Gut Hormones and Inflammatory Bowel Disease
Abstract
1. Obesity, Inflammation, and Inflammatory Bowel Disease
2. Glucagon-like Peptide
3. Glucose-Dependent Insulinotropic Polypeptide
4. Peptide YY
5. Cholecystokinin
6. Apolipoprotein A4
7. Synergistic Actions of Gut Hormones in IBD Regulation
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APOA4 | Apolipoprotein A4 |
| BAT | Brown adipose tissue |
| BMI | Body mass index |
| CCK | Cholecystokinin |
| CCK-1R | CCK1 receptor |
| CCK-2R | CCK2 receptor |
| CD | Crohn’s disease |
| cAMP | Cyclic adenosine monophosphate |
| DPP-4 | Dipeptidyl peptidase-4 |
| FA | Fatty acid |
| FOXP3 | Forkhead box protein P3 |
| GIP | Glucose-dependent insulinotropic polypeptide |
| GIP-R | GIP receptor |
| GLP-1 | Glucagon-like peptide-1 |
| GLP-2 | Glucagon-like peptide-2 |
| GLP-1R | GLP-1 receptor |
| HDL | High-density lipoprotein |
| HFD | High-fat diet |
| IBD | Inflammatory bowel disease |
| IFN-γ | Interferon-γ |
| IL-1α | Interleukin-1α |
| JNK | c-Jun N-terminal kinase |
| KO | Knockout |
| LRP1 | Low-density lipoprotein receptor-related protein 1 |
| NF-κB | Nuclear factor kappa B |
| NPY-Y2 | NPY receptor type 2 |
| PI3K | Phosphoinositide 3-kinase |
| PYY | Peptide YY |
| SCFA | Short-chain fatty acid |
| TG | Triglyceride |
| TNF-α | Tumor necrosis factor-α |
| UC | Ulcerative colitis |
| VIP | Vasoactive intestinal polypeptide |
| WAT | White adipose tissue |
References
- Centers for Disease Control and Prevention. Adult Obesity Prevalence USA; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2023.
- Arvanitakis, K.; Koufakis, T.; Popovic, D.; Maltese, G.; Mustafa, O.; Doumas, M.; Giouleme, O.; Kotsa, K.; Germanidis, G. GLP-1 Receptor Agonists in Obese Patients with Inflammatory Bowel Disease: From Molecular Mechanisms to Clinical Considerations and Practical Recommendations for Safe and Effective Use. Curr. Obes. Rep. 2023, 12, 61–74. [Google Scholar] [CrossRef]
- Calkins, B.M.; Mendeloff, A.I. Epidemiology of Inflammatory Bowel Disease. Epidemiol. Rev. 1986, 8, 60–91. [Google Scholar] [CrossRef]
- Lynn, A.; Harmsen, W.; Tremaine, W.; Loftus, E. Su1872-Trends in the Prevalence of Overweight and Obesity at the Time of Inflammatory Bowel Disease Diagnosis: A Population-Based Study. Gastroenterology 2018, 154, S-614–S-615. [Google Scholar] [CrossRef]
- Kim, J.H.; Yoo, J.H.; Oh, C.M. Obesity and Novel Management of Inflammatory Bowel Disease. World J. Gastroenterol. 2023, 29, 1779–1794. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dulai, P.S.; Zarrinpar, A.; Ramamoorthy, S.; Sandborn, W.J. Obesity in IBD: Epidemiology, Pathogenesis, Disease Course and Treatment Outcomes. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.J.; West, N.P.; Cripps, A.W. Obesity, Inflammation, and the Gut Microbiota. Lancet Diabetes Endocrinol. 2015, 3, 207–215. [Google Scholar] [CrossRef]
- Gallagher, E.J.; Leroith, D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol. Rev. 2015, 95, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary Intake and Risk of Developing Inflammatory Bowel Disease: A Systematic Review of the Literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic Inflammation in Fat Plays a Crucial Role in the Development of Obesity-Related Insulin Resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity Is Associated with Macrophage Accumulation in Adipose Tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory Mechanisms Linking Obesity and Metabolic Disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Paik, J.; Fierce, Y.; Treuting, P.M.; Brabb, T.; Maggio-Price, L. High-Fat Diet-Induced Obesity Exacerbates Inflammatory Bowel Disease in Genetically Susceptible Mdr1a-/- Male Mice. J. Nutr. 2013, 143, 1240–1247. [Google Scholar] [CrossRef]
- Kim, S.; Seo, S.U.; Kweon, M.N. Gut Microbiota-Derived Metabolites Tune Host Homeostasis Fate. Semin. Immunopathol 2024, 46, 2. [Google Scholar] [CrossRef] [PubMed]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef] [PubMed]
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermúdez-Humarán, L.G.; Smirnova, N.; Bergé, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal Mucosal Adherence and Translocation of Commensal Bacteria at the Early Onset of Type 2 Diabetes: Molecular Mechanisms and Probiotic Treatment. EMBO Mol. Med. 2011, 3, 559–572. [Google Scholar] [CrossRef]
- Kim, C.S.; Lee, S.C.; Kim, Y.M.; Kim, B.S.; Choi, H.S.; Kawada, T.; Kwon, B.S.; Yu, R. Visceral Fat Accumulation Induced by a High-Fat Diet Causes the Atrophy of Mesenteric Lymph Nodes in Obese Mice. Obesity 2008, 16, 1261–1269. [Google Scholar] [CrossRef]
- Osborn, O.; Olefsky, J.M. The Cellular and Signaling Networks Linking the Immune System and Metabolism in Disease. Nat. Med. 2012, 18, 363–374. [Google Scholar] [CrossRef]
- Drouet, M.; Dubuquoy, L.; Desreumaux, P.; Bertin, B. Visceral Fat and Gut Inflammation. Nutrition 2012, 28, 113–117. [Google Scholar] [CrossRef]
- Reilly, S.M.; Saltiel, A.R. Adapting to Obesity with Adipose Tissue Inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef]
- Crewe, C.; An, Y.A.; Scherer, P.E. The Ominous Triad of Adipose Tissue Dysfunction: Inflammation, Fibrosis, and Impaired Angiogenesis. J. Clin. Investig. 2017, 127, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Geerling, B.J.; Dagnelie, P.C.; Badart-Smook, A.; Russel, M.G.; Stockbrügger, R.W.; Brummer, R.-J.M. Diet as a Risk Factor for the Development of Ulcerative Colitis. Am. J. Gastroenterol. 2000, 95, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Yap, Y.A.; Mariño, E. An Insight into the Intestinal Web of Mucosal Immunity, Microbiota, and Diet in Inflammation. Front. Immunol. 2018, 9, 2617. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Nakae, J.; Watanabe, N.; Kikuchi, T.; Tateya, S.; Tamori, Y.; Kaneko, M.; Abe, T.; Onodera, M.; Itoh, H. Colonic Pro-Inflammatory Macrophages Cause Insulin Resistance in an Intestinal Ccl2/Ccr2-Dependent Manner. Cell Metab. 2016, 24, 295–310. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative Colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s Disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Mishra, S.P.; Wang, B.; Jain, S.; Ding, J.; Rejeski, J.; Furdui, C.M.; Kitzman, D.W.; Taraphder, S.; Brechot, C.; Kumar, A.; et al. A Mechanism by Which Gut Microbiota Elevates Permeability and Inflammation in Obese/Diabetic Mice and Human Gut. Gut 2023, 72, 1848–1865. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, C.; Heo, S.; Kim, B.; Hyun, C.K. DSS-Induced Colitis Is Associated with Adipose Tissue Dysfunction and Disrupted Hepatic Lipid Metabolism Leading to Hepatosteatosis and Dyslipidemia in Mice. Sci. Rep. 2021, 11, 5283. [Google Scholar] [CrossRef]
- Acciarino, A.; Diwakarla, S.; Handreck, J.; Bergola, C.; Sahakian, L.; McQuade, R.M. The Role of the Gastrointestinal Barrier in Obesity-Associated Systemic Inflammation. Obes. Rev. 2024, 25, e13673. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, C.R.; Caruso, C.; Candore, G. The Role of Adipose Tissue and Adipokines in Obesity-Related Inflammatory Diseases. Mediat. Inflamm. 2010, 2010, 802078. [Google Scholar] [CrossRef] [PubMed]
- Olefsky, J.M.; Glass, C.K. Macrophages, Inflammation, and Insulin Resistance. Annu. Rev. Physiol. 2009, 72, 219–246. [Google Scholar] [CrossRef]
- Kredel, L.; Batra, A.; Siegmund, B. Role of Fat and Adipokines in Intestinal Inflammation. Curr. Opin. Gastroenterol. 2014, 30, 559–565. [Google Scholar] [CrossRef]
- Zulian, A.; Cancello, R.; Micheletto, G.; Gentilini, D.; Gilardini, L.; Danelli, P.; Invitti, C. Visceral Adipocytes: Old Actors in Obesity and New Protagonists in Crohn’s Disease? Gut 2012, 61, 86–94. [Google Scholar] [CrossRef]
- Batra, A.; Zeitz, M.; Siegmund, B. Adipokine Signaling in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2009, 15, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; Scott, C.L.; Uronen-Hansson, H.; Gudjonsson, S.; Jansson, O.; Grip, O.; Guilliams, M.; Malissen, B.; Agace, W.W.; Mowat, A.M.I. Resident and Pro-Inflammatory Macrophages in the Colon Represent Alternative Context-Dependent Fates of the Same Ly6C Hi Monocyte Precursors. Mucosal Immunol. 2013, 6, 498–510. [Google Scholar] [CrossRef]
- Reinecker, H.-C.; Steffen, M.; Witthoeft, T.; Pflueger, I.; Schreiber, S.; Macdermottt, R.P.; Raedler, A. Enhanced Secretion of Tumour Necrosis Factor-Alpha, IL-6, and IL-1fi by Isolated Lamina Propria Mononuclear Cells from Patients with Ulcerative Colitis and Crohn’s Disease. Clin. Exp. Immunol. 1993, 94, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Reimund, J.; Wittersheim, C.; Dumont, S.; Muller, C.D.; Kenney, J.S.; Baumann, R.; Poindron, P.; Reimund Dumont, P.; Poindron, J.S.; Muller J S Kenney Antibody Solutions, C.D.; et al. Increased Production of Tumour Necrosis Factor-OL, Interleukin-11, and Interleukin-6 by Morphologically Normal Intestinal Biopsies from Patients with Crohn’s Disease. Gut 1996, 39, 684–689. [Google Scholar] [CrossRef]
- Kamada, N.; Hisamatsu, T.; Okamoto, S.; Chinen, H.; Kobayashi, T.; Sato, T.; Sakuraba, A.; Kitazume, M.T.; Sugita, A.; Koganei, K.; et al. Unique CD14+ Intestinal Macrophages Contribute to the Pathogenesis of Crohn Disease via IL-23/IFN-γ Axis. J. Clin. Investig. 2008, 118, 2269–2280. [Google Scholar] [CrossRef]
- Takayama, T.; Kamada, N.; Chinen, H.; Okamoto, S.; Kitazume, M.T.; Chang, J.; Matuzaki, Y.; Suzuki, S.; Sugita, A.; Koganei, K.; et al. Imbalance of NKp44+NKp46− and NKp44 −NKp46+ Natural Killer Cells in the Intestinal Mucosa of Patients with Crohn’s Disease. Gastroenterology 2010, 139, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Mitsialis, V.; Wall, S.; Liu, P.; Ordovas-Montanes, J.; Parmet, T.; Vukovic, M.; Spencer, D.; Field, M.; McCourt, C.; Toothaker, J.; et al. Single-Cell Analyses of Colon and Blood Reveal Distinct Immune Cell Signatures of Ulcerative Colitis and Crohn’s Disease. Gastroenterology 2020, 159, 591–608.e10. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, T.; West, G.A.; Youngman, K.R.; Klein, J.S.; Fiocchi, C. Immune Activation Genes in Inflammatory Bowel Disease. Gastroenterology 1993, 104, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, C.; Battisto, J.R.; Farmer, R.G. Studies on Isolated Gut Mucosal Lymphocytes in Inflammatory Bowel Disease. Dig. Dis. Sci. 1981, 26, 728–736. [Google Scholar] [CrossRef]
- Alexander, K.L.; Zhao, Q.; Reif, M.; Rosenberg, A.F.; Mannon, P.J.; Duck, L.W.; Elson, C.O. Human Microbiota Flagellins Drive Adaptive Immune Responses in Crohn’s Disease. Gastroenterology 2021, 161, 522–535.e6. [Google Scholar] [CrossRef]
- Calderón-Gómez, E.; Bassolas-Molina, H.; Mora-Buch, R.; Dotti, I.; Planell, N.; Esteller, M.; Gallego, M.; Martí, M.; Garcia-Martín, C.; Martínez-Torró, C.; et al. Commensal-Specific CD4+ Cells from Patients with Crohn’s Disease Have a T-Helper 17 Inflammatory Profile. Gastroenterology 2016, 151, 489–500.e3. [Google Scholar] [CrossRef]
- Pedersen, T.K.; Brown, E.M.; Plichta, D.R.; Johansen, J.; Twardus, S.W.; Delorey, T.M.; Lau, H.; Vlamakis, H.; Moon, J.J.; Xavier, R.J.; et al. The CD4+ T Cell Response to a Commensal-Derived Epitope Transitions from a Tolerant to an Inflammatory State in Crohn’s Disease. Immunity 2022, 55, 1909–1923.e6. [Google Scholar] [CrossRef]
- Panda, S.K.; Colonna, M. Innate Lymphoid Cells in Mucosal Immunity. Front. Immunol. 2019, 10, 861. [Google Scholar] [CrossRef]
- Caron, B.; Honap, S.; Peyrin-Biroulet, L. Epidemiology of Inflammatory Bowel Disease across the Ages in the Era of Advanced Therapies. J. Crohns Colitis 2024, 18, ii3–ii15. [Google Scholar] [CrossRef]
- Bhagavathula, A.S.; Clark, C.C.T.; Rahmani, J.; Chattu, V.K. Impact of Body Mass Index on the Development of Inflammatory Bowel Disease: A Systematic Review and Dose-Response Analysis of 15.6 Million Participants. Healthcare 2021, 9, 35. [Google Scholar] [CrossRef]
- Sehgal, P.; Shen, B.; Li, J.; Freedberg, D.E. Obesity among Those Newly Diagnosed with Crohn’s Disease and Ulcerative Colitis Compared with the General Population. Frontline Gastroenterol. 2023, 14, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Schölmerich, J. Extraintestinal Manifestations and Complications in IBD. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 585–595. [Google Scholar] [CrossRef]
- Fakhoury, M.; Negrulj, R.; Mooranian, A.; Al-Salami, H. Inflammatory Bowel Disease: Clinical Aspects and Treatments. J. Inflamm. Res. 2014, 7, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Iacucci, M.; Santacroce, G.; Majumder, S.; Morael, J.; Zammarchi, I.; Maeda, Y.; Ryan, D.; Di Sabatino, A.; Rescigno, M.; Aburto, M.R.; et al. Opening the Doors of Precision Medicine: Novel Tools to Assess Intestinal Barrier in Inflammatory Bowel Disease and Colitis-Associated Neoplasia. Gut 2024, 73, 1749–1762. [Google Scholar] [CrossRef]
- Kuhn, R.; Löhler, I.; Rennick, D.; Rajewsky, K.; Moiler, W. Interleukin-LO-Deficient Mice Develop Chronic Enterocolitis. Cell 1993, 75, 263–274. [Google Scholar] [CrossRef]
- Monteleone, G.; Macdonald, T.T.; Wathen, N.C.; Pallone, F.; Pender, S.L.F. Enhancing Lamina Propria Th1 Cell Responses with Interleukin 12 Produces Severe Tissue Injury. Gastroenterology 1999, 117, 1069–1077. [Google Scholar] [CrossRef]
- Powrie, F.; Leach, M.W.; Mauze, S.; Menon, S.; Barcomb Caddle, L.; Coffman, R.L. Inhibition of Thl Responses Prevents Inflammatory Bowel Disease in Scid Mice Reconstituted with CD45RBhi CD4+ T Cells. Immunity 1994, 1, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Isaacs, K.L.; Schneider, Y.; Siddique, S.M.; Falck-Ytter, Y.; Singh, S.; Chachu, K.; Day, L.; Lebwohl, B.; Muniraj, T.; et al. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020, 158, 1450–1461. [Google Scholar] [CrossRef]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European Evidence-Based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohns Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology Consensus Guidelines on the Management of Inflammatory Bowel Disease in Adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef]
- Neurath, M.F. Current and Emerging Therapeutic Targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 269–278. [Google Scholar] [CrossRef] [PubMed]
- McLean, L.P.; Cross, R.K. Adverse Events in IBD: To Stop or Continue Immune Suppressant and Biologic Treatment. Expert. Rev. Gastroenterol. Hepatol. 2014, 8, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Donaldson, T.; Lasch, K.; Yajnik, V. Management of Inflammatory Bowel Disease in the Elderly Patient. Inflamm. Bowel Dis. 2017, 23, 882–893. [Google Scholar] [CrossRef]
- Woods, S.C.; D’Alessio, D.A. Central Control of Body Weight and Appetite. J. Clin. Endocrinol. Metab. 2008, 93, s37–s50. [Google Scholar] [CrossRef]
- Woods, S.C.; May-Zhang, A.A.; Begg, D.P. How and Why Do Gastrointestinal Peptides Influence Food Intake? Physiol. Behav. 2018, 193, 218–222. [Google Scholar] [CrossRef]
- Wren, A.M.; Bloom, S.R. Gut Hormones and Appetite Control. Gastroenterology 2007, 132, 2116–2130. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Sun, E.W.; Keating, D.J. Mechanisms Controlling Hormone Secretion in Human Gut and Its Relevance to Metabolism. J. Endocrinol. 2020, 244, R1–R15. [Google Scholar] [CrossRef]
- Murphy, K.G.; Bloom, S.R. Gut Hormones and the Regulation of Energy Homeostasis. Nature 2006, 444, 854–859. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Woods, S.C.; Porte, D.; Seeley, R.J.; Baskin, D.G. Central Nervous System Control of Food Intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef]
- Koliaki, C.; Liatis, S.; Dalamaga, M.; Kokkinos, A. The Implication of Gut Hormones in the Regulation of Energy Homeostasis and Their Role in the Pathophysiology of Obesity. Curr. Obes. Rep. 2020, 9, 255–271. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like Peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Qu, J.; Ko, C.W.; Tso, P.; Bhargava, A. Apolipoprotein A-IV: A Multifunctional Protein Involved in Protection against Atherosclerosis and Diabetes. Cells 2019, 8, 319. [Google Scholar] [CrossRef]
- Qi, K.K.; Wu, J.; Wan, J.; Men, X.M.; Xu, Z.W. Purified PEGylated Porcine Glucagon-like Peptide-2 Reduces the Severity of Colonic Injury in a Murine Model of Experimental Colitis. Peptides 2014, 52, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Moran, G.W.; Leslie, F.C.; McLaughlin, J.T. Crohn’s Disease Affecting the Small Bowel Is Associated with Reduced Appetite and Elevated Levels of Circulating Gut Peptides. Clin. Nutr. 2013, 32, 404–411. [Google Scholar] [CrossRef]
- Vowinkel, T.; Mori, M.; Krieglstein, C.F.; Russell, J.; Saijo, F.; Bharwani, S.; Turnage, R.H.; Davidson, W.S.; Tso, P.; Granger, D.N.; et al. Apolipoprotein A-IV Inhibits Experimental Colitis. J. Clin. Investig. 2004, 114, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J.; Andersen, D.B.; Grunddal, K.V. Actions of Glucagon-like Peptide-1 Receptor Ligands in the Gut. Br. J. Pharmo. 2022, 179, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.; Göke, R.; Richter, G.; Fehmann, H.-C.; Arnold, R.; Göke, B. Glucagon-Like Peptide-1 and Glucose-Dependent Insulin-Releasing Polypeptide Plasma Levels in Response to Nutrients. Digestion 1995, 56, 117–126. [Google Scholar] [CrossRef]
- Holst, J.J. The Physiology of Glucagon-like Peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef]
- Ritzel, U.; Fromme, A.; Ottleben, M.; Leonhardt, U.; Ramadori, G. Release of Glucagon-like Peptide-1 (GLP-1) by Carbohydrates in the Perfused Rat Ileum. Acta Diabetol. 1997, 34, 18–21. [Google Scholar] [CrossRef]
- Lu, W.J.; Yang, Q.; Yang, L.; Lee, D.; D’Alessio, D.; Tso, P. Chylomicron Formation and Secretion Is Required for Lipid-Stimulated Release of Incretins GLP-1 and GIP. Lipids 2012, 47, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Boushey, R.P.; Drucker, D.J.; Brubaker, P.L. Secretion of the Intestinotropic Hormone Glucagon-like Peptide 2 Is Differentially Regulated by Nutrients in Humans. Gastroenterology 1999, 117, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Yazbeck, R.; Howarth, G.S.; Abbott, C.A. Growth Factor Based Therapies and Intestinal Disease: Is Glucagon-like Peptide-2 the New Way Forward? Cytokine Growth Factor. Rev. 2009, 20, 175–184. [Google Scholar] [CrossRef]
- Morrow, N.M.; Hanson, A.A.; Mulvihill, E.E. Distinct Identity of GLP-1R, GLP-2R, and GIPR Expressing Cells and Signaling Circuits Within the Gastrointestinal Tract. Front. Cell Dev. Biol. 2021, 9, 703966. [Google Scholar] [CrossRef]
- Hansen, L.; Deacon, C.F.; Ørskov, C.; Holst, J.J. Glucagon-Like Peptide-1-(7–36)Amide Is Transformed to Glucagon-Like Peptide-1-(9–36)Amide by Dipeptidyl Peptidase IV in the Capillaries Supplying the L Cells of the Porcine Intestine. Endocrinology 1999, 140, 5356–5363. [Google Scholar] [CrossRef]
- D’Alessio, D.; Lu, W.; Sun, W.; Zheng, S.; Yang, Q.; Seeley, R.; Woods, S.C.; Tso, P. Fasting and Postprandial Concentrations of GLP-1 in Intestinal Lymph and Portal Plasma: Evidence for Selective Release of GLP-1 in the Lymph System. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, 2163–2169. [Google Scholar] [CrossRef] [PubMed]
- Ghorpade, D.S.; Ozcan, L.; Zheng, Z.; Nicoloro, S.M.; Shen, Y.; Chen, E.; Blüher, M.; Czech, M.P.; Tabas, I. Hepatocyte-Secreted DPP4 in Obesity Promotes Adipose Inflammation and Insulin Resistance. Nature 2018, 555, 673–677. [Google Scholar] [CrossRef]
- Fadini, G.P.; Avogaro, A. Cardiovascular Effects of DPP-4 Inhibition: Beyond GLP-1. Vasc. Pharmacol. 2011, 55, 10–16. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Q.; Zhong, J.; Liu, C.; Zheng, B.; Gong, Q. DPP-4 Inhibitors as Potential Candidates for Antihypertensive Therapy: Improving Vascular Inflammation and Assisting the Action of Traditional Antihypertensive Drugs. Front. Immunol. 2019, 10, 1050. [Google Scholar] [CrossRef]
- Júnior, W.S.S.; Maria das Graças, C.S.; Kraemer-Aguiar, L.G. Dipeptidyl Peptidase 4 (DPP4), Adipose Inflammation, and Insulin Resistance: Is It Time to Look to the Hepatocyte? Hepatobiliary Surg. Nutr. 2018, 7, 499–500. [Google Scholar] [CrossRef] [PubMed]
- Kamrul-Hasan, A.B.M.; Dutta, D.; Nagendra, L.; Sharma, M.; Patra, S.; Bhattacharya, S. Role of Anagliptin, a Dipeptidyl Peptidase-4 Inhibitor, in Managing Type 2 Diabetes: A Systematic Review and Meta-Analysis. Medicine 2024, 103, e38870. [Google Scholar] [CrossRef]
- Chiba, Y.; Yamakawa, T.; Tsuchiya, H.; Oba, M.; Suzuki, D.; Danno, H.; Takatsuka, Y.; Shigematsu, H.; Kaneshiro, M.; Terauchi, Y. Effect of Anagliptin on Glycemic and Lipid Profile in Patients with Type 2 Diabetes Mellitus. J. Clin. Med. Res. 2018, 10, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Vardarli, I.; Arndt, E.; Deacon, C.F.; Holst, J.J.; Nauck, M.A. Effects of Sitagliptin and Metformin Treatment on Incretin Hormone and Insulin Secretory Responses to Oral and Isoglycemic Intravenous Glucose. Diabetes 2014, 63, 663–674. [Google Scholar] [CrossRef]
- Balas, B.; Baig, M.R.; Watson, C.; Dunning, B.E.; Ligueros-Saylan, M.; Wang, Y.; He, Y.L.; Darland, C.; Holst, J.J.; Deacon, C.F.; et al. The Dipeptidyl Peptidase IV Inhibitor Vildagliptin Suppresses Endogenous Glucose Production and Enhances Islet Function after Single-Dose Administration in Type 2 Diabetic Patients. J. Clin. Endocrinol. Metab. 2007, 92, 1249–1255. [Google Scholar] [CrossRef]
- Chong, S.C.; Sukor, N.; Robert, S.A.; Ng, K.F.; Kamaruddin, N.A. Endogenous GLP-1 Levels Play an Important Role in Determining the Efficacy of DPP-IV Inhibitors in Both Prediabetes and Type 2 Diabetes. Front. Endocrinol. 2022, 13, 1012412. [Google Scholar] [CrossRef]
- Holst, J.J.; Deacon, C.F. Glucagon-like Peptide-1 Mediates the Therapeutic Actions of DPP-IV Inhibitors. Diabetologia 2005, 48, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. The Incretin Effect in Healthy Individuals and Those with Type 2 Diabetes: Physiology, Pathophysiology, and Response to Therapeutic Interventions. Lancet Diabetes Endocrinol. 2016, 4, 525–536. [Google Scholar] [CrossRef]
- Yazbeck, R. Inhibiting Dipeptidyl Peptidase Activity Partially Ameliorates Colitis in Mice. Front. Biosci. 2008, 13, 6850–6858. [Google Scholar] [CrossRef]
- Salaga, M.; Binienda, A.; Draczkowski, P.; Kosson, P.; Kordek, R.; Jozwiak, K.; Fichna, J. Novel Peptide Inhibitor of Dipeptidyl Peptidase IV (Tyr-Pro-D-Ala-NH2) with Anti-Inflammatory Activity in the Mouse Models of Colitis. Peptides 2018, 108, 34–45. [Google Scholar] [CrossRef]
- Mimura, S.; Ando, T.; Ishiguro, K.; Maeda, O.; Watanabe, O.; Ujihara, M.; Hirayama, Y.; Morise, K.; Maeda, K.; Matsushita, M.; et al. Dipeptidyl Peptidase-4 Inhibitor Anagliptin Facilitates Restoration of Dextran Sulfate Sodium-Induced Colitis. Scand. J. Gastroenterol. 2013, 48, 1152–1159. [Google Scholar] [CrossRef]
- Drucker, D.J. Perspectives in Diabetes Glucagon-Like Peptides. Diabetes 1998, 47, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Radel, J.A.; Pender, D.N.; Shah, S.A. Dipeptidyl Peptidase-4 Inhibitors and Inflammatory Bowel Disease Risk: A Meta-Analysis. Ann. Pharmacother. 2019, 53, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Schneeweiss, S.; Glynn, R.J.; Doherty, M.; Goldfine, A.B.; Solomon, D.H. Dipeptidyl Peptidase-4 Inhibitors in Type 2 Diabetes May Reduce the Risk of Autoimmune Diseases: A Population-Based Cohort Study. Ann. Rheum. Dis. 2015, 74, 1968–1975. [Google Scholar] [CrossRef] [PubMed]
- Abrahami, D.; Douros, A.; Yin, H.; Yu, O.H.Y.; Renoux, C.; Bitton, A.; Azoulay, L. Dipeptidyl Peptidase-4 Inhibitors and Incidence of Inflammatory Bowel Disease among Patients with Type 2 Diabetes: Population Based Cohort Study. BMJ 2018, 360, k872. [Google Scholar] [CrossRef]
- Kridin, K.; Amber, K.; Khamaisi, M.; Comaneshter, D.; Batat, E.; Cohen, A.D. Is There an Association between Dipeptidyl Peptidase-4 Inhibitors and Autoimmune Disease? A Population-Based Study. Immunol. Res. 2018, 66, 425–430. [Google Scholar] [CrossRef]
- Shinzaki, S.; Sato, T.; Fukui, H. Antidiabetic Drugs for IBD: A Long but Promising Road Ahead for Drug Repositioning to Target Intestinal Inflammation. J. Gastroenterol. 2023, 58, 598–599. [Google Scholar] [CrossRef]
- Brierley, D.I.; Holt, M.K.; Singh, A.; de Araujo, A.; McDougle, M.; Vergara, M.; Afaghani, M.H.; Lee, S.J.; Scott, K.; Maske, C.; et al. Central and Peripheral GLP-1 Systems Independently Suppress Eating. Nat. Metab. 2021, 3, 258–273. [Google Scholar] [CrossRef]
- Abbott, C.R.; Monteiro, M.; Small, C.J.; Sajedi, A.; Smith, K.L.; Parkinson, J.R.C.; Ghatei, M.A.; Bloom, S.R. The Inhibitory Effects of Peripheral Administration of Peptide YY3-36 and Glucagon-like Peptide-1 on Food Intake Are Attenuated by Ablation of the Vagal-Brainstem-Hypothalamic Pathway. Brain Res. 2005, 1044, 127–131. [Google Scholar] [CrossRef]
- Labouesse, M.A.; Stadlbauer, U.; Weber, E.; Arnold, M.; Langhans, W.; Pacheco-López, G. Vagal Afferents Mediate Early Satiation and Prevent Flavour Avoidance Learning in Response to Intraperitoneally Infused Exendin-4. J. Neuroendocrinol. 2012, 24, 1505–1516. [Google Scholar] [CrossRef]
- Hayes, M.R.; Kanoski, S.E.; De Jonghe, B.C.; Leichner, T.M.; Alhadeff, A.L.; Fortin, S.M.; Arnold, M.; Langhans, W.; Grill, H.J. The Common Hepatic Branch of the Vagus Is Not Required to Mediate the Glycemic and Food Intake Suppressive Effects of Glucagon-like-Peptide-1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, 1479–1485. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Fortin, S.M.; Arnold, M.; Grill, H.J.; Hayes, M.R. Peripheral and Central GLP-1 Receptor Populations Mediate the Anorectic Effects of Peripherally Administered GLP-1 Receptor Agonists, Liraglutide and Exendin-4. Endocrinology 2011, 152, 3103–3112. [Google Scholar] [CrossRef]
- da Silva, R.S.; de Paiva, I.H.R.; Mendonça, I.P.; de Souza, J.R.B.; Lucena-Silva, N.; Peixoto, C.A. Anorexigenic and Anti-Inflammatory Signaling Pathways of Semaglutide via the Microbiota–Gut––Brain Axis in Obese Mice. Inflammopharmacology 2024, 33, 845–864. [Google Scholar] [CrossRef] [PubMed]
- Kakei, M.; Yada, T.; Nakagawa, A.; Nakabayashi, H. Glucagon-like Peptide-1 Evokes Action Potentials and Increases Cytosolic Ca2+ in Rat Nodose Ganglion Neurons. Auton. Neurosci. 2002, 102, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, A.; Satake, H.; Nakabayashi, H.; Nishizawa, M.; Furuya, K.; Nakano, S.; Kigoshi, T.; Nakayama, K.; Uchida, K. Receptor Gene Expression of Glucagon-like Peptide-1, but Not Glucose-Dependent Insulinotropic Polypeptide, in Rat Nodose Ganglion Cells. Auton. Neurosci. 2004, 110, 36–43. [Google Scholar] [CrossRef]
- Nakabayashi, H.; Nishizawa, M.; Nakagawa, A.; Takeda, R.; Niijima, A. Vagal Hepatopancreatic Reflex Effect Evoked by Intraportal Appearance of TGLP-1. Am. J. Physiol. Endocrinol. Metab. 1996, 271, E808–E813. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meier, J.J.; Gethmann, A.; Götze, O.; Gallwitz, B.; Holst, J.J.; Schmidt, W.E.; Nauck, M.A. Glucagon-like Peptide 1 Abolishes the Postprandial Rise in Triglyceride Concentrations and Lowers Levels of Non-Esterified Fatty Acids in Humans. Diabetologia 2006, 49, 452–458. [Google Scholar] [CrossRef]
- Zander, M.; Madsbad, S.; Madsen, J.L.; Holst, J.J. Effect of 6-Week Course of Glucagon-like Peptide 1 on Glycaemic Control, Insulin Sensitivity, and Beta-Cell Function in Type 2 Diabetes: A Parallel-Group Study. Lancet 2002, 359, 824–830. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Incretin Hormones: Their Role in Health and Disease. Diabetes Obes. Metab. 2018, 20, 5–21. [Google Scholar] [CrossRef]
- Heise, T.; Mari, A.; DeVries, J.H.; Urva, S.; Li, J.; Pratt, E.J.; Coskun, T.; Thomas, M.K.; Mather, K.J.; Haupt, A.; et al. Effects of Subcutaneous Tirzepatide versus Placebo or Semaglutide on Pancreatic Islet Function and Insulin Sensitivity in Adults with Type 2 Diabetes: A Multicentre, Randomised, Double-Blind, Parallel-Arm, Phase 1 Clinical Trial. Lancet Diabetes Endocrinol. 2022, 10, 418–429. [Google Scholar] [CrossRef]
- Anvari, M.; Paterson, C.A.; Daniel, E.E.; Mcdonald, T.J. Effects of GLP-1 on Gastric Emptying, Antropyloric Motility, and Transpyloric Flow in Response to a Nonnutrient Liquid. Dig. Dis. Sci. 1998, 43, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, D.J.; Nikou, G.C.; Kreymann, B.; Ghatei, M.A.; Bloom, S.R. Glucagon-like Peptide-1 (7–36)-NH2: A Physiological Inhibitor of Gastric Acid Secretion in Man. J. Endocrinol. 1990, 126, 169–173. [Google Scholar] [CrossRef]
- Qin, X.; Shen, H.; Liu, M.; Yang, Q.; Zheng, S.; Sabo, M.; D’Alessio, D.A.; Tso, P. GLP-1 Reduces Intestinal Lymph Flow, Triglyceride Absorption, and Apolipoprotein Production in Rats GLP-1 Re-Duces Intestinal Lymph Flow, Triglyceride Absorption, and Apoli-Poprotein Production in Rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, 943–949. [Google Scholar] [CrossRef]
- Schwartz, E.A.; Koska, J.; Mullin, M.P.; Syoufi, I.; Schwenke, D.C.; Reaven, P.D. Exenatide Suppresses Postprandial Elevations in Lipids and Lipoproteins in Individuals with Impaired Glucose Tolerance and Recent Onset Type 2 Diabetes Mellitus. Atherosclerosis 2010, 212, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Sonne, D.P.; Vilsbøll, T.; Knop, F.K. Pancreatic Amylase and Lipase Plasma Concentrations Are Unaffected by Increments in Endogenous GLP-1 Levels Following Liquid Meal Tests. Diabetes Care 2015, 38, e71–e72. [Google Scholar] [CrossRef]
- Hsieh, J.; Longuet, C.; Baker, C.L.; Qin, B.; Federico, L.M.; Drucker, D.J.; Adeli, K. The Glucagon-like Peptide 1 Receptor Is Essential for Postprandial Lipoprotein Synthesis and Secretion in Hamsters and Mice. Diabetologia 2010, 53, 552–561. [Google Scholar] [CrossRef]
- Vendrell, J.; El Bekay, R.; Peral, B.; García-Fuentes, E.; Megia, A.; Macias-Gonzalez, M.; Real, J.F.; Jimenez-Gomez, Y.; Escoté, X.; Pachón, G.; et al. Study of the Potential Association of Adipose Tissue GLP-1 Receptor with Obesity and Insulin Resistance. Endocrinology 2011, 152, 4072–4079. [Google Scholar] [CrossRef]
- van Eenige, R.; Ying, Z.; Tramper, N.; Wiebing, V.; Siraj, Z.; de Boer, J.F.; Lambooij, J.M.; Guigas, B.; Qu, H.; Coskun, T.; et al. Combined Glucose-Dependent Insulinotropic Polypeptide Receptor and Glucagon-like Peptide-1 Receptor Agonism Attenuates Atherosclerosis Severity in APOE*3-Leiden.CETP Mice. Atherosclerosis 2023, 372, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Joharapurkar, A.; Shah, G.; Jain, M. Effect of GLP-1 Based Therapies on Diabetic Dyslipidemia. Curr. Diabetes Rev. 2014, 10, 238–250. [Google Scholar] [CrossRef]
- Liu, L.; Yan, H.; Xia, M.F.; Zhao, L.; Lv, M.; Zhao, N.; Rao, S.; Yao, X.; Wu, W.; Pan, B.; et al. Efficacy of Exenatide and Insulin Glargine on Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes. Diabetes Metab. Res. Rev. 2020, 36, e3292. [Google Scholar] [CrossRef]
- Levine, I.; Sekhri, S.; Schreiber-Stainthorp, W.; Locke, B.; Delau, O.; Elhawary, M.; Pandit, K.; Meng, X.; Axelrad, J. GLP-1 Receptor Agonists Confer No Increased Rates of IBD Exacerbation Among Patients with IBD. Inflamm. Bowel Dis. 2025, 31, 467–475. [Google Scholar] [CrossRef]
- Ma, T.; Lu, W.; Wang, Y.; Qian, P.; Tian, H.; Gao, X.; Yao, W. An Oral GLP-1 and GIP Dual Receptor Agonist Improves Metabolic Disorders in High Fat-Fed Mice. Eur. J. Pharmacol. 2022, 914, 174635. [Google Scholar] [CrossRef] [PubMed]
- Vilsbøll, T.; Christensen, M.; Junker, A.E.; Knop, F.K.; Gluud, L.L. Effects of Glucagon-like Peptide-1 Receptor Agonists on Weight Loss: Systematic Review and Meta-Analyses of Randomised Controlled Trials. BMJ 2012, 344, d7771. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.M.; Tronieri, J.S.; Amaro, A.; Wadden, T.A. Semaglutide for the Treatment of Obesity. Trends Cardiovasc. Med. 2023, 33, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Rubino, D.M.; Greenway, F.L.; Khalid, U.; O’Neil, P.M.; Rosenstock, J.; Sørrig, R.; Wadden, T.A.; Wizert, A.; Garvey, W.T. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults with Overweight or Obesity Without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA 2022, 327, 138–150. [Google Scholar] [CrossRef]
- Suran, M. As Ozempic’s Popularity Soars, Here’s What to Know About Semaglutide and Weight Loss. JAMA 2023, 329, 1627–1629. [Google Scholar] [CrossRef]
- Campbell, J.E.; Drucker, D.J. Pharmacology, Physiology, and Mechanisms of Incretin Hormone Action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef]
- Bendotti, G.; Montefusco, L.; Lunati, M.E.; Usuelli, V.; Pastore, I.; Lazzaroni, E.; Assi, E.; Seelam, A.J.; El Essawy, B.; Jang, Y.; et al. The Anti-Inflammatory and Immunological Properties of GLP-1 Receptor Agonists. Pharmacol. Res. 2022, 182, 106320. [Google Scholar] [CrossRef]
- Lee, Y.S.; Park, M.S.; Choung, J.S.; Kim, S.S.; Oh, H.H.; Choi, C.S.; Ha, S.Y.; Kang, Y.; Kim, Y.; Jun, H.S. Glucagon-like Peptide-1 Inhibits Adipose Tissue Macrophage Infiltration and Inflammation in an Obese Mouse Model of Diabetes. Diabetologia 2012, 55, 2456–2468. [Google Scholar] [CrossRef]
- Pugazhenthi, U.; Velmurugan, K.; Tran, A.; Mahaffey, G.; Pugazhenthi, S. Anti-Inflammatory Action of Exendin-4 in Human Islets Is Enhanced by Phosphodiesterase Inhibitors: Potential Therapeutic Benefits in Diabetic Patients. Diabetologia 2010, 53, 2357–2368. [Google Scholar] [CrossRef]
- Yusta, B.; Baggio, L.L.; Koehler, J.; Holland, D.; Cao, X.; Pinnell, L.J.; Johnson-Henry, K.C.; Yeung, W.; Surette, M.G.; Bang, K.W.A.; et al. GLP-1R Agonists Modulate Enteric Immune Responses through the Intestinal Intraepithelial Lymphocyte GLP-1R. Diabetes 2015, 64, 2537–2549. [Google Scholar] [CrossRef]
- Abadie, V.; Discepolo, V.; Jabri, B. Intraepithelial Lymphocytes in Celiac Disease Immunopathology. Semin. Immunopathol. 2012, 34, 551–556. [Google Scholar] [CrossRef]
- Kronenberg, M.; Havran, W.L. Frontline T Cells: Γδ T Cells and Intraepithelial Lymphocytes. Immunol. Rev. 2007, 215, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, C.; Zhang, H.; Li, L.; Fan, T.; Jin, Z. The Alleviating Effect and Mechanism of GLP-1 on Ulcerative Colitis. Aging 2023, 15, 8044–8060. [Google Scholar] [CrossRef] [PubMed]
- Rosario, W.; D’Alessio, D. An Innate Disposition for a Healthier Gut: Glp-1r Signaling in Intestinal Epithelial Lymphocytes. Diabetes 2015, 64, 2329–2331. [Google Scholar] [CrossRef] [PubMed]
- Abdalqadir, N.; Adeli, K. GLP-1 and GLP-2 Orchestrate Intestine Integrity, Gut Microbiota, and Immune System Crosstalk. Microorganisms 2022, 10, 2061. [Google Scholar] [CrossRef]
- Al-Dwairi, A.; Alqudah, T.E.; Al-Shboul, O.; Alqudah, M.; Mustafa, A.G.; Alfaqih, M.A. Glucagon-like Peptide-1 Exerts Anti-Inflammatory Effects on Mouse Colon Smooth Muscle Cells through the Cyclic Adenosine Monophosphate/ Nuclear Factor-ΚB Pathway in Vitro. J. Inflamm. Res. 2018, 11, 95–109. [Google Scholar] [CrossRef]
- Bang-Berthelsen, C.H.; Holm, T.L.; Pyke, C.; Simonsen, L.; Søkilde, R.; Pociot, F.; Heller, R.S.; Folkersen, L.; Kvist, P.H.; Jackerott, M.; et al. GLP-1 Induces Barrier Protective Expression in Brunnerʼs Glands and Regulates Colonic Inflammation. Inflamm. Bowel Dis. 2016, 22, 2078–2097. [Google Scholar] [CrossRef]
- Morrow, N.M.; Morissette, A.; Mulvihill, E.E. Immunomodulation and Inflammation: Role of GLP-1R and GIPR Expressing Cells within the Gut. Peptides 2024, 176, 171200. [Google Scholar] [CrossRef]
- Anbazhagan, A.N.; Thaqi, M.; Priyamvada, S.; Jayawardena, D.; Kumar, A.; Gujral, T.; Chatterjee, I.; Mugarza, E.; Saksena, S.; Onyuksel, H.; et al. GLP-1 Nanomedicine Alleviates Gut Inflammation. Nanomedicine 2017, 13, 659–665. [Google Scholar] [CrossRef]
- Schirra, J.; Nicolaus, M.; Woerle, H.J.; Struckmeier, C.; Katschinski, M.; Göke, B. GLP-1 Regulates Gastroduodenal Motility Involving Cholinergic Pathways. Neurogastroenterol. Motil. 2009, 21, 609. [Google Scholar] [CrossRef] [PubMed]
- Hellström, P.M.; Näslund, E.; Edholm, T.; Schmidt, P.T.; Kristensen, J.; Theodorsson, E.; Holst, J.J.; Efendic, S. GLP-1 Suppresses Gastrointestinal Motility and Inhibits the Migrating Motor Complex in Healthy Subjects and Patients with Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2008, 20, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Schirra, J.; Houck, P.; Wank, U.; Arnold, R.; Göke, B.; Katschinski, M. EVects of Glucagon-like Peptide-1(7-36)Amide on Antro-Pyloro-Duodenal Motility in the Interdigestive State and with Duodenal Lipid Perfusion in Humans. Gut 2000, 46, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Thazhath, S.S.; Marathe, C.S.; Wu, T.; Chang, J.; Khoo, J.; Kuo, P.; Checklin, H.L.; Bound, M.J.; Rigda, R.S.; Crouch, B.; et al. The Glucagon-like Peptide 1 Receptor Agonist Exenatide Inhibits Small Intestinal Motility, Flow, Transit, and Absorption of Glucose in Healthy Subjects and Patients with Type 2 Diabetes: A Randomized Controlled Trial. Diabetes 2016, 65, 269–275. [Google Scholar] [CrossRef]
- Nakatani, Y.; Maeda, M.; Matsumura, M.; Shimizu, R.; Banba, N.; Aso, Y.; Yasu, T.; Harasawa, H. Effect of GLP-1 Receptor Agonist on Gastrointestinal Tract Motility and Residue Rates as Evaluated by Capsule Endoscopy. Diabetes Metab. 2017, 43, 430–437. [Google Scholar] [CrossRef]
- Jalleh, R.J.; Marathe, C.S.; Rayner, C.K.; Jones, K.L.; Umapathysivam, M.M.; Wu, T.; Quast, D.R.; Plummer, M.P.; Nauck, M.A.; Horowitz, M. Physiology and Pharmacology of Effects of GLP-1-Based Therapies on Gastric, Biliary and Intestinal Motility. Endocrinology 2024, 166, bqae155. [Google Scholar] [CrossRef]
- Imeryüz, N.; Yeğen, B.C.; Bozkurt, A.; Coṣkun, T.; Villanueva-Penñacarrillo, M.L.; Ulusoy, N.B. Glucagon-like Peptide-1 Inhibits Gastric Emptying via Vagal Afferent-Mediated Central Mechanisms. Am. J. Physiol. Gastrointest. Liver Physiol. 1997, 273, G920–G927. [Google Scholar] [CrossRef]
- Krieger, J.P.; Arnold, M.; Pettersen, K.G.; Lossel, P.; Langhans, W.; Lee, S.J. Knockdown of GLP-1 Receptors in Vagal Afferents Affects Normal Food Intake and Glycemia. Diabetes 2016, 65, 34–43. [Google Scholar] [CrossRef]
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-Obesity Drug Discovery: Advances and Challenges. Nat. Rev. Drug Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef]
- Bassotti, G.; Antonelli, E.; Villanacci, V.; Salemme, M.; Coppola, M.; Annese, V. Gastrointestinal Motility Disorders in Inflammatory Bowel Diseases. World J. Gastroenterol. 2014, 20, 37–44. [Google Scholar] [CrossRef]
- Dreja, J.; Ekberg, O.; Leander, P.; Månsson, S.; Ohlsson, B. Volumetric Analysis of Small Bowel Motility in an Unselected Cohort of Patients with Crohn’s Disease. Neurogastroenterol. Motil. 2020, 32, e13909. [Google Scholar] [CrossRef] [PubMed]
- Åkerman, A.; Månsson, S.; Fork, F.T.; Leander, P.; Ekberg, O.; Taylor, S.; Menys, A.; Ohlsson, B. Computational Postprocessing Quantification of Small Bowel Motility Using Magnetic Resonance Images in Clinical Practice: An Initial Experience. J. Magn. Reson. Imaging 2016, 44, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Ganga Pathirana, W.W.; Paul Chubb, S.; Gillett, M.J.; Vasikaran, S.D. Faecal Calprotectin. Clin. Biochem. Rev. 2018, 39, 77. [Google Scholar]
- Bickelhaupt, S.; Pazahr, S.; Chuck, N.; Blume, I.; Froehlich, J.M.; Cattin, R.; Raible, S.; Bouquet, H.; Bill, U.; Rogler, G.; et al. Crohn’s Disease: Small Bowel Motility Impairment Correlates with Inflammatory-related Markers C-reactive Protein and Calprotectin. Neurogastroenterol. Motil. 2013, 25, 467. [Google Scholar] [CrossRef]
- Gajendran, M.; Loganathan, P.; Catinella, A.P.; Hashash, J.G. A Comprehensive Review and Update on Crohn’s Disease. Dis. Mon. 2018, 64, 20–57. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.; Wang, L.; Chen, C.; Chen, L. Association between Different GLP-1 Receptor Agonists and Gastrointestinal Adverse Reactions: A Real-World Disproportionality Study Based on FDA Adverse Event Reporting System Database. Front. Endocrinol. 2022, 13, 1043789. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, T.D.; Panagiotopoulou, T.V.; Elisaf, M.S. Adverse Effects of GLP-1 Receptor Agonists. Rev. Diabet. Stud. 2014, 11, 202–230. [Google Scholar] [CrossRef]
- Weiss, T.; Yang, L.; Carr, R.D.; Pal, S.; Sawhney, B.; Boggs, R.; Rajpathak, S.; Iglay, K. Real-World Weight Change, Adherence, and Discontinuation among Patients with Type 2 Diabetes Initiating Glucagon-like Peptide-1 Receptor Agonists in the UK. BMJ Open Diabetes Res. Care 2022, 10, e002517. [Google Scholar] [CrossRef]
- Faillie, J.; Yin, H.; Yu, O.H.Y.; Herrero, A.; Altwegg, R.; Renoux, C.; Azoulay, L. Incretin-Based Drugs and Risk of Intestinal Obstruction Among Patients with Type 2 Diabetes. Clin. Pharmacol. Ther. 2022, 111, 272–282. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, Y.; Shi, Y.; Yu, K.; Zhao, M.; Liu, S.; Zhao, Z. Safety of Glucagon-Like Peptide-1 Receptor Agonists: A Real-World Study Based on the US FDA Adverse Event Reporting System Database. Clin. Drug Investig. 2022, 42, 965–975. [Google Scholar] [CrossRef]
- Gudin, B.; Ladhari, C.; Robin, P.; Laroche, M.-L.; Babai, S.; Hillaire-Buys, D.; Faillie, J.-L. Incretin-Based Drugs and Intestinal Obstruction: A Pharmacovigilance Study. Therapies 2020, 75, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, M.; Rezaeianzadeh, R.; Kezouh, A.; Etminan, M. Risk of Gastrointestinal Adverse Events Associated with Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss. JAMA 2023, 330, 1795. [Google Scholar] [CrossRef]
- Nielsen, J.; Friedman, S.; Nørgård, B.M.; Knudsen, T.; Kjeldsen, J.; Wod, M. Glucagon-Like Peptide 1 Receptor Agonists Are Not Associated with an Increased Risk of Ileus or Intestinal Obstruction in Patients with Inflammatory Bowel Disease—A Danish Nationwide Cohort Study. Inflamm. Bowel Dis. 2024, 31, 1961–1965. [Google Scholar] [CrossRef]
- Anderson, S.R.; Ayoub, M.; Coats, S.; McHenry, S.; Tan, T.; Deepak, P. Safety and Effectiveness of Glucagon-like Peptide-1 Receptor Agonists in Inflammatory Bowel Disease. Am. J. Gastroenterol. 2025, 120, 1152–1155. [Google Scholar] [CrossRef]
- Wøjdemann, M.; Wettergren, A.; Hartmann, B.; Hilsted, L.; Holst, J.J. Inhibition of Sham Feeding-Stimulated Human Gastric Acid Secretion by Glucagon-Like Peptide-2. J. Clin. Endocrinol. Metab. 1999, 84, 2513–2517. [Google Scholar] [CrossRef]
- Nagell, C.F.; Wettergren, A.; Pedersen, J.F.; Mortensen, D.; Holst, J.J. Glucagon-like Peptide-2 Inhibits Antral Emptying in Man, but Is Not as Potent as Glucagon-like Peptide-1. Scand. J. Gastroenterol. 2004, 39, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Burrin, D.G.; Stoll, B.; Jiang, R.; Petersen, Y.; Elnif, J.; Buddington, R.K.; Schmidt, M.; Holst, J.J.; Hartmann, B.; Sangild, A.P.T.; et al. GLP-2 Stimulates Intestinal Growth in Premature TPN-Fed Pigs by Suppressing Proteolysis and Apoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G1249–G1256. [Google Scholar] [CrossRef]
- Yusta, B.; Boushey, R.P.; Drucker, D.J. The Glucagon-like Peptide-2 Receptor Mediates Direct Inhibition of Cellular Apoptosis via a CAMP-Dependent Protein Kinase-Independent Pathway. J. Biol. Chem. 2000, 275, 35345–35352. [Google Scholar] [CrossRef]
- Ramsanahie, A.; Duxbury, M.S.; Grikscheit, T.C.; Perez, A.; Rhoads, D.B.; Gardner-Thorpe, J.; Ogilvie, J.; Ashley, S.W.; Vacanti, J.P.; Whang, E.E. Effect of GLP-2 on Mucosal Morphology and SGLT1 Expression in Tissue-Engineered Neointestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G1345–G1352. [Google Scholar] [CrossRef]
- Cheeseman, C.I. Upregulation of SGLT-1 Transport Activity in Rat Jejunum Induced by GLP-2 Infusion in Vivo. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1997, 273, R1965–R1971. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, C.; O’Neill, D. Basolateral D-glucose Transport Activity along the Crypt-villus Axis in Rat Jejunum and Upregulation Induced by Gastric Inhibitory Peptide and Glucagon-like Peptide−2. Exp. Physiol. 1998, 83, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Grande, E.M.; Raka, F.; Hoffman, S.; Adeli, K. GLP-2 Regulation of Dietary Fat Absorption and Intestinal Chylomicron Production via Neuronal Nitric Oxide Synthase (NNOS) Signaling. Diabetes 2022, 71, 1388–1399. [Google Scholar] [CrossRef]
- Guan, X.; Karpen, H.E.; Stephens, J.; Bukowski, J.T.; Niu, S.; Zhang, G.; Stoll, B.; Finegold, M.J.; Holst, J.J.; Hadsell, D.L.; et al. GLP-2 Receptor Localizes to Enteric Neurons and Endocrine Cells Expressing Vasoactive Peptides and Mediates Increased Blood Flow. Gastroenterology 2006, 130, 150–164. [Google Scholar] [CrossRef]
- Guan, X.; Stoll, B.; Lu, X.; Tappenden, K.A.; Holst, J.J.; Hartmann, B.; Burrin, D.G. GLP-2-Mediated up-Regulation of Intestinal Blood Flow and Glucose Uptake Is Nitric Oxide-Dependent in TPN-Fed Piglets. Gastroenterology 2003, 125, 136–147. [Google Scholar] [CrossRef]
- Stephens, J.; Stoll, B.; Cottrell, J.; Chang, X.; Helmrath, M.; Burrin, D.G. Glucagon-like Peptide-2 Acutely Increases Proximal Small Intestinal Blood Flow in TPN-Fed Neonatal Piglets. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Burrin, D.G.; Stoll, B.; Guan, X.; Cui, L.; Chang, X.; Holst, J.J. Glucagon-like Peptide 2 Dose-Dependently Activates Intestinal Cell Survival and Proliferation in Neonatal Piglets. Endocrinology 2005, 146, 22–32. [Google Scholar] [CrossRef]
- Tsai, C.H.; Hill, M.; Asa, S.L.; Brubaker, P.L.; Drucker, D.J. Intestinal Growth-Promoting Properties of Glucagon-like Peptide-2 in Mice. Am. J. Physiol. Endocrinol. Metab. 1997, 273, E77–E84. [Google Scholar] [CrossRef]
- Kouris, G.J.; Liu, Q.; Rossi, H.; Djuricin, G.; Gattuso, P.; Nathan, C.; Weinstein, R.A.; Prinz, R.A. The Effect of Glucagon-like Peptide 2 on Intestinal Permeability and Bacterial Translocation in Acute Necrotizing Pancreatitis. Am. J. Surg. 2001, 181, 571–575. [Google Scholar] [CrossRef]
- Benjamin, M.A.; Mckay, M.; Yang, P.-C.; Cameron, H.; Perdue, M.H. Glucagon-like Peptide-2 Enhances Intestinal Epithelial Barrier Function of Both Transcellular and Paracellular Pathways in the Mouse. Gut 2000, 47, 112–119. [Google Scholar] [CrossRef]
- Drucker, D.J.; Ehrlich, P.; Asat, S.L.; Brubaker, P.L.; Steiner, D.F. Induction of Intestinal Epithelial Proliferation by Glucagon-like Peptide 2. Proc. Natl. Acad. Sci. 1996, 93, 7911–7916. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J.; Deforest, L.; Brubaker, P.L. Intestinal Response to Growth Factors Administered Alone or in Combination with Human [Gly 2 ]Glucagon-like Peptide 2. Am. J. Physiol. Gastrointest. Liver Physiol. 1997, 273, G1252–G1262. [Google Scholar] [CrossRef]
- Pedersen, J.; Pedersen, N.B.; Brix, S.W.; Grunddal, K.V.; Rosenkilde, M.M.; Hartmann, B.; Erskov, C.; Poulsen, S.S.; Holst, J.J. The Glucagon-like Peptide 2 Receptor Is Expressed in Enteric Neurons and Not in the Epithelium of the Intestine. Peptides 2015, 67, 20–28. [Google Scholar] [CrossRef]
- Leen, J.L.S.; Izzo, A.; Upadhyay, C.; Rowland, K.J.; Dubé, P.E.; Gu, S.; Heximer, S.P.; Rhodes, C.J.; Storm, D.R.; Lund, P.K.; et al. Mechanism of Action of Glucagon-Like Peptide-2 to Increase IGF-I MRNA in Intestinal Subepithelial Fibroblasts. Endocrinology 2011, 152, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.A.; Yusta, B.; DaCambra, M.P.; Anini, Y.; Drucker, D.J.; Brubaker, P.L. Glucagon-Like Peptide-2 Receptor Activation in the Rat Intestinal Mucosa. Endocrinology 2003, 144, 4385–4392. [Google Scholar] [CrossRef]
- Sigalet, D.L.; Wallace, L.E.; Holst, J.J.; Martin, G.R.; Kaji, T.; Tanaka, H.; Sharkey, K.A.; Enteric, S.K. Enteric Neural Pathways Mediate the Anti-Inflammatory Actions of Glucagon-like Peptide 2. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Ivory, C.P.A.; Wallace, L.E.; McCafferty, D.M.; Sigalet, D.L. Interleukin-10-Independent Anti-Inflammatory Actions of Glucagon-like Peptide 2. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G1202–G1210. [Google Scholar] [CrossRef]
- L’Heureux, M.C.; Brubaker, P.L. Glucagon-like Peptide-2 and Common Therapeutics in a Murine Model of Ulcerative Colitis. J. Pharmacol. Exp. Ther. 2003, 306, 347–354. [Google Scholar] [CrossRef]
- Drucker, D.J.; Yusta, B.; Boushey, R.P.; Deforest, L.; Brubaker, P.L. Human [Gly2]GLP-2 Reduces the Severity of Colonic Injury in a Murine Model of Experimental Colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 1999, 276, G79–G91. [Google Scholar] [CrossRef]
- Alavi, K.; Schwartz, M.Z.; Palazzo, J.P.; Prasad, R. Treatment of Inflammatory Bowel Disease in a Rodent Model with the Intestinal Growth Factor Glucagon-like Peptide-2. J. Pediatr. Surg. 2000, 35, 847–851. [Google Scholar] [CrossRef]
- Arthur, G.L.; Schwartz, M.Z.; Kuenzler, K.A.; Birbe, R. Glucagonlike Peptide-2 Analogue: A Possible New Approach in the Management of Inflammatory Bowel Disease. J. Pediatr. Surg. 2004, 39, 448–452. [Google Scholar] [CrossRef]
- Buchman, A.L.; Katz, S.; Fang, J.C.; Bernstein, C.N.; Abou-Assi, S.G. Teduglutide, a Novel Mucosally Active Analog of Glucagon-like Peptide-2 (GLP-2) for the Treatment of Moderate to Severe Crohn’s Disease. Inflamm. Bowel Dis. 2010, 16, 962–973. [Google Scholar] [CrossRef]
- Finan, B.; Müller, T.D.; Clemmensen, C.; Perez-Tilve, D.; DiMarchi, R.D.; Tschöp, M.H. Reappraisal of GIP Pharmacology for Metabolic Diseases. Trends Mol. Med. 2016, 22, 359–376. [Google Scholar] [CrossRef]
- Lu, W.J.; Yang, Q.; Sun, W.; Woods, S.C.; Tso, P. Using the Lymph Fistula Rat Model to Study the Potentiation of GIP Secretion by the Ingestion of Fat and Glucose. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, 1130–1138. [Google Scholar] [CrossRef]
- Takeda, J.; Seinot, Y.; Tanaka, K.-I.; Fukumoto, H.; Kayanot, T.; Takahashi, H.; Mitani, T.; Kurono, M.; Suzuki, T.; Takayoshi, T.; et al. Sequence of an Intestinal CDNA Encoding Human Gastric Inhibitory Polypeptide Precursor (Glucose-Dependent Insulin-Releasing Peptide/Monobasic Processing/Preprohormone/Glucagon Superfamily). Proc. Natl. Acad. Sci. USA 1987, 84, 7005–7008. [Google Scholar] [CrossRef]
- Higashimoto, Y.; Simchock, J.; Liddle, R. Molecular Cloning of Rat Glucose-Dependent Insulinotropic Peptide (GIP). Biochim Biophys Acta 1993, 1, 72–74. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Jarboe, L.A.; Landau, S.B.; Williams, E.K.; Wolfe, M.M. Glucose-Dependent Insulinotropic Peptide: Structure of the Precursor and Tissue-Specific Expression in Rat. Proc. Natl. Acad. Sci. USA 1993, 90, 1992–1996. [Google Scholar] [CrossRef]
- Fujita, Y.; Asadi, A.; Yang, G.K.; Kwok, Y.N.; Kieffer, T.J. Differential Processing of Pro-Glucose-Dependent Insulinotropic Polypeptide in Gut. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G608–G614. [Google Scholar] [CrossRef]
- Ugleholdt, R.; Poulsen, M.L.H.; Holst, P.J.; Irminger, J.C.; Orskov, C.; Pedersen, J.; Rosenkilde, M.M.; Zhu, X.; Steiner, D.F.; Holst, J.J. Prohormone Convertase 1/3 Is Essential for Processing of the Glucose-Dependent Insulinotropic Polypeptide Precursor. J. Biol. Chem. 2006, 281, 11050–11057. [Google Scholar] [CrossRef]
- Brown, J.C.; Mutt, V.; Pederson, R.A. Further Purification of a Polypeptide Demonstrating Enterogastrone Activity. J. Physiol. 1970, 209, 57–64. [Google Scholar] [CrossRef]
- Brown, J.C.; Dryburgh, J.R. A Gastric Inhibitory Polypeptide II: The Complete Amino Acid Sequence. Can. J. Biochem. 1971, 49, 867–872. [Google Scholar] [CrossRef]
- Wolfe, M.M.; Reel, G.M. Inhibition of Gastrin Release by Gastric Inhibitory Peptide Mediated by Somatostatin. Am. J. Physiol. Gastrointest. Liver Physiol. 1986, 250, G331–G335. [Google Scholar] [CrossRef]
- Meier, J.J.; Goetze, O.; Anstipp, J.; Hagemann, D.; Holst, J.J.; Schmidt, W.E.; Gallwitz, B.; Nauck, M.A. Gastric Inhibitory Polypeptide Does Not Inhibit Gastric Emptying in Humans. Am. J. Physiol. Endocrinol. Metab. 2004, 286, 621–625. [Google Scholar] [CrossRef]
- Christensen, M.B.; Calanna, S.; Holst, J.J.; Vilsbøll, T.; Knop, F.K. Glucose-Dependent Insulinotropic Polypeptide: Blood Glucose Stabilizing Effects in Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2014, 99, E418–E426. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Christensen, M.; Vedtofte, L.; Holst, J.J.; Vilsbøll, T.; Knop, F.K. Glucose-Dependent Insulinotropic Polypeptide: A Bifunctional Glucose-Dependent Regulator of Glucagon and Insulin Secretion in Humans. Diabetes 2011, 60, 3103–3109. [Google Scholar] [CrossRef]
- Zaïmia, N.; Obeid, J.; Varrault, A.; Sabatier, J.; Broca, C.; Gilon, P.; Costes, S.; Bertrand, G.; Ravier, M.A. GLP-1 and GIP Receptors Signal through Distinct β-Arrestin 2-Dependent Pathways to Regulate Pancreatic β Cell Function. Cell Rep. 2023, 42, 113326. [Google Scholar] [CrossRef]
- Jones, B.; McGlone, E.R.; Fang, Z.; Pickford, P.; Corrêa, I.R.; Oishi, A.; Jockers, R.; Inoue, A.; Kumar, S.; Görlitz, F.; et al. Genetic and Biased Agonist-Mediated Reductions in β-Arrestin Recruitment Prolong CAMP Signaling at Glucagon Family Receptors. J. Biol. Chem. 2021, 296, 100133. [Google Scholar] [CrossRef]
- Asmar, M.; Asmar, A.; Simonsen, L.; Gasbjerg, L.S.; Sparre-Ulrich, A.H.; Rosenkilde, M.M.; Hartmann, B.; Dela, F.; Holst, J.J.; Bülow, J. The Gluco-and Liporegulatory and Vasodilatory Effects of Glucose-Dependent Insulinotropic Polypeptide (GIP) Are Abolished by an Antagonist of the Human GIP Receptor. Diabetes 2017, 66, 2363–2371. [Google Scholar] [CrossRef]
- Mroz, P.A.; Finan, B.; Gelfanov, V.; Yang, B.; Tschöp, M.H.; DiMarchi, R.D.; Perez-Tilve, D. Optimized GIP Analogs Promote Body Weight Lowering in Mice through GIPR Agonism Not Antagonism. Mol. Metab. 2019, 20, 51–62. [Google Scholar] [CrossRef]
- Ceperuelo-Mallafré, V.; Duran, X.; Pachón, G.; Roche, K.; Garrido-Sánchez, L.; Vilarrasa, N.; Tinahones, F.J.; Vicente, V.; Pujol, J.; Vendrell, J.; et al. Disruption of GIP/GIPR Axis in Human Adipose Tissue Is Linked to Obesity and Insulin Resistance. J. Clin. Endocrinol. Metab. 2014, 99, E908–E919. [Google Scholar] [CrossRef]
- Wasada, T.; McCorkle, K.; Harris, V.; Kawai, K.; Howard, B.; Unger, R.H. Effect of Gastric Inhibitory Polypeptide on Plasma Levels of Chylomicron Triglycerides in Dogs. J. Clin. Investig. 1981, 68, 1106–1107. [Google Scholar] [CrossRef]
- Kim, S.J.; Nian, C.; McIntosh, C.H.S. Activation of Lipoprotein Lipase by Glucose-Dependent Insulinotropic Polypeptide in Adipocytes: A Role for a Protein Kinase B, LKB1, and AMP-Activated Protein Kinase Cascade. J. Biol. Chem. 2007, 282, 8557–8567. [Google Scholar] [CrossRef]
- Widenmaier, S.B.; Kim, S.J.; Yang, G.K.; De Los Reyes, T.; Nian, C.; Asadi, A.; Seino, Y.; Kieffer, T.J.; Kwok, Y.N.; McIntosh, C.H.S. A GIP Receptor Agonist Exhibits β-Cell Anti-Apoptotic Actions in Rat Models of Diabetes Resulting in Improved β-Cell Function and Glycemic Control. PLoS ONE 2010, 5, e9590. [Google Scholar] [CrossRef]
- Eckel, R.; Fujimoto, W.; Brunzell, J. Gastric Inhibitory Polypeptide Lipoprotein Lipase Activity in Cultured Preadipocytes. Diabetes 1979, 12, 1141–1142. [Google Scholar] [CrossRef]
- Beck, B.; Max, J.-P. Gastric Inhibitory Polypeptide Enhancement of the Insulin Effect on Fatty Acid Incorporation into Adipose Tissue in the Rat. Regul. Pept. 1983, 7, 3–8. [Google Scholar] [CrossRef]
- Asmar, M.; Simonsen, L.; Madsbad, S.; Stallknecht, B.; Holst, J.J.; Bülow, J. Glucose-Dependent Insulinotropic Polypeptide May Enhance Fatty Acid Re-Esterification in Subcutaneous Abdominal Adipose Tissue in Lean Humans. Diabetes 2010, 59, 2160–2163. [Google Scholar] [CrossRef]
- Frias, J.P.; Nauck, M.A.; Van, J.; Kutner, M.E.; Cui, X.; Benson, C.; Urva, S.; Gimeno, R.E.; Milicevic, Z.; Robins, D.; et al. Efficacy and Safety of LY3298176, a Novel Dual GIP and GLP-1 Receptor Agonist, in Patients with Type 2 Diabetes: A Randomised, Placebo-Controlled and Active Comparator-Controlled Phase 2 Trial. The Lancet 2018, 392, 2180–2193. [Google Scholar] [CrossRef]
- Bartelt, A.; Bruns, O.T.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; Tromsdorf, U.I.; Weller, H.; Waurisch, C.; et al. Brown Adipose Tissue Activity Controls Triglyceride Clearance. Nat. Med. 2011, 17, 200–206. [Google Scholar] [CrossRef]
- Carpentier, A.C.; Blondin, D.P.; Virtanen, K.A.; Richard, D.; Haman, F.; Turcotte, É.E. Brown Adipose Tissue Energy Metabolism in Humans. Front. Endocrinol. 2018, 9, 447. [Google Scholar] [CrossRef]
- Khedoe, P.P.S.J.; Hoeke, G.; Kooijman, S.; Dijk, W.; Buijs, J.T.; Kersten, S.; Havekes, L.M.; Hiemstra, P.S.; Berbée, J.F.P.; Boon, M.R.; et al. Brown Adipose Tissue Takes up Plasma Triglycerides Mostly after Lipolysis. J. Lipid Res. 2015, 56, 51–59. [Google Scholar] [CrossRef]
- Langin, D. Recruitment of Brown Fat and Conversion of White into Brown Adipocytes: Strategies to Fight the Metabolic Complications of Obesity? Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 372–376. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Villarroya, F.; Cereijo, R.; Gavaldà-Navarro, A.; Villarroya, J.; Giralt, M. Inflammation of Brown/Beige Adipose Tissues in Obesity and Metabolic Disease. J. Intern. Med. 2018, 284, 492–504. [Google Scholar] [CrossRef]
- Heimbürger, S.M.N.; Hoe, B.; Nielsen, C.N.; Bergman, N.C.; Skov-Jeppesen, K.; Hartmann, B.; Holst, J.J.; Dela, F.; Overgaard, J.; Størling, J.; et al. GIP Affects Hepatic Fat and Brown Adipose Tissue Thermogenesis but Not White Adipose Tissue Transcriptome in Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2022, 107, 3261–3274. [Google Scholar] [CrossRef]
- Varol, C.; Zvibel, I.; Spektor, L.; Mantelmacher, F.D.; Vugman, M.; Thurm, T.; Khatib, M.; Elmaliah, E.; Halpern, Z.; Fishman, S. Long-Acting Glucose-Dependent Insulinotropic Polypeptide Ameliorates Obesity-Induced Adipose Tissue Inflammation. J. Immunol. 2014, 193, 4002–4009. [Google Scholar] [CrossRef]
- Ohneda, A.; Kobayashi, T.; Nihei, J. Response of Gastric Inhibitory Polypeptide to Fat Ingestion in Normal Dogs. Regul. Pept. 1984, 8, 123–130. [Google Scholar] [CrossRef]
- Samms, R.J.; Coghlan, M.P.; Sloop, K.W. How May GIP Enhance the Therapeutic Efficacy of GLP-1? Trends Endocrinol. Metab. 2020, 31, 410–421. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; D’Angelo, A.; Cipolla, G.; Moro, E.; Crema, F. Effects of Experimental Colitis in Rats on Incretin Levels, Inflammatory Markers, and Enteric Neuronal Function. Arch. Med. Sci. 2021, 17, 1087–1092. [Google Scholar] [CrossRef]
- Efimova, I.; Steinberg, I.; Zvibel, I.; Neumann, A.; Mantelmacher, D.F.; Drucker, D.J.; Fishman, S.; Varol, C. GIPR Signaling in Immune Cells Maintains Metabolically Beneficial Type 2 Immune Responses in the White Fat from Obese Mice. Front. Immunol. 2021, 12, 643144. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Grimelius, L.; Wilander, E.; Ryberg, B.; Terenius, L.; Lundberg, J.M.; Tatemoto, K. Immunocytochemical Identification of Polypeptide YY (PYY) Cells in the Human Gastrointestinal Tract. Histochemistry 1983, 77, 15–23. [Google Scholar] [CrossRef]
- Tatemoto, K.; Mutt, V. Isolation of Two Novel Candidate Hormones Using a Chemical Method for Finding Naturally Occurring Polypeptides. Nature 1980, 285, 417–418. [Google Scholar] [CrossRef]
- Tatemoto, K. Isolation and Characterization of Peptide YY (PYY), a Candidate Gut Hormone That Inhibits Pancreatic Exocrine Secretion (Chemical Assay/COOH-Terminal Xx-Amide/Amino Acid Sequence/Pancreatic Polypeptide Family). Proc. Natl. Acad. Sci. USA 1982, 79, 2514–2518. [Google Scholar] [CrossRef] [PubMed]
- Hill, F.L.C.; Zhang, T.; Gomez, G.; Greeley, G.H. Peptide YY, a New Gut (a Mini-Review) Hormone. Steroids 1991, 56, 77–82. [Google Scholar] [CrossRef]
- Ballantyne, G. Peptide YY(1-36) and Peptide YY(3-36): Part, I.; Distribution, Release and Actions. Obes. Surg. 2006, 16, 651–658. [Google Scholar] [CrossRef]
- Beglinger, C.; Degen, L. Gastrointestinal Satiety Signals in Humans—Physiologic Roles for GLP-1 and PYY ? Physiol. Behav. 2006, 89, 460–464. [Google Scholar] [CrossRef]
- Grandt A’, D.; Schimiczek, M.; Beglinger, C.; Layer, P.; Goebell, H.; Eysselein, V.E.; Reeve, J.R. Two Molecular Forms of Peptide YY (PYY) Are Abundant in Human Blood: Characterization of a Radioimmunoassay Recognizing PYY 1-36 and PYY 3-36. Regul. Pept. 1994, 51, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Neary, M.T.; Batterham, R.L. Peptide YY: Food for Thought. Physiol. Behav. 2009, 97, 616–619. [Google Scholar] [CrossRef]
- Walther, C.; Mörl, K.; Beck-Sickinger, A.G. Neuropeptide Y Receptors: Ligand Binding and Trafficking Suggest Novel Approaches in Drug Development. J. Pept. Sci. 2011, 17, 233–246. [Google Scholar] [CrossRef]
- Stadlbauer, U.; Woods, S.C.; Langhans, W.; Meyer, U. PYY3-36: Beyond Food Intake. Front. Neuroendocrinol. 2015, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pittner, R.A.; Moore, C.X.; Bhavsar, S.P.; Gedulin, B.R.; Smith, P.A.; Jodka, C.M.; Parkes, D.G.; Paterniti, J.R.; Srivastava, V.P.; Young, A.A. Effects of PYY[3-36] in Rodent Models of Diabetes and Obesity. Int. J. Obes. 2004, 28, 963–971. [Google Scholar] [CrossRef]
- Tm, J.T.C.; Sahu, A.; Kalra, P.S.; Balasubramaniam, A.; Kalra, S.P. Neuropeptide Y (NPY)-Induced Feeding Behavior in Female Rats: Comparison with Human NPY ([Met 17]NPY), NPY Analog ([NorLeu4]NpY) and Peptide YY*. Regul. Pept. 1987, 17, 31–39. [Google Scholar]
- Chelikani, P.K.; Haver, A.C.; Heidelberger, R.D. Intravenous Infusion of Peptide YY(3-36) Potently Inhibits Food Intake in Rats. Endocrinology 2005, 146, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Sloth, B.; Holst, J.J.; Flint, A.; Gregersen, N.T.; Astrup, A. Effects of PYY 1-36 and PYY 3-36 on Appetite, Energy Intake, Energy Expenditure, Glucose and Fat Metabolism in Obese and Lean Subjects. Am. J. Physiol. Endocrinol. Metab. 2007, 292, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Cohen, M.A.; Ellis, S.M.; Le Roux, C.W.; Withers, D.J.; Frost, G.S.; Ghatei, M.A.; Bloom, S.R. Inhibition of Food Intake in Obese Subjects by Peptide YY 3-36. N. Engl. J. Med. 2003, 349, 941–949. [Google Scholar] [CrossRef]
- Rahardjo, G.L.; Huang, X.-F.; Tan, Y.Y.; Deng, C. Decreased Plasma Peptide YY Accompanied by Elevated Peptide YY and Y2 Receptor Binding Densities in the Medulla Oblongata of Diet-Induced Obese Mice. Endocrinology 2007, 148, 4704–4710. [Google Scholar] [CrossRef]
- Le Roux, C.W.; Batterham, R.L.; Aylwin, S.J.B.; Patterson, M.; Borg, C.M.; Wynne, K.J.; Kent, A.; Vincent, R.P.; Gardiner, J.; Ghatei, M.A.; et al. Attenuated Peptide YY Release in Obese Subjects Is Associated with Reduced Satiety. Endocrinology 2006, 147, 3–8. [Google Scholar] [CrossRef]
- Batterham, R.L.; Heffron, H.; Kapoor, S.; Chivers, J.E.; Chandarana, K.; Herzog, H.; Le Roux, C.W.; Thomas, E.L.; Bell, J.D.; Withers, D.J. Critical Role for Peptide YY in Protein-Mediated Satiation and Body-Weight Regulation. Cell Metab. 2006, 4, 223–233. [Google Scholar] [CrossRef]
- Yan, G.; Lijun, M.; Enriori, P.J.; Koska, J.; Franks, P.W.; Brookshire, T.; Cowley, M.A.; Salbe, A.D.; DelParigi, A.; Tataranni, P.A. Physiological Evidence for the Involvement of Peptide YY in the Regulation of Energy Homeostasis in Humans. Obesity 2006, 14, 1562–1570. [Google Scholar] [CrossRef]
- Bartolomé, M.; Borque, M.; Martinez-Sarmiento, J.; Aparicio, E.; Hernández, C.; Cabrerizo, L.; Fernández-Represa, J. Peptide YY Secretion in Morbidly Obese Patients before and after Vertical Banded Gastroplasty. Obes. Surg. 2002, 12, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.H.; Lei, C.; Jodka, C.M.; Nikoulina, S.E.; Hoyt, J.A.; Gedulin, B.; Mack, C.M.; Kendall, E.S.; Modeling, V. PYY[3-36] Administration Decreases the Respiratory Quotient and Reduces Adiposity in Diet-Induced Obese Mice. J. Nutr. 2006, 136, 195–201. [Google Scholar] [CrossRef]
- Van Den Hoek, A.M.; Heijboer, A.C.; Voshol, P.J.; Havekes, L.M.; Romijn, J.A.; Corssmit, E.P.M.; Pijl, H. Chronic PYY3-36 Treatment Promotes Fat Oxidation and Ameliorates Insulin Resistance in C57BL6 Mice. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E238–E245. [Google Scholar] [CrossRef]
- Boey, D.; Lin, S.; Enriquez, R.F.; Lee, N.J.; Slack, K.; Couzens, M.; Baldock, P.A.; Herzog, H.; Sainsbury, A. PYY Transgenic Mice Are Protected against Diet-Induced and Genetic Obesity. Neuropeptides 2008, 42, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Radziszewska, M.; Ostrowska, L.; Smarkusz-Zarzecka, J. The Impact of Gastrointestinal Hormones on Human Adipose Tissue Function. Nutrients 2024, 16, 3245. [Google Scholar] [CrossRef]
- Strober, W.; Fuss, I.J.; Blumberg, R.S. The Immunology of Mucosal Models of Inflammation. Annu. Rev. Immunol. 2002, 20, 495–549. [Google Scholar] [CrossRef]
- Li, Z.; Kuang, X.; Chen, T.; Shen, T.; Wu, J. Peptide YY 3–36 Attenuates Trinitrobenzene Sulfonic Acid-Induced Colitis in Mice by Modulating Th1/Th2 Differentiation. Bioengineered 2022, 13, 10144–10158. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Suo, Z.; Lin, J.; Cong, Y.; Liu, Z. Monocyte-Macrophages Modulate Intestinal Homeostasis in Inflammatory Bowel Disease. Biomark. Res. 2024, 12, 76. [Google Scholar] [CrossRef]
- Macia, L.; Yulyaningsih, E.; Pangon, L.; Nguyen, A.D.; Lin, S.; Shi, Y.C.; Zhang, L.; Bijker, M.; Grey, S.; Mackay, F.; et al. Neuropeptide Y1 Receptor in Immune Cells Regulates Inflammation and Insulin Resistance Associated with Diet-Induced Obesity. Diabetes 2012, 61, 3228–3238. [Google Scholar] [CrossRef]
- De La Fuente, M.; Bernaez, I.; Del, M.; Hernanz, A. Stimulation of Murine Peritoneal Macrophage Functions by Neuropeptide Y and Peptide YY. Involvement of Protein Kinase C. Immunology 1993, 80, 259. [Google Scholar]
- El-Salhy, M.; Danielsson, Å.; Stenling, R.; Grimelius, L. Colonic Endocrine Cells in Inflammatory Bowel Disease. J. Intern. Med. 1997, 242, 413–419. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Mazzawi, T.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. The Role of Peptide YY in Gastrointestinal Diseases and Disorders (Review). Int. J. Mol. Med. 2013, 31, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Mannon, P.J.; Mele, J.M. Peptide YY Y1 Receptor Activates Mitogen-Activated Protein Kinase and Proliferation in Gut Epithelial Cells via the Epidermal Growth Factor Receptor. Biochem. J. 2000, 350, 655–661. [Google Scholar] [CrossRef]
- Mannon, P.J. Peptide YY as a Growth Factor for Intestinal Epithelium. Peptides 2002, 23, 383–388. [Google Scholar] [CrossRef]
- Crawley, J.N.; Corwin, R.L. Biological Actions of Cholecystokinin. Peptides 1994, 15, 731–755. [Google Scholar] [CrossRef]
- Liddle, R.A.; Goldfine, I.D.; Rosen, M.S.; Taplitz, R.A.; Williams, J.A. Cholecystokinin Bioactivity in Human Plasma. Molecular Forms, Responses to Feeding, and Relationship to Gallbladder Contraction. J. Clin. Investig. 1985, 75, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Mclaughlin, J.; Grazia Luca, M.; Jones, M.N.; Dockray, G.J.; Thompson, D.G. Fatty Acid Chain Length Determines Cholecystokinin Secretion and Effect on Human Gastric Motility. Gastroenterology 1999, 116, 46–53. [Google Scholar] [CrossRef]
- Dockray, G.J. Cholecystokinin and Gut-Brain Signaling. Regul. Pept. 2009, 155, 6–10. [Google Scholar] [CrossRef]
- Raybould, H.E.; Tache, Y. Cholecystokinin Inhibits Gastric Motility and Emptying via a Capsaicin-Sensitive Vagal Pathway in Rats. Am. J. Physiol. Gastrointest. Liver Physiol. 1988, 255, G242–G246. [Google Scholar] [CrossRef]
- Meyer, B.M.; Werth, B.; Beglinger, J.; Hildebrand, P.; Jansen, J.; Zach, D.; Rovati, L.; Stalder, G.A. Role of Cholecystokin in Regulation of Gastrointestinal Motor Functions. Lancet 1989, 2, 12–15. [Google Scholar] [CrossRef]
- Raybould, H.E. Capsaicin-Sensitive Vagal Afferents and CCK in Inhibition of Gastric Motor Function Induced by Intestinal Nutrients. Peptides 1991, 12, 1279–1283. [Google Scholar] [CrossRef]
- Smith, G.P.; Jerome, C.; Cushin, B.J.; Eterno, R.; Simansky, K.J. Abdominal Vagotomy Blocks the Satiety Effect of Cholecystokinin in the Rat. Science 1981, 213, 1036–1037. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.M.; King, A.; Samuelson, L.C.; Kindel, T.L.; Rider, T.; Jandacek, R.J.; Raybould, H.E.; Woods, S.C.; Tso, P. Cholecystokinin Knockout Mice Are Resistant to High-Fat Diet-Induced Obesity. Gastroenterology 2010, 138, 1997–2005. [Google Scholar] [CrossRef]
- Kopin, A.S.; Lee, Y.M.; Mcbride, E.W.; Miller, L.J.; Lu, M.; Lin, H.Y.; Kolakowski, L.F.; Beinborn, M. Expression Cloning and Characterization of the Canine Parietal Cell Gastrin Receptor. Proc. Natl. Acad. Sci. USA 1992, 89, 3605–3609. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.; Moran, T.H.; Coyle, J.T.; Kuhar, M.; O’Donahue, T.; McHugh, P. Anatomic Localization of Cholecystokinin Receptors to the Pyloric Sphincter. Am. J. Physiol. 1984, 246, R127–R130. [Google Scholar] [CrossRef]
- Cheng, C.A.; Geoghegan, J.G.; Lawson, D.C.; Berlangieri, S.U.; Akwari, O.; Pappas, T.N. Central and Peripheral Effects of CCK Receptor Antagonists on Satiety in Dogs. Am. J. Physiol. Gastrointest. Liver Physiol. 1993, 265, G219–G223. [Google Scholar] [CrossRef] [PubMed]
- Mongeau, R.; Marsden, C.A. Effect of Central and Peripheral Administrations of Cholecystokinin—Tetrapeptide on Panic-like Reactions Induced by Stimulation of the Dorsal Periaqueductal Grey Area in the Rat. Biol. Psychiatry 1997, 42, 335–344. [Google Scholar] [CrossRef]
- Lique Sebret, A.; Lé Na, I.; Cré Té, D.; Matsui, T.; Roques, B.P.; Rie Daugé, V. Rat Hippocampal Neurons Are Critically Involved in Physiological Improvement of Memory Processes Induced by Cholecystokinin-B Receptor Stimulation. J. Neurosci. 1999, 19, 7230–7237. [Google Scholar] [CrossRef]
- Pérez de la Mora, M.; Hernández-Gómez, A.M.; Arizmendi-García, Y.; Jacobsen, K.X.; Lara-García, D.; Flores-Gracia, C.; Crespo-Ramírez, M.; Gallegos-Cari, A.; Nuche-Bricaire, A.; Fuxe, K. Role of the Amygdaloid Cholecystokinin (CCK)/Gastrin-2 Receptors and Terminal Networks in the Modulation of Anxiety in the Rat. Effects of CCK-4 and CCK-8S on Anxiety-like Behaviour and [3H]GABA Release. Eur. J. Neurosci. 2007, 26, 3614–3630. [Google Scholar] [CrossRef]
- Moran, T.H.; Robinson, P.H.; Goldrich, M.S.; McHugh, P.R. Two Brain Cholecystokinin Receptors: Implications for Behavioral Actions. Brain Res. 1986, 362, 175–179. [Google Scholar] [CrossRef]
- Hill, D.R.; Campbell, N.J.; Shaw, T.M.; Woodruff, G.N. Autoradiographic Localization and Biochemical Characterization of Peripheral Type CCK Receptors in Rat CNS Using Highly Selective Nonpeptide CCK Antagonists. J. Neurosci. 1987, 7, 2967–2976. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, J.F. Cholecystokinin and the Hormone Concept. Endocr. Connect. 2021, 10, R139–R150. [Google Scholar] [CrossRef]
- Kopin, A.S.; Beinborn, M.; Lee, Y.-M.; McBride, E.W.; Qqinn, S.M. The CCK-B/Gastrin Receptor. Identification of Amino Acids That Determine Nonpeptide Antagonist Affinity. Ann. N.Y. Acad. Sci. 1994, 713, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Kopin, A.S.; Mathes, W.F.; Mcbride, E.W.; Nguyen, M.; Al-haider, W.; Schmitz, F.; Bonner-weir, S.; Kanarek, R.; Beinborn, M. The Cholecystokinin-A Receptor Mediates Inhibition of Food Intake yet Is Not Essential for the Maintenance of Body Weight. J. Clin. Invest. 1999, 103, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.J.; Paulino, G.; Raybould, H.E. CCK1 Receptor Is Essential for Normal Meal Patterning in Mice Fed High Fat Diet. Physiol. Behav. 2007, 92, 969–974. [Google Scholar] [CrossRef]
- Clerc, P.; Constans, M.G.C.; Lulka, H.; Broussaud, S.; Guigné, C.; Leung-Theung-Long, S.; Perrin, C.; Knauf, C.; Carpéné, C.; Pénicaud, L.; et al. Involvement of Cholecystokinin 2 Receptor in Food Intake Regulation: Hyperphagia and Increased Fat Deposition in Cholecystokinin 2 Receptor-Deficient Mice. Endocrinology 2007, 148, 1039–1049. [Google Scholar] [CrossRef]
- Weiland, T.; Voudouris, N.; Kent, S. The Role of CCK2 Receptors in Energy Homeostasis: Insights from the CCK2 Receptor-Deficient Mouse. Physiol. Behav. 2004, 82, 471–476. [Google Scholar] [CrossRef]
- Miyasaka, K.; Ichikawa, M.; Ohta, M.; Kanai, S.; Yoshida, Y.; Masuda, M.; Nagata, A.; Matsui, T.; Noda, T.; Takiguchi, S.; et al. Energy Metabolism and Turnover Are Increased in Mice Lacking the Cholecystokinin-B Receptor. J. Nutr. 2002, 132, 739–741. [Google Scholar] [CrossRef]
- Williams, J.A. Intracellular Signaling Mechanisms Activated by Cholecystokinin- Regulating Synthesis and Secretion of Digestive Enzymes in Pancreatic Acinar Cells. Annu. Rev. Physiol. 2001, 63, 77–97. [Google Scholar] [CrossRef]
- Ahrén, B.; Lundquist, I. Effects of Two Cholecystokinin Variants CCK-39 and CCK-8, on Basal and Stimulated Insulin Secretion. Acta Diabetol. 1981, 18, 345–356. [Google Scholar] [CrossRef]
- Rushakoff, R.J.; Goldfine, I.D.; Carter, J.D.; Liddle, R.A. Physiological Concentrations of Cholecystokinin Stimulate Amino-Acid Induced Insulin Release in Humans. J. Clin. Endocrinol. Metab. 1987, 65, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, L.; Shulman, G.I.; Zawalich, W.S. Physiological Role of Cholecystokinin in Meal-Induced Insulin Secretion in Conscious Rats. Studies with L 364718, a Specific Inhibitor of CCK-Receptor Binding. Diabetes. Diabetes 1987, 36, 1212–1215. [Google Scholar] [CrossRef]
- Ahren, B.; Hedner, P.; Lundquist, I. Interaction of Gastric Inhibitory Polypeptide (GIP) and Cholecystokinin (CCK-8) with Basal and Stimulated Insulin Secretion in Mice. Acta Endocrinol. 1983, 102, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Verspohl, E.J.; Ammon, H.P.T. Cholecystokinin (CCK8) Regulates Glucagon, Insulin, and Somatostatin Secretion from Isolated Rat Pancreatic Islets: Interaction with Glucose. Pflugers Arch. 1987, 410, 284–287. [Google Scholar] [CrossRef]
- Karlsson, S.; Ahrén, B. CCK-8-Stimulated Insulin Secretion in Vivo Is Mediated by CCK A Receptors. Eur. J. Pharmacol. 1992, 213, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Rushakoff, R.A.; Goldfine, I.D.; Beccaria, L.J.; Mathur, A.S.H.W.I.N.I.; Brand, R.J.; Liddle, R.A. Reduced Postprandial Cholecystokinin (CCK) Secretion in Patients with Noninsulin-Dependent Diabetes Mellitus: Evidence for a Role for CCK in Regulating Postprandial Hyperglycemia. J. Clin. Endocrinol. Metab. 1993, 76, 489–493. [Google Scholar]
- Liddle, R.A.; Rushakoff, R.J.; Morita, E.T.; Beccaria, L.; Carter, J.D.; Goldfine, I.D. Physiological Role for Cholecystokinin in Reducing Postprandial Hyperglycemia in Humans. J. Clin. Investig. 1988, 81, 1675–1681. [Google Scholar] [CrossRef]
- Ahrén, B.; Holst, J.J.; Efendic, S. Antidiabetogenic Action of Cholecystokinin-8 in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2000, 85, 1043–1048. [Google Scholar] [CrossRef]
- Yue, C.; Ma, B.; Zhao, Y.; Li, Q.; Li, J. Lipopolysaccharide-Induced Bacterial Translocation Is Intestine Site-Specific and Associates with Intestinal Mucosal Inflammation. Inflammation 2012, 35, 1880–1888. [Google Scholar] [CrossRef]
- Weiland, T.J.; Kent, S.; Voudouris, N.J.; Shulkes, A. The Effect of Lipopolysaccharide on Cholecystokinin in Murine Plasma and Tissue. Peptides 2005, 26, 447–455. [Google Scholar] [CrossRef]
- Saia, R.S.; Ribeiro, A.B.; Giusti, H. Cholecystokinin Modulates the Mucosal Inflammatory Response and Prevents the Lipopolysaccharide-Induced Intestinal Epithelial Barrier Dysfunction. Shock 2020, 53, 242–251. [Google Scholar] [CrossRef]
- Bozkurt, A.; Çakir, B.; Ercan, F.; Yeǧen, B.Ç. Anti-Inflammatory Effects of Leptin and Cholecystokinin on Acetic Acid-Induced Colitis in Rats: Role of Capsaicin-Sensitive Vagal Afferent Fibers. Regul. Pept. 2003, 116, 109–118. [Google Scholar] [CrossRef]
- Ling, Y.-L.; Meng, A.-H.; Zhao, X.-Y.; Shan, B.-E.; Zhang, J.-L.; Zhang, X.-P. Effect of Cholecystokinin on Cytokines during Endotoxic Shock in Rats. World J. Gastroenterol. 2001, 7, 667. [Google Scholar] [CrossRef][Green Version]
- Konturek, S.J.; Brzozowski, T.; Pytko-Polonczyk, J.; Drozdowicz, D. Comparison of Cholecystokinin, Pentagastrin, and Duodenal Oleate in Gastroprotection in Rats. Scand. J. Gastroenterol. 1995, 30, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, T.; Konturek, P.C.; Konturek, S.J.; Pajdo, R.; Drozdowicz, D.; Kwiecien, S.; Hahn, E.G. Acceleration of Ulcer Healing by Cholecystokinin (CCK): Role of CCK-A Receptors, Somatostatin, Nitric Oxide and Sensory Nerves. Regul. Pept. 1999, 82, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D.W.; Klemm, K.; Cross, J.M.; Smith, G.S.; Cashman, M.; Miller, T.A. Cholecystokinin-Induced Protection against Gastric Injury Is Independent of Endogenous Somatostatin. Am. J. Physiol.-Gastrointest. Liver Physiol. 1996, 271, G692–G700. [Google Scholar] [CrossRef]
- Mercer, D.W.; Cross, J.M.; Barreto, J.C.; Strobel, N.H.P.; Russell, D.H.; Miller, T.A. Cholecystokinin Is a Potent Protective Agent against Alcohol-Induced Gastric Injury in the Rat. Dig. Dis. Sci. 1995, 40, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Stroff, T.; Lambrecht, N.; Peskar, B.M. Nitric Oxide as Mediator of the Gastroprotection by Cholecystokinin-8 and Pentagastrin. Eur. J. Pharmacol. 1994, 260, R1–R2. [Google Scholar] [CrossRef]
- Evangelista, S.; Maggi, C.A. Protection Induced by Cholecystokinin-8 (CCK-8) in Ethanol-Induced Gastric Lesions Is Mediated via Vagal Capsaicin-Sensitive Fibres and CCK(A) Receptors. Br. J. Pharmacol. 1991, 102, 119–122. [Google Scholar] [CrossRef]
- Elitsur, Y.; Luk, G.D. The Inhibition Effect of Cholecystokinin in Human Colonic Lamina Propria Lymphocyte Proliferation, and Reversal by the Cholecystokinin Receptor Antagonist L-364718. Neuropeptides 1991, 20, 41–47. [Google Scholar] [CrossRef]
- McMillen, M.; Ferrara, A.; Adrian, T.; Margolis, D.; Schaefer, H.; Zucker, K. Cholecystokinin Effect on Human Lymphocyte Ionized Calcium and Mitogenesis. J. Surg. Res. 1995, 58, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Luyer, M.D.; Greve, J.W.M.; Hadfoune, M.; Jacobs, J.A.; Dejong, C.H.; Buurman, W.A. Nutritional Stimulation of Cholecystokinin Receptors Inhibits Inflammation via the Vagus Nerve. J. Exp. Med. 2005, 202, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Ramanathan, V.; Quante, M.; Baik, G.H.; Yang, X.; Wang, S.S.W.; Tu, S.; Gordon, S.A.K.; Pritchard, D.M.; Varro, A.; et al. Inactivating Cholecystokinin-2 Receptor Inhibits Progastrin-Dependent Colonic Crypt Fission, Proliferation, and Colorectal Cancer in Mice. J. Clin. Investig. 2009, 119, 2691–2701. [Google Scholar] [CrossRef]
- Zhang, J.-G.; Liu, J.-X.; Jia, X.-X.; Geng, J.; Yu, F.; Cong, B. Cholecystokinin Octapeptide Regulates the Differentiation and Effector Cytokine Production of CD4+ T Cells in Vitro. Int. Immunopharmacol. 2014, 20, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ni, Z.; Cong, B.; Gao, W.; Xu, S.; Wang, C.; Yao, Y.; Ma, C.; Ling, Y. CCK-8 Inhibits LPS-Induced IL-1β Production in Pulmonary Interstitial Macrophages by Modulating PKA, P38, and NF-ΚB Pathway. Shock 2007, 27, 678–686. [Google Scholar] [CrossRef]
- Apfelbaum, T.F.; Davidson, N.O.; Glickman, R.M. Apolipoprotein A-IV Synthesis in Rat Intestine: Regulation by Dietary Triglyceride. Am. J. Physiol.-Gastrointest. Liver Physiol. 1987, 252, G662–G666. [Google Scholar] [CrossRef]
- Wang, F.; Kohan, A.B.; Lo, C.M.; Liu, M.; Howles, P.; Tso, P. Apolipoprotein A-IV: A Protein Intimately Involved in Metabolism. J. Lipid Res. 2015, 56, 1403–1418. [Google Scholar] [CrossRef]
- Ghiselli, G.; Krishnan, S.; Beigel, Y.; Gotto, A.M. Plasma Metabolism of Apolipoprotein A-IV in Humans. J. Lipid Res. 1986, 27, 813–827. [Google Scholar] [CrossRef]
- Ghiselli, G.; Crump, W.L.; Musanti, R.; Sherrill, B.C.; Gotto, A.M. Metabolism of Apolipoprotein A-IV in Rat. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1989, 1006, 26–34. [Google Scholar] [CrossRef]
- Kuo, H.C.N.; LaRussa, Z.; Xu, F.M.; West, K.; Consitt, L.; Davidson, W.S.; Liu, M.; Coschigano, K.T.; Shi, H.; Lo, C.C. Apolipoprotein A4 Elevates Sympathetic Activity and Thermogenesis in Male Mice. Nutrients 2023, 15, 2486. [Google Scholar] [CrossRef]
- Lo, C.C.; Langhans, W.; Georgievsky, M.; Arnold, M.; Caldwell, J.L.; Cheng, S.; Liu, M.; Woods, S.C.; Tso, P. Apolipoprotein AIV Requires Cholecystokinin and Vagal Nerves to Suppress Food Intake. Endocrinology 2012, 153, 5857–5865. [Google Scholar] [CrossRef]
- Weinberg, R.B.; Gallagher, J.W.; Fabritius, M.A.; Shelness, G.S. ApoA-IV Modulates the Secretory Trafficking of ApoB and the Size of Triglyceride-Rich Lipoproteins. J. Lipid Res. 2012, 53, 736–743. [Google Scholar] [CrossRef]
- Kohan, A.B.; Wang, F.; Li, X.; Vandersall, A.E.; Huesman, S.; Xu, M.; Yang, Q.; Lou, D.; Tso, P. Is Apolipoprotein A-IV Rate Limiting in the Intestinal Transport and Absorption of Triglyceride? Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G1128–G1135. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Weng, J.; Shen, M.; Fish, J.; Shen, Z.; Coschigano, K.T.; Davidson, W.S.; Tso, P.; Shi, H.; Lo, C.C. Apolipoprotein A-IV Enhances Fatty Acid Uptake by Adipose Tissues of Male Mice via Sympathetic Activation. Endocrinology 2020, 161, bqaa042. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.N.; LaRussa, Z.; Xu, F.M.; Consitt, L.A.; Liu, M.; Davidson, W.S.; Puri, V.; Coschigano, K.T.; Shi, H.; Lo, C.C. Attenuation of High-fat Diet-induced Weight Gain by Apolipoprotein A4. Obesity 2024, 32, 2321–2333. [Google Scholar] [CrossRef]
- Ezeh, B.; Haiman, M.; Alber, H.F.; Kunz, B.; Paulweber, B.; Lingenhel, A.; Kraft, H.G.; Weidinger, F.; Pachinger, O.; Dieplinger, H.; et al. Plasma Distribution of ApoA-IV in Patients with Coronary Artery Disease and Healthy Controls. J. Lipid Res. 2003, 44, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F.; Stühlinger, M.; Trenkwalder, E.; Geethanjali, F.S.; Pachinger, O.; Von Eckardstein, A.; Dieplinger, H. Low Apolipoprotein A-IV Plasma Concentrations in Men with Coronary Artery Disease. J. Am. Coll. Cardiol. 2000, 36, 751–757. [Google Scholar] [CrossRef]
- Wong, W.M.R.; Hawe, E.; Li, L.K.; Miller, G.J.; Nicaud, V.; Pennacchio, L.A.; Humphries, S.E.; Talmud, P.J. Apolipoprotein AIV Gene Variant S347 Is Associated with Increased Risk of Coronary Heart Disease and Lower Plasma Apolipoprotein AIV Levels. Circ. Res. 2003, 92, 969–975. [Google Scholar] [CrossRef]
- LaRussa, Z.; Kuo, H.C.N.; West, K.; Shen, Z.; Wisniewski, K.; Tso, P.; Coschigano, K.T.; Lo, C.C. Increased BAT Thermogenesis in Male Mouse Apolipoprotein A4 Transgenic Mice. Int. J. Mol. Sci. 2023, 24, 4231. [Google Scholar] [CrossRef]
- Cohen, R.D.; Castellani, L.W.; Qiao, J.H.; Van Lenten, B.J.; Lusis, A.J.; Reue, K. Reduced Aortic Lesions and Elevated High Density Lipoprotein Levels in Transgenic Mice Overexpressing Mouse Apolipoprotein A-IV. J. Clin. Investig. 1997, 99, 1906–1916. [Google Scholar] [CrossRef] [PubMed]
- Shearston, K.; Tan, J.T.M.; Cochran, B.J.; Rye, K.A. Inhibition of Vascular Inflammation by Apolipoprotein A-IV. Front. Cardiovasc. Med. 2022, 9, 901408. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, A.; Utermann, G. Activation of Lecithin: Cholesterol Acyltransferase by Human Apolipoprotein A-IV. J. Biol. Chem. 1985, 260, 2258–2264. [Google Scholar] [CrossRef]
- Steinmetz, A.; Barbaras, R.; Ghalim, N.; Clavey, V.; Fruchart, J.C.; Ailhaud, G. Human Apolipoprotein A-IV Binds to Apolipoprotein A-I/A-II Receptor Sites and Promotes Cholesterol Efflux from Adipose Cells. J. Biol. Chem. 1990, 265, 7859–7863. [Google Scholar] [CrossRef]
- Stein, O.; Stein, Y.; Lefevre, M.; Roheim, P.S. The Role of Apolipoprotein A-IV in Reverse Cholesterol Transport Studied with Cultured Cells and Liposomes Derived from an Ether Analog of Phosphatidylcholine. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1986, 878, 7–13. [Google Scholar] [CrossRef]
- Dvorin, E.; Gorder, N.L.; Benson, D.M.; Gotto, A.M. Apolipoprotein A-IV. A Determinant for Binding and Uptake of High Density Lipoproteins by Rat Hepatocytes. J. Biol. Chem. 1986, 261, 15714–15718. [Google Scholar] [CrossRef]
- Emmanuel, F.; Steinmetz, A.; Rosseneu, M.; Brasseur, R.; Gosselet, N.; Attenot, F.; Cuiné, S.; Séguret, S.; Latta, M.; Fruchart, J.C.; et al. Identification of Specific Amphipathic α-Helical Sequence of Human Apolipoprotein A-IV Involved in Lecithin: Cholesterol Acyltransferase Activation. J. Biol. Chem. 1994, 269, 29883–29890. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Liu, X.H.; He, J.; Gao, J.; Zhou, J.T.; Fan, J.N.; Jin, X.; Zhang, J.; Chang, L.; Xiong, Z.; et al. Apolipoprotein A4 Restricts Diet-Induced Hepatic Steatosis via SREBF1-Mediated Lipogenesis and Enhances IRS-PI3K-Akt Signaling. Mol. Nutr. Food Res. 2022, 66, 2101034. [Google Scholar] [CrossRef]
- Recalde, D.; Ostos, M.A.; Badell, E.; Garcia-Otin, A.L.; Pidoux, J.; Castro, G.; Zakin, M.M.; Scott-Algara, D. Human Apolipoprotein A-IV Reduces Secretion of Proinflammatory Cytokines and Atherosclerotic Effects of a Chronic Infection Mimicked by Lipopolysaccharide. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 756–761. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhang, Y.; Chang, L.; Wei, Y.; Huang, N.; Zhou, J.T.; Cheng, C.; Zhang, J.; Xu, J.; Li, Z.; et al. Apolipoprotein A-IV Reduced Metabolic Inflammation in White Adipose Tissue by Inhibiting IKK and JNK Signaling in Adipocytes. Mol. Cell Endocrinol. 2023, 559, 111813. [Google Scholar] [CrossRef]
- Qin, X.; Swertfeger, D.K.; Zheng, S.; Hui, D.Y.; Tso, P. Apolipoprotein AIV: A Potent Endogenous Inhibitor of Lipid Oxidation. Am. J. Physiol. 1998, 274, H1836–H1840. [Google Scholar] [CrossRef]
- Ostos, M.A.; Conconi, M.; Vergnes, L.; Baroukh, N.; Ribalta, J.; Girona, J.; Caillaud, J.-M.; Ochoa, A.; Zakin, M.M. Antioxidative and Antiatherosclerotic Effects of Human Apolipoprotein A-IV in Apolipoprotein E-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1023–1028. [Google Scholar] [CrossRef]
- Peng, J.; Li, X. ping Apolipoprotein A-IV: A Potential Therapeutic Target for Atherosclerosis. Prostaglandins Other Lipid Mediat. 2018, 139, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Zhang, Y.; Cheng, C.; Fan, J.; Zhou, J.; Garstka, M.A.; Li, Z. Hepatoprotective Effect of Apolipoprotein A4 against Carbon Tetrachloride Induced Acute Liver Injury through Mediating Hepatic Antioxidant and Inflammation Response in Mice. Biochem. Biophys. Res. Commun. 2021, 534, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, H.L.; Saijo, F.; Turnage, R.H.; Alexander, J.S.; Aw, T.Y.; Kalogeris, T.J. Apolipoprotein A-IV Attenuates Oxidant-Induced Apoptosis in Mitotic Competent, Undifferentiated Cells by Modulating Intracellular Glutathione Redox Balance. Am. J. Physiol. Cell Physiol. 2006, 290, 95–103. [Google Scholar] [CrossRef]
- Orsó, E.; Moehle, C.; Boettcher, A.; Szakszon, K.; Werner, T.; Langmann, T.; Liebisch, G.; Buechler, C.; Ritter, M.; Kronenberg, F.; et al. The Satiety Factor Apolipoprotein A-IV Modulates Intestinal Epithelial Permeability through Its Interaction with α-Catenin: Implications for Inflammatory Bowel Diseases. Horm. Metab. Res. 2007, 39, 601–611. [Google Scholar] [CrossRef]
- Kim, M.; Lee, S.; Yang, S.; Song, K.; Lee, I. Differential Expression in Histologically Normal Crypts of Ulcerative Colitis Suggests Primary Crypt Disorder. Oncol. Rep. 2006, 16, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Broedl, U.C.; Schachinger, V.; Lingenhel, A.; Lehrke, M.; Stark, R.; Seibold, F.; Göke, B.; Kronenberg, F.; Parhofer, K.G.; Konrad-Zerna, A. Apolipoprotein A-IV Is an Independent Predictor of Disease Activity in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2007, 13, 391–397. [Google Scholar] [CrossRef]
- Kristensen, T.; Moestrup, S.K.; Gliemann, J.; Bendtsen, L.; Sand, O.; Sottrup-Jensen, L. Evidence That the Newly Cloned Low-Density-Lipoprotein Receptor Related Protein (LRP) Is the A2-Macroglobulin Receptor. FEBS Lett. 1990, 276, 151–155. [Google Scholar] [CrossRef]
- Mineo, C. Lipoprotein Receptor Signalling in Atherosclerosis. Cardiovasc. Res. 2021, 116, 1254–1274. [Google Scholar] [CrossRef]
- Hofmann, S.M.; Zhou, L.; Perez-Tilve, D.; Greer, T.; Grant, E.; Wancata, L.; Thomas, A.; Pfluger, P.T.; Basford, J.E.; Gilham, D.; et al. Adipocyte LDL Receptor-Related Protein-1 Expression Modulates Postprandial Lipid Transport and Glucose Homeostasis in Mice. J. Clin. Investig. 2007, 117, 3271–3282. [Google Scholar] [CrossRef] [PubMed]
- Dato, V.A.; Chiabrando, G.A. The Role of Low-Density Lipoprotein Receptor-Related Protein 1 in Lipid Metabolism, Glucose Homeostasis and Inflammation. Int. J. Mol. Sci. 2018, 19, 1780. [Google Scholar] [CrossRef] [PubMed]
- Terrand, J.; Bruban, V.; Zhou, L.; Gong, W.; El Asmar, Z.; May, P.; Zurhove, K.; Haffner, P.; Philippe, C.; Woldt, E.; et al. LRP1 Controls Intracellular Cholesterol Storage and Fatty Acid Synthesis through Modulation of Wnt Signaling. J. Biol. Chemistry 2009, 284, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Rohlmann, A.; Gotthardt, M.; Hammer, R.E.; Herz, J. Inducible Inactivation of Hepatic LRP Gene by Cre-Mediated Recombination Confirms Role of LRP in Clearance of Chylomicron Remnants. J. Clin. Investig. 1998, 101, 689–695. [Google Scholar] [CrossRef]
- Kowal, R.C.; Herz, J.; Goldstein, J.L.; Esser, V.; Brown, M.S. Low Density Lipoprotein Receptor-Related Protein Mediates Uptake of Cholesteryl Esters Derived from Apoprotein E-Enriched Lipoproteins. Proc. Natl. Acad. Sci. USA 1989, 86, 5810–5814. [Google Scholar] [CrossRef]
- Kowal, R.C.; Herz, J.; Weisgraber, K.H.; Mahley, R.W.; Brown, M.S.; Goldstein, J.L. Opposing Effects of Apolipoproteins E and C on Lipoprotein Binding to Low Density Lipoprotein Receptor-Related Protein. J. Biol. Chem. 1990, 265, 10771–10779. [Google Scholar] [CrossRef]
- Ishibashi, S.; Herz, J.; Maedat, N.; Goldstein, J.L.; Brown, M.S. The Two-Receptor Model of Lipoprotein Clearance: Tests of the Hypothesis in “Knockout” Mice Lacking the Low Density Lipoprotein Receptor, Apolipoprotein, E., or Both Proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 4431–4435. [Google Scholar] [CrossRef]
- Linton, M.F.; Hasty, A.H.; Babaev, V.R.; Fazio, S. Hepatic Apo E Expression Is Required for Remnant Lipoprotein Clearance in the Absence of the Low Density Lipoprotein Receptor. J. Clin. Investig. 1998, 101, 1726–1736. [Google Scholar] [CrossRef]
- El Asmar, Z.; Terrand, J.; Jenty, M.; Host, L.; Mlih, M.; Zerr, A.; Justiniano, H.; Matz, R.L.; Boudier, C.; Scholler, E.; et al. Convergent Signaling Pathways Controlled by LRP1 (Receptor-Related Protein 1) Cytoplasmic and Extracellular Domains Limit Cellular Cholesterol Accumulation. J. Biol. Chem. 2016, 291, 5116–5127. [Google Scholar] [CrossRef]
- Muratoglu, S.C.; Belgrave, S.; Lillis, A.P.; Migliorini, M.; Robinson, S.; Smith, E.; Zhang, L.; Strickland, D.K. Macrophage LRP1 Suppresses Neo-Intima Formation during Vascular Remodeling by Modulating the TGF-β Signaling Pathway. PLoS ONE 2011, 6, e28846. [Google Scholar] [CrossRef]
- Overton, C.D.; Yancey, P.G.; Major, A.S.; Linton, M.F.; Fazio, S. Deletion of Macrophage LDL Receptor-Related Protein Increases Atherogenesis in the Mouse. Circ. Res. 2007, 100, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Boucher, P.; Gotthardt, M.; Li, W.-P.; Anderson, R.G.W.; Herz, J. LRP: Role in Vascular Wall Integrity and Protection from Atherosclerosis. Science 2003, 300, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Choi, H.Y.; Li, W.P.; Xu, F.; Herz, J. LRP1 Controls CPLA2 Phosphorylation, ABCA1 Expression and Cellular Cholesterol Export. PLoS ONE 2009, 4, e6853. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Takayama, Y.; Boucher, P.; Tallquist, M.D.; Herz, J. LRP1 Regulates Architecture of the Vascular Wall by Controlling PDGFRβ-Dependent Phosphatidylinositol 3-Kinase Activation. PLoS ONE 2009, 4, e6922. [Google Scholar] [CrossRef]
- Xian, X.; Ding, Y.; Dieckmann, M.; Zhou, L.; Plattner, F.; Liu, M.; Parks, J.S.; Hammer, R.E.; Boucher, P.; Tsai, S.; et al. LRP1 Integrates Murine Macrophage Cholesterol Homeostasis and Inflammatory Responses in Atherosclerosis. eLife 2024, 13, e104437. [Google Scholar] [CrossRef]
- Lillis, A.P.; Muratoglu, S.C.; Au, D.T.; Migliorini, M.; Lee, M.J.; Fried, S.K.; Mikhailenko, I.; Strickland, D.K. LDL Receptor-Related Protein-1 (LRP1) Regulates Cholesterol Accumulation in Macrophages. PLoS ONE 2015, 10, e0128903. [Google Scholar] [CrossRef]
- Yancey, P.G.; Blakemore, J.; Ding, L.; Fan, D.; Overton, C.D.; Zhang, Y.; Linton, M.F.; Fazio, S. Macrophage LRP-1 Controls Plaque Cellularity by Regulating Efferocytosis and Akt Activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 787–795. [Google Scholar] [CrossRef]
- García-Fernández, P.; Üçeyler, N.; Sommer, C. From the Low-Density Lipoprotein Receptor-Related Protein 1 to Neuropathic Pain: A Potentially Novel Target. Pain. Rep. 2021, 6, E898. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.; Zhang, J.; Wang, R.; Cheng, B.; Kalambhe, D.; Wang, Y.; Gu, Z.; Chen, D.; Wang, B.; et al. Lactoferrin-Mediated Macrophage Targeting Delivery and Patchouli Alcohol-Based Therapeutic Strategy for Inflammatory Bowel Diseases. Acta Pharm. Sin. B 2020, 10, 1966–1976. [Google Scholar] [CrossRef]
- Lillis, A.P.; Van Duyn, L.B.; Murphy-Ullrich, J.E.; Strickland, D.K. LDL Receptor-Related Protein 1: Unique Tissue-Specific Functions Revealed by Selective Gene Knockout Studies. Physiol. Rev. 2008, 88, 887–918. [Google Scholar] [CrossRef]
- Gonias, S.L.; Campana, W.M. LDL Receptor-Related Protein-1: A Regulator of Inflammation in Atherosclerosis, Cancer, and Injury to the Nervous System. Am. J. Pathol. 2014, 184, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Shen, H.; Wang, B.; He, Y.; Chen, J.; Ren, J.; Zhang, Z.; Jian, X. LRP1 as a Promising Therapeutic Target for Gastrointestinal Tumors: Inhibiting Proliferation, Invasion and Migration of Cancer Cells. Oncol. Lett. 2023, 26, 432. [Google Scholar] [CrossRef]
- Bain, C.C.; Schridde, A. Origin, Differentiation, and Function of Intestinal Macrophages. Front. Immunol. 2018, 9, 2733. [Google Scholar] [CrossRef] [PubMed]
- Gaultier, A.; Arandjelovic, S.; Niessen, S.; Overton, C.D.; Linton, M.F.; Fazio, S.; Campana, W.M.; Cravatt, B.F.; Gonias, S.L. Regulation of Tumor Necrosis Factor Receptor-1 and the IKK-NF-ΚB Pathway by LDL Receptor-Related Protein Explains the Antiinflammatory Activity of This Receptor. Blood 2008, 111, 5316–5325. [Google Scholar] [CrossRef]
- Na, Y.R.; Stakenborg, M.; Seok, S.H.; Matteoli, G. Macrophages in Intestinal Inflammation and Resolution: A Potential Therapeutic Target in IBD. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.; Ackermann, E.J.; Van Der Geer, P. V-Src Induces Shc Binding to Tyrosine 63 in the Cytoplasmic Domain of the LDL Receptor-Related Protein 1. Oncogene 2003, 22, 3589–3597. [Google Scholar] [CrossRef]
- Gotthardt, M.; Trommsdorff, M.; Nevitt, M.F.; Shelton, J.; Richardson, J.A.; Stockinger, W.; Nimpf, J.; Herz, J. Interactions of the Low Density Lipoprotein Receptor Gene Family with Cytosolic Adaptor and Scaffold Proteins Suggest Diverse Biological Functions in Cellular Communication and Signal Transduction. J. Biol. Chem. 2000, 275, 25616–25624. [Google Scholar] [CrossRef]
- Lutz, C.; Nimpf, J.; Jenny, M.; Boecklinger, K.; Enzinger, C.; Utermann, G.; Baier-Bitterlich, G.; Baier, G. Evidence of Functional Modulation of the MEKK/JNK/CJun Signaling Cascade by the Low Density Lipoprotein Receptor-Related Protein (LRP). J. Biol. Chem. 2002, 277, 43143–43151. [Google Scholar] [CrossRef]
- Mantuano, E.; Brifault, C.; Lam, M.S.; Azmoon, P.; Gilder, A.S.; Gonias, S.L. LDL Receptor-Related Protein-1 Regulates NFκB and MicroRNA-155 in Macrophages to Control the Inflammatory Response. Proc. Natl. Acad. Sci. USA 2016, 113, 1369–1374. [Google Scholar] [CrossRef]
- Qu, J.; Fourman, S.; Fitzgerald, M.; Liu, M.; Nair, S.; Oses-Prieto, J.; Burlingame, A.; Morris, J.H.; Davidson, W.S.; Tso, P.; et al. Low-Density Lipoprotein Receptor-Related Protein 1 (LRP1) Is a Novel Receptor for Apolipoprotein A4 (APOA4) in Adipose Tissue. Sci. Rep. 2021, 11, s41598-s021. [Google Scholar] [CrossRef]
- Rehfeld, J.F. Cholecystokinin-From Local Gut Hormone to Ubiquitous Messenger. Front. Endocrinol. 2017, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.; Bocsik, A.; Katona, G.; Gróf, I.; Deli, M.A.; Csóka, I. Encapsulation in Polymeric Nanoparticles Enhances the Enzymatic Stability and the Permeability of the Glp-1 Analog, Liraglutide, across a Culture Model of Intestinal Permeability. Pharmaceutics 2019, 11, 599. [Google Scholar] [CrossRef]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory Controls and Physiological Roles in Eating and Glycemia in Health, Obesity, and after RYGB. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.; Nauck, M.A.; Van, J.; Benson, C.; Bray, R.; Cui, X.; Milicevic, Z.; Urva, S.; Haupt, A.; Robins, D.A. Efficacy and Tolerability of Tirzepatide, a Dual Glucose-Dependent Insulinotropic Peptide and Glucagon-like Peptide-1 Receptor Agonist in Patients with Type 2 Diabetes: A 12-Week, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate Different Dose-Escalation Regimens. Diabetes Obes. Metab. 2020, 22, 938–946. [Google Scholar] [CrossRef]
- Neary, N.M.; Small, C.J.; Druce, M.R.; Park, A.J.; Ellis, S.M.; Semjonous, N.M.; Dakin, C.L.; Filipsson, K.; Wang, F.; Kent, A.S.; et al. Peptide YY3-36 and Glucagon-like Peptide-17-36 Inhibit Food Intake Additively. Endocrinology 2005, 146, 5120–5127. [Google Scholar] [CrossRef]
- Lo, C.M.; Zhang, D.M.; Pearson, K.; Ma, L.; Sun, W.; Sakai, R.R.; Davidson, W.S.; Liu, M.; Raybould, H.E.; Woods, S.C.; et al. Interaction of Apolipoprotein AIV with Cholecystokinin on the Control of Food Intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1490–R1494. [Google Scholar] [CrossRef] [PubMed]
- Willard, F.S.; Douros, J.D.; Gabe, M.B.N.; Showalter, A.D.; Wainscott, D.B.; Suter, T.M.; Capozzi, M.E.; van der Velden, W.J.C.; Stutsman, C.; Cardona, G.R.; et al. Tirzepatide Is an Imbalanced and Biased Dual GIP and GLP-1 Receptor Agonist. JCI Insight 2020, 5, e140532. [Google Scholar] [CrossRef]
- Thomas, M.K.; Nikooienejad, A.; Bray, R.; Cui, X.; Wilson, J.; Duffin, K.; Milicevic, Z.; Haupt, A.; Robins, D.A. Dual GIP and GLP-1 Receptor Agonist Tirzepatide Improves Beta-Cell Function and Insulin Sensitivity in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2021, 106, 388–396. [Google Scholar] [CrossRef]
- Samms, R.J.; Christe, M.E.; Collins, K.A.L.; Pirro, V.; Droz, B.A.; Holland, A.K.; Friedrich, J.L.; Wojnicki, S.; Konkol, D.L.; Cosgrove, R.; et al. GIPR Agonism Mediates Weight-Independent Insulin Sensitization by Tirzepatide in Obese Mice. J. Clin. Investig. 2021, 131, e146353. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; le Roux, C.W.; Stefanski, A.; Aronne, L.J.; Halpern, B.; Wharton, S.; Wilding, J.P.H.; Perreault, L.; Zhang, S.; Battula, R.; et al. Tirzepatide for Obesity Treatment and Diabetes Prevention. N. Engl. J. Med. 2024, 392, 958–971. [Google Scholar] [CrossRef]
- Karrar, H.R.; Nouh, M.I.; Nouh, Y.I.; Nouh, M.I.; Khan Alhindi, A.S.; Hemeq, Y.H.; Aljameeli, A.M.; Aljuaid, J.A.; Alzahrani, S.J.; Alsatami, A.A.; et al. Tirzepatide-Induced Gastrointestinal Manifestations: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e46091. [Google Scholar] [CrossRef]
- Hammoud, R.; Kaur, K.D.; Koehler, J.A.; Baggio, L.L.; Wong, C.K.; Advani, K.E.; Yusta, B.; Efimova, I.; Gribble, F.M.; Reimann, F.; et al. Glucose-Dependent Insulinotropic Polypeptide Receptor Signaling Alleviates Gut Inflammation in Mice. JCI Insight 2024, 10, e17482. [Google Scholar] [CrossRef]
- Schmidt, J.B.; Gregersen, N.T.; Pedersen, S.D.; Arentoft, J.L.; Ritz, C.; Schwartz, T.W.; Holst, J.J.; Astrup, A.; Sjödin, A.; Schmidt, J.B. Effects of PYY 3-36 and GLP-1 on Energy Intake, Energy Expenditure, and Appetite in Overweight Men. Am. J. Physiol. Endocrinol. Metab. 2014, 306, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.; Salem, V.; Long, C.J.; Makwana, A.; Newbould, R.D.; Rabiner, E.A.; Ghatei, M.A.; Bloom, S.R.; Matthews, P.M.; Beaver, J.D.; et al. The Gut Hormones PYY 3-36 and GLP-1 7-36 Amide Reduce Food Intake and Modulate Brain Activity in Appetite Centers in Humans. Cell Metab. 2011, 14, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Boland, B.B.; Laker, R.C.; O’Brien, S.; Sitaula, S.; Sermadiras, I.; Nielsen, J.C.; Barkholt, P.; Roostalu, U.; Hecksher-Sørensen, J.; Sejthen, S.R.; et al. Peptide-YY3-36/Glucagon-like Peptide-1 Combination Treatment of Obese Diabetic Mice Improves Insulin Sensitivity Associated with Recovered Pancreatic β-Cell Function and Synergistic Activation of Discrete Hypothalamic and Brainstem Neuronal Circuitries. Mol. Metab. 2022, 55, 101392. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.J.; Qin, X.; Chey, W.Y.; Tso, P. PYY Stimulates Synthesis and Secretion of Intestinal Apolipoprotein AIV without Affecting MRNA Expression. Am. J. Physiol. 1998, 275, G668–G674. [Google Scholar] [CrossRef]
- Zhan, J.; Weng, J.; Hunt, B.G.; Sean Davidson, W.; Liu, M.; Lo, C.C. Apolipoprotein A-IV Enhances Cholecystokinin Secretion. Physiol. Behav. 2018, 188, 11–17. [Google Scholar] [CrossRef]
- Kohan, A.B.; Wang, F.; Lo, C.M.; Liu, M.; Tso, P. ApoA-IV: Current and Emerging Roles in Intestinal Lipid Metabolism, Glucose Homeostasis, and Satiety. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G472–G481. [Google Scholar] [CrossRef]
- Weng, J.; Lou, D.; Benoit, S.C.; Coschigano, N.; Woods, S.C.; Tso, P.; Lo, C.C. Energy Homeostasis in Apolipoprotein AIV and Cholecystokinin-Deficient Mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, 535–548. [Google Scholar] [CrossRef]
- Pence, S.; Larussa, Z.; Shen, Z.; Liu, M.; Coschigano, K.T.; Shi, H.; Lo, C.C. Central Apolipoprotein A-Iv Stimulates Thermogenesis in Brown Adipose Tissue. Int. J. Mol. Sci. 2021, 22, 1221. [Google Scholar] [CrossRef]
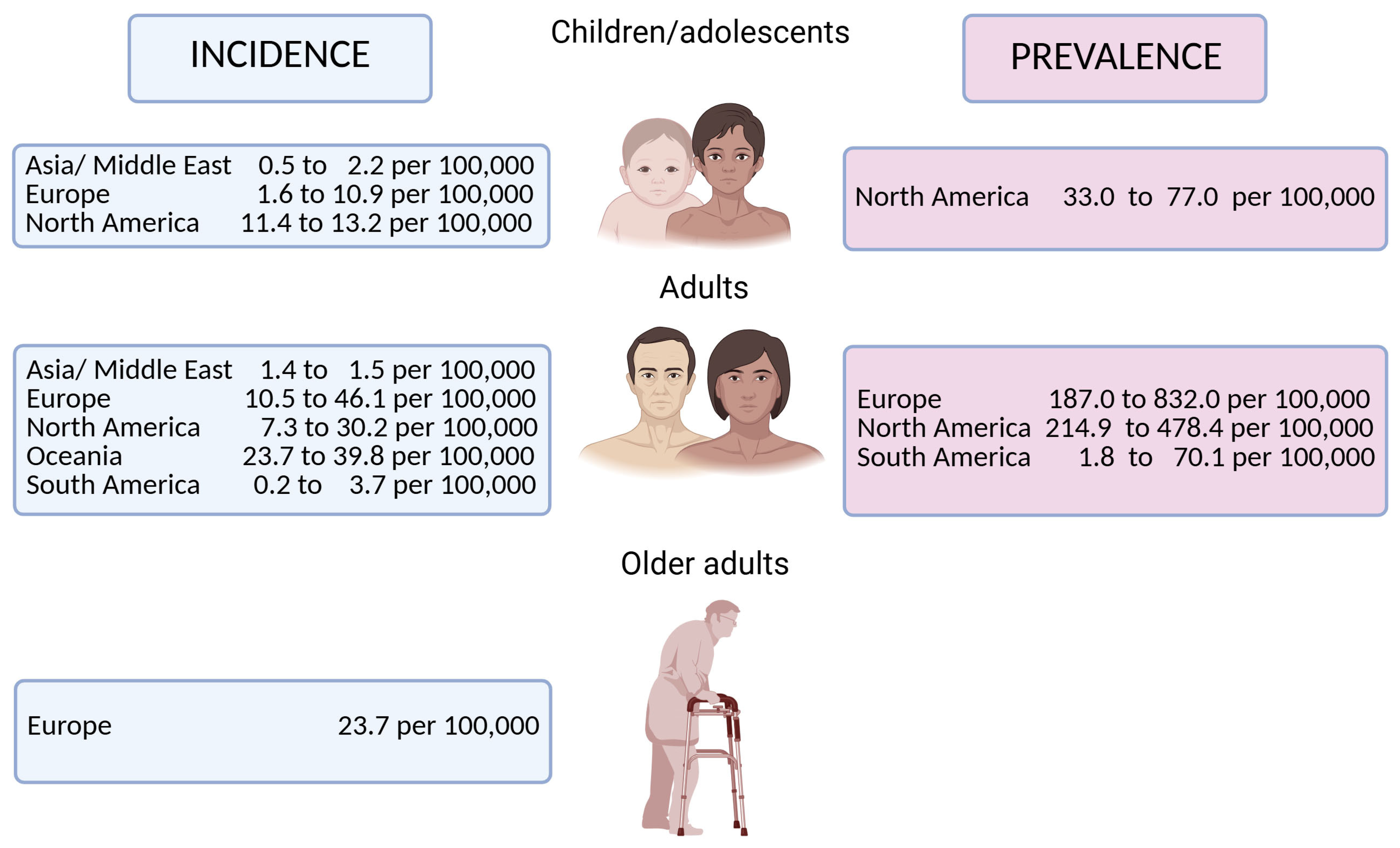
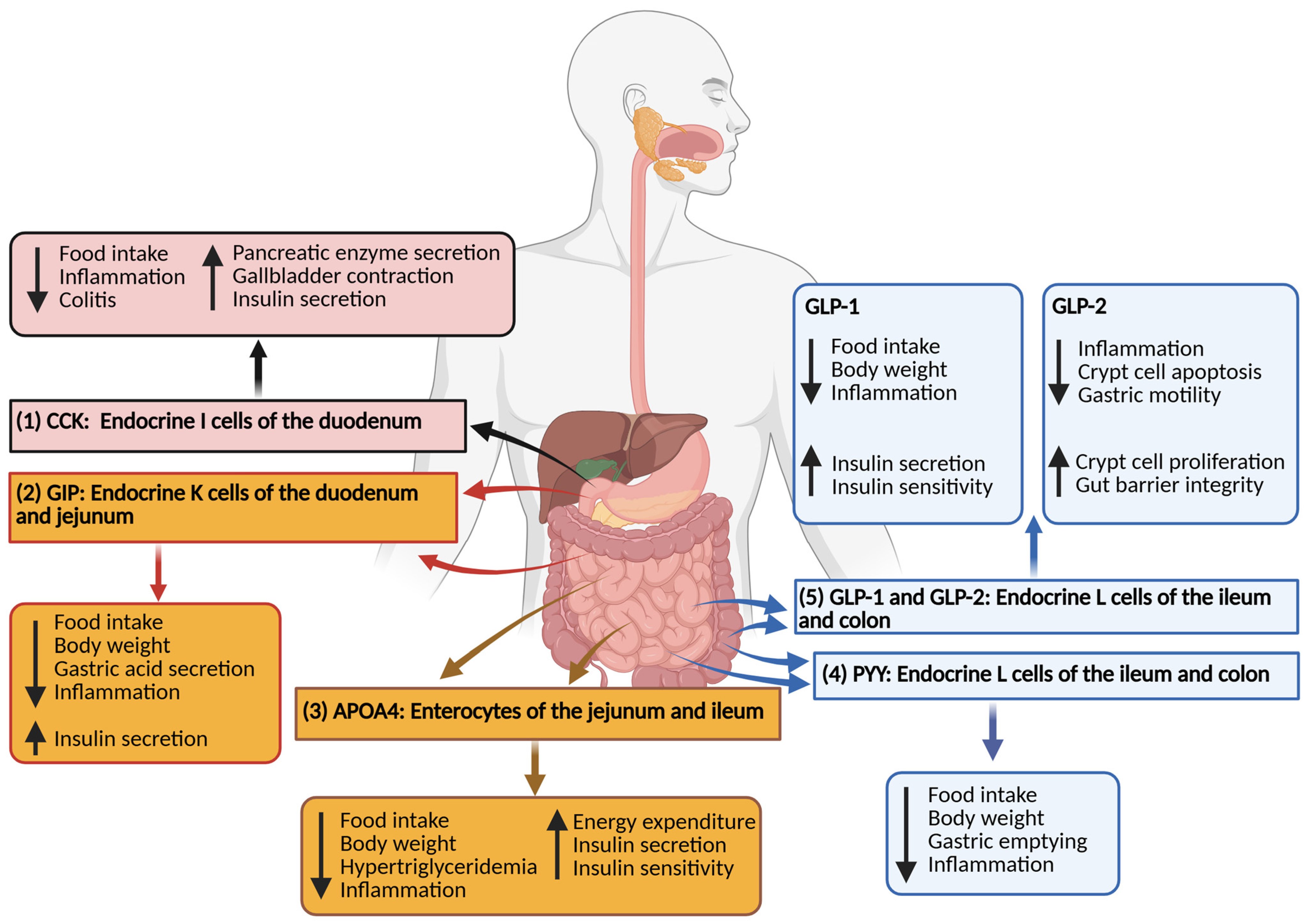
| CD | UC | IBD | |
|---|---|---|---|
| Age, median (IQR) | 40.4 (28.0–62.2) | 56.9 (36.0–70.5) | 48.0 (30.5–67.0) |
| Gender | |||
| Female, n (%) | 737 (46.9) | 649 (52.6) | 1386 (49.4) |
| Male, n (%) | 836 (53.1) | 585 (47.4) | 1421 (50.6) |
| Age at diagnosis, median (IQR) | 36.8 (23.9–58.9) | 52.8 (32.3–66.4) | 44.2 (27.0–63.3) |
| BMI at diagnosis, median (IQR) | 24.3 (21.1–28.0) | 26.0 (22.4–29.6) | 25.0 (22.0–29.0) |
| <18 years, median (IQR) | 20.0 (18.0–23.6) | 20.0 (17.5–24.2) | 20.0 (18.0–24.0) |
| 18–24 years, median (IQR) | 22.0 (20.0–25.1) | 23.0 (21.0–26.5) | 22.3 (20.0-25.9) |
| 25–44 years, median (IQR) | 24.4 (22.0–28.5) | 25.0 (22.0–29.0) | 25.0 (22.0–28.6) |
| 45–64 years, median (IQR) | 26.0 (22.2–30.0) | 27.0 (24.0–30.2) | 26.5 (23.0–30.0) |
| ≥65 years | 25.7 (23.0–29.4) | 27.0 (23.4–30.2) | 26.9 (23.1–30.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, J.; Lo, C.C. Gut Hormones and Inflammatory Bowel Disease. Biomolecules 2025, 15, 1013. https://doi.org/10.3390/biom15071013
Weng J, Lo CC. Gut Hormones and Inflammatory Bowel Disease. Biomolecules. 2025; 15(7):1013. https://doi.org/10.3390/biom15071013
Chicago/Turabian StyleWeng, Jonathan, and Chunmin C. Lo. 2025. "Gut Hormones and Inflammatory Bowel Disease" Biomolecules 15, no. 7: 1013. https://doi.org/10.3390/biom15071013
APA StyleWeng, J., & Lo, C. C. (2025). Gut Hormones and Inflammatory Bowel Disease. Biomolecules, 15(7), 1013. https://doi.org/10.3390/biom15071013







