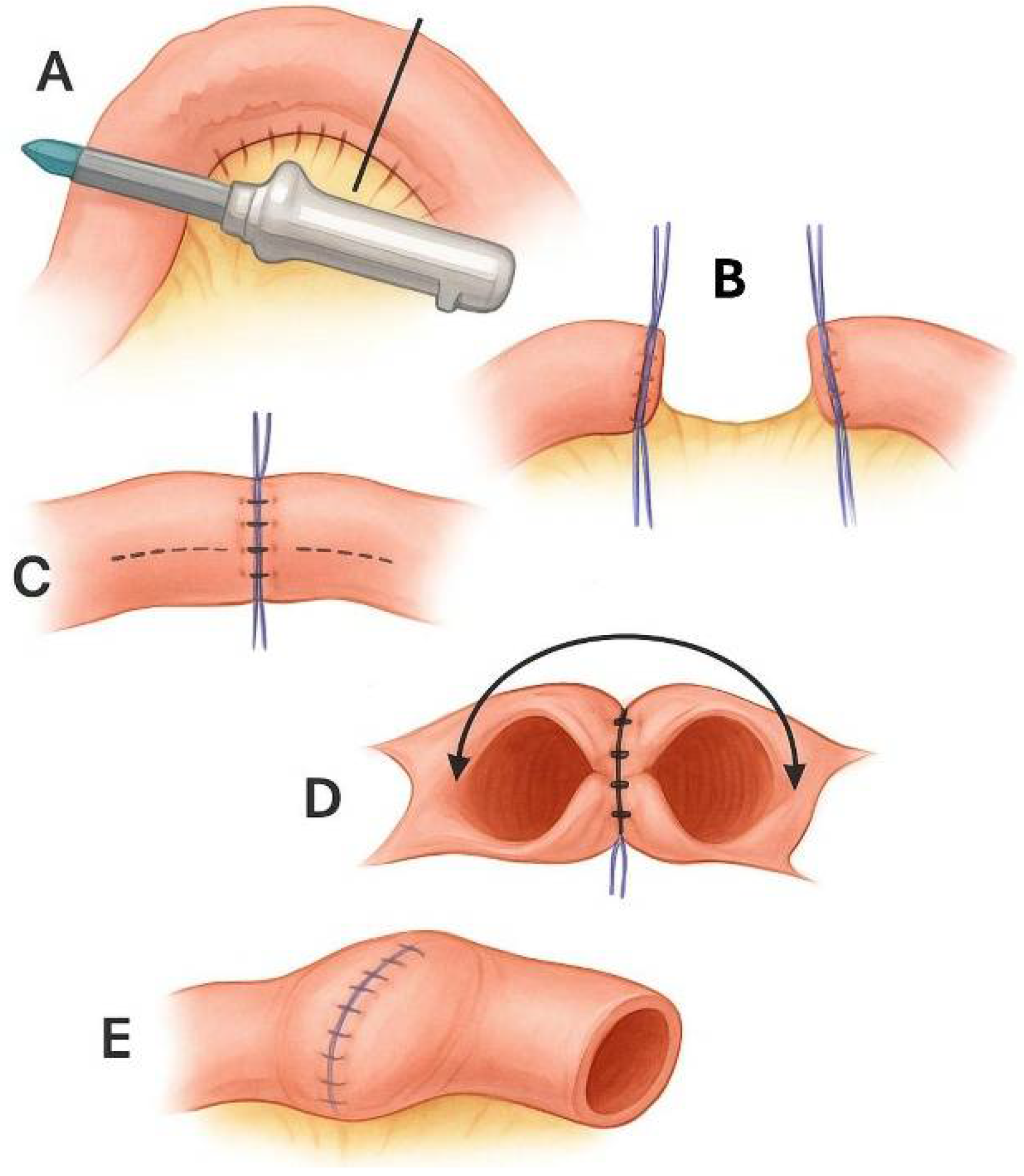
6. Mesenteric Surgery: Unfulfilled Promises
7. Clinical Implications: Therapeutic Options Related to Obesity and Metabolic Syndrome in IBD
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IBD | Inflammatory Bowel Disease |
| CD | Crohn’s Disease |
| UC | Ulcerative Colitis |
| SCFAs | Short-Chain Fatty Acids |
| TNF | Tumor Necrosis Factor |
| IL | Interleukin |
| BMI | Body Mass Index |
References
- Gros, B.; Kaplan, G.G. Ulcerative Colitis in Adults: A Review. JAMA 2023, 330, 951–965. [Google Scholar] [CrossRef]
- Dolinger, M.; Torres, J.; Vermeire, S. Crohn’s disease. Lancet 2024, 403, 1177–1191. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Kuenzig, M.E.; Fung, S.G.; Marderfeld, L.; Mak, J.W.; Kaplan, G.G.; Ng, S.C.; Wilson, D.C.; Cameron, F.; Henderson, P.; Kotze, P.G.; et al. Twenty-first Century Trends in the Global Epidemiology of Pediatric-Onset Inflammatory Bowel Disease: Systematic Review. Gastroenterology 2022, 162, 1147–1159.e4. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the in-flammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef]
- Milajerdi, A.; Abbasi, F.; Esmaillzadeh, A. A systematic review and meta-analysis of prospective studies on obesity and risk of inflammatory bowel disease. Nutr. Rev. 2021, 80, 479–487. [Google Scholar] [CrossRef]
- Rahmani, J.; Kord-Varkaneh, H.; Hekmatdoost, A.; Thompson, J.; Clark, C.; Salehisahlabadi, A.; Day, A.S.; Jacobson, K. Body mass index and risk of inflammatory bowel disease: A systematic review and dose-response meta-analysis of cohort studies of over a million participants. Obes. Rev. 2019, 20, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Bhagavathula, A.S.; Clark, C.C.; Rahmani, J.; Chattu, V.K. Impact of Body Mass Index on the Development of Inflammatory Bowel Disease: A Systematic Review and Dose-Response Analysis of 15.6 Million Participants. Healthcare 2021, 9, 35. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, C.M.; Yoo, J.H. Obesity and novel management of inflammatory bowel disease. World J. Gastroenterol. 2023, 29, 1779–1794. [Google Scholar] [CrossRef] [PubMed]
- Adolph, T.E.; Meyer, M.; Schwärzler, J.; Mayr, L.; Grabherr, F.; Tilg, H. The metabolic nature of inflammatory bowel diseases. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.W.; Zisman, T.L. Interaction of obesity and inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 7868–7881. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, A.; Wyse, J.; Abdulezer, J. Dietary Relationships between Obesity and Inflammatory Bowel Diseases: A Narrative Review of Diets Which May Promote Both Diseases. Curr. Gastroenterol. Rep. 2025, 27, 29. [Google Scholar] [CrossRef]
- Argollo, M.; Gilardi, D.; Peyrin-Biroulet, C.; Chabot, J.-F.; Peyrin-Biroulet, L.; Danese, S. Comorbidities in inflammatory bowel disease: A call for action. Lancet Gastroenterol. Hepatol. 2019, 4, 643–654. [Google Scholar] [CrossRef]
- Mahmoud, M.; Syn, W.K. Impact of Obesity and Metabolic Syndrome on IBD Outcomes. Dig. Dis. Sci. 2024, 69, 2741–2753. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.M.; Ríos, M.S. A New International Diabetes Federation (IDF) Worldwide Definition of the Metabolic Syndrome: The Rationale and the Results. Rev. Espanola. Cardiol. 2005, 58, 1371–1375. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Ni, Y.; Yi, C.; Fang, Y.; Ning, Q.; Shen, B.; Zhang, K.; Liu, Y.; Yang, L.; et al. Global Prevalence of Overweight and Obesity in Children and Adolescents. JAMA Pediatr. 2024, 178, 800–813. [Google Scholar] [CrossRef]
- Jia, P.; Shi, Y.; Jiang, Q.; Dai, S.; Yu, B.; Yang, S.; Qiu, G.; Yang, S. Environmental determinants of childhood obesity: A meta-analysis. Lancet Glob. Health 2023, 11, S7. [Google Scholar] [CrossRef]
- Rodriguez-Duque, J.C.; Calleja, J.L.; Iruzubieta, P.; Hernández-Conde, M.; Rivas-Rivas, C.; Vera, M.I.; Garcia, M.J.; Pascual, M.; Castro, B.; García-Blanco, A.; et al. Increased risk of MAFLD and Liver Fibrosis in Inflammatory Bowel Disease Independent of Classic Metabolic Risk Factors. Clin. Gastroenterol. Hepatol. 2022, 21, 406–414.e7. [Google Scholar] [CrossRef]
- Singh, S.; Dulai, P.S.; Zarrinpar, A.; Ramamoorthy, S.; Sandborn, W.J. Obesity in IBD: Epidemiology, pathogenesis, disease course and treatment outcomes. Nat. Rev. Gastroenterol. Hepatol. 2016, 14, 110–121. [Google Scholar] [CrossRef]
- Duan, M.; Coffey, J.C.; Li, Y. Mesenteric-based surgery for Crohn’s disease: Evidence and perspectives. Surgery 2024, 176, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Adolph, E.; Meyer, T.; Jukic, A.; Tilg, H. Heavy arch: From inflammatory bowel diseases to metabolic disorders. Gut 2024, 73, 1376–1387. [Google Scholar] [CrossRef]
- Sankararaman, S.; Noriega, K.; Velayuthan, S.; Sferra, T.; Martindale, R. Gut Microbiome and Its Impact on Obesity and Obesity-Related Disorders. Curr. Gastroenterol. Rep. 2023, 25, 31–44. [Google Scholar] [CrossRef]
- Geng, J.; Ni, Q.; Sun, W.; Li, L.; Feng, X. The links between gut microbiota and obesity and obesity related diseases. Biomed. Pharmacother. 2022, 147, 112678. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837–3850. [Google Scholar] [CrossRef]
- Li, G.; Lin, J.; Zhang, C.; Gao, H.; Lu, H.; Gao, X.; Zhu, R.; Li, Z.; Li, M.; Liu, Z. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes 2021, 13, 1968257. [Google Scholar] [CrossRef] [PubMed]
- Dowdell, A.S.; Colgan, S.P. Metabolic Host–Microbiota Interactions in Autophagy and the Pathogenesis of Inflammatory Bowel Disease (IBD). Pharmaceuticals 2021, 14, 708. [Google Scholar] [CrossRef]
- Machate, D.J.; Figueiredo, P.S.; Marcelino, G.; Guimarães, R.d.C.A.; Hiane, P.A.; Bogo, D.; Pinheiro, V.A.Z.; de Oliveira, L.C.S.; Pott, A. Fatty Acid Diets: Regulation of Gut Microbiota Composition and Obesity and Its Related Metabolic Dysbiosis. Int. J. Mol. Sci. 2020, 21, 4093. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Physiol. 2020, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ambrosi, J. Adipose Tissue Inflammation. Cells 2023, 12, 1484. [Google Scholar] [CrossRef]
- Schäffler, A.; Schölmerich, J. Innate immunity and adipose tissue biology. Trends Immunol. 2010, 31, 228–235. [Google Scholar] [CrossRef]
- Karaskova, E.; Velganova-Veghova, M.; Geryk, M.; Foltenova, H.; Kucerova, V.; Karasek, D. Role of Adipose Tissue in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 4226. [Google Scholar] [CrossRef]
- Wen, L.; Yang, K.; Wang, J.; Zhou, H.; Ding, W. Gut microbiota-mitochondrial crosstalk in obesity: Novel mechanistic insights and therapeutic strategies with traditional Chinese medicine. Front. Pharmacol. 2025, 16, 1574887. [Google Scholar] [CrossRef]
- Guney, E.; Arruda, A.P.; Parlakgul, G.; Cagampan, E.; Min, N.; Lee, G.Y.; Greene, L.; Tsaousidou, E.; Inouye, K.; Han, M.S.; et al. Aberrant Ca2+ signaling by IP3Rs in adipocytes links inflammation to metabolic dysregulation in obesity. Sci. Signal. 2021, 14, eabf2059. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Turovskaya, M.V.; Dynnik, V.V. Deregulation of Ca2+-Signaling Systems in White Adipocytes, Manifested as the Loss of Rhythmic Activity, Underlies the Development of Multiple Hormonal Resistance at Obesity and Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 5109. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Varlamova, E.G.; Turovskaya, M.V. Activation of Cx43 Hemichannels Induces the Generation of Ca2+ Oscillations in White Adipocytes and Stimulates Lipolysis. Int. J. Mol. Sci. 2021, 22, 8095. [Google Scholar] [CrossRef]
- Bertin, B.; Desreumaux, P.; Dubuquoy, L. Obesity, visceral fat and Crohn’s disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Barbier, M.; Vidal, H.; Desreumaux, P.; Dubuquoy, L.; Bourreille, A.; Colombel, J.F.; Cherbut, C.; Galmiche, J.P. Overexpression of leptin mRNA in mesenteric adipose tissue in inflammatory bowel diseases. Gastroentérol. Clin. Biol. 2003, 27, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; Sánchez-Margalet, V. Role of Leptin in Inflammation and Vice Versa. Int. J. Mol. Sci. 2020, 21, 5887. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.S.; Milanski, M.; Fagundes, J.J.; Torsoni, A.S.; Ayrizono, M.L.S.; Nunez, C.E.C.; Dias, C.B.; Meirelles, L.R.; Dalal, S.; Coy, C.S.R.; et al. Serum levels and mesenteric fat tissue expression of adiponectin and leptin in patients with Crohn’s disease. Clin. Exp. Immunol. 2012, 170, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Karmiris, K.; Koutroubakis, I.E.; Xidakis, C.; Polychronaki, M.; Voudouri, T.; Kouroumalis, E.A. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm. Bowel. Dis. 2006, 12, 100–105. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kiyohara, T.; Murayama, Y.; Kihara, S.; Okamoto, Y.; Funahashi, T.; Ito, T.; Nezu, R.; Tsutsui, S.; Miyagawa, J.I.; et al. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn’s disease. Gut 2005, 54, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Konrad, A.; Lehrke, M.; Schachinger, V.; Seibold, F.; Stark, R.; Ochsenkühn, T.; Parhofer, K.G.; Göke, B.; Broedl, U.C. Resistin is an inflammatory marker of inflammatory bowel disease in humans. Eur. J. Gastroenterol. Hepatol. 2007, 19, 1070–1074. [Google Scholar] [CrossRef]
- Schaeffler, A.; Gross, P.; Buettner, R.; Bollheimer, C.; Buechler, C.; Neumeier, M.; Kopp, A.; Schoelmerich, J.; Falk, W. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology 2009, 126, 233–245. [Google Scholar] [CrossRef]
- Camilleri, M. Is intestinal permeability increased in obesity? A review including the effects of dietary, pharmacological and surgical interventions on permeability and the microbiome. Diabetes, Obes. Metab. 2022, 25, 325–330. [Google Scholar] [CrossRef]
- Cox, A.J.; West, N.P.; Cripps, A.W. Obesity, inflammation, and the gut microbiota. Lancet. Diabetes. Endocrinol. 2015, 3, 207–215. [Google Scholar] [CrossRef]
- Szilagyi, A. Relationship(s) between obesity and inflammatory bowel diseases: Possible intertwined pathogenic mechanisms. Clin. J. Gastroenterol. 2019, 13, 139–152. [Google Scholar] [CrossRef]
- Gérard, P. Gut microbiota and obesity. Cell. Mol. Life. Sci. 2016, 73, 147–162. [Google Scholar] [CrossRef]
- Johnson, A.M.; Harmsen, W.S.; Aniwan, S.; Tremaine, W.J.; Raffals, L.E.; Abu Dayyeh, B.K.; Loftus, E.V.J. Prevalence and Impact of Obesity in a Population-Based Cohort of Patients With Crohn’s Disease. J. Clin. Gastroenterol. 2022, 58, 176–182. [Google Scholar] [CrossRef]
- Nic Suibhne, T.; Raftery, T.C.; McMahon, O.; Walsh, C.; O’MOrain, C.; O’SUllivan, M. High prevalence of overweight and obesity in adults with Crohn’s disease: Associations with disease and lifestyle factors. J. Crohn’s Colitis 2013, 7, e241–e248. [Google Scholar] [CrossRef]
- Khalili, H.; Ananthakrishnan, A.N.; Konijeti, G.G.; Higuchi, L.M.; Fuchs, C.S.; Richter, J.M.; Chan, A.T. Measures of obesity and risk of Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2015, 21, 361–368. [Google Scholar] [CrossRef]
- Mendall, M.; Harpsøe, M.C.; Kumar, D.; Andersson, M.; Jess, T.; Ahmad, R. Relation of body mass index to risk of developing inflammatory bowel disease amongst women in the Danish National Birth Cohort. PLoS ONE 2018, 13, e0190600. [Google Scholar] [CrossRef]
- Jensen, C.B.; Ängquist, L.H.; Mendall, M.A.; Sørensen, T.I.A.; Baker, J.L.; Jess, T. Childhood body mass index and risk of inflammatory bowel disease in adulthood: A population-based cohort study. Am. J. Gastroenterol. 2018, 113, 694–701. [Google Scholar] [CrossRef]
- Chan, S.S.M.; Luben, R.; Olsen, A.; Tjonneland, A.; Kaaks, R.; Teucher, B.; Lindgren, S.; Grip, O.; Key, T.; Crowe, F.L.; et al. Body Mass Index and the Risk for Crohn’s Disease and Ulcerative Colitis: Data From a European Prospective Cohort Study (The IBD in EPIC Study). Am. J. Gastroenterol. 2013, 108, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Harpsøe, M.C.; Basit, S.; Andersson, M.; Nielsen, N.M.; Frisch, M.; Wohlfahrt, J.; Nohr, E.A.; Linneberg, A.; Jess, T. Body mass index and risk of autoimmune diseases: A study within the Danish National Birth Cohort. Leuk. Res. 2014, 43, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Cañete, F.; Vela, E.; Calafat, M.; Piera, J.; Mañosa, M.; Domènech, E. Severe obesity, a susceptibility factor for developing inflammatory bowel disease: Results of a population-based study. J. Crohn’s Colitis. 2025, 19, jjaf010. [Google Scholar] [CrossRef]
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; Brown, W.A.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J.; et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025, 13, 221–262. [Google Scholar] [CrossRef] [PubMed]
- Pringle, P.L.; Stewart, K.O.; Peloquin, J.M.; Sturgeon, H.C.; Nguyen, D.D.; Sauk, J.; Garber, J.; Yajnik, V.; Ananthakrishnan, A.N.; Chan, A.T.; et al. Sa1230 Body Mass Index, Genetic Susceptibility, and Risk of Complications Among Individuals With Crohn’s Disease. Gastroenterology 2015, 148, S-264. [Google Scholar] [CrossRef]
- Flores, A.; Burstein, E.; Cipher, D.J.; Feagins, L.A. Obesity in Inflammatory Bowel Disease: A Marker of Less Severe Disease. Dig. Dis. Sci. 2015, 60, 2436–2445. [Google Scholar] [CrossRef]
- Stabroth-Akil, D.; Leifeld, L.; Pfützer, R.; Morgenstern, J.; Kruis, W. The effect of body weight on the severity and clinical course of ulcerative colitis. Int. J. Color. Dis. 2014, 30, 237–242. [Google Scholar] [CrossRef] [PubMed]
- García-Mateo, S.; Martínez-Domínguez, S.J.; Gargallo-Puyuelo, C.J.; Villarino, M.T.A.; De La Torre, V.L.; Gallego, B.; Alfambra, E.; Gomollón, F. Lifestyle Can Exert a Significant Impact on the Development of Metabolic Complications and Quality Life in Patients with Inflammatory Bowel Disease. Nutrients 2023, 15, 3983. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, K.-Q.; Qin, X.-R.; Lu, W.; Liu, Y.; Wang, X.-Y. Association between physical activity and inflammatory bowel disease risk: A meta-analysis. Dig. Liver Dis. 2016, 48, 1425–1431. [Google Scholar] [CrossRef]
- Chicco, F.; Magrì, S.; Cingolani, A.; Paduano, D.; Pesenti, M.; Zara, F.; Tumbarello, F.; Urru, E.; Melis, A.; Casula, L.; et al. Multidimensional Impact of Mediterranean Diet on IBD Patients. Inflamm. Bowel Dis. 2020, 27, 1–9. [Google Scholar] [CrossRef]
- Aya, V.; Flórez, A.; Perez, L.; Ramírez, J.D.; Foster, J. Association between physical activity and changes in intestinal microbiota composition: A systematic review. PLoS ONE 2021, 16, e0247039. [Google Scholar] [CrossRef]
- Dahiya, D.S.; Kichloo, A.; Wani, F.; Singh, J.; Solanki, D.; Shaka, H. A nationwide analysis on the influence of obesity in inflammatory bowel disease hospitalizations. Intest. Res. 2021, 20, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Hass, D.; Brensinger, C.; Lewis, J.; Lichtenstein, G. The Impact of Increased Body Mass Index on the Clinical Course of Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2006, 4, 482–488. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Kumar, V.; Al-Abbasi, F.A.; Kamal, M.A.; Anwar, F. Risk of colorectal cancer in inflammatory bowel diseases. Semin. Cancer Biol. 2020, 64, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Blain, A.; Cattan, S.; Beaugerie, L.; Carbonnel, F.; Gendre, J.; Cosnes, J. Crohn’s disease clinical course and severity in obese patients. Clin. Nutr. 2002, 21, 51–57. [Google Scholar] [CrossRef]
- Yin, Y.; Xie, Y.; Ge, W.; Li, Y. Creeping fat formation and interaction with intestinal disease in Crohn’s disease. United Eur. Gastroenterol. J. 2022, 10, 1077–1084. [Google Scholar] [CrossRef]
- Dickson, I. Creeping fat in Crohn’s disease explained. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 713. [Google Scholar] [CrossRef]
- Hwang, N.; Kang, D.; Shin, S.-J.; Yoon, B.K.; Chun, J.; Kim, J.-W.; Fang, S. Creeping fat exhibits distinct Inflammation-specific adipogenic preadipocytes in Crohn’s disease. Front. Immunol. 2023, 14, 1198905. [Google Scholar] [CrossRef]
- Kim, K.; Park, S.; Lee, Y.; Baek, J.; Kim, Y.; Hwang, S.W.; Lee, J.L.; Park, S.H.; Yang, S.-K.; Han, B.; et al. Transcriptomic Profiling and Cellular Composition of Creeping Fat in Crohn’s disease. J. Crohn’s Colitis 2023, 18, 223–232. [Google Scholar] [CrossRef]
- Shu, W.; Wang, Y.; Li, C.; Zhang, L.; Zhuoma, D.; Yang, P.; Yan, G.; Chen, C.; Ba, Y.; Du, P.; et al. Single-cell Expression Atlas Reveals Cell Heterogeneity in the Creeping Fat of Crohn’s Disease. Inflamm. Bowel Dis. 2023, 29, 850–865. [Google Scholar] [CrossRef]
- Ha, C.W.; Martin, A.; Sepich-Poore, G.D.; Shi, B.; Wang, Y.; Gouin, K.; Humphrey, G.; Sanders, K.; Ratnayake, Y.; Chan, K.S.; et al. Translocation of Viable Gut Microbiota to Mesenteric Adipose Drives Formation of Creeping Fat in Humans. Cell 2020, 183, 666–683.e17. [Google Scholar] [CrossRef]
- Kredel, L.I.; Batra, A.; Stroh, T.; Kühl, A.A.; Zeitz, M.; Erben, U.; Siegmund, B. Adipokines from local fat cells shape the macrophage compartment of the creeping fat in Crohn’s disease. Gut 2012, 62, 852–862. [Google Scholar] [CrossRef]
- Paul, G.; Schäffler, A.; Neumeier, M.; Fürst, A.; Bataillle, F.; Buechler, C.; Müller-Ladner, U.; Schölmerich, J.; Rogler, G.; Herfarth, H. Profiling adipocytokine secretion from creeping fat in Crohn’s disease. Inflamm. Bowel. Dis. 2006, 12, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Doyon, G.; Gordon, I.O.; Li, J.; Lin, S.; Wang, J.; Le, T.H.N.; Elias, M.; Kurada, S.; Southern, B.; et al. Activated intestinal muscle cells promote preadipocyte migration: A novel mechanism for creeping fat formation in Crohn’s disease. Gut 2021, 71, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Kurada, S.; Gordon, I.O.; Baker, M.E.; Gandhi, N.; McDonald, C.; Coffey, J.C.; Rieder, F. The Mesenteric Fat and Intestinal Muscle Interface: Creeping Fat Influencing Stricture Formation in Crohn’s Disease. Inflamm. Bowel Dis. 2018, 25, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Mao, R.; Le, T.H.N.; West, G.; Varadharajan, V.; Banerjee, R.; Doyon, G.; Mukherjee, P.; Nguyen, Q.T.; Mulya, A.; et al. Creeping Fat–Derived Free Fatty Acids Induce Hyperplasia of Intestinal Muscularis Propria Muscle Cells: A Novel Link Between Fat and Intestinal Stricture Formation in Crohn’s Disease. Gastroenterology 2024, 168, 508–524. [Google Scholar] [CrossRef] [PubMed]
- Atreya, R.; Siegmund, B. Location is important: Differentiation between ileal and colonic Crohn’s disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 544–558. [Google Scholar] [CrossRef]
- Shu, W.; Wang, Y.; Chen, M.; Zhu, X.; Wang, F.; Chen, C.; Du, P.; Bartolomucci, A.; Su, X.; Wang, X. Extracellular vesicles derived from creeping fat stem cells promote lymphatic function and restrain inflammation of Crohn’s disease. Clin. Transl. Med. 2024, 14, e70086. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Xu, Y.; Wen, W.; Huang, L.; Guo, Z.; Zhu, W.; Li, Y. Exosomal miR-103a-3p from Crohn’s Creeping Fat-Derived Adipose-Derived Stem Cells Contributes to Intestinal Fibrosis by Targeting TGFBR3 and Activating Fibroblasts. J. Crohn’s Colitis 2023, 17, 1291–1308. [Google Scholar] [CrossRef]
- Serena, C.; Queipo-Ortuño, M.; Millan, M.; Sanchez-Alcoholado, L.; Caro, A.; Espina, B.; Menacho, M.; Bautista, M.; Monfort-Ferré, D.; Terrón-Puig, M.; et al. Microbial Signature in Adipose Tissue of Crohn’s Disease Patients. J. Clin. Med. 2020, 9, 2448. [Google Scholar] [CrossRef] [PubMed]
- Heinzel, S.; Jureczek, J.; Kainulainen, V.; Nieminen, A.I.; Suenkel, U.; von Thaler, A.-K.; Kaleta, C.; Eschweiler, G.W.; Brockmann, K.; Aho, V.T.E.; et al. Elevated fecal calprotectin is associated with gut microbial dysbiosis, altered serum markers and clinical outcomes in older individuals. Sci. Rep. 2024, 14, 13513. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, W.; Xie, H.; Cao, M.; Yang, J.; Xie, Y.; Luo, Z.; Zhang, Z.; Xu, H.; Huang, W.; et al. Microbiota-induced alteration of kynurenine metabolism in macrophages drives formation of creeping fat in Crohn’s disease. Cell Host Microbe 2024, 32, 1927–1943.e9. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Ashida, T.; Ebisawa, Y.; Chisato, N.; Okamoto, K.; Katsuno, H.; Maeda, K.; Fujiya, M.; Kohgo, Y.; Furukawa, H. A New Antimesenteric Functional End-to-End Handsewn Anastomosis: Surgical Prevention of Anastomotic Recurrence in Crohn’s Disease. Dis. Colon Rectum 2011, 54, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.H.; Chin, Y.H.; Lin, S.Y.; Koh, J.W.H.; Lieske, B.; Koh, F.H.; Chong, C.S.; Foo, F.J. Kono-S anastomosis for Crohn’s disease: A systemic review, meta-analysis, and meta-regression. Surg. Today 2021, 51, 493–501. [Google Scholar] [CrossRef]
- Luglio, G.; Rispo, A.; Imperatore, N.; Giglio, M.C.; Amendola, A.; Tropeano, F.P.; Peltrini, R.; Castiglione, F.; De Palma, G.D.; Bucci, L. Surgical Prevention of Anastomotic Recurrence by Excluding Mesentery in Crohn’s Disease: The SuPREMe-CD Study—A Randomized Clinical Trial. Ann. Surg. 2020, 272, 210–217. [Google Scholar] [CrossRef]
- Alibert, L.; Betton, L.; Falcoz, A.; Manceau, G.; Benoist, S.; Zerbib, P.; Podevin, J.; Maggiori, L.; Brouquet, A.; Tyrode, G.; et al. Does Kono-S Anastomosis Reduce Recurrence in Crohn’s Disease Compared with Conventional Ileocolonic Anastomosis? A Nationwide Propensity Score-matched Study from GETAID Chirurgie Group [KoCoRICCO Study]. J. Crohn’s Colitis 2023, 18, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Haanappel, A.E.G.; Bellato, V.; Buskens, C.J.; Armuzzi, A.; van der Bilt, J.D.W.; de Boer, N.K.H.; Danese, S.; Willebois, E.M.L.v.d.D.d.; Duijvestein, M.; van der Horst, D.; et al. Optimising surgical anastomosis in ileocolic resection for Crohn’s disease with respect to recurrence and functionality: Two international parallel randomized controlled trials comparing handsewn (END-to-end or Kono-S) to stapled anastomosis (HAND2END and the End2End STUDIES). BMC Surg. 2024, 24, 71. [Google Scholar] [CrossRef]
- Trencheva, K.; Spinelli, A.; Kienle, P.; D’HOore, A.; Luglio, G.; Flemming, S.; Scaringi, S.; Tropeano, F.; Christos, P.; Michelassi, F. OP20 Postoperative endoscopic recurrence after resection of Crohn’s terminal ileitis with Kono-S or side-to-side functional end anastomosis: Results of a Multicenter Prospective Randomized Trial. J. Crohn’s Colitis 2024, 18, i37. [Google Scholar] [CrossRef]
- Pompeu, B.F.; Marcolin, P.; Marques, F.I.L.C.B.; Soares, G.A.d.R.; e Silva, A.L.C.; Pigossi, B.D.; de Figueiredo, S.M.P.; Formiga, F.B. Extended versus limited mesenteric excision in bowel resection for Crohn’s disease: A meta-analysis and systematic review. Tech. Coloproctol. 2025, 29, 80. [Google Scholar] [CrossRef]
- Vaghiri, S.; Alipouriani, A.; Knoefel, W.T.; Kessler, H.; Prassas, D. Extended mesenteric resection reduces the rate of surgical recurrence in Crohn’s disease: A systematic review and meta-analysis. Int. J. Color. Dis. 2025, 40, 51. [Google Scholar] [CrossRef]
- Mostafa, O.E.S.; Zaman, S.; Malik, M.; Kumar, P.; Kumar, L.; Akingboye, A.; Sarma, D.; Peravali, R. Clinical outcomes of conventional versus extended mesenteric resection in limited ileo-colonic Crohn’s disease: A systematic review and meta-analysis. Int. J. Color. Dis. 2025, 40, 144. [Google Scholar] [CrossRef]
- Willebois, E.M.L.v.d.D.d.; Bellato, V.; Duijvestein, M.; van der Bilt, J.D.W.; van Dongen, K.; Spinelli, A.; D’HAens, G.R.; Mundt, M.W.; Furfaro, F.; Danese, S.; et al. Effect of mesenteric sparing or extended resection in primary ileocolic resection for Crohn’s disease on postoperative endoscopic recurrence (SPICY): An international, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2024, 9, 793–801. [Google Scholar] [CrossRef]
- Peppas, S.; Piovani, D.; Peyrin-Biroulet, L.; Danese, S.; Bonovas, S. Statins and inflammatory bowel disease: Where do we stand? Eur. J. Intern. Med. 2020, 75, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Wanchaitanawong, W.; Thinrungroj, N.; Chattipakorn, S.C.; Chattipakorn, N.; Shinlapawittayatorn, K. Repurposing metformin as a potential treatment for inflammatory bowel disease: Evidence from cell to the clinic. Int. Immunopharmacol. 2022, 112, 109230. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Cai, S.; Chen, Y.; Hou, X.; Zhang, J.; Bi, C.; Gu, P.; Yang, M.; Zhang, H.; Zhong, W.; et al. Atorvastatin improved ulcerative colitis in association with gut microbiota-derived tryptophan metabolism. Life Sci. 2024, 351, 122790. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.K.; Cho, J.H.; Kim, E.J.; Kim, E.-K.; Park, D.K.; Kwon, K.A.; Chung, J.-W.; Kim, K.O.; Kim, Y.J. Anti-inflammatory and anti-apoptotic effects of rosuvastatin by regulation of oxidative stress in a dextran sulfate sodium-induced colitis model. World J. Gastroenterol. 2017, 23, 4559–4568. [Google Scholar] [CrossRef]
- Naito, Y.; Katada, K.; Takagi, T.; Tsuboi, H.; Isozaki, Y.; Handa, O.; Kokura, S.; Yoshida, N.; Ichikawa, H.; Yoshikawa, T. Rosuvastatin, a new HMG-CoA reductase inhibitor, reduces the colonic inflammatory response in dextran sulfate sodium-induced colitis in mice. Int. J. Mol. Med. 2006, 17, 997–1004. [Google Scholar] [CrossRef][Green Version]
- Côté-Daigneault, J.; Mehandru, S.; Ungaro, R.; Atreja, A.; Colombel, J.F. Potential Immunomodulatory Effects of Statins in Inflammatory Bowel Disease. Inflamm. Bowel. Dis. 2016, 22, 724–732. [Google Scholar] [CrossRef]
- Lochhead, P.; Khalili, H.; Sachs, M.C.; Chan, A.T.; Olén, O.; Ludvigsson, J.F. Association Between Statin Use and Inflammatory Bowel Diseases: Results from a Swedish, Nationwide, Population-based Case-control Study. J. Crohn’s Colitis 2020, 15, 757–765. [Google Scholar] [CrossRef]
- Ungaro, R.; Chang, H.L.; Côté-Daigneault, J.; Mehandru, S.; Atreja, A.; Colombel, J.F. Statins Associated With Decreased Risk of New Onset Inflammatory Bowel Disease. Am. J. Gastroenterol. 2016, 111, 1416–1423. [Google Scholar] [CrossRef]
- Khalil, D.; Boktor, M.; Mortensen, E.M.; Frei, C.R.; Mansi, I. Comparison of Frequency of Inflammatory Bowel Disease and Noninfectious Gastroenteritis Among Statin Users Versus Nonusers. Am. J. Cardiol. 2015, 115, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, S.H.; Yang, E.J.; Kim, E.K.; Kim, J.K.; Shin, D.Y.; Cho, M.L. Metformin Ameliorates Inflammatory Bowel Disease by Suppression of the STAT3 Signaling Pathway and Regulation of the between Th17/Treg Balance. PLoS ONE 2015, 10, e0135858. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, S.; Liu, Q.; Shan, T.; Wang, Y. Metformin Protects against LPS-Induced Intestinal Barrier Dysfunction by Activating AMPK Pathway. Mol. Pharm. 2018, 15, 3272–3284. [Google Scholar] [CrossRef] [PubMed]
- Takahara, M.; Takaki, A.; Hiraoka, S.; Takei, K.; Yasutomi, E.; Igawa, S.; Yamamoto, S.; Oka, S.; Ohmori, M.; Yamasaki, Y.; et al. Metformin ameliorates chronic colitis in a mouse model by regulating interferon-γ-producing lamina propria CD4+ T cells through AMPK activation. FASEB J. 2022, 36, e22139. [Google Scholar] [CrossRef]
- Pandey, A.; Verma, S.; Kumar, V.L. Metformin maintains mucosal integrity in experimental model of colitis by inhibiting oxidative stress and pro-inflammatory signaling. Biomed. Pharmacother. 2017, 94, 1121–1128. [Google Scholar] [CrossRef]
- Tseng, C.-H. Metformin Use Is Associated with a Lower Risk of Inflammatory Bowel Disease in Patients with Type 2 Diabetes Mellitus. J. Crohn’s Colitis 2020, 15, 64–73. [Google Scholar] [CrossRef]
- Petrov, J.C.; Desai, A.A.; Kochhar, G.S.; Crosby, S.K.; Kinnucan, J.A.; Picco, M.F.; Hashash, J.G.; Farraye, F.A. Metformin Is Associated With Improved Inflammatory Bowel Disease Outcomes in Patients With Type 2 Diabetes Mellitus: A Propensity-Matched Cohort Study. Inflamm. Bowel Dis. 2024, 31, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Allin, K.H.; Jensen, C.B.; Jacobsen, R.K.; Jess, T. Metformin use is not associated with reduced risk of older onset inflammatory bowel disease: A Danish nationwide population-based study. J. Gastroenterol. 2022, 57, 761–769. [Google Scholar] [CrossRef]
- Gravina, A.G.; Pellegrino, R.; Izzo, M.; De Costanzo, I.; Imperio, G.; Landa, F.; Tambaro, A.; Federico, A. Relevance of Glucagon-Like Peptide 1 (GLP-1) in Inflammatory Bowel Diseases: A Narrative Review. Curr. Issues Mol. Biol. 2025, 47, 383. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef]
- Bang-Berthelsen, C.H.; Holm, T.L.; Pyke, C.; Simonsen, L.; Søkilde, R.; Pociot, F.; Heller, R.S.; Folkersen, L.; Kvist, P.H.; Jackerott, M.; et al. GLP-1 Induces Barrier Protective Expression in Brunnerʼs Glands and Regulates Colonic Inflammation. Inflamm. Bowel Dis. 2016, 22, 2078–2097. [Google Scholar] [CrossRef]
- Kato, S.; Sato, T.; Fujita, H.; Kawatani, M.; Yamada, Y. Effects of GLP-1 receptor agonist on changes in the gut bacterium and the underlying mechanisms. Sci. Rep. 2021, 11, 9167. [Google Scholar] [CrossRef]
- Bray, J.J.H.; Foster-Davies, H.; Salem, A.; Hoole, A.L.; Obaid, D.R.; Halcox, J.P.J.; Stephens, J.W. Glucagon-like peptide-1 receptor agonists improve biomarkers of inflammation and oxidative stress: A systematic review and meta-analysis of randomised controlled trials. Diabetes. Obes. Metab. 2021, 23, 1806–1822. [Google Scholar] [CrossRef]
- Villumsen, M.; Schelde, A.B.; Jimenez-Solem, E.; Jess, T.; Allin, K.H. GLP-1 based therapies and disease course of inflammatory bowel disease. eClinicalMedicine 2021, 37, 100979. [Google Scholar] [CrossRef]
- Desai, A.; Sehgal, P.; Khataniar, H.; Lewis, J.D.; Farraye, F.A.; Lichtenstein, G.R.; Kochhar, G.S. Obesity Is Associated With Worsened Outcomes in Patients With Ulcerative Colitis on Advanced Therapies: A Propensity Matched Cohort Study From the U.S. Aliment. Pharmacol. Ther. 2025, 61, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, Y.; Ghersin, I.; Lujan, R.; Shlon, D.; Weisband, Y.L.; Ben-Tov, A.; Matz, E.; Zacay, G.; Dotan, I.; Turner, D.; et al. GLP-1 Analog Use is Associated With Improved Disease Course in Inflammatory Bowel Disease: A Report from the Epi-IIRN. J. Crohn’s Colitis 2024, 19, jjae160. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Friedman, S.; Nørgård, B.M.; Knudsen, T.; Kjeldsen, J.; Wod, M. Glucagon-Like Peptide 1 Receptor Agonists Are Not Associated With an Increased Risk of Ileus or Intestinal Obstruction in Patients with Inflammatory Bowel Disease—A Danish Nationwide Cohort Study. Inflamm. Bowel Dis. 2024, 31, 1961–1965. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Klein, J.; Choi, N.K.; Fear, E.; Pannain, S.; Rubin, D.T. Efficacy and Safety of GLP-1 Agonists on Metabolic Parameters in Non-diabetic Patients with Inflammatory Bowel Disease. Dig. Dis. Sci. 2024, 69, 4437–4445. [Google Scholar] [CrossRef] [PubMed]
- Levine, I.; Sekhri, S.; Schreiber-Stainthorp, W.; Locke, B.; Delau, O.; Elhawary, M.; Pandit, K.; Meng, X.; Axelrad, J. GLP-1 Receptor Agonists Confer No Increased Rates of IBD Exacerbation Among Patients With IBD. Inflamm. Bowel Dis. 2024, 31, 467–475. [Google Scholar] [CrossRef]
- Anderson, S.R.; Ayoub, M.; Coats, S.; McHenry, S.; Tan, T.; Deepak, P. Safety and Effectiveness of Glucagon-like Peptide-1 Receptor Agonists in Inflammatory Bowel Disease. Am. J. Gastroenterol. 2025, 120, 1152–1155. [Google Scholar] [CrossRef]
- Ramos Belinchón, C.; Martínez-Lozano, H.; Serrano Moreno, C.; Hernández Castillo, D.; Lois Chicharro, P.; Ferreira Ocampo, P.; Marín-Jiménez, I.; Bretón Lesmes, I.; Menchén, L. Effectiveness and safety of a GLP-1 agonist in obese patients with inflammatory bowel disease. Rev. Esp. Enferm. Dig. 2024, 116, 478–483. [Google Scholar] [CrossRef]

| Author | Year | Study Design | Data Source | N | Obesity Measurement | Patient Characteristics | Outcome for Inflammatory Bowel Disease |
|---|---|---|---|---|---|---|---|
| Cañete [58] | 2025 | Population-based retrospective cohort | Catalan Health Surveillance System (CHSS) | 1,117,427 | Clinical diagnosis of obesity/severe obesity and bariatric surgery | General adult population | Increased risk of severe obesity with CD and UC |
| Chan [56] | 2013 | Prospective cohort | European Prospective Investigation into Cancer and Nutrition (EPIC) | 300,724 | BMI measured at baseline | General adult population | No association |
| Harpsoe [57] | 2014 | Prospective cohort | Danish National Birth Cohort (DNBC) | 75,008 | Self-reported pre-pregnancy BMI | General adult population | No association |
| Jensen [55] | 2018 | Prospective cohort | Copenhagen School Health Records Register (CSHRR) | 316,799 | BMI z-score between ages 7 and 13 | Pediatric population | Inverse association with UC and direct association with CD |
| Khalili [53] | 2015 | Prospective cohort | Nurses’ Health Study II (EE.UU.) | 111,498 | Current BMI, BMI at age 18, weight, body shape, and waist and hip measurements | Nurses | Increased risk of CD |
| Mendall [54] | 2018 | Prospective cohort | Danish National Birth Cohort (DNBC) | 74,512 | Pre-pregnancy BMI and BMI 18 months postpartum | Pregnant women | Increased risk of CD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas-Deza, D.; García-López, S.; Bernal-Monterde, V.; Polo-Cuadro, C.; Yagüe-Caballero, C.; Arbones-Mainar, J.M. Obesity-Mediated Inflammation and Its Influence on Inflammatory Bowel Disease: Pathophysiology, Clinical Impact, and Therapeutic Implications. Biomolecules 2025, 15, 1185. https://doi.org/10.3390/biom15081185
Casas-Deza D, García-López S, Bernal-Monterde V, Polo-Cuadro C, Yagüe-Caballero C, Arbones-Mainar JM. Obesity-Mediated Inflammation and Its Influence on Inflammatory Bowel Disease: Pathophysiology, Clinical Impact, and Therapeutic Implications. Biomolecules. 2025; 15(8):1185. https://doi.org/10.3390/biom15081185
Chicago/Turabian StyleCasas-Deza, Diego, Santiago García-López, Vanesa Bernal-Monterde, Cristina Polo-Cuadro, Carmen Yagüe-Caballero, and José M. Arbones-Mainar. 2025. "Obesity-Mediated Inflammation and Its Influence on Inflammatory Bowel Disease: Pathophysiology, Clinical Impact, and Therapeutic Implications" Biomolecules 15, no. 8: 1185. https://doi.org/10.3390/biom15081185
APA StyleCasas-Deza, D., García-López, S., Bernal-Monterde, V., Polo-Cuadro, C., Yagüe-Caballero, C., & Arbones-Mainar, J. M. (2025). Obesity-Mediated Inflammation and Its Influence on Inflammatory Bowel Disease: Pathophysiology, Clinical Impact, and Therapeutic Implications. Biomolecules, 15(8), 1185. https://doi.org/10.3390/biom15081185







